Abstract
Natural antioxidants applied as feed additives can improve not only animals’ health and overall performance but also increase their resistance to environmental stress such as heat stress, bad housing conditions, diseases, etc. Marine organisms, for example seaweeds – red, brown, and green macroalgae contain a plethora of biologically active substances, including phenolic compounds, polysaccharides, pigments, vitamins, micro- and macroelements, and proteins known for their antioxidant activity, which can help in the maintenance of appropriate redox status in animals and show pleiotropic effects for enhancing good health, and productivity. The dysregulated production of free radicals is a marked characteristic of several clinical conditions, and antioxidant machinery plays a pivotal role in scavenging the excessive free radicals, thereby preventing and treating infections in animals. Supplementation of seaweeds to animal diet can boost antioxidant activity, immunity, and the gut environment. Dietary supplementation of seaweeds can also enhance meat quality due to the deposition of marine-derived antioxidant components in muscles. The use of natural antioxidants in the meat industry is a practical approach to minimize or prevent lipid oxidation. However, overconsumption of seaweeds, especially brown macroalgae, should be avoided because of their high iodine content. An important point to consider when including seaweeds in animal feed is their variable composition which depends on the species, habitat, location, harvest time, growing conditions such as nutrient concentration in water, light intensity, temperature, etc. This review highlights the beneficial applications of seaweeds and their extracted compounds, which have antioxidant properties as feed additives and impact animal health and production.
Keywords: Seaweeds, antioxidants, polysaccharides, animal production, health, diseases
1. Introduction
Seaweeds, also called macroalgae, are multicellular large-size marine organisms, which are classified into three main groups, according to their pigments – green (Chlorophyta), red (Rhodophyta), and brown seaweeds (Phaeophyceae) (Makkar et al. 2016; Corino et al. 2019; Alboofetileh et al. 2021). This biomass has been used for centuries in many coastal communities and played an important role as a component of food, natural fertilizer or animal feed (Mac Monagail et al. 2017). According to the latest Food and Agriculture Organization (FAO) report (2018), in 2015, the total world seaweed production was about 30.4 million tonnes – 29.4 million tonnes from aquaculture (seaweed cultivation) and 1.1 million tonnes harvested from the wild. Cultivation of seaweeds dominates in China, Indonesia, the Republic of Korea, and the Philippines, whereas the leading producers for wild species are Chile, China, and Norway. It is assumed that 221 species of seaweeds have a commercial value. Intensively cultivated are species of brown seaweeds – Saccharina japonica, Sargassum fusiforme, Undaria pinnatifia, red seaweeds – Eucheuma spp., Gracilaria spp., Porphyra spp., Kappaphycus alvarezii and green seaweeds such as Caulerpa spp., Enteromorpha clathrata, Monostroma nitidum. Among them, the most popular is Saccharina japonica (earlier classified as Laminaria japonica), which production constitutes 33%.
Seaweeds are known to be rich in both primary and secondary metabolites. The primary metabolites, which are directly involved in processes such as growth, development, reproduction to perform physiological functions, can be divided into several classes: carbohydrates (fucoidan, alginate, laminarin in brown seaweeds, agar, carrageenan in red seaweeds, and ulvan in green seaweeds), lipids (fatty acids: saturated, mono-, di-, and polyunsaturated, waxes, acylglycerols: mono-, di-, and triacylglycerols, phospholipids, glycoglycerolipids, fat-soluble vitamins, e.g., A and E, sterols, and carotenoids), and proteins (combination of different amino acids). In addition to the primary metabolites, seaweeds can accumulate minerals (microelements, macroelements, and trace elements) that are also essential to their life (Makkar et al. 2016; Salehi et al. 2019; Matos et al. 2021). Seaweeds can also synthesize a vast number of secondary metabolites, which largely determine their bioactive potential (Øverland et al. 2019; Salehi et al. 2019). In contrast to primary metabolites, secondary metabolites are not necessary for tissue growth. They are formed in the biomass as a response to exposure to biotic (e.g., fungi, bacteria, viruses, insects, etc.) and abiotic stress (e.g., UV radiation, drought, salinity, high temperature) (Metsämuuronen and Sirén 2019; Matos et al. 2021). An important group of seaweeds’ secondary metabolites are phenolics, which involve simple phenols like phenolic acids and polyphenols, including flavonoids and non-flavonoids – e.g., tannins. Carotenoids and sterols listed above as primary metabolites are often included in the secondary metabolite class (Salehi et al. 2019). Most of the listed compounds have antioxidant activity. Seaweed antioxidants play the function of “free radical scavengers” – they prevent or repair damages caused by oxidative stress and have a high potential for treating various diseases (Liu and Sun 2020). This unique composition of seaweeds results from their growth in an ever-changing environment. They are constantly exposed to stress conditions such as salinity, changes in temperature, nutrient enrichment, UV radiation exposure, presence of herbivores or pollutants. The resistance of seaweeds to biotic and abiotic stress is due to the production of physiologically active substances such as oligosaccharides, amino acids, vitamins, and phytohormones (Farvin and Jacobsen 2013; Manlusoc et al. 2019; Matos et al. 2021).
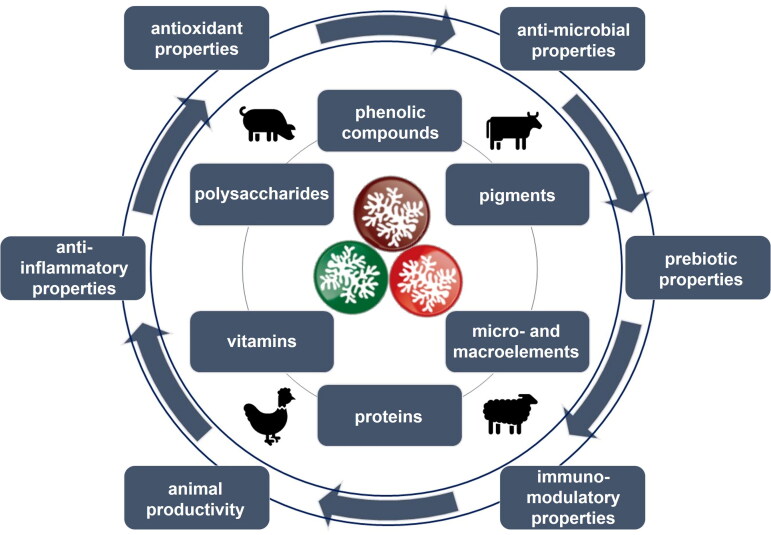
Seaweed-based antioxidants are discussed mainly in terms of cosmetic, pharmaceutical, and biomedical applications, food applications (stabilizer and preservative), and agriculture (biostimulants of plant growth) (Cotas et al. 2020), but less in terms of feed and animal health and production. Antioxidants derived from seaweeds can have many beneficial effects on animal health (de Quiros et al. 2010; Liu and Sun 2020), such as antioxidant, anti-inflammatory, antimicrobial, immunomodulatory, and prebiotic effects (Corino et al. 2019; Liu and Sun 2020). The supplementation of animal feed with prebiotics based on seaweed biomass has recently gained much attention due to their positive effect on gut health and immune system (Ruiz et al. 2018). The general scheme showing the seaweed antioxidants and their effect on animal health and performance is presented in Figure 1.
Figure 1.
The general scheme of seaweed antioxidants and their effect on animal health and performance.
This review focuses on the application of seaweeds, seaweed extracts, and extracted compounds in animal feed. Special attention has been paid to antioxidants and their effects on animal health and production performance. Furthermore, the significance of seaweeds’ antioxidants, their different forms and use in animal nutrition, and their influence on the quality of animal-derived products have been discussed.
2. Seaweeds as a source of antioxidants
Seaweeds can serve as a source of antioxidants as they are produced by the biomass as a result of exposure to external environmental factors such as herbivory, salinity, nutrients availability, light, depth, seasonality as well as intrinsic factors such as age, length, and type of tissues (Farvin and Jacobsen 2013; Manlusoc et al. 2019). The main groups of antioxidants in seaweeds are phenolics, polysaccharides, and pigments.
Phenolic compounds found in seaweeds include (a) simple phenols such as phenolic acids (de Quiros et al. 2010): hydroxycinnamic acids – caffeic, p-coumaric, ferulic, sinapic acid and hydroxybenzoic acids – gallic, vanilic, 4-hydroxybenzoic, protocatechuic, syringic, gentisic acid (Farvin and Jacobsen 2013) and (b) polyphenols covering flavonoids and non-flavonoids. Flavonoids have several subgroups, which include flavones (e.g., rhoifolin), flavanol (e.g., catechins, epicatechin, gallocatechin, epigallocatechin), flavanones, flavonols (e.g., quercetin), anthocyanins (e.g., delphinidin, isopeonidin, malvidin), and isoflavones (e.g., sativanone, dalbergin) (de Quiros et al. 2010; Farvin and Jacobsen 2013; Cotas et al. 2020; Zhong et al. 2020). Non-flavonoids are divided into tannins (de Quiros et al. 2010; Cotas et al. 2020) such as phlorotannins (phloroglucinol and eckol) (Cotas et al. 2020), lignans (lignan derivatives: 2′-hydroxyenterolactone, arctigenin, dimethylmatairesinol, deoxyschisandrin), and stilbenes (Zhong et al. 2020). Phlorotannins are the major phenolic compounds in brown seaweeds, which are unique to this seaweed class (de Quiros et al. 2010; Holdt and Kraan 2011; Ford et al. 2020a). Other polyphenols which can be distinguished in seaweeds are phenolic terpenes (rosmanol, carnosol, carnosic acid [Zhong et al. 2020]) and terpenoids (chromene, chromanol, plastoquinone [Cotas et al. 2020]). Phenolic terpenoids were determined and characterized in red and brown seaweeds (Stengel et al. 2011). Seaweeds also contain bromophenols like 2,4-bromophenol, 2,6-bromophenol, 2,4,6 tribromophenol (de Quiros et al. 2010; Cotas et al. 2020). Besides antioxidant properties (prevention and/or reduction in oxidative damage), most phenolic compounds possess a wide variety of biological activities such as antibacterial, antiviral, anti-inflammatory, antidiabetic, antimethanogenic, etc. (Corino et al. 2019; Campbell et al. 2020; Cotas et al. 2020; Ford et al. 2020a). Phenolic compounds are considered an alternative to antimicrobials in feed, which can help reduce antibiotic resistance in livestock (Campbell et al. 2020; Ford et al. 2020b). It is suggested that the antibacterial activity of extract produced from brown seaweed (Ascophyllum nodosum) can be attributed to the presence of phlorotannins (Nagayama et al. 2002).
Furthermore, seaweeds are known to contain unique polysaccharides, which are specific to a given algal group. The content of carbohydrates in all seaweeds is recognized as high (Ford et al. 2020a; Matos et al. 2021). Øverland et al. (2019) presented the ranges of their content – brown seaweeds 380–610 g/kg of dry mass (d.m.); green seaweeds 150–650 g/kg d.m. and red seaweeds 360–660 g/kg d.m. Polysaccharides like fucoidan, alginate, laminarin are found in brown seaweeds, agar and carrageenan in red seaweeds, and ulvan in green seaweeds (Kraan 2012; Corino et al. 2019; Øverland et al. 2019; Liu and Sun 2020; Morais et al. 2020). Sulfated polysaccharides from marine algae such as fucoindan, carragenan, and ulvan possess antioxidative activity and can enhance the antioxidant status in animals (Salehi et al. 2019; Liu and Sun 2020). Seaweeds are rich not only in polysaccharides acting as antioxidants but also in the undigestible polysaccharides, thereby serving as a potential source of dietary fibers (O’Doherty et al. 2010). The dietary fibers are classified into two groups (1) insoluble such as cellulose, mannan, and xylan, and (2) water-soluble such as alginic acid, furonan, laminarin, agar, and porphyran (Kraan 2012). Polysaccharides applied as prebiotics in animal feed positively affect the cellulolytic and lactic acid bacteria present in the gastrointestinal tract (GIT) and inhibit the occurrence of pathogenic bacteria such as Escherichia coli and Clostridium difficile. Maintenance of the adequate gut microbiome and reduced risk of inflammation enhance gut morphology and nutrient absorption from the feed (Lynch et al. 2010; Kraan 2012). Polysaccharides, especially laminarin and fucoidan, have a positive effect on animal health – modulate the gut environment, stimulate the innate immune system, reduce the risk of diarrhea, enhance growth performance and promote productivity (also in the absence of in-feed antibiotics) (Lynch et al. 2010; Kraan 2012; Del Tuffo et al. 2019).
Natural pigments are another group of bioactive compounds with antioxidant properties (de Quiros et al. 2010; Holdt and Kraan 2011; Øverland et al. 2019; Matos et al. 2021). Seaweeds contain chlorophylls, mainly green seaweeds (de Quiros et al. 2010; Øverland et al. 2019; Morais et al. 2020), β-carotene, lutein, zeaxanthin, phycobiliproteins – red seaweeds (de Quiros et al. 2010; Øverland et al. 2019), carotenoids (α- and β-carotene, lutein, zeaxanthin, and fucoxanthin) – brown seaweeds (de Quiros et al. 2010; Farvin and Jacobsen 2013; Øverland et al. 2019; Morais et al. 2020), whereas fucoxanthin is typical to brown seaweeds (Cotas et al. 2020) and masks chlorophyll, β-carotene, violaxanthin and diatoxanthin (Corino et al. 2019).
Seaweeds also contain a wide array of vitamins, including vitamin E (α-tocopherol) (de Quiros et al. 2010; Farvin and Jacobsen 2013; Morais et al. 2020), vitamin C (ascorbic acid) (de Quiros et al. 2010; Morais et al. 2020; Matos et al. 2021; Nielsen et al. 2021), A, B1, B2, B3, B5, B6, and B8 (Corino et al. 2019; Morais et al. 2020). In addition, some carotenoids also act like vitamins, for example, β-carotene with the provitamin A activity (Holdt and Kraan 2011).
Micro- and macroelements (e.g., iodine, zinc, iron, manganese, selenium, sodium, calcium, potassium, and magnesium) are other seaweed constituents important for the proper development and health of animals. Seaweeds easily bioaccumulate minerals from seawater and contain 10–20 times more minerals than land plants (Makkar et al. 2016; Matos et al. 2021). Micro- and macroelements are present in a form, which is highly bioavailable to animals. Some microelements – e.g., selenium, zinc, and manganese demonstrate strong antioxidant properties (Corino et al. 2019; Øverland et al. 2019; Liu and Sun 2020; Morais et al. 2020). Feed for animals containing seaweeds are usually higher in ash than the feed/concentrates used in the control group (Rjiba et al. 2010). Seaweeds possess the ability to accumulate micro- and macroelements and toxic metal ions such as arsenic, cadmium, and lead, which can limit their use as a component of animal feed (Corino et al. 2019; Øverland et al. 2019). The same is the case of high iodine content (Øverland et al. 2019).
Seaweeds constitute a valuable source of proteins (Farvin and Jacobsen 2013; Makkar et al. 2016; Corino et al. 2019; Morais et al. 2020; Matos et al. 2021) with red seaweeds as the richest source of protein − 64–376 g/kg d.m. (crude protein), then green seaweeds 32–352 g/kg d.m. and finally brown seaweeds 24–168 g/kg d.m. (Øverland et al. 2019). Seaweeds can provide animals with all essential and many non-essential amino acids. Therefore, a detailed amino acid analysis of seaweeds should be performed for nutritional purposes (Øverland et al. 2019). Essential amino acids in Sargassum latifolium represent 42% of the total amino acids content (12 mg/g d.m.). Therefore, they are suggested as a complementary source of proteins for animal nutrition (Ramadan et al. 2020).
An important point to consider when including seaweeds in animal feed is their variable composition which depends on the species, habitat, location, time of harvest, growing conditions such as nutrient concentration in water, light intensity, temperature, etc. (Makkar et al. 2016; Del Tuffo et al. 2019). It is recommended to harvest seaweeds when active compounds are the most abundant to reduce the amount of algal biomass supplemented to the feed (Ford et al. 2020a). Therefore, the use of seaweeds in animal nutrition as a source of antioxidants should be preceded by a detailed analysis of the algae composition, depending on the species, location, harvest date, etc.
3. Significance of antioxidants to animals
Oxidative stress and increased production of reactive oxygen species (ROS) regulate several organs and tissues' metabolic activities under diverse veterinary conditions and play a significant role in the productive output of livestock. The oxidative damage caused by the increased generation of unnecessary ROS due to the activity of NADPH-oxidases results from the exposure to multiple stress factors, including biotic and abiotic factors (Kaur et al. 2014). In the pathophysiology of various infections and diseases, such as foot and mouth viral infection and interdigital dermatitis, the weakening of antioxidant defense has an essential role in the prognosis of the disease (Khoshvaghti et al. 2014; Hayat et al. 2020).
Enzymatic antioxidant machinery comprises catalase (CAT), glutathione peroxidase (GPX), and superoxide dismutase (SOD), and other enzymes (Kaur et al. 2016). At the same time, the non-enzymatic antioxidants include vitamins A, C, and E, thiol antioxidants (thioredoxin, glutathione (GSH) and lipoic acid), carotenoids, melatonin, natural flavonoids, etc. (Rahman 2007; Valko et al. 2007; Kurutas 2016; Changxing et al. 2018a, 2018b). Several biotic and abiotic stress factors (e.g., bacteria, environmental pollutants, radiation, chemicals, medications, food, diseases, and temperature) may influence the concentration of multiple antioxidant enzymes like CAT, GPX, SOD, and GSH, MDA (malondialdehyde – a product of lipid peroxidation in animal blood), as well as their activities. The reduction in the concentration of antioxidant enzymes and their activities increases free radical’s generation levels. The virulence effect of pathogenic microorganisms is heightened by the presence of elevated amounts of free radicals in macrophages, which are released in large numbers when bacterial infections arise. CAT, GPX, and GSH are essential components of the cell's mechanism of protection against oxidative stress, which help sustain the immune cells' functions against infectious diseases (Rahman 2007; Valko et al. 2007; Rahal et al. 2014).
Several studies in various clinical manifestations have explored the role of antioxidant enzymes and their functioning. A study was conducted to evaluate the relationship between antioxidant enzymes such as SOD, GPX, MDA, CAT, and foot and mouth disease (FMD) in cattle. Forty diseased cows affected with FMD virus were compared with ten healthy adult cattle as a control. SOD and GPX activities were significantly reduced by FMD virus infection as compared to the control group. MDA activity was significantly higher in infected animals compared with healthy cows. It has been reported that the antioxidant enzymatic machinery significantly reduced its functioning in viral infection in cattle, which postulates the possible roles of oxidative stress in clinical manifestation (Khoshvaghti et al. 2014). In another study, the correlation between acute bovine laminitis and antioxidant enzymatic activities of MDA, CAT, GSH, and SOD was measured in dairy heifers. A significant reduction in SOD concentration was recorded when compared to the control group, whereas MDA activities were significantly higher in treated heifers than in the healthy heifers. Non-significant differences were recorded in GSH and CAT activities between the control and experimental group. It has been postulated that inadequate levels of antioxidants (enzymatic and non-enzymatic) may be linked to oxidative stress in sick heifers. Antioxidant enzymes such as SOD, GSH, CAT, and MDA activity might play a crucial role in the pathogenesis of acute laminitis in dairy heifers (Hayat et al. 2020). Further, another study reported that the enhanced production of MDA and NO in the case of interdigital dermatitis infection. CAT levels were significantly lower in serum compared to the control group in several conditions, including interdigital dermatitis, footrot, and interdigital pouch infections. GPX and GSH concentrations in serum were also reported significantly lower in interdigital dermatitis infections. Further, it has been recorded that MDA and NO concentrations were significantly higher in sheep with foot disease whereas, CAT concentrations were significantly lower in serum. It has been postulated that there is a strong correlation between foot infectious diseases and antioxidant machinery in animals. Hence, oxidative stress and increased ROS levels lead to lipid peroxidation and protein and DNA damage, which enhance the severity of the infection (Yurdakul and Yildirim 2018).
Antioxidants in the body cells are present in small amounts, which positively limit the oxidation of important cellular components such as proteins, DNA or RNA. Additionally, natural or synthetic antioxidants may be used either endogenously or exogenously as a component in livestock diet or as a feed additive. Ascorbic acid, among other vitamins and nutrients, has been believed to be an antioxidant agent, which can reduce and neutralize reactive oxygen species. This vitamin, being a cofactor for several enzymes, is required for normal growth and development (Eryilmaz 2017; Nielsen et al. 2021). Seaweeds can serve as a source of vitamin C – the average content is 0.773 mg/g d.m. The highest mean value of this vitamin was observed in brown seaweeds − 0.815 mg/g, then green seaweeds − 0.781 mg/g, and finally in red seaweeds − 0.720 mg/g d.m. (Nielsen et al. 2021). Physiologically, strong antioxidants can be readily absorbed and effectively remove free radicals while also removing redox metals at appropriate amounts. The endogenous antioxidant serves a key role in helping the cells work more efficiently (Arain et al. 2018a, 2018b; Waheed Janabi et al. 2020; Naiel et al. 2021). Recently, it has been suggested that persistent oxidative stress may contribute to chronic inflammation, which in turn mediate a variety of chronic diseases in animals (Juárez-Portilla et al. 2019). For example, a link between tumor initiation and progression and oxidative stress has been demonstrated either by genetic mutations or DNA injury (Visconti and Grieco 2009). In comparison, the release of histamine or other inflammatory molecules and the increase in the inflammation-inducing white blood cells, like mast cells and neutrophils, resulting in a rise of the blood supply to the affected region, thereby enhancing the oxygen influx, which in turn leads to oxidative stress at the site of infection. Therefore, increased development and concentration of oxidative stress leads to the infection's severity in various clinical manifestations. Proinflammatory cytokines, as well as specific mediators of inflammatory cells, including the derivatives of fatty acids, cytokines, and chemokines, can also respond through growing the number of immune cells present at the injury or disease site and thereby augmenting the output of ROS (Coussens and Werb 2002; Hussain et al. 2003).
Besides, free radicals are frequently found in animals' neuropathological conditions. Hence, the defense against oxidative damage could serve as a means of preventing disease progression such as neurodegeneration in animals. Among other aspects, researchers found that ROS production is closely linked to inflammation, and marine algae compounds containing both antioxidant and anti-inflammatory properties may be excellent choices for treating hyperinflammatory responses in several animal diseases due to their complex and multifaceted properties (Barbalace et al. 2019). Barbalace et al. (2019), in their comprehensive literature survey, showed that seaweed chemical components such as antioxidants, fatty acids, polysaccharides, sterols, carotenoids, phycobilins, phycocolloids, etc. possess the potential in reducing proinflammatory cytokines such as NO, TNF-α, IL-6, and IL-1β and in downregulating the inflammatory enzymes such as iNOS and COX-2. Further, the antioxidant properties of several seaweed extracts have been reported to attenuate the signaling mechanisms contributing to inflammatory enzymes activation (Barbalace et al. 2019).
4. Forms of seaweeds’ antioxidants in animal nutrition
Seaweeds as a feed/feed additive can be used in the form of intact natural biomass (fresh or dried) or in the form of extract (Dierick et al. 2009; Michiels et al. 2012; Michalak and Mahrose 2020). The intact seaweeds are usually incorporated into feed pellets, whereas extracts into solutions (drinking water) (Abbott et al. 2020). The biomass after collection from the coastal zone is, in most cases, washed with tap water to remove sodium and impurities, dried, grounded, and finally used as a seaweed powder in animal diet (Abdoun et al. 2014; Ramadan et al. 2020). In this case, not only antioxidants but also other seaweed bioactive compounds are supplemented to animals. Campbell et al. (2020) proposed ensiling of seaweeds before application in livestock feeding, which is a suitable method of biomass preservation. Ensiling of brown seaweeds – Saccharina latissimi and Fucus vesiculosus with or without lactic acid bacteria inoculant (Lactobacillus plantarum) had a limited effect on the phenolic content. Fermentation for 90-days did not influence the chemical composition of F. vesiculosus, but for S. latissimi the content of crude protein, ash, and fiber was decreased.
To make seaweeds’ biomass a valuable source of antioxidants for animals, it should be subjected to the extraction process (Table 1). Before extraction, this biomass is usually pretreated (washing, drying, milling or grinding, pre-extraction) to obtain a higher yield of seaweed antioxidants (Cotas et al. 2020). The content of antioxidants in the produced extract depends on different extraction methods, as well as on the tested seaweed species (Farvin and Jacobsen 2013). Usually, seaweed extracts are a concentrate of many bioactive compounds. But appropriate extraction method can be selected aimed at extracting a given compound. For example, to extract polysaccharide – ulvan from green seaweed (Ulva sp.) used as a feed additive for laying hens, extraction with cellulase and later with hydrogen peroxide was applied (Li et al. 2018). However, the most appropriate method of seaweeds delivery to animals is not possible to predict at this moment. It will require animal feeding trials suitable for the animal production method used in a given country (Abbott et al. 2020).
Table 1.
The examples of the effect of seaweeds antioxidants on animals.
| Seaweed | Tested antioxidant/dose/animal | Effect on animals (as compared to the control group) | Reference |
|---|---|---|---|
| Polysaccharides | |||
| Laminaria digitata (B) | extract containing laminarin − 0.5 g/kg feed and fucoidan − 0.42 g/kg feed, pigs | no effect on the plasma total antioxidant status, increase in antioxidant activity in animals – lowering levels of lipid oxidation in porcine muscle | Moroney et al. 2012 |
|
Laminaria digitata (B), Bioatlantis Ltd (Tralee, Ireland) |
extract containing laminarin (112 g/kg) and fucoidan (89 g/kg), dose 2.8 g/kg, newly weaned pigs | improved performance, higher average daily gain, gain to feed ratio, increase in the coefficient of total tract apparent digestibility of N, gross energy, reduction in the counts of E. coli in faeces | O’Doherty et al. 2010 |
|
Laminaria hyperborea (B) Laminaria digitata (B) |
extract containing laminarin and fucoidan, 1.5 g/kg, weaned pigs | L. hyperborea extract – lower population of Bifidobacteria in the colon and Lactobacilli in the caecum and colon, increase in total monocyte number, L. digitata extract – lower population of Enterobacteria in the caecum and colon, Bifidobacteria in the caecum, Lactobacilli in the caecum and colon, for both extracts – reduced ammonia concentration in the colon | Reilly et al. 2008 |
|
Laminaria spp. (B), Bioatlantis Ltd (Tralee, Ireland) |
extract containing laminarin (112 g/kg) and fucoidan (89 g/kg), dose 1, 2, 4 g/kg, weanling piglets | at low (60 g/kg) and medium (150 g/kg) levels of lactose in diet – increase in average daily gain with the increase in extract to 2 g/kg, at low level of lactose – significant improvement in food conversion ratio as the levels of seaweed extract increased to 4 g/kg, for medium level – improvement till 2 g/kg of extract | Gahan et al. 2009 |
| Laminaria spp. (B) | extract containing laminarin and fucoidan, 300 mg/kg of laminarin, 240 mg/kg of fucoidan, mixture of 300 mg/kg of laminarin and 240 mg/kg of fucoidan, weanling piglet | laminarin – increase in daily gain and gain-to-feed ratio, increase in faecal dry matter, reduced diarrhea, reduced faecal E. coli, fucoidan – increase in Lactobacilli, no effect of fucoidan on faecal Lactobacilli when laminarin was added, a combination of laminarin and fucoidan reduced post-weaning diarrhea | McDonnell et al. 2010 |
| Laminaria hyperborea (B) | extract containing laminarin and fucoidan, 0.7, 1.4, 2.8, 5.6 g/kg, pigs | increase in urine output, water intake with an increase in extract inclusion, effect on colonic Bifidobacterium spp. (higher than in control, decrease with a rise in extract dose) and on caecal Enterobacterium spp. (higher for 0.7 g/kg than in the control), decrease in caecal Bifidobacterium spp. and colonic Lactobacilli spp. as the level of seaweed extract increased | Lynch et al. 2010 |
| Laminaria hyperborea (B) | extract containing laminarin and fucoidan, 300 mg/kg of laminarin, 240 mg/kg of fucoidan, mixture of 300 mg/kg of laminarin and 240 mg/kg of fucoidan, pigs | laminarin – reduced Enterobacterium spp. compared to the control diet, the combination of laminarin and fucoidan increased Enterobacterium spp. compared with alone polysaccharides, fucoidan – increased Lactobacilli spp. in the proximal colon and distal colon as compared with non-fucoidan diet | Lynch et al. 2010 |
|
Laminaria spp. (B), Bioatlantis Ltd (Tralee, Ireland) |
seaweed extract containing laminarin (1.0 g), fucoidan (0.8 g), and ash (8.2 g), sows and post-weaned pigs, 2.8 g/kg diet | higher average daily gain (0–21 post-weaning day) in pigs weaned from extract-supplemented sows, increase in average daily gain during the grower–finisher phase, decreased colonic E. coli population in pigs feed with post-weaning diets with extract | Leonard et al. 2011a |
|
Laminaria spp. (B), Bioatlantis Ltd (Tralee, Ireland) |
seaweed extract containing laminarin (1.0 g), fucoidan (0.8 g), and ash (8.2 g), 10 g/d, suckling piglet | increase in immunoglobulin G (IgG) and immunoglobulin A (IgA) in sow colostrum, an increase in piglet serum IgG, reduction in fecal Enterobacteriaceae numbers in sows at parturition, decrease in colonic E. coli population at weaning in piglets suckling extract-supplemented sows | Leonard et al. 2012 |
|
Ascophyllum nodosum (B), Tasco-14 (Acadian, Nova Scotia, Canada) |
2%, steers and heifers | antimicrobial properties, reduction in the prevalence of enterohemorrhagic E. coli on hide swabs and in fecal samples, possible inhibition of the growth of Salmonella spp. | Braden et al. 2004 |
|
Ascophyllum nodosum (B), Tasco (Acadian, Nova Scotia, Canada) |
extract, 1.7 and 3.4 kg/ha of fescue, grazing lambs | Increase in antioxidant activity in grazing ruminant, for summer lambs grazing period – increase in lamb gain, serum vit. A and Se concentration with increase in Tasco dose, no effect on vit. E | Fike et al. 2001 |
|
Ascophyllum nodosum (B), Tasco-Forage (Acadian, Nova Scotia, Canada) |
tall fescue pastures infected with endophyte (Neotyphodium coenophialum) treated with Tasco-Forage (3.4 kg/ha), weaned beef steers | increase in rectal temperatures due to endophyte infection, decrease in temperature of steers grazing infected fescue treated with Tasco, alleviation of rough hair coats and loss of hair color, mitigation of the adverse effects of endophytes on immune function | Saker et al. 2001 |
|
Ascophyllum nodosum (B), TascoTM (Acadian Sealants Ltd, Nova Scotia, Canada) |
wether lambs, heat stress | supplementation of Tasco to post-harvest fescue hay – enhancement of immune function (increased phagocytic activity, increase in red and white blood cell glutathione peroxidase), protection against prolonged heat-induced oxidative stress, enhancement of monocyte oxidative burst through short and long duration heat stress | Saker et al. 2004 |
| Ascophyllum nodosum (B) | intact dried biomass – source of dietary fiber, 2.5, 5, 10 g/kg, weaned piglets | no significant effect on daily weight gain, feed conversion ratio, performances of piglets, gut health parameters and plasma oxidative status (measurements of TBARS, FRAP, GSH-Px), increase in plasma α-tocopherol in all experimental groups, no changes in the microbial ecology in the foregut and in the caecum | Michiels et al. 2012 |
| Ulva armoricana (G) | algal extract containing sulfated polysaccharide, 2, 8 and 16 g/day (two periods: before the last atrophic rhinitis vaccine booster and a week before farrowing), pig, sows – the end of gestation | 16 g/day – an increase in specific IgG in sow’s blood and colostrum, 8 g/day – an increase in the level of total IgA in milk as compared to the control group | Bussy et al. 2019 |
| brown seaweeds (Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian, China) | alginic acid oligosaccharide, 100 mg/kg, weaned pigs | enhancement of the average daily body weight gain, increase in the concentrations of IL-10, IgG, and IgA, increase in SOD, CAT activity, and total antioxidant capacity in the serum, decrease in serum MDA, increase in the population of Bifidobacterium and Lactobacillus, decrease in the populations of E. coli in the intestine | Wan et al. 2016 |
| brown seaweeds (Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian, China) | alginate oligosaccharide, 50, 100 and 200 mg/kg, weaned pigs | 100 and 200 mg/kg – significant increase in the average daily body weight gain, 100 mg/kg – promotion of antioxidant defense properties (enhanced serum CAT activity and GSH content), improvement of serum hormone levels (insulin and insulin-like growth factor-1), increase in the nutrient digestibility (crude ash, protein, and fat), maltase and sucrase activities in the duodenal and jejunal mucosa | Wan et al. 2017 |
| brown seaweeds (Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian, China) | alginate oligosaccharide, 100 mg/kg, weaned pigs | enhanced the intestinal integrity, increase in intestinal occludin protein abundance, increase in the jejunal and ileal catalase activity, decrease in the duodenal and jejunal tumor necrosis factor-α concentration, inhibition in the pro-inflammatory cytokines production | Wan et al. 2018 |
| Sargassum latifolium (B) | seaweed powder containing mainly carbohydrates (41.4%), ash (26.2%), amino acids, phenolic compounds (carotenes, flavonoids, kaempherol, alkaloids), 2 and 4%, bacterial endotoxin (bacterial lipopolysaccharides)-challenged sheep | increase in the thermo-respiratory response (skin and rectal temperatures, respiration rate), the obtained systemic inflammation (blood leukocytosis, the increase in the erythrocyte sedimentation rate, in serum concentrations of proinflammatory cytokines, heat shock protein-70), improvement of the total antioxidant capacity of the blood (increase in the CAT and SOD activity), decrease in the blood markers of tissue damage (MDA concentration and the activity of alanine aminotransferase and lactate dehydrogenase) | Ramadan et al. 2020 |
| Sargassum latifolium (B) | seaweed powder containing mainly carbohydrates (41.4%), ash (26.2%), amino acids, phenolic compounds (carotenes, flavonoids, kaempherol, alkaloids), 2 and 4%, environmental heat stress-induced toxicity, sheep | increase in the thermo-respiratory responses (skin and rectal temperatures, respiration rate) and the resulted dyslipidemia, anemia, systemic inflammation (blood leukocytosis, the increase in the erythrocyte sedimentation rate, in serum concentration of proinflammatory cytokines, heat shock protein-70), significant improvement of the body-weight gain, kidney functions (especially for 4%), blood antioxidant defense system (total antioxidant capacity, the activity of CAT and SOD), protection of animals from oxidative tissue damage and the risk of atherosclerosis | Ellamie et al. 2020 |
| OceanFeed Swine® (mixture of green, brown and red seaweeds)(Milltown, Ireland) | carbohydrates (43%), also proteins (8%), vitamins and minerals, 5 g/kg, nursery and fattening pigs | improvement of average daily weight gain and feed efficiency, increase in the slaughter weight, a reduction of E. coli CFU, increase in Lactobacillus sp. in faeces samples | Ruiz et al. 2018 |
| OceanFeed Swine® (mixture of green, brown and red seaweeds)(Milltown, Ireland) | carbohydrates (43%), proteins (8%), vitamins and minerals, 0.5% in gestation and 0.66% in lactation, 0.75% in nursery diet, sows and their offspring | no statistically significant impact on sow body weight during gestation and lactation, no differences in colostrum yield, composition of colostrum and milk, no effect on growth performance during nursery period, lower number of pathogenic bacteria (Fusobacteriaceae) for pigs from sows and higher of beneficial bacteria (Ruminoccocaceae and Lachnospiraceae) | Del Tuffo et al. 2019 |
| Phenolic compounds | |||
| Ascophyllum nodosum (B), Fucus serratus (B) | phlorotannin extract (0.1, 0.781, 1.56, 3.125, 20, 50 mg/mL) and whole seaweeds (1, 3, 5, 10, 20%), in vitro (pig digestibility model) | phlorotannin extract – significant decrease in the digestibility of feed for both seaweeds, smaller decrease for whole seaweeds (no significant difference was observed for inclusion rates till 5%), difference in digestibility for the same species and inclusion rate, but collected from different seasons (effect of seasonality on chemical composition) | Ford et al. 2020a |
| Vitamins | |||
|
Ascophyllum nodosum (B) (Kerry Enhancer, Kerry Algae, Curraheen, Tralee Co. Kerry, Ireland) |
extract, vitamins E, K, A, B1, B2, B12, niacin and elements: Cu, Fe, Mn, I, Zn, Se, Ca, Mg, Na, K (3, 6 and 9 g/kg), grower–finisher pigs | increase in the dose of extract resulted in reduced daily gain, carcass weight, and kill-out yield, increase in adherent Lactobacilli in the colon but decrease in caecal Bifidobacteria, decrease in ileal coliform counts, no effect of treatment on feed intake, feed conversion ratio or carcass characteristics, intestinal pH | Gardiner et al. 2008 |
| Microelements | |||
|
Ulva rigida (G), Sargasum muticum (B), Saccorhiza polyschides (B) |
I in the biomass, 100 g/animal per day, Holstein Friesian lactating cows | improvement of the mineral status of dairy cattle, especially I and Se, increase in the concentration of Co, Cr, Fe, I, Se, and Zn in milk | Rey-Crespo et al. 2014 |
| Chaetomorpha linum (G) | Zn, Mn, Fe, Ca and Na in the biomass, 20%, lambs fattening during drought periods | feed conversion ratio was higher than for the control group, no effect on organic matter digestibility, faecal N, urinary N, and N retention, lower final body weight | Rjiba et al. 2010 |
| Ulva spp. (G) | seaweed meal (ash 375 g/kg d.m., crude protein 176 g/kg d.m., crude fat 339 g/kg d.m., neutral detergent fibre 322 g/kg d.m., 171 g/kg d.m. acid detergent fibre), 20, 25, 30 and 35 g/kg, hens | increase in feed intake, overall body weight gain, no effect on feed conversion eff ciency, lack of improvement of apparent nutrient digestibility, no dietary effects on serum biochemical indices (glucose, urea, phosphorus, calcium, total protein, albumin, bilirubin, amylase, creatinine, globulin) |
Nhlane et al. 2020 |
| Ascophyllum nodosum (B) | iodine-rich intact seaweed, 10 and 20 g/kg, weaned piglets | beneficial effect on microbial population – reduction in E. coli load in the stomach and small intestine, enhancement of Lactobacilli/E. coli ratio in the small intestine, increase in iodine content in several tissues in piglets | Dierick et al. 2009 |
|
Ascophyllum nodosum (B) kelp meal (Thorvin Inc., New Castle, VA) |
seaweed meal iodine rich (820 mg/kg d.m.), 57, 113, 170 g/d, early lactation dairy cows | no effect of seaweed on milk yield, concentration of milk components (fat, protein, lactose, milk urea N), serum concentrations of thyroxine and triiodothyronine, linear increase in concentration of milk I with increased dose of seaweed, reduction in the plasma concentration of non-esterified fatty acids | Antaya et al. 2015 |
Where: B – brown, G – green seaweed
There are several methods used for the extraction of antioxidants such as Soxhlet, maceration, percolation, microwave-, ultrasounds-, enzyme-assisted extraction, subcritical, and supercritical fluid extraction, accelerated extraction, etc. (Cotas et al. 2020; Liu and Sun 2020; Monteiro et al. 2020; Matos et al. 2021). The simplest extraction methods can be applied for the isolation of antioxidants, such as maceration or shaking of seaweeds. Considering the potential use of algal extracts in animal nutrition, water as a solvent may be recommended. In the literature, for the extraction of phenolic compounds from seaweeds, usually, polar solvents are used, mainly aqueous mixtures of ethanol, methanol, acetone, or ethyl acetate (Monteiro et al. 2020). For example, Hwang and Thi (2014) compared the extraction method and antioxidant compound content in extracts obtained from edible red seaweed – Porphyra tenera. The extraction yield for water as a solvent at 37 °C was 25.5%, and 70% ethanol at 37 °C − 17.9%. The total phenolic content from water extraction (28.7 ± 0.5 mg GAE/g extract) was comparable to the ethanolic extract (30.2 ± 0.4 mg GAE/g extract). Phlorotannins from brown seaweeds can be extracted with a mixture of acetone and water (Ford et al. 2020a). As the main component in seaweeds, polysaccharides can be readily extracted with water or acetone (Liu and Sun 2020). Alginate oligosaccharides, which have great potential as a novel feed supplement, are produced via depolymerization of alginic acid polysaccharides from brown seaweeds using alginate lyases (Wan et al. 2016; Wan et al. 2017; Wan et al. 2018).
One seaweed preparation with antioxidant properties is Tasco®, produced by Acadian AgriTech™ (Dartmouth, Nova Scotia, Canada). This product is available in two forms: Tasco-Forage and Tasco-EX. The first one is used as an extract applied to foliage, which is grazed by livestock, and the second is used for direct feeding. Both forms are responsible for antioxidant responses measured in livestock (Allen et al. 2001; Saker et al. 2001; Saker et al. 2004).
The key issue in the use of seaweeds as animal feed/feed additive is their composition (especially the content of toxic elements that easily accumulate in the biomass, but also bacterial load, pathogens, mycotoxins, pesticides, dioxins, polychlorinated biphenyls) and palatability for the animals (seaweed supplement is usually given to animals in order to verify the feed acceptance) (Dierick et al. 2009; Michiels et al. 2012; Rey-Crespo et al. 2014). In addition, pelleting is recommended to avoid the rejection of seaweeds added to the feed by animals (Rjiba et al. 2010).
5. The effect of seaweed antioxidants on animal health and production
Table 1 presents examples of the effect of seaweed antioxidants on animal health and productivity. The antioxidant effect of seaweeds can be evaluated by measuring the total antioxidant capacity, the activity of primary antioxidant enzymes (superoxide dismutase, glutathione, and catalase), and the level of malondialdehyde (Wan et al. 2016; Corino et al. 2019; Liu and Sun 2020; Ramadan et al. 2020). As shown in Table 1, the inclusion of seaweeds in the animal diet can help maintain the appropriate redox status in animals, good health, and productivity. In addition, supplementation of seaweeds can boost antioxidant activity, immunity, and the gut environment. However, this effect has not been observed in all animal studies performed (it depends on the seaweed species, their doses in the diet, animal species, etc.).
Seaweeds and their active compounds can enhance growth performance (O’Doherty et al. 2010; Leonard et al. 2011a, 2011b) and increase the average daily body weight gain of animals (Wan et al. 2016; Wan et al. 2017; Ruiz et al. 2018; Ellamie et al. 2020; Nhlane et al. 2020). Brown seaweeds’ polysaccharides like alginate, laminarin, fucoidan, being soluble dietary fiber, increase feed intake (Allen et al. 2001). This effect was not confirmed in green seaweeds (Ulva lactuca) supplemented to the diet of lambs reared under heat stress conditions. This inclusion did not show any beneficial effect on body weight gain or feed conversion efficiency (Abdoun et al. 2014). The species of algae thus plays a key role in its impact on animal health and performance.
There are several seaweed-based preparations on the market like OceanFeed® (Milltown, Ireland) designed for nutrition of swine, equine, and bovine. For example, OceanFeed® Swine brings many benefits for gestating and lactating sows, post-wean pigs, and growing-finishing pigs – improved feed intake, feed conversion ratio, digestive function, nutrient use, enhanced performance, weight gain, gut health, and reduce diarrhea in pigs (Ruiz et al. 2018; Del Tuffo et al. 2019).
Finally, seaweed feed additives can biofortify animal products with algal active compounds (Dierick et al. 2009; Moroney et al. 2012; Rey-Crespo et al. 2014; Moroney et al. 2015), improve carcass quality, and prolong the retail shelf life (Braden et al. 2007; Moroney et al. 2015). Dietary supplementation of laminarin and fucoidan extracted from Laminaria digitata resulted in enhanced pork meat due to deposition of marine-derived bioactive antioxidant components in longissimus thoracis et lumborum muscle (Moroney et al. 2015). Moroney et al. (2012) found that the use of seaweed extracts containing fucoidan and laminarin reduced lipid oxidation to the minimum level in liver tissue homogenates. Thus, the use of natural antioxidants in the meat industry is a practical approach to minimize or prevent lipid oxidation (Moroney et al. 2015).
5.1. The main seaweed antioxidants examined in animal feeding
As shown in Table 1, mainly brown seaweeds were used in animal feeding as a source of antioxidants. Their main representatives were Ascophyllum nodosum, Sargassum sp. and Laminaria sp. The antioxidant function is played dominantly by polysaccharides – laminarin, fucoidan, and alginate. It is hypothesized that these polysaccharides positively influence the gut environment, immunoglobulins output, and the growth performance of animals (Del Tuffo et al. 2019).
The beneficial effect of Ascophyllum nodosum extract on animals is attributed to its chemical composition. Dierick et al. (2009) showed that the dried intact A. nodosum contains 503 g/kg of the total dietary fiber, whose main components are galactose 6.9 g/kg, xylose 16.8 g/kg, mannose 38.4 g/kg, glucose 44.8 g/kg, fucose 66.0 g/kg, uronic acids 144.4 g/kg and klason lignin 185.8 g/kg. These compounds have an additional prebiotic effect. Ascophyllum nodosum biomass also contains crude ash 211 g/kg, crude protein 49.0 g/kg, crude fat 50.0 g/kg, and iodine at the 400 mg/kg level. Ascophyllum nodosum extract is also a source of minerals and vitamins in animal diet – micro- and macroelements (Se 2–3 mg/kg, Mn 15–30 mg/kg, Cu 20–45 mg/kg, Zn 50–170 mg/kg, I 250–500 mg/kg, Fe 250–1000 mg/kg, Mg 2–5 g/kg, Ca 15–20 g/kg, Na 20–40 g/kg, K 110–130 g/kg), as well as vitamins (B12 < 1 mg/kg, B1 0.1–0.3 mg/kg, B2 4–8 mg/kg, K 4–9 mg/kg, niacin 8–25 mg/kg, A 20–40 mg/kg, E 100–250 mg/kg) (Gardiner et al. 2008). Many microelements derived from Ascophyllum nodosum biomass like Cu, Fe, and Zn are known to participate in animal antioxidant responses (Allen et al. 2001).
Another representative of brown seaweeds is Sargassum latifolium having a significant amount of carbohydrates (41.4%), ash (26.2%), and essential amino acids, which represent 42% of the total amino acids content (12 mg/g d.m.). Therefore, this alga is considered as a complementary source of protein in animal feed. Flavonoids, kaempherol, and carotenes are the main phenolic compounds in this biomass (Ramadan et al. 2020).
The popularity of Tasco® application (Acadian AgriTech™, Dartmouth, Nova Scotia Canada) in animal feeding results from its rich composition, which involves polysaccharide and oligosaccharide alginate 52% (alginic acid 25%, mannitol 5%, laminarin 3%, fucose containing sulfated polysaccharide 15%, other carbohydrates 4%), other proximate constituents like crude fat min 2%, crude protein 6%, crude fibre 6%, minerals – macro (P 0.1–0.2%, Mg 0.5–1%, Ca 1–3%, S 2–2.3%, K 2–3%, Na 2.4–4%) and microelements (Se < 1 mg/kg, Cu 4–15 mg/kg, Mn 10–50 mg/kg, Zn 35–100 mg/kg, I < 1000 mg/kg). It can contain toxic metals like As but below 3 mg/kg. Ash content in this preparation is about 22%. Tasco® also provides animals with amino acids (in g/100 g of protein): cystine trace amounts, methionine 0.7, tyrosine 0.9, histidine 1.3, phenylalanine 2.3, proline 2.6, isoleucine 2.8, threonine 2.8, serine 3, valine 3.7, leucine 4.6, lysine 4.9, glycine 5, alanine 5.3, aspartic acid 6.9, arginine 8, glutamic acid 10 (Allen et al. 2001; Fike et al. 2001). This product is known to enhance immune function and improve carcass characteristics (Allen et al. 2001).
Popular products based on seaweed biomass are OceanFeed® Swine, OceanFeed® Equine, OceanFeed® Bovine, produced by Ocean Harvest Technology Company Ltd. (Tuam, Co Galway, Milltown, Ireland). These products contain multiple green, brown and red seaweeds species and constitute a 100% natural macroalgal blend. The proximate composition of OceanFeedTM products (14.5% moisture) includes carbohydrates 43.2%, ash 30.4%, protein 8.0%, fibre 4.88% and fat 0.35%. The main vitamins are vit. C 80 mg/kg, niacin 6.3 mg/kg, vit. E 4 mg/kg, A 0.86 mg/kg, K3 0.3 mg/kg, thiamine 0.1 mg/kg, as well as folic acid 165 μg/kg, biotin 21.8 μg/kg and cyanocobalamin 1.4 μg/kg. This preparation contains also a wide range of minerals such as S 5.0%, Cl 4.0%, Ca 2.8%, K 2.7%, Na 2.3%, P 1.22 g/kg, Fe 1440 mg/kg, Mn 715 mg/kg, I 64 mg/kg, Zn 15.2 mg/kg, Cu 5.1 mg/kg, Mg 2.15 mg/kg and Se 0.13 mg/kg.
5.2. Effect of seaweed antioxidants on animal health and infectious diseases
Seaweeds and their active molecules with antioxidant and immunomodulatory activities can reduce oxidative damage, which might play a crucial role in the prognosis and amelioration of several infectious diseases, thereby safeguarding animal and human health (Shi et al. 2017; Juárez-Portilla et al. 2019). In addition, the biologically active compounds function as modulators of various cellular signaling mechanisms implicated in a plethora of infectious diseases (Juárez-Portilla et al. 2019). Furthermore, distinct extracts from different seaweeds can promote growth efficiency and gastrointestinal tract health by changing its anatomy and thus enhancing the intestinal absorption and utilization of nutrients and by modifying intestinal flora and attenuating the immune system and hence strengthening the gut structural integrity (Reilly et al. 2008; McDonnell et al. 2010; Sweeney et al. 2012).
Seaweeds have been found as a beneficial diet supplement as they enhanced pig growth and improved the digestibility of animal feed due to the content of bioactive phytochemicals (Corona et al. 2017). Further, the efficacy of the seaweeds diet was linked with its antioxidant compounds as seaweed can enhance the immune system functioning by reducing oxidative damage. The highest content of phenols and phlorotannins (up to 12–14%) is observed in brown seaweeds – Ascophyllum nodosum, Sargassum spp., and Fucus spp. In green and red seaweeds, this content is below 1% (Holdt and Kraan 2011).
Based on the data collected in Table 1, it can be concluded that seaweeds with antioxidant compounds used in feed improved mainly gut health and enhanced the function of the immune system. Seaweeds’ polysaccharides can improve the composition of animal’s gastro-intestinal microbiota without disrupting their performance (Reilly et al. 2008). Laminarin and fucoidan extracted from Laminaria sp. may provide a dietary means to improve gut health as these polysaccharides with antioxidant and antimicrobial properties can reduce the intestinal population of Enterobacterium spp. and increase the population of Lactobacilli spp. (Lynch et al. 2010; McDonnell et al. 2010; O’Doherty et al. 2010). Gahan et al. (2009) suggested that the extract obtained from Laminaria spp., rich in laminarin and fucoidan, can be used as a substitute for lactose in piglet diets, being the substrate for microbial fermentation in the large intestine. This approach could reduce the level of lactose in the diet and maintain post-weaned piglet performance fed with the diet free from growth promoters. In addition, extracts from Laminaria hyperborea and L. digitata, containing laminarin and fucoidan reduced Enterobacteria, Bifidobacteria, and Lactobacilli in the caecum and colon of the weaned pigs (Reilly et al. 2008).
Recently, brown seaweed-derived laminarin has been shown to possess a considerable level of antioxidant activity (Rajauria et al. 2021), which directly affected gut mucous membranes or gut-associated lymph nodes and lymphoid tissues, improving the integrity of the gut and stimulating the immune function of the digestive tract. Further, it has been reported that dietary laminarin enhanced the expression of several genes encoding mucin production while feeding, specifically MUC2, an important mucin-producing gene in the intestine, resulting in increased mucin production in the digestive tract (Ryan et al. 2010). Laminarin is processed by the GIT’s cells and then transferred to certain immunologically important cells such as dendritic cells. Thus, it works on cytokine output to influence the digestive system employing an immunomodulatory effect. In other studies, it has been reported that the diet augmentation with laminarin and other extracts resulted in the downregulation of developing a network of pro-inflammatory chemokines and cytokines in intestinal and hepatic cells (Sweeney et al. 2012; Walsh et al. 2013). The ability to lower the levels of pro-inflammatory cytokines can enable the animal to receive more nutrients and thus develop at a greater rate by distributed nutrients apart from strengthening the immune system (Walsh et al. 2013). Because of these immunomodulatory impacts, one might predict that they may regulate hyperinflammatory responses in various autoimmune disorders and viral infections.
The second crucial polysaccharide of brown seaweeds – fucoidan, is a critical immunomodulatory compound with many biological functions that have not yet been completely understood. However, it was discovered that the diet supplemented with a consortium of two different macroalgae-derived compounds, including fucoidan, laminarin, and their mixture to pregnant sows, enhanced the antibodies production rate in the breast milk, which, in turn, increased the IgG concentrations in the new-born piglets' serum. The fecal enterobacterial levels in sows shortly before parturition were significantly lower, and an additional observation confirmed the reduced number of coliform bacteria in the suckling pigs, which was correlated with piglets dramatically increasing the length of villi compared to the number of crypts in the upper small intestine, as well as increased growth rate. It is possible that reduced numbers of Escherichia coli and greater pathogen effects in the intestinal tracts of weaned piglets can be driven by the accumulation of mammary laminarin of low-molecular-weight in piglets' gastrointestinal tract. Additionally, algal extract intake induces enhanced TNF and TFF (trefoil factor) production in the gastrointestinal tract, which indicates their beneficial effects in newborn pigs (Leonard et al. 2011b). Moreover, laminarin supplementation to the diet of pregnant sows following by infection of Salmonella Typhimurium resulted in an increase in size, significant increase in the availability of nutrients, and reduced expression of certain interleukins such as IL-22, which is a crucial interleukin or mediator to maintain the gastrointestinal tract integrity and tissue generation (Bouwhuis et al. 2017). Seaweed extract, obtained from Laminaria sp., containing laminarin and fucoidan increased immunoglobulin G (IgG), immunoglobulin A (IgA) in sow’s colostrum and serum IgG in piglets (Leonard et al. 2012). Also, extracts from green seaweeds – Ulva armoricana containing sulfated polysaccharide increased IgG in sow’s blood and colostrum and the level of total IgA in milk (Bussy et al. 2019). Antioxidant enzymes (CAT and SOD) and immune-related activities (lysozyme, anti-protease, and myeloperoxidase) were higher in Sargassum sp. (2.5% and 5%) groups compared with control in intestinal mucus, several tissues, and serum of goat (Angulo et al. 2020). The use of seaweeds in goat diets is a good strategy to strengthen the antioxidant system and stimulate immunity, which helps to control pathogens (Angulo et al. 2020). Overall, dietary supplementation of seaweeds (Sargassum spp.) and their derivatives enhanced the immune responses and antioxidant capabilities (Telles et al. 2018).
Interesting research was conducted by Vizzari et al. (2021), who evaluated the effect of dietary seaweed extract (containing polysaccharides from Laminaria digitata and Laminaria hyperborea) on male-rabbit semen. It was found that after 90 days of the experiment, a decrease in aspartate aminotransferase (AST) and increase in alanine aminotransferase (ALT) and glutathione peroxidase (GPX) activities, ferric reducing ability of seminal plasma were observed with a dietary mixture of plant polyphenols and seaweed extract. Based on these results, it can be reported that natural polysaccharides extracted from brown seaweeds plus hydroxycinnamic acids, phenolic acid, flavonoids, and tannins from plant extracts mix (0.3 and 0.6%) didn’t have any adverse effect on the male rabbit reproductive parameters, but improved the animal antioxidant status, enhanced the antioxidant capability of the seminal plasma and can have a positive impact on growth, health, and development of animals.
The second most studied macroalga in animal nutrition is Ascophyllum nodosum. This seaweed was used as a feed additive for pigs and participated in pathogen reduction (Allen et al. 2001; Gardiner et al. 2008). Ford et al. (2020b) tested in vitro the antimicrobial potential of extracts obtained from Ascophyllum nodosum and Fucus serratus containing phlorotannins against foodborne pathogens such as Escherichia coli O157, Salmonella agona, and Streptococcus suis, which often colonize weaning piglets. These extracts were very effective, and for A. nodosum polyphenol extract, the minimum inhibitory concentration was between 1.56 and 0.78 mg/mL for all tested pathogens, whereas for F. serratus was 3.13 mg/mL. In the work of Bach et al. (2008), sun-dried Ascophyllum nodosum (Tasco-14TM) was administered at a dose of 20 g/kg diet of feedlot cattle and was efficient in the reduction in fecal shedding of the pathogen – E. coli O157:H7. In the in vivo conditions, Turner et al. (2002) examined the effect of Ascophyllum nodosum extract on the immune function of young pigs challenged with enteric disease caused by Salmonella Typhimurium. This extract had very little effect on growth performance and a positive effect on the immune function in pigs. Dietary supplementation with seaweeds (Ascophyllum nodosum) at 1.5% or 0.75% for 451 days improved the kidney function of minks infected with Aleutian mink disease virus (AMDV) with no impact on liver function and immunity (Farid and Smith 2020). No differences among groups were observed for antibody titer determined by the counter-immunoelectrophoresis, total serum protein, globulins, albumin, gamma-glutamyl transferase and alkaline phosphatase activities, but blood levels of creatine and urea were lower in the 1.5% Ascophyllum nodosum supplemented group than in the unsupplemented group (Farid and Smith 2020).
The high content of antioxidants in A. nodosum is responsible for the improved antioxidant status and immune functions in animals (Allen et al. 2001; Turner et al. 2002; Saker et al. 2004). In addition, Ascophyllum nodosum has a positive effect on the gut microbiota, translating into increased immunity (Gardiner et al. 2008; Dierick et al. 2009). In animals, modulation of gut microbiota can stimulate immunity and decrease the risk of diarrhea (Gardiner et al. 2008). According to this research, the supplement of A. nodosum can increase nutrient digestibility, improve nutrient availability from the gastrointestinal tract, stimulate the immune system and promote a healthy intestinal flora. When fed with a mixture of laminarin and fucoidan reduce in the growth rate appeared, but feeding purified laminarin emerged to improve health and productivity. Moreover, pigs fed with a laminarin and fucoidan enriched diet also reported increased growth productivity and good health (Gardiner et al. 2008).
When considering poultry research, the addition of A. nodosum extract to the broilers diet had beneficial effects on the gastrointestinal tract's integrity. It reduced the infection rates of newly born chickens colonized with Campylobacter jejuni in the cecum (Sweeney et al. 2016). Dietary addition of marine brown seaweeds (sodium alginate oligosaccharides) significantly reduced the mortality rate and increased body weight gain of chickens diseased with Salmonella Enteritidis (Yan et al. 2011). In layer diets, the use of red seaweeds (Chondrus crispus and Sarcodiotheca gaudichaudii) as prebiotics could enhance the productivity, criteria of eggs, and gut status as well as short-chain fatty acids (acetic acid, propionic acid, n-butyric acid, and butyric acid) in eggs (Kulshreshtha et al. 2014). In broiler chicken rations, Choi et al. (2014) found positive impacts on growth rate and immune response when used by-products of seaweed fusiforme and brown seaweed.
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is mainly a disease affecting humans, and its pandemic is ongoing. However, few reports of SARS-CoV-2 infection have been reported from animals such as dogs, cats, lions, tigers, mink (Tiwari et al. 2020; Sharun et al. 2021). Dietary seaweeds contain several nutrients and components such as angiotensin-converting enzyme 2 (ACE2) inhibitory peptides, n-3 fatty acids, soluble dietary fibers (fucoidan and porphyran), fucosterol, fucoxanthin, phlorotannins, and some vitamins (D3 and cobalamin). In addition, inhibitory action on ACE2 receptor binding of SARS-CoV-2 in the host cells and anti-inflammatory activities of seaweed extracts may also be explored in the treatment of COVID-19, which is marked by a severe hyperinflammatory response that plays a key role in the severity of infection and prognosis of the disease. Both of red algae and brown algae could be potential antiviral therapeutic agents against SARS-CoV-2 and can be used to reduce the deleterious consequences of SARS-CoV-2 infection (Pereira and Critchley 2020; Rauf et al. 2021). Dietary seaweeds and their derivatives can effectively inhibit SARS-CoV-2 entry and may provide protection against COVID-19 through different mechanisms in humans and hypothetically could render protection in animals too, for which purpose further investigation and clinical trials are required to conclude their efficacy (Li et al. 2020; Tamama 2021; Kuznetsova et al. 2021; Yim et al. 2021).
Further, several extracts of well-known invasive macroalgae, Codium fragile, have been reported to inhibit the inflammatory cascade in human cell lines and mice models. These studies suggest that C. fragile extracts interrupt the release of several proinflammatory cytokines such as TNF-α, IL-1β, and IL-6 through impairing the mechanisms of a plethora of crucial inflammatory enzymes, including COX-2, iNOS, NF-κB, and MAPK (Lee et al. 2013; Lee et al. 2017). The release of inflammatory mediators, including PGE2, NO, TNF-α, and other inflammatory mediators, such as several interleukins like 1β, and IL-6, is suppressed by seaweed-derived compounds (Hwang et al. 2015, Hwang et al. 2017; Lee et al. 2016; Sadeeshkumar et al. 2017). Additionally, fucoidan, a well-known biologically active compound from L. japonica, has been reported to attenuate the inflammatory response through decreased pro-inflammatory cytokines such as TNF-α, IL-6, and IL-1β. The decrease in pro-inflammatory cytokines in rat hepatic ischemia-reperfusion damage was reported to attenuate via the downregulation of several important pathways, including ERK1/2, p38, and JNK MAPK (Li and Ye 2015).
Some emerging or re-emerging viral illnesses have caused significant harm to human health in recent years, owing to the continual occurrence of these diseases. During the previous two decades, the number of antiviral agents that have been approved for clinical use has expanded from five to more than thirty medications. The polysaccharides extracted from the red alga Gelidium robustum (formerly Gelidium cartilagineum) (Rhodophyta) were found to have antiviral activity in embryonic eggs when tested against influenza B or mumps viruses, as reported by Gerber et al. (1958). In large amounts, many species of sea algae contain complex structural sulphated polysaccharides, which have been demonstrated to hinder the replication of enveloped viruses, particularly those belonging to the Nidovirales family. Other compounds, including those derived from red algae (e.g., the lectin griffithsin) and other sulphated polysaccharides derived from green algae (e.g., ulvans) and brown algae (e.g., fucoidans), have been proposed as potential antiviral therapeutic agents against SARS-CoV-2 (Pereira 2018; Barre et al. 2019; Lee 2019; Rosa et al. 2019).
5.3. Effect of seaweed antioxidants on animals exposed to stressful conditions
Seaweeds and their compounds are also beneficial to animals exposed to stress. Saker et al. (2004) stated that natural extracts from brown seaweeds might be used to optimize the immunocompetence of stressed animals (e.g., heat-stressed lambs) kept under suboptimal production conditions. Increased antioxidant activity in animals diminishes oxidative stress and enhances tolerance to stress (Fike et al. 2001). Antioxidants contained in the biomass of A. nodosum (phlorotannins, vitamins – ascorbic acid, tocopherol, and pigments – carotenoids) can scavenge peroxyl radicals and may prevent or diminish oxidative stress in weaned piglets (Michiels et al. 2012). Seaweeds’ minerals with antioxidant properties – for example, zinc and selenium also protect animals against oxidant stress (Rey-Crespo et al. 2014). Wan et al. (2017) showed that alginate oligosaccharide extracted from brown seaweeds has the potential to reverse weaning-induced oxidative stress and intestinal digestive disorders in pigs. But in case of dietary inclusion of green seaweeds (Ulva lactuca) to the feed of lambs reared under heat stress conditions, blood constituents and antioxidant capacity were not affected by this supplementation (Abdoun et al. 2014). Seaweed powder obtained from Sargassum latifolium may protect sheep from heat stress (Ellamie et al. 2020), as well as stress caused by bacterial endotoxins (Ramadan et al. 2020) and alleviate their harmful effects. Seaweed’s supplementation may improve the antioxidant defense system of animals and regulate their inflammatory and thermo-respiratory responses (Ellamie et al. 2020; Ramadan et al. 2020).
5.4. Effect of seaweed antioxidants on the quality of animal-derived products
Seaweeds are a rich source of important elements and compounds such as iodine, protein, and sulfated polysaccharides, which are responsible for their bioactivity in the body (Tanna and Mishra 2019; Cermeño et al. 2020; Darias-Rosales et al. 2020). Polysaccharides like laminarin and fucoidan can be used as alternatives to commercial antioxidants incorporated into animal feed (Moroney et al. 2012). An additional advantage of seaweeds, used as a source of antioxidants in animal nutrition, is the production of food enriched with these ingredients. Moroney et al. (2012) showed that there is a possibility to incorporate seaweed antioxidants and polysaccharides into animal muscle through the animal's diet. Meat and meat products enriched with antioxidants can be used to produce functional food with health-promoting properties (Holdt and Kraan 2011; Michalak et al. 2011).
Marine macroalgae can improve the lipid profile – the content of polyunsaturated fatty acids (PUFA) (e.g., González-Esquerra and Leeson 2001; Carrillo et al. 2008), the color of animal-derived products (Bonos et al. 2017), and also their multielemental composition (Michalak et al. 2011; Al-Harthi and El-Deek 2012). Rey-Crespo at al. (2014) showed that green – Ulva rigida and brown seaweeds – Sargasum muticum and Saccorhiza polyschides could significantly improve the mineral status of animals and the content of microelements in animal products (e.g., milk, meat). Michalak et al. (2011) found that seaweeds enriched with microelements such as Zn(II), Cu(II), Co(II), Cr(III), and Mn(II) via biosorption – increased the elements content in eggs when compared to the control group. Dierick et al. (2009) demonstrated that dried and intact Ascophyllum nodosum rich in iodine improved not only pig health and performance but also enriched with iodine porcine tissues (M. psoas, M. longissimus dorsi, back fat, liver, kidney, heart) and blood serum.
The supplementation of Ascophyllum nodosum to the diet of early lactation dairy cows significantly increased the concentration of iodine in milk (without effect on animal performance), which reached the level that may be toxic to humans, particularly children (Antaya et al. 2015). High consumption of iodine can cause damages to animal and human health. The use of analyzed seaweeds at 4 g per day did not pose a health risk (Darias-Rosales et al. 2020).
The use of seaweed feed additives can also improve the quality of animal-derived products. Braden et al. (2007) showed that supplementation of Ascophyllum nodosum biomass (2% Tasco) to the diet of crossbred cattle increased the amount of intramuscular fat, the marbling score, and additionally may increase meat shelf life. No effect on sensory attributes and visual color of strip-loin steaks was observed. Moroney et al. (2015) examined the effect of extract from Laminaria digitata containing polysaccharides (laminarin and fucoidan) on quality indices of fresh pork. This extract did not affect the plasma total antioxidant status, pH of muscle Longissimus thoracis et lumborum, color, microbiology, and sensory parameters, enhanced the visual sensory descriptors (pinkness, whiteness, drip, overall acceptability), and reduced saturated fatty acids and decreased lipid oxidation in fresh muscle. The content of cholesterol tended to be lower in rabbit muscle fed with the diet containing Laminaria spp. (0.3 and 0.6%) when compared to the control group. Also, the content of retinol and α-tocopherol was improved in both muscles (Longissimus lumborum and Semimembranosus) of rabbits receiving the algal feed additives. The sensory attributes of texture were improved in both muscles with brown seaweeds compared to control (Rossi et al. 2020). The use of 0.5, 1, and 2% of Ascophyllum nodosum in broiler diets did not affect the total saturated, polyunsaturated, and monounsaturated (MFA) fatty acids in the breast or the thigh muscle (Bonos et al. 2017). While the birds fed with 2% A. nodosum had a higher content of n-6 (gamma-linolenic fatty acid) in the breast muscle and a lower content of n-9 (eicosenoic fatty acid) when compared to the unsupplemented group (Bonos et al. 2017). The positive effect of seaweed polysaccharides on enhancing meat quality can result from the health-promoting effects of gut-associated immunity (Moroney et al. 2015).
Carrillo et al. (2012) stated that seaweeds could protect polyunsaturated fatty acids accumulated in egg yolks and can increase the storage time of these eggs. Green alga – Enteromorpha spp. had a protective impact on the level of docosahexaenoic acid (DHA) in eggs. In addition, the brown algae had a similar impact on the level of eicosapentaenoic acid (EPA). Furthermore, the antioxidant compounds of seaweeds like phenolic compounds, carotenoids, and vitamins (A, C, and E) play an antioxidant role in eggs enriched with n-3 PUFAs (Carrillo et al. 2012).
The use of seaweeds in poultry diet has a positive effect on PUFA and can improve the color of meat and yolk due to the deposition of carotenoids (Herber-McNeill and Van Elswyk 1998; Saeed et al. 2021). These pigments are important and beneficial for animals and humans due to their antioxidant activities (Al-Harthi and El-Deek 2012). Zeaxanthin, fucoxanthin, and lutein are the main carotenoids of algae that improve egg quality by increasing the yolk color (Carrillo et al. 2012). In the same context, Al-Harthi and El-Deek (2012) found that fucoxanthin improved the coloring of yolks (Al-Harthi and El-Deek 2012). On the contrary, in white leghorn laying hens, Strand et al. (1998) stated that fucoxanthin as the major carotenoid in seaweed meal was not transferred to egg yolks but gave rise to fucoxanthinol sulfate, fucoxanthinol, and paracentrone. In general, the carotenoid content in yolks increased about 12–15 times compared to the control (Strand et al. 1998). The use of Sargassum dentifebium (brown algae) in different sources (sun-dried, autoclaved, or boiled) at levels of 3 and 6% increased the content of lutein and zeaxanthin in egg yolks as compared to control (Al-Harthi and El-Deek 2012).
Conclusions and future prospects
The use of natural antioxidants as feed supplements can enhance not only animal’s productivity and health but also increase their ability and resistance to many stresses like diseases, stocking density, high ambient temperatures, inadequate housing systems, and others. Seaweeds as a feed/feed additive can be used in the form of intact natural biomass or in the form of extract. The different types of seaweeds play a vital role in improving the antioxidant system in the body due to their contents of biologically active molecules like phenolic compounds, flavonoids, polysaccharides, pigments, vitamins, minerals, micro-, and macroelements. Dietary bioactive compounds in seaweeds demonstrate potential to improve animal health, productivity, and welfare. Seaweeds’ polysaccharides can improve the composition of animal’s gastro-intestinal microbiota without disrupting their performance. Also, the use of seaweeds in animal diets can boost antioxidant activity, immunity, and the gut environment. Furthermore, seaweeds have antimicrobial properties; they played an essential role in reducing the prevalence of enterohemorrhagic E. coli on hide swabs and in fecal samples, as well as the growth of Salmonella spp. It can be concluded that seaweeds with antioxidant compounds used in feed improve mainly gut health and enhance the function of the immune system. Hence, due to the available evidence of health benefits related to macroalgae and their extracts, researchers are exploring their relevant biological activities in several pathological conditions. Supplementing specific extracts of seaweeds such as laminarin in the animal feed could act as immunomodulators in many clinical conditions, including viral and bacterial infections.
Moreover, in the future, there is an urgent need to explore the biological activities of specific compounds, specifically exhibiting the antioxidant properties present in seaweeds for the enhanced production of livestock via shielding farmed animals from infections and diseases. Additionally, to acquire the efficient biological active molecules from seaweeds, there is a need to develop techniques to extract and purify biomolecules from seaweeds so that the extraction methods can be incorporated to yield macroalgae extracts with higher proportions to be utilized in several in vitro experiments to prevent and treat several infections in animals. However, in vitro screening studies and in vivo animal studies are needed to identify potential prebiotics from seaweeds, alongside untargeted metabolomics, to decipher microbial-derived metabolites from seaweeds. Furthermore, controlled animal intervention studies with health-related endpoints to elucidate prebiotic efficacy are required.
Acknowledgments
All the authors acknowledge and thank their respective Institutes and Universities. This review was prepared as a part of the project entitled: “The effect of bioactive algae enriched by biosorption in the certain minerals such as Cr(III), Mg(II) and Mn(II) on the status of glucose in the course of metabolic syndrome in horses. Evaluation “in vitro and in vivo” (No 2015/18/E/NZ9/00607) from the National Science Center in Poland.
Funding Statement
This compilation is a review article written by its authors and required no substantial funding to be stated.
Disclosure statement
All authors declare that there exist no commercial or financial relationships that could, in any way, lead to a potential conflict of interest.
References
- Abbott DW, Aasen IM, Beauchemin KA, Grondahl F, Gruninger R, Hayes M, Huws S, Kenny DA, Krizsan SJ, Kirwan SE, et al. 2020. Seaweed and seaweed bioactives for mitigation of enteric methane: challenges and opportunities. Animals. 10(12):2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdoun KA, Okab AB, El-Waziry AM, Samara EM, Aa A-H.. 2014. Dietary supplementation of seaweed (Ulva lactuca) to alleviate the impact of heat stress in growing lambs. Pak Vet J. 34(1):108–111. [Google Scholar]
- Alboofetileh M, Hamzeh A, Abdollahi M.. 2021. Seaweed proteins as a source of bioactive peptides. Curr Pharm Des. 27(11):1342–1352. [DOI] [PubMed] [Google Scholar]
- Al-Harthi MA, El-Deek AA.. 2012. Effect of different dietary concentrations of brown marine algae (Sargassum dentifebium) prepared by different methods on plasma and yolk lipid profiles, yolk total carotene and lutein plus zeaxanthin of laying hens. Ital J Anim Sci. 11:347–353. [Google Scholar]
- Allen VG, Pond KR, Saker KE, Fontenot JP, Bagley CP, Ivy RL, Evans RR, Schmidt RE, Fike JH, Zhang X, et al. 2001. Tasco: influence of a brown seaweed on antioxidants in forages and livestock–a review. J Anim Sci. 79 (E-Suppl):E21–E31. [Google Scholar]
- Angulo C, Chavez-Infante L, Reyes-Becerril M, Angulo M, Romero-Geraldo R, Llinas-Cervantes X, Cepeda-Palacios R.. 2020. Immunostimulatory and antioxidant effects of supplemental feeding with macroalga Sargassum spp. on goat kids. Trop Anim Health Prod. 52(4):2023–2033. [DOI] [PubMed] [Google Scholar]
- Antaya NT, Soder KJ, Kraft J, Whitehouse NL, Guindon NE, Erickson PS, Conroy AB, Brito AF.. 2015. Incremental amounts of Ascophyllum nodosum meal do not improve animal performance but do increase milk iodine output in early lactation dairy cows fed high-forage diets. J Dairy Sci. 98(3):1991–2004. [DOI] [PubMed] [Google Scholar]
- Arain MA, Mei Z, Hassan FU, Saeed M, Alagawany M, Shar AH, Rajput IR.. 2018a. Lycopene: a natural antioxidant for prevention of heat-induced oxidative stress in poultry. World Poult Sci J. 74(1):89–100. [Google Scholar]
- Bach SJ, Wang Y, McAllister TA.. 2008. Effect of feeding sun-dried seaweed (Ascophyllum nodosum) on fecal shedding of Escherichia coli O157:H7 by feedlot cattle and on growth performance of lambs. Anim Feed Sci Technol. 142(1-2):17–32. [Google Scholar]
- Barbalace MC, Malaguti M, Giusti L, Lucacchini A, Hrelia S, Angeloni C.. 2019. Anti-inflammatory activities of marine algae in neurodegenerative diseases. IJMS. 20(12):3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barre A, Simplicien M, Benoist H, Van Damme EJM, Rouge P.. 2019. Mannose-specific lectins from marine algae: diverse structural scaffolds associated to common virucidal and anti-cancer properties. Mar Drugs. 17(8):440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonos E, Kargopoulos A, Nikolakakis I, Florou-Paneri P, Christaki E.. 2017. The seaweed Ascophyllum nodosum as a potential functional ingredient in chicken nutrition. J Oceanogr Mar Res. 04(01):140. [Google Scholar]
- Bouwhuis MA, Sweeney T, Mukhopadhya A, McDonnell MJ, O’Doherty JV.. 2017. Maternal laminarin supplementation decreases Salmonella Typhimurium shedding and improves intestinal health in piglets following an experimental challenge with S. Typhimurium post-weaning. Anim Feed Sci Technol. 223:156–168. [Google Scholar]
- Braden KW, Blanton JR, Allen VG, Pond KR, Miller MF.. 2004. Ascophyllum nodosum supplementation: a preharvest intervention for reducing Escherichia coli O157:H7 and Salmonella spp. in feedlot steers. J Food Prot. 67(9):1824–1828. [DOI] [PubMed] [Google Scholar]
- Braden KW, Blanton JR, Montgomery JL, van Santen E, Allen VG, Miller MF.. 2007. Tasco supplementation: effects on carcass characteristics, sensory attributes, and retail display shelf-life. J Anim Sci. 85(3):754–768. [DOI] [PubMed] [Google Scholar]
- Bussy F, Le Goff M, Salmon H, Delaval J, Berri M, Pi NC.. 2019. Immunomodulating effect of a seaweed extract from Ulva armoricana in pig: Specific IgG and total IgA in colostrum, milk, and blood. Vet Anim Sci. 7:100051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell M, Ortuño J, Ford L, Davies DR, Koidis A, Walsh PJ, Theodoridou K.. 2020. The effect of ensiling on the nutritional composition and fermentation characteristics of brown seaweeds as a ruminant feed ingredient. Animals. 10(6):1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrillo S, Bahena A, Casas M, Carranco ME, Calvo CC, Ávila E, Pérez–Gi F.. 2012. The alga Sargassum spp. as alternative to reduce egg cholesterol content. Cuban J Agricult Sci. 46:181–186. [Google Scholar]
- Carrillo S, López E, Casas MM, Avila E, Castillo RM, Carranco ME, Calvo C, Pérez-Gil F.. 2008. Potential use of seaweeds in the laying hen ration to improve the quality of n-3 fatty acid enriched eggs. J Appl Phycol. 20(5):721–728. [Google Scholar]
- Cermeño M, Kleekayai T, Amigo-Benavent M, Harnedy-Rothwell P, FitzGerald RJ.. 2020. Current knowledge on the extraction, purification, identification, and validation of bioactive peptides from seaweed. Electrophoresis. 41(20):1694–1717. [DOI] [PubMed] [Google Scholar]
- Changxing L, Saeed M, Kamboh AA, Alagawany MA, El-Hack ME, Arain MA, Babazadeh D, Dhama K, Mo C.. 2018a. Reconsidering a citrus flavonoid naringin as a promising nutritional supplement and its beneficial health applications in humans, animals and poultry. Int J Pharm. 12(8): 874–883. [Google Scholar]
- Changxing L, Chenling M, Alagawany M, Jianhua L, Dongfang D, Gaichao W, Wenyin Z, Syed SF, Arain MA, Saeed M, et al. 2018b. Health benefits and potential applications of anthocyanins in poultry feed industry. World Poult Sci J. 74(2):251–264. [Google Scholar]
- Choi Y, Lee S, Oh J.. 2014. Effects of dietary fermented seaweed and seaweed fusiforme on growth performance, carcass parameters and immunoglobulin concentration in broiler chicks. Asian Australas J Anim Sci. 27(6):862–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corino C, Modina SC, Di Giancamillo A, Chiapparini S, Rossi R.. 2019. Seaweeds in pig nutrition. Animals. 9(12):1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corona G, Coman MM, Guo Y, Hotchkiss S, Gill C, Yaqoob P, Spencer JPE, Rowland I.. 2017. Effect of simulated gastrointestinal digestion and fermentation on polyphenolic content and bioactivity of brown seaweed phlorotannin-rich extracts. Mol Nutr Food Res. 61(11):1700223. [DOI] [PubMed] [Google Scholar]
- Cotas J, Leandro A, Monteiro P, Pacheco D, Figueirinha A, Gonçalves AMM, da Silva GJ, Pereira L.. 2020. Seaweed phenolics: from extraction to applications. Mar Drugs. 18(8):384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussens LM, Werb Z.. 2002. Inflammation and cancer. Nature. 420(6917):860–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darias-Rosales J, Rubio C, Gutiérrez ÁJ, Paz S, Hardisson A.. 2020. Risk assessment of iodine intake from the consumption of red seaweeds (Palmaria palmata and Chondrus crispus). Environ Sci Pollut Res Int. 27(36):45737–45741. [DOI] [PubMed] [Google Scholar]
- de Quiros ARB, Frecha-Ferreiro S, Vidal-Perez AM, Lopez-Hernandez J.. 2010. Antioxidant compounds in edible brown seaweeds. Eur Food Res Technol. 231(3):495–498. [Google Scholar]
- Del Tuffo L, Laskoski F, Vier CM, Tokach MD, Dritz SS, Woodworth JC, DeRouchey JM, Goodband RD, Constance LA, Niederwerder M, et al. 2019. Effects of Oceanfeed Swine feed additive on performance of sows and their offspring. Kansas Agricult Exp Stat Res Rep. 5(8): 1–22. [Google Scholar]
- Dierick N, Ovyn A, De Smet S.. 2009. Effect of feeding intact brown seaweed Ascophyllum nodosum on some digestive parameters and on iodine content in edible tissues in pigs. J Sci Food Agric. 89(4):584–594. [Google Scholar]
- Ellamie AM, Fouda WA, Ibrahim WM, Ramadan G.. 2020. Dietary supplementation of brown seaweed (Sargassum latifolium) alleviates the environmental heat stress-induced toxicity in male Barki sheep (Ovis aries). J Thermal Biol. 89:102561. [DOI] [PubMed] [Google Scholar]
- Eryilmaz F. 2017. Vitamin C: an antioxidant agent. Hamza Amal H., Rijeka, Croatia: IntechOpen, Available from: https://www.intechopen.com/chapters/56013 [accessed on 21 July 2021]. [Google Scholar]
- FAO (Food and Agriculture Organization). 2018. The global status of seaweed production, trade and utilization. Globefish Research Programme, 124, Rome, Italy. [Google Scholar]
- Farid AH, Smith NJ.. 2020. Dietary supplementation of Ascophylum nodosum improved kidney function of mink challenged with Aleutian mink disease virus. BMC Vet Res. 16(1):465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farvin KHS, Jacobsen C.. 2013. Phenolic compounds and antioxidant activities of selected species of seaweeds from Danish coast. Food Chem. 138(2–3):1670–1681. [DOI] [PubMed] [Google Scholar]
- Fike JH, Allen VG, Schmidt RE, Zhang X, Fontenot JP, Bagley CP, Ivy RL, Evans RR, Coelho RW, Wester DB.. 2001. Tasco-forage: I. Influence of a seaweed extract on antioxidant activity in tall fescue and in ruminants. J Anim Sci. 79(4):1011–1021. [DOI] [PubMed] [Google Scholar]
- Ford L, Curry C, Campbell M, Theodoridou K, Sheldrake G, Dick J, Stella L, Walsh PJ.. 2020a. Effect of phlorotannins from brown seaweeds on the in vitro digestibility of pig feed. Animals. 10(11):2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford L, Stratakos ACh, Theodoridou K, Dick JTA, Sheldrake GN, Linton M, Corcionivoschi N, Walsh PJ.. 2020b. Polyphenols from brown seaweeds as a potential antimicrobial agent in animal feeds. ACS Omega. 5(16):9093–9103. − [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahan DA, Lynch MB, Callan JJ, O’Sullivan TO, O’Doherty JV.. 2009. Performance of weanling piglets offered low-, medium- or high-lactose diets supplemented with a seaweed extract from Laminaria spp. Animal. 3(1):24–31. [DOI] [PubMed] [Google Scholar]
- Gardiner GE, Campbell AJ, O’Doherty JV, Pierce E, Lynch PB, Leonard FC, Stanton C, Ross RP, Lawlor PG.. 2008. Effects of Ascophyllum nodosum extract on growth performance, digestibility, carcass characteristics and selected intestinal microflora populations of grower-finisher pigs. Anim Feed Sci Technol. 141(3–4):259–273. [Google Scholar]
- Gerber P, Dutcher JD, Adams EV, Sherman JH.. 1958. Protective effect of seaweed extracts for chicken embryos infected with influenza B or mumps virus. Proc Soc Exp Biol Med. 99(3):590–593. [DOI] [PubMed] [Google Scholar]
- González-Esquerra R, Leeson S.. 2001. Alternatives for enrichment of eggs and chicken meat with omega-3 fatty acids. Can J Anim Sci. 81(3):295–305. [Google Scholar]
- Hayat MA, Ding J, Li Y, Zhang X, Zhang J, Li S, Wang HB.. 2020. Determination of the activity of selected antioxidant enzymes during bovine laminitis, induced by oligofructose overload. Med Weter. 76(5):289–295. [Google Scholar]
- Herber-McNeill SM, Van Elswyk ME.. 1998. Dietary marine algae maintains egg consumer acceptability while enhancing yolk color. Poult Sci. 77(3):493–496. [DOI] [PubMed] [Google Scholar]
- Holdt SL, Kraan S.. 2011. Bioactive compounds in seaweed: Functional food applications and legislation. J Appl Phycol. 23(3):543–597. [Google Scholar]
- Hussain SP, Hofseth LJ, Harris CC.. 2003. Radical causes of cancer. Nat Rev Cancer. 3(4):276–285. [DOI] [PubMed] [Google Scholar]
- Hwang PA, Hung YL, Chien SY.. 2015. Inhibitory activity of Sargassum hemiphyllum sulfated polysaccharide in arachidonic acid-induced animal models of inflammation. J Food Drug Anal. 23(1):49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang JH, Kim KJ, Lee BY.. 2017. Crude Ecklonia cava flake extracts attenuate inflammation through the regulation of TLR4 signaling pathway in LPS-Induced RAW264.7 cells. Molecules. 22(5):777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang ES, Thi ND.. 2014. Effects of extraction and processing methods on antioxidant compound contents and radical scavenging activities of laver (Porphyra tenera). Prev Nutr Food Sci. 19(1):40–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juárez-Portilla C, Olivares-Bañuelos T, Molina-Jiménez T, Sánchez-Salcedo JA, Moral DID, Meza-Menchaca T, Flores-Muñoz M, López-Franco Ó, Roldán-Roldán G, Ortega A, et al. 2019. Seaweeds-derived compounds modulating effects on signal transduction pathways: a systematic review. Phytomedicine. 63:153016. [DOI] [PubMed] [Google Scholar]
- Kaur N, Dhawan M, Sharma I, Pati PK.. 2016. Interdependency of reactive oxygen species generating and scavenging system in salt sensitive and salt tolerant cultivars of rice. BMC Plant Biol. 16(1):131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur G, Sharma A, Guruprasad K, Pati PK.. 2014. Versatile roles of plant NADPH oxidases and emerging concepts. Biotechnol Adv. 32(3):551–563. [DOI] [PubMed] [Google Scholar]
- Khoshvaghti A, Askari A, Nazifi S, Ghane M.. 2014. Evaluation of some antioxidant enzymes in cattle infected with Foot and Mouth Virus. İstanbul Üniv Vet Fak Derg. 40(1):70–75. [Google Scholar]
- Kraan S. 2012. Algal polysaccharides, novel applications and outlook. In: Chang Chuan-Fa, editor. Carbohydrates – comprehensive studies on glycobiology and glycotechnology. Rijeka, Croatia: IntechOpen. Available from: https://www.intechopen.com/books/carbohydrates-comprehensive-studies-on-glycobiology-and-glycotechnology/algal-polysaccharides-novel-applications-and-outlook. [accessed on 3rd February, 2021]. [Google Scholar]
- Kulshreshtha G, Rathgeber B, Stratton G, Thomas N, Evans F, Critchley A, Hafting J, Prithiviraj B.. 2014. Feed supplementation with red seaweeds, Chondrus crispus and Sarcodiotheca gaudichaudii, affects performance, egg quality, and gut microbiota of layer hens. Poult Sci. 93(12):2991–3001. [DOI] [PubMed] [Google Scholar]
- Kurutas EB. 2016. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: current state. Nutr J. 15(1):71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuznetsova TA, Andryukov BG, Makarenkova ID, Zaporozhets TS, Besednova NN, Fedyanina LN, Kryzhanovsky SP, Shchelkanov MY.. 2021. The potency of seaweed sulfated polysaccharides for the correction of hemostasis disorders in COVID-19. Molecules. 26(9):2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. 2019. Griffithsin, a highly potent broad-spectrum antiviral lectin from red algae: from discovery to clinical application. Mar Drugs. 17:567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SY, Lee J, Lee H, Kim B, Lew J, Baek N, Kim SH.. 2016. MicroRNA134 mediated upregulation of JNK and downregulation of NFkB signalings are critically involved in dieckol induced antihepatic fibrosis. J Agric Food Chem. 64(27):5508–5514. [DOI] [PubMed] [Google Scholar]
- Lee SA, Moon SM, Choi YH, Han SH, Park BR, Choi MS, Kim JS, Kim YH, Kim DK, Kim CS.. 2017. Aqueous extract of Codium fragile suppressed inflammatory responses in lipopolysaccharide-stimulated RAW264.7 cells and carrageenan-induced rats. Biomed Pharmacother. 93:1055–1064. [DOI] [PubMed] [Google Scholar]
- Lee C, Park GH, Ahn EM, Kim BA, Park CI, Jang JH.. 2013. Protective effect of Codium fragile against UVB-induced pro-inflammatory and oxidative damages in HaCaT cells and BALB/c mice. Fitoterapia. 86:54–63. [DOI] [PubMed] [Google Scholar]
- Leonard SG, Sweeney T, Bahar B, Lynch BP, O'Doherty JV.. 2011a. Effects of dietary seaweed extract supplementation in sows and post-weaned pigs on performance, intestinal morphology, intestinal microflora and immune status. Br J Nutr. 106(5):688–699. [DOI] [PubMed] [Google Scholar]
- Leonard SG, Sweeney T, Bahar B, Lynch BP, O'Doherty JV.. 2011b. Effect of dietary seaweed extracts and fish oil supplementation in sows on performance, intestinal microflora, intestinal morphology, volatile fatty acid concentrations and immune status of weaned pigs. Br J Nutr. 105(4):549–560. [DOI] [PubMed] [Google Scholar]
- Leonard SG, Sweeney T, Bahar B, O'Doherty JV.. 2012. Effect of maternal seaweed extract supplementation on suckling piglet growth, humoral immunity, selected microflora, and immune response after an ex vivo lipopolysaccharide challenge. J Anim Sci. 90(2):505–514. [DOI] [PubMed] [Google Scholar]
- Li J, Guo C, Wu J.. 2020. Fucoidan: biological activity in liver diseases. Am J Chin Med. 48(7):1617–1632. [DOI] [PubMed] [Google Scholar]
- Li Q, Luo J, Wang C, Ta W, Wang H, Zhang X, Liu K, Jia Y, Lyv X, Wang L, et al. 2018. Ulvan extracted from green seaweeds as new natural additives in diets for laying hens. J Appl Phycol. 30(3):2017–2027. [Google Scholar]
- Li XJ, Ye QF.. 2015. Fucoidan reduces inflammatory response in a rat model of hepatic ischemia-reperfusion injury. Can J Physiol Pharmacol. 93(11):999–1005. [DOI] [PubMed] [Google Scholar]
- Liu ZW, Sun X.. 2020. A critical review of the abilities, determinants, and possible molecular mechanisms of seaweed polysaccharides antioxidants. IJMS. 21(20):7774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch MB, Sweeny T, Callan JJ, O’Sullivan JT, O’Doherty JV.. 2010. The effect of dietary laminaria derived laminarin and fucoidan on nutrient digestibility, nitrogen utilisation, intestinal microflora and volatile fatty acid concentration in pigs. J Sci Food Agric. 90(3):430–437. [DOI] [PubMed] [Google Scholar]
- Mac Monagail M, Cornish L, Morrison L, Araújo R, Critchley AT.. 2017. Sustainable harvesting of wild seaweed resources. Eur J Phycol. 52(4):371–390. [Google Scholar]
- Makkar HPS, Tran G, Heuzé V, Giger-Reverdin S, Lessire M, Lebas F, Ankers P.. 2016. Seaweeds for livestock diets: a review. Anim Feed Sci Technol. 212:1–17. [Google Scholar]
- Manlusoc JKT, Hsieh CL, Hsieh CY, Salac ESN, Lee YT, Tsai PW.. 2019. Pharmacologic application potentials of sulfated polysaccharide from marine algae. Polymers (Basel). 11(7):1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matos GS, Pereira SG, Genisheva ZA, Gomes AM, Teixeira JA, Rocha CMR.. 2021. Advances in extraction methods to recover added-value compounds from seaweeds: sustainability and functionality. Foods. 10(3):516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonnell P, Figat S, O’Doherty JV.. 2010. The effect of dietary laminarin and fucoidan in the diet of the weanling piglet on performance, selected faecal microbial populations and volatile fatty acid concentrations. Animal. 4(4):579–585. [DOI] [PubMed] [Google Scholar]
- Metsämuuronen S, Sirén H.. 2019. Bioactive phenolic compounds, metabolism and properties: a review on valuable chemical compounds in Scots pine and Norway spruce. Phytochem Rev. 18(3):623–664. [Google Scholar]
- Michalak I, Chojnacka K, Dobrzański Z, Górecki H, Zielińska A, Korczyński M, Opaliński S.. 2011. Effect of macroalgae enriched with microelements on egg quality parameters and mineral content of eggs, eggshell, blood, feathers and droppings. J Anim Physiol Anim Nutr (Berl)). 95(3):374–387. [DOI] [PubMed] [Google Scholar]
- Michalak I, Mahrose K.. 2020. Seaweeds, intact and processed, as a valuable component of poultry feeds. JMSE. 8(8):620. [Google Scholar]
- Michiels J, Skrivanova E, Missotten J, Ovyn A, Mrazek J, De Smet S, Dierick N.. 2012. Intact brown seaweed (Ascophyllum nodosum) in diets of weaned piglets: effects on performance, gut bacteria and morphology and plasma oxidative status. J Anim Physiol Anim Nutr (Berl)). 96(6):1101–1111. [DOI] [PubMed] [Google Scholar]
- Monteiro M, Santos RA, Iglesias P, Couto A, Serra CR, Gouvinhas I, Barros A, Oliva-Teles A, Enes P, Díaz-Rosales P.. 2020. Effect of extraction method and solvent system on the phenolic content and antioxidant activity of selected macro and microalgae extracts. J Appl Phycol. 32(1):349–362. [Google Scholar]
- Morais T, Inácio A, Coutinho T, Ministro M, Cotas J, Pereira L, Bahcevandziev K.. 2020. Seaweed potential in the animal feed: a review. JMSE. 8(8):559. [Google Scholar]
- Moroney NC, O'Grady MN, O'Doherty JV, Kerry JP.. 2012. Addition of seaweed (Laminaria digitata) extracts containing laminarin and fucoidan to porcine diets: influence on the quality and shelf-life of fresh pork. Meat Sci. 92(4):423–429. [DOI] [PubMed] [Google Scholar]
- Moroney NC, O'Grady MN, Robertson RC, Stanton C, O'Doherty JV, Kerry JP.. 2015. Influence of level and duration of feeding polysaccharide (laminarin and fucoidan) extracts from brown seaweed (Laminaria digitata) on quality indices of fresh pork. Meat Sci. 99:132–141. [DOI] [PubMed] [Google Scholar]
- Nagayama K, Iwamura Y, Shibata T, Hirayama I, Nakamura T.. 2002. Bactericidal activity of phlorotannins from the brown alga Ecklonia kurome. J Antimicrob Chemother. 50(6):889–893. [DOI] [PubMed] [Google Scholar]
- Naiel MAE, Alagawany M, Patra AK, El-Kholy AI, Amer MS, Abd El-Hack ME.. 2021. Beneficial impacts and health benefits of macroalgae phenolic molecules on fish production. Aquaculture. 534:736186. [Google Scholar]
- Nhlane LT, Mnisi CM, Mlambo V, Madibana M.. 2020. Nutrient digestibility, growth performance, and blood indices of Boschveld chickens fed seaweed-containing diets. Animals. 10(8):1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen CW, Rustad T, Holdt SL.. 2021. Vitamin C from seaweed: a review assessing seaweed as contributor to daily intake. Foods. 10(1):198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Doherty JV, Dillon S, Figat S, Callan JJ, Sweeney T.. 2010. The effects of lactose inclusion and seaweed extract derived from Laminaria spp. on performance, digestibility of diet components and microbial populations in newly weaned pigs. Anim Feed Sci Technol. 157(3-4):173–180. [Google Scholar]
- Øverland M, Mydland LT, Skrede A.. 2019. Marine macroalgae as sources of protein and bioactive compounds in feed for monogastric animals. J Sci Food Agric. 99(1):13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira L. 2018. Biological and therapeutic properties of the seaweed polysaccharides. Int Biol Rev. 2:1–50. [Google Scholar]
- Pereira L, Critchley AT.. 2020. The COVID 19 novel coronavirus pandemic 2020: seaweeds to the rescue? Why does substantial, supporting research about the antiviral properties of seaweed polysaccharides seem to go unrecognized by the pharmaceutical community in these desperate times? J Appl Phycol.. 32(3):1875–1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahal A, Kumar A, Singh V, Yadav B, Tiwari R, Chakraborty S, Dhama K.. 2014. Oxidative stress, prooxidants and antioxidants: the interplay. Biomed Res Int. 2014:761264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman K. 2007. Studies on free radicals, antioxidants, and co-factors. Clin Interv Aging. 2(2):219–236. [PMC free article] [PubMed] [Google Scholar]
- Rajauria G, Ravindran R, Garcia-Vaquero M, Rai DK, Sweeney T, O'Doherty J.. 2021. Molecular characteristics and antioxidant activity of laminarin extracted from the seaweed species Laminaria hyperborea, using hydrothermal-assisted extraction and a multi-step purification procedure. Food Hydrocoll. 112:106332. [Google Scholar]
- Ramadan G, Fouda WA, Ellamie AM, Ibrahim WM.. 2020. Dietary supplementation of Sargassum latifolium modulates thermo-respiratory response, inflammation, and oxidative stress in bacterial endotoxin-challenged male Barki sheep. Environ Sci Pollut Res. 27(27):33863–33871. [DOI] [PubMed] [Google Scholar]
- Rauf A, Rashid U, Khalil AA, Khan SA, Anwar S, Alafnan A, Alamri A, Rengasamy KR.. 2021. Docking-based virtual screening and identification of potential COVID-19 main protease inhibitors from brown algae. S Afr J Bot. 143:428–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly P, O’Doherty JV, Pierce KM, Callan JJ, O’Sullivan JT, Sweeney T.. 2008. The effects of seaweed extract inclusion on gut morphology, selected intestinal microbiota, nutrient digestibility, volatile fatty acid concentrations and the immune status of the weaned pig. Animal. 2(10):1465–1473. [DOI] [PubMed] [Google Scholar]
- Rey-Crespo F, López-Alonso M, Miranda M.. 2014. The use of seaweed from the Galician coast as a mineral supplement in organic dairy cattle. Animal. 8(4):580–586. [DOI] [PubMed] [Google Scholar]
- Rjiba SK, Chermiti A, Mahouachi M.. 2010. The use of seaweeds (Ruppia maritima and Chaetomorpha linum) for lamb fattening during drought periods. Small Rum Res. 91(1):116–119. [Google Scholar]
- Rosa GP, Tavares WR, Sousa PMC, Pages AK, Seca AML, Pinto D.. 2019. Seaweed secondary metabolites with beneficial health effects: an overview of successes in in vivo studies and clinical trials. Mar Drugs. 18(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi R, Vizzarri F, Ratti S, Palazzo M, Casamassima D, Corino C.. 2020. Effects of long-term supplementation with brown seaweeds and polyphenols in rabbit on meat quality parameters. Animals (Basel). 10(12):2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz ÁR, Gadicke P, Andrades SM, Cubillos R.. 2018. Supplementing nursery pig feed with seaweed extracts increases final body weight of pigs. Austral j Vet Sci. 50(2):83–87. [Google Scholar]
- Ryan MT, Smith AG, O'Doherty JV, Bahar B, Reilly P, Sweeney T.. 2010. Effects of nutrient supplementation with laminarin derived from Laminaria hyperborea and Laminaria digitata on mucin gene expression in the porcine ileum. Livest Sci. 133(1-3):236–238. [Google Scholar]
- Sadeeshkumar V, Duraikannu A, Ravichandran S, Kodisundaram P, Fredrick WS, Gobalakrishnan R.. 2017. Modulatory efficacy of dieckol on xenobiotic-metabolizing enzymes, cell proliferation, apoptosis, invasion and angiogenesis during NDEA-induced rat hepatocarcinogenesis. Mol Cell Biochem. 433(1–2):195–204. [DOI] [PubMed] [Google Scholar]
- Saeed M, Arain MA, Fazlani SA, Marghazani IB, Umar M, Soomro J, Bhutto ZA, Soomro F, Noreldin AE, Abd El-Hack ME, et al. 2021. A comprehensive review on the health benefits and nutritional significance of fucoidan polysaccharide derived from brown seaweeds in human, animals and aquatic organisms. Aquac Nutr. 27(3):633–654. [Google Scholar]
- Saker KE, Allen VG, Fontenot JP, Bagley CP, Ivy RL, Evans RR, Wester DB.. 2001. Tasco-Forage: II. Monocyte immune cell response and performance of beef steers grazing tall fescue treated with a seaweed extract. J Anim Sci. 79(4):1022–1031. [DOI] [PubMed] [Google Scholar]
- Saker KE, Fike JH, Veit H, Ward DL.. 2004. Brown seaweed-(TascoTM) treated conserved forage enhances antioxidant status and immune function in heat-stressed wether lambs. J Anim Physiol Anim Nutr. 88(3–4):122–130. [DOI] [PubMed] [Google Scholar]
- Salehi B, Sharifi-Rad J, Seca AML, Pinto DCGA, Michalak I, Trincone A, Mishra AP, Nigam M, Zam W, Martins N.. 2019. Current trends on seaweeds: looking at chemical composition, phytopharmacology, and cosmetic applications. Molecules. 24:4182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharun K, Dhama K, Pawde AM, Gortázar C, Tiwari R, Bonilla-Aldana DK, Rodriguez-Morales AJ, de la Fuente J, Michalak I, Attia YA.. 2021. SARS-CoV-2 in animals: potential for unknown reservoir hosts and public health implications. Vet Q. 41(1):181–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Q, Wang A, Lu Z, Qin C, Hu J, Yin J.. 2017. Overview on the antiviral activities and mechanisms of marine polysaccharides from seaweeds. Carbohydr Res. 453-454:1–9. [DOI] [PubMed] [Google Scholar]
- Stengel DB, Connan S, Popper ZA.. 2011. Algal chemodiversity and bioactivity: sources of natural variability and implications for commercial application. Biotechnol Adv. 29(5):483–501. [DOI] [PubMed] [Google Scholar]
- Strand A, Herstad O, Liaaen-Jensen S.. 1998. Fucoxanthin metabolites in egg yolks of laying hens. Comp Biochem Physiol Part A Mol Integr Physiol. 119(4):963–974. [DOI] [PubMed] [Google Scholar]
- Sweeney T, Collins CB, Reilly P, Pierce KM, Ryan M, O'Doherty JV.. 2012. Effect of purified beta-glucans derived from Laminaria digitata, Laminaria hyperborea and Saccharomyces cerevisiae on piglet performance, selected bacterial populations, volatile fatty acids and pro-inflammatory cytokines in the gastrointestinal tract of pigs. Br J Nutr. 108(7):1226–1234. [DOI] [PubMed] [Google Scholar]
- Sweeney T, Meredith H, Ryan MT, Gath V, Thornton K, O'Doherty JV.. 2016. Effects of Ascophyllum nodosum supplementation on Campylobacter jejuni colonisation, performance and gut health following an experimental challenge in 10 days old chicks. Innov Food Sci Emerg Technol. 37:247–252. [Google Scholar]
- Tamama K. 2021. Potential benefits of dietary seaweeds as protection against COVID-19. Nutr Rev. 79(7):814–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanna B, Mishra A.. 2019. Nutraceutical potential of seaweed polysaccharides: structure, bioactivity, safety, and toxicity. Compr Rev Food Sci Food Saf. 18(3):817–831. [DOI] [PubMed] [Google Scholar]
- Telles CBS, Mendes-Aguiar C, Fidelis GP, Frasson AP, Pereira WO, Scortecci KC, Camara RBG, Nobre LTDB, Costa LS, Tasca T, et al. 2018. Immunomodulatory effects and antimicrobial activity of heterofucans from Sargassum filipendula. J Appl Phycol. 30(1):569–578. [Google Scholar]
- Tiwari R, Dhama K, Sharun K, Iqbal Yatoo M, Malik YS, Singh R, Michalak I, Sah R, Bonilla-Aldana DK, Rodriguez-Morales AJ.. 2020. COVID-19: animals, veterinary and zoonotic links. Vet Q. 40(1):169–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner JL, Dritz SS, Higgins JJ, Minton JE.. 2002. Effects of Ascophyllum nodosum extract on growth performance and immune function of young pigs challenged with Salmonella typhimurium. J Anim Sci. 80(7):1947–1953. [DOI] [PubMed] [Google Scholar]
- Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J.. 2007. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 39(1):44–84. [DOI] [PubMed] [Google Scholar]
- Visconti R, Grieco D.. 2009. New insights on oxidative stress in cancer. Curr Opin Drug Discov Devel. 12(2):240–245. [PubMed] [Google Scholar]
- Vizzari F, Massányi M, Knížatová N, Corino C, Rossi R, Ondruška Ľ, Tirpák F, Halo M, Massányi P.. 2021. Effects of dietary plant polyphenols and seaweed extract mixture on male-rabbit semen: Quality traits and antioxidant markers. Saudi J Biol Sci. 28(1):1017–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waheed Janabi AH, Kamboh AA, Saeed M, Xiaoyu L, BiBi J, Majeed F, Naveed M, Mughal MJ, Korejo NA, Kamboh R, et al. 2020. Flavonoid-rich foods (FRF): A promising nutraceutical approach against lifespan-shortening diseases. Iran J Basic Med Sci. 23:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh AM, Sweeney T, O'Shea CJ, Doyle DN, O'Doherty JV.. 2013. Effect of dietary laminarin and fucoidan on selected microbiota, intestinal morphology and immune status of the newly weaned pig. Br J Nutr. 110(9):1630–1638. [DOI] [PubMed] [Google Scholar]
- Wan J, Jiang F, Xu QS, Chen DW, He J.. 2016. Alginic acid oligosaccharide accelerates weaned pig growth through regulating antioxidant capacity, immunity and intestinal development. RSC Adv. 6(90):87026–87035. [Google Scholar]
- Wan J, Zhang J, Chen DW, Yu B, He J.. 2017. Effects of alginate oligosaccharide on the growth performance, antioxidant capacity and intestinal digestion–absorption function in weaned pigs. Anim Feed Sci Technol. 234:118–127. [Google Scholar]
- Wan J, Zhang J, Chen DW, Yu B, Huang Z, Mao X, Zheng P, Yu J, He J.. 2018. Alginate oligosaccharide enhances intestinal integrity of weaned pigs through altering intestinal inflammatory responses and antioxidant status. RSC Adv. 8(24):13482–13492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan G, Guo Y, Yuan J, Liu D, Zhang B.. 2011. Sodium alginate oligosaccharides from brown algae inhibit Salmonella Enteritidis colonization in broiler chickens. Poult Sci. 90(7):1441–1448. [DOI] [PubMed] [Google Scholar]
- Yim SK, Kim K, Kim IH, Chun SH, Oh TH, Kim JU, Kim JW, Jung WH, Moon HS, Ku BS, Jung KJ.. 2021. Inhibition of SARS-CoV-2 virus entry by the crude polysaccharides of seaweeds and abalone viscera in vitro. Mar Drugs. 19(4):219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurdakul I, Yildirim BA.. 2018. Assessment of oxidative status in foot diseases of sheep. Acta Scientiae Vet. 46(1):6. [Google Scholar]
- Zhong B, Robinson NA, Warner RD, Barrow CJ, Dunshea FR, Suleria HAR.. 2020. LC-ESI-QTOF-MS/MS characterization of seaweed phenolics and their antioxidant potential. Mar Drugs. 18(6):331. [DOI] [PMC free article] [PubMed] [Google Scholar]