ABSTRACT
The transmissible locus of stress tolerance (tLST) confers resistance to multiple stresses in E. coli. Utilizing 18,959 E. coli genomes available in the NCBI database, we investigated the prevalence, phylogenetic distribution, and configuration patterns of tLST, and correlations between tLST, and virulence and antimicrobial resistance (AMR) genes in E. coli. Four tLST variants were found in 2.7% of E. coli, with the most prevalent (77.1%) variant being tLST1 followed by tLST2 (8.3%), tLST3b (8.3%) and tLST3a (6.3%). The majority (93%) of those tLST were in E. coli belonging to phylogroup A in which the prevalence was 10.4%. tLST was also found in phylogroup B1 (0.5%) and C (0.5%) but not found in B2 or D-G. An additional 1% of the 18,959 E. coli genomes harbored tLST fragments to various extent. Phylogenetic analysis revealed both intra- and interspecies transmission of both chromosomal and plasmid-borne tLST, with E. coli showing a preference of chromosomal over plasmid-borne tLST. The presence of tLST and virulence genes in E. coli was overall negatively correlated, but tLST was found in all genomes of a subgroup of enterotoxigenic E. coli (ST2332). Of note, no Shiga toxin-producing E. coli (n = 3,492) harbored tLST. The prevalence of tLST and AMR genes showed different temporal trends over the period 1985 to 2019. However, a substantial fraction of tLST positive E. coli harbor AMR genes, posing a threat to public health. In conclusion, this study improves our understanding of the genetic characteristics of tLST and E. coli harboring tLST.
IMPORTANCE This study, through a large-scale genomic analysis, demonstrated that the genomic island tLST related to multiple stress resistance (such as extreme heat resistance and oxidative stress tolerance) in E. coli is differentially present in subgroups of E. coli and is strongly associated with certain phylogenetic background of the host strain. The study also shows the transmission mechanisms of tLST in E. coli and other bacterial species. The overall negative association of tLST, and virulence genes and antimicrobial (AMR) genes suggest the selective pressures for the acquisition and transmission of these traits likely differ. Even so, the high prevalence of tLST in the enterotoxigenic E. coli clone ST2332 and co-occurrence of tLST and AMR genes in E. coli are concerning. Thus, the findings better our understanding of tLST evolution and provide information for risk assessment of tLST harboring bacteria.
KEYWORDS: E. coli, tLST, heat resistance, virulence genes, antimicrobial resistance, STEC, ETEC
INTRODUCTION
Escherichia coli is a commensal of the gastrointestinal tracts of warm blooded animals and is also able to survive in various environmental niches. It is categorized into eight phylogroups, namely, A, B1, B2 and C-G (1). Most E. coli are harmless; however, certain strains can cause intestinal and extra-intestinal diseases in humans, and they are termed as diarrheagenic E. coli (DEC) and extra-intestinal pathogenic E. coli (ExPEC). DEC is further classified into 6 pathotypes: Shiga toxin-producing E. coli (STEC), enteropathogenic E. coli (EPEC), enteroinvasive E. coli (EIEC), enterotoxigenic E. coli (ETEC), enteroaggregative E. coli (EAEC), and adherent-invasive E. coli (AIEC). The infectious doses of these pathotypes vary largely, from as few as 10 cells for STEC (2) to up to 1010 cells for EPEC (3, 4), primarily due to the differences in their key virulence genes. ExPEC are often associated with urinary tract infection, meningitis and septicemia, and are regarded as opportunistic pathogens to humans (5, 6).
Horizontal gene transfer plays an important role in the evolution of E. coli and its adaptation to various habitats. The acquisition and deletion of DNA fragments in E. coli genomes are influenced by both phylogenetic background and habitat source of the corresponding strains (7). Commensal E. coli strains mainly belong to phylogroups A and B1, while pathogenic strains are often of phylogroups B2, D, E and F (8–11). A genomic island associated with heat resistance was found in various species of proteobacteria (12–15), which was termed as the locus of heat resistance in E. coli (14), but as the transmissible locus of quality control in Pseudomonas aeruginosa (15). Those terms have recently been consolidated to the transmissible locus of stress tolerance (tLST) (12), due to its involvement in tolerance to multiple stresses and its transmissibility (13, 16, 17).
The tLST in E. coli AW1.7, the first reported E. coli strain harboring tLST, has 16 open reading frames (ORFs) (14, 18). A tLST variant (tLST2) was later found in dairy E. coli strains (19), with four unique ORFs orfA and orfC-E, missing orf4 and disrupted orf11 (19), compared to the E. coli AW1.7 tLST (tLST1). Another tLST variant, likely a hybrid of tLST1 and tLST2, has recently been reported (20). The overall prevalence of tLST in E. coli is estimated to be about 2% (13, 14), but information on the distribution of tLST variants or novel variants is limited. Despite the low overall prevalence of tLST in E. coli, tLST seems to be more prevalent in some niches than others (20–24), e.g., raw milk cheese (36.3%; 93/256) and wastewater (59%; 42/70). In addition, the reported heat resistance of tLST positive E. coli strains varied among studies, with D60°C from ∼2 min to >70 min (14, 20, 23, 25–28), the reason for which is not well understood.
Pathogenic E. coli harboring tLST would pose threats to public health, for example, the foodborne pathogen STEC, the infectious does of which is low. The compatibility between tLST and Shiga toxin genes (stx), however, has been a matter of debate. On the one hand, it is argued that the environmental pressure maintaining Shiga toxin prophages selects against tLST or tLST negatively affects the expression of Shiga toxins, i.e., tLST and Shiga toxins are ecologically or molecularly incompatible (12, 13, 16). This is supported by studies in which cloning of tLST reduced stx-converting prophage induction in E. coli O104:H4 (16) and all STEC (n = 160) investigated for the presence of tLST were negative for the trait (16, 26). On the other hand, a plasmid harboring tLST (pFAM21805) has been found highly transferrable to other E. coli strains, including STEC under laboratory settings (19). Ma et al. (24) found tLST in 0.5% (3/613) of clinical E. coli isolates by PCR, and 2/3 of the tLST positive isolates were reported to be STEC.
Antimicrobial resistance (AMR) of bacteria is another concern to public health and the World Health Organization has declared it is one of the top 10 public health threats facing humanity. E. coli harboring AMR genes and tLST may require greater effort and more hurdles to control. However, information on the co-occurrence of tLST and AMR genes in E. coli in the literature was largely lacking. Antibiotics is one of the important solutions for human infections by pathogenic E. coli and are often used in the treatment of most E. coli pathotypes except for STEC (29, 30).
To better understand the evolution of tLST and its implications to public health, we performed a large-scale genomic analysis to investigate (i) the prevalence of tLST and its variants in E. coli; (ii) the phylogenetic distribution of tLST in E. coli; (iii) the relationships of the presence of virulence and AMR genes, and tLST.
RESULTS
Prevalence of tLST in E. coli.
A total of 18,959 E. coli genomes were retained for analysis after quality control (Table S1). The genomes ranged from 4.3 to 6.3 Mbp with a median of 5.1 Mbp (Fig. S1). An ORF was regarded as present in a genome if the query sequence covered >80% of the reference ORF (Table S2) with >80% identity. A cutoff value of 80% of ORFs (13 for tLST1 and 14 for tLST2) was used to define complete (≥80%) or partial (<80%) tLST. In this study, tLST refers to complete tLST unless otherwise stated. In total, 706 (3.7%) E. coli genomes were positive for at least one tLST ORF. Of these genomes, 514 (2.7%) had complete tLST (Table S1). Of the genomes positive for tLST, 35 were complete and 479 were draft genomes (Table S1 and Fig. S2-S4).
In 363 of the 479 draft genomes, tLST was fully assembled into one piece (referred to as tidy group) whereas for the remaining 116 genomes, it was scattered on different contigs (messy group) (Fig. S3-S4). Both the median number of contigs and the number of tLST ORFs were significantly larger in the messy group (Fig. 1). This indicates the fragmented tLST assembly in some genomes may be attributed to relatively poor assembly quality and/or redundant tLST ORFs.
FIG 1.

Violin plots comparing the numbers of contigs (panel a) and tLST ORFs (panel b) between genomes with tLST scattered into different contigs/scaffolds (“Messy”; n = 116) and with tLST assembled together (“Tidy”; n = 363). The boxes in each plot show the ends of the first and third quartile. Whiskers indicate the lowest data point within 1.5 interquartile range (IQR) of the first quartile and the highest data point within 1.5 IQR of the third quartile. Central horizontal lines and “x” in the plot indicate medians and means, respectively.
tLST variants in E. coli.
Four tLST variants were identified (Fig. 2, S2 and S3), one of which, tLST3a, has not been previously reported. The tLST variants shared orf1-3, ftsH, orf7-10 and orf12-16 and occasionally had ORF deletion and gene insertion(s). In contrast to tLST1, other variants had an orfA instead of orf4 between orf3 and ftsH. This is the only feature differentiating tLST3a from tLST1. Mobile element proteins were found in upstream and/or downstream regions of all tLST or the majority of partial tLST in complete genomes (Fig. S3 and S5).
FIG 2.
Schematic representations of tLST variants. The NCBI GenBank accession numbers of representative nucleotide sequences harboring tLST are on the left. Differential presence of ORFs is indicated as follows: present in tLST1 but absent in tLST2, purple; present in tLST2 but absent in tLST1, blue; shared by both tLST1 and tLST2, gray; ORF remnants (orf11 in tLST2), yellow. The figure was plotted using the package “gggenes” in R v4.0.2.
Complete E. coli genomes (n = 1,324) were used to estimate the prevalence of each tLST variant in E. coli. Overall, 35 (2.6%) of those genomes harbor tLST, containing 48 complete tLST and three partial ones (Fig. S2). Most tLST positive genomes (23/65.7%) had only one complete tLST, while six (17.1%), two (5.7%) and one (2.9%) genome(s) each had two, three or four complete tLST, respectively. The remaining three genomes each had one complete tLST and one partial tLST2. Among the 48 complete tLST, tLST1 was predominant (37, 77.1%), followed by tLST2 (4, 8.3%), tLST3b (4, 8.3%) and tLST3a (3, 6.3%), all of which were found on both chromosomes and plasmids. Of the 37 complete tLST1, 32 were on chromosomes and 5 on plasmids, respectively. Further, a majority of the chromosome-harbored tLST1 (31/32) had a complete ftsH gene, while all plasmid-harbored tLST1 had interrupted/truncated ftsH.
Intra- and inter-species transmission of chromosomal and plasmid tLST.
We investigated the phylogenetic relatedness of tLST in both E. coli (n = 432; 398 tLST1, 21 tLST2, 6 tLST3a and 7 tLST3b) and representative non-E. coli species (n = 40; 23 tLST1, 5 tLST2, 11 tLST3a and 1 tLST3b) whose genomes were available in the NCBI Nucleotide database as of September 2021 (Table S3). In the phylogenetic tree, all tLST1 and some of tLST3a were clustered into one large clade regardless of bacterial species, although no intermingling between the two variants was observed (Fig. 3a, position 2:00 to 12:00). tLST3b and tLST2 were mixed in one clade (Fig. 3a, 0:00 to 2:00). This is consistent with the configuration pattern of the four tLST variants (Fig. 2).
FIG 3.
The maximum likelihood tree (midpoint rooted) of tLST. Panel a shows all tLST while panel b collapses 310 tLST1 to show a better resolution of the tree for other tLST variants and tLST in non-E. coli species. The tree includes 472 tLST from E. coli (n = 432) and other species (n = 40), among which 404, 21, 6 and 7 are tLST1, tLST2, tLST3a and tLST3b, respectively. The tree was constructed based on the alignment of nucleotide sequences of tLST core ORFs, including orf2, orf3, ftsH, orf7, orf8, orf9, orf10, orf13 and orf14. The tips for tLST from complete genomes of E. coli and non-E. coli are labeled using “EC” and species names, respectively; black and red indicate chromosome and plasmid harbored tLST, respectively. The tips for tLST from draft genomes are not labeled. The first inner rings around the tree in both panel a and b show tLST variants. The middle ring in panel a shows the phylogroup of E. coli harboring tLST. The outer rings in both panel a and b show the type of plasmids harboring tLST, with “Conj” (black), “Mob_unconj” (organge) and “Unmob” (blue) representing conjugative plasmids, mobile but unconjugative plasmids and unmobile plasmids, respectively. The insertion sequences (ISs) in the upstream and downstream of tLST from complete genomes are shown as stars. The tree was midpoint rooted and displayed using iTOL (https://itol.embl.de/).
Four plasmid-harbored tLST1 clustered together with tLST1 in AW1.7 (Fig. 3b, 4:00 to 6:00). Another plasmid borne tLST1 in E. coli clustered together with 22 tLST1 from non-E. coli genomes (Fig. 3b, 10:00 to 12:00). The non-E. coli tLST mainly clustered together instead of scattered in different clades in the tree dominated by E. coli tLST. The plasmids harboring tLST were predicted to be “conjugative,” “mobilizable but unconjugative” or “unmobilizable” (Table S4 and Fig. 3).
A total of nine groups of insertion sequences (ISs) were found in the 5 kb upstream and/or downstream of tLST in complete genomes, including IS1, IS110, IS256, IS3, IS30, IS4, IS5, IS6 and IS66. The number and type of IS varied with bacterial strain/species. IS256 (24/39; 61.5%) and IS6 (6/9; 66.7%) are the most abundant families for chromosome- and plasmid-harbored tLST in E. coli genomes, respectively, while in non-E. coli genomes, IS5 (chromosome, 10/23, 43.5%; plasmid, 12/17, 70.6%) was the most abundant family regardless of the location of tLST (Table S5 and Fig. 3).
Differential prevalence of tLST in E. coli subgroups.
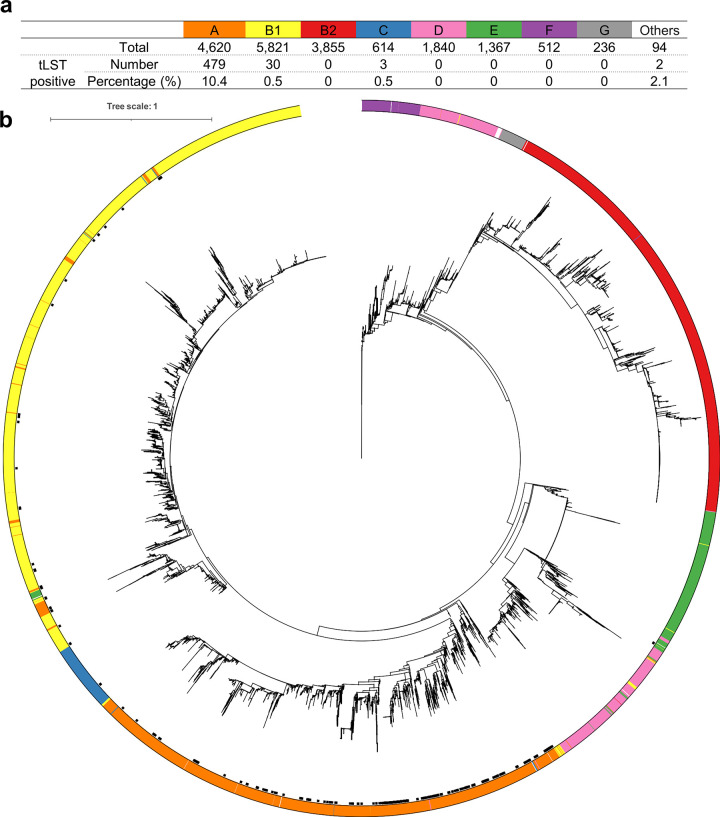
The E. coli genomes belonged to phylogroups A (n = 4,620), B1 (n = 5,821), B2 (n = 3,855), C (n = 614), D (n = 1,840), E (n = 1,367), F (n = 512), G (n = 236) and Others (n = 94, including 39 genomes identified as clade I and 55 genomes with unknown designation) (Fig. 4a and Table S1). The distribution of tLST positive genomes among the groups differed significantly (P < 0.001), with group A having the highest frequency of tLST at 10.4%, and B2 or E-G having no tLST identified (Fig. 4). Similarly, tLST was also unevenly distributed in different serotypes (P < 0.001). Notably, tLST was prevalent in -:H12 (-, unknown O type, 18.2%, 10/55), -:H30 (9%, 6/67) and O9:H30 (8.4%, 8/95), but was not found in O25:H4 (0/1,120), O157:H7 (0/923) or O26:H11 (0/663) (Table S6). A similar uneven distribution was noted for individual O and H antigens (P < 0.001) (Tables S7 and S8).
FIG 4.
The proportions of tLST positive isolates in each phylogroup of E. coli (panel a) and the maximum likelihood tree of 18,959 E. coli isolates (panel b). “Others” in panel a includes 39 genomes identified as clade I and 55 genomes with unknown phylogroups. The tree in panel b was rooted using the genome of Salmonella enterica subsp. enterica serovar Typhimurium str. LT2 (Assembly accession no. in NCBI, GCF_000006945.2). The ring around the tree shows the phylogroup of each isolate, with color consistent with that used in panel a and the black dots inside the ring representing tLST positive isolates. The tree was outgroup-rooted and displayed using iTOL (https://itol.embl.de/).
Surprisingly, all E. coli (n = 89) of ST2332 (Achtman’s scheme) contained tLST (Table S9). The meta information of these genomes in NCBI (Table S10) showed that a majority (93.3%, 83/89) of these E. coli were ETEC recovered from stool samples of people from diverse countries. For other MLST types/clonal complexes (STc) (Achtman’s scheme) often associated with pathogenic E. coli (reviewed by Denamur et al. in 2021) (11), tLST was found in ST88 (2/112, ExPEC and APEC associated), ST1788 (2/4, EPEC associated), STc10 (54/1816, EPEC associated), and ST301 (2/139, Hybrid DEC–ExPEC associated) (Table S11). In Pasteur Institute’ scheme, tLST was most prevalent in ST132 (4/80) compared to other types (Table S12).
Differential gene content of tLST positive and negative E. coli genomes.
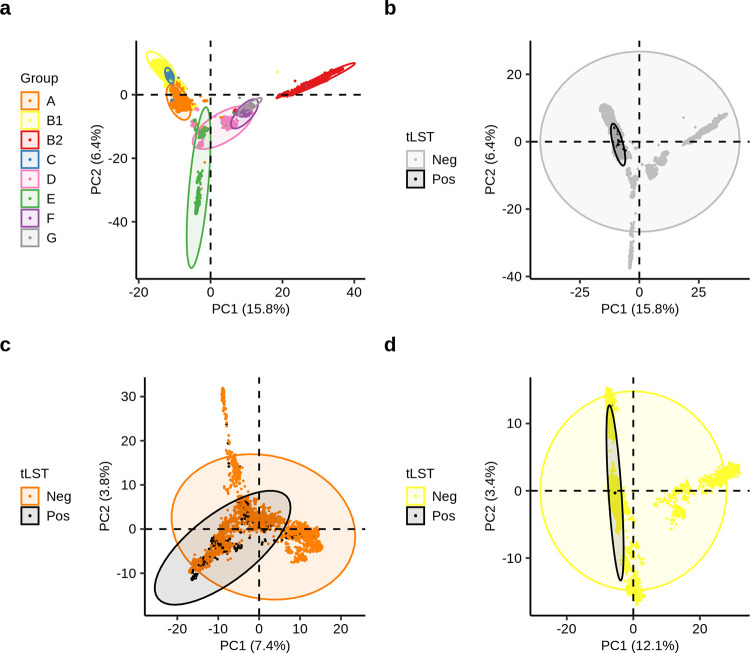
In total, 449,221 genes were found in the pan-genome and 1,836 genes were present in ≥95% of the 18,959 E. coli genomes. PCA of gene content revealed clustering of different groups of E. coli (Fig. 5a) and differing distribution of tLST (Fig. 5b to d). We hence further interrogated for genes that were positively or negatively correlated with the presence of tLST.
FIG 5.
Principal-component analysis (PCA) of gene content of E. coli of phylogroups A-G (panels a and b), group A (panel c) and group B1 (panel d). A total of 103,032, 64,903 and 80,943 genes were included in the PCA for groups A-G, A, and B1, respectively. Each ellipse contains 95% of data points within each group.
In total, 22 genes were positively correlated with tLST (Table S13), half of which are markers of cryptic prophage CP4-6 or CP4-57. Six CP4-6/57 prophage marker genes, encoding homocysteine methyltransferase (mmuM), S-methyl-L-methionine transporter (mmuP), type IV toxin-antitoxin system antitoxin (yafW) and toxin (ykfI), and proteins with unknown functions (ykfB and ypiK), were present in ≥70% of tLST positive E. coli. The CP4-6/4-57 prophage related genes were present in both 20 kb upstream and downstream of 26/32 of chromosomal tLST1 (Table S14 and Fig. S2), but were rarely found in corresponding regions of plasmid tLST1 or other tLST variants. In addition, a mhp gene cluster (responsible for propionic acid degradation) (31) was found in upstream of 24/32 of chromosome tLST1 (Fig. S2). The complete genomes (n = 9) solely harboring partial tLST had all partial tLST (n = 18) on chromosomes (Fig. S5). Only 3/18 of them had CP4-6/57 prophage marker genes in their upstream and/or downstream regions.
A larger number (n = 71) of genes were negatively correlated with tLST, with more than half encoding virulence factors, including Shiga toxin genes (stx), urease gene cluster (ureA-G), genes involved in the heme/iron acquisition system and type 3 secretion system (T3SS). These genes were present in ≤2.7% of 514 tLST positive genomes (Table S13).
Overall negative correlation of virulence genes and tLST in E. coli genomes.
To investigate the compatibility between tLST and virulence genes in E. coli genomes in general, we scanned all E. coli genomes for virulence genes. A total of 153 virulence genes were found in at least one genome (Table S15). The median number of virulence genes was largest in group E, B2 and G and smallest in group A where 93.2% of tLST were identified, respectively (Fig. S6a). In both phylogroup A and B1 in which 99.0% of tLST were identified, tLST negative genomes had larger median numbers of virulence genes than tLST positive genomes (P < 0.001) (Fig. S6b and S6c).
In total, 86 and 29 virulence genes showed significantly different prevalence between tLST positive and tLST negative E. coli phylogroup A and B1genomes, respectively (Table S15). The majority (group A, 87.2%; group B1, 89.7%) of those genes were more prevalent in tLST negative than positive genomes. Of the 18,959 E. coli genomes, 3,492 had stx, two of which also had orf16 and one of which (GCF_009767355.1) had tLST. However, upon further analysis, the stx genes in GCF_009767355.1 were likely contamination as the K-mer coverages of the contigs containing stx genes were only 1.8–2.9 (Fig. S7). Genes that were more prevalent in tLST positive than tLST negative group A E. coli included eatA (encoding serine protease autotransporters), lngA (longus type IV pilus), cfaC (CFA/I fimbrial subunit C), fyuA (siderophore receptor), irp2 (high molecular weight protein 2 nonribosomal peptide synthetase), and lpfA (long polar fimbriae) (Table S15). The former three contribute to the virulence of ETEC (32–34). These three genes were found in 18, 15.9 and 18.4% of tLST positive E. coli genomes, with most of those tLST positive genomes being ETEC ST2322 (eatA, 82/86; lngA, 72/76; cfaC, 84/89), and 2.7, 1.8 and 0.9% of tLST negative E. coli genomes. FyuA and irp2, involved in iron acquisition, are associated with the virulence of uropathogenic E. coli (UPEC, an ExPEC pathotype) (35) and avian pathogenic E. coli (APEC) (36), and are also often found in ETEC (37, 38). They both were present in 31.7% of tLST positive genomes, and the tLST negative genomes had fyuA and irp2 at 22% and 21.6%. The genomes harboring fyuA and irp2 included those of 90 (59.2%) ETEC, one UPEC, one AIEC and 61 of unknown pathogenicity based on the meta information in the NCBI Biosample database. The tLST positive genomes harboring lpfA (n = 100), a gene potentially associated with intestinal colonization (39), were mainly from humans (n = 48) with unclear disease causing ability or from other resources (n = 52) (Table S10).
Co-occurrence of tLST and AMR genes.
ResFinder database contained genes associated with 16 antibiotics classes. The AMR genes for fusidic acid, nitroimidazole, pseudomonicacid, glycopeptide, and oxazolidinone resistance were not found in any of the E. coli genomes. A total of 340 genes involved in resistance to 11 classes of antibiotics were found (Table S16). The frequency of AMR genes for these 11 antibiotic classes in the genomes of E. coli collected between 1980s and 2020s trended upwards (P < 0.001), while that of tLST was largely unchanged (P = 0.53; Fig. 6).
FIG 6.
The proportion of tLST or antimicrobial resistance (AMR) genes harboring E. coli collected between 1985 and 2019. For the convenience of data visualization, the proportion of E. coli positive for tLST and AMR genes is shown as percent*10 and percent, respectively. The years that had fewer than 100 genomes collected were excluded from the analysis. Correlations between positive rates and years of collection were examined using Spearman’s rank correlation test, and were regarded as significant if P < 0.05. The correlation coefficients (R) and P-values for tLST and AMR genes are shown in the same order as they are in the legend. For each of tLST and antimicrobial classes, the data points and linear regression line are shown in filled shapes and solid line, or unfilled shapes and dotted line, respectively.
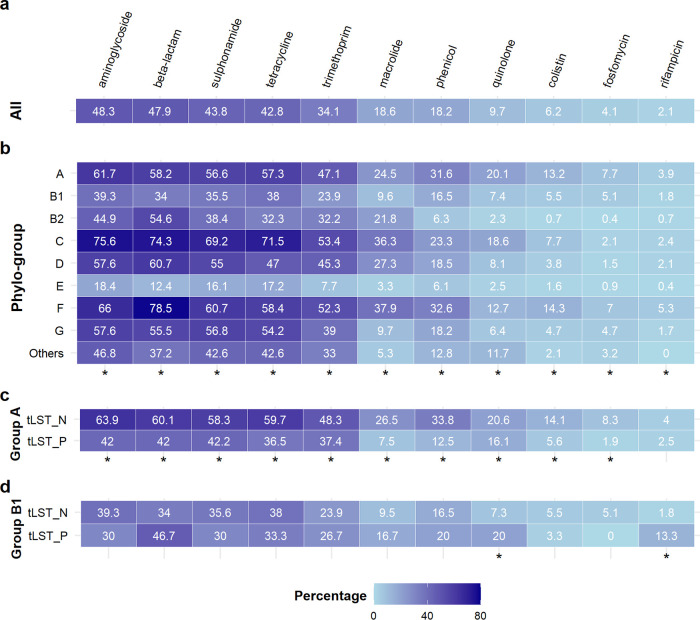
Genes associated with resistance to aminoglycoside, β-lactam, sulfonamide and tetracycline were present in >40% of all genomes, while <10% of genomes had resistance genes to quinolone, colistin, fosfomycin and rifampicin (Fig. 7a). The prevalence of AMR genes in different phylogroups differed significantly (Fig. 7b). Phylogroups C and F, and group E and B1 had the largest and smallest mean number of resistance genes, compared to other phylogroups (Fig. S8a). For phylogroup A, the median of AMR genes was significantly higher in tLST negative than positive genomes (Fig. S8b); no such difference was observed for phylogroup B1 (Fig. S8c). Among phylogroup A, resistance genes were more prevalent in tLST negative genomes compared to tLST positive ones for all 11 antibiotics classes except for rifampicin (Fig. 7c). Among group B1, quinolone and rifampicin showed significantly higher prevalence in tLST positive genomes than in negative ones, and no significant difference was found for other antibiotics (Fig. 7d).
FIG 7.
Antimicrobial resistance (AMR) genes in E. coli genomes. Panels show the proportions (%) of E. coli harboring at least one gene associated with resistance to each of 11 antimicrobial classes in all 18,959 E. coli genomes (panel a), in each phylogroup (panel b), and in tLST positive (“tLST_P”) and negative (“tLST_N”) genomes of group A (panel c) and B1 (panel d). “Others” in panel b represent 39 genomes identified as clade I and 55 isolates with unknown phylogroup. The 11 classes of antimicrobials to which at least one associated AMR gene was found in the E. coli genomes are shown. The contingency tables of presence of AMR genes versus phylogroup (panel b) or the presence of tLST (panels c and d) were analyzed using Fisher’s exact test and a relationship between two variables was regarded as significant if P < 0.05 (“*” on the bottom of each panel).
Although AMR genes were, overall, less prevalent in tLST positive genomes than in tLST negative ones, a considerable proportion of tLST positive genomes harbored AMR genes. AMR genes associated with β-lactam, aminoglycoside, sulfonamide, trimethoprim and tetracycline were present in >30% of tLST positive genomes (Table S17). Genes encoding extended-spectrum β-lactamase (ESBL) were in 40.5% of tLST positive genomes.
Differential prevalence of tLST in E. coli from various sources.
The sources from which the 18,959 E. coli were collected are stratified into 12 categories: human, cattle, chicken, pig, water, dog, caprid, horse, other animals (mainly wide animals), other birds (mainly wild birds), “others” (sources each containing <100 E. coli) and Unknown (Table S18). The prevalence of tLST in E. coli from these sources differed significantly (P < 0.001), with E. coli from water (8.1%), other birds (4.6%), “others” (4.4%), and humans (3.3%) having slightly higher prevalence than E. coli overall (2.7%). Of note, 11 of 43 (25.6%) genomes of E. coli from cheese product (“others”) were tLST positive.
DISCUSSION
The genomic island tLST, associated with increased tolerance to elevated temperature, oxidative stress and pressure (13, 16, 17), has been found in various classes of proteobacteria, including E. coli (12). The prevalence of tLST in E. coli in the present study, 2.7%, is slightly higher than the previously reported 2% (13, 14). This difference is likely attributable to the slight difference in the scanning methods for tLST and the fact that most E. coli genomes in NCBI are draft assemblies from short reads. The present study regarded a harborage of >80% of tLST ORFs as positive. The earlier studies (13, 14) regarded >80% coverage of the nucleotide sequence of tLST as positive, which may have overlooked the draft genomes with tLST ORFs scattered on different contigs. Noncontiguous tLST fragments were also found in the draft genome of Salmonella enterica ATCC43845 while a full-length tLST was found in the complete genome of this strain (40, 41). Extra copy of tLST tends to confer extra thermal tolerance for E. coli under some circumstances (19, 20) and E. coli harboring up to four copies of tLST have been reported. A considerable proportion (34.3%, 12/35) of tLST positive E. coli (complete) genomes had more than one tLST in this study. However, E. coli containing four copies tLST seem to be rare, with two of the 18,959 E. coli genomes being of this type. By PCR assays, tLST was found in E. coli isolated from raw milk cheese at 36.3% (93/256) (21), wastewater at 59% (41/70) (22), meat of diverse livestock species at 11.4% (470/4,123) (20), beef production chain at 1.97% (28/1,450) (23), and clinical samples at 0.5% (3/613) (24). The present study found tLST in about 8% of genomes of E. coli from water and 25.6% from cheese, much higher than the overall prevalence of tLST in E. coli. It has been suggested the mild thermal treatment for raw milk cheese and chlorination for municipal wastewater may select for tLST positive strains (21, 22). These findings highlight the importance of monitoring tLST in environments where potential selective pressure could be exerted.
The top non-E. coli BLAST hits of tLST against the NCBI nucleotide database had more gamma-- than betaproteobacteria, which is more or less in agreement with previous studies (12, 14, 41). The harborage of tLST on both chromosomes and plasmids in E. coli has been previously reported (19, 41); however, the predominant chromosomal origin of E. coli tLST is different from the primarily plasmid located tLST in K. pneumonia (41). Under laboratory conditions, a chromosomal tLST in the dairy E. coli strain FAM21805 transferred at a much lower rate than a plasmid harbored tLST (19). The intermingling of plasmid-borne tLST in different E. coli strains, and in E. coli and other species on the tLST phylogenetic tree (Fig. 4) supports both intra- and interspecies transmission of plasmid-borne tLST. However, the predominance of the chromosomal tLST in E. coli seems to suggest there could be mechanisms in E. coli limiting the transmission of plasmid-borne tLST within the species. Mobile element genes were found in all 48 tLST in the complete genomes in this study, consistent with the transmissibility nature of tLST (12, 14). It is interesting that the cryptic prophage CP4-6/4-57 associated genes (mmuM, mmuP, yafw-ykfI, ypiK and ykfB) were primarily found in the flanking region of the most prevalent tLST variant, chromosomal tLST1, and in its complete form, rarely in other variants or tLST negative E. coli. Interactions between these genes and tLST are unknown. Studies have indicated that the cryptic prophage CP4-6 and/or CP4-57 can enhance the resistance of E. coli to nalidixic acid, oxidative stress and acid stress (42, 43). It seems plausible that some of these genes may contribute to the dominance and the completeness of chromosomal tLST1 in E. coli.
tLST is frequently present in Enterobacteriaceae species with “blended lifestyle” including colonization in plants and persistence in vertebrates (13). The meta information on the E. coli genomes shows that A and B1 are the most prevalent phylogroups among E. coli collected from both animal (n = 13,678; A, 22%, B1, 29%) and plant (n = 86; A, 16%, B1, 59%) sourced samples (data not shown), indicating the “blended lifestyle” of both groups. However, the present study demonstrates that tLST is predominantly (93%) present in phylogroup A, but much less in B1 (6%), which is corroborated with a number of reports involving a small number of E. coli from beef, raw milk cheese and wastewater (14, 21, 22, 44). Very few studies have reported on serotype or MLST type of tLST positive E. coli. This study found uneven distribution of tLST in E. coli serotypes and MLST types. In a study of Yang et al. (44), tLST positive E. coli (n = 25) from beef were also predominated by certain serotypes/MLST types. This is also in line with the high prevalence of tLST in P. aeruginosa clone C, which is represented by MLST subtypes of ST17, ST845, ST2691 and ST2894 (15). Together, these findings suggest genetic background plays an important role in the acquisition and maintenance of tLST by bacteria.
Interestingly, the present study demonstrates that tLST and the majority of virulence genes do not seem to prefer the same genetic background, evidenced by the lack of correlation of the presence of tLST and the pathogen-associated phylogroups (B2, D, E and F) or the majority of pathogen-associated serotypes or MLST types, and the negative correlation of tLST and the majority of virulence genes. Zhi et al. (22) investigated the presence of 28 virulence genes associated with E. coli pathogenesis in humans and animals in tLST positive isolates from municipal wastewater and only found fimH, an adhesive protein gene for type 1 fimbriae. Also, Wang et al. (16) showed that tLST was found negatively correlated with UPEC associated virulence genes and was exclusively present with stx (n = 160). tLST has been found to reduce the peroxide-induced induction of Shiga toxin-converting prophages in STEC O104:H4 (16). However, tLST positive STEC determined by PCR have been occasionally reported (20, 24, 45) for which unspecific binding of primers in PCR could be a factor. The hypothesis of the incompatibility of stx and tLST is supported by the present study that none of 3,492 STEC genomes were found to harbor tLST. Despite the overall low prevalence of virulence genes in tLST positive E. coli genomes, strong positive correlation of tLST with five virulence factors in ETEC was noted in this study. Those ETEC genomes were mostly of one particular subtype, ST2332, although serotypes/sequence types of ETEC are very diverse and depend on geographical regions (11, 29, 46). The overall negative correlation of tLST and virulence genes suggests the acquisition of tLST is driven more by secondary rather than primary habitats of E. coli.
Despite the lower prevalence of AMR genes in tLST positive than negative E. coli genomes, a considerable proportion of tLST positive E. coli carry AMR genes involved in resistance to 11 antibiotic classes. Of heat resistant raw milk cheese E. coli isolates, 20.5% are resistant to at least one antimicrobial agent (21). Approximately 24% (19/80) of ESBL encoding K. pneumonia bloodstream infection isolates harbored clpK gene (orf3), one of marker genes of tLST (47). Natural co-occurrence of tLST and ESBL genes has not been reported for E. coli. The present study found 40.5% of tLST positive E. coli harbored ESBL genes. Mechanisms for the differential harborage of AMR genes by tLST positive and negative E. coli or different phylogroups of E. coli would warrant further investigation. The different temporal trend of the prevalence of tLST and AMR genes in E. coli genomes suggest the use of antibiotics has unlikely contributed to the emergence and spread of tLST. Nevertheless, multistress resistant AMR E. coli are very concerning to public health.
Taken together, the large-scale genomic analysis demonstrated subgroup specific distribution and the transmission mechanisms of tLST in E. coli. The acquisition and maintenance of tLST in E. coli is strongly associated with the strain’s genetic background. In addition, tLST positive ETEC (ST2332) and E. coli harboring both tLST and AMR genes should be closely monitored.
MATERIALS AND METHODS
E. coli genome sequence retrieval and scanning for tLST ORFs.
The E. coli genomes deposited in the National Center for Biotechnology Information (NCBI) were downloaded on February 6, 2021, and examined for quality using PanACoTA v1.1.0 (48) (Table S1 and Fig. S1). All genomes were annotated using Prokka v1.14.6 (49). The tLST ORFs in these genomes were scanned using ABRicate v1.0.1 (https://github.com/tseemann/abricate) with default settings with published tLST (14, 19, 44) as references (Table S2).
Phylogenetic analysis of tLST.
The nucleotide sequences of tLST were extracted from each tLST positive genome with tLST assembled into one piece using Seqkit v0.15.0 (50). The sequences of four tLST variants in E. coli identified in this study were aligned against the NCBI Nucleotide database using Megablast (51) with default settings with E. coli excluded. The sequences that had <80% coverage or identity were removed from the top 100 best hits (lowest E value). One representative tLST was selected at random from the above retained sequences for each tLST variant and bacterial species.
The tLST (n = 472) from both E. coli and non-E. coli species were included in the phylogenetic analysis. Nine ORFs (orf2-3, orf5, orf7-10, orf13-14) which were present in all tLST variants were extracted from the nucleotide sequence of each tLST using Seqkit and were each aligned using MAFFT v7.475. The alignments were concatenated using Seqkit. A maximum likelihood tree was constructed using RaxML v8.2.12 with general time reversible gamma nucleotide model and 1000 bootstrap analyses (52). The tree was midpoint rooted and displayed using iTOL (53).
Plasmids harboring tLST were classified as “conjugative,” “mobilizable but unconjugative” or “unmobilizable” using Plascad v1.17 (54). Insertion-sequence elements (ISs) related to tLST were searched by scanning the 5 kb upstream and downstream of tLST against ISfinder database (v2020-Oct) (55) using ABRicate with default settings.
Phylogenetic analysis of E. coli.
A concatenated alignment of amino acid sequences of 3,184 core genes of E. coli was created using PhyloPhlAn v3.0 (56). Five Salmonella enterica genomes were included as outgroups (Table S1). IQ-TREE v2.0.3 (57) was used to construct trees with the best protein model identified by ModelFinder (58) and 1000 iterations of ultrafast bootstrap analysis (59).
Genotyping and extracting the meta information of E. coli.
All E. coli genomes were phylotyped using ClermonTyping v20.03 (60) and serotyped using ECTyper v1.0.0 (https://github.com/phac-nml/ecoli_serotyping) with default settings. The multilocus sequence typing (MLST) for each genome was carried out using mlst v2.19.0 (https://github.com/tseemann/mlst). The source and date of collection (where and when an isolate for a genome was recovered) was extracted from GenBank file associated with each E. coli genome using an in-house python script or directly from NCBI by interrogating the BioSample database using Entrez-direct v13.9.
Pan-genome and principal-component analysis (PCA).
The pan-genome of 18,959 E. coli was parsed using Roary v3.13.0 with identity threshold at 80% for clustering proteins and paralogs not split. PCA was performed for the gene content in all E. coli with clear phylogroup designation, in E. coli of phylogroup A and B, respectively, using the prcomp function and the package “factoextra” in R. For each analysis, the rare genes (present in <0.1% of included genomes) and persistent genes (absent in <0.1% of genomes) were excluded. The genes that are negatively or positively correlated with tLST were investigated. Genes that were in more than 10% of tLST negative/positive genomes and 10 times more abundant in tLST negative/positive than positive/negative genomes were regarded as tLST negatively/positively correlated genes.
Virulence and AMR genes.
The nucleotide sequence of each genome was scanned for virulence and AMR genes using the VirulenceFinder (v2021-05-06) (https://bitbucket.org/genomicepidemiology/virulencefinder_db/src/master/virulence_ecoli.fsa) and ResFinder database (v2021-04-20) (https://cge.cbs.dtu.dk/services/ResFinder/) as references, respectively. ABRicate was used for scanning with default settings.
Statistical analysis.
Wilcoxon test was performed to determine differences in the number of contigs and the number of tLST ORFs between genomes with tLST fully assembled and those with tLST scattered into different contigs; and to assess differences in the number of virulence and AMR genes between tLST positive and negative genomes, respectively. Kruskal-Wallis rank sum test followed by post hoc pairwise comparison with Dunn’s test was performed to compare the number of virulence and AMR genes between different phylogroups of E. coli. Fisher’s exact test was performed to determine whether there is any relation between the presence of tLST and the genotype (phylogroup/MLST type/sero-group) of E. coli, between tLST harboring E. coli and collection source, and between the presence of virulence/AMR genes and the phylogroup of/the presence of tLST in E. coli. Correlation between the proportion of E. coli harboring tLST/AMR genes and isolate collection years was examined via Spearman’s rank correlation test. For all analyses, a significance level of 0.05 was used.
ACKNOWLEDGMENTS
Funding was provided by the Beef Cattle Research Council in Canada, Alberta Innovates and Alberta Agriculture and Forestry. Bioinformatics analyses were performed using Compute Canada and Biocluster. We are grateful to Arun Kommadath for providing helpful suggestions in data analysis and manuscript proof reading.
We declare no conflict of interest.
Footnotes
Supplemental material is available online only.
Contributor Information
Xianqin Yang, Email: xianqin.yang@agr.gc.ca.
Johanna Björkroth, University of Helsinki.
REFERENCES
- 1.Clermont O, Dixit OVA, Vangchhia B, Condamine B, Dion S, Bridier-Nahmias A, Denamur E, Gordon D. 2019. Characterization and rapid identification of phylogroup G in Escherichia coli, a lineage with high virulence and antibiotic resistance potential. Environ Microbiol 21:3107–3117. 10.1111/1462-2920.14713. [DOI] [PubMed] [Google Scholar]
- 2.Hara-Kudo Y, Takatori K. 2011. Contamination level and ingestion dose of foodborne pathogens associated with infections. Epidemiol Infect 139:1505–1510. 10.1017/S095026881000292X. [DOI] [PubMed] [Google Scholar]
- 3.Kothary MH, Babu US. 2001. Infective dose of foodborne pathogens in volunteers: a review. J Food Saf 21:49–68. 10.1111/j.1745-4565.2001.tb00307.x. [DOI] [Google Scholar]
- 4.DuPont HL, Formal SB, Hornick RB, Snyder MJ, Libonati JP, Sheahan DG, LaBrec EH, Kalas JP. 1971. Pathogenesis of Escherichia coli diarrhea. N Engl J Med 285:1–9. 10.1056/NEJM197107012850101. [DOI] [PubMed] [Google Scholar]
- 5.Robins-Browne RM, Holt KE, Ingle DJ, Hocking DM, Yang J, Tauschek M. 2016. Are Escherichia coli pathotypes still relevant in the era of whole-genome sequencing? Front Cell Infect Microbiol 6:141. 10.3389/fcimb.2016.00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sarowska J, Futoma-Koloch B, Jama-Kmiecik A, Frej-Madrzak M, Ksiazczyk M, Bugla-Ploskonska G, Choroszy-Krol I. 2019. Virulence factors, prevalence and potential transmission of extraintestinal pathogenic Escherichia coli isolated from different sources: recent reports. Gut Pathog 11:10. 10.1186/s13099-019-0290-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Touchon M, Perrin A, de Sousa JAM, Vangchhia B, Burn S, O'Brien CL, Denamur E, Gordon D, Rocha EP. 2020. Phylogenetic background and habitat drive the genetic diversification of Escherichia coli. PLoS Genet 16:e1008866. 10.1371/journal.pgen.1008866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Picard B, Garcia JS, Gouriou S, Duriez P, Brahimi N, Bingen E, Elion J, Denamur E. 1999. The link between phylogeny and virulence in Escherichia coli extraintestinal infection. Infect Immun 67:546–553. 10.1128/IAI.67.2.546-553.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moulin-Schouleur M, Reperant M, Laurent S, Bree A, Mignon-Grasteau S, Germon P, Rasschaert D, Schouler C. 2007. Extraintestinal pathogenic Escherichia coli strains of avian and human origin: link between phylogenetic relationships and common virulence patterns. J Clin Microbiol 45:3366–3376. 10.1128/JCM.00037-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson JR, Delavari P, Kuskowski M, Stell AL. 2001. Phylogenetic distribution of extraintestinal virulence-associated traits in Escherichia coli. J Infect Dis 183:78–88. 10.1086/317656. [DOI] [PubMed] [Google Scholar]
- 11.Denamur E, Clermont O, Bonacorsi S, Gordon D. 2021. The population genetics of pathogenic Escherichia coli. Nat Rev Microbiol 19:37–54. 10.1038/s41579-020-0416-x. [DOI] [PubMed] [Google Scholar]
- 12.Kamal SM, Simpson DJ, Wang Z, Gänzle M, Römling U. 2021. Horizontal transmission of stress resistance genes shape the ecology of beta- and gamma-proteobacteria. Front Microbiol 12:696522. 10.3389/fmicb.2021.696522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Z, Hu H, Zhu T, Zheng J, Ganzle MG, Simpson DJ. 2021. Ecology and function of the transmissible locus of stress tolerance in Escherichia coli and plant-associated Enterobacteriaceae. mSystems 6:e0037821. 10.1128/mSystems.00378-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mercer RG, Zheng J, Garcia-Hernandez R, Ruan L, Ganzle MG, McMullen LM. 2015. Genetic determinants of heat resistance in Escherichia coli. Front Microbiol 6:932. 10.3389/fmicb.2015.00932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee C, Wigren E, Trcek J, Peters V, Kim J, Hasni MS, Nimtz M, Lindqvist Y, Park C, Curth U, Lunsdorf H, Romling U. 2015. A novel protein quality control mechanism contributes to heat shock resistance of worldwide-distributed Pseudomonas aeruginosa clone C strains. Environ Microbiol 17:4511–4526. 10.1111/1462-2920.12915. [DOI] [PubMed] [Google Scholar]
- 16.Wang Z, Fang Y, Zhi S, Simpson DJ, Gill A, McMullen LM, Neumann NF, Ganzle MG. 2020. The locus of heat resistance confers resistance to chlorine and other oxidizing chemicals in Escherichia coli. Appl Environ Microbiol 86. 10.1128/AEM.02123-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li H, Mercer R, Behr J, Heinzlmeir S, McMullen LM, Vogel RF, Ganzle MG. 2020. Heat and pressure resistance in Escherichia coli relates to protein folding and aggregation. Front Microbiol 11:111. 10.3389/fmicb.2020.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mercer R, Nguyen O, Ou Q, McMullen L, Ganzle MG. 2017. Functional analysis of genes comprising the locus of heat resistance in Escherichia coli. Appl Environ Microbiol 83. 10.1128/AEM.01400-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boll EJ, Marti R, Hasman H, Overballe-Petersen S, Stegger M, Ng K, Knochel S, Krogfelt KA, Hummerjohann J, Struve C. 2017. Turn up the heat-food and clinical Escherichia coli isolates feature two transferrable loci of heat resistance. Front Microbiol 8:579. 10.3389/fmicb.2017.00579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guragain M, Brichta-Harhay DM, Bono JL, Bosilevac JM. 2021. Locus of heat resistance (LHR) in meat-borne Escherichia coli: screening and genetic characterization. Appl Environ Microbiol 87. 10.1128/AEM.02343-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marti R, Muniesa M, Schmid M, Ahrens CH, Naskova J, Hummerjohann J. 2016. Short communication: heat-resistant Escherichia coli as potential persistent reservoir of extended-spectrum beta-lactamases and Shiga toxin-encoding phages in dairy. J Dairy Sci 99:8622–8632. 10.3168/jds.2016-11076. [DOI] [PubMed] [Google Scholar]
- 22.Zhi S, Banting G, Li Q, Edge TA, Topp E, Sokurenko M, Scott C, Braithwaite S, Ruecker NJ, Yasui Y, McAllister T, Chui L, Neumann NF. 2016. Evidence of naturalized stress-tolerant strains of Escherichia coli in municipal wastewater treatment plants. Appl Environ Microbiol 82:5505–5518. 10.1128/AEM.00143-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang P, Tran F, Stanford K, Yang X. 2020. Are antimicrobial interventions sssociated with heat-resistant Escherichia coli on meat? Appl Environ Microbiol 86. 10.1128/AEM.00512-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma A, Chui L. 2017. Identification of heat resistant Escherichia coli by qPCR for the locus of heat resistance. J Microbiol Methods 133:87–89. 10.1016/j.mimet.2016.12.019. [DOI] [PubMed] [Google Scholar]
- 25.Ma A, Glassman H, Chui L. 2020. Characterization of Escherichia coli possessing the locus of heat resistance isolated from human cases of acute gastroenteritis. Food Microbiol 88:103400. 10.1016/j.fm.2019.103400. [DOI] [PubMed] [Google Scholar]
- 26.Yang X, Tran F, Klassen MD. 2020. Heat resistance in Escherichia coli and its implications on ground beef cooking recommendations in Canada. J Food Saf 40:e12769. 10.1111/jfs.12769. [DOI] [Google Scholar]
- 27.Dlusskaya EA, McMullen LM, Ganzle MG. 2011. Characterization of an extremely heat-resistant Escherichia coli obtained from a beef processing facility. J Appl Microbiol 110:840–849. 10.1111/j.1365-2672.2011.04943.x. [DOI] [PubMed] [Google Scholar]
- 28.Stanford K, Reuter T, Bach SJ, Chui L, Ma A, Conrad CC, Tostes R, McAllister TA. 2017. Effect of severe weather events on the shedding of Shiga toxigenic Escherichia coli in slaughter cattle and phenotype of serogroup O157 isolates. FEMS Microbiol Ecol 93. [DOI] [PubMed] [Google Scholar]
- 29.Croxen MA, Law RJ, Scholz R, Keeney KM, Wlodarska M, Finlay BB. 2013. Recent advances in understanding enteric pathogenic Escherichia coli. Clin Microbiol Rev 26:822–880. 10.1128/CMR.00022-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pitout JD. 2012. Extraintestinal pathogenic Escherichia coli: an update on antimicrobial resistance, laboratory diagnosis and treatment. Expert Rev Anti Infect Ther 10:1165–1176. 10.1586/eri.12.110. [DOI] [PubMed] [Google Scholar]
- 31.Burlingame RP, Wyman L, Chapman PJ. 1986. Isolation and characterization of Escherichia coli mutants defective for phenylpropionate degradation. J Bacteriol 168:55–64. 10.1128/jb.168.1.55-64.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kumar P, Luo Q, Vickers TJ, Sheikh A, Lewis WG, Fleckenstein JM. 2014. EatA, an immunogenic protective antigen of enterotoxigenic Escherichia coli, degrades intestinal mucin. Infect Immun 82:500–508. 10.1128/IAI.01078-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mazariego-Espinosa K, Cruz A, Ledesma MA, Ochoa SA, Xicohtencatl-Cortes J. 2010. Longus, a type IV pilus of enterotoxigenic Escherichia coli, is involved in adherence to intestinal epithelial cells. J Bacteriol 192:2791–2800. 10.1128/JB.01595-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li YF, Poole S, Nishio K, Jang K, Rasulova F, McVeigh A, Savarino SJ, Xia D, Bullitt E. 2009. Structure of CFA/I fimbriae from enterotoxigenic Escherichia coli. Proc Natl Acad Sci U S A 106:10793–10798. 10.1073/pnas.0812843106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hancock V, Ferrieres L, Klemm P. 2008. The ferric yersiniabactin uptake receptor FyuA is required for efficient biofilm formation by urinary tract infectious Escherichia coli in human urine. Microbiology (Reading) 154:167–175. 10.1099/mic.0.2007/011981-0. [DOI] [PubMed] [Google Scholar]
- 36.Tu J, Xue T, Qi K, Shao Y, Huang B, Wang X, Zhou X. 2016. The irp2 and fyuA genes in High Pathogenicity Islands are involved in the pathogenesis of infections caused by avian pathogenic Escherichia coli (APEC). Pol J Vet Sci 19:21–29. 10.1515/pjvs-2016-0004. [DOI] [PubMed] [Google Scholar]
- 37.Guerra JA, Romero-Herazo YC, Arzuza O, Gomez-Duarte OG. 2014. Phenotypic and genotypic characterization of enterotoxigenic Escherichia coli clinical isolates from northern Colombia, South America. Biomed Res Int 2014:236260. 10.1155/2014/236260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hajihosein-Tabrizi A, Habibi M, Tabasi M, Karam MRA, Department of Molecular Biology, Pasteur Institute of Iran, Tehran, Iran. 2018. Distribution of genes encoding iron uptake systems among the Escherichia coli isolates from diarrheal patients of Iran. JoMMID 6:25–30. 10.29252/JoMMID.6.1.25. [DOI] [Google Scholar]
- 39.Cordonnier C, Etienne-Mesmin L, Thevenot J, Rougeron A, Renier S, Chassaing B, Darfeuille-Michaud A, Barnich N, Blanquet-Diot S, Livrelli V. 2017. Enterohemorrhagic Escherichia coli pathogenesis: role of Long polar fimbriae in Peyer's patches interactions. Sci Rep 7:44655. 10.1038/srep44655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mercer RG, Walker BD, Yang X, McMullen LM, Ganzle MG. 2017. The locus of heat resistance (LHR) mediates heat resistance in Salmonella enterica, Escherichia coli and Enterobacter cloacae. Food Microbiol 64:96–103. 10.1016/j.fm.2016.12.018. [DOI] [PubMed] [Google Scholar]
- 41.Nguyen SV, Harhay GP, Bono JL, Smith TP, Harhay DM. 2017. Genome sequence of the thermotolerant foodborne pathogen Salmonella enterica serovar Senftenberg ATCC 43845 and phylogenetic analysis of loci encoding increased protein auality control Mechanisms. mSystems 2. 10.1128/mSystems.00190-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang X, Kim Y, Ma Q, Hong SH, Pokusaeva K, Sturino JM, Wood TK. 2010. Cryptic prophages help bacteria cope with adverse environments. Nat Commun 1:147. 10.1038/ncomms1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wen Z, Wang P, Sun C, Guo Y, Wang X. 2017. Interaction of Type IV toxin/antitoxin systems in cryptic prophages of Escherichia coli K-12. Toxins 9:77. 10.3390/toxins9030077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang X, Tran F, Zhang P, Wang H. 2021. Genomic and phenotypic analysis of heat and sanitizer resistance in Escherichia coli from beef in relation to the locus of heat resistance. Appl Environ Microbiol 87. 10.1128/AEM.01574-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Figueiredo DS, Ciol MA, da Conceicao Dos Santos M, de Araujo Silva L, Bidin Brooks JB, Santos Diniz RA, Tucci HT. 2020. Comparison of the effect of nocturnal use of commercial versus custom-made wrist orthoses, in addition to gliding exercises, in the function and symptoms of carpal tunnel syndrome: a pilot randomized trial. Musculoskelet Sci Pract 45:102089. 10.1016/j.msksp.2019.102089. [DOI] [PubMed] [Google Scholar]
- 46.Qadri F, Svennerholm A-M, Faruque ASG, Sack RB. 2005. Enterotoxigenic Escherichia coli in developing countries: epidemiology, microbiology, clinical features, treatment, and prevention. Clin Microbiol Rev 18:465–483. 10.1128/CMR.18.3.465-483.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bojer MS, Hammerum AM, Jorgensen SL, Hansen F, Olsen SS, Krogfelt KA, Struve C. 2012. Concurrent emergence of multidrug resistance and heat resistance by CTX-M-15-encoding conjugative plasmids in Klebsiella pneumoniae. APMIS 120:699–705. 10.1111/j.1600-0463.2012.02885.x. [DOI] [PubMed] [Google Scholar]
- 48.Perrin A, Rocha EPC. 2021. PanACoTA: a modular tool for massive microbial comparative genomics. NAR Genomics and Bioinformatics 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Seemann T. 2014. Prokka: rapid prokaryotic genome annotation. Bioinformatics 30:2068–2069. 10.1093/bioinformatics/btu153. [DOI] [PubMed] [Google Scholar]
- 50.Shen W, Le S, Li Y, Hu F. 2016. SeqKit: a cross-platform and ultrafast toolkit for FASTA/Q file manipulation. PLoS One 11:e0163962. 10.1371/journal.pone.0163962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen Y, Ye W, Zhang Y, Xu Y. 2015. High speed BLASTN: an accelerated MegaBLAST search tool. Nucleic Acids Res 43:7762–7768. 10.1093/nar/gkv784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stamatakis A. 2014. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30:1312–1313. 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Letunic I, Bork P. 2019. Interactive tree of life (iTOL) v4: recent updates and new developments. Nucleic Acids Res 47:W256–W259. 10.1093/nar/gkz239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Che Y, Yang Y, Xu X, Brinda K, Polz MF, Hanage WP, Zhang T. 2021. Conjugative plasmids interact with insertion sequences to shape the horizontal transfer of antimicrobial resistance genes. Proc Natl Acad Sci USA 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Siguier P, Perochon J, Lestrade L, Mahillon J, Chandler M. 2006. ISfinder: the reference centre for bacterial insertion sequences. Nucleic Acids Res 34:D32–6. 10.1093/nar/gkj014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Asnicar F, Thomas AM, Beghini F, Mengoni C, Manara S, Manghi P, Zhu Q, Bolzan M, Cumbo F, May U, Sanders JG, Zolfo M, Kopylova E, Pasolli E, Knight R, Mirarab S, Huttenhower C, Segata N. 2020. Precise phylogenetic analysis of microbial isolates and genomes from metagenomes using PhyloPhlAn 3.0. Nat Commun 11:2500. 10.1038/s41467-020-16366-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nguyen LT, Schmidt HA, von Haeseler A, Minh BQ. 2015. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol Biol Evol 32:268–274. 10.1093/molbev/msu300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kalyaanamoorthy S, Minh BQ, Wong TKF, von Haeseler A, Jermiin LS. 2017. ModelFinder: fast model selection for accurate phylogenetic estimates. Nat Methods 14:587–589. 10.1038/nmeth.4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hoang DT, Chernomor O, von Haeseler A, Minh BQ, Vinh LS. 2018. UFBoot2: improving the ultrafast bootstrap approximation. Mol Biol Evol 35:518–522. 10.1093/molbev/msx281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Beghain J, Bridier-Nahmias A, Le Nagard H, Denamur E, Clermont O. 2018. ClermonTyping: an easy-to-use and accurate in silico method for Escherichia genus strain phylotyping. Microb Genom 4:e000192. 10.1099/mgen.0.000192. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 to S8. Download aem.02185-21-s0001.pdf, PDF file, 14.5 MB (14.8MB, pdf)
Tables S1 to S18. Download aem.02185-21-s0002.xlsx, XLSX file, 1.8 MB (1.8MB, xlsx)