Abstract
Background.
Lung nociceptor neurons amplify immune cell activity and mucus metaplasia in response to an inhaled allergen challenge in sensitized mice.
Objective.
We now sought to identify the cellular mechanisms by which these sensory neurons are activated upon allergen exposure.
Methods.
We used calcium microscopy and electrophysiological recording to assess whether vagal neurons directly respond to the model allergen ovalbumin (OVA). Next, we generated the first nociceptor specific FcεR1γ knockdown (TRPV1Cre::FcεR1γfl/fl) mice to assess whether this targeted invalidation would impact the severity of allergic inflammation in response to allergen challenges.
Results.
Lung-innervating jugular nodose complex ganglion (JNC) neurons express the high-affinity IgE receptor FcεR1 and the levels of this receptor increase in OVA-sensitized mice. FcεR1γ-expressing vagal nociceptor neurons respond directly to OVA complexed with IgE, with depolarization, action potential firing, calcium influx, and neuropeptide release. Activation of vagal neurons by IgE/allergen immune complexes, through the release of substance P (SP) from their peripheral terminals, directly amplifies TH2 cell influx and polarization in the airways. Allergic airway inflammation is decreased in TRPV1cre::FcεR1γfl/fl mice or in bone marrow-transplanted FcsR1α−/− mice. Finally, increased in vivo circulating levels of IgE following allergen sensitization enhances the responsiveness of FcεR1 to immune complexes in both mouse JNC neurons and human iPSC-derived nociceptors.
Conclusions.
Allergen-sensitization triggers a feedforward inflammatory loop between IgE-producing plasma cells, FcεR1 expressing vagal sensory neurons, and TH2 cells, which helps both initiate and amplify allergic airway inflammation. These data highlight a novel target for reducing allergy; FcεR1γ expressed by nociceptors.
Keywords: Neuro-immunity, nociceptor neurons, allergy, asthma, TH2, FcεR1, TRPV1, allergen detection, vagal sensing, Substance P
Introduction.
Trigeminal and lumbar nociceptor somatosensory neurons express antibody-sensing Fc receptors (FcR) 1 for both immunoglobulin type G (IgG), FcγR; and immunoglobulin type E (IgE), FcεR 2, 3. This phenomenon may explain why sensory neurons in a sensitized animal fire action potentials when exposed to an allergen 4,5. Upon binding to the antigen for which they were raised, antibodies form immune complexes that activate FcR expressed by nociceptors. Such stimuli generate calcium flux, action potential firing, and neuropeptide release, triggering pain or itching 6,7,8. However, whether vagal sensory neurons innervating visceral organs also sense immune complexes, and if they do, what the consequences are, is unknown. We set out to examine this question.
Nociceptor neurons respond to immune cell cues, such as cytokines, and drive immune responses 9–12,13. For example, in the ovalbumin (OVA) and house dust mite (HDM) mouse models of allergic airway inflammation (AAI), sensory neurons innervating the lung drive CD4+ T and ILC2 cell activation through a VIP-VPAC2 neuropeptide axis 14–16. In turn, these immune cells release the cytokines IL-5 and IL-13, which activate lung sensory neurons as part of a feed-forward pro-inflammatory loop that amplifies the adaptive immune response to allergen exposure 14 and also drives mucus metaplasia 17. However, the specific mechanisms by which an antigen initiates type 2 airway inflammation, and at what point nociceptors are engaged, remains to be determined. To address this issue, we have now used genetic approaches and allergic airway inflammation models to examine i) whether and how jugular/nodose complex ganglion (JNC) nociceptor neurons sense allergens, ii) if they do, what the consequences on immune cells may be, and iii) whether allergen sensitization sets off an interaction between adaptive immune responses and nociceptor activity that triggers allergic airway inflammation.
Methods.
Detailed information on materials and methods are available in the supplementary Methods in this article’s Online Repository (available at www.jacionline.org). In brief, all procedures were approved by the Institutional Animal Care and Use Committees of Boston Children’s Hospital. Allergic airway inflammation was studied in an ovalbumin (OVA) based model 18. On days 0 and 7, mice were sensitized by a 200 μl i.p. injections of a solution containing 1 mg/ml ovalbumin (Sigma, #A5503–25G) and 5 mg/ml aluminum hydroxide (Sigma, #239186–500G). On days 14–17 (10:00 am), mice were exposed to 6% OVA aerosol for 25 min or intranasal OVA instillation (50 μg /50 μl). Mice were sacrificed on days 0, 3, 6, 9, 13, 14, 15, 18, 20, 21 or 26; JNC neurons were harvested, cultured, and analyzed using calcium microscopy, electrophysiology 14, and single-cell qPCR 19. Culture media were analyzed using ELISA 14. BALF was harvested; cells isolated, counted, and immunophenotyped using FACS 14. Alternatively, QX-314 (100 μM) and capsaicin (1 μM) were nebulized on day 14 and typically sacrificed on day 15. Another type 2 inflammation model was induced via house dust mites (HDM). Lightly anesthetized (isoflurane) mice were sensitized (day 1–5) and challenged (day 8–10) with house dust mites (CiteQ, #15J01; 20 μg/50μl, intranasal), sacrificed on day 11, BALF was harvested; cells were isolated, counted, and immunophenotyped by flow cytometry.
Results.
Vagal nociceptors initiate airway inflammation.
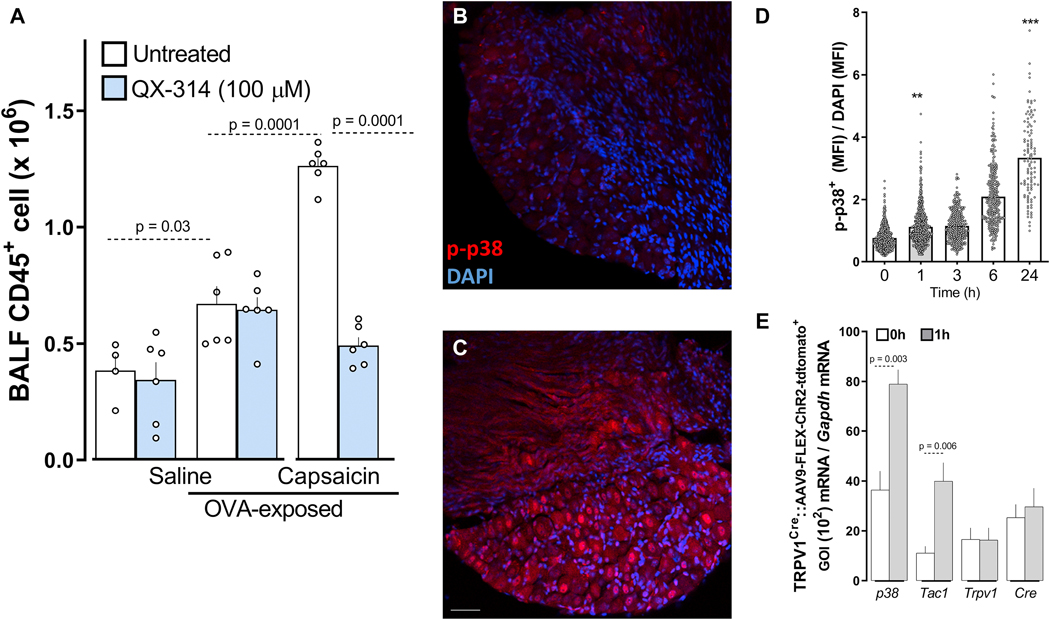
Type 2 allergic inflammation was induced in mice by an initial sensitization to ovalbumin (OVA) with aluminum hydroxide as an adjuvant (i.p. days 0 and 7), followed two weeks later by inhaled OVA challenges 14. A single inhaled OVA exposure on day 14 after sensitization results in increased CD45+ cells in bronchoalveolar lavage fluid (BALF) when tested 24 hours later (Figure 1A). To establish whether nociceptor activation at the time of the allergen exposure contributes to the inflammatory response, we tested if capsaicin (1 μM; intranasal (i.n.)), an exogenous TRPV1 ligand that activates lung afferents nociceptors, would modify the extent of the immune infiltrate. Capsaicin, given 3 hours before the initial allergen challenge, doubled the OVA-mediated influx of CD45+ cell count in the BALF (Figure 1A).
Figure 1: Vagal nociceptors initiate airway inflammation.
An acute inhaled ovalbumin challenge (day 14) increased CD45+ cells in the BALF of previously sensitized mice. A 3h pre-treatment with intranasal 1μM capsaicin led to a further increase in CD45+ cells over OVA treatment alone. QX-314 treatment 3h before the first OVA challenge did not alter the allergen-induced inflammation, but co-treatment with capsaicin to open TRP channels, and allowed entry of QX-314 in airway nociceptors to silence their electrical activity, prevented the onset of allergic airway inflammation (A). Allergen challenge (day 14) with ovalbumin time-dependently increased the number of neurons that were positive for p-p38, an activity marker in JNC neurons (B-D). To track airway-innervating neurons, a cre-dependent AAV9-FLEX-Tdtomato virus (109 titers, 50 μL) was injected intranasally two weeks prior to sensitization. Using single-cell qPCR, p38 and Tac1 transcript expression were increased in airway-innervating neurons (Td-tomato+) from OVA-challenged mice (1h). TRPV1 and cre expression were not impacted (E). Mean ± S.E.M; One-way ANOVA post-hoc Bonferroni (A); two-tailed unpaired Student’s t-test (D-E); n = 4 – 10 animals/group, 1–3 cohorts. In (B-C) p-p38 are labeled in red, and nuclei are labeled in blue; scale bar = 50 ym.
Next, to specifically explore if nociceptors have a role in initiating the inflammation resulting from allergen exposure in sensitized mice, we used a strategy to selectively silence lung nociceptors before the allergen exposure. This approach uses non-selective large pore ion channels (TRPA1 and TRPV1) as cell-specific drug-entry ports to deliver a charged, membrane-impermeable form of lidocaine (QX-314) into pulmonary sensory fibers, to block sodium currents and thereby, action potentials 14. A 3h pre-treatment with QX-314, in the presence of capsaicin as a TRPV1 channel opener, reduced the OVA-mediated airway inflammatory response to the subsequent allergen inhalation (Figure 1A). QX-314 pre-treatment without capsaicin, which does not silence nociceptors, did not affect BALF CD45+ cell numbers (Figure 1A).
To test for nociceptor activation by an allergen-challenge, we probed p-p38 MAPK activation levels as an activity marker in JNC neurons 20–24. An acute inhaled OVA challenge in sensitized mice produced a time-dependent increase in phosphorylated p38 (p-p38) positive JNC neurons one-hour post-OVA challenges and continuing for 24hrs (Figure 1B–D). To determine whether this effect occurred in the airway-innervating neurons, TRPV1Cre mice were injected intranasally with AAV9-FLEX-ChR2-Tdtomato (109 titers, 50μL) virus two weeks prior to allergen sensitization. Using single-cell qPCR, we found that p38 and Tac1 transcript expression increased in airway-innervating neurons (Td-tomato+) obtained from OVA-challenged mice (1h; Figure 1E). The finding that silencing sensory neurons before the first allergen exposure reduced the inflammatory response, while activating the nociceptors had the opposite effect, raises the possibility that vagal nociceptors might be directly engaged by the allergen challenge and that such activation may contribute to immune cell recruitment/activation.
Vagal nociceptors express FcєR1
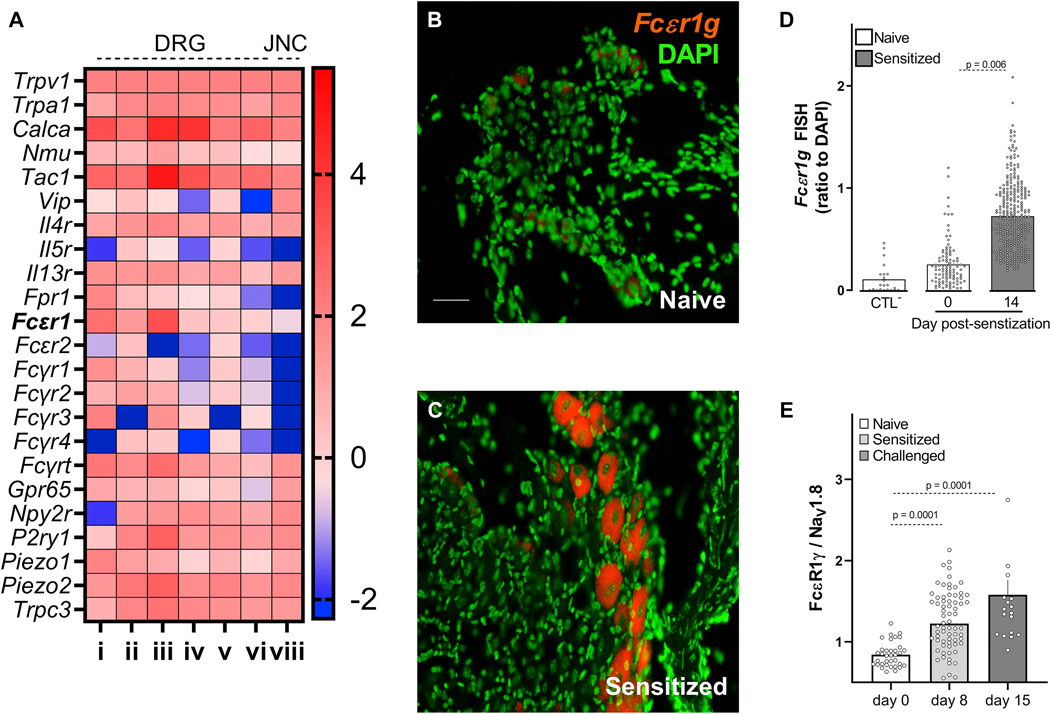
In-silico analysis of seven previously published expression profiling datasets 25 of TRPV1+ neurons shows that, in addition to sensory neuron markers (TRPV1, TRPA1) and nociceptor neuropeptides (SP, VIP, NMU, CGRP), these afferents express the immunoglobulin receptors FcεR1, FcεR2, FcγR1, FcγR2, and FcγR3. In the case of FcεR1, we found higher relative expression levels than for the pattern recognition receptor Fpr1, or the P2Y purinoceptor 1 (P2YR1; Figure 2A); all of which were found to be functional on these neurons 11, 26. Next, ex vivo JNC neurons from naïve and allergen-sensitized animals (Tac1cre::GCaMP6fl/wt reporter) were co-cultured (1:1 mix). In this context, we found that 87% of all FcεR1γ transcript expressing neurons originated from allergen sensitized mice (GCaMP6+, supplementary Figure 1A). We then measured the level of FcεR1 expression on vagal sensory neurons using fluorescent in-situ hybridization (Figure 2B–D), immunofluorescence (Figure 2E, supplementary Figure 1B–H), and qPCR performed on FACS-purified TRPV1+ JNC neurons (TRPV1cre::td-tomatofl/wt mouse, supplementary Figure 1I). As in the co-culture setting (supplementary Figure 1A), we found that FcεR1α/γ transcript and protein levels were expressed in naïve mouse JNC neurons, but that this level is further increased in neurons from allergen-sensitized mice (Figure 2B–E; supplementary Figure 1B–I).
Figure 2: Vagal nociceptors express FctR1.
A meta-analysis of seven published nociceptor expression profiling datasets79 showed basal expression of sensory neuron markers (TRPV1, TRPA1), neuropeptides (SP, VIP, NMU, CGRP), asthma-driving cytokine receptors (IL-4R, IL-5R, IL-13R), and the immunoglobulin receptor FcεR1 (A). Fluorescent in situ hybridization and immunohistochemistry was used to analyze the levels of FcεR1 transcript (B-D) and protein (E) expression in JNC neurons (day 0 and 14 (B-D); day 0, 8, and 15 (E)). The data reveal that these levels increased in allergen-sensitized mice neurons relative to those in naïve mice (B-E). Mean ± S.E.M; Two-tailed unpaired Student’s t-test (D-E); n = 8 animals/group, 2 cohorts. i) RNA-sequencing of human lumbar neurons 80; ii) microarrays of mouse FACS-sorted NaV1.8+ neurons81; iii) and iv) single-cell RNA- sequencing of mouse lumbar neurons 2‘83; v) refers to microarray profiling of mouse whole DRG8 ; vi) refers to performed RNA-sequencing of mouse TRPV1+ neurons84; vii) single-cell RNA sequencing of mouse vagal ganglia85. Expression across datasets was ratioed over Trpv1, multiplied by 100, and the Log10 of these values were presented as a heatmap (A). FceR1 are labeled in red, and nuclei are labeled in green (B-C); scale bar = 50 μm.
FcεR1-expressing vagal nociceptors respond to immune complexes.
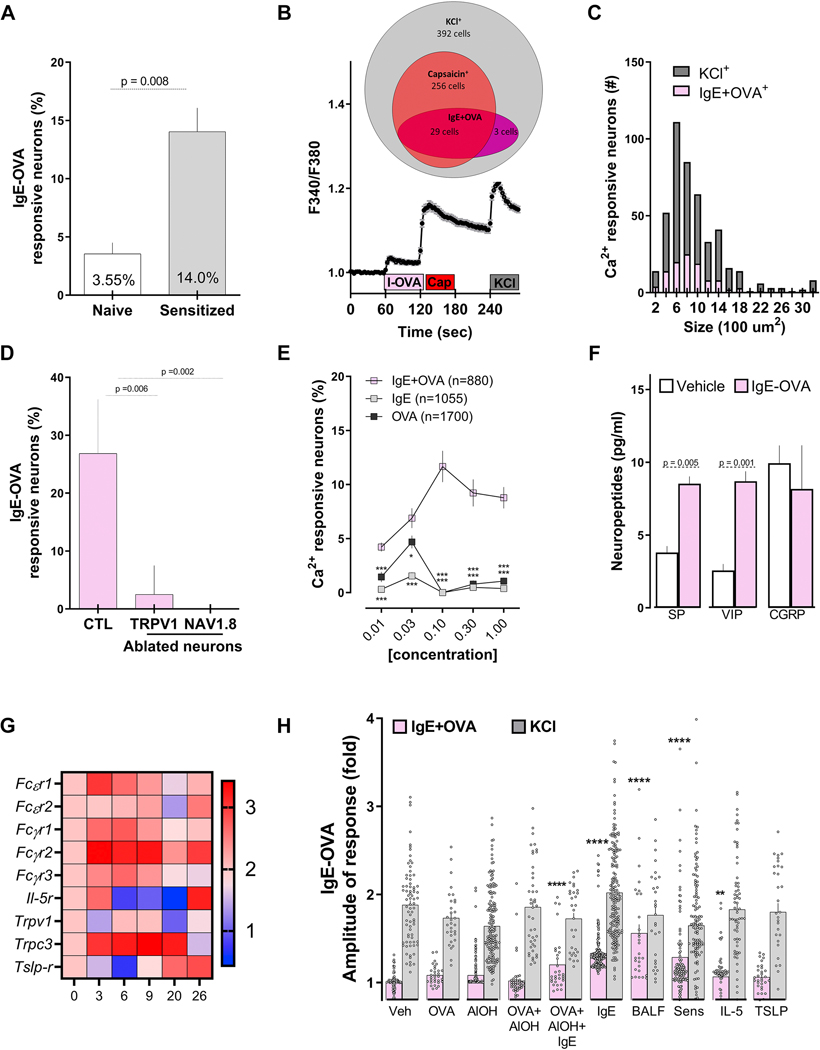
To assess if vagal sensory neurons directly respond to IgE-OVA complexes, we exposed primary JNC neuron cultures to a range of concentrations of IgE, ovalbumin and ovalbumin-IgE complex (IC) and tested calcium flux as a proxy of neuronal activation. ~12–15% of isolated neurons from a sensitized mouse responded to the allergen-antibody complex (>10% increase in Fura-2AM fluorescence), with a significantly lower response in non-sensitized JNC neurons (Figure 3A). Increased immune complex sensitivity of JNC neurons from isolated allergen-sensitized mice was confirmed in NaV1.8Cre::GCaMP6fl/wt and Fura-2AM-loaded neurons (supplementary Figure 2A). Immune complex-responsive neurons were mostly small-diameter AITC- and capsaicin-sensitive neurons (TRPA1 and TRPV1 responsive respectively; Figure 3B–D; supplementary Figure 2B). The identity of the responsive neurons as nociceptors was confirmed using cultured JNC neurons from TRPV1Cre::DTAfl/wt or NaV1.8 Cre::DTAfl/wt nociceptor depleted mice, where the response to IgE-OVA was absent in the latter and massively reduced in the former (Figure 3D). The IgE-OVA induced calcium flux was dose-dependent and higher than that produced by exposure to IgE or OVA alone (~1 – 4%; Figure 3E). Immune complex-stimulated allergen- sensitized JNC neurons release substance P (SP) and vasoactive intestinal peptides (VIP) but not calcitonin gene-related peptide (CGRP; Figure 3F). IgE or OVA exposure alone had no impact on SP secretion from allergen-sensitized mouse neurons (supplementary figure 2C).
Figure 3: IgE-Ovalbumin complex evokes calcium flux in vagal nociceptors.
Ultra-pure ovalbumin (1%), when complexed with mouse recombinant immunoglobulin E (IgE, 0.001%), triggered larger calcium transients in cultured JNC neurons obtained from allergen-sensitized (day 14) than from naïve mice (A). Shown is an example of the response evoked in capsaicin-sensitive neurons (B). IgE-OVA-induced calcium influx was mostly observed in small-sized neurons (C) and capsaicin-sensitive neurons and absent in cultured neurons obtained from allergen-sensitized (day 14) TRPV1 (TRPV1creDTAfl/wt) or NaV1.8 (NaV1.8creDTAfl/wt) nociceptor ablated mice. (D). The IgE-OVA complex dose-dependently evoked a calcium response greater than that for IgE or OVA alone (E) and triggered the release of substance P (SP) and vasoactive intestinal peptide (VIP; F). Capsaicin- responsive neurons were picked at various time points of the allergy-induction protocol and analyzed by single-cell qPCR. Allergen sensitization transiently drove the expression of FcsR1, which peaked on day 3 (G). Compared with naïve vagal neurons, we found an increased number of immune complex-responsive neurons in cells pre-treated (24h) with IgE, OVA+AlOH+IgE, IL-5, or asthmatic BALF or harvested from allergen-sensitized (day 14) mice (H). Mean ± S.E.M; Two-tailed unpaired Student’s t-test (A, D, F); two-tailed Mann-Whitney U test (H), one-way ANOVA post-hoc Bonferroni (E); n = 3–8 animals/group, 2–3 cohorts. In (B), IgE-OVA (1%+0.001%; 60–75 sec) is represented by the pink box, capsaicin (1 jM; 120–135 sec) is represented by the red box, and the gray box represents KCl (40 mM; 240–255 sec). Relative gene expression was ratioed over day 0, multiplied by 1000, and the Log10 of these values were presented as a heatmap (G).
Next, naïve and allergen-sensitized mice isolated neurons were co-cultured (1:1 mix) to directly compare their responsiveness to immune complex exposure in the same experimental conditions. In this setting, we found ~3 fold more IgE-OVA responsive neurons and higher amplitude of response in allergen-sensitized neurons from a reporter mouse (TRPV1Cre::Td-tomatofl/wt) than in non-sensitized (no reporter) naïve neurons (supplementary Figure 2D–E; supplementary movie 1 showing decay in unbound cytoplasmic Ca2+ in response to IC).
IgE controls FcєR1 expression.
Given the increased immune complex-mediated calcium flux in JNC neurons from sensitized mice, we set out to test what allergen-sensitization component might be responsible for this effect. To do this, we used unbiased single-cell qPCR to probe changes in transcript expression in TRPV1+ JNC neurons (capsaicin-responsive) at various times during the asthma protocol. We found elevated FcεR1γ transcript expression as early as three days post-sensitization, as well as elevated IL-5R and TSLPR levels peaking on day 26 (Figure 3G). Given that in addition to immunologically “arming” mast cells to undergo antigen-mediated activation, IgE stabilizes and enhances FcεR1 expression on immune cells 27–29; we posited that circulating IgE might be responsible for the neuronal expression of FcεR1. We tested whether immunoglobulins, interleukins, the adjuvant, the model allergen or a combination of these, induced the expression of functional FcεR1 in primary cultured JNC neurons. While KCl responses were similar across the various treatment groups, we found an increased response to the immune complex when JNC neurons were pre-exposed to IgE, IgE+OVA+AlOH, or BALF extracted from an asthmatic mouse (Figure 3H). As expected, allergen-sensitization prior to harvesting the neurons had a similar effect (Figure 3H). Interestingly, human iPSC-derived nociceptor neurons (iNoc) that were pre-exposed to IgE for 24h, also showed a calcium flux (11 %) in response to the immune complex, an effect that was absent in non-IgE exposed iNoc neurons (supplementary Figure 2F–G). These findings reveal the context-dependent expression of FcεRI on nociceptor neurons, which is modulated by IgE upon allergen-sensitization, highlighting a linkage between plasma B cells and vagal nociceptors.
Immune complex modulation of neuron excitability.
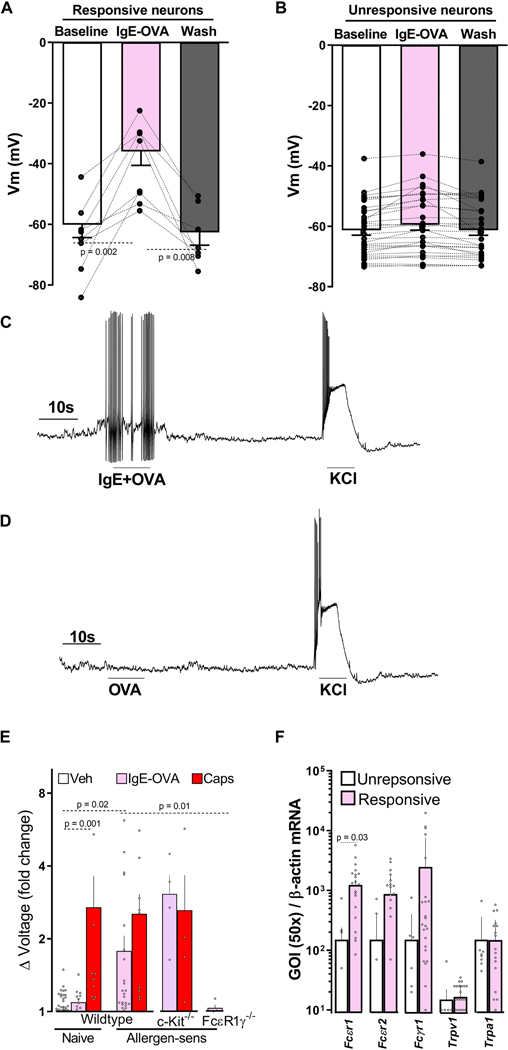
To probe whether the immune complex modulates nociceptor neurons’ excitability, we first identified those allergen-sensitized cultured JNC neurons that respond to the immune complex with a calcium flux (supplementary Figure 3A). We then tested their electrophysiological response to the complex by whole-cell patch recording. While having similar baseline resting membrane potentials (~−60mV), the responder group became depolarized when re-exposed to the allergen-antibody complex (8/8) (Figure 4A), an effect virtually absent in the non-responder group (1/24) (Figure 4B). Furthermore, the depolarization was sufficient to evoke action potential firing in some allergen-antibody sensitive neurons (3/8; Figure 4C), an effect never observed when the cells were treated with OVA or IgE alone (Figure 4D).
Figure 4: Direct detection of allergens by nociceptors.
In comparison to IC-unresponsive neurons, IgE-OVA sensitive neurons (day 14) showed increased depolarization of the membrane potential (A-B), and action potential firing following IgE-OVA re-exposure (C). OVA alone did not induce action potential firing (D). Whole- nerve electrophysiology was used to assess in vivo allergen sensing by vagal neurons and revealed that allergen-sensitized wild type and mast cell-depleted mice (c-Kit−/−) demonstrated compound action potential when exposed to IgE-OVA (1%, i.p.), effects that were absent in naïve or allergen-sensitized FcεR1γ−/− mice. Capsaicin (1 μM) showed compound action potential in all tested mice lines (E). IgE-OVA responsive neurons overexpressed FcεR1 transcript, as evidenced by single-cell qPCR (F). Mean ± S.E.M; Two-tailed paired (A, B), or unpaired (F) Student’s t-test; one-way ANOVA post-hoc Bonferroni (E); n = 8–24 animals/group, 2–8 cohorts.
To test whether allergen sensing by vagal nociceptors increases lung allergic responses, we first used whole nerve electrophysiological recording of vagal neurons to check whether immune complexes were sensed in vivo. Similar to the findings in an in vitro setting, allergen sensitized wildtype mice showed vagal compound action potential responses to the immune complex when injected intraperitoneally. Importantly, a similar effect was also found in allergen-sensitized mast cell-depleted mice (KitW-sh/W-sh, 30), supporting a mast cell-independent effect. The immune complex had no effect in naïve wildtype mice or allergen-sensitized FcsR1γ−/− mice (Figure 4E; supplementary Figure 3B–E).
FcєR1-TRPC3 axis controls IC-induced calcium flux.
To specifically explore whether the activation of vagal nociceptors by the IgE-OVA complex occurred though FcεR1, we identified small diameter (≤25um) immune complex-responsive and non-responsive neurons in culture from sensitized and challenged mice (supplementary Figure 3A) and then profiled them using single-cell qPCR. The responsive neurons showed higher FcεR1 transcript expression than those in non-responsive neurons, even though both expressed similar transcript levels of TRPV1 and TRPA1 (Figure 4F). Next, we used FcεR1 a null mice to confirm the role of FcεR1 in sensing the immune complex. We then found that while cultured JNC neurons from FcεR1α−/− mice respond normally to KCl or capsaicin, they were virtually insensitive to the immune complex (supplementary Figure 4A–D). Similarly, two different FcεR1α blocking antibodies abolished the neuronal response to the immune complex, but not to KCl or capsaicin (MAR1 clone, supplementary Figure 4E; CRA1 clone, supplementary Figure 4F).
Immune complex-induced calcium flux was absent when allergen-sensitized neurons were cultured in the absence of extracellular calcium (supplementary Figure 4G–H) and when treated with the non-selective TRP channel blocker ruthenium red (supplementary Figure 4I) or with a TRPC3 antagonist (supplementary Figure 4J–K). Similar to the IgG-antigen complex activation of lumbar DRG neurons 31 and in accordance with their co-expression in a subset of peptidergic neurons (supplementary Figure 5) 32–34, our data indicate that the allergen-IgE complex produces a calcium influx through an FcεR1-TRPC3 axis.
Neuron-sensing allergen helps prime TH2 cell activity,
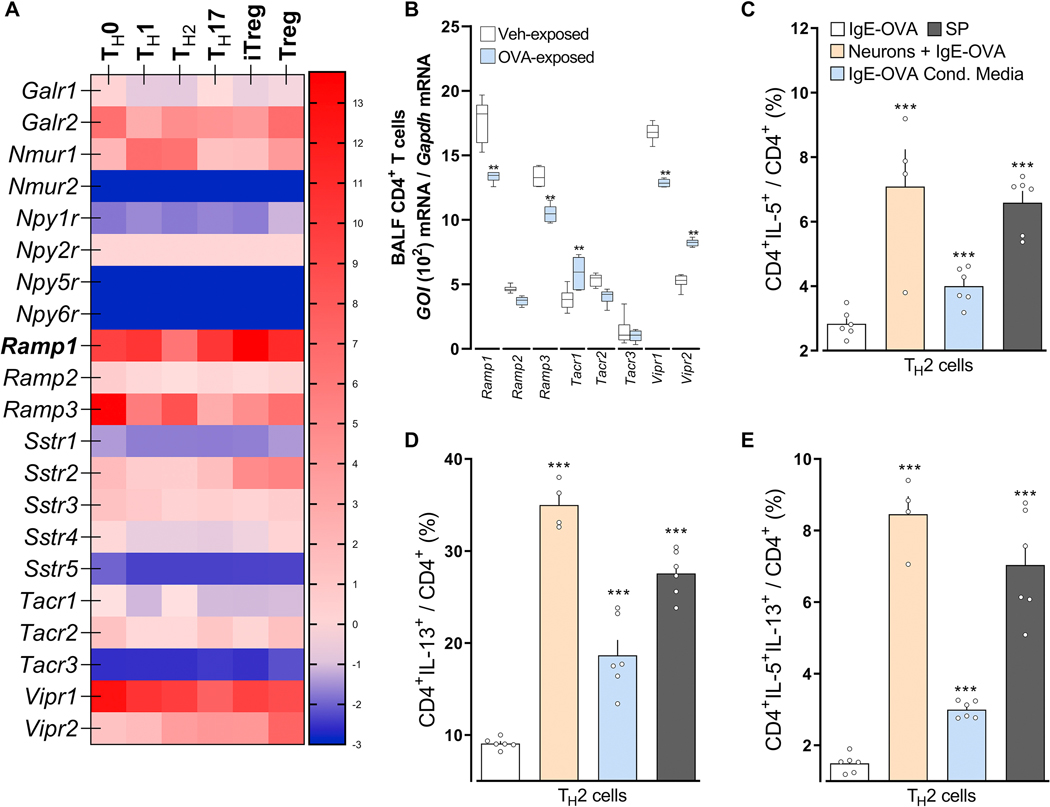
Although detection of allergen immune complexes by vagal neurons constitute a novel feature of danger detection and may elicit sensations and reflexes, we tested whether such early sensing also impacts local immune responses. To do this, we focused on ILC2 and TH2 cells, which are early drivers of allergic airway inflammation that are modulated by sensory neurons 14, 35, 36. TH2 (Figure 5A; supplementary Figure 6A, B) or BALF CD4 T (Figure 5B) cells harvested from OVA-challenged mice showed increased Tacrl transcript expression compared to those from naïve mice. Therefore, these cells are poised to respond to nociceptor cues.
Figure 5: Following allergen sensing, FctR1+ nociceptor neurons drive TH2 cell polarization.
In comparison to naïve, TH1, TH17, iTreg and Treg (A), or naïve CD4 T cells (B), TH2 cells (A; In-silico analysis of Immgen’s RNA sequencing data) or BALF CD4 T cells harvested from OVA-challenged mice (day 15; B) showed increased Tacrl transcript expression (A, B). Purified naïve CD4 spleen cells were polarized into TH2 cells and then either: co-cultured with allergen-sensitized mice JNC neurons (beige bar), stimulated with conditioned media harvested from IC-stimulated allergen-sensitized JNC neurons (blue bar) or with Substance P (C-E; gray bar). In comparison to IgE-OVA-stimulated TH2 cells, TH2 cells co-cultured with neurons then stimulated with neuron-conditioned media or Substance P, showed increased levels of IL-5+ (C), IL-13+ (D), and IL-5+IL-13+ (E). Mean ± S.E.M; Two-tailed unpaired Student’s t-test (B-E). Transcript expression are shown as DESeq2 normalized counts (A). Stimulation and co-culture were done in the presence of a cocktail of protease inhibitors (1/1000; C-E); n = 5–6 animals/group, 2 cohorts.
Splenocyte-harvested naïve CD4+ T cells were polarized into TH2 cells. In the presence of peptidase inhibitor, the cells were then either co-cultured with JNC neurons from allergen-sensitized mice JNC neurons, stimulated with conditioned media harvested from IC-stimulated allergen-sensitized JNC neurons (1:2 dilution) or exposed to substance P (1μM) and TH2 cell activation, then profiled by flow cytometry. In comparison to IgE-OVA stimulated TH2 cells, TH2 cells co-cultured with IgE-OVA-stimulated neurons, TH2 cells exposed to IgE-OVA- induced neuron conditioned media or TH2 cells exposed to substance P shows increased levels of IL-5+ (Figure 5C), IL-13+ (Figure 5D), and IL-5+IL-13+ (Figure 5E). Also, substance P or JNC neuron co-culture increased TH2 expression of IL-4+IL-13+ and IL-4+IL-5+ (supplementary Figure 6C, D; supplementary Figure 7). These treatments had no impact on the proportion of GATA3+ and IL-4+ in CD4+ T cells (supplementary Figure 6E, F; supplementary Figure 7). TH2 cells polarization was also not impacted when co-cultured with naive JNC neurons (supplementary Figure 8). While ILC2 also express various neuropeptide receptors (supplementary Figure 9A), IL-7 stimulated lung ILC2 cells were insensitive both to IgE-OVA triggered neuron conditioned media or substance P (supplementary Figure 9B–D).
Allergen sensing by vagal nociceptors amplifies allergic lung inflammation.
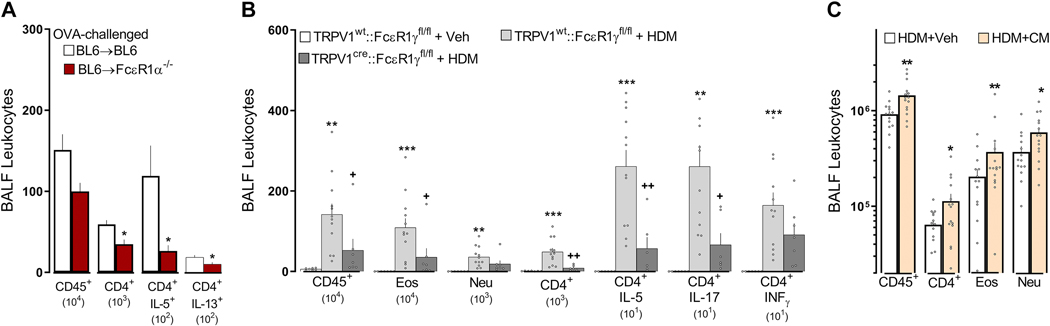
To assess the impact of vagal neuronal sensing of allergens, we transplanted C57BL6 bone marrow into whole-body irradiated C57BL6 (BL6→BL6) and FcεR1a−/− (BL6→FcsR1a−/−) mice. We found that spleen CD45+ immunocytes had a similar expression of FcεR1α (supplementary Figure 10A) in allergen-sensitized BL6→FcεR1α−/− chimeric mice, and the JNC neurons were insensitive to immune complex (supplementary Figure 10B). We also found that one day after an acute OVA-challenge, allergen-sensitized BL6→FcεR1α−/− chimeric mice had reduced numbers of BALF-infiltrating CD45+, total CD4+, and CD4+IL-5+ cells, in comparison to control chimeric mice (Figure 6A). These findings support a key upstream role for FcεR1+ non-hematopoietic cells (e.g., nociceptors) in inducing allergic airway inflammation.
Figure 6: FcεR1+ nociceptor neurons initiate type 2 airway inflammation.
One day after an acute OVA challenge, allergen-sensitized FcεR1α−/− chimeric mice (BL6⟶FcεR1α−/−) displayed a reduced level of AAI (lowered numbers of BALF-infiltrating leukocytes, CD45+, CD4+) and polarization (IL-5+, IL-13+) in comparison with allergen-sensitized control (BL6⟶BL6) mice (A). Next, we used the cre/lox toolbox to generate the first cell- specific FcεR1γ knockdown (TRPV1Cre::FcεR1γfl/fl) and littermate control (TRPV1wt::FcεR1γfl/fl) mice. TRPV1Cre::FcεR1γfl/fl mice were protected from HDM-challenge-induced BALF infiltration of CD45+, eosinophils, and CD4+ as well as CD4 polarization (IL-5, IL-17; B). HDM-challenged mice exposed to neuron-conditioned media harvested from IgE-OVA-stimulated allergen-sensitized JNC neurons demonstrated increased AAI (BALF influx of CD45+, CD4+, eosinophils, neutrophils; C). Mean ± S.E.M; Two-tailed unpaired Student’s t-test (A, C); one-way ANOVA post-hoc Bonferroni (B); n = 5–14 animals/group, 2 cohorts.
To directly pinpoint a role for Fcε1γ+ nociceptors from radio-resistant hematopoietic cells, we used cre/lox technology to generate nociceptor specific Fcε1γ knockdown (TRPV1Cre::FcεR1γfl/fl) mice. The TRPV1Cre::FcεR1γ fl/fl mice showed a 3.2-fold decrease in HDM-induced AAI (BALF numbers of CD45+, eosinophils, total and IL-5+ CD4+ T cells; Figure 6B; supplementary figure 12, supplementary figure 13), and BALF cytokines levels (IL-13; supplementary Figure 11A) when compared to littermate control mice (TRPV1wt::FcεR1γfl/fl). Heterozygote knockout mice (TRPV1Cre::FcεR1γfl/wt) also showed a significant, albeit lower, reduction in HDM-induced AAI (~1.58-fold) and OVA-induced AAI (~1.65-fold; supplementary Figure 11B, C). We then tested whether the TRPV1cre mice nocifensive behavior remained intact. We confirmed that cre recombinase expression in TRPV1+ neurons had no impact on the mice’s thermal nociceptive threshold nor on neurons’ sensitivity to noxious stimuli (supplementary figure 14).
An in-silico analysis of five mouse expression profiling datasets (single-cell RNA-sequencing, bulk RNA sequencing, microarray; supplementary Figure 15–18) revealed that TRPV1 is not expressed by airway stromal or immune cells (including TH2 and mast cells). In addition, TRPV1creFCεR1γfl/fl peritoneal mast cells degranulate normally in response to IgE-OVA (supplementary Figure 18C). TRPV1creDTAfl/wt mice have the same proportion of peritoneal mast cells as controls (supplementary Figure 18D; supplementary figure 19), whereas flow cytometry analysis of peritoneal mast cells from TRPV1creTd-tomato/wt mice showed no cre expression (supplementary Figure 18E; supplementary figure 20). While TRPV1creFCεR1γfl/fl mouse TH2 and mast cells are intact, their JNC neurons do not express FCεR1γ following in vivo allergen-sensitization (supplementary Figure 21A–D), nor do they respond to IgE-OVA (supplementary Figure 21E). However, capsaicin-induced calcium flux responses are still intact (supplementary Figure 21F).
The reduction in the influx of lung immunocytes in TRPV1Cre::FCεR1γfl/fl mice was accompanied by similar levels of IgE (not shown), suggesting that while the capacity to mount an allergic antibody response is present in these mice, allergic airway inflammation is reduced. The diminished inflammatory phenotype in the TRPV1Cre::FcεR1γfl/fl mice was phenocopied by mice whose TRPV1+ sensory neurons were genetically ablated (TRPV1Cre::DTAfl/wt; supplementary Figure 22A–C). While the specific knockdown of FcεR1γ on TRPV1+ sensory neurons did not prevent OVA- and HDM-influx and polarization of ILC2 cells (not shown), the genetic ablation of TRPV1+ neurons did (supplementary Figure 22D–F). Finally, co-exposing HDM-challenged mice on days 8 and 9 to neuron-conditioned media (supernatant from immune complex-stimulated allergen-sensitized JNC neurons), further augmented the HDM evoked increases in BALF CD45+, CD4+, eosinophil, and neutrophil cell numbers (Figure 6C).
Discussion.
Sensory neurons’ involvement in allergic airway inflammation was first suggested by the finding that TRPA1 genetic ablation reversed OVA-induced AAI 37. How are these neurons activated? IL-5, a type II effector cytokine produced by multiple immune cells during allergy, directly activates airway sensory neurons, leading to the secretion of the neuropeptide VIP. VIP, in turn, acts on ILC2 and CD4+ cells to induce cytokine production, including IL-5 and IL-13, to initiate a positive feedforward pro-inflammatory cycle14, 15, 38 In parallel, neuromedin U (NMU), the ligand of NMUR1, activates ILC2s. Acting with IL-25, it also amplifies allergic inflammation 35, 36, 39. In the airway, NMU is found exclusively in lung afferent DRG neurons, and a loss of NMU-NMUR1 signaling reduces ILC2 function by altering transcriptional programs following an allergen-challenge in vivo, and this signaling loss prevents the development of allergic airway inflammation 35.
What sets off the neuro-immune cycle in the setting of allergic airway inflammation? Does it begin with the activation of immune cells or nociceptors by allergen exposure in sensitized animals? The data presented here point to an upstream detection role of allergen-IgE complexes by nociceptors in driving TH2 cell activation and downstream allergic inflammation. Following allergen sensitization, immune-complex-sensing by FcεR1γ+ nociceptors sets off the airway neuro-immune interactions that trigger and maintain allergic airway inflammation.
JNC neurons express FcεR1.
We identified a subset of mouse JNC neurons that express functional FcεR1. Studies have reported neuronal-Ig interactions in Alzheimer’s disease, chronic pain, arthritis-induced hypersensitivity, allergy, and antigen-specific autoimmune diseases 2, 3, 6, 7, 40–46. IgG and IgE activate motor neuron release of neurotransmitters and help control antigen trafficking in lymph nodes 45, 47–50. These findings are in line with the expression by neurons of receptors typically expressed by immune cells, such as MHCI on CNS neurons, as well as nociceptor expression of the immune checkpoint receptor PD1 51, 52.
IgE drives neuronal expression of FcεR1.
Sensory neuron responsiveness is highly tunable, changing in response to diverse insults 15, 53. Similar to FcεR1 expression on hematopoietic cells 54, 27–29, we now reveal that IgE drives FcεR1 overexpression in cultured murine JNC nociceptors and in human iPSC-derived nociceptors. FcεR1 is also upregulated in the abdominal vagus in food-allergic mice 49; in trigeminal ganglion neurons of allergen-sensitized mice 8, and in lumbar DRG neurons following exposure to ragweed pollens or during cutaneous anaphylaxis 43. Overall, these data support a mechanistically conserved mechanism for the neuronal sensing of immune complexes during allergy.
TRPC3 mediates IC-FcεR1-induced calcium influx.
Immune complex sensing leads to TRPC3-dependant calcium influx and the subsequent release of neuropeptides. TRPV1, TRPC3, and FcεR1 are co-expressed in nociceptors, as revealed by the single-cell RNA-sequencing of sciatic nerve crushed mouse peptidergic neurons (cluster 2) 55. The FcγR-syk-TRPC3 axis is also responsible for IgG-IC induced hyperalgesia 31.
Nociceptor-ILC2 crosstalk.
ST2+ ILC2 cells express transcripts for Rampl, Nmurl, and Tacrl. Previous reports have shown that vagal neurons produce VIP 14, 56 and that lumbar neurons release NMU, both of which control ILC2 activation 35, 36, 39. In addition, while CGRP supports ILC2 production of IL-5 57, it also inhibits alarmin-driven type 2 cytokine production, constrains IL-13 expression, and blocks ILC2 proliferation 57–59. We found here that the complete ablation of peptidergic neurons (in TRPV1Cre::DTAfl/wt mice) prevents type 2 cytokine production and infiltration of ILC2. Such findings phenocopy our earlier data obtained with ablation of all nociceptors in NaV1.8Cre::DTAfl/wt mice or nociceptor silencing pharmacologically with QX-314 14. While immune-complex-sensing promotes SP and VIP (but not CGRP) production, IgE-allergen complex-stimulated JNC neurons fail to modulate ILC2 cell function. Immune complex-FcϵR1-induced VIP release (0.1 ng/ml) is, however, lower than that produced by IL-5 (0.8 ng/ml) or capsaicin (40 ng/ml). In addition, the ILC2 cells used here were co-stimulated with IL-7, paralleling some studies that found a broader co-stimulation, including IL-25 and IL-33, is necessary for these cells to respond to CGRP and NMU. This result suggests that neuron-ILC2 crosstalk requires the maximal engagement of multiple peptidergic neurons to trigger a broad neuropeptide release, including NMU and CGRP, for their activation, and such a pattern of neuropeptide release may not occur upon allergen sensing.
Neuropeptides drive TH2 polarization.
Conditioned media harvested from IgE-OVA-stimulated JNC neurons obtained from allergen-sensitized mice drives TH2 cell polarization in vitro and enhances HDM-mediated airway inflammation with increased BALF influx of CD45+, CD4+, eosinophils in vivo. The neuronal detection of immune complexes leads to the release of SP and VIP in the mucosa 60, 61, which can facilitate the local influx and the polarization of these immune cells. Indeed, TH2 cells and OVA-exposed BALF CD4+ T cells express the receptors for these peptides, Vipr2 and Tacrl. While we previously found that recombinant VIP increased TH2 cell activation and polarization, we now show that the SP-Tacr1 axis increases the production of IL-5+, IL-13+, and IL-5+IL-13+ TH2 cells in vitro. In addition, we also have found that SP contributes to mucus hyperplasia, mucin imbalance (Muc5AC/Muc5B), and metaplasia 17. Overall, all our current and past data indicate that antigen detection by lung nociceptors sets off systemic defensive reflex responses like cough or mucus secretion17 and, by mobilizing TH2 cells, prompts the engagement of the adaptive immune system.
Neurons control allergic airway inflammation.
Fcεrlγ acts as an ITAM adapter protein transducing signals of immunoreceptors like FcRs and IL-3R 62, 63. Consistent with the immune complex-sensing by FcϵR1-nociceptors as a major upstream driver of TH2-mediated allergic airway inflammation, we found that nociceptors lacking FcεR1γ (TRPV1cre::FcεR1γfl/fl) are protected from IgE-OVA-induced calcium flux. In addition, mice whose nociceptors lack FcεR1γ are protected from airway inflammation induced by ovalbumin, a classic mouse model of allergic asthma, and house dust mites, a more clinically relevant model of patient allergy. These effects phenocopy the findings we observed in irradiated bone- marrow transplanted FcsR1a knockout mice, a model resistant to IgE-induced systemic anaphylaxis. In addition, the detection of allergens by nociceptors preceded an immune response because silencing nociceptors prior to the allergen challenge reduced the inflammation. In vivo immune-complex challenges in sensitized mice, triggered action potentials in vagal neurons within 5 minutes of exposure, an effect independent of mast cells. These findings are similar to the role of IL-4R+ skin nociceptors in atopic dermatitis-induced skin inflammation via infiltration of TH2 cells and basophils 9.
TRPV1 does not impact mast cell function.
Along with the irradiated bone-marrow transplanted mice, we generated nociceptors lacking FcεR1γ using a well-characterized TRPV1cre mouse line 64,65,66,15,37,68,69,70. While immortalized human mast cells (from mast cell leukemia) might bear TRPV1, detailed genetic findings reveal that mouse mast cells express do not express this ion channel in functional assays 71 and that mouse mast cells do not express either the TRPV1 transcript or protein 72,73,74,65, and do not degranulate in response to heat 73 or capsaicin 73,75 and are insensitive to resiniferatoxin chemo-ablation, which occurs through TRPV1 activation 72.
From five unbiased expression profiling datasets (microarray, bulk RNA sequencing, and single-cell RNA- sequencing), we now demonstrate that mouse mast cell populations do not express TRPV1. In addition, peritoneal mast cells from TRPV1creFCεR1γfl/fl mice degranulate normally in response to IgE-OVA, and TRPV1creDTAfl/wt mice have the same proportion of peritoneal mast cells as controls, while flow cytometry analysis of peritoneal mast cell from TRPV1creTd-tomatofl/wt mouse shows no Cre expression. Overall, while an indirect activation of nociceptors by mast cell-produced histamine is likely76, our data suggest that the earliest activation of nociceptors by allergens is actually direct - via the immune-complex. Both IgE-OVA and capsaicin (TRPV1 agonist) induces vagal nerve compound action potentials in vivo, and this activity is present in mast cell-depleted mice (KitW-sh/W-sh) 30 but absent in the FCεR1γ−/− mouse.
Immunological sensitization drives B cells to initiate antibody class switching, the generation of memory B cells, and the release of specific immunoglobulins 77. Upon antigen recall, the specific antibodies bind to Fc receptors on innate immune cells allowing for a swift and precise response 78. We have now uncovered a novel aspect of allergen detection, that by vagal nociceptors expressing FcεR1, which detect allergens complexed to IgE, and show that this detection leads to the activation of lung TH2 cells. Preventing immune-complex sensing by FcεR1-nociceptors reduces antigen-induced airway inflammation. These findings uncover a novel potential therapeutic avenue for the early treatment or prevention of allergic airway disease, one that targets direct sensory neuron activation by allergens in an immune complex.
Supplementary Material
Key Messages
Vagal nociceptor neurons overexpress FcεR1γ during allergy
FcεR1γ-expressing neurons sense invading allergens which initiate allergic inflammation
Nociceptor neurons released Substance P drive TH2 cell polarization.
Capsule summary
Airway-innervating vagal nociceptor neurons sense invading allergens, which, in turn, drives TH2 cells polarization. Fcε1γ+ neurons, therefore, constitute a novel target to prevent the development of allergy.
Acknowledgements.
This work was supported by the National Institute of Health (NIH) R37NS039518 (CJW), R01-HL122531 (BDL); the Canadian Institutes of Health Research (ST, JT), the Canadian Foundation for Innovation (ST, JT), Canada Research Chair program (ST), Natural Sciences and Engineering Research Council of Canada (ST) and the Brain Canada Foundation, Health Canada and the Azrieli Foundation (ST). JCW received from the. REA and JCW respectively hold fellowships from the NIH (K08 HL130540) and a Bastable-Potts Graduate research award from the Canadian Allergy, Asthma and Immunology Foundation. NIH-P30- HD18655 supported the cores used. We would like to thank Pr. Bruce P. Bean, Dr. Yung-Chih Cheng, Dr. Seungkyu Lee and Dr. Andrew Snavely for discussion, Catherine Ward and Elaria Meshreky for their assistance with mice genotyping.
Abbreviations.
- AAI
allergic airway inflammation
- AITC
mustard oil
- BALF
bronchoalveolar lavage fluid
- BL6
C57BL6
- CGRP
calcitonin gene-related peptide
- DTA
Diptheria toxin
- FcR
Fc receptors
- HDM
house dust mite
- IC
ovalbumin-IgE complex
- IgE
immunoglobulin type E
- IL
Interleukin
- ILC2
innate lymphoid type 2 cells
- i.n.
intranasal
- JNC
nodose complex ganglion
- KCl
potassium chloride
- Muc5AC
Mucin 5AC
- NMU
neuromedin U
- OVA
ovalbumin
- QX-314
membrane-impermeable form of lidocaine
- SP
substance P
- TRPC3
Transient Receptor Potential Cation Channel Subfamily C Member 3
- TRPV1
transient receptor potential vanilloid subtype 1
- VIP
vasoactive intestinal peptide
Footnotes
Disclosure. ST, BDL and CJW have an equity stake in Nocion Therapeutics. The rest of the authors declare that they have no relevant conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References.
- 1.Kinet JP. The high-affinity IgE receptor (Fc epsilon RI): from physiology to pathology. Annu Rev Immunol 1999; 17:931–72. [DOI] [PubMed] [Google Scholar]
- 2.Andoh T, Kuraishi Y. Direct action of immunoglobulin G on primary sensory neurons through Fc gamma receptor I. FASEB J 2004; 18:182–4. [DOI] [PubMed] [Google Scholar]
- 3.van der Kleij H, Charles N, Karimi K, Mao YK, Foster J, Janssen L, et al. Evidence for neuronal expression of functional Fc (epsilon and gamma) receptors. J Allergy Clin Immunol 2010; 125:757–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chuaychoo B, Hunter DD, Myers AC, Kollarik M, Undem BJ. Allergen-induced substance P synthesis in large-diameter sensory neurons innervating the lungs. J Allergy Clin Immunol 2005; 116:325–31. [DOI] [PubMed] [Google Scholar]
- 5.Potenzieri C, Meeker S, Undem BJ. Activation of mouse bronchopulmonary C-fibres by serotonin and allergen-ovalbumin challenge. J Physiol 2012; 590:5449–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bersellini Farinotti A, Wigerblad G, Nascimento D, Bas DB, Morado Urbina C, Nandakumar KS, et al. Cartilage-binding antibodies induce pain through immune complex-mediated activation of neurons. J Exp Med 2019; 216:1904–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang L, Jiang X, Zheng Q, Jeon SM, Chen T, Liu Y, et al. Neuronal FcgammaRI mediates acute and chronic joint pain. J Clin Invest 2019; 130:3754–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu F, Xu L, Chen N, Zhou M, Li C, Yang Q, et al. Neuronal Fc-epsilon receptor I contributes to antigen- evoked pruritus in a murine model of ocular allergy. Brain Behav Immun 2017; 61:165–75. [DOI] [PubMed] [Google Scholar]
- 9.Lk Oetjen, Mack MR, Feng J, Whelan TM, Niu H, Guo CJ, et al. Sensory Neurons Co-opt Classical Immune Signaling Pathways to Mediate Chronic Itch. Cell 2017; 171:217–28 e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilson SR, The L, Batia LM, Beattie K, Katibah GE, McClain SP, et al. The epithelial cell-derived atopic dermatitis cytokine TSLP activates neurons to induce itch. Cell 2013; 155:285–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chiu IM, Heesters BA, Ghasemlou N, Von Hehn CA, Zhao F, Tran J, et al. Bacteria activate sensory neurons that modulate pain and inflammation. Nature 2013; 501:52–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kashem SW, Riedl MS, Yao C, Honda CN, Vulchanova L, Kaplan DH. Nociceptive Sensory Fibers Drive Interleukin-23 Production from CD301b+ Dermal Dendritic Cells and Drive Protective Cutaneous Immunity. Immunity 2015; 43:515–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Riol-Blanco L, Ordovas-Montanes J, Perro M, Naval E, Thiriot A, Alvarez D, et al. Nociceptive sensory neurons drive interleukin-23-mediated psoriasiform skin inflammation. Nature 2014; 510:157–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Talbot S, Abdulnour RE, Burkett PR, Lee S, Cronin SJ, Pascal MA, et al. Silencing Nociceptor Neurons Reduces Allergic Airway Inflammation. Neuron 2015; 87:341–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Foster SL, Seehus CR, Woolf CJ, Talbot S. Sense and Immunity: Context-Dependent Neuro-Immune Interplay. Front Immunol 2017; 8:1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee S, Jo S, Talbot S, Zhang HB, Kotoda M, Andrews NA, et al. Novel charged sodium and calcium channel inhibitor active against neurogenic inflammation. Elife 2019; 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Talbot S, Doyle B, Huang J, Wang JC, Ahmadi M, Roberson DP, et al. Vagal sensory neurons drive mucous cell metaplasia. J Allergy Clin Immunol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haworth O, Cernadas M, Yang R, Serhan CN, Levy BD. Resolvin E1 regulates interleukin 23, interferongamma and lipoxin A4 to promote the resolution of allergic airway inflammation. Nat Immunol 2008; 9:873–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chiu IM, Barrett LB, Williams EK, Strochlic DE, Lee S, Weyer AD, et al. Transcriptional profiling at whole population and single cell levels reveals somatosensory neuron molecular diversity. Elife 2014; 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ji RR, Samad TA, Jin SX, Schmoll R, Woolf CJ. p38 MAPK activation by NGF in primary sensory neurons after inflammation increases TRPV1 levels and maintains heat hyperalgesia. Neuron 2002; 36:57–68. [DOI] [PubMed] [Google Scholar]
- 21.Jin X, Gereau RW. Acute p38-mediated modulation of tetrodotoxin-resistant sodium channels in mouse sensory neurons by tumor necrosis factor-alpha. J Neurosci 2006; 26:246–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kondo T, Sakurai J, Miwa H, Noguchi K. Activation of p38 MAPK through transient receptor potential A1 in a rat model of gastric distension-induced visceral pain. Neuroreport 2013; 24:68–72. [DOI] [PubMed] [Google Scholar]
- 23.Mizushima T, Obata K, Katsura H, Yamanaka H, Kobayashi K, Dai Y, et al. Noxious cold stimulation induces mitogen-activated protein kinase activation in transient receptor potential (TRP) channels TRPA1- and TRPM8-containing small sensory neurons. Neuroscience 2006; 140:1337–48. [DOI] [PubMed] [Google Scholar]
- 24.Mizushima T, Obata K, Yamanaka H, Dai Y, Fukuoka T, Tokunaga A, et al. Activation of p38 MAPK in primary afferent neurons by noxious stimulation and its involvement in the development of thermal hyperalgesia. Pain 2005; 113:51–60. [DOI] [PubMed] [Google Scholar]
- 25.Crosson T, Roversi K, Balood M, Othman R, Ahmadi M, Wang JC, et al. Profiling of how nociceptor neurons detect danger - new and old foes. J Intern Med 2019; 286:268–89. [DOI] [PubMed] [Google Scholar]
- 26.Chang RB, Strochlic DE, Williams EK, Umans BD, Liberles SD. Vagal Sensory Neuron Subtypes that Differentially Control Breathing. Cell 2015; 161:622–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Furuichi K, Rivera J, Isersky C. The receptor for immunoglobulin E on rat basophilic leukemia cells: effect of ligand binding on receptor expression. Proc Natl Acad Sci U S A 1985; 82:1522–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hsu C, MacGlashan D, Jr. IgE antibody up-regulates high affinity IgE binding on murine bone marrow- derived mast cells. Immunol Lett 1996; 52:129–34. [DOI] [PubMed] [Google Scholar]
- 29.Yamaguchi M, Lantz CS, Oettgen HC, Katona IM, Fleming T, Miyajima I, et al. IgE enhances mouse mast cell Fc(epsilon)RI expression in vitro and in vivo: evidence for a novel amplification mechanism in IgE-dependent reactions. J Exp Med 1997; 185:663–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grimbaldeston MA, Chen CC, Piliponsky AM, Tsai M, Tam SY, Galli SJ. Mast cell-deficient W-sash c-kit mutant Kit W-sh/W-sh mice as a model for investigating mast cell biology in vivo. Am J Pathol 2005; 167:835–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qu L, Li Y, Pan X, Zhang P, LaMotte RH, Ma C. Transient receptor potential canonical 3 (TRPC3) is required for IgG immune complex-induced excitation of the rat dorsal root ganglion neurons. J Neurosci 2012; 32:9554–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alkhani H, Ase AR, Grant R, O’Donnell D, Groschner K, Seguela P. Contribution of TRPC3 to store-operated calcium entry and inflammatory transductions in primary nociceptors. Mol Pain 2014; 10:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Quick K, Zhao J, Eijkelkamp N, Linley JE, Rugiero F, Cox JJ, et al. TRPC3 and TRPC6 are essential for normal mechanotransduction in subsets of sensory neurons and cochlear hair cells. Open Biol 2012; 2:120068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Renthal W, Tochitsky I, Yang L, Cheng YC, Li E, Kawaguchi R, et al. Transcriptional Reprogramming of Distinct Peripheral Sensory Neuron Subtypes after Axonal Injury. Neuron 2020; 108:128–44 e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wallrapp A, Riesenfeld SJ, Burkett PR, Abdulnour RE, Nyman J, Dionne D, et al. The neuropeptide NMU amplifies ILC2-driven allergic lung inflammation. Nature 2017; 549:351–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Klose cSn, Mahlakoiv T, Moeller JB, Rankin LC, Flamar AL, Kabata H, et al. The neuropeptide neuromedin U stimulates innate lymphoid cells and type 2 inflammation. Nature 2017; 549:282–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Caceres AI, Brackmann M, Elia MD, Bessac BF, del Camino D, D’Amours M, et al. A sensory neuronal ion channel essential for airway inflammation and hyperreactivity in asthma. Proc Natl Acad Sci U S A 2009; 106:9099–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Talbot S, Foster SL, Woolf CJ. Neuroimmunity: Physiology and Pathology. Annu Rev Immunol 2016; 34:421–47. [DOI] [PubMed] [Google Scholar]
- 39.Cardoso V, Chesne J, Ribeiro H, Garcia-Cassani B, Carvalho T, Bouchery T, et al. Neuronal regulation of type 2 innate lymphoid cells via neuromedin U. Nature 2017; 549:277–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.He L, Zhang G, Liu W, Gao T, Sheikh KA. Anti-Ganglioside Antibodies Induce Nodal and Axonal Injury via Fcgamma Receptor-Mediated Inflammation. J Neurosci 2015; 35:6770–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Conti G, Vedeler C, Bannerman P, Rostami A, Pleasure D. Peripheral nervous system (PNS) expression of mRNAs encoding myelin proteins and Fc gamma RIII during experimental allergic neuritis. J Neuroimmunol 1992; 41:43–9. [DOI] [PubMed] [Google Scholar]
- 42.Rijnierse A, Kroese AB, Redegeld FA, Blokhuis BR, van der Heijden MW, Koster AS, et al. Immunoglobulin-free light chains mediate antigen-specific responses of murine dorsal root ganglion neurons. J Neuroimmunol 2009; 208:80–6. [DOI] [PubMed] [Google Scholar]
- 43.Mack M, Tonc E, Ashbaugh A, Wetzel A, Sykes A, Engblom C, et al. Clonal differences in IgE antibodies affect cutaneous anaphylaxis-associated thermal sensitivity in mice. Immunol Lett 2014; 162:149–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wigerblad G, Bas DB, Fernades-Cerqueira C, Krishnamurthy A, Nandakumar KS, Rogoz K, et al. Autoantibodies to citrullinated proteins induce joint pain independent of inflammation via a chemokine- dependent mechanism. Ann Rheum Dis 2016; 75:730–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Okun E, Mattson MP, Arumugam TV. Involvement of Fc receptors in disorders of the central nervous system. Neuromolecular Med 2010; 12:164–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kam TI, Song S, Gwon Y, Park H, Yan JJ, Im I, et al. FcgammaRIIb mediates amyloid-beta neurotoxicity and memory impairment in Alzheimer’s disease. J Clin Invest 2013; 123:2791–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hanes WM, Olofsson PS, Talbot S, Tsaava T, Ochani M, Imperato GH, et al. Neuronal circuits modulate antigen flow through lymph nodes. Bioelectronic Medicine 2016; 3:18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Harada N, Zhao J, Kurihara H, Nakagata N, Okajima K. Stimulation of Fc gammaRI on primary sensory neurons increases insulin-like growth factor-I production, thereby reducing reperfusion-induced renal injury in mice. J Immunol 2010; 185:1303–10. [DOI] [PubMed] [Google Scholar]
- 49.Liang H, Xu L, Zhou C, Zhang Y, Xu M, Zhang C. Vagal activities are involved in antigen-specific immune inflammation in the intestine. J Gastroenterol Hepatol 2011; 26:1065–71. [DOI] [PubMed] [Google Scholar]
- 50.Mohamed HA, Mosier DR, Zou LL, Siklos L, Alexianu ME, Engelhardt JI, et al. Immunoglobulin Fc gamma receptor promotes immunoglobulin uptake, immunoglobulin-mediated calcium increase, and neurotransmitter release in motor neurons. J Neurosci Res 2002; 69:110–6. [DOI] [PubMed] [Google Scholar]
- 51.Chen G, Kim YH, Li H, Luo H, Liu DL, Zhang ZJ, et al. PD-L1 inhibits acute and chronic pain by suppressing nociceptive neuron activity via PD-1. Nat Neurosci 2017; 20:917–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shatz CJ. MHC class I: an unexpected role in neuronal plasticity. Neuron 2009; 64:40–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mazzone SB, Undem BJ. Vagal Afferent Innervation of the Airways in Health and Disease. Physiol Rev 2016; 96:975–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Borkowski TA, Jouvin MH, Lin SY, Kinet JP. Minimal requirements for IgE-mediated regulation of surface Fc epsilon RI. J Immunol 2001; 167:1290–6. [DOI] [PubMed] [Google Scholar]
- 55.Renthal W, Tochitsky I, Yang L, Cheng YC, Li E, Kawaguchi R, et al. Transcriptional Reprogramming of Distinct Peripheral Sensory Neuron Subtypes after Axonal Injury. Neuron 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nussbaum JC, Van Dyken SJ, von Moltke J, Cheng LE, Mohapatra A, Molofsky AB, et al. Type 2 innate lymphoid cells control eosinophil homeostasis. Nature 2013; 502:245–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wallrapp A, Burkett PR, Riesenfeld SJ, Kim SJ, Christian E, Abdulnour RE, et al. Calcitonin Gene- Related Peptide Negatively Regulates Alarmin-Driven Type 2 Innate Lymphoid Cell Responses. Immunity 2019; 51:709–23 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xu H, Ding J, Porter CBM, Wallrapp A, Tabaka M, Ma S, et al. Transcriptional Atlas of Intestinal Immune Cells Reveals that Neuropeptide alpha-CGRP Modulates Group 2 Innate Lymphoid Cell Responses. Immunity 2019; 51:696–708 e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nagashima H, Mahlakoiv T, Shih HY, Davis FP, Meylan F, Huang Y, et al. Neuropeptide CGRP Limits Group 2 Innate Lymphoid Cell Responses and Constrains Type 2 Inflammation. Immunity 2019; 51:682–95 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ludwig M, Sabatier N, Bull PM, Landgraf R, Dayanithi G, Leng G. Intracellular calcium stores regulate activity-dependent neuropeptide release from dendrites. Nature 2002; 418:85–9. [DOI] [PubMed] [Google Scholar]
- 61.Sudhof TC. Calcium control of neurotransmitter release. Cold Spring Harb Perspect Biol 2012; 4:a011353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sakurai D, Yamasaki S, Arase K, Park SY, Arase H, Konno A, et al. Fc epsilon RI gamma-ITAM is differentially required for mast cell function in vivo. J Immunol 2004; 172:2374–81. [DOI] [PubMed] [Google Scholar]
- 63.Takai T, Li M, Sylvestre D, Clynes R, Ravetch JV. FcR gamma chain deletion results in pleiotrophic effector cell defects. Cell 1994; 76:519–29. [DOI] [PubMed] [Google Scholar]
- 64.Michoud F, Seehus C, Schonle P, Brun N, Taub D, Zhang Z, et al. Epineural optogenetic activation of nociceptors initiates and amplifies inflammation. Nat Biotechnol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Solinski HJ, Kriegbaum MC, Tseng PY, Earnest TW, Gu X, Barik A, et al. Nppb Neurons Are Sensors of Mast Cell-Induced Itch. Cell Rep 2019; 26:3561–73 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Trankner D, Hahne N, Sugino K, Hoon MA, Zuker C. Population of sensory neurons essential for asthmatic hyperreactivity of inflamed airways. Proc Natl Acad Sci U S A 2014; 111:11515–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Talbot S, Doyle B, Huang J, Wang JC, Ahmadi M, Roberson DP, et al. Vagal sensory neurons drive mucous cell metaplasia. J Allergy Clin Immunol 2020; 145:1693–6 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lai NY, Ma Musser, Pinho-Ribeiro FA, Baral P, Jacobson A, Ma P, et al. Gut-Innervating Nociceptor Neurons Regulate Peyer’s Patch Microfold Cells and SFB Levels to Mediate Salmonella Host Defense. Cell 2020; 180:33–49 e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cohen JA, Edwards TN, Liu AW, Hirai T, Jones MR, Wu J, et al. Cutaneous TRPV1(+) Neurons Trigger Protective Innate Type 17 Anticipatory Immunity. Cell 2019; 178:919–32 e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Baral P, Umans BD, Li L, Wallrapp A, Bist M, Kirschbaum T, et al. Nociceptor sensory neurons suppress neutrophil and gammadelta T cell responses in bacterial lung infections and lethal pneumonia. Nat Med 2018; 24:417–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Huber M, Cato ACB, Ainooson GK, Freichel M, Tsvilovskyy V, Jessberger R, et al. Regulation of the pleiotropic effects of tissue-resident mast cells. J Allergy Clin Immunol 2019; 144:S31–S45. [DOI] [PubMed] [Google Scholar]
- 72.Serhan N, Basso L, Sibilano R, Petitfils C, Meixiong J, Bonnart C, et al. House dust mites activate nociceptor-mast cell clusters to drive type 2 skin inflammation. Nat Immunol 2019; 20:1435–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Solis-Lopez A, Kriebs U, Marx A, Mannebach S, Liedtke WB, Caterina MJ, et al. Analysis of TRPV channel activation by stimulation of FCepsilonRI and MRGPR receptors in mouse peritoneal mast cells. PLoS One 2017; 12:e0171366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Buhner S, Schemann M. Mast cell-nerve axis with a focus on the human gut. Biochim Biophys Acta 2012; 1822:85–92. [DOI] [PubMed] [Google Scholar]
- 75.Magadmi R, Meszaros J, Damanhouri ZA, Seward EP. Secretion of Mast Cell Inflammatory Mediators Is Enhanced by CADM1-Dependent Adhesion to Sensory Neurons. Front Cell Neurosci 2019; 13:262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Daeron M, Malbec O, Latour S, Arock M, Fridman WH. Regulation of high-affinity IgE receptor-mediated mast cell activation by murine low-affinity IgG receptors. J Clin Invest 1995; 95:577–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Galli SJ, Tsai M. IgE and mast cells in allergic disease. Nat Med 2012; 18:693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kurosaki T, Kometani K, Ise W. Memory B cells. Nat Rev Immunol 2015; 15:149–59. [DOI] [PubMed] [Google Scholar]
- 79.Crosson T, Roversi K, Balood M, Othman R, Ahmadi M, Wang JC, et al. Profiling of how nociceptor neurons detect danger-new and old foes. Journal of internal medicine 2019; 286:268–89. [DOI] [PubMed] [Google Scholar]
- 80.Ray P, Torck A, Quigley L, Wangzhou A, Neiman M, Rao C, et al. Comparative transcriptome profiling of the human and mouse dorsal root ganglia: an RNA-seq-based resource for pain and sensory neuroscience research. Pain 2018; 159:1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chiu IM, Barrett LB, Williams EK, Strochlic DE, Lee S, Weyer AD, et al. Transcriptional profiling at whole population and single cell levels reveals somatosensory neuron molecular diversity. Elife 2014; 3:e04660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Usoskin D, Furlan A, Islam S, Abdo H, Lönnerberg P, Lou D, et al. Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing. Nature neuroscience 2015; 18:145–53. [DOI] [PubMed] [Google Scholar]
- 83.Li C, Wang S, Chen Y, Zhang X. Somatosensory neuron typing with high-coverage single-cell RNA sequencing and functional analysis. Neuroscience bulletin 2018; 34:200–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Goswami SC, Mishra SK, Maric D, Kaszas K, Gonnella GL, Clokie SJ, et al. Molecular signatures of mouse TRPV1-lineage neurons revealed by RNA-Seq transcriptome analysis. The Journal of Pain 2014; 15:1338–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kupari J, Häring M, Agirre E, Castelo-Branco G, Ernfors P. An atlas of vagal sensory neurons and their molecular specialization. Cell reports 2019; 27:2508–23. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.