Abstract
Introduction
Thyroid nodules are exceedingly common, occurring in up to 76% of adults. Less than 10% are palpable, and the majority are detected incidentally with an estimated prevalence of 68%, 25%, and 18% using ultrasound (US), CT, and MRI, respectively. The rising use of imaging over the last four decades has led to a significant increase in nodule detection or ‘over-identification,’ fine-needle aspiration (FNA), a higher reported incidence of thyroid cancer, and thyroidectomy. The purpose of this study is to provide a descriptive experience with thyroid nodule FNAs one year prior and one year after the implementation of the American College of Radiology (ACR) Thyroid Imaging Reporting and Data System (TI-RADS) at a prototypical community hospital.
Methods
A total of 104 patients with 114 thyroid nodules underwent US-guided FNA at Bluewater Health from January 1, 2018, to March 31, 2020, with available cytological results (The Bethesda System). The study population was divided into two cohorts (January 1, 2018, to December 31, 2018 - ‘local best practice cohort’, and March 1, 2019, to March 31, 2020 - ‘ACR TI-RADS cohort’) based on the implementation of the ACR TI-RADS guidelines in March 2019.
Results
The local best practice cohort (January 1, 2018, to December 31, 2018) comprised 57 thyroid nodules in 52 patients (mean age 66 ± 12; 40 Women). The ACR TI-RADS cohort (March 1, 2019, to March 31, 2020) comprised 57 thyroid nodules in 52 patients (mean age 61 ± 16; 41 Women). There were no statistical differences with respect to age, gender, or thyroid nodule location. Our results show a dramatic decrease in the number of unnecessary FNAs if ACR TI-RADS was implemented from January to December 2018. Thirty (52.6%) of the previously sampled thyroid nodules using the local best practice guidelines would have been followed as per ACR TI-RADS.
Conclusion
ACR TI-RADS is a reliable classification system in routine practice that significantly reduces the number of unnecessary thyroid FNAs with higher specificity compared to local best practice guidelines.
Keywords: american college of radiology, thyroid imaging reporting and data system, ultrasonography, thyroid cancer, thyroid nodule, biopsy, imaging, ultrasound, nodule, thyroid
Introduction
Thyroid nodules are exceedingly common, occurring in up to 76% of adults [1]. Less than 10% are palpable, and the majority are detected incidentally with an estimated prevalence of 68%, 25%, and 18% using ultrasound (US), CT, and MRI, respectively [1-4]. The rising use of imaging over the last four decades has led to a significant increase in nodule detection or ‘over-identification,’ fine-needle aspiration (FNA), a higher reported incidence of thyroid cancer, and thyroidectomy [1,4-5].
Papillary carcinoma is the most common malignancy, occurring in 80-90% of all cases, and has a 30-year survival rate of 95% [6]. Follicular thyroid carcinoma is the second most frequent malignancy, accounting for 10-20% of all thyroid neoplasms, and although the prognosis is not as favorable as papillary carcinoma, 10-year survival can be expected for up to 90% of patients [7]. Both of those differentiated thyroid cancers account for the greatest rise in incidence; in Canada, thyroid cancer incidence has dramatically increased by nearly five times for men and six times for women from 1970 to 2012 [8]. Despite the increasing radiological, pathological, and surgical interventions associated with thyroid nodules, low thyroid cancer mortality rates worldwide have not significantly changed [1].
Many risk-stratification systems were developed to address the over-diagnosis ‘epidemic,’ particularly given the associated substantial human and financial costs [9]. They include the American Thyroid Association (ATA) risk stratification system [10], Korean Society of Thyroid Radiology Thyroid Imaging Reporting and Data System (K-TIRADS) [11], American Association of Clinical Endocrinologists (AACE) [12], European Thyroid Association TIRADS (EU-TIRADS) [13], and the American College of Radiology (ACR) TI-RADS [14]. Recent studies have shown the ACR TI-RADS classification to be a reliable, non-invasive, and practical method for assessing thyroid nodules in routine practice, as well as outperforming the other classification in systems by allowing for the largest reduction of unnecessary thyroid nodule FNAs with the lowest negative predictive value at 2.2% [15-20].
The purpose of this study is to provide a descriptive experience with thyroid nodule FNAs one year prior to and one year after the implementation of the ACR TI-RADS at a prototypical community hospital.
Materials and methods
Study setting
Bluewater Health (BWH) is a dual-site secondary care center with 330 beds serving the county of Sarnia-Lambton, Ontario, Canada, with a catchment area of 150,000 people. Prior to the implementation of the ACR TI-RADS stratification system in March 2019, US-guided thyroid nodule FNAs were performed according to local best practice (LBP) inspired by the ATA guidelines. While the sonographic suspicion categories were the same as outlined in the ATA guidelines, the size criteria for biopsy were more conservative (e.g., local best practice recommends FNA at >2 cm for an intermediate sonographic pattern compared to ATA recommendations at >1 cm) [10,21].
The research ethics board approved this study, and the principles of the Declaration of Helsinki were followed. Informed signed consent was not required.
Study population
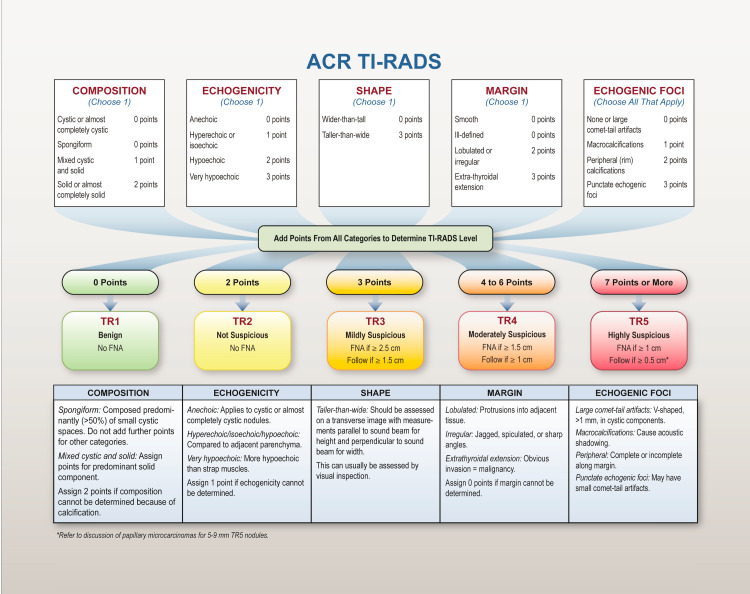
A total of 104 patients with 114 thyroid nodules underwent US-guided FNA at Bluewater Health from January 1, 2018, to March 31, 2020, with available cytological results (The Bethesda System) [22]. Patients with incomplete clinical information and, or absent cytological results were excluded from the analysis. The study population was divided into two cohorts (January 1, 2018, to December 31, 2018 - ‘Local Best Practice cohort’, and March 1, 2019, to March 31, 2020 - ‘ACR TI-RADS cohort’) based on the implementation of the ACR TI-RADS guidelines in March 2019 (Figure 1).
Figure 1. American College of Radiology Thyroid Imaging Reporting and Data System risk stratification system and management recommendations.
ACR TI-RADS = American College of Radiology Thyroid Imaging Reporting and Data System; FNA = fine-needle aspiration.
Adapted from the White Paper of the ACR TI-RADS committee [23]
Image interpretation and ACR TI-RADS categorization
A total of eight radiologists with between two and 20 years of post-training experience routinely interpret thyroid US exams at Bluewater Health. Recommendations for thyroid nodule FNA prior to the implementation of the ACR TI-RADS criteria were based on local best practice guidelines motivated by ATA guidelines (Table 1), as discussed previously [10,21].
Table 1. Comparison of ATA and local best practice recommendations.
ATA = American Thyroid Association; LBP = local best practice; FNA = fine-needle aspiration
Adapted from the Princess Margaret Cancer Centre Clinical Practice Guidelines [10,21]
| Sonographic Pattern | Risk of Malignancy | ATA Recommendations | LBP Recommendations |
| Benign | < 1% | No biopsy | FNA at > 4 cm |
| Very Low Risk | < 3% | Consider at > 2 cm | FNA at > 4 cm |
| Low Risk | 5 - 10% | FNA at > 1.5 cm | FNA at > 2 cm |
| Intermediate | 10 - 20% | FNA at > 1 cm | FNA at > 2 cm |
| High Risk | 70 - 90% | FNA at > 1 cm | FNA at > 1 cm |
All thyroid nodules that were sampled between January 1, 2018, and December 31, 2018, were reclassified based on their sonographic morphology and maximal size using the ACR TI-RADS algorithm with structured reporting similar to the Cancer Care Ontario (CCO) thyroid ultrasound reporting template and blinded from the cytopathology results [24]. This was performed to explore any impact ACR TI-RADS would have on the FNA rate at Bluewater Health. Cohen’s kappa was calculated at 0.56 correlating to 93% inter-rater reliability. Any discrepancy was rectified by consensus.
Starting from March 1, 2019, all thyroid US assessments and management recommendations were standardized to the ACR TI-RADS guidelines.
Statistics
Microsoft Excel (Microsoft Corporation, Redmond, WA) was used for data tabulation. Categorical data were described as counts. Statistical analysis was performed using GraphPad Prism (GraphPad LLC, San Diego, California). The z-score test for two population proportions was used to determine the level of significance between population characteristics for categorical data. A two-tailed t-test was used to compare normally distributed data to determine statistical significance. p≤0.05 was deemed statistically significant.
Results
Data related to the study population are presented in Table 2. Briefly, the local best practice cohort (January 1, 2018, to December 31, 2018) comprised 57 thyroid nodules in 52 patients (mean age 66 ± 12; 40 Women). The ACR TI-RADS cohort (March 1, 2019, to March 31, 2020) comprised 57 thyroid nodules in 52 patients (mean age 61 ± 16; 41 Women). There were no statistical differences with respect to age, gender, or thyroid nodule location.
Table 2. Study Population.
ACR TI-RADS = American College of Radiology Thyroid Imaging Reporting and Data System; LBP = local best practice
| LBP Cohort | ACR TI-RADS Cohort | P-value | |
| Patients | 52 | 52 | - |
| Thyroid nodules | 57 | 57 | - |
| Women | 40 | 40 | 0.86 |
| Age (mean ± range) | 67 ± 12 | 62 ± 17 | 0.09 |
| Location | |||
| Right lobe | 31 | 33 | 0.70 |
| Left lobe | 24 | 24 | 1 |
| Isthmus | 2 | 0 | 0.15 |
Thyroid nodules sampled between January 1, 2018, and December 31, 2018, were reclassified according to the ACR TI-RADS guidelines and presented in Table 3 with Bethesda System correlation. Thirty out of 57 thyroid nodules would have been followed using the ACR TI-RADS guidelines (TI-RADS 3 or less), and all of them were benign by pathologic assessment. Twenty-six out of 57 thyroid nodules would have required tissue sampling and all of them were TI-RADS 4 or 5 lesions. One thyroid nodule was categorized as benign (TI-RADS 2) with no follow-up or FNA recommendation.
Table 3. Reclassified LBP Cohort (January 2018 - December 2018) using ACR TI-RADS with Bethesda System correlation.
ACR TI-RADS = American College of Radiology Thyroid Imaging Reporting and Data System
| ACR TI-RADS Classification | Bethesda System Results | ||||||
| 1 | 2 | 3 | 4 | 5 | 6 | Total | |
| 2 | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| 3 | 6 | 15 | 3 | 0 | 0 | 0 | 24 |
| 4 | 4 | 14 | 4 | 0 | 0 | 0 | 22 |
| 5 | 3 | 6 | 1 | 0 | 0 | 0 | 10 |
| Total | 14 | 35 | 8 | 0 | 0 | 0 | 57 |
Thyroid nodules sampled between March 1, 2019, and March 31, 2020, according to the ACR TI-RADS guidelines are presented in Table 4 with Bethesda System correlation. There were significantly more thyroid nodules with Bethesda III or above (malignant risk >10%) (21 in the ACR TI-RADS cohort versus 8 in the local best practice cohort, p = 0.005) and Bethesda IV or above (malignant risk > 25%) (7 in the ACR TI-RADS cohort versus 0 in the local best practice cohort, p = 0.006). There were similar rates of non-diagnostic/unsatisfactory samples (17.5% for the ACR TI-RADS group versus 24.6% for the local best practice group, p = 0.36).
Table 4. ACR TI-RADS cohort (March 2019 - March 2020) with Bethesda System correlation.
ACR TI-RADS = American College of Radiology Thyroid Imaging Reporting and Data System
| ACR TI-RADS Classification | Bethesda System Results | ||||||
| 1 | 2 | 3 | 4 | 5 | 6 | Total | |
| 3 | 0 | 3 | 0 | 1 | 0 | 0 | 4 |
| 4 | 5 | 12 | 5 | 3 | 2 | 0 | 27 |
| 5 | 5 | 11 | 9 | 1 | 0 | 0 | 26 |
| Total | 10 | 26 | 14 | 5 | 2 | 0 | 57 |
Discussion
Multiple thyroid nodule classification systems were developed to minimize the number of unnecessary FNAs while maintaining reasonable to high negative predictive values. ACR TI-RADS has been validated and shown to outperform the other classification systems in minimizing unnecessary FNAs with negative predictive values as low as 2.2% [15-20]. Starting from March 2019, Bluewater Health has transitioned from utilizing the LBP guidelines, which were largely based on the ATA classification system, to the ACR TI-RADS criteria for FNA recommendation.
Our results show a dramatic decrease in the number of unnecessary FNAs if the ACR TI-RADS guidelines were implemented from January to December 2018. Thirty (52.6%) of the previously sampled thyroid nodules using the LBP guidelines would have been followed as per ACR TI-RADS. This is a considerable amount given the amount of resources, costs, and patient anxiety that could have been averted. Our results also show that higher ACR TI-RADS corresponds to higher Bethesda grades. There were significantly more thyroid nodules with Bethesda III (malignant risk > 10%) in the ACR TI-RADS cohort versus the LBP cohort (21 versus 8, respectively, p = 0.005), as well as Bethesda IV or above (7 versus 0, respectively, p = 0.006). These findings are consistent with the literature demonstrating that ACR TI-RADS is reliable, specific, and outperforms the other classification systems in reducing unnecessary FNAs [15-20].
There is still room for improvement for ACR TI-RADS. Forty-five point six percent (45.6%) to 61.6% of the sampled thyroid nodules as per ACR TI-RADS showed benign cytology. Interobserver variability among radiologists and sonographers is an important consideration to improve TI-RADS performance. In a retrospective analysis of 127 nodules using ACR TI-RADS, Sahli et al. (2019) showed that while TI-RADS interobserver variability was fair (0.6 to 0.74), shape and margin criteria were the biggest sources of disagreement (poor; 0.359 and 0.192, respectively) [25]. Interestingly, in a separate study exploring sonographer performance and interobserver variability, Wildman-Tobriner et al. (2020) showed that sonographers also struggle with margins. Compared to the other sonographic criteria, shape and margin are scored either 0 or 3, and 0, 2, or 3, respectively. Differences in opinion with respect to shape and margin can certainly have a big impact on the overall TI-RADS score and, ultimately, whether a patient will need FNA or not [26].
A study suggested that decreasing the point assignment for punctate echogenic foci, particularly for mixed solid and cystic thyroid nodules, can also reduce the number of benign nodules [27]. Teefey et al. (2021) showed that of 287 mixed thyroid nodules, reducing the points assigned to punctate echogenic foci from 3 to 1 caused the overall TI-RADS score to change for 198 mixed nodules. Forty-four (44) benign nodules would not have been sampled. Although seven carcinomas would not have been sampled as well, six of them would have received follow-up. If the points assigned were changed from 3 to 2, eight benign nodules would not have been sampled and three carcinomas would have been followed instead of sampled [27].
Shape is currently a binary criterion in ACR TI-RADS (0 for wider-than-tall and 3 for taller-than wide). Grani et al. (2020) showed that applying a more specific AP/T ratio ≥1.2 would decrease unnecessary FNAs by up to 58.2% without a negative impact on sensitivity or diagnostic odds ratio [28].
Real-time tissue elastography can also improve the diagnostic performance of ACR TI-RADS [29-30]. Pei et al. (2020) showed that the elasticity score of real-time elastography and the malignant risk stratification of TI-RADS showed a strong correlation, particularly in the size intervals of 0.5 < D ≤ 1.0 cm, 1.0 < D ≤ 2.0 cm, and 2.0 < D ≤ 2.5 cm (r = 0.768, 0.711, and 0.743, respectively). The diagnostic performance of real-time tissue elastography in combination with ACR TI-RADS was consistently better than elastography or TI-RADS alone (p<0.001) [29].
The limitations of this study include the inherent biases of retrospective analysis, small sample size, as well as interobserver variability for both sonographers and radiologists.
Conclusions
ACR TI-RADS significantly reduces the number of unnecessary thyroid FNAs compared to local best practice guidelines. A review of the literature suggests that further modifications to ACR TI-RADS may be helpful to improve overall diagnostic performance.
Acknowledgments
We would like to acknowledge Cancer Care Ontario for developing the local best practice guidelines for the management of thyroid nodules.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
The authors have declared that no competing interests exist.
Human Ethics
Consent was obtained or waived by all participants in this study. The Bluewater Health Research Subcommittee issued approval NA. The Bluewater Health Research Subcommittee recommends that this project be approved with no requirement for Research Ethics Board approval on June 22, 2021
Animal Ethics
Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue.
References
- 1.The global epidemic of thyroid cancer overdiagnosis illustrated using 18 months of consecutive nodule biopsy correlating clinical priority, ACR-TIRADS and Bethesda scoring. Hawkins SP, Jamieson SG, Coomarasamy CN, Low IC. J Med Imaging Radiat Oncol. 2021;65:309–316. doi: 10.1111/1754-9485.13161. [DOI] [PubMed] [Google Scholar]
- 2.Very high prevalence of thyroid nodules detected by high frequency (13 MHz) ultrasound examination. Guth S, Theune U, Aberle J, Galach A, Bamberger CM. Eur J Clin Invest. 2009;39:699–706. doi: 10.1111/j.1365-2362.2009.02162.x. [DOI] [PubMed] [Google Scholar]
- 3.The incidental thyroid nodule. Fisher SB, Perrier ND. CA Cancer J Clin. 2018;68:97–105. doi: 10.3322/caac.21447. [DOI] [PubMed] [Google Scholar]
- 4.ACR TI-RADS: pitfalls, solutions, and future directions. Tappouni RR, Itri JN, McQueen TS, Lalwani N, Ou JJ. Radiographics. 2019;39:2040–2052. doi: 10.1148/rg.2019190026. [DOI] [PubMed] [Google Scholar]
- 5.Current thyroid cancer trends in the United States. Davies L, Welch HG. JAMA Otolaryngol Head Neck Surg. 2014;140:317–322. doi: 10.1001/jamaoto.2014.1. [DOI] [PubMed] [Google Scholar]
- 6.Recent incidences and differential trends of thyroid cancer in the USA. Mao Y, Xing M. Endocr Relat Cancer. 2016;23:313–322. doi: 10.1530/ERC-15-0445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diagnosis and treatment of patients with thyroid cancer. Nguyen QT, Lee EJ, Huang MG, Park YI, Khullar A, Plodkowski RA. https://pubmed.ncbi.nlm.nih.gov/25964831/ Am Health Drug Benefits. 2015;8:30–40. [PMC free article] [PubMed] [Google Scholar]
- 8.Thyroid cancer incidence in Canada: a national cancer registry analysis. Topstad D, Dickinson JA. CMAJ Open. 2017;5:0–6. doi: 10.9778/cmajo.20160162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Annual financial impact of well-differentiated thyroid cancer care in the United States. Lubitz CC, Kong CY, McMahon PM, et al. Cancer. 2014;120:1345–1352. doi: 10.1002/cncr.28562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.2015 American Thyroid Association Management Guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on thyroid nodules and differentiated thyroid cancer. Haugen BR, Alexander EK, Bible KC, et al. Thyroid. 2016;26:1–133. doi: 10.1089/thy.2015.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ultrasonography diagnosis and imaging-based management of thyroid nodules: revised Korean Society of Thyroid Radiology Consensus Statement and Recommendations. Shin JH, Baek JH, Chung J, et al. Korean J Radiol. 2016;17:370–395. doi: 10.3348/kjr.2016.17.3.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.American Association of Clinical Endocrinologists and Associazione Medici Endocrinologi medical guidelines for clinical practice for the diagnosis and management of thyroid nodules. Gharib H, Papini E, Valcavi R, et al. Endocr Pract. 2006;12:63–102. doi: 10.4158/EP.12.1.63. [DOI] [PubMed] [Google Scholar]
- 13.European Thyroid Association Guidelines for ultrasound malignancy risk stratification of thyroid nodules in adults: the EU-TIRADS. Russ G, Bonnema SJ, Erdogan MF, Durante C, Ngu R, Leenhardt L. Eur Thyroid J. 2017;6:225–237. doi: 10.1159/000478927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thyroid Ultrasound Reporting Lexicon: white paper of the ACR Thyroid Imaging, Reporting and Data System (TIRADS) committee. Grant EG, Tessler FN, Hoang JK, et al. J Am Coll Radiol. 2015;12:1272–1279. doi: 10.1016/j.jacr.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 15.Reducing the number of unnecessary thyroid biopsies while improving diagnostic accuracy: toward the "Right" TIRADS. Grani G, Lamartina L, Ascoli V, et al. J Clin Endocrinol Metab. 2019;104:95–102. doi: 10.1210/jc.2018-01674. [DOI] [PubMed] [Google Scholar]
- 16.Correlation of Thyroid Imaging Reporting and Data System [TI-RADS] and fine needle aspiration: experience in 1,000 nodules. Rahal A Junior, Falsarella PM, Rocha RD, et al. Einstein (Sao Paulo) 2016;14:119–123. doi: 10.1590/S1679-45082016AO3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Diagnostic reliability of the Thyroid Imaging Reporting and Data System (TI-RADS) in routine practice. Jabar AS, Koteshwara P, Andrade J. Pol J Radiol. 2019;84:0–80. doi: 10.5114/pjr.2019.86823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Retrospective cohort study of 1947 thyroid nodules: a comparison of the 2017 American College of Radiology TI-RADS and the 2015 American Thyroid Association Classifications. Pandya A, Caoili EM, Jawad-Makki F, et al. AJR Am J Roentgenol. 2020;214:900–906. doi: 10.2214/AJR.19.21904. [DOI] [PubMed] [Google Scholar]
- 19.Comparison of diagnostic performance between the American College of Radiology Thyroid Imaging Reporting and Data System and American Thyroid Association guidelines: a systematic review. Zhang Q, Ma J, Sun W, Zhang L. Endocr Pract. 2020;26:552–563. doi: 10.4158/EP-2019-0237. [DOI] [PubMed] [Google Scholar]
- 20.Diagnostic Performance of American College of Radiology TI-RADS: A Systematic Review and Meta-Analysis. Li W, Wang Y, Wen J, Zhang L, Sun Y. AJR Am J Roentgenol. 2021;216:38–47. doi: 10.2214/AJR.19.22691. [DOI] [PubMed] [Google Scholar]
- 21.Differentiated thyroid cancer. Princess Margaret Cancer Centre clinical practice guidelines. Sewell A, Sawka A, Mete O, et al. https://www.uhn.ca/PrincessMargaret/Health_Professionals/Programs_Departments/Documents/CPG_Endocrine_Thyroid.pdf 2019
- 22.The Bethesda system for reporting thyroid cytopathology. Cibas ES, Ali SZ. Am J Clin Pathol. 2009;132:658–665. doi: 10.1309/AJCPPHLWMI3JV4LA. [DOI] [PubMed] [Google Scholar]
- 23.ACR Thyroid Imaging, Reporting and Data System (TI-RADS): white paper of the ACR TI-RADS Committee. Tessler FN, Middleton WD, Grant EG, et al. J Am Coll Radiol. 2017;14:587–595. doi: 10.1016/j.jacr.2017.01.046. [DOI] [PubMed] [Google Scholar]
- 24.Ontario Health (Cancer Care Ontario). Thyroid ultrasound - radiologist reporting template. Version 1.0. Ontario Health (Cancer Care Ontario) https://www.cancercareontario.ca/sites/ccocancercare/files/assets/RadiologistsReportingTemplate.pdf 2020
- 25.TIRADS interobserver variability among indeterminate thyroid nodules: a single-institution study. Sahli ZT, Sharma AK, Canner JK, et al. J Ultrasound Med. 2019;38:1807–1813. doi: 10.1002/jum.14870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Using the American College of Radiology Thyroid Imaging Reporting and Data System at the point of care: sonographer performance and interobserver variability. Wildman-Tobriner B, Ahmed S, Erkanli A, Mazurowski MA, Hoang JK. Ultrasound Med Biol. 2020;46:1928–1933. doi: 10.1016/j.ultrasmedbio.2020.04.019. [DOI] [PubMed] [Google Scholar]
- 27.Effect of decreasing the ACR TI-RADS point assignment for punctate echogenic foci when they occur in mixed solid and cystic thyroid nodules. Teefey SA, Middleton WD, Reading CC, Langer JE, Beland MD, Szabunio MM, Desser TS. AJR Am J Roentgenol. 2021;216:479–485. doi: 10.2214/AJR.20.22793. [DOI] [PubMed] [Google Scholar]
- 28.Taller-than-wide shape: a new definition improves the specificity of TIRADS systems. Grani G, Lamartina L, Ramundo V, et al. Eur Thyroid J. 2020;9:85–91. doi: 10.1159/000504219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ultrasound real-time tissue elastography improves the diagnostic performance of the ACR Thyroid Imaging Reporting and Data System in differentiating malignant from benign thyroid nodules: a summary of 1525 thyroid nodules. Pei S, Zhang B, Cong S, et al. Int J Endocrinol. 2020;2020:1749351. doi: 10.1155/2020/1749351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.American College of Radiology thyroid imaging report and data system combined with K-RAS mutation improves the management of cytologically indeterminate thyroid nodules. Wu H, Zhang B, Cai G, Li J, Gu X. PLoS One. 2019;14:0. doi: 10.1371/journal.pone.0219383. [DOI] [PMC free article] [PubMed] [Google Scholar]