ABSTRACT
The second messenger cyclic di-GMP (c-di-GMP) is a key molecule that controls different physiological and behavioral processes in many bacteria, including motile-to-sessile lifestyle transitions. Although the external stimuli that modulate cellular c-di-GMP contents are not fully characterized, there is growing evidence that certain amino acids act as environmental cues for c-di-GMP turnover. In the plant-beneficial bacterium Pseudomonas putida KT2440, both arginine biosynthesis and uptake influence second messenger contents and the associated phenotypes. To further understand this connection, we have analyzed the role of ArgR, which in different bacteria is the master transcriptional regulator of arginine metabolism but had not been characterized in P. putida. The results show that ArgR controls arginine biosynthesis and transport, and an argR-null mutant grows poorly with arginine as the sole carbon or nitrogen source and also displays increased biofilm formation and reduced surface motility. Modulation of c-di-GMP levels by exogenous arginine requires ArgR. The expression of certain biofilm matrix components, namely, the adhesin LapF and the exopolysaccharide Pea, as well as the diguanylate cyclase CfcR is influenced by ArgR, likely through the alternative sigma factor RpoS. Our data indicate the existence of a regulatory feedback loop between ArgR and c-di-GMP mediated by FleQ.
IMPORTANCE Identifying the molecular mechanisms by which metabolic and environmental signals influence the turnover of the second messenger c-di-GMP is key to understanding the regulation of bacterial lifestyles. The results presented here point at the transcriptional regulator ArgR as a central node linking arginine metabolism and c-di-GMP signaling and indicate the existence of a complex balancing mechanism that connects cellular arginine contents and second messenger levels, ultimately controlling the lifestyles of Pseudomonas putida.
KEYWORDS: arginine synthesis, transport, biofilm, second messenger, Pseudomonas, amino acid biosynthesis, amino acid transport, c-di-GMP, cell signaling, gene regulation, metabolism
INTRODUCTION
Second messengers are intracellular signaling molecules that modulate cell responses to environmental and metabolic stimuli. Among them, cyclic di-GMP (c-di-GMP) plays a relevant role in bacterial fitness, from cell division to antibiotic tolerance and secondary metabolism (1–4), although it has been particularly studied in connection with its effect on bacterial motility and biofilm development (5, 6). In many bacteria, increased levels of this second messenger lead to the transition from a motile planktonic lifestyle to a sessile state, promoting surface attachment and biofilm formation, while c-di-GMP degradation favors biofilm dispersal (6–8). Proteins containing diguanylate cyclase (DGC) or phosphodiesterase (PDE) activity domains are involved in the turnover of c-di-GMP (9, 10). Sometimes, these domains appear together in one protein and may be combined with sensory and/or response regulator domains (9).
The environmental signals that modulate c-di-GMP levels and the subsequent changes in gene expression are being unraveled, an intricate task due to the frequent presence of several proteins with potential DGC and/or PDE activity in a single microorganism. Such redundancy suggests the existence of complex and tight regulation of c-di-GMP levels and biofilm formation in response to different cues. In some bacterial species, amino acids, and particularly arginine, function as modulators of c-di-GMP turnover through different mechanisms. At certain concentrations, arginine promotes biofilm formation and suppresses surface motility in Pseudomonas aeruginosa PA14 (11), processes that require the DGCs RoeA and SadC. In contrast, arginine binding to RmcA, a multidomain transmembrane protein with PDE activity, leads to reduced c-di-GMP levels in P. aeruginosa PAO1 (12). This protein was recently shown to play a role in biofilm maintenance under nutrient-limiting conditions (13). Arginine and glutamate have also been found to modulate swimming motility in Burkholderia cenocepacia through a c-di-GMP PDE (14). In Salmonella enterica serovar Typhimurium, c-di-GMP synthesis is induced by arginine through its binding to the periplasmic protein ArtI, a process that involves the DGC STM1987 (15).
We have reported that l-arginine has a positive influence on c-di-GMP synthesis and biofilm formation in the plant-beneficial bacterium Pseudomonas putida KT2440, whereas l-aspartic acid causes the opposite effect (16, 17). Periplasmic amino acid binding proteins associated with arginine transport systems participate in this response (17), but no homolog of the DGC STM1987 of S. Typhimurium is present in P. putida. In this bacterium, arginine not only is an environmental signal but also seems to function as a metabolic indicator. Mutants in the last two genes of the arginine biosynthesis pathway (argG and argH, encoding the enzymes that convert aspartate and citrulline into arginine and fumarate) (see Fig. S1 in the supplemental material) show lower c-di-GMP contents than the parental strain, a defect that is partially restored by the exogenous addition of arginine, which also increases second messenger production in the wild type in a dose-dependent manner (17).
In P. putida KT2440, the response regulator with DGC activity CfcR, exerts a fundamental contribution to c-di-GMP levels in the stationary phase of growth (18, 19). The overexpression of CfcR causes a large increase in c-di-GMP, which in turn leads to a pleiotropic phenotype that includes cell aggregation, enhanced biofilm formation, and crinkly colony morphology (16, 18). This phenotype and the increase in c-di-GMP associated with CfcR overexpression are lost in ΔargG and ΔargH mutants and greatly reduced in arginine transport mutants (16, 17).
Based on currently existing data, we have proposed a model in which cellular arginine pools act as a metabolic signal that translates into c-di-GMP variations, thus modulating biofilm formation (17). However, the molecular mechanisms that connect arginine transport and metabolism with second messenger turnover and signaling in P. putida remain unknown; therefore, an alternative model where exogenous and endogenous l-arginine are sensed through different pathways cannot be excluded.
In bacteria such as P. aeruginosa and Escherichia coli, arginine metabolism and transport are controlled by the transcriptional regulator ArgR. In the presence of the amino acid, ArgR functions as a repressor of genes involved in arginine biosynthesis and activates the expression of genes in the arginine succinyltransferase (AST) catabolic pathway (Fig. S1) as well as operons encoding basic amino acid transport systems (20, 21). ArgR of E. coli forms trimers in the absence of arginine that are stabilized as hexamers by l-arginine (22). This conformational change has been proposed to alter the interaction of the protein with its DNA binding site, the ARG box, present in the operator of genes regulated by ArgR (22).
In this work, we have focused on ArgR, which had not been previously studied in P. putida, and its potential role in arginine sensing, transport, and metabolism as modulators of second messenger levels. Our results indicate that ArgR is a central node in the connection between arginine and c-di-GMP.
RESULTS
Identification and characterization of the arginine metabolism regulator ArgR.
Based on the sequence similarity with ArgR of P. aeruginosa, we identified locus PP_4482 as the one encoding this protein in P. putida KT2440. This protein shares 82% identical residues with ArgR of P. aeruginosa PAO1, and the genetic organization of the surrounding chromosomal region in P. putida is similar to that of the aotJQMOP-argR operon in P. aeruginosa (23) (see Fig. S1 in the supplemental material): upstream of argR, the argT-hisQ-hisM-hisP genes encode the components of an ABC transport system involved in l-arginine uptake (16). A gene cluster encoding the enzymes of the AST catabolic pathway for arginine utilization is located 335 bp downstream of argR (Fig. S1).
An argR-null mutant was generated by the complete removal of the open reading frame, as described in Materials and Methods. The mutant grew normally in LB or minimal medium with glucose as the carbon and energy source and ammonia as the nitrogen source, but its growth in minimal medium with l-arginine as the sole nitrogen or carbon and energy source was impaired (Fig. 1). These data indicate that ArgR is necessary for l-arginine transport and utilization in P. putida KT2440. The growth of the ΔargR strain with other basic amino acids revealed that ArgR is also involved in l-ornithine and l-lysine (but not l-histidine) uptake and/or metabolism (Fig. S2).
FIG 1.
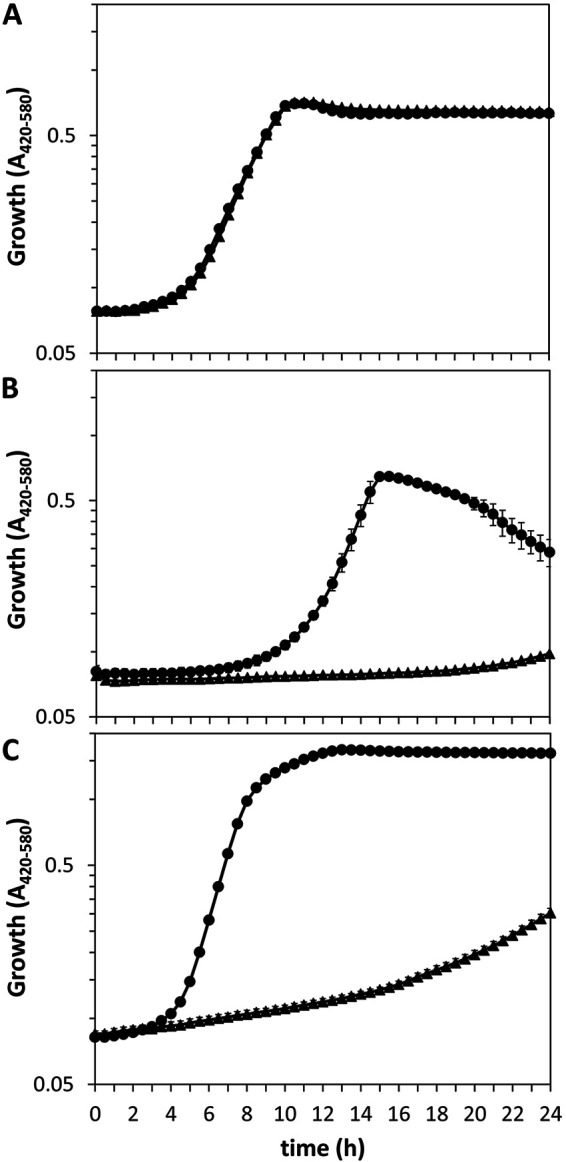
Growth of KT2440 (circles) and the ΔargR mutant (triangles) in M9 minimal medium with 10 mM glucose (A) or 10 mM l-arginine (B) as the carbon source or in M8 medium with 10 mM glucose as the carbon source and 5 mM l-arginine as the nitrogen source (C). Growth was monitored during incubation at 30°C with continuous shaking for 24 h using a Bioscreen apparatus, measuring the absorbance in the 420- to 580-nm range every 30 min. Averages and standard deviations from one representative experiment with three replicates are shown.
A recognition sequence for ArgR, termed the ARG box, has been identified in the promoter regions of P. aeruginosa genes regulated by this protein (24, 25). It consists of two half-sites, each with the consensus sequence TGTCGC[N6]GNAA[N5], with the second half-site being generally more conserved. Analysis of the upstream regions of genes related to arginine transport and metabolism in P. putida indicates the presence of putative ARG boxes in argG and argT, although in the latter, there is only one clearly conserved half-site, with an additional potential half-site 15 bp downstream and a less obvious one 5 bp upstream (Fig. S3). ARG boxes could also be present in astC (encoding succinylornithine aminotransferase, the third enzyme in the AST pathway for arginine catabolism), where three potential half-sites are found; spuE (part of a polyamine transporter); and the Arg-tRNA ligase gene argS (Fig. S3). Of these, argG and astC (annotated in P. aeruginosa as aruC) are among the genes identified as being regulated by ArgR in P. aeruginosa (25).
Arginine and ArgR modulate the expression of argT and argR.
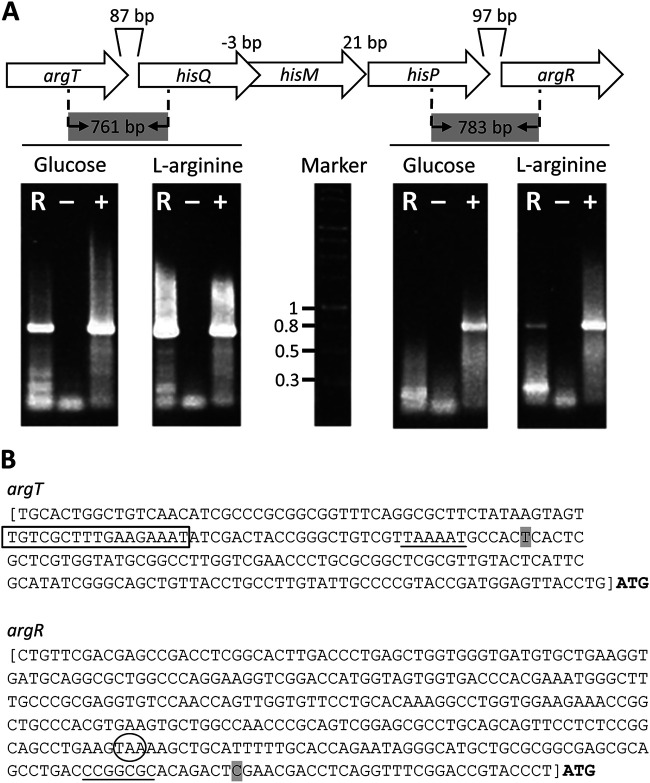
Previous transcriptional profiling of P. putida has shown that the PP_4486 (argT)-PP_4483 (hisP) cluster forms an operon (26). Based on the genetic organization, it seemed likely that argR was also cotranscribed with the upstream genes, as is the case in P. aeruginosa, although this was not observed in the above-mentioned study, done in cultures grown in minimal medium with glucose or citrate as the carbon source (26). To test this, primer pairs were designed for the amplification of fragments including the argT-hisQ and hisP-argR intergenic regions (Fig. 2A), and reverse transcription coupled with PCR (RT-PCR) was performed using RNA extracted from P. putida KT2440 cultures grown in minimal medium with either glucose or l-arginine as the carbon source. Electrophoresis of the reaction products showed a band of the expected size if argT and hisQ are cotranscribed under all conditions tested (Fig. 2A), further supporting that argT and hisQMP form an operon, as described previously (26). No amplification was observed for the region between hisP and argR in RNA obtained from cultures grown with glucose, whereas a band was observed in the case of arginine-grown cultures (Fig. 2A), indicative of argR coexpression with upstream genes, at least in response to the amino acid.
FIG 2.
(A) Analysis of the cotranscription of argT and argR with the hisQMP cluster. Electrophoresis was performed on RT-PCR products amplified with primers designed to detect transcripts containing the intergenic regions between argT and hisQ (761 bp) (left) and between hisP and argR (783 bp) (right). Lanes: R, template RNA obtained from P. putida KT2440 cultures; −, negative control without the reverse transcriptase reaction; +, positive control using DNA as the template for the reaction. (B) Determination of the transcription initiation sites for argT and argR. The base corresponding to the +1 site is shaded. The conserved −10 region for the argT promoter and the GC-rich region in the argR promoter are underlined, and the predicted ARG site overlapping the −35 site of argT is boxed. The stop codon for hisP is circled. The square brackets indicate the fragments used to construct transcriptional fusions in plasmids pLBM21 and pLBM20.
To further characterize the expression pattern of the operon, the transcriptional start site for argT was determined by 5′ rapid amplification of cDNA ends (RACE) to be 116 bp upstream of the ATG codon (Fig. 2B). A −10 sequence similar to the consensus sequence for σ70-dependent promoters could be identified, with an ARG box half-site overlapping the −35 region, which lacks a clear resemblance to the σ70 consensus (Fig. 2B). An argT::lacZ transcriptional fusion was then constructed by cloning a 229-bp region upstream of argT into pMP220 (27). The resulting construct (pLBM21) was introduced into KT2440 and the ΔargR mutant, and β-galactosidase activity was analyzed during growth in M9 medium with glucose, supplemented or not with l-arginine. As shown in Fig. 3A, activity remained more or less stable in the wild-type strain during growth in the absence of l-arginine, whereas the addition of the amino acid caused a large increase in activity. In contrast, expression was completely abolished in the ΔargR mutant, regardless of the presence of the amino acid.
FIG 3.
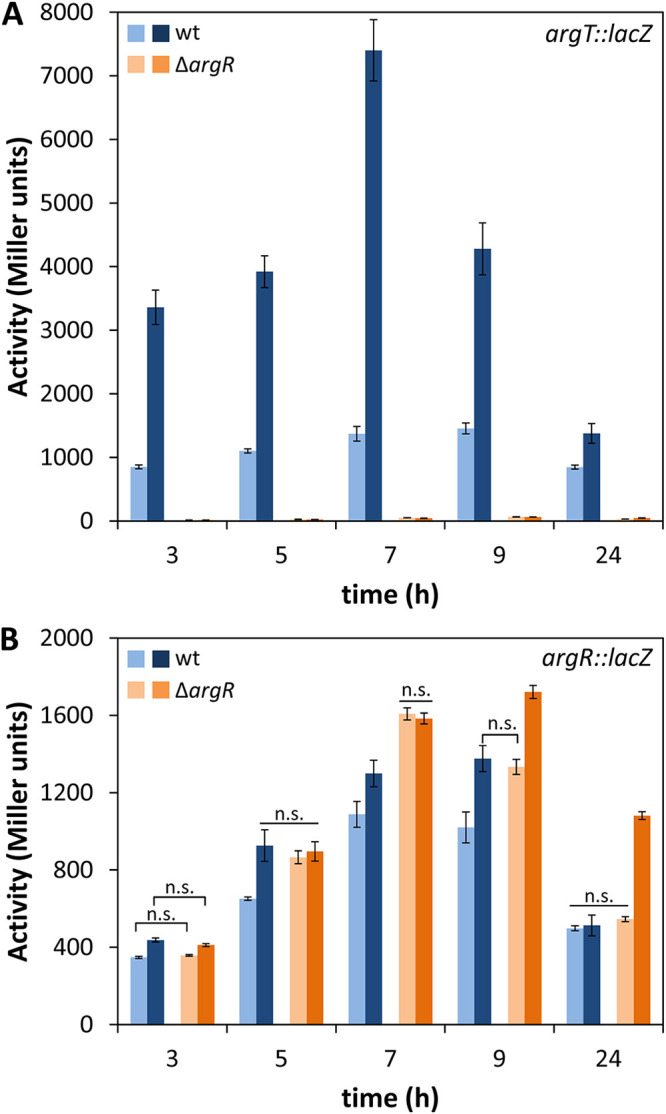
Expression of argT::lacZ (pLBM21) (A) and argR::lacZ (pLBM20) (B) during growth in minimal medium with glucose in the absence or presence of l-arginine. β-Galactosidase activity (Miller units) was assessed in KT2440 (wild type [wt]) and the ΔargR mutant harboring pLBM21 or pLBM20 during growth in minimal medium with glucose (light bars) or glucose and 10 mM l-arginine (dark bars). The data correspond to averages and standard deviations from two experiments with three technical replicates each (n = 6). Differences in panel A between the wild type and the mutant and in the wild type in the presence and absence of arginine were statistically significant at all time points, while samples not showing significant differences in panel B at a given time point are indicated as n.s. (not significant) (P ≤ 0.05 by ANOVA).
Such a strict dependence on ArgR for the activity of the argT promoter suggested that at least basal expression of argR must take place in the absence of l-arginine and therefore would not be due solely to arginine-dependent transcription from the argT promoter. To check if an additional promoter could be present upstream of argR, a transcriptional fusion was generated by cloning a 362-bp fragment containing the 97-bp intergenic region between hisP and argR into pMP220. The construct (pLBM20) was introduced into KT2440 and the ΔargR mutant, and β-galactosidase activity was assayed during growth in minimal medium with glucose and in the absence or presence of l-arginine. The results presented in Fig. 3B indicate the existence of an additional promoter in this region, with increased expression in the late exponential to early stationary phases (5 to 9 h of growth). Activity was enhanced in the presence of the amino acid and was increased in the ΔargR mutant with respect to the wild type, indicating that ArgR exerts a certain level of self-repression. The transcriptional start site for the argR promoter was determined by 5′ RACE to be located 29 bp upstream of the ATG codon (Fig. 3B). In this case, the −10 and −35 regions do not show a clear resemblance to σ70-dependent promoters, and no clear ARG box is found, although a GC-rich sequence similar to one of the overrepresented motifs identified in P. putida promoters (26) is present.
All these data suggest that the expression of argR is subject to complex control that includes a certain degree of self-regulation and an arginine-dependent response.
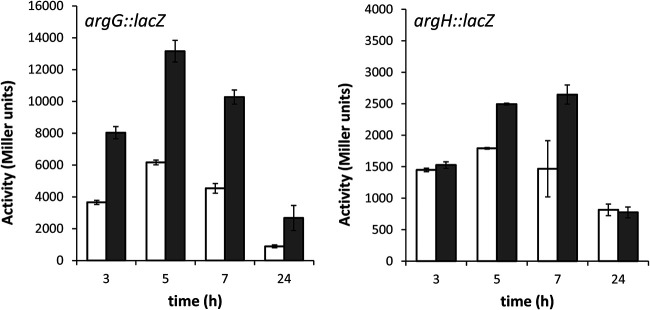
ArgR controls arginine biosynthesis through the expression of argG.
To define the role of ArgR in the regulation of l-arginine biosynthesis in P. putida, transcriptional fusions of the argG and argH promoter regions to the reporter gene lacZ were constructed in pMP220. These constructs, named pLBM13 and pLBM14, respectively, were introduced into the wild type and the ΔargR mutant, and β-galactosidase activity was assayed during growth in LB. As shown in Fig. 4, the activity of the argG::lacZ fusion was higher in the mutant than in the wild type at all times, whereas increased expression of the argH::lacZ fusion was observed only in the mutant at 5 to 7 h of growth. These results indicate that ArgR functions as a negative regulator of arginine synthesis mainly through argG, as described previously for P. aeruginosa (24), having a weaker effect on argH.
FIG 4.
ArgR functions as a repressor of l-arginine biosynthesis genes. β-Galactosidase activities (Miller units) of argG::lacZ (pLBM13) (A) and argH::lacZ (pLBM14) (B) transcriptional fusions were measured in KT2440 (white bars) and the ΔargR mutant (gray bars) in LB at the indicated times. The data are averages and standard deviations from two independent experiments with three technical repetitions each (n = 6). Statistically significant differences between the wild type and the ΔargR mutant were detected from 3 h onward (A) and at 5 h and 7 h (B), respectively (P ≤ 0.05 by Student’s t test).
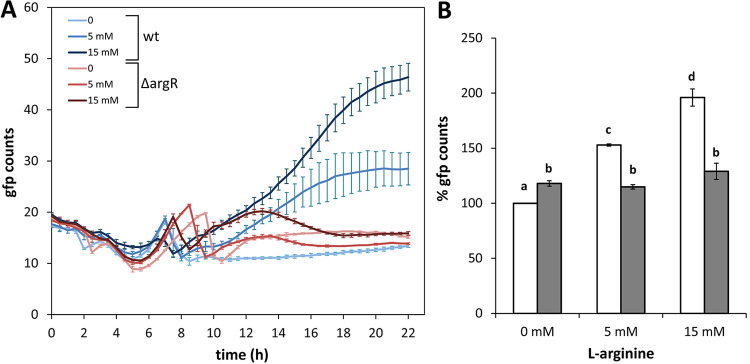
ArgR is required for the increase in c-di-GMP levels in response to exogenous l-arginine.
Given the role of ArgR as a positive regulator of argT and a negative regulator of argG, we predicted that the ΔargR mutant would show higher c-di-GMP levels than the wild type due to increased arginine synthesis but a reduced response to exogenous l-arginine due to limited transport of the amino acid. To test these predictions, the c-di-GMP bioreporter plasmid pCdrA::gfpC (28) was transferred to KT2440 and the ΔargR mutant, and fluorescence was measured during growth in 1/3 LB with increasing concentrations of l-arginine. As shown in Fig. 5A, the fluorescence profile (corrected by growth) of the mutant was similar to that of the wild type in the absence of l-arginine supplementation, but the dose-dependent increase in c-di-GMP levels in the presence of the amino acid observed in KT2440 was greatly reduced in the ΔargR mutant. These results were similar in minimal medium (Fig. S4) and indicate that functional ArgR is required for the response to environmental l-arginine in terms of c-di-GMP synthesis. In contrast, only a slight overall increase in c-di-GMP (around 15%) was observed in the mutant with respect to the wild type throughout growth in the absence of exogenously added amino acid (Fig. 5B).
FIG 5.
Influence of ArgR on the changes in cellular c-di-GMP contents in response to exogenous l-arginine. (A) Strain KT2440 (wild type) and the ΔargR mutant harboring pCdrA::gfpC were inoculated into microtiter plates containing diluted LB (1:3) supplied with different final concentrations of l-arginine (0, 5, and 15 mM). Fluorescence and turbidity were recorded every 30 min for 24 h using a Varioskan Lux fluorimeter. Data (GFP counts) correspond to fluorescence values normalized by growth (OD600) and are averages and standard deviations from one representative experiment using three experimental replicates under each condition. The line color intensity indicates increasing concentrations of the amino acid. (B) Values corresponding to the area under the curve derived from fluorescence measurements normalized by culture growth (OD600) were calculated for KT2440 (white bars) and the ΔargR mutant (gray bars), to obtain a global overview of fluorescence along the whole growth curve, where 100% corresponds to the wild type without l-arginine supplementation. Statistically significant differences between groups (P ≤ 0.01 by ANOVA) are indicated by different lowercase letters.
ArgR influences surface motility and biofilm formation.
Based on the above-described results, surface motility and biofilm formation were analyzed in the ΔargR mutant, given the opposing role of c-di-GMP levels in these phenotypes. The mutant showed reduced surface motility on PG agar plates (Fig. 6A), whereas biofilm formation on microtiter plates was enhanced with respect to the parental strain in the absence of exogenously added l-arginine (Fig. 6B). However, while the addition of the amino acid caused an increase in the attached biomass in the wild type, this was not the case for the ΔargR mutant (Fig. 6C). These results are consistent with the observed influence of ArgR on c-di-GMP levels and its role in the response to exogenous l-arginine.
FIG 6.
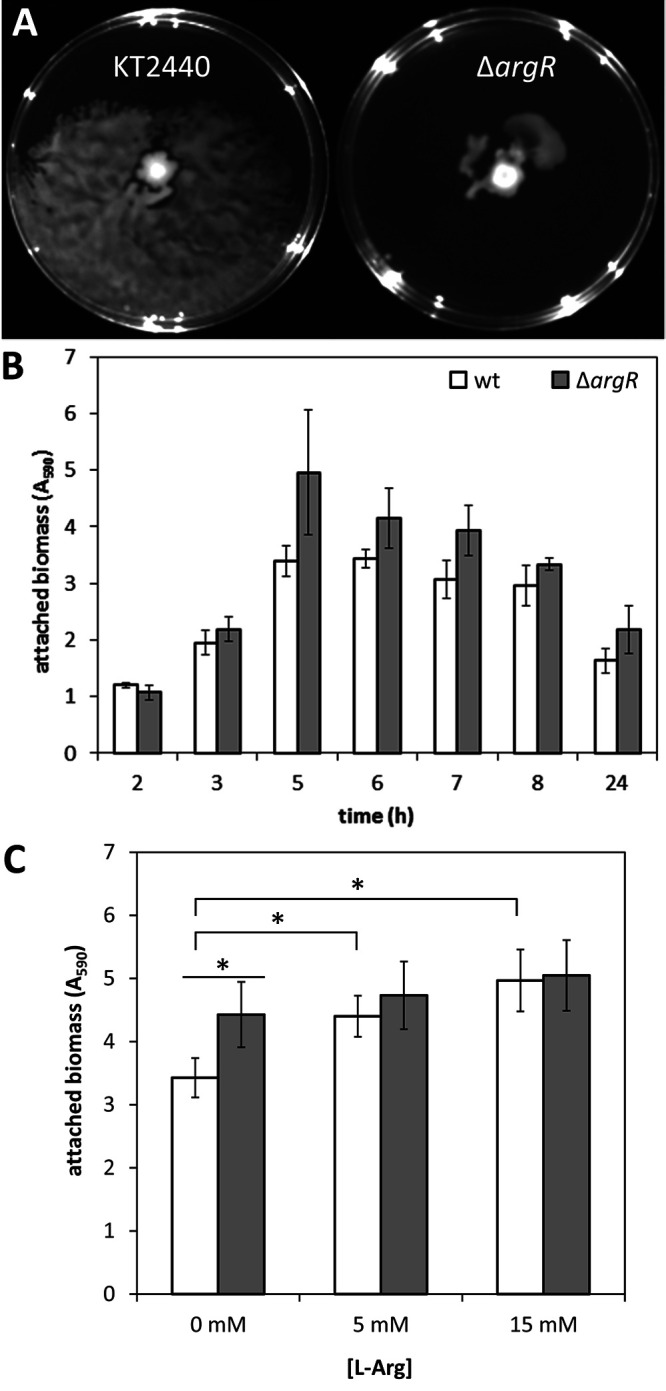
(A) Surface motility of KT2440 and the ΔargR mutant. Cultures grown overnight were diluted to an OD660 of 1, 2 μL was spotted onto the center of the plate, and images were taken after 72 h of growth at 25°C. The assay was done in duplicate, with spotting on six plates in each case, and one representative image is shown for each strain. (B) Biofilm formation by KT2440 (wild type) and its ΔargR derivative during growth in LB in 96-well polystyrene plates under static conditions. At the indicated times, adhered biomass was quantified after crystal violet staining (A595). Data correspond to averages and standard deviations from two independent experiments with 15 replicates per time point. Statistically significant differences between KT2440 and the ΔargR mutant were detected from 5 h onward (P ≤ 0.05 by Student’s t test). (C) Effect of exogenous arginine on biofilm formation by KT2440 (white bars) and the ΔargR mutant (gray bars) after 10 h of growth in FAB medium with glucose as the carbon source supplied with 0, 5, or 15 mM l-arginine. The setup and attached biomass quantification were done as described above for panel B. Data correspond to averages and standard deviations from three independent experiments with three technical replicates. Statistically significant differences (P ≤ 0.05 by ANOVA) are indicated (*).
We next checked if the increase in attachment could correlate with changes in the expression of structural components of the biofilm matrix, namely, the two large adhesins LapF and LapA and the exopolysaccharides (EPSs) Pea, Peb, and cellulose (Bcs). KT2440 and its ΔargR derivative harboring plasmids pMMG1, pMMGA, pMP220-pea, pMP220-peb, and pMP220-bcs, which carry transcriptional fusions of these elements to lacZ (29–31), were grown in LB, and β-galactosidase activity was measured. Differences were observed for the lapF::lacZ and pea::lacZ fusions, which showed higher activity in the mutant than in the wild type, whereas the expression of the other fusions was not significantly influenced by ArgR under these conditions (Fig. 7 and Fig. S5). These data correlate with the observed differences in biofilm formation, which are evident at relatively advanced times of growth (Fig. 6B), when Pea and LapF are expressed (Fig. S5).
FIG 7.
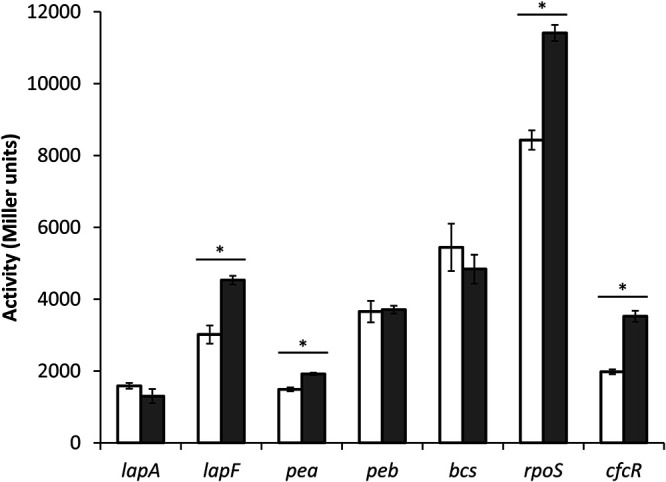
Influence of argR mutation on the expression of adhesin- and EPS-encoding genes, rpoS and cfcR. Plasmids pMMGA, pMMG1, pMP220-bcs, pMP220-pea, pMP220-peb, and pMIR200, harboring transcriptional fusions of lapA, lapF, pea, peb, bcs, and cfcR to ′lacZ, and pMAMV21, harboring an rpoS-lacZ protein fusion, were introduced into KT2440 (white bars) and the ΔargR mutant (gray bars), and β-galactosidase activity was measured after 10 h of growth in LB. The data are averages and standard deviations from two biological replicates with three technical repetitions each. Statistically significant differences (P ≤ 0.05 by Student’s t test) are indicated (*).
The transcription of pea and lapF has been shown to be under the control of the alternative sigma factor RpoS (32, 33), which in turn is influenced by mutations in the arginine synthesis genes argG and argH as well as by exogenous l-arginine within a certain concentration range (17). All this prompted us to consider a potential regulatory connection between ArgR and RpoS. Plasmid pMAMV21, harboring an rpoS-lacZ protein fusion (18), was introduced into KT2440 and the ΔargR mutant, and β-galactosidase activity was analyzed during growth in LB. As shown in Fig. 7, activity was higher in the ΔargR mutant. Given that RpoS is involved in the control of cfcR (18), the influence of ArgR on the expression of a cfcR::lacZ transcriptional fusion harbored in plasmid pMIR200 (19) was also tested (Fig. 7). As expected, β-galactosidase activity was higher in the ΔargR strain than in the wild type. All these data place ArgR in the regulatory cascade that connects arginine and biofilm formation.
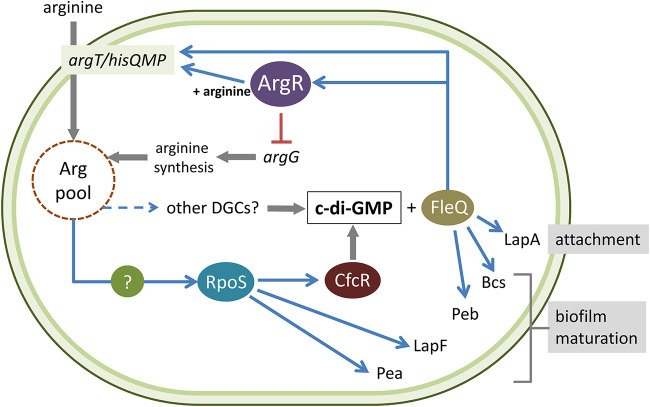
A regulatory feedback loop between arginine and c-di-GMP.
In Pseudomonas, the transduction of c-di-GMP signaling takes place through the binding of this second messenger to the transcriptional regulator FleQ, which suffers a conformational change that results in changes in the expression of its target genes (34). Interestingly, the region upstream of argT was found among those bound by FleQ in a chromatin immunoprecipitation sequencing (ChIP-Seq) analysis of the regulon in P. putida (35; our unpublished results). This made us consider the existence of a potential feedback loop between c-di-GMP and arginine signaling. To test this possibility, the argT::lacZ and argR::lacZ fusions were introduced into strain cfcK-77, a fleQ mutant derivative of KT2440 (16), and β-galactosidase activity was analyzed. The results indicate that FleQ acts as a positive regulator of the expression of both argR (Fig. 8A) and the whole argT-hisQMP-argR cluster (Fig. 8B), suggesting that arginine transport and the regulation of arginine metabolism are modulated by c-di-GMP. To confirm this idea, the expression of the argR::lacZ fusion was evaluated in KT2440 and the fleQ mutant harboring pMAMV1, a multicopy plasmid that carries cfcR under the control of its own promoter, rendering high levels of c-di-GMP (18). As shown in Fig. 8C, the activity of the argR::lacZ fusion increased by 2-fold in KT2440(pMAMV1) with respect to the control harboring the empty vector pBBR1-MCS5, whereas no differences were found in the fleQ mutant. These data indicate that the expression of ArgR is modulated by c-di-GMP via FleQ.
FIG 8.
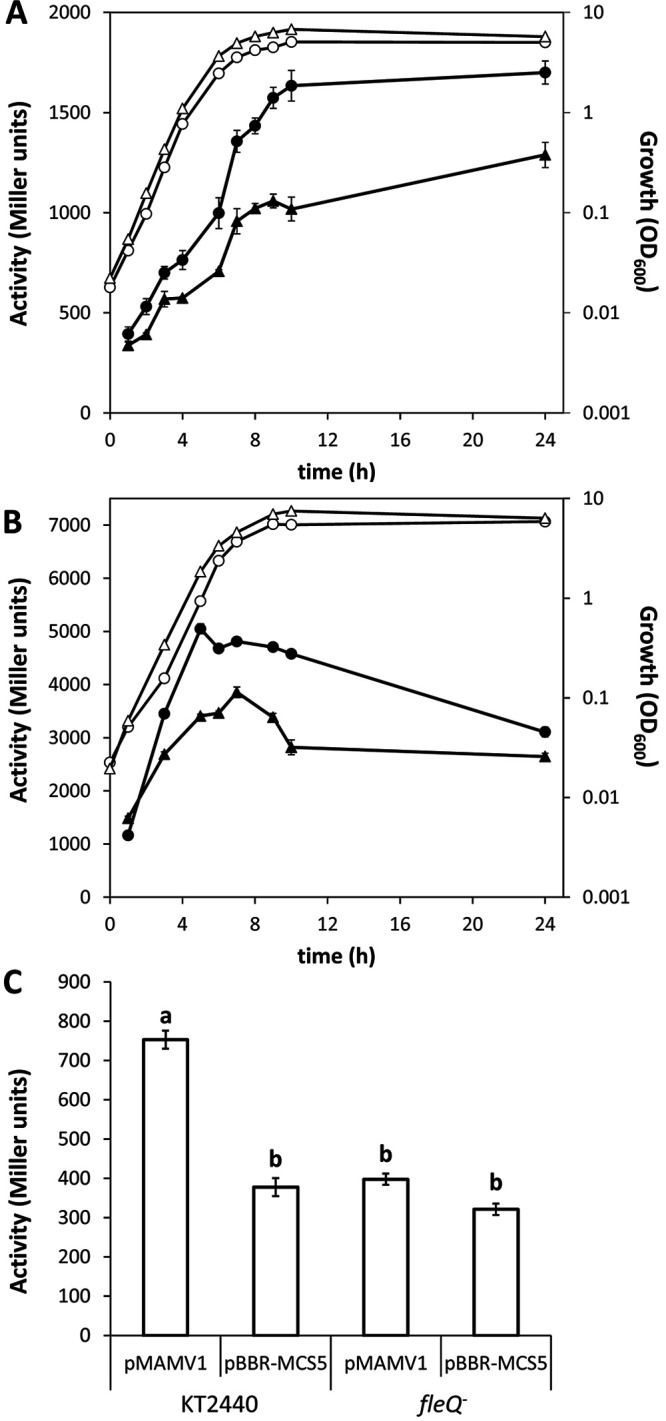
Expression of argR and argT is modulated by c-di-GMP via fleQ. (A and B) Plasmids harboring argR::lacZ (pLBM20) (A) and argT::lacZ (pLBM21) (B) were introduced into KT2440 (circles) and its fleQ mutant derivative cfcK-77 (triangles). Growth (OD600) (open symbols) and β-galactosidase activity (Miller units) (closed symbols) were measured over time in LB. The data are averages and standard deviations from two biological replicates with three technical repetitions each. (C) Activity of the argR::lacZ fusion in KT2440 and cfcK-77 harboring pMAMV1 (cfcR in multicopy) or pBBR1-MCS5 (empty vector) after growth overnight in LB. Cultures were treated with glass beads (diameter of 425 to 600 μm) for 1 min to disrupt cell aggregates formed as a consequence of high c-di-GMP levels due to the presence of pMAMV1. The results correspond to averages and standard deviations from four biological replicates with three technical repetitions each. Statistically significant differences (P ≤ 0.05 by ANOVA) are indicated by different lowercase letters.
DISCUSSION
Besides being required for protein synthesis, l-arginine has other important functions in bacteria. It can be efficiently used as a carbon, energy, and nitrogen source; it is a precursor for the synthesis of other amino acids, polyamines, and nitric oxide; and it is involved in siderophore production and the oxidative stress response in P. putida (21, 36–39). In recent years, l-arginine has also emerged as a modulator of c-di-GMP signaling and social behaviors in different bacteria, both directly and indirectly (11–15, 40). Given its multiple roles, the complexity of arginine metabolism and its tight regulation (21, 24) are not surprising. In many bacteria, the transcriptional regulator ArgR is the key element controlling the metabolism and transport of this amino acid. We have identified locus PP_4482 as the one encoding ArgR in P. putida KT2440, being the last gene of a cluster with argT-hisQMP, the main arginine transport system in this bacterium (17). This function and the similarity to the aotJQMOP-argR operon in P. aeruginosa (23) may call for a reannotation of the gene nomenclature in P. putida. Our results show that ArgR acts as a negative regulator of l-arginine synthesis, at least through argG, encoding argininosuccinate synthase, and as a positive regulator of l-arginine transport and utilization as a carbon or nitrogen source, with the argT promoter being strictly dependent on ArgR. In addition, it is required for l-ornithine utilization as a carbon source. Similar results have been reported for an argR mutant of P. aeruginosa, which was unable to grow with arginine or ornithine as the sole carbon source (40).
Our data indicate that ArgR is required for the arginine-dependent increase in c-di-GMP contents reported previously (16) and exerts an opposing effect on surface motility and biofilm formation. It also influences the expression of at least two elements of the extracellular matrix of P. putida biofilms, the cell-cell adhesion protein LapF and the EPS Pea. These two elements are regulated by the stationary-phase sigma factor RpoS, and we have shown that ArgR acts as a negative modulator of rpoS expression. These results are consistent with our previous data indicating that the expression of pea and lapF is reduced in the ΔargG and ΔargH arginine biosynthesis mutants (17) since these genes (mainly argG) are negatively regulated by ArgR. This can also explain the increase in cfcR expression observed in the ΔargR mutant given that RpoS controls the transcription of cfcR (18). In fact, a cfcR argR double mutant shows very low c-di-GMP levels, and the response to the amino acid is completely lost (see Fig. S6 in the supplemental material), which confirms the involvement of both elements in the response to exogenous l-arginine.
Putative ArgR recognition sites (ARG boxes) (22, 41) can be found in the promoter regions of argG and argT, indicating a direct effect of ArgR on their expression. No obvious ARG box seems to be present upstream of other genes related to arginine metabolism that are directly regulated by ArgR in P. aeruginosa, such as carA or argF (25). Similarly, argH and rpoS do not show clear ARG boxes in their upstream regions. This could imply that the influence of ArgR on these genes is indirect, although a more in-depth analysis combined with DNA-protein binding assays will be required to fully characterize the recognition sequence for ArgR in P. putida.
The expression of argR is in turn subject to complex regulation. It is cotranscribed with the upstream genes in the presence of the amino acid, while no cotranscription is observed in its absence despite the fact that the argT promoter is active under these conditions (Fig. 4). In P. aeruginosa, the transcription of argR occurs from two promoters, P1 and P2, upstream of aotJ (23). The expression of the whole operon takes place from the distal promoter P1 in the absence of l-arginine, whereas transcription from the proximal promoter P2 is highly increased when the amino acid is present, with this induction being mediated by ArgR (23). In contrast to P. aeruginosa, in P. putida, under the tested conditions, we have been able to detect only one transcriptional start site upstream of argT, with an additional arginine-responsive promoter upstream of argR, although no clear ARG box is found in this region, and its activity is lower than that of the promoter upstream of argT. The expression pattern of the argR promoter indicates complex regulation: increased activity is observed in the ΔargR mutant, indicative of self-regulation, but the response to l-arginine is still observed at late times of growth, suggesting the existence of additional arginine-dependent regulatory elements active in stationary phase, which are yet to be identified.
Perhaps the most intriguing result is the positive regulation of argR and argT expression exerted by c-di-GMP through FleQ. The interpretation of these data from a physiological point of view is not trivial. Exogenous arginine augments c-di-GMP levels in a dose-dependent way (16, 17), and in turn, the second messenger would stimulate arginine transport, thus leading to further increases in c-di-GMP and related phenotypes. This could be a way to amplify the signal and quickly modify the planktonic or sessile lifestyle of P. putida in response to an environmental abundance of l-arginine, a readily usable nitrogen, carbon, and energy source. On the other hand, the increased expression of ArgR would lead to the shutdown of arginine synthesis. Reduced c-di-GMP is observed in arginine biosynthesis mutants (16, 17), and the effect of the second messenger on ArgR through FleQ would likely also imply an accumulation of aspartate and citrulline (the substrates for ArgG). l-Aspartate has the opposite effect of l-arginine in terms of c-di-GMP levels and the crinkly colony morphology associated with its overproduction (16, 17). Therefore, the positive-feedback loop between l-arginine transport and c-di-GMP levels would be countered by the associated reduction in the synthesis of the amino acid. This apparent contradiction might reflect a mechanism to prevent the bacterium from “overreacting” to environmental arginine, finely tuning c-di-GMP signaling with respect to cellular pools of the amino acid.
The proposed model, based on our current knowledge regarding the connections between arginine and c-di-GMP in P. putida, is summarized in Fig. 9. Although this complex system deserves further analysis for its detailed characterization, the results presented here reveal the key role of ArgR in the regulatory network that links central metabolism with second messenger synthesis and signal transduction.
FIG 9.
Current model of the regulatory network connecting l-arginine metabolism, c-di-GMP signaling, and biofilm formation in P. putida KT2440. ArgR influences c-di-GMP levels (and, therefore, biofilm formation) indirectly through its positive regulatory role in arginine transport and negative effect on arginine synthesis. Arginine and c-di-GMP signaling are connected through the RpoS-dependent transcription of cfcR and potentially via other DGCs. In turn, c-di-GMP modulates the expression of argR via FleQ, thus establishing a feedback loop between arginine pools and c-di-GMP. See the text for further details.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The strains and plasmids used in this work are detailed in Table 1. P. putida KT2440 is a plasmid-free derivative of P. putida mt-2 (42). P. putida strains were routinely grown at 30°C in LB (43) or in M9 minimal medium (44) with 10 or 20 mM glucose or citrate as the carbon and energy source. Escherichia coli strains were grown at 37°C in LB. When appropriate, the following antibiotics were used at the indicated final concentrations: chloramphenicol (Cm) at 30 μg/mL, kanamycin (Km) at 25 μg/mL, rifampin (Rif) at 10 μg/mL, piperacillin (Pip) at 30 μg/mL, ampicillin (Ap) at 100 μg/mL, streptomycin (Sm) at 50 μg/mL (for E. coli) and 100 μg/mL (for P. putida), tetracycline (Tc) at 10 μg/mL, and gentamicin (Gm) at 10 μg/mL (for E. coli) and 50 or 100 μg/mL (for P. putida). When fitting, l-arginine (Sigma-Aldrich) was added at the indicated concentrations.
TABLE 1.
Strains and plasmids used in this work
| Strain or plasmid | Genotype or relevant characteristic(s)a | Source or referenceb |
|---|---|---|
| Strains | ||
| E. coli | ||
| CC118λpir | Rifr λpir | PRCC |
| DH5α | supE44 lacU169(ϕ80lacZΔM15) hsdR17(rK− mK−) recA1 endA1 gyrA96 thi-1 relA1 | PRCC |
| P. putida | ||
| KT2440 | Wild type; derivative of P. putida mt-2, cured of pWWO | 41 |
| ΔargR | Null mutant derivative of KT2440 in PP_4482 | This work |
| cfcK-77 | Kmr; fleQ mutant derivative of KT2440 obtained by random transposon mutagenesis with mini-Tn5[Km1] | 16 |
| Plasmids | ||
| pCR2.1-TOPO | Kmr; PCR cloning vector with β-galactosidase α-complementation | Invitrogen |
| pBBR1-MCS5 | Gmr; broad-host-range cloning vector; mobilizable | PRCC |
| pRK600 | Helper plasmid for conjugation; Cmr mob tra | PRCC |
| pCdrA::gfpC | Apr (Pipr) Gmr; FleQ-dependent c-di-GMP bioreporter | 27 |
| pKNG101 | Smr; oriR6K mobRK2 sacBR | 47 |
| pMP220 | Tcr; oriRK2 ′lacZ | 24 |
| pLBM9 | pCR2.1-TOPO derivative with a 375-bp BglII/PstI fragment containing the argG promoter | This work |
| pLBM11 | pCR2.1-TOPO derivative with a 369-bp BglII/PstI fragment containing the argH promoter | This work |
| pLBM13 | Tcr; transcriptional fusion argG::lacZ in pMP220 | This work |
| pLBM14 | Tcr; transcriptional fusion argH::lacZ in pMP220 | This work |
| pLBM17 | Kmr; pCR2.1-TOPO derivative with a 362-bp BglII/EcoRI fragment containing the argR upstream region | This work |
| pLBM18 | Kmr; pCR2.1-TOPO derivative with a 229-bp BglII/PstI fragment containing the argT promoter | This work |
| pLBM20 | Tcr; transcriptional fusion argR::lacZ in pMP220 | This work |
| pLBM21 | Tcr; transcriptional fusion argT::lacZ in pMP220 | This work |
| pLBM36 | Kmr; pCR2.1-TOPO derivative with a 1.5-kb NotI fragment containing the argR-null allele | This work |
| pLBM37 | Smr; pKNG101 derivative for argR-null allele replacement with the 1.5-kb NotI fragment of pLBM36 cloned into pKNG101 | This work |
| pMAMV1 | Gmr; derivative plasmid of pBBR1-MCS5 containing cfcR; confers high c-di-GMP levels to KT2440 | 18 |
| pMP220-bcs | Tcr; transcriptional fusion PP_2629::lacZ | 30 |
| pMP220-pea | Tcr; transcriptional fusion PP_3132::lacZ | 30 |
| pMP220-peb | Tcr; transcriptional fusion PP_1795::lacZ | 30 |
| pMMG1 | Tcr; transcriptional fusion lapF::lacZ | 28 |
| pMMGA | Tcr; transcriptional fusion lapA::lacZ | 29 |
| pMAMV21 | Tcr; translational fusion rpoS-lacZ in pMP220-BamHI | 18 |
| pMIR200 | Tcr; transcriptional fusion cfcR::lacZ in pMP220 | 16 |
Rifr, rifampin resistance; Cmr, chloramphenicol resistance; Kmr, kanamycin resistance; Tcr, tetracycline resistance; Smr, streptomycin resistance; Pipr, piperacillin resistance; Apr, ampicillin resistance; Gmr, gentamicin resistance.
PRCC, Pseudomonas Reference Culture Collection.
For growth curves, cultures grown overnight on glucose-M9 agar were scraped off in 1 mL of M9 salts and washed twice in the same medium. Cultures were adjusted to a final optical density at 660 nm (OD660) of 0.02 in M9 minimal medium, and 150 μL per well was distributed into 100-well plates (Honeycomb). l-Arginine, l-lysine, l-histidine, or l-ornithine (Sigma-Aldrich) was added as a carbon source at a final concentration of 10 mM. Plates were incubated at 30°C with continuous shaking (200 rpm), and the growth of the cultures (OD420–580) was monitored every 30 min for 24 h in an automated Bioscreen C MBR apparatus equipped with a wide-band filter (420 to 580 nm). Alternatively, M8 medium (i.e., M9 medium without ammonia [44]) with 10 mM glucose as the carbon source and the different amino acids supplied as the nitrogen source was used.
Molecular biology techniques.
The primers used are detailed in Table 2. DNA preparation, digestion with restriction enzymes, plasmid dephosphorylation, adenylation, ligation, and bacterial transformations were carried out using standard methods (44, 45). Kits for plasmid purification and the extraction of DNA amplicons from agarose gels were used according to the manufacturers’ indications (Qiagen and NZYTech, respectively). PCR amplifications were performed using Taq DNA polymerase (Roche) or Phusion high-fidelity DNA polymerase (Thermo Fisher Scientific). Plasmid transfer to P. putida KT2440 cells was carried out by triparental conjugation or electrotransformation, as described previously (46).
TABLE 2.
Primers used in this work
| Primer name | Sequence (5′–3′)a | Use |
|---|---|---|
| argR-UpF | ATTGCGGCCGCGGTTCGGGCAAGTCGACCTT | argR-null mutant construction |
| argR-UpR | ATACACCCCGAACCCACCCGAAATCAGGGTACGGTCCGAAACCTG | |
| argR-DwF | GACCTCAGGTTTCGGACCGTACCCTGATTTCGGGTGGGTTCGGG | |
| argR-DwR | ATTGCGGCCGCACGCTGACGGTAAACAGGGT | |
| RT-PCR hisP-F | CCTGCGCTGCATCAACCTGC | Cotranscription analysis; region hisP–argR |
| RT-PCR argR-R | GGTCAAAGGCCTGGTGCTGG | |
| RT-PCR argT-F | GTCGATCACCGATGACCGCAAG | Cotranscription analysis; region argT–hisQ |
| RT-PCR hisQ-R | GGAATACAGATCGCCCAGCCAG | |
| TGSP1b | CGACTTCTTGCGGTCATCGG | 5′-RACE analysis of argT |
| TGSP2b | CATGGACGACAGGATGGCGT | |
| RGSP1 | GAGGAGAAGCCACAGGACAG | 5′-RACE analysis of argR |
| RGSP2 | CCTTGTTCAACCGCAACTCC | |
| argG-BglII | CTGAGATCTGGTCGGTGCAT | argG::lacZ transcriptional fusion |
| argG-PstI | CTGCAGCACTCCACGGGGTTGTACG | |
| argH-BglII | AGATCTCTCCAGTTCGCCGAGCAG | argH::lacZ transcriptional fusion |
| argH-PstI | CTGCAGTGCAGGCGTGAACGAAAAAGTG | |
| argT-BglII | AGATCTTGCACTGGCTGTCAACATC | argT::lacZ transcriptional fusion |
| argT-PstI | CTGCAGCAGGTAACTCCATCGGTACG | |
| argR-BglII | AGATCTCTGTTCGACGAGCCGACCT | argR::lacZ transcriptional fusion |
| argR-EcoRI | GAATTCAGGGTACGGTCCGAAACCTG | |
Restriction sites inserted into the primer for the cloning strategy are underlined. Bases complementary between upstream and downstream fragments of the gene to be replaced are shown in boldface type.
Generation of an argR-null mutant.
A derivative mutant of P. putida KT2440 was obtained by the complete removal of the argR open reading frame via homologous recombination. The strategy followed was similar to that previously described (38). Briefly, fragments corresponding to the upstream and downstream regions surrounding argR were amplified by overlapping PCR using Phusion high-fidelity DNA polymerase (Thermo Fisher Scientific) in two steps. In the first PCR round, the products incorporated a NotI restriction site on one end and a complementary tail on the other end. These fragments were used as the template for a second PCR, obtaining one amplicon flanked with NotI restriction sites. The final PCR product was cloned into the pCR2.1-TOPO vector and transferred to E. coli DH5α. The absence of mutations was confirmed by sequencing the resulting plasmid (pLBM36). Subsequently, the fragment was cloned into the NotI site of suicide vector pKNG101 (47) to generate plasmid pLBM37, which was introduced into E. coli CC118λpir and mobilized to P. putida KT2440 by triparental conjugation, with HB101 harboring pRK600 as a helper strain. Merodiploid exconjugants were selected first in M9 minimal medium with citrate as the carbon source supplied with streptomycin and then in LB medium with 14% sucrose, obtaining a sucrose-resistant and streptomycin-sensitive mutant strain, indicative of vector loss after double homologous recombination (47). The null mutant was checked by PCR and further sequencing of the corresponding chromosomal region.
Construction of transcriptional fusions.
To generate transcriptional fusions to the reporter gene lacZ devoid of its own promoter, fragments of 375, 369, 362, and 229 bp containing the upstream regions of argG, argH, argR, and argT, respectively, were amplified by PCR and cloned into pCR2.1-TOPO to generate plasmids pLBM9, pLBM11, pLBM17, and pLBM18. These constructs were introduced into E. coli DH5α, and the absence of mutations was assessed by sequencing. Subsequently, pLBM9, pLBM11, and pLBM18 were digested with BglII/PstI, and pLBM17 was digested with BglII/EcoRI. The resulting fragments were cloned into pMP220 (24) to yield pLBM13, pLBM14, pLBM20, and pLBM21, where the ribosome binding site (RBS) and ATG for ′lacZ correspond to those of the cat gene in pMP220. After sequencing to confirm their integrity, plasmids were transferred to P. putida by triparental conjugation as previously described (38).
RNA purification.
Bacterial cells were grown at 30°C at 200 rpm in triplicate in M9 minimal medium with glucose or l-arginine (20 mM) as the carbon source to mid-exponential phase (OD660 = 0.3). Cells were harvested by centrifugation, immediately frozen in liquid nitrogen, and stored at −80°C. Total RNA was extracted using Tri reagent (Ambion) according to the manufacturer’s protocol, with a slight modification: the reagent was preheated at 65°C before being added to the samples, which were subsequently incubated at 65°C for 10 min. The samples were treated with RNase-free DNase I (Turbo DNA-free kit; Invitrogen) and RNaseOUT (Invitrogen), followed by treatment with inactivation reagent (Invitrogen). RNA quality was verified by agarose gel electrophoresis, the RNA concentration was determined using a NanoDrop ND1000 spectrophotometer (NanoDrop Technologies), and the absence of any residual DNA was checked by PCR.
Reverse transcription coupled to PCR.
Cotranscription analyses were performed by using the Titan one-tube reverse transcription-PCR (RT-PCR) system (Roche) according to the manufacturer’s recommendations. For each reaction, 0.5 μg of total RNA was used. Positive and negative controls were included. Total DNA (0.2 μg) was used as a positive control, while the negative control was a reaction with the same RNA but omitting the cDNA synthesis step. Primers were designed for the amplification of a 783-bp fragment containing the intergenic region between PP_4482 (argR) and PP_4483 (hisP) and a 761-bp fragment containing the intergenic region between PP_4485 (hisQ) and PP_4486 (argT).
Determination of transcription initiation sites.
The rapid amplification of cDNA ends (RACE) system (Invitrogen) was used to determine the 5′ ends of argT and argR transcripts. Total RNA was extracted from P. putida KT2440 cultures grown in M9 medium with glucose or arginine as the carbon source to an OD600 of 0.8, and the RACE technique was carried out, according to the manufacturer’s instructions. Clear bands were obtained for cultures grown in arginine. These amplified fragments were cloned into pCR2.1-TOPO (Invitrogen), and six clones corresponding to each transcript were sequenced to identify the +1 site.
β-Galactosidase activity assays.
β-Galactosidase activity was assayed during growth in LB as previously described (48). Where appropriate, glucose-M9 minimal medium with or without l-arginine was used. Briefly, cultures grown overnight were diluted (1:100) into fresh medium supplemented with 10 μg mL−1 Tc and grown at 30°C with orbital shaking (200 rpm) for 1 h. Next, cultures were diluted 1:10 to minimize β-galactosidase that may have accumulated during growth overnight, and incubation continued as described above. This dilution step was omitted when cultures were grown in minimal medium. At the indicated times, samples were collected, and culture growth (OD600) and β-galactosidase activity were measured. Activity data are presented in Miller units and correspond to averages and standard deviations from at least two independent experiments with three technical replicates per sample.
Bioreporter-based c-di-GMP quantification assays.
The bioreporter plasmid pCdrA::gfpC (27) was used to indirectly quantify c-di-GMP levels based on fluorescence. Cultures grown overnight harboring the bioreporter were inoculated into freshly diluted LB (1:3) medium supplied with 50 μg mL−1 Gm to a final OD600 of 0.02 and distributed into 96-well black microplates (Greiner). Where indicated, l-arginine (Sigma-Aldrich) was added at final concentrations of 5 and 15 mM. Growth (OD600) and fluorescence (excitation at 485 nm and emission at 515 nm) were monitored at 30°C under static conditions every 30 min for 24 h using a Varioskan Lux microplate reader. A shaking pulse of 5 s was done before each measurement. Data are indicated as green fluorescent protein (GFP) counts, which correspond to fluorescence values corrected by culture growth.
Biofilm and motility assays.
Biofilm formation was analyzed in polystyrene 96-well microtiter plates as previously described (49). Briefly, cultures grown overnight were diluted to an OD600 of 0.02 in fresh medium, distributed (150 μL per well), and incubated at 30°C under static conditions. At the indicated times, the liquid was transferred to a new plate, and planktonic growth was measured (OD600). Plates were washed twice with distilled water and stained with crystal violet (0.4%, vol/vol) for 15 min. After dye solubilization with glacial acetic acid (30%, vol/vol), the biomass attached to the surface was quantified by measuring the absorbance at 595 nm using a Tecan Sunrise microplate reader. Experiments were done in LB or modified FAB minimal medium [34 mM Na2HPO4·2H2O, 15 mM (NH4)2SO4, 22 mM KH2PO4, 51 mM NaCl, 1 mM MgCl2, 0.1 mM CaCl2, trace metals solution] (50) with glucose as a carbon source and l-arginine (5 or 15 mM), as indicated.
Surface motility assays were performed as previously described (51) on PG agar medium (0.5% [wt/vol] proteose peptone, 0.2% [wt/vol] glucose, 0.5% [wt/vol] agar).
Statistical analyses.
Student’s t test or analysis of variance (ANOVA) with a Tukey-Kramer post hoc test was applied as appropriate for all statistical analyses.
ACKNOWLEDGMENTS
L.B.-M. is the recipient of a postdoctoral fellowship from the Fundación Alfonso Martín Escudero. This work was supported by grants P11-CVI-7391 from the Junta de Andalucía, BFU2016-80122-P and PID2019-109372GB-I00 funded by MCIN/AEI/10.13039/501100011033, and ERDF: a Way of Making Europe by the European Union.
Footnotes
Supplemental material is available online only.
Contributor Information
Manuel Espinosa-Urgel, Email: manuel.espinosa@eez.csic.es.
Maia Kivisaar, University of Tartu.
REFERENCES
- 1.Römling U, Galperin MY, Gomelsky M. 2013. Cyclic di-GMP: the first 25 years of a universal bacterial second messenger. Microbiol Mol Biol Rev 77:1–52. 10.1128/MMBR.00043-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chua SL, Tan SY, Rybtke MT, Chen Y, Rice SA, Kjelleberg S, Tolker-Nielsen T, Yang L, Givskov M. 2013. Bis-(3′-5′)-cyclic dimeric GMP regulates antimicrobial peptide resistance in Pseudomonas aeruginosa. Antimicrob Agents Chemother 57:2066–2075. 10.1128/AAC.02499-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gupta KR, Baloni P, Indi SS, Chatterji D. 2016. Regulation of growth, cell shape, cell division, and gene expression by second messengers (p)ppGpp and cyclic di-GMP in Mycobacterium smegmatis. J Bacteriol 198:1414–1422. 10.1128/JB.00126-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liang F, Zhang B, Yang Q, Zhang Y, Zheng D, Zhang L, Yan Q, Wu X. 2020. Cyclic-di-GMP regulates the quorum-sensing system and biocontrol activity of Pseudomonas fluorescens 2P24 through the RsmA and RsmE proteins. Appl Environ Microbiol 86:e02016-20. 10.1128/AEM.02016-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simm R, Morr M, Kader A, Nimtz M, Römling U. 2004. GGDEF and EAL domains inversely regulate cyclic di-GMP levels and transition from sessility to motility. Mol Microbiol 53:1123–1134. 10.1111/j.1365-2958.2004.04206.x. [DOI] [PubMed] [Google Scholar]
- 6.Valentini M, Filloux A. 2016. Biofilms and cyclic di-GMP (c-di-GMP) signaling: lessons from Pseudomonas aeruginosa and other bacteria. J Biol Chem 291:12547–12555. 10.1074/jbc.R115.711507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hengge R. 2009. Principles of c-di-GMP signalling in bacteria. Nat Rev Microbiol 7:263–273. 10.1038/nrmicro2109. [DOI] [PubMed] [Google Scholar]
- 8.Jonas K, Melefors O, Römling U. 2009. Regulation of c-di-GMP metabolism in biofilms. Future Microbiol 4:341–358. 10.2217/fmb.09.7. [DOI] [PubMed] [Google Scholar]
- 9.Schirmer T. 2016. c-di-GMP synthesis: structural aspects of evolution, catalysis and regulation. J Mol Biol 428:3683–3701. 10.1016/j.jmb.2016.07.023. [DOI] [PubMed] [Google Scholar]
- 10.Chou SH, Galperin MY. 2016. Diversity of cyclic di-GMP-binding proteins and mechanisms. J Bacteriol 198:32–46. 10.1128/JB.00333-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bernier SP, Ha DG, Khan W, Merritt JH, O’Toole GA. 2011. Modulation of Pseudomonas aeruginosa surface-associated group behaviors by individual amino acids through c-di-GMP signaling. Res Microbiol 162:680–688. 10.1016/j.resmic.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paiardini A, Mantoni F, Giardina G, Paone A, Janson G, Leoni L, Rampioni G, Cutruzzolà F, Rinaldo S. 2018. A novel bacterial L-arginine sensor controlling c-di-GMP levels in Pseudomonas aeruginosa. Proteins 86:1088–1096. 10.1002/prot.25587. [DOI] [PubMed] [Google Scholar]
- 13.Katharios-Lanwermeyer S, Whitfield GB, Howell PL, O’Toole GA. 2021. Pseudomonas aeruginosa uses c-di-GMP phosphodiesterases RmcA and MorA to regulate biofilm maintenance. mBio 12:e03384-20. 10.1128/mBio.03384-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kumar B, Sorensen JL, Cardona ST. 2018. A c-di-GMP-modulating protein regulates swimming motility of Burkholderia cenocepacia in response to arginine and glutamate. Front Cell Infect Microbiol 8:56. 10.3389/fcimb.2018.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mills E, Petersen E, Kulasekara BR, Miller SI. 2015. A direct screen for c-di-GMP modulators reveals a Salmonella Typhimurium periplasmic L-arginine-sensing pathway. Sci Signal 8:ra57. 10.1126/scisignal.aaa1796. [DOI] [PubMed] [Google Scholar]
- 16.Ramos-González MI, Travieso ML, Soriano MI, Matilla MA, Huertas-Rosales Ó, Barrientos-Moreno L, Tagua VG, Espinosa-Urgel M. 2016. Genetic dissection of the regulatory network associated with high c-di-GMP levels in Pseudomonas putida KT2440. Front Microbiol 7:1093. 10.3389/fmicb.2016.01093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barrientos-Moreno L, Molina-Henares MA, Ramos-González MI, Espinosa-Urgel M. 2020. Arginine as an environmental and metabolic cue for cyclic diguanylate signalling and biofilm formation in Pseudomonas putida. Sci Rep 10:13623. 10.1038/s41598-020-70675-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matilla MA, Travieso ML, Ramos JL, Ramos-González MI. 2011. Cyclic diguanylate turnover mediated by the sole GGDEF/EAL response regulator in Pseudomonas putida: its role in the rhizosphere and an analysis of its target processes. Environ Microbiol 13:1745–1766. 10.1111/j.1462-2920.2011.02499.x. [DOI] [PubMed] [Google Scholar]
- 19.Huertas-Rosales Ó, Romero M, Heeb S, Espinosa-Urgel M, Cámara M, Ramos-González MI. 2017. The Pseudomonas putida CsrA/RsmA homologues negatively affect c-di-GMP pools and biofilm formation through the GGDEF/EAL response regulator CfcR. Environ Microbiol 19:3551–3566. 10.1111/1462-2920.13848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caldara M, Charlier D, Cunin R. 2006. The arginine regulon of Escherichia coli: whole-system transcriptome analysis discovers new genes and provides an integrated view of arginine regulation. Microbiology (Reading) 152:3343–3354. 10.1099/mic.0.29088-0. [DOI] [PubMed] [Google Scholar]
- 21.Charlier D, Bervoets I. 2019. Regulation of arginine biosynthesis, catabolism and transport in Escherichia coli. Amino Acids 51:1103–1127. 10.1007/s00726-019-02757-8. [DOI] [PubMed] [Google Scholar]
- 22.Van Duyne GD, Ghosh G, Maas WK, Sigler PB. 1996. Structure of the oligomerization and L-arginine binding domain of the arginine repressor of Escherichia coli. J Mol Biol 256:377–391. 10.1006/jmbi.1996.0093. [DOI] [PubMed] [Google Scholar]
- 23.Nishijyo T, Park SM, Lu CD, Itoh Y, Abdelal AT. 1998. Molecular characterization and regulation of an operon encoding a system for transport of arginine and ornithine and the ArgR regulatory protein in Pseudomonas aeruginosa. J Bacteriol 180:5559–5566. 10.1128/JB.180.21.5559-5566.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu CD, Yang Z, Li W. 2004. Transcriptome analysis of the ArgR regulon in Pseudomonas aeruginosa. J Bacteriol 186:3855–3861. 10.1128/JB.186.12.3855-3861.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park SM, Lu CD, Abdelal AT. 1997. Purification and characterization of an arginine regulatory protein, ArgR, from Pseudomonas aeruginosa and its interactions with the control regions for the car, argF, and aru operons. J Bacteriol 179:5309–5317. 10.1128/jb.179.17.5309-5317.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.D’Arrigo I, Bojanovič K, Yang X, Holm Rau M, Long KS. 2016. Genome-wide mapping of transcription start sites yields novel insights into the primary transcriptome of Pseudomonas putida. Environ Microbiol 18:3466–3481. 10.1111/1462-2920.13326. [DOI] [PubMed] [Google Scholar]
- 27.Spaink HP, Okker RJ, Wijffelman CA, Pees E, Lugtenberg BJ. 1987. Promoters in the nodulation region of the Rhizobium leguminosarum Sym plasmid pRL1JI. Plant Mol Biol 9:27–39. 10.1007/BF00017984. [DOI] [PubMed] [Google Scholar]
- 28.Rybtke MT, Borlee BR, Murakami K, Irie Y, Hentzer M, Nielsen TE, Givskov M, Parsek MR, Tolker-Nielsen T. 2012. Fluorescence-based reporter for gauging cyclic di-GMP levels in Pseudomonas aeruginosa. Appl Environ Microbiol 78:5060–5069. 10.1128/AEM.00414-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martínez-Gil M, Yousef-Coronado F, Espinosa-Urgel M. 2010. LapF, the second largest Pseudomonas putida protein, contributes to plant root colonization and determines biofilm architecture. Mol Microbiol 77:549–561. 10.1111/j.1365-2958.2010.07249.x. [DOI] [PubMed] [Google Scholar]
- 30.Martínez-Gil M, Quesada JM, Ramos-González MI, Soriano MI, de Cristóbal RE, Espinosa-Urgel M. 2013. Interplay between extracellular matrix components of Pseudomonas putida biofilms. Res Microbiol 164:382–389. 10.1016/j.resmic.2013.03.021. [DOI] [PubMed] [Google Scholar]
- 31.Molina-Henares MA, Ramos-González MI, Daddaoua A, Fernández-Escamilla AM, Espinosa-Urgel M. 2017. FleQ of Pseudomonas putida KT2440 is a multimeric cyclic diguanylate binding protein that differentially regulates expression of biofilm matrix components. Res Microbiol 168:36–45. 10.1016/j.resmic.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 32.Martínez-Gil M, Ramos-González MI, Espinosa-Urgel M. 2014. Roles of cyclic di-GMP and the Gac system in transcriptional control of the genes coding for the Pseudomonas putida adhesins LapA and LapF. J Bacteriol 196:1484–1495. 10.1128/JB.01287-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu H, Yan H, Xiao Y, Nie H, Huang Q, Chen W. 2019. The exopolysaccharide gene cluster pea is transcriptionally controlled by RpoS and repressed by AmrZ in Pseudomonas putida KT2440. Microbiol Res 218:1–11. 10.1016/j.micres.2018.09.004. [DOI] [PubMed] [Google Scholar]
- 34.Matsuyama BY, Krasteva PV, Baraquet C, Harwood CS, Sondermann H, Navarro MV. 2016. Mechanistic insights into c-di-GMP-dependent control of the biofilm regulator FleQ from Pseudomonas aeruginosa. Proc Natl Acad Sci USA 113:E209–E218. 10.1073/pnas.1523148113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blanco-Romero E, Redondo-Nieto M, Martínez-Granero F, Garrido-Sanz D, Ramos-González MI, Martín M, Rivilla R. 2018. Genome-wide analysis of the FleQ direct regulon in Pseudomonas fluorescens F113 and Pseudomonas putida KT2440. Sci Rep 8:13145. 10.1038/s41598-018-31371-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Llácer JL, Fita I, Rubio V. 2008. Arginine and nitrogen storage. Curr Opin Struct Biol 18:673–681. 10.1016/j.sbi.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 37.Crane BR, Sudhamsu J, Patel BA. 2010. Bacterial nitric oxide synthases. Annu Rev Biochem 79:445–470. 10.1146/annurev-biochem-062608-103436. [DOI] [PubMed] [Google Scholar]
- 38.Morris SM, Jr. 2016. Arginine metabolism revisited. J Nutr 146:2579S–2586S. 10.3945/jn.115.226621. [DOI] [PubMed] [Google Scholar]
- 39.Barrientos-Moreno L, Molina-Henares MA, Pastor-García M, Ramos-González MI, Espinosa-Urgel M. 2019. Arginine biosynthesis modulates pyoverdine production and release in Pseudomonas putida as part of the mechanism of adaptation to oxidative stress. J Bacteriol 201:e00454-19. 10.1128/JB.00454-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rinaldo S, Giardina G, Mantoni F, Paone A, Cutruzzolà F. 2018. Beyond nitrogen metabolism: nitric oxide, cyclic-di-GMP and bacterial biofilms. FEMS Microbiol Lett 365:fny029. 10.1093/femsle/fny029. [DOI] [PubMed] [Google Scholar]
- 41.Park SM, Lu CD, Abdelal AT. 1997. Cloning and characterization of argR, a gene that participates in regulation of arginine biosynthesis and catabolism in Pseudomonas aeruginosa PAO1. J Bacteriol 179:5300–5308. 10.1128/jb.179.17.5300-5308.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Regenhardt D, Heuer H, Heim S, Fernández DU, Strömpl C, Moore ERB, Timmis KN. 2002. Pedigree and taxonomic credentials of Pseudomonas putida strain KT2440. Environ Microbiol 4:912–915. 10.1046/j.1462-2920.2002.00368.x. [DOI] [PubMed] [Google Scholar]
- 43.Lennox ES. 1955. Transduction of linked genetic characters of the host by bacteriophage P1. Virology 1:190–206. 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- 44.Sambrook J, Russell DW. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- 45.Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K (ed). 1987. Current protocols in molecular biology. Wiley, New York, NY. [Google Scholar]
- 46.Enderle PJ, Farwell MA. 1998. Electroporation of freshly plated Escherichia coli and Pseudomonas aeruginosa cells. Biotechniques 25:954–956. 10.2144/98256bm05. [DOI] [PubMed] [Google Scholar]
- 47.Kaniga K, Delor I, Cornelis GR. 1991. A wide-host-range suicide vector for improving reverse genetics in gram-negative bacteria: inactivation of the blaA gene of Yersinia enterocolitica. Gene 109:137–141. 10.1016/0378-1119(91)90599-7. [DOI] [PubMed] [Google Scholar]
- 48.Miller JH. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY. [Google Scholar]
- 49.O’Toole GA, Kolter R. 1998. Initiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signalling pathways: a genetic analysis. Mol Microbiol 28:449–461. 10.1046/j.1365-2958.1998.00797.x. [DOI] [PubMed] [Google Scholar]
- 50.Heydorn A, Nielsen AT, Hentzer M, Sternberg C, Givskov M, Ersbøll BK, Molin S. 2000. Quantification of biofilm structures by the novel computer program COMSTAT. Microbiology (Reading) 146:2395–2407. 10.1099/00221287-146-10-2395. [DOI] [PubMed] [Google Scholar]
- 51.Matilla MA, Ramos JL, Duque E, Alché JD, Espinosa-Urgel M, Ramos-González MI. 2007. Temperature and pyoverdine-mediated iron acquisition control surface motility of Pseudomonas putida. Environ Microbiol 9:1842–1850. 10.1111/j.1462-2920.2007.01286.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 to S6. Download aem.00064-22-s0001.pdf, PDF file, 0.5 MB (548.5KB, pdf)