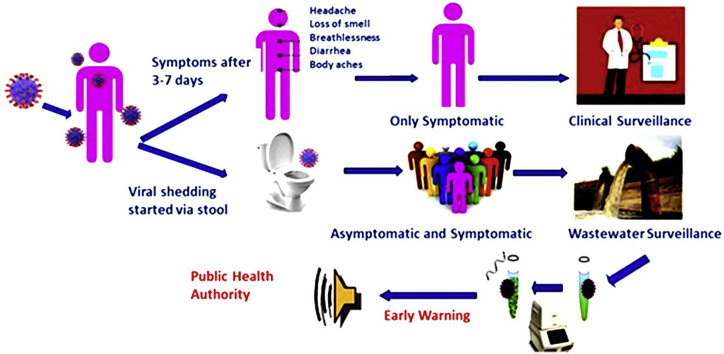
Abstract
The ongoing pandemic of the coronavirus disease 2019 (COVID-19) is a public health crisis of global concern. The progression of the COVID-19 pandemic has been monitored in the first place by testing symptomatic individuals for SARS-CoV-2 virus in the respiratory samples. Concurrently, wastewater carries feces, urine, and sputum that potentially contains SARS-CoV-2 intact virus or partially damaged viral genetic materials excreted by infected individuals. This brings significant opportunities for understanding the infection dynamics by environmental surveillance. It has advantages for the country, especially in densely populated areas where individual clinical testing is difficult. However, there are several challenges including: 1) establishing a sampling plan and schedule that is representative of the various catchment populations 2) development and validation of standardized protocols for the laboratory analysis 3) understanding hydraulic flows and virus transport in complex wastewater drainage systems and 4) collaborative efforts from government agencies, NGOs, public health units and academia.
Keywords: Sanitation, WASH, Wastewater, Sewage, SARS-CoV-2, Low-income countries
Graphical abstract
Introduction
Wastewater surveillance
As a result of the COVID-19 outbreak, wastewater surveillance has garnered considerable attention worldwide. Wastewater surveillance can be used to monitor wastewater released from individual household septic tanks to community drains, surface water, and sewage treatment plants, capturing the occurrence of SARS-CoV-2 and other enteric pathogens trends [1]. There is evidence that COVID-19 infected person shed SARS-CoV-2 viral RNA through the stool and urine before the symptoms appear and persist longer [2]. The presence of SARS-CoV-2 in the feces was first reported in early February 2020 by a number of investigators and demonstrated that about 48% of patients with COVID-19, irrespective of clinical severity or symptoms, have detectable SARS-CoV-2 virus RNA in their feces [3]. Notably, viral RNA was detected in fecal samples from patients even after their respiratory specimens tested negative. Other investigators have also reported longer persistence of SARS-CoV-2 RNA in the feces than in the respiratory and serum samples [2, 3, 4]. The viral load in the feces of persons testing positive for SARS-CoV-2 was estimated to be between 5 × 103-107.6 genome copies/mL, depending on the infection course [5]. Through statistical modeling, viral RNA concentrations in wastewater samples can predict symptomatic and asymptomatic COVID infections within a community [6]. Therefore, one of the motivations to use a wastewater surveillance system is to detect viral RNA and other enteric pathogens to understand the presence of infections in the community ahead of any outbreaks.
Sanitation challenges in Bangladesh
Approximately 72% of people in Asia do not have access to appropriate sanitation and in South Asia alone over 610 million people still practice open defecation [7]. Since the independence of Bangladesh in 1971, the country has made significant progress in providing access to sanitation. Between 2000 and 2015, Bangladesh took concerted actions to become an open defecation free (ODF) country, and as of 2018, open defecation in Bangladesh has been virtually non-existent [8]. However, Bangladesh faces considerable socio-ecological challenges for safe disposal of fecal waste generated in urban areas. Dhaka, the capital of Bangladesh, has the highest population density in the country with 47,400 people/square kilometer. Approximately 6 million people reside in urban slums in Dhaka, and an estimated population of 4.3 million people use shared sanitation facilities [9,10]. With limited sewage treatment, the management of fecal sludge has become a major challenge. There is only one wastewater treatment plant (WWTP) in Dhaka that is serves 20 percent of the population (∼5 million people) of Dhaka city [9]. The WWTP is connected to 935 km of sewer networks with 26 sewage lift stations and 4 storm sewage stations [9]. According to the WHO/UNICEF report [8], an estimated 80% of the neighborhoods in Dhaka city are located outside the coverage of this sewerage network. Furthermore, 97% of the fecal waste in Dhaka reaches the environment untreated, with 71% of households using pit and septic tank that discharges untreated fecal waste directly or indirectly into an open drains or ditches [11, 12, 13]. Our recent findings showed that the performance of on-site sanitation (OSS) was not adequate to remove pathogens of public health significance and failure to ensure strong links throughout the fecal sludge management (FSM) service chain resulted in untreated sewage contaminating the environment and surface water [14]. Frequent flooding in areas with poor drainage systems has far-reaching deleterious impacts on human health [15]. Similarly, groundwater sources in the city are impacted by fecal contamination and there is potential for viral contamination during groundwater recharge, which increases the risk of waterborne infections. This situation mirrors Bangladesh's rural areas, where OSS is the primary sanitation option [12].
Wastewater surveillance with low sanitation coverage
Most studies of SARS-CoV-2 in wastewater are in high-income countries where sanitation systems are well-structured and population catchment areas are specific (Table 1 ) [16∗, 17∗∗, 18, 19, 20, 21, 22, 23]. Bangladesh, and most low- and middle-income countries, have mixed sanitation systems, with subsequent varied characteristics of sewage-sludge. Therefore, the approaches used in high-income countries will need to be adapted for shared and private latrines, on-site sanitation, and direct dumping of fecal waste into the environment. Nevertheless, two studies from Bangladesh can support the successful identification and quantification of SARS-CoV-2 viral RNA from the wastewater collected from drains, surface water, and effluent of the septic tank samples [18,24]. Data are still limited from Bangladesh to implement wastewater surveillance at the national level. Wastewater monitoring output should present integrated rather individual research outcomes. Also, the impact of climatic variability on wastewater-based surveillance should be explored in sub-tropical environments because rainfall, humidity, and temperature may affect the viability, degradation, mutation and detection of the virus in the environment. There is evidence of SARS-CoV-2 mobility in the subsurface water and possible leaching into the groundwater [25]. Developing a common platform from the different environmental data banks would be helpful to compare, sharing each other's expertise, wastewater monitoring tools, and standard method to replicate the wastewater surveillance even in a country with inadequate sanitation facilities [26].
Table 1.
CoV-2 viral RNA in wastewater surveillance.
| Country | Sources | No of samples | Method | Kit used for RT-PCR | Target gene | References |
|---|---|---|---|---|---|---|
| India | WWTP1,2,3 aeration pond | <20 | qPCR | TaqPathTM Covid-19 RT-PCR Kit | N, S, ORF1 ab |
[16] |
| Australia | Pumping station3, WWTP3 | 4–5 | qPCR | iTaq™ Universal Probes One–Step Reaction Mix | N protein | [17] |
| France | WWTP4,6 | 31 | qPCR/RNA | – | RdRP, E protein |
[19] |
| Spain | WWTP1,2 | 72 | qPCR | One Step PrimeScript™ RT-PCR Kit | N protein | [21] |
| USA | WWTP1 | 10 | RT-PCR qPCR Genome sequencing | – | S and N protein | [37] |
| Italy | River, WWTP4,6 |
– | qPCR, Genome sequencing, cell culture |
2019-nCoV Real-Time RT-PCR Diagnostic Panel | ORF1 ab, N and E protein |
[20] |
| Netherlands | WWTP1 | 29 | qPCR | EvoScript RNA Probes Master | E and N protein | [23] |
| Italy | WWTP1 | 12 | RT-PCR, qPCR | Kit Platinum™ SuperFi™ Green PCR Master Mix, Thermo | S protein, ORF1 aba RdRP | [38] |
| Japan | River, WWTP1 | 13 | Nested PCR qPCR, (IDEXX) | Premix Ex Taq Hot Start Version | ORF1ab, S and E protein, E. colia |
[22] |
| Israel | Raw sewage WWTP | 26 | qPCR | StepOnePlus™ Real-Time PCR System | – | [39] |
| Bangladesh | Drain, Canal, Sewer | 16 | RT-PCR (CFX96, BioRad) | Sansure RT-PCR kit | ORF1ab, N genes, and RNase | [18] |
1 = influent; 2 = effluent; 3 = sludge; 4 = raw sewage; 5 = Pre-treated; 6 = treated ∗ “-” not available strong evidence.
A summary of potential opportunities and challenges of implementing wastewater surveillance
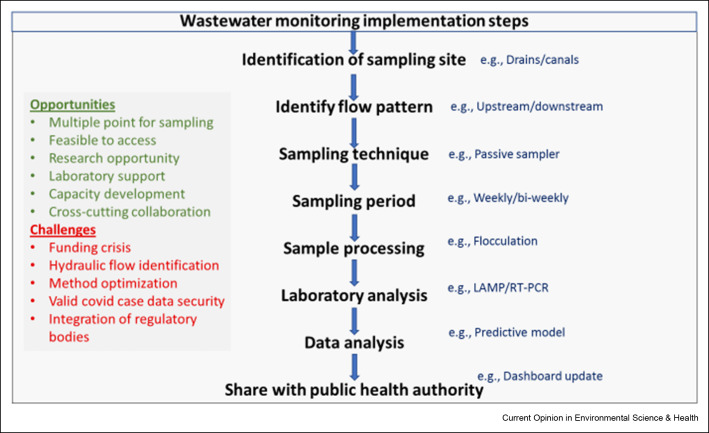
The purpose of this present paper is to encourage researchers, governments, local and international development organizations, and practitioners to use wastewater surveillance as a low-cost approach for better understanding COVID-19 and other enteric pathogens (Figure 1 ).
-
•
Sampling points: In countries like Bangladesh, it is challenging to develop a city-wide wastewater surveillance strategy and identify sample collection sites that represent population-level feces in communities where the COVID-19 burden is largely unknown due to lack of diagnostic testing centers. Designing city-wide wastewater surveillance will need to include sampling from the wastewater treatment plant (WWTP) and pumping stations for the parts of the city that have a sewerage network and sample sites at major drains and canals that capture the wastewater from septic tanks and pit latrines [27]. Understanding hydraulic flows and virus transport in complex wastewater drainage systems in Dhaka city including a mix of sewered and non-sewered areas, is challenging [28]. The present paper posits that wastewaters both from sewer network and on-site sanitation facilities receive and harbor SARS-CoV-2 from various sources in a community or catchment. The sewersheds and catchment areas can be estimated using GIS tools and sewerage network maps. Examining drainage and flow patterns such as manholes or up and downstream drains will lead to the identification of representative sampling points for periodic sampling in the next step.
-
•
Sampling technique: Wastewater surveillance requires significant logistical and financial support in dispersed communities. Traditional sampling methods, such as autosamplers, were a decent choice in affluent countries. However, not suitable for large rapid monitoring at these low-resources settings. Therefore, Passive samplers can aid with low-cost sample collecting. Passive sampling presents a cheap, safe, and easy alternative to traditional wastewater sampling within the sewage catchment for wastewater monitoring. The installation of the passive sampler in waterbodies is simple (i.e., no specific skills are necessary), quick, and usually does not necessitate space limitations. Also, it is continuously exposed to the water column, therefore the sampling error rate is low even when single water samples are taken [29].
-
•
Laboratory methods optimization: Sample preparation and analysis methods need to be developed and validated for treated and untreated wastewater. Accredited laboratories with skilled personnel and high biosafety protocols are needed to implement this method. Because of the funding constraint, the laboratory method also needs to be cost-effective, at the same time sensitive and reliable. Loop-Mediated Isothermal Amplification (LAMP) method can be suitable in this context. LAMP reactions are capable of detecting even a few copies of target nucleic acid sequences under isothermal conditions (usually 60–65 °C) with the help of specially designed primer sets [30,31]. Due to its simplicity, it is gaining more popularity in the diagnosis of various viral diseases. Unlike PCR, this technique can be performed in a low-resource setting by merely heating the samples and reagents in a single reaction tube. Currently, Bangladesh has developed about 118 PCR laboratories since the pandemic hit in the country [32]. Once a valid method is optimized, there are opportunities to roll over the wastewater surveillance all over the country [33]. As a result, many researchers are concentrating on improving and modifying the RT-LAMP methodology to meet the needs of COVID-19 diagnosis in laboratories with limited resources in underdeveloped nations, where uncontrolled and undetected COVID-19 spread can have unforeseeable consequences. Furthermore, mobile testing units and rapid, low-cost sensors need to be developed to support the surveillance system for any future outbreak.
-
•
End-user: The necessity for low-cost, quick monitoring of COVID-19 prevalence and trends has long been acknowledged. One example is environmental surveillance, which includes the proposed wastewater monitoring for determining COVID-19 frequency in the community. The Directorate General of Health Services (DGHS) is the key player and leads the national technical committee that manages, implements, guides, and supports COVID-19 management in Bangladesh. The technical committee at DGHS endorses all research related to COVID-19 and uses the research findings for implementation and incorporation in the national guidelines. The DGHS plays a key role in the implementation of public health interventions [32]. Sharing the results of the environmental surveillance in near real-time with health authorities, including outbreak investigators, epidemiologists, public health engineers, and sanitary inspectors, to enable a more targeted public health response to current outbreaks and endemic disease. An online platform for sharing the weekly/biweekly/monthly wastewater surveillance findings, including SARS-CoV-2 temporal trends, spatial trends, and detection of variants of concern, with designated public health entities, will support decision making prior to outbreak take place. Currently, the COVID-19 prevalence rate is determined by the number of clinical tests that are positive every day and the COVID-19 case fatality rate. By establishing wastewater surveillance, it will be able to predict trends in COVID-19 prevalence 2–6 weeks ahead of changes in healthcare utilization and mortality associated with COVID-19 [34,35]. This dashboard will help key partners to make critical public health response decisions based on viral infection dynamics reflected by trends in the environmental surveillance data.
-
•
Implementation with collaborative effort: Departments from the Ministry of Health and Family Welfare (MoH&FW) and Ministry of Local Government Rural Development and Cooperatives (MoLGRD&C) are the key partners from the government; Dhaka Water Supply and Sewerage Authority (DWASA) is responsible for water supply, sanitation and stormwater disposal services in the capital, and serves around 8.6 million of the 11 million people in the Dhaka Metropolitan Area (DMA). Support from DWASA is essential to explore the diversity of the sanitation system in Dhaka City and establish wastewater surveillance; Dhaka North City Corporation (DNCC) and Dhaka South City Corporation (DSCC) are responsible for the management of drainage canal and system, and maintenance of the Sewerage Lifting Station (SLS) and The Institute of Epidemiology, Disease Control, and Research (IEDCR) is responsible for conducting communicable disease outbreak and surveillance in Bangladesh [36]. The Institute also contributes research on communicable diseases as well as the functioning of disease control programs mainly in the form of parasitic and entomological containment of vector-borne diseases, emerging and re-emerging diseases, and their response through the application of epidemiological studies. Their engagement and collaboration are crucial to implementing and scaling up Bangladesh's environmental surveillance system.
Figure 1.
Wastewater surveillance proposed implementation steps in Bangladesh.
Concluding remarks
Wastewater monitoring is a noninvasive, sensitive, and more cost-effective approach to adopt in low-to middle-income countries (LMICs) to examine changes in total COVID-19 burden and other waterborne diseases in community and can inform public health decision making for responding to the current COVID-19 pandemic as well as future health threats. The COVID-19 pandemic highlighted that monitoring wastewater and other relevant environmental samples for SARS-CoV-2 and other pathogens provides a sensitive signal of the presence of the pathogens in entire communities and can also indicate whether infection rates are increasing or declining. Data from the surveillance can be used as a valuable complement to epidemiological case data. Where no such epidemiological data are available, surveillance for the presence and concentration of pathogens in wastewater can be a very effective noninvasive public health tool in low-resource countries like Bangladesh.
Editorial disclosure statements
Given their role as Guest Editor, Prosun Bhattacharya had no involvement in the peer-review of this article and has no access to information regarding its peer-review. Full responsibility for the editorial process for this article was delegated to Payal Mazumder.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Dr. Rehnuma Haque is supported by the Global Health Equity Scholars Program NIH FIC and NIEHS D43 TW010540. The authors thank to UNICEF/SDC and the Life science technology platform, Science for Life Laboratory for the funding to initiate the SARS-CoV-2 WBE project at icddr, b, Bangladesh. icddr, b gratefully acknowledge our core donors including the Governments of Bangladesh, Canada, Sweden, and the UK, for their support and commitment to icddr, b research efforts.
This review comes from a themed issue on Occupational Safety and Health 2022: COVID-19 in environment: Treatment, Infectivity, Monitoring, Estimation
Edited by Manish Kumar, Ryo Honda, Prosun Bhattacharya, Dan Snow and Payal Mazumder
References
- Meng X., Wang X., Meng S., Wang Y., Liu H., Liang D., Fan W., Min H., Huang W., Chen A., et al. A global overview of SARS-CoV-2 in wastewater: detection, treatment, and prevention. ACS EST Water. 2021;1:2174–2185. doi: 10.1021/acsestwater.1c00146. [DOI] [PubMed] [Google Scholar]; This study demonstrated the significant global overview of implementing SARS-CoV-2 wastewater surveillance.
- 2.Wu Y., Guo C., Tang L., Hong Z., Zhou J., Dong X., Yin H., Xiao Q., Tang Y., Qu X., et al. Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. Lancet Gastroenterol Hepatol. 2020;5:434–435. doi: 10.1016/S2468-1253(20)30083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheung K.S., Hung I.F.N., Chan P.P.Y., Lung K.C., Tso E., Liu R., Ng Y.Y., Chu M.Y., Chung T.W.H., Tam A.R., et al. Gastrointestinal manifestations of SARS-CoV-2 infection and virus load in fecal samples from a Hong Kong cohort: systematic review and meta-analysis. Gastroenterology. 2020;159:81–95. doi: 10.1053/j.gastro.2020.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]; This meta-analysis detected SARS-CoV-2 genetic materials in stool from 48.1% of patients, even after respiratory samples were found negative thus, concluded fecal shedding is longer than the respiratory samples.
- 4.Zheng S., Fan J., Yu F., Feng B., Lou B., Zou Q., Xie G., Lin S., Wang R., Yang X., et al. Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang province, China, January-March 2020: retrospective cohort study. BMJ. 2020;369:m1443. doi: 10.1136/bmj.m1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Foladori P., Cutrupi F., Segata N., Manara S., Pinto F., Malpei F., Bruni L., La Rosa G. SARS-CoV-2 from faeces to wastewater treatment: what do we know? A review. Sci Total Environ. 2020;743:140444. doi: 10.1016/j.scitotenv.2020.140444. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article estimates the fecal shedding from a COVID-19 infected patient is 5•103–107.6 copies/mL and reduced at least 2 copies before entering the WWTP.
- 6.Vallejo J.A., Trigo N., Rumbo-Feal S., Conde-Pérez K., Lopez-Oriona Á., Barbeito I., Vaamonde M., Tarrío-Saavedra J., Reif R., Ladra S., et al. Modeling the number of people infected with SARS-COV-2 from wastewater viral load in Northwest Spain. Sci Total Environ. 2021 doi: 10.1016/j.scitotenv.2021.152334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.A snapshot of sanitation, hygiene and drinking water safety in South Asia. 2019. [Google Scholar]
- 8.Progress on drinking water, sanitation and hygiene 2000–2017. UNICEF; 2019. [Google Scholar]
- 9.Bhaban W., Avenue K.N.I., Bazar K. 2020. Dhaka water supply and sewerage authority. Annual report 2018-19. [Google Scholar]
- 10.Yeasmin F., Rahman M., Luby S.P., Das J.B., Begum F., Saxton R.E., Nizame F.A., Hwang S.T., Alam M.-U., Hossain M.K., et al. Landlords' and compound managers' role in improving and sustaining shared latrines in three Dhaka city slums. Water. 2020;12:2073. [Google Scholar]
- 11.Ross A.G., Rahman M., Alam M., Zaman K., Qadri F. Can we “WaSH” infectious diseases out of slums? Int J Infect Dis IJID Off Publ Int Soc Infect Dis. 2020;92:130–132. doi: 10.1016/j.ijid.2020.01.014. [DOI] [PubMed] [Google Scholar]; This opinion paper highlighted current sanitation situation of Dhaka City slum areas.
- 12.WHO/UNICEF joint monitoring programme for water supply and sanitation: Progress on sanitation and drinking-water: 2010 update. World Health Organization; 2010. [Google Scholar]
- 13.Bangladesh – water & sanitation for the urban poor. 2017. [Google Scholar]
- 14.Amin N., Liu P., Foster T., Rahman M., Miah M.R., Ahmed G.B., Kabir M., Raj S., Moe C.L., Willetts J. Pathogen flows from on-site sanitation systems in low-income urban neighborhoods, Dhaka: a quantitative environmental assessment. Int J Hyg Environ Health. 2020;230:113619. doi: 10.1016/j.ijheh.2020.113619. [DOI] [PubMed] [Google Scholar]
- 15.Amin N., Rahman M., Raj S., Ali S., Green J., Das S., Doza S., Mondol M.H., Wang Y., Islam M.A., et al. Quantitative assessment of fecal contamination in multiple environmental sample types in urban communities in Dhaka, Bangladesh using SaniPath microbial approach. PLoS One. 2019;14 doi: 10.1371/journal.pone.0221193. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study highlighted wastewater fromdrain water, sediment, canal water, floodwater, sludge, supernatant, and effluent samples from septic tanks, and ABRs identified high concentrations of enteric pathogens, particularly NoV-GII, V.cholerae, and Shigella in Dhaka, Bangladesh.
- Kumar M., Patel A.K., Shah A.V., Raval J., Rajpara N., Joshi M., Joshi C.G. First proof of the capability of wastewater surveillance for COVID-19 in India through detection of genetic material of SARS-CoV-2. Sci Total Environ. 2020;746:141326. doi: 10.1016/j.scitotenv.2020.141326. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article from India that shares a similar sanitation system like 274 Bangladesh confirmed the presenceof wastewater surveillance for the genetic materials of SARS-CoV-2 in municipal wastewater.
- Ahmed W., Angel N., Edson J., Bibby K., Bivins A., O'Brien J.W., Choi P.M., Kitajima M., Simpson S.L., Li J., et al. First confirmed detection of SARS-CoV-2 in untreated wastewater in Australia: a proof of concept for the wastewater surveillance of COVID-19 in the community. Sci Total Environ. 2020;728:138764. doi: 10.1016/j.scitotenv.2020.138764. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper is published earlier in 2020 as the first proof of concept and successful detection of SARS-CoV-2 genetic materials in the wastewater through wastewater-based epidemiology (WBE) surveillance in the community at Brisbane, Australia.
- 18.Ahmed F., Islam M.A., Kumar M., Hossain M., Bhattacharya P., Islam M.T., Hossen F., Hossain M.S., Islam M.S., Uddin M.M., et al. First detection of SARS-CoV-2 genetic material in the vicinity of COVID-19 isolation Centre in Bangladesh: variation along the sewer network. Sci Total Environ. 2021;776:145724. doi: 10.1016/j.scitotenv.2021.145724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wurtzer S., Marechal V., Mouchel J.M., Maday Y., Teyssou R., Richard E., Almayrac J.L., Moulin L. 2020. Evaluation of lockdown impact on SARS-CoV-2 dynamics through viral genome quantification in Paris wastewaters. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rimoldi S.G., Stefani F., Gigantiello A., Polesello S., Comandatore F., Mileto D., Maresca M., Longobardi C., Mancon A., Romeri F., et al. Presence and infectivity of SARS-CoV-2 virus in wastewaters and rivers. Sci Total Environ. 2020;744:140911. doi: 10.1016/j.scitotenv.2020.140911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Randazzo W., Truchado P., Cuevas-Ferrando E., Simón P., Allende A., Sánchez G. SARS-CoV-2 RNA in wastewater anticipated COVID-19 occurrence in a low prevalence area. Water Res. 2020;181:115942. doi: 10.1016/j.watres.2020.115942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haramoto E., Malla B., Thakali O., Kitajima M. First environmental surveillance for the presence of SARS-CoV-2 RNA in wastewater and river water in Japan. Sci Total Environ. 2020;737:140405. doi: 10.1016/j.scitotenv.2020.140405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Medema G., Heijnen L., Elsinga G., Italiaander R., Brouwer A. Presence of SARS-coronavirus-2 RNA in sewage and correlation with reported COVID-19 prevalence in the early stage of the epidemic in The Netherlands. Environ Sci Technol Lett. 2020 doi: 10.1021/acs.estlett.0c00357. [DOI] [PubMed] [Google Scholar]
- 24.Haque Rehnuma, Rahman Md Mahbubur, Amin Nuhu, Rahman Mohammed Ziaur Rahman, Mahmud Zahid Hayat, Sarker Protim, Raqib Rubhana, et al. CUGH 2021 virtual conference. CUGH; 2021. Inference and forecasting of SARS-CoV-2 wastewater surveillance in Bangladesh. [Google Scholar]
- 25.Kumar M., Thakur A.K., Mazumder P., Kuroda K., Mohapatra S., Rinklebe J., Ramanathan Al, Cetecioglu Z., Jain S., Tyagi V.K., et al. Frontier review on the propensity and repercussion of SARS-CoV-2 migration to aquatic environment. J Hazard Mater Lett. 2020;1:100001. doi: 10.1016/j.hazl.2020.100001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bhattacharya P., Kumar M., Islam MdT., Haque R., Chakraborty S., Ahmad A., Niazi N.K., Cetecioglu Z., Nilsson D., Ijumulana J., et al. Prevalence of SARS-CoV-2 in communities through wastewater surveillance—a potential approach for estimation of disease burden. Curr Pollut Rep. 2021 doi: 10.1007/s40726-021-00178-4. [DOI] [PMC free article] [PubMed] [Google Scholar]; This opinion paper emphasized the importance of wastewater-based epidemiology surveillance for SARS-CoV-2 in the LMIC and prioritized the low sanitation areas, proposing standard international tools for surveillance methods and data interpretation.
- 27.Wang Y., Mairinger W., Raj S.J., Yakubu H., Siesel C., Green J., Durry S., Joseph G., Rahman M., Amin N., et al. Quantitative assessment of exposure to fecal contamination in urban environment across nine cities in low-income and lower-middle-income countries and a city in the United States. Sci Total Environ. 2022;806:151273. doi: 10.1016/j.scitotenv.2021.151273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Foster T., Falletta J., Amin N., Rahman M., Liu P., Raj S., Mills F., Petterson S., Norman G., Moe C., et al. Modelling faecal pathogen flows and health risks in urban Bangladesh: implications for sanitation decision making. Int J Hyg Environ Health. 2021;233:113669. doi: 10.1016/j.ijheh.2020.113669. [DOI] [PubMed] [Google Scholar]; This paper described about city-wide wastewater sampling modelling technique in the low sanitation areas among Africa, Asia and Atlanta.
- 29.Schang C., Crosbie N.D., Nolan M., Poon R., Wang M., Jex A., John N., Baker L., Scales P., Schmidt J., et al. Passive sampling of SARS-CoV-2 for wastewater surveillance. Environ Sci Technol. 2021;55:10432–10441. doi: 10.1021/acs.est.1c01530. [DOI] [PubMed] [Google Scholar]
- 30.Augustine R., Hasan A., Das S., Ahmed R., Mori Y., Notomi T., Kevadiya B.D., S. Thakor A. Loop-mediated isothermal amplification (LAMP): a rapid, sensitive, specific, and cost-effective point-of-care test for coronaviruses in the context of COVID-19 pandemic. Biology. 2020;9:182. doi: 10.3390/biology9080182. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article demonstrated losw cost point of care test for COVID-19 by using LAMP method.
- 31.Kaya D., Niemeier D., Ahmed W., Kjellerup B.V. Evaluation of multiple analytical methods for SARS-CoV-2 surveillance in wastewater samples. Sci Total Environ. 2022;808:152033. doi: 10.1016/j.scitotenv.2021.152033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anwar S., Nasrullah M., Hosen M.J. COVID-19 and Bangladesh: challenges and how to address them. Front Public Health. 2020;8:154. doi: 10.3389/fpubh.2020.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ahmed W., Simpson S.L., Bertsch P.M., Bibby K., Bivins A., Blackall L.L., Bofill-Mas S., Bosch A., Brandão J., Choi P.M., et al. Minimizing errors in RT-PCR detection and quantification of SARS-CoV-2 RNA for wastewater surveillance. Sci Total Environ. 2022;805:149877. doi: 10.1016/j.scitotenv.2021.149877. [DOI] [PMC free article] [PubMed] [Google Scholar]; This review paper drew the technical factors of minimizing laboratory methodological errors and ensuring quality control while conducting SARS-CoV-2 wastewater surveillance.
- 34.Kirby A.E. Using wastewater surveillance data to support the COVID-19 response — United States, 2020–2021. MMWR Morb Mortal Wkly Rep. 2021;70 doi: 10.15585/mmwr.mm7036a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Larsen DA., Wigginton KR. Tracking COVID-19 with wastewater. Nat Biotechnol. 2020;38:1151–1153. doi: 10.1038/s41587-020-0690-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.IEDCR https://iedcr.gov.bd/
- 37.Holshue M.L., DeBolt C., Lindquist S., Lofy K.H., Wiesman J., Bruce H., Spitters C., Ericson K., Wilkerson S., Tural A., et al. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382:929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.La Rosa G., Iaconelli M., Mancini P., Bonanno Ferraro G., Veneri C., Bonadonna L., Lucentini L., Suffredini E. First detection of SARS-CoV-2 in untreated wastewaters in Italy. Sci Total Environ. 2020;736:139652. doi: 10.1016/j.scitotenv.2020.139652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bar-Or I., Weil M., Indenbaum V., Bucris E., Bar-Ilan D., Elul M., Levi N., Aguvaev I., Cohen Z., Shirazi R., et al. Detection of SARS-CoV-2 variants by genomic analysis of wastewater samples in Israel. Sci Total Environ. 2021;789:148002. doi: 10.1016/j.scitotenv.2021.148002. [DOI] [PMC free article] [PubMed] [Google Scholar]