SUMMARY
Evidence of clinical and/or biochemical androgen excess poses a unique differential in postmenopausal women. Some signs and symptoms of postmenopausal hyperandrogenism can be normal and attributed to the natural aging process. However, the causes of androgen excess in this group include both nontumorous and tumorous causes. Treatment of androgen excess in postmenopausal women may improve both quality of life and long-term metabolic outcomes.
Keywords: Postmenopausal, Hyperandrogenism, Virilization, Hyperthecosis, Adrenal tumor(s), Ovarian tumor(s)
INTRODUCTION
Postmenopausal androgen excess often occurs from the imbalance of rapidly decreasing ovarian estrogen with a relatively gradual decline in androgen secretion.1 Symptoms of postmenopausal hyperandrogenism, such as increases in hirsutism or changes in hair patterns, are common and mild. Because these changes are attributed to the natural aging process with declines in sex-hormone binding globulin (SHBG) and subsequent increase in free androgen index,2 clinicians often do not consider further clinical or biochemical evaluation. However, the development of rapid and true hirsutism, alopecia, and acne is rare and warrants further investigation. In the presence of virilizing signs, clinicians should consider an underlying malignancy.3 A review of findings during the history, physical examination, and appropriate laboratory and radiologic evaluation will help guide in differentiating tumorous from nontumorous causes of postmenopausal hyperandrogenism, leading to optimal treatment strategies.
PRESENTING SIGNS AND SYMPTOMS
The diagnosis of postmenopausal androgen excess is based on patient presentation, thorough history, and physical examination. The most common complaints are facial or truncal hirsutism as well as loss of hair on the head. However, a detailed history is crucial in differentiating progressive hirsutism from true virilization. Hirsutism is defined as excessive terminal hair that appears in androgen-dependent areas (ie, in a male pattern) in women, such as on the chin, upper lip, and abdomen.4 The Ferriman-Gallwey score has traditionally been used to quantify hirsutism in premenopausal women by assigning a score of 0 to 4 to describe hair patterns in the 9 body areas most sensitive to androgens,5 but has not been validated for use in postmenopausal women. Virilization includes severe hirsutism along with male pattern balding, anabolic appearance or increased muscularity, lowering of the voice, and clitoromegaly (>1.5 × 2.5 cm).4 Rapid progression of hirsutism along with virilization suggests severe hyperandrogenism and should trigger an evaluation for an androgen-secreting neoplasm.
A full menstrual history, including timing of menarche and menopause, prior irregular menses or premenopausal hyperandrogenism (ie, hirsutism, acne, and/or male pattern hair loss), should be obtained. The timing of acneiform lesions (ie, pubertal, perimenopausal, or sudden onset) may also be useful information. A personal history of headaches or visual disturbances may point to a pituitary disorder. A weight history, including timing of weight gain and loss, should also be elicited, particularly related to the onset of hirsutism and/or acne. A list of medications and supplements should be carefully obtained. A family history of endocrine disorders, hirsutism, or balding should also be recorded.6
Physical examination should include an evaluation of the extent of excess hair growth as well as acneiform lesions. Hair along the linea alba below the umbilicus is common in up to 20% of women. It is more concerning when hair is present on the upper chest and back and is associated with male pattern balding or clitoromegaly. Often, viewing pictures of the patient before symptom onset can be helpful. Because endocrinopathies can lead to postmenopausal hyperandrogenism, visual field testing for a pituitary tumor, evaluating for stigmata of hypercortisolism, and examining for galactorrhea are all indicated.
DIFFERENTIAL DIAGNOSIS
Broadly speaking, the differential diagnosis for postmenopausal hyperandrogenism can be divided into tumorous versus nontumorous causes. These causes and their presentations are outlined in Table 1 and discussed later in detail.
Table 1.
Differential diagnosis and presentation of androgen excess in postmenopausal women
| Diagnosis | Presentation in Postmenopausal Women |
|
|---|---|---|
| Nontumorous hyperandrogenism Endogenous causes |
||
| Inherited disorders | PCOS |
Sx: Premenopausal history of anovulatory cycles and hyperandrogenism Data: Elevated T and DHEAS |
| NCCAH |
Sx: Premenopausal history of anovulatory cycles and hyperandrogenism Data: Elevated 17-hydroxyprogesterone after ACTH stimulation |
|
| Obesity-induced hyperandrogenism |
Sx: Progressive weight gain without return to baseline weight; normal menses until reaching threshold weight Data: Elevated T but otherwise normal laboratory tests and imaging studies |
|
| Hyperthecosis |
Sx: Premenopausal history of anovulatory cycles and hyperandrogenism; postmenopausal virilization Data: Very high T, modest elevations in DHEAS; pelvic US with bilateral ovarian enlargement (≥10 cm3) |
|
| Endocrinopathies | Cushing syndrome |
Sx: Stigmata of glucocorticoid excess Data: Elevated cortisol on UFC, 1 mg DST, and/or salivary cortisol |
| Hyperprolactinemia |
Sx: Galactorrhea; headaches and/or visual disturbances with prolactinoma Data: Elevated serum prolactin level |
|
| Acromegaly |
Sx: Stigmata of GH excess Data: Elevated serum insulin-like growth factor-1, nonsuppressed GH with oral glucose tolerance testing |
|
| Nontumorous hyperandrogenisms Iatrogenic causes |
||
| Medication use | Glucocorticoids |
Sx: Stigmata of glucocorticoid excess Data: Elevated cortisol on laboratory tests, suppressed gonadotropins |
| Androgens Antiepileptics |
Sx: Rapid virilization temporally related to medication/supplement start Data: Severe elevations in T and/or DHEAS; suppressed gonadotropins |
|
| Medication abuse | Anabolic steroids Pellets |
Sx: Rapid virilization temporally related to medication/supplement start Data: Severe elevations in T and/or DHEAS; suppressed gonadotropins |
| Tumorous hyperandrogenism Adrenal causes |
||
| Adrenal adenomas |
Sx: mild to moderate hyperandrogenism and hypercortisolism Data: Elevated T, usually with concurrent mild to moderate hypercortisolism; suppressed ACTH and DHEAS |
|
| Adrenal carcinomas |
Sx: Rapid virilization often with stigmata of hypercortisolism Data: T ≥150 ng/dL, DHEAS >800 ng/mL, with cosecretion of other adrenal hormones, usually glucocorticoids; >8- to 10-cm mass seen on imaging |
|
| Tumorous hyperandrogenism Ovarian causes |
||
| Sertoli-Leydig cell tumors |
Sx: Rapid virilization Data: T 150 ng/dL; larger tumors (3–12 cm) at presentation, unilateral |
|
| Granulosa cell tumors |
Sx: Predominantly estrogen-secreting but 10% cosecrete androgens leading to rapid virilization; postmenopausal bleeding, endometrial hyperplasia, and endometrial carcinoma Data: T ≥150 ng/dL; larger tumors (3–12 cm) at presentation, cysticappearing |
|
| Metastatic tumors cystadenomas |
Sx: Rapid virilization; in metastatic tumors, signs of systemic illness Data: Paracrine action of β-hCG stimulates androgen secretion, stromal hyperplasia |
The most common cause of hyperandrogenism in premenopausal women is the polycystic ovarian syndrome (PCOS), occurring in up to 12% of reproductive-aged women worldwide using the widely accepted 2003 Rotterdam Criteria.7,8 The diagnosis of PCOS using the Rotterdam Criteria requires the presence of 2 of the 3 criteria after exclusion of other causes: oligomenorrhea/amenorrhea, clinical or biochemical hyperandrogenism, and polycystic morphology of ovaries on ultrasound. The 2009 Androgen Excess Society definition emphasizes the key role of hyperandrogenism and thus requires the presence clinical and/or biochemical hyperandrogenism in the setting of ovarian dysfunction (oligoanovulation and/or polycystic ovaries on ultrasound) after excluding related disorders.9 The 1990 National Institutes of Health criteria are less commonly used and exclude ultrasound findings.7,10 Although the signs and symptoms of PCOS are most notable during the reproductive years, diagnosis in the postmenopausal period may also occur. However, there is no established phenotype for PCOS in postmenopausal women.11 Although signs and symptoms of hyperandrogenism may improve in PCOS patients after menopause, postmenopausal women with PCOS continue to have higher levels of both adrenal and ovarian androgen levels than women without PCOS.7,12 Weight gain may also exacerbate hyperandrogenic symptoms in this population.10,11 A careful menstrual history focusing on cycle regularity, timing of onset and progression of hyperandrogenic symptoms, and weight gain before menopause will help to elucidate this diagnosis.
Congenital Adrenal Hyperplasia
Like PCOS, congenital adrenal hyperplasia (CAH) is another potential cause of postmenopausal hyperandrogenism that typically presents before the menopausal transition. Both classical (rare) and nonclassical (more common) CAH should be on the differential for postmenopausal hyperandrogenism, particularly in individuals with a family history of early pubarche, short stature, and certain ethnic backgrounds.13 However, it is important to note that screening for classical CAH owing to 21-hydroxylase deficiency is routinely done in newborns in the United States.
Obesity-Induced Hyperandrogenism
Nearly 40% of all postmenopausal women may be overweight/obese, but not all have PCOS.14 A common presentation within the authors’ clinical practice that has not been rigorously defined is “obesity-induced hyperandrogenism.” Obesity-induced hyperandrogenism is distinguishable from PCOS, as these women usually have normal menstrual cyclicity throughout life, but have a history of progressive weight gain, often with successive pregnancies, without a return to baseline weight. At a certain weight threshold (differing for each individual), the menstrual cycle becomes irregular with development of hyperandrogenic signs. Development of hyperandrogenic symptoms is in part due to reduced levels of SHBG in obesity leading to increased circulating free androgens.15 The authors further hypothesize that excess aromatase and 5-alpha-reductase in adipose tissue cause increased local estrogens and androgens that lead to irregular menses, hirsutism, and acne.6 A similar cascade of events may also occur in postmenopausal women if their weight reaches the individual threshold after the menopausal transition. Therefore, a careful weight history focusing on the trajectory and timing of weight gain will help differentiate between PCOS and obesity-induced hyperandrogenism. Another clue is whether hyperandrogenic signs and symptoms might improve with weight loss, as the hyperandrogenic symptoms of obesity-induced hyperandrogenism may be more responsive to weight loss. Unfortunately, there are no head-to-head studies comparing changes in androgen levels in women with PCOS after menopause versus women with obesity with similar body mass index without a prior history of PCOS.
Hyperthecosis
A rare, nontumorous cause of hyperandrogenism is hyperthecosis, although this condition is often hard to differentiate from both PCOS and tumorous causes. In a 2018 study by Elhassan and colleagues,16 hyperthecosis accounted for up to 9.3% of all cases of postmenopausal hyperandrogenism in 1205 consecutively recruited women. Obtaining a history of PCOS signs and symptoms is important, as hyperthecosis is considered a severe form of PCOS with considerable overlap in clinical manifestations and metabolic consequences.17 Although the extent of symptoms with hyperthecosis can be as extreme as those with a tumor, they typically develop over several years, often beginning in the premenopausal period. The exact cause is unknown but results from the overproduction of androgens in the ovarian stromal cells and relates to elevated gonadotropins characteristic of menopause.18,19 In addition, women with hyperthecosis have high degrees of insulin resistance that further drives production of ovarian androgens.20 The ovarian volume in women with hyperthecosis generally exceeds 10 cm3, which may be a normal ovarian size in premenopausal but not in postmenopausal women (where the typical volume is between 2.5 and 3.7 cm3).19 Therefore, it is important to consider both age and menopausal status when interpreting pelvic ultrasound findings. Serum levels of testosterone (>150 ng/dL) and estrogen are elevated, which may confer an increased risk of endometrial hyperplasia and carcinoma.19,21 In addition, women with postmenopausal ovarian hyperthecosis are at risk for developing the metabolic syndrome, with conditions such as obesity, hypertension, hyperlipidemia, insulin resistance, hyperinsulinemia, and type 2 diabetes.19,20,22 Ultimately, the diagnosis of hyperthecosis is made on histopathology.
Tumors of the Adrenal or Ovary
There may be considerable overlap in hyperandrogenic and virilizing symptoms between hyperthecosis and tumorous causes. However, severe postmenopausal hyperandrogenism that is rapid in onset generally points toward a neoplastic process. These women have evidence of virilization owing to high levels of circulating androgens. Old photographs may be helpful to review. Androgen-secreting tumors may be either adrenal or ovarian in origin.
Adrenal adenomas
Adrenal adenomas are the most common type of adrenal tumors, and they may be malignant or benign and secretory or nonfunctional.23 Androgen-secreting tumors are ones that produce androgenic prohormones: dehydroepiandrostenedione (DHEA), dehydroepiandrostenedione sulfate (DHEAS), glucocorticoids, and/or estrogens; they rarely secrete testosterone.24 A 2004 study by Moreno and colleagues25 found that out of 801 consecutive adrenal operations from 1970 through 2003, only 21 patients had a pure androgen-secreting tumor. Adrenal carcinomas represent a rare cause of androgen-secreting adrenal tumors (annual incidence of 0.7–2 cases per million) and are more likely to lead to hyperandrogenism in postmenopausal women through cosecretion of DHEAS and glucocorticoids; secretion of androstenedione and testosterone is also common in postmenopausal women with adrenal carcinomas.16,26,27 These tumors grow slowly and are typically diagnosed once they are larger than 8 to 10 cm.28 In contrast, patients with adrenal Cushing syndrome may present with subtle hypercortisolism, elevated testosterone levels, but low DHEAS levels, as the tumor will shut off the normal hypothalamic-pituitary-adrenal axis.29
Ovarian androgen-secreting tumors
Ovarian androgen-secreting tumors arise from either the sex cord cells surrounding the oocyte (theca and granulosa cells) or from stromal cells. Sex cord–stromal tumors are rare and represent approximately 5% to 8% of all ovarian tumors. They are further divided based on their cell of origin and include Leydig cell tumors, Sertoli cell tumors, granulosa cell tumors, ovarian thecomas, and steroid cell tumors, not otherwise specified; less than half are androgen secreting. The Sertoli-Leydig tumors, for example, comprise 0.5% of all ovarian tumors and approximately one-fourth present after menopause.21,27 Pure Leydig cell tumors secrete androgens, whereas pure Sertoli cells secrete estrogens. Both Sertoli and Leydig cell tumors are often relatively large in size, unilateral, and confined to the ovary at the time of diagnosis.21 They are rarely malignant, although this depends on the degree of differentiation at diagnosis, but may exhibit early recurrence after resection.30 Granulosa cell tumors similarly present at an early stage and exhibit good prognosis, although late recurrences and metastases requiring chemotherapy can occur. They account for 2% to 3% of all ovarian tumors and typically present in the sixth decade of life.31 Granulosa cell tumors primarily secrete estrogens and lead to postmenopausal bleeding, endometrial hyperplasia, and endometrial carcinoma. However, up to 10% may secrete androgens leading to virilization.31,32 Among epithelial tumors, serous cystadenomas are the most common, presenting in the fourth or fifth decade of life. Although not steroidogenic, they can secrete β-human chorionic gonadotropin (β-hCG), which can stimulate androgen secretion via paracrine action. Stromal hyperplasia can occur as a result.21,27,33 Rarely, androgen secretion can occur from similar paracrine activity from metastases of neuroendocrine tumors to the ovaries.
Iatrogenic Hyperandrogenism
Medications and/or supplements may contribute to postmenopausal hyperandrogenism.4 Most common are anabolic (eg, danazol) or androgenic steroids (eg, testosterone, DHEA, androstenedione) that may be used by athletes, included in dietary supplements, or used for low energy or sexual dysfunction.27 Some clinicians are prescribing compounded combinations of estrogens with testosterone or DHEA for hypoactive sexual desire disorder. The Endocrine Society recommends against the routine diagnosis of androgen deficiency syndrome, as there are limited data correlating androgen levels with specific symptoms.34 Although a high physiologic dose of testosterone in postmenopausal women has shown short-term efficacy and safety, the use of non–Food and Drug Administration–approved “bioidentical hormonal therapy” is thought to cause more harm than benefit.35 The use of such therapies as pellets containing testosterone, estrogen, and progesterone, which are often promoted on the Internet or inserted at antiaging clinics, can cause serum testosterone levels to escalate into the male range, leading to rapid onset of acne, virilization, mood changes, male pattern balding, and hyperlipidemia. Furthermore, it may take many months for testosterone levels to return to the normal postmenopausal range after discontinuation.36 Exposure to a partner’s testosterone gel may lead to milder symptoms of androgen excess. Antiepileptics, such as valproic acid, or glucocorticoids may be additional iatrogenic causes of hyperandrogenism. A careful review of all medications, supplements, and partner’s hormonal therapies is key in detecting these reversible causes of postmenopausal hyperandrogenism.
Endocrinopathies
The major endocrinopathies to consider in postmenopausal women that may lead to androgen excess are Cushing syndrome, hyperprolactinemia, and acromegaly. Half of the patients with Cushing syndrome may have hirsutism from adrenal androgen excess; hypercortisolism also correlates with increased free androgens, likely because of decreased SHBG.21,37 As previously discussed, androgens may also be cosecreted with cortisol in adrenal carcinomas. In contrast, women with adrenocorticotropin-dependent Cushing disease have mild hirsutism. Hyperprolactinemia from medications or thyroid disorders also can cause hirsutism in premenopausal women that has been shown to improve with dopamine agonist therapy, suggesting that prolactin may lead to increased adrenal androgen production.38,39 Because high estrogen levels can stimulate prolactin secretion, elevated prolactin levels and associated symptoms may improve after menopause.40
Acromegaly is a rare cause of postmenopausal hyperandrogenism, although up to 50% of women with growth hormone (GH) excess can have hirsutism.41 In premenopausal women with acromegaly, GH hypersecretion leads to increased insulin-like growth factor-1 (IGF-1) levels along with hyperinsulinemia that stimulates ovarian androgen production.41,42 There are also several, rarer endocrinopathies, such as glucocorticoid-resistant states, altered glucocorticoid metabolism, and androgen insensitivity states, that can present in postmenopausal women, but these are typically diagnosed earlier in life.21 It is important to keep the list of endocrinopathies in the differential framework for postmenopausal hyperandrogenism, as it will impact the diagnostic workup.
DIAGNOSTIC EVALUATION
The history and physical examination help direct appropriate steps in laboratory and radiologic evaluation (outlined in Fig. 1). It is useful to start with laboratory evaluation to help select imaging modality, particularly with suspected adrenal causes, because an adrenal adenoma can be detected in up to 7% of the elderly population.29
Fig. 1.
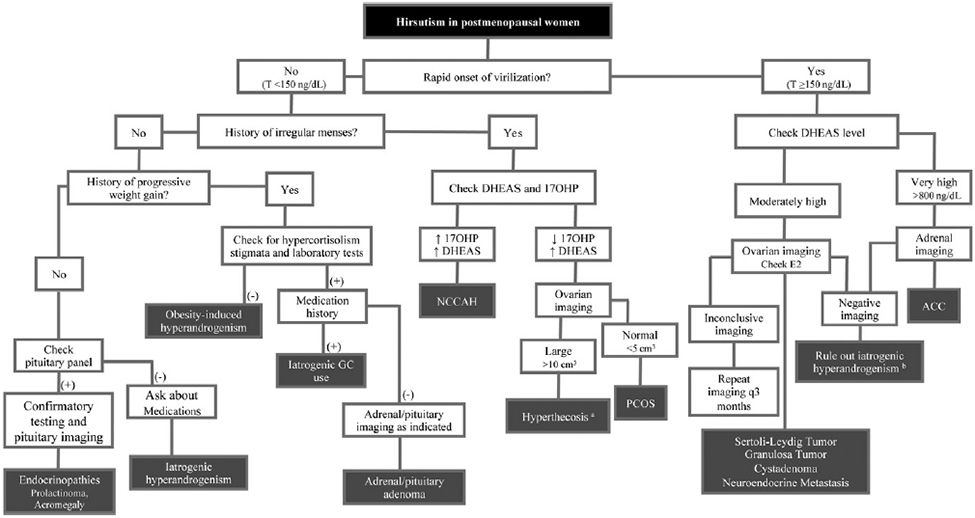
Flowchart of workup of postmenopausal androgen excess. ACC, adrenal carcinoma; GC, glucocorticoid; E2, estradiol; NCCAH, nonclassical congenital adrenal hyperplasia; T, testosterone. a In many cases of hyperthecosis, T may be ≥150 ng/dL. b If negative for iatrogenic hyperandrogenism, consider performing ovarian-adrenal vein sampling. (Data from Refs6,21,27)
Laboratory Testing
The most important androgens to check are total testosterone level and DHEAS, although androstenedione may be a useful prohormone to measure. The normal concentrations of these androgens in postmenopausal women vary by age and laboratory normal ranges. Measuring DHEA is not recommended, as it is a pulsatile hormone. Most commercial total testosterone assays are radioimmunoassays developed for testing levels in men and are frequently insensitive for detection of lower levels of testosterone in women.43 Liquid chromatography–tandem mass spectrometry is considered the gold standard for measuring total testosterone level in women given its high sensitivity and specificity.44 Free testosterone measured by direct assays are generally unreliable but can be useful if measured by equilibrium dialysis.43 Hyperandrogenism in postmenopausal women should be interpreted in the context that both adrenal and ovarian androgens decline with age in women.45
There are some general guidelines on distinguishing tumorous and nontumorous causes of androgen excess on laboratory tests. For example, a total testosterone level less than 150 ng/dL (5.2 nmol/L) generally indicates a nontumorous cause, especially when virilization is not present. However, no threshold definitively separates benign from malignant processes.21,46 It can be difficult to differentiate hyperthecosis from ovarian tumors on laboratory evaluation alone.47 Although a retrospective study of 34 patients showed that postmenopausal women with ovarian tumors had higher testosterone/estradiol levels and lower gonadotropins compared with women with hyperthecosis, there was a great deal of overlap in hormonal profiles between the 2 conditions.18 Both groups had total testosterone levels greater than 150 ng/dL, although levels in women with ovarian tumors were nearly 3-fold higher (560 ± 434 ng/dL in tumors vs 182 ± 89 ng/dL in hyperthecosis). The gonadotropin-releasing hormone (GnRH) analogue suppression test has been used to determine if testosterone excess originates from the ovaries; however, its utility in distinguishing hyperthecosis from an ovarian tumor is limited. In small studies of women with hyperthecosis or ovarian tumors, the GnRH suppression test resulted in suppression of gonadotropins as well as a reduction in testosterone levels by at least 50% in both groups.18,48 Historically, a DHEAS level greater than 800 ng/mL (2171 nmol/L) with a total testosterone level greater than 200 ng/dL (6.94 nmol/L) pointed toward a tumorous cause.6 In Elhassan’s 2018 study, severe DHEAS elevations were found exclusively in adrenal carcinoma.16 Interestingly, very high DHEAS in premenopausal women was predominantly due to PCOS. Severe androstenedione excess was also a marker of adrenal carcinoma in postmenopausal women, whereas mild elevations in androstenedione were more likely related to PCOS.
Other laboratory tests should be obtained based on clinical suspicion from the history and physical examination. These tests primarily include ruling out CAH or the list of endocrinopathies. A prolactin level can be obtained in the setting of associated galactorrhea or medications that may cause hyperprolactinemia. A borderline 17-hydroxyprogesterone (17OHP) level should be followed by testing 30 minutes after intravenous cosyntropin to rule out CAH.13 Cushing syndrome should also be excluded with 2 of the 3 of the following tests: 24-hour urinary free cortisol, 1-mg overnight dexamethasone suppression test, and salivary free cortisol.23,49 If there is suspicion of acromegaly, an IGF-1 level should be drawn, and if elevated, confirmation testing can be performed with an oral glucose tolerance test.50 Based on laboratory findings, specific imaging modalities can be used for localization of potential causes.
Radiologic Evaluation
When history/physical examination and laboratory evaluation point to an adrenal or ovarian cause of androgen excess, imaging studies should be the next step. Ovarian tumors are often small but can be identified using transvaginal ultrasonography (TVUS) or MRI. In a study of 22 postmenopausal women with both tumorous and nontumorous causes of androgen excess, MRI was found to be more sensitive and specific with higher positive predictive (78%) and negative predictive (100%) values versus TVUS in detecting ovarian tumors.21,46 In cases whereby a clear ovarian tumor cannot be identified, asymmetry of the ovaries may suggest a lesion, and imaging should be repeated at 3-month intervals.6
As previously mentioned, adrenal tumors that cause hyperandrogenism are typically greater than 8 to 10 cm at the time of presentation. However, computed axial tomography (CT) or MRI of the adrenals is indicated in a hyperandrogenic woman with a high DHEAS and other clinical concerns for Cushing syndrome. Measuring Hounsfield units (HU) in a noncontrast CT scan can help distinguish adrenal carcinomas from benign adenomas. Low fat content (high density; >10 HU) correlates with malignancy with a sensitivity and specificity close to 71% and 98%, respectively. On a CT scan with delayed contrast media washout, a 50% washout and absolute value of 35 HU after 10 to 15 minutes have superior diagnostic accuracy. With MRI, adrenal carcinomas appear heterogenous, often with necrosis or calcification, and bright because of their high water content.26,27 A pituitary MRI with and without contrast is indicated when history and data point to endocrinopathies, such as acromegaly or hyperprolactinemia.
If TVUS, CT, and MRI are unable to localize the androgen-secreting tumor, combined ovarian and adrenal vein sampling can be considered. However, the data do not support its ability to reliably change management. The ovary is likely to be the source in these cases given their small size at diagnosis. The process is tedious and requires catheterization of all 4 veins (right/left adrenal and right/left ovarian) to detect a left-to-right difference in androgen concentrations.3,51 In a series of 38 patients from St Bartholomew’s Hospital in London, successful catheterization of all 4 veins was only 27%. In 2 of the 38 patients whereby vein sampling indicated a tumor, the final pathology was consistent with PCOS. Adrenal and ovarian vein sampling is not widely performed and should be considered only in centers with expertise.51
TREATMENT BENEFITS AND OPTIONS
Treatments to reduce androgen levels may be important for metabolic and cardiovascular concerns as well as for quality of life. However, the degree to which elevated androgens confer risk of cardiovascular disease (CVD) has been debated. Although women with PCOS often have accompanying insulin resistance and obesity along with dyslipidemia and hyperandrogenism, increased cardiovascular risk has not been well established in this population.52,53 In studies of transgender men, who have been exposed to androgens long term, excess risk for CVD has not been consistently demonstrated.54 However, much of these data is in younger patients where cardiovascular risk is generally low, and longer-term prospective studies are needed. In postmenopausal women with virilizing tumors and very high androgen levels, hypertension and dyslipidemia have been reported, but it remains unclear whether these abnormalities increase CVD risk and how effectively tumor removal will optimize metabolic alterations.55 Nonetheless, treatment is frequently used to remove the inciting cause and improve patient distress related to androgenic symptoms.
Treatment strategies depend on the cause of androgen excess. Tumorous causes generally require surgical resection. Although the diagnosis of hyperthecosis is ultimately made by histopathology, many of the sequelae in this condition are driven by insulin resistance. Lifestyle changes, weight loss, and insulin sensitizers can improve testosterone to normal postmenopausal levels,17,47,56 but more commonly, bilateral oophorectomy is indicated. GnRH analogues can be used as a medical castration to treat hyperthecosis or ovarian tumors if the patient is not a surgical candidate or if a tumor is not identified on imaging.19,20,57 Insulin sensitizers can suppress androgens in premenopausal women with PCOS,58 although data in postmenopausal women are lacking. Postmenopausal women with nonclassical CAH may be treated similarly to PCOS patients, although the addition of hydrocortisone may sometimes be indicated, similar to classical CAH patients.13 Iatrogenic causes of hyperandrogenism can be ameliorated by discontinuation of the causative medication/supplement. Hyperandrogenism from endocrinopathies should be approached using the diagnostic and treatment guidelines for the specific condition. Finally, treatment can also target symptom reduction. For example, hirsutism may be ameliorated with antiandrogens, such as spironolactone or flutamide. Cyproterone acetate can also be used, although it is not available in the United States.4
KEY POINTS.
Postmenopausal hyperandrogenism can present with hirsutism and virilization.
Changes are often mild and attributed to the normal aging process, but causes span both nontumorous and tumorous causes.
This review details the evaluation and treatment of the unique differential of androgen excess in postmenopausal women.
CLINICS CARE POINTS.
Symptoms of mild postmenopausal hyperandrogenism are common; however, a workup is often indicated to assess the cause.
The development of rapid and true virilizing signs signals a need to quickly assess for malignancy.
A focused history and physical examination with appropriate laboratory and radiologic evaluation can differentiate tumorous from nontumorous causes of postmenopausal hyperandrogenism, leading to optimal treatment strategies.
DISCLOSURE
Dr A. Zaman has received grant F32 DK123878 from NIH. The other author has nothing to disclose.
REFERENCES
- 1.Fogle RH, Stanczyk FZ, Zhang X, et al. Ovarian androgen production in postmenopausal women. J Clin Endocrinol Metab 2007;92(8):3040–3. [DOI] [PubMed] [Google Scholar]
- 2.Gershagen S, Doeberl A, Jeppsson S, et al. Decreasing serum levels of sex hormone-binding globulin around the menopause and temporary relation to changing levels of ovarian steroids, as demonstrated in a longitudinal study. Fertil Steril 1989;51(4):616–21. [DOI] [PubMed] [Google Scholar]
- 3.Alpanes M, Gonzalez-Casbas JM, Sanchez J, et al. Management of postmenopausal virilization. J Clin Endocrinol Metab 2012;97(8):2584–8. [DOI] [PubMed] [Google Scholar]
- 4.Martin KA, Anderson RR, Chang RJ, et al. Evaluation and treatment of hirsutism in premenopausal women: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2018;103(4):1233–57. [DOI] [PubMed] [Google Scholar]
- 5.Ferriman D, Gallwey JD. Clinical assessment of body hair growth in women. J Clin Endocrinol Metab 1961;21:1440–7. [DOI] [PubMed] [Google Scholar]
- 6.Rothman MS, Wierman ME. How should postmenopausal androgen excess be evaluated? Clin Endocrinol (Oxf) 2011;75(2):160–4. [DOI] [PubMed] [Google Scholar]
- 7.Azziz R, Carmina E, Chen Z, et al. Polycystic ovary syndrome. Nat Rev Dis Primers 2016;2:16057. [DOI] [PubMed] [Google Scholar]
- 8.Bozdag G, Mumusoglu S, Zengin D, et al. The prevalence and phenotypic features of polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod 2016;31(12):2841–55. [DOI] [PubMed] [Google Scholar]
- 9.Azziz R, Carmina E, Dewailly D, et al. The Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome: the complete task force report. Fertil Steril 2009;91(2):456–88. [DOI] [PubMed] [Google Scholar]
- 10.Rotterdam EA-SPCWG. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril 2004;81(1):19–25. [DOI] [PubMed] [Google Scholar]
- 11.Legro RS, Arslanian SA, Ehrmann DA, et al. Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2013;98(12):4565–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Markopoulos MC, Rizos D, Valsamakis G, et al. Hyperandrogenism in women with polycystic ovary syndrome persists after menopause. J Clin Endocrinol Metab 2011;96(3):623–31. [DOI] [PubMed] [Google Scholar]
- 13.Speiser PW, Arlt W, Auchus RJ, et al. Congenital adrenal hyperplasia due to steroid 21-hydroxylase deficiency: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2018;103(11):4043–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hales CM, Fryar CD, Carroll MD, et al. Trends in obesity and severe obesity prevalence in US youth and adults by sex and age, 2007-2008 to 2015-2016. JAMA 2018;319(16):1723–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pasquali R. Obesity and androgens: facts and perspectives. Fertil Steril 2006;85(5):1319–40. [DOI] [PubMed] [Google Scholar]
- 16.Elhassan YS, Idkowiak J, Smith K, et al. Causes, patterns, and severity of androgen excess in 1205 consecutively recruited women. J Clin Endocrinol Metab 2018;103(3):1214–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vaikkakara S, Al-Ozairi E, Lim E, et al. The investigation and management of severe hyperandrogenism pre- and postmenopause: non-tumor disease is strongly associated with metabolic syndrome and typically responds to insulinsensitization with metformin. Gynecol Endocrinol 2008;24(2):87–92. [DOI] [PubMed] [Google Scholar]
- 18.Yance VRV, Marcondes JAM, Rocha MP, et al. Discriminating between virilizing ovary tumors and ovary hyperthecosis in postmenopausal women: clinical data, hormonal profiles and image studies. Eur J Endocrinol 2017;177(1):93–102. [DOI] [PubMed] [Google Scholar]
- 19.Krug E, Berga SL. Postmenopausal hyperthecosis: functional dysregulation of androgenesis in climacteric ovary. Obstet Gynecol 2002;99(5 Pt 2):893–7. [DOI] [PubMed] [Google Scholar]
- 20.Barth JH, Jenkins M, Belchetz PE. Ovarian hyperthecosis, diabetes and hirsuties in post-menopausal women. Clin Endocrinol (Oxf) 1997;46(2):123–8. [DOI] [PubMed] [Google Scholar]
- 21.Markopoulos MC, Kassi E, Alexandraki Kl, et al. Elyperandrogenism after menopause. Eur J Endocrinol 2015;172(2):R79–91. [DOI] [PubMed] [Google Scholar]
- 22.Rittmaster RS. Polycystic ovary syndrome, hyperthecosis and the menopause. Clin Endocrinol (Oxf) 1997;46(2):129–30. [DOI] [PubMed] [Google Scholar]
- 23.Nieman LK. Approach to the patient with an adrenal incidentaloma. J Clin Endocrinol Metab 2010;95(9):4106–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou WB, Chen N, Li CJ. A rare case of pure testosterone-secreting adrenal adenoma in a postmenopausal elderly woman. BMC Endocr Disord 2019;19(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moreno S, Montoya G, Armstrong J, et al. Profile and outcome of pure androgen-secreting adrenal tumors in women: experience of 21 cases. Surgery 2004; 136(6):1192–8. [DOI] [PubMed] [Google Scholar]
- 26.Kassi E, Angelousi A, Zografos G, et al. Current issues in the diagnosis and management of adrenocortical carcinomas. [Updated 2016 Mar 6], In: Feingold KR, Anawalt B, Boyce A, et al. editors. Endotext [Internet], South Dartmouth (MA): MDText.com, Inc.; 2000. Available at: https://www.ncbi.nlm.nih.gov/books/NBK279009/. [Google Scholar]
- 27.Ervin K, Kostakis LNG, Macut D, et al. Androgens in menopausal women: not only polycystic ovary syndrome. In: Renato Pasquali DP, editor. Hyperandrogenism in women: beyond polycystic ovary syndrome, vol. 53. Basel: Karger; 2019. p. 135–61. [DOI] [PubMed] [Google Scholar]
- 28.Cordera F, Grant C, van Heerden J, et al. Androgen-secreting adrenal tumors. Surgery 2003;134(6):874–80 [discussion: 880]. [DOI] [PubMed] [Google Scholar]
- 29.Young WF Jr. Clinical practice. The incidentally discovered adrenal mass. N Engl J Med 2007;356(6):601–10. [DOI] [PubMed] [Google Scholar]
- 30.Gui T, Cao D, Shen K, et al. A clinicopathological analysis of 40 cases of ovarian Sertoli-Leydig cell tumors. Gynecol Oncol 2012;127(2):384–9. [DOI] [PubMed] [Google Scholar]
- 31.Sekkate S, Kairouani M, Serji B, et al. Ovarian granulosa cell tumors: a retrospective study of 27 cases and a review of the literature. World J Surg Oncol 2013;11:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakashima N, Young RH, Scully RE. Androgenic granulosa cell tumors of the ovary. A clinicopathologic analysis of 17 cases and review of the literature. Arch Pathol Lab Med 1984;108(10):786–91. [PubMed] [Google Scholar]
- 33.Heinonen PK, Morsky P, Aine R, et al. Hormonal activity of epithelial ovarian tumours in post-menopausal women. Maturitas 1988;9(4):325–38. [DOI] [PubMed] [Google Scholar]
- 34.Wierman ME, Arlt W, Basson R, et al. Androgen therapy in women: a reappraisal: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2014; 99(10):3489–510. [DOI] [PubMed] [Google Scholar]
- 35.Santoro N, Braunstein GD, Butts CL, et al. Compounded bioidentical hormones in endocrinology practice: an Endocrine Society Scientific Statement. J Clin Endocrinol Metab 2016;101(4):1318–43. [DOI] [PubMed] [Google Scholar]
- 36.Seaborg E. Targeting patients with pellets: a look at bioidentical hormones. In: Newman MA, Bagley D, editors. Endocrine News. Washington, DC: Endocrine Society; 2019. p. 22–7. [Google Scholar]
- 37.Kaltsas GA, Korbonits M, Isidori AM, et al. How common are polycystic ovaries and the polycystic ovarian syndrome in women with Cushing’s syndrome? Clin Endocrinol (Oxf) 2000;53(4):493–500. [DOI] [PubMed] [Google Scholar]
- 38.Tirgar-Tabari S, Sharbatdaran M, Manafi-Afkham S, et al. Hyperprolactinemia and hirsutism in patients without polycystic ovary syndrome. Int J Trichology 2016; 8(3): 130–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hagag P, Hertzianu I, Ben-Shlomo A, et al. Androgen suppression and clinical improvement with dopamine agonists in hyperandrogenic-hyperprolactinemic women. J Reprod Med 2001;46(7):678–84. [PubMed] [Google Scholar]
- 40.Faje AT, Klibanski A. The treatment of hyperprolactinemia in postmenopausal women with prolactin-secreting microadenomas: cons. Endocrine 2015;48(1):79–82. [DOI] [PubMed] [Google Scholar]
- 41.Kaltsas GA, Mukherjee JJ, Jenkins PJ, et al. Menstrual irregularity in women with acromegaly. J Clin Endocrinol Metab 1999;84(8):2731–5. [DOI] [PubMed] [Google Scholar]
- 42.Kaltsas GA, Androulakis II, Tziveriotis K, et al. Polycystic ovaries and the polycystic ovary syndrome phenotype in women with active acromegaly. Clin Endocrinol (Oxf) 2007;67(6):917–22. [DOI] [PubMed] [Google Scholar]
- 43.Rosner W, Auchus RJ, Azziz R, et al. Position statement: utility, limitations, and pitfalls in measuring testosterone: an Endocrine Society position statement. J Clin Endocrinol Metab 2007;92(2):405–13. [DOI] [PubMed] [Google Scholar]
- 44.Pugeat M, Plotton I, de la Perriere AB, et al. Management of endocrine disease hyperandrogenic states in women: pitfalls in laboratory diagnosis. Eur J Endocrinol 2018;178(4):R141–54. [DOI] [PubMed] [Google Scholar]
- 45.Davison SL, Bell R, Donath S, et al. Androgen levels in adult females: changes with age, menopause, and oophorectomy. J Clin Endocrinol Metab 2005;90(7):3847–53. [DOI] [PubMed] [Google Scholar]
- 46.Sarfati J, Bachelot A, Coussieu C, Meduri G, Touraine P, Study Group Hyperandrogenism in Postmenopausal Women. Impact of clinical, hormonal, radiological, and immunohistochemical studies on the diagnosis of postmenopausal hyperandrogenism. Eur J Endocrinol 2011;165(5):779–88. [DOI] [PubMed] [Google Scholar]
- 47.Mamoojee Y, Ganguri M, Taylor N, et al. Clinical case seminar: postmenopausal androgen excess-challenges in diagnostic work-up and management of ovarian thecosis. Clin Endocrinol (Oxf) 2018;88(1):13–20. [DOI] [PubMed] [Google Scholar]
- 48.Pascale MM, Pugeat M, Roberts M, et al. Androgen suppressive effect of GnRH agonist in ovarian hyperthecosis and virilizing tumours. Clin Endocrinol (Oxf) 1994;41(5):571–6. [DOI] [PubMed] [Google Scholar]
- 49.Thompson GB, Young WF Jr. Adrenal incidentaloma. Curr Opin Oncol 2003; 15(1):84–90. [DOI] [PubMed] [Google Scholar]
- 50.Katznelson L, Laws ER Jr, Melmed S, et al. Acromegaly: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2014;99(11):3933–51. [DOI] [PubMed] [Google Scholar]
- 51.Kaltsas GA, Mukherjee JJ, Kola B, et al. Is ovarian and adrenal venous catheterization and sampling helpful in the investigation of hyperandrogenic women? Clin Endocrinol (Oxf) 2003;59(1):34–43. [DOI] [PubMed] [Google Scholar]
- 52.Torchen LC. Cardiometabolic risk in PCOS: more than a reproductive disorder. Curr Diab Rep 2017;17(12):137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Anagnostis P, Tarlatzis BC, Kauffman RP. Polycystic ovarian syndrome (PCOS): long-term metabolic consequences. Metabolism 2018;86:33–43. [DOI] [PubMed] [Google Scholar]
- 54.Irwig MS. Cardiovascular health in transgender people. Rev Endocr Metab Disord 2018:19(3):243–51. [DOI] [PubMed] [Google Scholar]
- 55.Rocha T, Crespo RP, Yance VVR, et al. Persistent poor metabolic profile in post-menopausal women with ovarian hyperandrogenism after testosterone level normalization. J Endocr Soc 2019;3(5):1087–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Al-Ozairi E, Michael E, Quinton R. Insulin resistance causing severe postmenopausal hyperandrogenism. Int J Gynaecol Obstet 2008;100(3):280–1. [DOI] [PubMed] [Google Scholar]
- 57.Vollaard ES, van Beek AP, Verburg FA, et al. Gonadotropin-releasing hormone agonist treatment in postmenopausal women with hyperandrogenism of ovarian origin. J Clin Endocrinol Metab 2011;96(5):1197–201. [DOI] [PubMed] [Google Scholar]
- 58.Katsiki N, Hatzitolios AI. Insulin-sensitizing agents in the treatment of polycystic ovary syndrome: an update. Curr Opin Obstet Gynecol 2010;22(6):466–76. [DOI] [PubMed] [Google Scholar]


