Abstract
Context
Recent recommendations from the American Heart Association aim to improve cardiovascular health by encouraging the general population to meet 7 cardiovascular health metrics: not smoking; being physically active; having normal blood pressure, blood glucose and total cholesterol levels, and weight; and eating a healthy diet.
Objective
To examine time trends in cardiovascular health metrics and to estimate joint associations and population-attributable fractions of these metrics in relation to all-cause and cardiovascular disease (CVD) mortality risk.
Design, Setting, and Participants
Study of a nationally representative sample of 44 959 US adults (≥20 years), using data from the National Health and Nutrition Examination Survey (NHANES) 1988–1994, 1999–2004, and 2005–2010 and the NHANES III Linked Mortality File (through 2006).
Main Outcome Measures
All-cause, CVD, and ischemic heart disease (IHD) mortality.
Results
Few participants met all 7 cardiovascular health metrics (2.0% [95% CI, 1.5%–2.5%] in 1988–1994, 1.2% [95% CI, 0.8%–1.9%] in 2005–2010). Among NHANES III participants, 2673 all-cause, 1085 CVD, and 576 IHD deaths occurred (median follow-up, 14.5 years). Among participants who met 1 or fewer cardiovascular health metrics, age- and sex-standardized absolute risks were 14.8 (95% CI, 13.2–16.5) deaths per 1000 person-years for all-cause mortality, 6.5 (95% CI, 5.5–7.6) for CVD mortality, and 3.7 (95% CI, 2.8–4.5) for IHD mortality. Among those who met 6 or more metrics, corresponding risks were 5.4 (95% CI, 3.6–7.3) for all-cause mortality, 1.5 (95% CI, 0.5–2.5) for CVD mortality, and 1.1 (95% CI, 0.7–2.0) for IHD mortality. Adjusted hazard ratios were 0.49 (95% CI, 0.33–0.74) for all-cause mortality, 0.24 (95% CI, 0.13–0.47) for CVD mortality, and 0.30 (95% CI, 0.13–0.68) for IHD mortality, comparing participants who met 6 or more vs 1 or fewer cardiovascular health metrics. Adjusted population-attributable fractions were 59% (95% CI, 33%–76%) for all-cause mortality, 64% (95% CI, 28%–84%) for CVD mortality, and 63% (95% CI, 5%–89%) for IHD mortality.
Conclusion
Meeting a greater number of cardiovascular health metrics was associated with a lower risk of total and CVD mortality, but the prevalence of meeting all 7 cardiovascular health metrics was low in the study population.
Cardiovascular disease (CVD) is the leading cause of deaths in the United States (>800 000, or about 1 in 3 overall deaths/y),1 with estimated annual direct and overall costs of $273 billion and $444 billion, respectively.2 Previous epidemiologic studies indicate that individuals who meet an increased number of cardiovascular health behaviors or factors (such as not smoking and achieving normal blood pressure, blood glucose level, and weight) have a significantly reduced risk of CVD incidence and mortality.3–9 Achieving and maintaining cardiovascular health behaviors and factors in individuals and communities could have significant effects on reducing CVD incidence and mortality at the population level.
Based on evidence from randomized clinical trials and epidemiologic studies, the American Heart Association (AHA) recently published recommendations aimed at improving cardiovascular health and reducing CVD mortality in the United States by encouraging the general population to meet 7 defined ideal cardiovascular health behaviors or factors, for the purpose of this study called cardiovascular health metrics (ie, not smoking; being physically active; having normal blood pressure, blood glucose and total cholesterol levels, and weight; and eating a healthy diet).10 Several studies have examined the prevalence and trends of CVD risk factors1,11 and some also reported the prevalence of cardiovascular health metrics,3,12,13 but none have examined time trends in all 7 AHA cardiovascular health metrics and their joint associations with CVD mortality using nationally representative data.
In this study, we examined the time trends of these 7 cardiovascular health metrics among 44 959 persons 20 years or older and estimated the joint associations and population-attributable fractions (PAFs) of these cardiovascular health metrics in relation to risk of all-cause and CVD mortality.
METHODS
National Health and Nutrition Examination Survey
The National Health and Nutrition Examination Survey (NHANES) comprises a series of cross-sectional, national, stratified, multistage probability surveys of the civilian, noninstitutionalized US population. Each survey participant completed a household interview and underwent a physical examination. NHANES III was conducted in 1988–1994; beginning in 1999, the survey became a continuous program, with every 2 years representing 1 cycle.
For trends in cardiovascular health metrics, we used data from NHANES III (1988–1994) (n = 16 215), 1999–2004 (n = 13 097), and 2005–2010 (n=15 647). All nonpregnant participants 20 years or older with available data on cardiovascular health metrics were included in trends analyses for each specific metric, whereas participants with complete information on all 7 cardiovascular health metrics were included for distribution of health metrics.
Detailed descriptions of the plan and operation of each survey have been published.14,15 NHANES III and NHANES 1999–2010 received institutional review board approval and required provision of written informed consent.
NHANES III (1988–1994) Linked Mortality File (2006)
To examine the association and PAFs of cardiovascular health metrics in relation to risk of all-cause and CVD mortality, we used data from the NHANES III Linked Mortality File, in which NHANES III–eligible participants were matched, using a probabilistic matching algorithm, to the National Death Index through December 31, 2006, to determine their mortality status.
International Classification of Diseases, Tenth Revision codes were used to identify participants for whom CVD (codes I00-I78) or ischemic heart disease (IHD) (codes I20-I25) were listed as the underlying cause of death. Follow-up of participants continued until death attributable to CVD, with censoring at the time of death for those who died from causes other than CVD. Participants not matched with a death record were considered alive through the entire follow-up period. A complete, detailed description of the methodology is available.16
Definition of Cardiovascular Health Metrics
The AHA definition of ideal, intermediate, and poor cardiovascular health metrics for adults and the modified definitions used in our trends and association analyses are presented in eTable 1, available at http://www.jama.com. Because duration of the physical activity was not ascertained in NHANES III, each physical activity was assigned an intensity value (metabolic equivalent tasks [METs]) that represents the ratio of the energy expenditure of the activity to the basal metabolic rate.17,18 We classified the participants as physically active (ideal category) if they engaged in any physical activity with 3 to 5.9 METs and 5 or more times per week or any physical activity with 6 or more METs and 3.0 or more times per week.18,19 The questionnaires for physical activity were consistent from NHANES 1999 through 2006 with duration (minutes) for each activity but changed substantially in the 2007–2008 cycle; thus, we restricted our trend analysis for physical activity using data from NHANES 1999–2004 and NHANES 2005–2006.
The healthy diet score from the AHA ranges from 0 to 5 and is calculated by summing the following components, assigning 1 point each for the consumption of fruits and vegetables (≥4.5 cups/d), fish (≥two 3.5-oz servings/wk), fiber-rich whole grains (≥three 1-oz–equivalent servings/d), sodium (<1500 mg/d), and sugar-sweetened beverages (≤36 oz/wk).10 We estimated the healthy diet score based on the food frequency questionnaire (FFQ) for all the dietary elements, with the exception of sodium, for which we used the National Cancer Institute methods to estimate the usual intakes of sodium.20 The FFQ was administrated in NHANES III, NHANES 2003–2004, and NHANES 2005–2006 cycles only. For trend and association analyses, we dichotomized the score as fewer than 2 vs 2 or more components, owing to the paucity of participants with a score of 4 or greater (<1.0%).
Fasting plasma glucose values were available for a subsample of NHANES participants (NHANES III, n = 6939; NHANES 1999–2004, n = 5635; and NHANES 2005–2010, n = 6679). For trend analysis, we presented the prevalence of fasting plasma glucose levels but used glycated hemoglobin (HbA1c) values less than 5.7% as a proxy for fasting glucose levels less than 100 mg/dL (to convert to mmol/L, multiply by 0.0555) for the distribution of 7 cardiovascular health metrics and the association study, as suggested by American Diabetes Association.21
The AHA definition of ideal category of smoking status included former smokers who had not smoked for 12 months or longer. Time since smoking cessation was not assessed in NHANES III. We used the categories of “never,” “former,” and “current” smoking across surveys for comparability. However, because the results from NHANES 1999–2004 and 2005–2010 indicated that only about 3% of former smokers had quit for less than 12 months,1 we combined “never” and “former” smoking as a single group in the association study.
We constructed a cardiovascular health metrics score (number of cardiovascular health metrics) by recoding the 7 metrics as dichotomous variables using 1 point for the AHA ideal category vs 0 points for the other categories, with the exceptions of the healthy diet score (<2 components [0 points] vs ≥2 [1 point]) and smoking (never and former [1 point] vs current smoking [0 points]) and calculating scores as (never and former smoking [1 point] vs current smoking [0 points]) + (ideal physical activity [1 point] vs others [0 points]) + (body mass index [BMI, calculated as weight in kilograms divided by height in meters squared] <25 [1 point] vs others [0 points]) + (healthy diet score ≥2 [1 point] vs <2 components [0 points]) + (total cholesterol <200 mg/dL [to convert to mmol/L, multiply by 0.0259] untreated [1 point] vs others [0 points]) + (blood pressure <120/<80 mm Hg untreated [1 point] vs others [0 points]) + (HbA1c <5.7% [1 point] vs others [0 points]). All participants were classified as meeting 0, 1, 2, 3, 4, 5, 6, or 7 cardiovascular health metrics. For this score, we excluded participants missing data on 1 or more of the cardiovascular health metrics. These dichotomized variables were used in the association study and calculation of PAFs.
Other covariates in our association analyses were race/ethnicity, education, alcohol consumption, and family history of CVD (yes/no). Race/ethnicity was classified as non-Hispanic white, non-Hispanic black, Mexican American, or other. Educational attainment was classified as less than 12 years, 12 to 15 years, or more than 15 years of formal education. Alcohol consumption was classified as 0, fewer than 3, or 3 or more drinks per week.
Statistical Analysis
We estimated the weighted means and percentages of the selected covariates for NHANES 1988–1994, 1999–2004, and 2005–2010. We calculated the age-standardized and weighted prevalence and 95% CIs of the cardiovascular health metrics and the cardiovascular health metrics score. We used logistic regression to test for linear trends in changes in cardiovascular health metrics by including a time variable corresponding to the approximate midpoint of the surveys and adjusted for age, sex, and race/ethnicity.
For the association analysis, we used Cox proportional hazards regression to estimate the hazard ratios (HRs) and 95% CIs for all-cause, CVD, and IHD mortality associated with each cardiovascular health metric as well as health metrics score as a single categorical variable. For the joint HRs, we combined the participants with 0 or 1 cardiovascular health metric as the reference group, because of the paucity of participants who met none of the defined health metrics. We also combined participants with 6 or 7 cardiovascular health metrics into a single group for a stable estimate. We presented age-, sex-, and race/ethnicity–adjusted HRs as well as fully adjusted HRs. The multivariable-adjusted HRs included age, sex, race/ethnicity, educational attainment, alcohol consumption, family history of CVD, and 7 cardiovascular health metrics. A P value for trends across the HRs for the categories of the health metrics score was calculated using a Satterthwaite adjusted F test.22
We calculated adjusted PAFs to provide an estimate of the proportion of all-cause and CVD mortality in this cohort that hypothetically would have been avoided or postponed, assuming a causal relationship, during the follow-up if all participants met all 7 cardiovascular health metrics.23 All-cause and CVD mortality rates (per 1000 person-years), adjusted for age and sex, were calculated by the number of cardiovascular health metrics using the direct method of standardization, with age and sex distribution of the whole cohort as the standard. The number of person-years of follow-up was calculated from the time of entry into the study until date of death or the termination date of the study. We also calculated Kaplan-Meier survival curves for cumulative all-cause, CVD, and IHD mortality by the cardiovascular health metrics score categories and log-rank tests for difference in cumulative mortality across cardiovascular health metrics score categories.
We conducted several sensitivity analyses. First, we conducted stratified analyses of cardiovascular health metrics and all-cause and CVD mortality by age (<60 vs ≥60 years), sex, race/ethnicity, and educational attainment (<12 vs ≥12 years). We tested for interactions of cardiovascular health metrics with these covariates by including respective interactions terms in the Cox models based on Satterthwaite-adjusted tests.22 Second, because BMI and total cholesterol levels showed a U-shaped relationship with all-cause and CVD mortality in this cohort,24,25 we used BMI less than 30 vs 30 or greater and total cholesterol levels less than 240 mg/dL vs 240 mg/dL or greater in the sensitivity analyses. Third, we estimated the associations of cardiovascular health metrics in relation to the risk of cancer mortality (eTables 2–8)
The proportional hazards assumption of the Cox models was evaluated with Schoenfeld residuals, which revealed no significant departures from proportionality in hazards over time (P > .05).26 Data were analyzed using SAS release 9.2 and SUDAAN release 10, taking into account the complex sampling design.22 All tests were 2-sided, and P < .05 was considered statistically significant.
RESULTS
Demographic characteristics of the study participants are reported in Table 1. As shown in Table 2, the prevalence of current smoking declined from 27.9% (95% CI, 26.4%–29.3%) in NHANES III (1988–1994) to 22.6% (95% CI, 21.3%–24.1%) in 2005–2010 (P < .001 for trend). The prevalence of adults with desirable total cholesterol levels (<200 mg/dL) and blood pressure (<120 mm Hg/<80 mm Hg) remained largely unchanged. However, the prevalence of consuming a healthy diet (≥2 healthy diet score components), having a BMI less than 25, and having a fasting glucose level less than 100 mg/dL declined significantly from 1988–1994 to 2005–2010 (P < .05).
Table 1.
Characteristics of US Adults—NHANES 1988–1994, 1999–2004, and 2005–2010
| Characteristic | NHANES 1988–1994a
|
NHANES 1999–2004a
|
NHANES 2005–2010a
|
|||
|---|---|---|---|---|---|---|
| No. | Prevalence, % (95% CI) | No. | Prevalence, % (95% CI) | No. | Prevalence, % (95% CI) | |
| Age, mean (95% CI), y | 16 215 | 45.0 (44.0–45.9) | 13 097 | 45.6 (45.1–46.2) | 15 647 | 46.8 (46.1–47.4) |
|
| ||||||
| Sex | ||||||
| Men | 7750 | 48.2 (47.4–49.0) | 6598 | 48.8 (48.1–49.6) | 7816 | 49.0 (48.3–49.6) |
|
| ||||||
| Women | 8465 | 51.8 (51.0–52.6) | 6499 | 51.2 (50.4–51.9) | 7831 | 51.1 (50.4–51.7) |
|
| ||||||
| Race/ethnicity | ||||||
| Non-Hispanic white | 6697 | 76.9 (74.4–9.2) | 6567 | 72.0 (68.7–75.1) | 7631 | 69.9 (66.2–73.4) |
|
| ||||||
| Non-Hispanic black | 4517 | 10.7 (9.6–11.9) | 2530 | 10.6 (8.8–12.7) | 3117 | 11.1 (9.4–13.0) |
|
| ||||||
| Mexican American | 4348 | 4.8 (4.1–5.6) | 2954 | 6.9 (5.5–8.7) | 2844 | 8.4 (6.7–10.4) |
|
| ||||||
| Other | 653 | 7.7 (6.2–9.4) | 1046 | 10.5 (8.2–13.3) | 2055 | 10.7 (9.0–12.6) |
Abbreviation: NHANES, National Health and Nutrition Examination Survey.
Age-standardized for the entire population by the direct method to the US 2000 Census population using the age groups 20–29, 30–39, 40–49, 50–59, 60–69, 70–79, and 80 years or older.
Table 2.
Age-Standardized and Weighted Prevalence of Meeting Cardiovascular Health Metrics in Adults—NHANES 1988–1994, 1999–2004, and 2005–2010
| Cardiovascular Health Metricc | NHANES 1988–1994a
|
NHANES 1999–2004a
|
NHANES 2005–2010a
|
P Value for Trendsb | |||
|---|---|---|---|---|---|---|---|
| No. | Prevalence, % (95% CI) | No. | Prevalence, % (95% CI) | No. | Prevalence, % (95% CI) | ||
| Smoking status | |||||||
| Never | 7932 | 45.3 (43.8–46.8) | 6604 | 50.0 (48.3–51.7) | 8232 | 53.2 (51.5–55.0) | <.001 |
|
| |||||||
| Former | 4041 | 26.8 (25.8–27.9) | 3486 | 25.0 (23.9–26.1) | 3891 | 24.1 (23.0–25.3) | .008 |
|
| |||||||
| Current | 4241 | 27.9 (26.4–29.3) | 2986 | 25.0 (23.7–26.4) | 3515 | 22.6 (21.3–24.1) | <.001 |
|
| |||||||
| Physical activityd | |||||||
| Ideal | 5932 | 41.1 (38.8–43.3) | 4679 | 42.6 (40.2–44.7) | 1694 | 45.2 (42.4–47.8) | .17 |
|
| |||||||
| Intermediate | 6624 | 43.4 (41.8–45.0) | 2436 | 21.6 (20.4–22.8) | 911 | 22.9 (21.5–24.6) | .51 |
|
| |||||||
| None | 3625 | 15.6 (14.2–17.1) | 5389 | 35.8 (34.5–37.5) | 1565 | 31.9 (29.1–35.1) | .02 |
|
| |||||||
| BMIe | |||||||
| <25.0 | 6413 | 44.1 (42.5–45.7) | 4122 | 35.3 (33.9–36.6) | 4541 | 32.5 (31.2–33.8) | <.001 |
|
| |||||||
| 25.0–29.9 | 5653 | 33.1 (32.0–34.3) | 4563 | 34.6 (33.3–35.9) | 5283 | 33.5 (32.5–34.5) | .41 |
|
| |||||||
| ≥30.0 | 4117 | 22.8 (21.5–24.1) | 3986 | 30.2 (28.7–31.6) | 5610 | 34.1 (32.7–35.4) | <.001 |
|
| |||||||
| Healthy diet scoref | |||||||
| ≥2 components | 4932 | 33.1 (31.6–34.6) | 709 | 19.2 (17.0–21.6) | 637 | 22.3 (19.5–25.4) | <.001 |
|
| |||||||
| <2 components | 11 283 | 66.9 (65.4–68.4) | 2413 | 80.8 (78.5–83.0) | 2148 | 77.7 (74.6–80.5) | <.001 |
|
| |||||||
| Total serum cholesterol, mg/dL | |||||||
| <200 (untreated)g | 7281 | 46.8 (45.2–48.4) | 5428 | 45.5 (44.3–46.6) | 6571 | 46.0 (44.9–47.0) | .20 |
|
| |||||||
| 200–239 or treated to goal | 5000 | 32.8 (31.4–34.2) | 4894 | 37.7 (36.7–38.8) | 6229 | 39.9 (38.9–40.9) | <.001 |
|
| |||||||
| ≥240 | 3167 | 20.5 (19.3–21.7) | 2101 | 16.8 (15.9–17.8) | 2115 | 14.2 (13.3–15.0) | <.001 |
|
| |||||||
| Blood pressure, mm Hg | |||||||
| <120/80 (untreated)g | 6170 | 42.4 (41.1–43.7) | 4322 | 40.1 (38.4–41.8) | 5675 | 42.8 (41.4–44.3) | .09 |
|
| |||||||
| 120–139/80–89 mm Hg or treated to goal | 6015 | 37.7 (36.7–38.8) | 5050 | 40.3 (39.3–41.4) | 6454 | 41.7 (40.4–42.9) | <.001 |
|
| |||||||
| ≥140/≥90 mm Hg | 4000 | 19.9 (18.9–20.9) | 3204 | 19.6 (18.4–20.9) | 2956 | 15.5 (14.8–6.2) | .04 |
|
| |||||||
| Fasting blood glucose, mg/dLh | |||||||
| <100 | 4352 | 67.3 (65.1–69.4) | 3408 | 65.3 (63.1–67.4) | 3572 | 59.5 (57.6–61.3) | <.001 |
|
| |||||||
| 100–<126 | 2038 | 27.1 (25.2–29.1) | 1729 | 27.8 (25.8–29.9) | 2380 | 33.3 (31.6–35.0) | <.001 |
|
| |||||||
| ≥126 | 549 | 5.7 (4.9–6.5) | 498 | 6.9 (6.1–7.9) | 727 | 7.3 (6.6–8.0) | .92 |
|
| |||||||
| No. of cardiovascular health metricsi | |||||||
| 0 | 166 | 0.8 (0.59–1.2) | 46 | 1.5 (1.2–1.9) | 54 | 1.4 (1.0–2.0) | .009 |
|
| |||||||
| 1 | 1188 | 6.4 (5.8–7.1) | 269 | 8.0 (6.7–9.4) | 280 | 7.3 (6.6–8.2) | .02 |
|
| |||||||
| 2 | 2953 | 16.1 (15.1–17.1) | 616 | 20.1 (18.0–22.5) | 595 | 18.0 (16.1–20.0) | .02 |
|
| |||||||
| 3 | 3943 | 24.8 (23.9–25.8) | 715 | 24.3 (22.6–26.1) | 771 | 25.5 (22.9–28.4) | .06 |
|
| |||||||
| 4 | 3584 | 23.9 (22.9–25.0) | 618 | 23.7 (22.4–25.0) | 642 | 22.4 (21.5–23.3) | .15 |
|
| |||||||
| 5 | 2371 | 17.6 (16.5–18.7) | 342 | 15.1 (13.3–17.0) | 419 | 16.6 (14.6–18.7) | .01 |
|
| |||||||
| 6 | 934 | 8.3 (7.5–9.3) | 128 | 6.1 (5.1–7.3) | 168 | 7.5 (6.2–9.1) | .14 |
|
| |||||||
| 7 | 166 | 2.0 (1.5–2.5) | 31 | 1.3 (0.87–1.9) | 26 | 1.2 (0.8–1.9) | .32 |
|
| |||||||
| 0–1 | 1354 | 7.2 (6.5–8.0) | 315 | 9.4 (8.1–10.9) | 334 | 8.8 (8.0–9.6) | .001 |
|
| |||||||
| ≥6 | 1100 | 10.3 (9.4–11.3) | 159 | 7.4 (6.3–8.6) | 194 | 8.8 (7.3–10.6) | .12 |
Abbreviations: BMI, body mass index; NHANES, National Health and Nutrition Examination Survey.
SI conversion factors: To convert total cholesterol values to mmol/L, multiply by 0.0259; to convert glucose values to mmol/L, multiply by 0.0555.
See Table 1, footnote “a.”
For trends across the surveys using logistic regression model adjusted for age, sex, and race/ethnicity; all tests 2-tailed.
All nonpregnant participants 20 years or older with available cardiovascular health metrics were included in trends analyses for each specific metric; therefore, sample sizes might vary by the cardiovascular health metrics.
Ideal: 150 minutes per week or more at moderate intensity or 75 minutes per week or more at vigorous intensity or 150 minutes per week or more at moderate + vigorous intensity. Intermediate: 1 to 149 minutes per week at moderate intensity or 1 to 74 minutes per week at vigorous intensity or 1 to 149 minutes per week at moderate + vigorous intensity. Duration (time) of the physical activity was not ascertained in NHANES III; see “Methods” for details of classification of physical activity. P value for trends compared NHANES 1999–2004 and NHANES 2005–2006.
Calculated as weight in kilograms divided by height in meters squared.
See “Methods” for details of calculation of healthy diet score. Trends may be attributed to change in questionnaires from NHANES III to NHANES 1999–2004 and NHANES 2005–2010.
Untreated value.
Fasting glucose level was available for the subsample of participants in NHANES III, NHANES 1999–2004, and NHANES 2005–2010.
See “Methods” for details of calculation of cardiovascular health metrics scores.
Few participants (<2%) met all 7 cardiovascular health metrics. The prevalence of meeting 6 or more cardiovascular health metrics was 10.3% (95% CI, 9.4%–11.3%) in 1988–1994 and 8.8% (95% CI, 7.3%–10.6%) in 2005–2010, and the prevalence of meeting 1 or fewer cardiovascular health metrics increased from 7.2% (95% CI, 6.5%–8.0%) to 8.8% (95% CI, 8.0%–9.6%) (P < .05 for trend).
Of the nonpregnant adults 20 years or older with complete data on all 7 cardiovascular health metrics and complete mortality follow-up information (n=15 305), we sequentially excluded 1262 participants who reported a history of myocardial infarction, stroke, or congestive heart failure, 465 with diagnosed cancer, and 266 with BMI less than 18.5 at baseline. After these exclusions, 13 312 NHANES III participants were available for the association study. There were 2673 total, 1085 CVD, and 576 IHD deaths over 182 352 person-years of follow-up (median follow-up, 14.5 years). Younger participants, women, non-Hispanic whites, and those with higher education levels tended to meet a greater number of cardiovascular health metrics (eTable 9).
After multivariable adjustment, not smoking, being physically active, having normal blood pressure, and having an HbA1c level less than 5.7% were independently associated with a significantly lower risk of all-cause mortality (Table 3). A similar pattern was observed for CVD mortality (Table 4). Not smoking, eating a healthy diet (≥2 healthy diet score components), and having an HbA1c level less than 5.7% were independently associated with a significantly reduced rate of IHD mortality (Table 5).
Table 3.
Adjusted Hazard Ratio and Population-Attributable Faction of Cardiovascular Health Metrics and Risk of All-Cause Mortality—NHANES III Linked Mortality File
| Cardiovascular Health Metrica | Cases/Participants | HR (95% CI)
|
Adjusted PAF (95% CI)c | |
|---|---|---|---|---|
| Age-, Sex-, and Race/Ethnicity–Adjusted | Fully Adjustedb | |||
| Current smoking | ||||
| Yes | 691/3542 | 1 [Reference] | 1 [Reference] | |
|
| ||||
| No | 1982/9770 | 0.46 (0.40–0.52) | 0.49 (0.42–0.57) | 24.5 (17.9–30.9) |
|
| ||||
| Physical activity | ||||
| No | 1722/8316 | 1 [Reference] | 1 [Reference] | |
|
| ||||
| Yes | 951/4996 | 0.79 (0.74–0.86) | 0.85 (0.77–0.93) | 10.9 (3.0–18.7) |
|
| ||||
| BMId | ||||
| ≥25 | 1678/8160 | 1 [Reference] | 1 [Reference] | |
|
| ||||
| <25 | 995/5152 | 1.04 (0.93–1.16) | 1.04 (0.93–1.17) | NA |
|
| ||||
| Healthy diet score | ||||
| <2 components | 1919/10 245 | 1 [Reference] | 1 [Reference] | |
|
| ||||
| ≥2 components | 754/3067 | 0.83 (0.73–0.95) | 0.94 (0.83–1.07) | 1.8 (0.0–15.2) |
|
| ||||
| Total serum cholesterol, mg/dL | ||||
| ≥200 | 1669/6831 | 1 [Reference] | 1 [Reference] | |
|
| ||||
| <200 (untreated) | 1004/6481 | 1.23 (1.11–1.37) | 1.28 (1.15–1.42) | NA |
|
| ||||
| Blood pressure, mm Hg | ||||
| ≥120/≥80 | 2297/7947 | 1 [Reference] | 1 [Reference] | |
|
| ||||
| <120/<80 (untreated) | 376/5365 | 0.79 (0.66–0.94) | 0.81 (0.68–0.95) | 30.4 (19.4–40.6) |
|
| ||||
| HbA1c, %e | ||||
| ≥5.7 | 1144/3233 | 1 [Reference] | 1 [Reference] | |
|
| ||||
| <5.7 | 1529/10 079 | 0.69 (0.63–0.75) | 0.73 (0.67–0.80) | 10.5 (6.2–14.7) |
Abbreviations: BMI, body mass index; CVD, cardiovascular disease; HbA1c, glycated hemoglobin; HR, hazard ratio; IHD, ischemic heart disease; NA, not available; NHANES, National Health and Nutrition Examination Survey; PAF, population-attributable fraction.
SI conversion factors: To convert total cholesterol values to mmol/L, multiply by 0.0259.
Each cardiovascular health metric was dichotomized by the ideal category of American Heart Association definitions, with the exceptions of the healthy diet score and smoking: (never and former smoking vs current smoking); (ideal physical activity vs others) + (BMI <25 vs others) + (healthy diet score ≥2 vs <2 components) + (total cholesterol <200 mg/dL untreated vs others) + (blood pressure <120/<80 mm Hg untreated vs others) + (HbA1c <5.7% vs others).
Adjusted for age, sex, race/ethnicity, educational attainment, alcohol intake, family history of CVD, smoking status, physical activity, BMI, healthy diet score, total cholesterol level, blood pressure, and HbA1cvalue.
Adjusted for age, sex, race/ethnicity, educational attainment, alcohol intake, family history of CVD, smoking status, physical activity, BMI, healthy diet score, total cholesterol level, blood pressure, and HbA1c value. Sum of individual PAFs can sum to more than the overall PAF (Table 3), because some individuals with more than 1 risk factor could have cardiovascular disease deaths postponed in more than 1 way, and the postponed deaths of these individuals could be counted more than once. The individual PAFs cannot be calculated for the cardiovascular health metrics with adjusted HRs of 1.0 or greater (eg, BMI or total cholesterol level), and the negative values of the lower 95% CI of the PAF were rounded to zero.
Calculated as weight in kilograms divided by height in meters squared.
An HbA1c value less than 5.7% was used as a proxy for a fasting glucose level less than 100 mg/dL.
Table 4.
Adjusted Hazard Ratio and Population-Attributable Faction of Cardiovascular Health Metrics and Risk of CVD Mortality—NHANES III Linked Mortality File
| Cardiovascular Health Metrica | Cases/Participants | HR (95% CI)
|
Adjusted PAF (95% CI)c | |
|---|---|---|---|---|
| Age-, Sex-, and Race/Ethnicity–Adjusted | Fully Adjustedb | |||
| Current smoking | ||||
| Yes | 229/3542 | 1 [Reference] | 1 [Reference] | |
|
| ||||
| No | 856/9770 | 0.46 (0.38–0.57) | 0.50 (0.39–0.64) | 13.7 (4.8–22.3) |
|
| ||||
| Physical activity | ||||
| No | 718/8316 | 1 [Reference] | 1 [Reference] | |
|
| ||||
| Yes | 367/4996 | 0.74 (0.63–0.87) | 0.77 (0.65–0.92) | 11.9 (1.3–22.3) |
|
| ||||
| BMId | ||||
| ≥25 | 694/8160 | 1 [Reference] | 1 [Reference] | |
|
| ||||
| <25 | 391/5152 | 1.00 (0.85–1.18) | 1.05 (0.88–1.25) | NA |
|
| ||||
| Healthy diet score | ||||
| <2 components | 763/10 245 | 1 [Reference] | 1 [Reference] | |
|
| ||||
| ≥2 components | 322/3067 | 0.74 (0.60–0.90) | 0.82 (0.67–1.02) | 13.2 (3.5–29.2) |
|
| ||||
| Total serum cholesterol, mg/dL | ||||
| ≥200 | 357/6481 | 1 [Reference] | 1 [Reference] | |
|
| ||||
| <200 (untreated) | 728/6831 | 0.98 (0.84–1.15) | 1.02 (0.87–1.21) | NA |
|
| ||||
| Blood pressure, mm Hg | ||||
| ≥120/≥80 | 981/7947 | 1 [Reference] | 1 [Reference] | |
|
| ||||
| <120/<80 (untreated) | 104/5365 | 0.61 (0.45–0.83) | 0.64 (0.47–0.86) | 40.6 (24.5–54.6) |
|
| ||||
| HbA1c, % e | ||||
| ≥5.7 | 497/3233 | 1 [Reference] | 1 [Reference] | |
|
| ||||
| <5.7 | 588/10 079 | 0.66 (0.54–0.80) | 0.71 (0.58–0.86) | 8.8 (2.1–15.4) |
Abbreviations: BMI, body mass index; CVD, cardiovascular disease; HbA1c, glycated hemoglobin; HR, hazard ratio; IHD, ischemic heart disease; NA, not available; NHANES, National Health and Nutrition Examination Survey; PAF, population-attributable fraction.
SI conversion factors: To convert total cholesterol values to mmol/L, multiply by 0.0259.
See Table 3, footnote “a.”
See Table 3, footnote “b.”
See Table 3, footnote “c.”
Calculated as weight in kilograms divided by height in meters squared.
An HbA1c value less than 5.7% was used as a proxy for a fasting glucose level less than 100 mg/dL.
Table 5.
Adjusted Hazard Ratio and Population-Attributable Fraction of Cardiovascular Health Metrics and Risk of IHD Mortality—NHANES III Linked Mortality File
| Cardiovascular Health Metrica | Cases/Participants | HR (95% CI)
|
Adjusted PAF (95% CI)c | |
|---|---|---|---|---|
| Age-, Sex-, and Race/Ethnicity–Adjusted | Fully Adjustedb | |||
| Current smoking | ||||
| Yes | 120/3542 | 1 [Reference] | 1 [Reference] | |
|
| ||||
| No | 456/9770 | 0.42 (0.32–0.56) | 0.48 (0.35–0.66) | 16.7 (6.4–26.6) |
|
| ||||
| Physical activity | ||||
| No | 377/8316 | 1 [Reference] | 1 [Reference] | |
|
| ||||
| Yes | 199/4996 | 0.76 (0.61–0.94) | 0.83 (0.65–1.06) | 7.8 (0.0–22.2) |
|
| ||||
| BMId | ||||
| ≥25 | 353/8160 | 1 [Reference] | 1 [Reference] | |
|
| ||||
| <25 | 223/5152 | 1.11 (0.87–1.42) | 1.16 (0.90–1.49) | NA |
|
| ||||
| Healthy diet score | ||||
| <2 components | 412/10 245 | 1 [Reference] | 1 [Reference] | |
|
| ||||
| ≥2 components | 164/3067 | 0.65 (0.48–0.87) | 0.74 (0.55–0.98) | 20.6 (1.2–38.6) |
|
| ||||
| Total serum cholesterol, mg/dL | ||||
| ≥200 | 391/6481 | 1 [Reference] | 1 [Reference] | |
|
| ||||
| <200 (untreated) | 185/6831 | 0.98 (0.78–1.22) | 1.00 (0.79–1.30) | NA |
|
| ||||
| Blood pressure, mm Hg | ||||
| ≥120/≥80 | 518/7947 | 1 [Reference] | 1 [Reference] | |
|
| ||||
| <120/<80 (untreated) | 58/5365 | 0.66 (0.38–1.12) | 0.67 (0.39–1.13) | 34.7 (6.6–57.7) |
|
| ||||
| HbA1c, % e | ||||
| ≥5.7 | 262/3233 | 1 [Reference] | 1 [Reference] | |
|
| ||||
| <5.7 | 314/10 079 | 0.66 (0.51–0.85) | 0.71 (0.55–0.90) | 7.5 (3.0–14.7) |
Abbreviations: BMI, body mass index; CVD, cardiovascular disease; HbA1c, glycated hemoglobin; HR, hazard ratio; IHD, ischemic heart disease; NA, not available; NHANES, National Health and Nutrition Examination Survey; PAF, population-attributable fraction.
SI conversion factors: To convert total cholesterol values to mmol/L, multiply by 0.0259.
See Table 3, footnote “a.”
See Table 3, footnote “b.”
See Table 3, footnote “c.”
Calculated as weight in kilograms divided by height in meters squared.
An HbA1c value less than 5.7% was used as a proxy for a fasting glucose level less than 100 mg/dL.
Adjusted PAFs for all-cause mortality ranged from 10.5% (95% CI, 6.2%–14.7%) for HbA1c level of 5.7% or greater to 30.4% (95% CI, 19.4%–40.6%) for elevated blood pressure (Table 3); PAFs for CVD mortality ranged from 8.8% (95% CI, 2.1%–15.4%) for HbA1c level of 5.7% or greater to 40.6% (95% CI, 24.5%–54.6%) for elevated blood pressure (Table 4); PAFs for IHD mortality ranged from 7.5% (95% CI, 3.0%–14.7%) for HbA1c level of 5.7% or greater to 34.7% (95% CI, 6.6%–57.7%) for elevated blood pressure (Table 5).
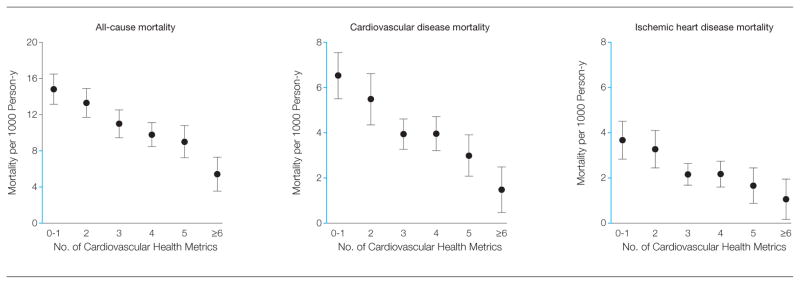
Among participants who met 1 or fewer cardiovascular health metrics, age- and sex-standardized absolute risks were 14.8 (95% CI, 13.2–16.5) deaths per 1000 person-years for all-cause mortality, 6.5 (95% CI, 5.5–7.6) for CVD mortality, and 3.7 (95% CI, 2.8–4.5) for IHD mortality. Among participants who met 6 or more cardiovascular health metrics, corresponding risks were 5.4 (95% CI, 3.6–7.3) deaths per 1000 person-years for all-cause mortality, 1.5 (95% CI, 0.5–2.5) for CVD mortality, and 1.1 (95% CI, 0.7–2.0) for IHD mortality (P < .001 for linear trends) (Figure 1).
Figure 1.
Age- and Sex-Standardized Mortality Rates per 1000 Person-Years of All-Cause, CVD, and IHD Mortality, by Number of Cardiovascular Health Metrics—NHANES III Linked Mortality File
Error bars indicate 95% CIs. Y-axis segments shown in blue indicate range from 0 to 8. CVD indicates cardiovascular disease; IHD, ischemic heart disease; NHANES, National Health and Nutrition Examination Survey.
Comparing participants with 6 or more vs 1 or fewer cardiovascular health metrics, the adjusted HR was 0.49 (95% CI, 0.33–0.74) for all-cause mortality, 0.24 (95% CI, 0.13–0.47) for CVD mortality, and 0.30 (95% CI, 0.13–0.68) for IHD mortality. Meeting a greater number of cardiovascular health metrics was associated with a significantly lower risk of all-cause, CVD, and IHD mortality (P < .001 for linear trends) (Table 6). Adjusted PAFs were 58.6% (95% CI, 33.2%–76.1%) for all-cause mortality, 63.9% (95% CI, 28.0%–84.1%) for CVD mortality, and 62.9% (95% CI, 5.3%–89.1%) for IHD mortality.
Table 6.
Adjusted Hazard Ratios of All-Cause and CVD Mortality by Cardiovascular Health Metrics—NHANES III Linked Mortality File
| Characteristic | No. of Cardiovascular Health Metricsb
|
P Value for Trendsc | Adjusted PAF (95% CI)d | |||||
|---|---|---|---|---|---|---|---|---|
| 0–1 | 2 | 3 | 4 | 5 | ≥6 | |||
| All-cause mortality | ||||||||
| All deaths | 405 | 723 | 760 | 490 | 226 | 69 | ||
|
| ||||||||
| No. of participants | 1236 | 2608 | 3370 | 3081 | 2060 | 957 | ||
|
| ||||||||
| Total person-years | 15 894 | 34 666 | 45 515 | 43 030 | 29 397 | 13 851 | ||
|
| ||||||||
| Age-, sex-, and race/ethnicity–adjusted HR | 1 [Reference] | 0.79 (0.67–0.94) | 0.69 (0.58–0.81) | 0.59 (0.51–0.69) | 0.57 (0.44–0.73) | 0.41 (0.28–0.61) | <.001 | |
|
| ||||||||
| Fully adjusted HRa | 1 [Reference] | 0.81 (0.68–0.97) | 0.71 (0.60–0.84) | 0.63 (0.54–0.74) | 0.64 (0.49–0.82) | 0.49 (0.33–0.74) | <.001 | 58.6 (33.2–76.1) |
|
| ||||||||
| CVD mortality | ||||||||
| CVD deaths | 183 | 303 | 300 | 201 | 77 | 21 | ||
|
| ||||||||
| No. of participants | 1236 | 2608 | 3370 | 3081 | 2060 | 957 | ||
|
| ||||||||
| Total person-years | 15 894 | 34 666 | 45 515 | 43 030 | 29 397 | 13 851 | ||
|
| ||||||||
| Age-, sex-, and race/ethnicity–adjusted HR | 1 [Reference] | 0.72 (0.58–0.89) | 0.55 (0.43–0.69) | 0.50 (0.40–0.62) | 0.41 (0.29–0.60) | 0.22 (0.11–0.41) | <.001 | |
|
| ||||||||
| Fully adjusted HRa | 1 [Reference] | 0.73 (0.59–0.91) | 0.56 (0.44–0.71) | 0.52 (0.41–0.66) | 0.44 (0.30–0.65) | 0.24 (0.13–0.47) | <.001 | 63.9 (28.0–84.1) |
|
| ||||||||
| IHD mortality | ||||||||
| IHD deaths | 94 | 163 | 158 | 108 | 40 | 13 | ||
|
| ||||||||
| No. of participants | 1236 | 2608 | 3370 | 3081 | 2060 | 957 | ||
|
| ||||||||
| Total person-years | 15 894 | 34 666 | 45 515 | 43 030 | 29 397 | 13 851 | ||
|
| ||||||||
| Age-, sex-, and race/ethnicity–adjusted HR | 1 [Reference] | 0.72 (0.54–0.98) | 0.52 (0.39–0.70) | 0.48 (0.35–0.67) | 0.39 (0.23–0.64) | 0.26 (0.11–0.58) | <.001 | |
|
| ||||||||
| Fully adjusted HRa | 1 [Reference] | 0.74 (0.55–1.00) | 0.54 (0.40–0.72) | 0.51 (0.37–0.72) | 0.43 (0.26–0.72) | 0.30 (0.13–0.68) | <.001 | 62.9 (5.3–89.1) |
Abbreviations: CVD, cardiovascular disease; HR, hazard ratio; IHD, ischemic heart disease; NHANES, National Health and Nutrition Examination Survey; PAF, population-attributable fraction.
Adjusted for age, sex, race/ethnicity, educational attainment, alcohol intake, family history of CVD, smoking status, physical activity, body mass index, health diet score, total cholesterol level, blood pressure, and glycated hemoglobin level.
See “Methods” for details of calculation of cardiovascular health metrics scores.
For trends across the categories of cardiovascular health metrics scores based on Satterthwaite adjusted F test; all tests 2-tailed.
Adjusted for age, sex, race/ethnicity, educational attainment, alcohol intake, and family history of CVD and cardiovascular health metrics.
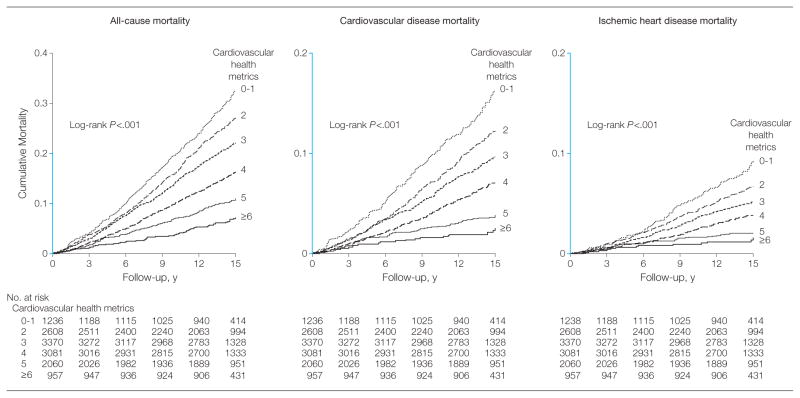
Figure 2 shows the Kaplan-Meier survival curves for cumulative all-cause, CVD, and IHD mortality among participants meeting 0–1, 2, 3, 4, 5, and 6 or more cardiovascular health metrics (P < .001 for all log-rank tests). The patterns of the reduced risk for all-cause, CVD, or IHD mortality, in association with meeting greater numbers of cardiovascular health metrics, remained largely consistent across subgroups defined by age, sex, race/ethnicity, and educational attainments (eTables 2–5). The significant interaction between cardiovascular health metrics and age group (<60 vs ≥60 years, P < .02) on CVD mortality suggested that meeting cardiovascular health metrics might offer greater protection against premature CVD deaths among younger participants (eTable 2). However, the absolute numbers of CVD deaths that would be avoided or postponed by meeting a greater number of cardiovascular health metrics would be substantially larger among older participants because of much higher baseline risk in the older population.
Figure 2.
Kaplan-Meier Curves for Cumulative All-Cause, CVD, and IHD Mortality, by Number of Cardiovascular Health Metrics—NHANES III Linked Mortality File
Y-axis segments shown in blue indicate range from 0 to 0.2. CVD indicates cardiovascular disease; IHD, ischemic heart disease; NHANES, National Health and Nutrition Examination Survey.
COMMENT
Using a series of nationally representative samples of the adult population, our results indicated that prevalence of current smoking continued to decline since 1988. However, the desirable level of untreated blood pressure (<120/<80 mm Hg) and total cholesterol level (<200 mg/dL) remained unchanged, and the prevalence of desirable levels of BMI (<25) and fasting glucose (<100 mg/dL) continued to decline for the study period. The prevalence of meeting all 7 cardiovascular health metrics was low.
During a median of 14.5 years of follow-up in the NHANES III Linked Mortality File cohort, participants who met 6 or more vs 1 or fewer cardiovascular health metrics had a 51% lower risk of all-cause mortality, a 76% lower risk of CVD mortality, and a 70% lower risk of IHD mortality. In addition, meeting a greater number of cardiovascular health metrics also appeared to be associated with lower risk for all-cancer mortality (eTable 6).
Elevated blood pressure, including prehypertension and hypertension, was associated with the largest adjusted PAFs for all-cause and CVD deaths in this cohort (30.4% and 40.6%, respectively). Hypertension affected approximately 68 million individuals in the United States in 2009.27 Studies suggest that for every 10% increase in hypertension treatment, an estimated 14 000 deaths would be prevented annually.28 Although the awareness, treatment, and management of hypertension are extremely important in prevention of CVD incidence and mortality, our results indicated that the desirable level of blood pressure (<120/<80 mm Hg) among the adult population remained unchanged since 1988. In addition to high sodium intake,29 overweight or obesity, lack of physical activity, high alcohol intake, and poor diet are other important modifiable risk factors for elevated blood pressure and hypertension, supporting the importance of primordial prevention of elevated blood pressure through behavioral and policy changes.30
Smoking was associated with the second-largest adjusted PAF for all-cause mortality and significantly contributed to the CVD mortality in our study. Although the prevalence of smoking among US adult populations declined over the last 40 years,31,32 about 1 in 5 US adults (23%) were current cigarette smokers in 2005–2010. Every 5% increase in smoking cessation would prevent 7000 deaths annually.28 Continuing to implement population-based prevention strategies, operationalize tobacco control policies, and promote clinical cessation interventions would further reduce the prevalence of smoking and improve cardiovascular health in the population at large.
Many observational studies provide strong evidence to support the benefits of healthy diets and physical activity on CVD incidence and mortality.33–35 Both diet and physical activity affect the presence and severity of CVD risk factors.4,36–43 Despite the recommendations and well-known benefits of eating fruits and vegetables daily and engaging in regular physical activity, only about 1 in 5 adults met the AHA intermediate level of healthy diet (≥2 healthy diet score components), less than 1% met the ideal level (≥4 components), and there was no evidence of increased prevalence of healthy diet over time. Although the prevalence of physical activity has increased slightly since the late 1980s,44–46 the majority of US adults are not physically active at levels that can promote health. Additionally, significant racial/ethnic disparities in physical activity levels have been observed.44–47 Federal, state, and local public health agencies should continue to implement evidence-based, culturally appropriate public education and policy initiatives to further increase physical activity levels and promote healthy diets among all adults in the United States.
The prevalence of BMI less than 25 and fasting glucose levels less than 100 mg/dL continues to decline significantly, whereas the prevalence of obesity and diabetes continues to increase among US adults, although the increase in obesity rates has slowed since mid-2000.48–52 It is estimated that 36% of US adults are obese, 8.3% have diabetes, and more than 50% of persons with diagnosed diabetes are also obese.51–55 The high prevalence of obesity and the increasing prevalence of diabetes could offset the positive effects of the decline in other CVD risk factors56,57 and continue to slow improvements in cardiovascular health. To address these competing influences, public health intervention programs aimed at improving lifestyle choices, eg, increasing physical activity and promoting healthy eating, should be made more widely available. For example, translating the Diabetes Prevention Program to community-based settings has shown promising results in improving diet and physical activity, reducing body weight, and improving cardiovascular health in general.58,59
Body mass index and total cholesterol level showed a U-shaped association with CVD mortality in this cohort.24,25 In our sensitivity analyses using BMI less than 30 vs 30 or greater and total cholesterol level less than 240 mg/dL vs 240 mg/dL or greater, these categories of BMI and total cholesterol level were associated with lower risk of CVD mortality (eTable 7), and adjusted HRs were 0.35 (95% CI, 0.26–0.46) for all-cause mortality, 0.21 (95% CI, 0.13–0.33) for CVD mortality, and 0.19 (95% CI, 0.10–0.34) for IHD mortality, comparing participants with 6 or more vs 1 or fewer cardiovascular health metrics (eTable 8). However, the prevalence of desirable total cholesterol level (<200 mg/dL) remained unchanged since 1988. Apart from actively managing cholesterol levels with medications, comprehensive primary prevention strategies promoting a healthy diet and lifestyle to increase the prevalence of desirable total cholesterol levels will improve the cardiovascular health status of the general population.
The major strengths of our study include the use of data from a series of nationally representative samples of US adults for trend analyses of cardiovascular health metrics and from a nationally representative cohort of US adults with a long duration of follow-up (median, 14.5 years) for mortality study; the availability of 7 cardiovascular health metrics measurements or appropriate proxy measurements; detailed data on potential confounders for CVD; and the estimation of adjusted PAFs for 7 cardiovascular risk factors.
Our study has several limitations. First, the NHANES III Linked Mortality File included only the baseline measurements of cardiovascular health metrics. We were not able to quantify the effects of changes in these factors over the life course on all-cause and CVD mortality. Second, the survey questionnaire about physical activity was consistent from NHANES 1999–2004 and 2005–2006 regarding duration (minutes) for each activity. However, NHANES III included no information on duration, and we thus used METs and number of times per week to approximate the AHA definition.
Third, the FFQ in NHANES III used “past month” as the reference period, but the FFQ in NHANES 2003–2004 and 2005–2006 used “previous 12 months.” The interpretation of trends should take into account this difference. Fourth, the analyses of the effects of some modifiable risk factors, especially obesity and mortality, are subject to confounding by smoking and reverse causality because of preexisting conditions, which might have led to an underestimate of these effects on mortality.60 In the multivariate analyses, the association for the healthy diet score was also underestimated because the effects of a healthy diet are partly mediated through intermediate cardiovascular risk factors such as blood pressure and levels of cholesterol and glucose.
Fifth, we used an HbA1c level less than 5.7% to approximate a fasting glucose level less than 100 mg/dL, as suggested by the American Diabetes Association.21 This might have resulted in underestimation of the prevalence of prediabetes. Sixth, PAFs derived from an observational study could lead to biased estimates of the effects of changing CVD risk factors on deaths avoided or postponed; a well-designed randomized clinical trial would have been ideal but not feasible. In addition, it is unlikely that in reality all participants would change to meet the ideal or desirable cardiovascular health metrics; the generalized impact fraction might be a more practical evaluation of the effects of changes in risk factors.61
Seventh, the NHANES III Linked Mortality File identified causes of death through the National Death Index; the index is based on death certificates, which are subject to errors in classification of the cause of death. Eighth, our study examined the association of cardiovascular health metrics with CVD mortality but not incidence. However, the pattern of associations observed in our study was consistent with that for CVD incidence.3
Healthy People 2020 and the AHA’s national strategy to reduce CVD morbidity and mortality by 20% by 2020 through promoting ideal cardiovascular health metrics represents a great challenge but also an achievable goal.10 Coordinated efforts, such as the recently announced Million Hearts initiative, align CVD prevention and control activities across the public and private sectors, creating opportunities to reduce the burden of CVD across a large segment of the population.62–64
In summary, our findings indicate that the presence of a greater number of cardiovascular health metrics was associated with a graded and significantly lower risk of total and CVD mortality. However, the prevalence of meeting all 7 cardiovascular health metrics was low in the adult population.
Supplementary Material
Footnotes
Author Contributions: Drs Yang and Zhang had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Yang, Hu.
Analysis and interpretation of the data: Yang, Cogswell, Flanders, Hong, Zhang, Loustalot, Gillespie, Merritt, Hu.
Drafting of the manuscript: Yang.
Critical revision of the manuscript for important intellectual content: Yang, Cogswell, Flanders, Hong, Zhang, Loustalot, Gillespie, Merritt, Hu.
Statistical expertise: Yang, Flanders.
Study supervision: Yang, Hu.
Conflict of Interest Disclosures: The authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest and none were reported.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Online-Only Material: eTables 1–9 are available at http://www.jama.com.
Additional Contributions: We thank Ellen Hertzmark, MS, and Donna Spiegelman, PhD (Department of Epidemiology, Harvard School of Public Health), for their help with the calculation of the adjusted population-attributable fraction and preparation of the Kaplan-Meier curves of cumulative mortality. We thank Paula Yoon, ScD (Division of Community Preventive Services), and Edward Gregg, PhD (Division of Diabetes Translation, Centers for Disease Control and Prevention [CDC]), for their helpful comments. We thank Tiebin Liu, MS (National Center on Birth Defects and Developmental Disabilities, CDC), for help with statistical analysis. None of the persons acknowledged received any compensation.
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation. 2012;125(1):e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heidenreich PA, Trogdon JG, Khavjou OA, et al. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation. 2011;123(8):933–944. doi: 10.1161/CIR.0b013e31820a55f5. [DOI] [PubMed] [Google Scholar]
- 3.Folsom AR, Yatsuya H, Nettleton JA, et al. Community prevalence of ideal cardiovascular health, by the American Heart Association definition, and relationship with cardiovascular disease incidence. J Am Coll Cardiol. 2011;57(16):1690–1696. doi: 10.1016/j.jacc.2010.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stampfer MJ, Hu FB, Manson JE, et al. Primary prevention of coronary heart disease in women through diet and lifestyle. N Engl J Med. 2000;343(1):16–22. doi: 10.1056/NEJM200007063430103. [DOI] [PubMed] [Google Scholar]
- 5.Stamler J, Stamler R, Neaton JD, et al. Low risk-factor profile and long-term cardiovascular and non-cardiovascular mortality and life expectancy. JAMA. 1999;282(21):2012–2018. doi: 10.1001/jama.282.21.2012. [DOI] [PubMed] [Google Scholar]
- 6.Daviglus ML, Stamler J, Pirzada A, et al. Favorable cardiovascular risk profile in young women and long-term risk of cardiovascular and all-cause mortality. JAMA. 2004;292(13):1588–1592. doi: 10.1001/jama.292.13.1588. [DOI] [PubMed] [Google Scholar]
- 7.Ford ES, Zhao G, Tsai J, Li C. Low-risk lifestyle behaviors and all-cause mortality. Am J Public Health. 2011;101(10):1922–1929. doi: 10.2105/AJPH.2011.300167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berry JD, Dyer A, Cai X, et al. Lifetime risks of cardiovascular disease. N Engl J Med. 2012;366(4):321–329. doi: 10.1056/NEJMoa1012848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ford ES, Greenlund KJ, Hong Y. Ideal cardiovascular health and mortality from all causes and diseases of the circulatory system among adults in the United States. Circulation. doi: 10.1161/CIRCULATIONAHA.111.049122. published online January 30, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lloyd-Jones DM, Hong Y, Labarthe D, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction. Circulation. 2010;121(4):586–613. doi: 10.1161/CIRCULATIONAHA.109.192703. [DOI] [PubMed] [Google Scholar]
- 11.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics—2011 update: a report from the American Heart Association. Circulation. 2011;123(4):e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ford ES, Li C, Zhao G, Pearson WS, Capewell S. Trends in the prevalence of low risk factor burden for cardiovascular disease among United States adults. Circulation. 2009;120(13):1181–1188. doi: 10.1161/CIRCULATIONAHA.108.835728. [DOI] [PubMed] [Google Scholar]
- 13.Shay CM, Ning H, Allen NB, et al. Status of cardiovascular health in US adults. Circulation. 2012;125(1):45–56. doi: 10.1161/CIRCULATIONAHA.111.035733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Center for Health Statistics, Centers for Disease Control and Prevention. The Third National Health and Nutrition Examination Survey (NHANES III, 1988–94) Reference Manuals and Reports: Plan and Operation of the Third National Health and Nutrition Examination Survey, 1988–94; Weighting and Estimation Methodology. Hyattsville, MD: US Dept of Health and Human Services; 1998. [Google Scholar]
- 15.National Center for Health Statistics. National Health and Nutrition Examination Survey: questionnaires, datasets, and related documentation. Centers for Disease Control and Prevention website. [Accessed January 8, 2012]. http://www.cdc.gov/nchs/nhanes/nhanes_questionnaires.htm .
- 16.The Third National Health and Nutrition Examination Survey (NHANES III) Linked Mortality File, mortality follow-up through 2006: matching methodology. Centers for Disease Control and Prevention website. 2009. [Accessed March 1, 2012]. http://www.cdc.gov/nchs/data/datalinkage/matching_methodology_nhanes3_final.pdf .
- 17.Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32(9 suppl):S498–S504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 18.Ainsworth BE, Haskell WL, Herrmann SD, et al. 2011 Compendium of Physical Activities: a second update of codes and MET values. Med Sci Sports Exerc. 2011;43(8):1575–1581. doi: 10.1249/MSS.0b013e31821ece12. [DOI] [PubMed] [Google Scholar]
- 19.Beddhu S, Baird BC, Zitterkoph J, et al. Physical activity and mortality in chronic kidney disease (NHANES III) Clin J Am Soc Nephrol. 2009;4(12):1901–1906. doi: 10.2215/CJN.01970309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tooze JA, Kipnis V, Buckman DW, et al. A mixed-effects model approach for estimating the distribution of usual intake of nutrients: the NCI method. Stat Med. 2010;29(27):2857–2868. doi: 10.1002/sim.4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.American Diabetes Association. Standards of medical care in diabetes—2010. Diabetes Care. 2010;33(suppl 1):S11–S61. doi: 10.2337/dc10-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shah VBBB, Bieler GS. SUDAAN User’s Manual, Release 9. Research Triangle Park, NC: Research Triangle Institute; 2005. [Google Scholar]
- 23.Spiegelman D, Hertzmark E, Wand HC. Point and interval estimates of partial population attributable risks in cohort studies: examples and software. Cancer Causes Control. 2007;18(5):571–579. doi: 10.1007/s10552-006-0090-y. [DOI] [PubMed] [Google Scholar]
- 24.Flegal KM, Graubard BI, Williamson DF, Gail MH. Excess deaths associated with underweight, overweight, and obesity. JAMA. 2005;293(15):1861–1867. doi: 10.1001/jama.293.15.1861. [DOI] [PubMed] [Google Scholar]
- 25.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143–3421. [PubMed] [Google Scholar]
- 26.Schoenfeld DA. Residuals for the proportional hazards regresssion model. Biometrika. 1982;69:239–241. [Google Scholar]
- 27.Centers for Disease Control and Prevention (CDC) Vital signs: prevalence, treatment, and control of hypertension—United States, 1999–2002 and 2005–2008. MMWR Morb Mortal Wkly Rep. 2011;60(4):103–108. [PubMed] [Google Scholar]
- 28.Farley TA, Dalal MA, Mostashari F, Frieden TR. Deaths preventable in the U.S. by improvements in use of clinical preventive services. Am J Prev Med. 2010;38(6):600–609. doi: 10.1016/j.amepre.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 29.Sacks FM, Campos H. Dietary therapy in hypertension. N Engl J Med. 2010;362(22):2102–2112. doi: 10.1056/NEJMct0911013. [DOI] [PubMed] [Google Scholar]
- 30.Appel LJ, Brands MW, Daniels SR, Karanja N, Elmer PJ, Sacks FM American Heart Association. Dietary approaches to prevent and treat hypertension. Hypertension. 2006;47(2):296–308. doi: 10.1161/01.HYP.0000202568.01167.B6. [DOI] [PubMed] [Google Scholar]
- 31.Centers for Disease Control and Prevention (CDC) Vital signs: current cigarette smoking among adults aged >or=18 years—United States, 2009. MMWR Morb Mortal Wkly Rep. 2010;59(35):1135–1140. [PubMed] [Google Scholar]
- 32.Garrett BE, Dube SR, Trosclair A, et al. Centers for Disease Control and Prevention (CDC) Cigarette smoking—United States, 1965–2008. MMWR Surveill Summ. 2011;60(suppl):109–113. [PubMed] [Google Scholar]
- 33.Lichtenstein AH, Appel LJ, Brands M, et al. American Heart Association Nutrition Committee. Diet and lifestyle recommendations revision 2006. Circulation. 2006;114(1):82–96. doi: 10.1161/CIRCULATIONAHA.106.176158. [DOI] [PubMed] [Google Scholar]
- 34.Artinian NT, Fletcher GF, Mozaffarian D, et al. American Heart Association Prevention Committee of the Council on Cardiovascular Nursing. Interventions to promote physical activity and dietary lifestyle changes for cardiovascular risk factor reduction in adults. Circulation. 2010;122(4):406–441. doi: 10.1161/CIR.0b013e3181e8edf1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buttar HS, Li T, Ravi N. Prevention of cardiovascular diseases: role of exercise, dietary interventions, obesity and smoking cessation. Exp Clin Cardiol. 2005;10(4):229–249. [PMC free article] [PubMed] [Google Scholar]
- 36.Mozaffarian D, Rimm EB. Fish intake, contaminants, and human health. JAMA. 2006;296(15):1885–1899. doi: 10.1001/jama.296.15.1885. [DOI] [PubMed] [Google Scholar]
- 37.Mozaffarian D, Katan MB, Ascherio A, Stampfer MJ, Willett WC. Trans fatty acids and cardiovascular disease. N Engl J Med. 2006;354(15):1601–1613. doi: 10.1056/NEJMra054035. [DOI] [PubMed] [Google Scholar]
- 38.Estruch R, Martínez-González MA, Corella D, et al. Effects of a Mediterranean-style diet on cardiovascular risk factors. Ann Intern Med. 2006;145(1):1–11. doi: 10.7326/0003-4819-145-1-200607040-00004. [DOI] [PubMed] [Google Scholar]
- 39.Appel LJ, Sacks FM, Carey VJ, et al. OmniHeart Collaborative Research Group. Effects of protein, monounsaturated fat, and carbohydrate intake on blood pressure and serum lipids. JAMA. 2005;294(19):2455–2464. doi: 10.1001/jama.294.19.2455. [DOI] [PubMed] [Google Scholar]
- 40.Bassuk SS, Manson JE. Epidemiological evidence for the role of physical activity in reducing risk of type 2 diabetes and cardiovascular disease. J Appl Physiol. 2005;99(3):1193–1204. doi: 10.1152/japplphysiol.00160.2005. [DOI] [PubMed] [Google Scholar]
- 41.Bassuk SS, Manson JE. Physical activity and cardiovascular disease prevention in women. Nutr Metab Cardiovasc Dis. 2010;20(6):467–473. doi: 10.1016/j.numecd.2009.12.015. [DOI] [PubMed] [Google Scholar]
- 42.Thompson PD, Buchner D, Pina IL, et al. Exercise and physical activity in the prevention and treatment of atherosclerotic cardiovascular disease: a statement from the Council on Clinical Cardiology (Subcommittee on Exercise, Rehabilitation, and Prevention) and the Council on Nutrition, Physical Activity, and Metabolism (Subcommittee on Physical Activity) Circulation. 2003;107(24):3109–3116. doi: 10.1161/01.CIR.0000075572.40158.77. [DOI] [PubMed] [Google Scholar]
- 43.Sacks FM, Svetkey LP, Vollmer WM, et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. N Engl J Med. 2001;344(1):3–10. doi: 10.1056/NEJM200101043440101. [DOI] [PubMed] [Google Scholar]
- 44.Centers for Disease Control and Prevention (CDC) Prevalence of regular physical activity among adults—United States, 2001 and 2005. MMWR Morb Mortal Wkly Rep. 2007;56(46):1209–1212. [PubMed] [Google Scholar]
- 45.Centers for Disease Control and Prevention (CDC) Prevalence of no leisure-time physical activity—35 States and the District of Columbia, 1988–2002. MMWR Morb Mortal Wkly Rep. 2004;53(4):82–86. [PubMed] [Google Scholar]
- 46.Centers for Disease Control and Prevention (CDC) Prevalence of physical activity, including lifestyle activities among adults—United States, 2000–2001. MMWR Morb Mortal Wkly Rep. 2003;52(32):764–769. [PubMed] [Google Scholar]
- 47.Haskell WL, Lee IM, Pate RR, et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc. 2007;39(8):1423–1434. doi: 10.1249/mss.0b013e3180616b27. [DOI] [PubMed] [Google Scholar]
- 48.Mokdad AH, Ford ES, Bowman BA, et al. Diabetes trends in the U.S.: 1990–1998. Diabetes Care. 2000;23(9):1278–1283. doi: 10.2337/diacare.23.9.1278. [DOI] [PubMed] [Google Scholar]
- 49.Mokdad AH, Ford ES, Bowman BA, et al. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA. 2003;289(1):76–79. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- 50.Cowie CC, Rust KF, Byrd-Holt DD, et al. Prevalence of diabetes and impaired fasting glucose in adults in the U.S. population. Diabetes Care. 2006;29(6):1263–1268. doi: 10.2337/dc06-0062. [DOI] [PubMed] [Google Scholar]
- 51.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303(3):235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 52.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA. 2012;307(5):491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 53.Cowie CC, Rust KF, Ford ES, et al. Full accounting of diabetes and pre-diabetes in the U.S. population in 1988–1994 and 2005–2006. Diabetes Care. 2009;32(2):287–294. doi: 10.2337/dc08-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Centers for Disease Control and Prevention (CDC) Vital signs: state-specific obesity prevalence among adults—United States, 2009. MMWR Morb Mortal Wkly Rep. 2010;59(30):951–955. [PubMed] [Google Scholar]
- 55.Cowie CC, Rust KF, Byrd-Holt DD, et al. Prevalence of diabetes and high risk for diabetes using A1C criteria in the U.S. population in 1988–2006. Diabetes Care. 2010;33(3):562–568. doi: 10.2337/dc09-1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang Y, Beydoun MA, Liang L, et al. Will all Americans become overweight or obese? estimating the progression and cost of the US obesity epidemic. Obesity (Silver Spring) 2008;16(10):2323–2330. doi: 10.1038/oby.2008.351. [DOI] [PubMed] [Google Scholar]
- 57.Boyle JP, Thompson TJ, Gregg EW, Barker LE, Williamson DF. Projection of the year 2050 burden of diabetes in the US adult population. Popul Health Metr. 2010;8:29. doi: 10.1186/1478-7954-8-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bazzano AT, Zeldin AS, Diab IR, et al. The Healthy Lifestyle Change Program: a pilot of a community-based health promotion intervention for adults with developmental disabilities. Am J Prev Med. 2009;37(6 suppl 1):S201–S208. doi: 10.1016/j.amepre.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 59.Jackson L. Translating the Diabetes Prevention Program into practice: a review of community interventions. Diabetes Educ. 2009;35(2):309–320. doi: 10.1177/0145721708330153. [DOI] [PubMed] [Google Scholar]
- 60.Hu FB. Obesity Epidemiology. New York, NY: Oxford University Press; 2008. [Google Scholar]
- 61.Drescher K, Becher H. Estimating the generalized impact fraction from case-control data. Biometrics. 1997;53(3):1170–1176. [PubMed] [Google Scholar]
- 62.Tomaselli GF, Harty MB, Horton K, Schoeberl M. The American Heart Association and the Million Hearts Initiative [published online September 13, 2011] Circulation. 2011;124(16):1795–1799. doi: 10.1161/CIR.0b013e3182327084. [DOI] [PubMed] [Google Scholar]
- 63.Centers for Disease Control and Prevention (CDC) Million Hearts: strategies to reduce the prevalence of leading cardiovascular disease risk factors—United States, 2011. MMWR Morb Mortal Wkly Rep. 2011;60(36):1248–1251. [PubMed] [Google Scholar]
- 64.Frieden TR, Berwick DM. The “Million Hearts” initiative—preventing heart attacks and strokes. N Engl J Med. 2011;365(13):e27. doi: 10.1056/NEJMp1110421. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.