Abstract
Carbohydrate recognition is crucial for biological processes ranging from development to immune system function to host-pathogen interactions. The proteins that bind glycans are faced with a daunting task — to coax these hydrophilic species out of water and into a binding site. Here, we examine the forces underlying glycan recognition by proteins. Our previous bioinformatic study of glycan-binding sites indicated that the most over-represented side chains are electron-rich aromatic residues, including tyrosine and tryptophan. These findings point to the importance of CH-π interactions for glycan binding. Studies of CH-π interactions show a strong dependence on the presence of an electron-rich π-system, and the data indicate binding is enhanced by complementary electronic interactions between the electron-rich aromatic ring and the partial positive charge of the carbohydrate C-H protons. This electronic dependence means that carbohydrate residues with multiple aligned highly polarized C-H bonds, such as β-galactose, form strong CH-π interactions, whereas less polarized residues such as α-mannose do not. This information can guide the design of proteins to recognize sugars and the generation of ligands for proteins, small molecules, or catalysts that bind sugars
Graphical Abstract
INTRODUCTION
Glycans coat the surface of cells,1 where they play crucial roles in biology. Many of these roles require protein recognition.2–4 For example, glycan recognition is critical for sperm-egg binding5 and host defense. Pathogens also generate lectins (carbohydrate-binding proteins) that influence their infectivity and virulence.6, 7 Indeed, the most common glycan binders are lectins. Lectins are non-antibody proteins that recognize glycans, and they are found in all dtomains of life.8, 9Some are toxic, such as cholera toxin10 or those in rattlesnake venom.11 Others are essential for life, such as XEEL, a lectin that forms a protective gel around Xenopus laevis eggs,12 or the mammalian cation-independent mannose-6-phosphate receptor, which is critical for targeting proteins to the lysosomes.
Lectins have been known since the late nineteenth century. Stillmark described ricin in his 1888 doctoral thesis, which is the earliest traceable description of a lectin.13 Ricin’s toxicity and hemagglutination activity were apparent, but its carbohydrate-binding ability was not. The knowledge that lectin hemagglutination activity is due to their ability to bind glycans did not emerge until 1936, when Sumner and Howell showed that soluble polysaccharides could inhibit concanavalin A-mediated agglutination of red blood cells.14 Sixteen years later, Watkins and Morgan demonstrated the specificity of lectins for glycan recognition.15 Studies of mammalian lectins have a much shorter history. The first mammalian lectin was identified in 1974 – the asialoglycoprotein receptor, a human lectin that binds terminal β-galactose residues but not sialylated galactose residues.16, 17 Although the structure of concanavalin A was determined in 1972,18 the first mammalian lectin structure, that of the rat C-type lectin mannose-binding protein A, did not appear until 1991.19 Access to lectin structures, Kurt Drickamer’s insight into animal lectin classification,20 and the development of glycan arrays21, 22 all have propelled our ability to identify lectins, find their target ligands, and understand how they recognize their glycan targets.
Before recombinant lectins were readily available, glycan recognition studies generally focused on the abundant and readily isolable plant lectins.23 These proteins have proved to be valuable tools. For example, the mannose-binding lectin concanavalin A can be used to visualize the endoplasmic reticulum; glycoproteins in this compartment have not undergone processing to mask the mannose residue core intrinsic to processed N-glycosylation sites.24 In contrast, wheat germ agglutinin binds to the cell membrane because it can recognize the sialylated glycans on the cell surface. Mammalian lectins are typically not used as tools, but their study has revealed their critical roles in processes ranging from proteostasis to immune function.25, 26 Thus, lectins are critical for biology and our ability to probe biology. Understanding how lectins recognize their cognate glycans can advance both their utility and knowledge of their function.
Most protein-ligand interactions benefit from the hydrophobic effect, as water is more polar than ligand binding sites; therefore, desolvation can drive ligand binding. As ligands, hydroxyl group-rich glycans pose a challenge because they are hydrophilic. Proteins that recognize glycans need to compete with ligand-water interactions in the unbound state. As a result, they must co-opt other forces to facilitate binding (Figure 1).27 To understand these forces, researchers have analyzed individual lectin-glycan structures determined by X-ray crystallography and conducted studies where point mutations were made to assess the contribution of the residue in question.28, 29 These studies have uncovered distinct features of protein-lectin complexes.
Figure 1. Themes in lectin–carbohydrate recognition.
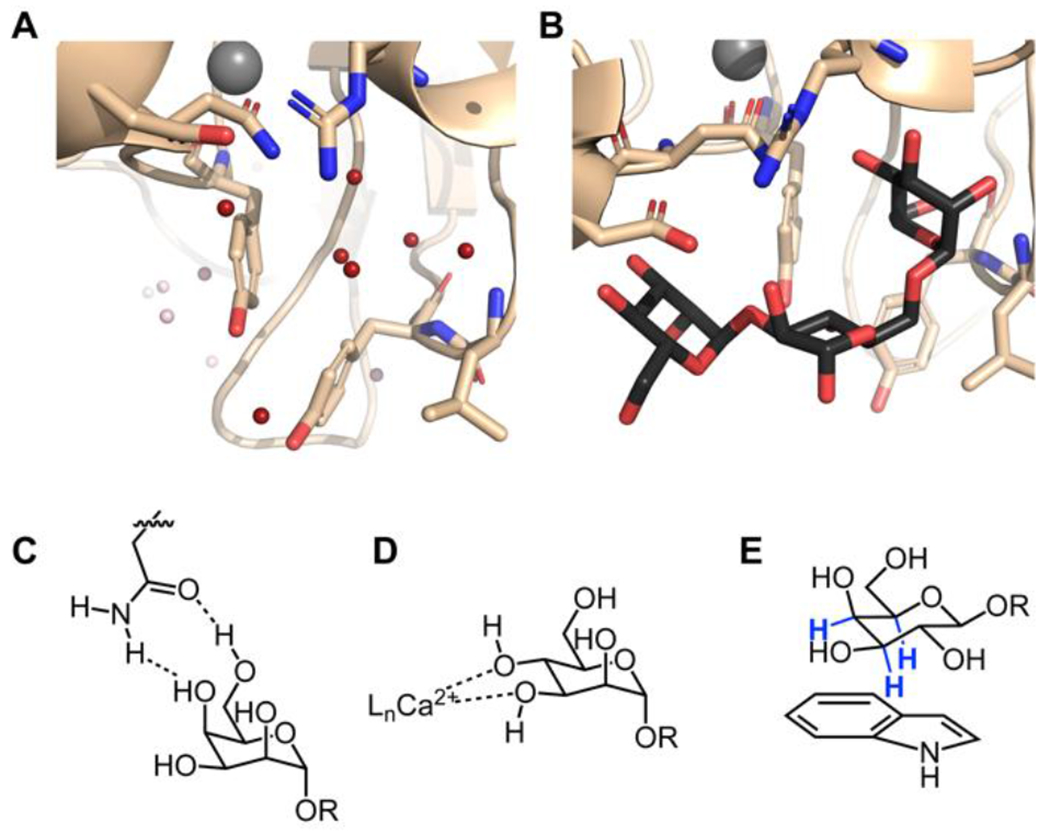
Structure of concanavalin A in the free (A) and bound (B) states. In view 1A, the water molecules displaced in the complex are highlighted in red (PDB code 1QNY).43 Concanavalin A bound to the core trimannoside is shown in B (PDB code 1CVN).34 C. Bifurcated hydrogen bonds orient the carbohydrate for binding. This binding can be entropically favorable because a single side-chain residue can displace two bound water molecules. D. Calcium ions coordinate hydroxyl groups and polarize the carbohydrate, enhancing the enthalpy of binding. This binding mode also displaces calcium ion-coordinated water molecules. C-type lectins and the intelectins use calcium coordination for ligand binding. E. CH-π interactions between the carbohydrate and aromatic residues in the protein are a crucial source of binding energy for highly polarized carbohydrates such as β-galactose. The C–H bonds highlighted in blue are those involved in the interaction.
Lectins generally have preorganized, solvent-accessible binding sites. Thus, protein–protein interaction and glycan-binding sites share some common attributes. When no glycan is bound, structured water molecules typically occupy positions that map onto the hydroxyl groups of the glycan ligand (Figure 1).30 As with all ligand binding, the release of these bound water molecules can contribute to the entropy of binding.31, 32 Analyses of lectin crystal structures have shown that, in some cases, such as the interaction of concanavalin-A with trimannose, this contribution can account for most of the binding energy.32–34 Concanavalin A’s affinity for the trimannoside is weaker than that of a typical protein-small molecule inhibitor complex but similar to a transient protein–protein interaction (Kd = 2 x 10−6 M).35 In most cases, however, water displacement from a protein binding site does not solely account for lectin binding.36
Many studies of lectin complexes have focused on hydrogen bonding because glycans are replete with hydroxyl groups. In isolation, hydrogen bond energies typically range from 3 to 7 kcal/mol. Because hydrogen bonding to glycan hydroxyl groups also occurs in water, there is no significant energetic gain upon protein binding. Still, failure to satisfy all hydrogen bonds of the carbohydrate can preclude binding.37 Thus, hydrogen bonding is critical for discriminating between carbohydrate ligands.38 Among hydrogen bonding amino acids, those with planar geometry and capable of bifurcated hydrogen bonds, such as aspartate and glutamine are favored, as are arginine and histidine, which can act as hydrogen bond donors and acceptors.39, 40 Meanwhile, those hydrogen bonding residues with flexible geometries, such as serine and lysine, are underrepresented, likely due to the entropic cost of fixing the rotamers of both the functional group and ligand.28 In many instances, side chains in carbohydrate-binding sites engage in bifurcated hydrogen bonds (Figure 1B). These interactions can constrain the ligand in a favorable conformation for binding, as in xylan carbohydrate-binding modules.41 Often, these interactions take the form of ambifunctional hydrogen bonding, where each group is both a hydrogen bond donor and a hydrogen bond acceptor. The strength of such interactions can approach, but not exceed, the additive value of the two individual hydrogen bonds.42
Another common feature in glycan-binding sites is calcium ions, which can coordinate to the vicinal hydroxyl groups of a sugar ligand (Figure 1C). The C-type lectins and intelectins exploit this binding mode.12, 26, 44–46 In these cases, the glycan can occupy two coordination sites on the calcium, which results in some release of water to the bulk solution. Protein side chains act to strengthen the ability of the glycan hydroxyl groups to chelate to the calcium ion. In lectins where calcium ions participate in binding the ligand, chelation of the ions by ethylenediaminetetraacetic acid abrogates carbohydrate-binding, demonstrating that the coordination of the calcium by hydroxyl groups is required. 46
As structures of protein–glycan complexes were determined by x-ray crystallography, researchers noted aromatic residues in glycan-binding sites. Specifically, structural studies of enzymes and periplasmic sugar-binding proteins revealed aromatic residues within the binding site.28, 50 For example, in the 1967 structure of hen egg white lysozyme,51 a tryptophan was found packing against a carbohydrate residue. Specifically, Trp62 is oriented over an N-acetylglucosamine residue.52 A tryptophan variant showed markedly decreased activity.53 Muraki et al. suggested that Trp63 engages in a stacking interaction with the GlcNAc residue.54 A key role for aromatic residues in sugar binding was also observed for bacterial periplasmic-binding proteins. The arabinose-binding protein has a tryptophan, Trp16, in the binding site that forms a close contact with arabinose. The structure shown shows C3, C4, and C5 in the vicinity of tryptophan, a favorable geometry for a CH-π interaction.55 Maltose binding protein has three tryptophan residues and a tyrosine residue in the binding site.56 The glucose/galactose-binding protein, meanwhile, features an aromatic sandwich involving both faces of β-D-glucose.57 A high resolution structure (0.92 Å) of this protein complexed to galactose suggested the bound conformation features a partial positive charge at the C-1 position.58 This feature should augment the contribution of CH-π interactions (vide infra). Similarly, the first structure of an antibody-glycan interaction also indicated many aromatic residues within the binding site.59
The structural studies above provided glimpses into protein – glycan recognition; however, determining which interactions are most prevalent requires statistical analyses. The growing collection of protein-glycan structures offers opportunities to assess protein-glycan interactions using bioinformatics,40, 47 a tool we deployed with the Woolfson group to determine which amino acid side chains were present in the binding sites (Figure 2).40, 47 Aromatic residues, particularly tyrosine and tryptophan, were dramatically enriched in carbohydrate-binding pockets..40 We detected only a modest increase in residues providing bifurcated hydrogen bonds. Non-aromatic, hydrophobic side chains were somewhat disfavored in glycan-binding sites. While aromatic groups can the facilitate binding of nonpolar ligands through the hydrophobic effect, the underrepresentation of nonpolar aliphatic residues in glycan binding sites indicates that another force is at work. 28, 40 In most earlier analyses of structures from x-ray crystallography, these aromatic residues are annotated as simply being in Van der Waals contact with the bound glycan. 48 Still, it was noted that they exhibit a high degree of evolutionary conservation.49 This high prevalence and conservation of aromatic residues in binding sites, combined with the depletion of aliphatic residues in similar positions, suggests that aromatic residues have properties beyond hydrophobicity that facilitate glycan recognition.
Figure 2. Aromatic residues are enriched in carbohydrate binding sites.
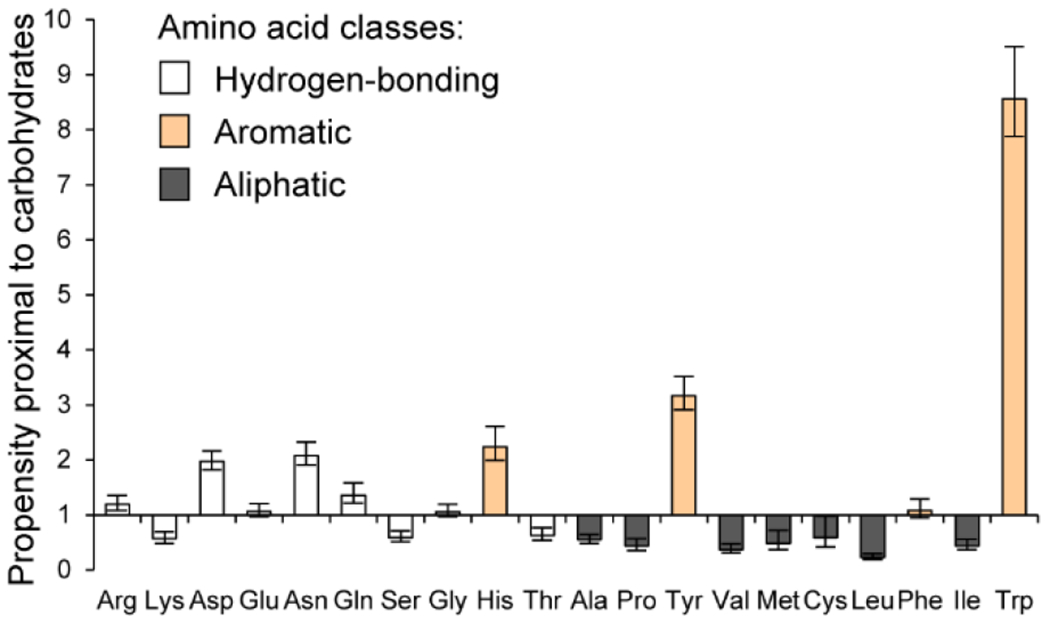
The horizontal axis is ordered with increasing hydrophobicity to the right,71 while the vertical axis shows the ratio between the occurrence of a given amino acid within 4 Å of a noncovalently bound glycan compared to the frequency in the UniProt database. Reproduced from Hudson, K. L.; Bartlett, G. J.; Diehl, R. C.; Agirre, J.; Gallagher, T.; Kiessling, L. L.; Woolfson, D. N., Carbohydrate-Aromatic Interactions in Proteins. J. Am. Chem. Soc. 2015, 137, 15152-15160. Copyright 2015 American Chemical Society.
The data from the bioinformatic study unites two distinct lines of research. First, it aligns with general information regarding the importance of CH-π interactions in glycan recognition.60, 61 Second, the preferences for aromatic residues detected in our bioinformatic analysis are reminiscent of those observed for lysine and arginine cation-π interactions.62 As with proteins recognizing their ligands through cation-π interactions, tryptophan residues are most enriched in lectin binding sites. Cation-π interactions have an electrostatic component in that the aromatic quadrupole can engage with the electrophilic cation.63 Similarly, C-H bonds can interact favorably with an aromatic quadrupole—especially that of tryptophan. Given that C-H bonds are preferentially localized over the aromatic residues in lectins, glycan stereochemistry should play a profound role.
Stereochemistry is not an issue in the cation-π interaction studied, but for CH-π interactions, especially those involved in glycan recognition, glycan stereochemistry could be crucial. Both bioinformatics and NMR experiments suggest glycan stereochemistry influences CH-π interactions. Many previous studies had focused on the recognition of glucose or N-acetylglucosamine.64, 65 Enzymes acting on their substrates may use CH-π interactions to facilitate catalysis, as these interactions would be enhanced in a transition state involving cationic character. With lectins, however, CH-π interactions of glucose or N-acetylglucosamine are not as energetically favorable as those found in saccharide complexes. Specifically, the data indicate that the monosaccharides that benefit most are β-mannosides and β-galactosides. Thus, the magnitude of CH-π interactions strongly depends on the stereochemistry of the glycan.
Although bioinformatic analysis of lectin-glycan interactions provided insight into glycan recognition, the analysis of glycosylated proteins (i.e., intramolecular interactions) failed to yield a statistically significant amino acid side chain enrichment near the glycan. Still, the Kelly Group has shown that aromatic groups in the N-glycosylation sequon can influence the efficiency of glycosylation.25, 65, 66 This review, however, focuses on data and experiments relevant to non-covalent glycan-protein interactions.
Here, we examine how CH-π interactions contribute to glycan recognition by lectins. First, we detail the factors that allow some aromatic groups to make stronger CH-π interactions than others. Second, we discuss analyses of CH-π interactions in the gas phase and solution, focusing on the balance between dispersive forces and electronics in powering the interaction. Third, we investigate trends among carbohydrates, describing the relative propensities of monosaccharides found in mammalian glycans to engage in CH-π interactions and the features of each glycan that lead to stronger or weaker interactions. Finally, we discuss the implications of these trends in enzymatic and chemical reactions that modify carbohydrates.
ELECTRON-RICH AROMATIC GROUPS IN CH-π INTERACTIONS
CH-π interactions in glycan recognition involve donation from an aromatic-π system to C–H σ* orbitals. In principle, four canonical aromatic amino acid side chains could contribute: phenylalanine, histidine, tyrosine, and tryptophan. 66 Of these, tryptophan is the most overrepresented in carbohydrate-binding sites, followed by tyrosine. In contrast, there is no significant enrichment of phenylalanine (Figure 2).40 Histidine is well represented; however, this residue more often engages in bifurcated hydrogen bonds than in CH-π interactions with aliphatic carbohydrate protons. 40, 47 Of the other two aromatic amino acids, both have electron-rich π electron clouds. While tryptophan possesses an aromatic system nearly twice as large as tyrosine, it is more than twice as prevalent in carbohydrate-binding sites as tyrosine. These data indicate that a difference in chemical properties beyond mere size explains the greater representation of tryptophan. 40
Several groups have examined small molecule model systems to assess the roles and contributions of different aromatic groups to CH-π interactions. One such system is the hairpin peptide, in which the propensity of a serine-substituted O-glycan to stack over an aromatic group elsewhere on the peptide is determined.64 Both canonical and non-canonical aromatic amino acids were included to assess which aromatic groups made the most robust intramolecular CH-π interactions with acetyl- or methyl-protected β-glucose or β-glucosamine residues.67 In this system, conformations consistent with CH-π interactions were only detected with protected sugars. The data indicate that tryptophan led to the most productive interactions compared to phenylalanine and cyclohexylalanine, but the basis for these interactions was difficult to ascertain, given that the glycans were protected.
Another method to compare the strengths of CH-π interactions involves dynamic combinatorial chemistry. Jimenez-Moreno et al. tested the likelihood of imine formation using a library of amine-containing disaccharides and aromatic aldehydes.68 Glycan stereochemistry had an impact, and adducts with extended aromatic systems were preferentially generated.69 However, the interpretation of these data is complicated because adduct geometry is constrained and the imine linker can protonate and may influence stacking. Despite the caveats in interpreting the results, the model systems show that large and electron-rich aromatic groups such as the indole group of tryptophan, make stronger CH-π interactions. In contrast, small, electron-poor groups such as the imidazole functional group of histidine make weak CH-π interactions.
A strategy for interrogating trends among CH-π donors and acceptors in solution is to use NMR spectroscopy. Vandenbussche et al. showed that 1H NMR could reveal an anisotropic shift in signals caused by the proximity of aromatic rings to carbohydrate protons in CH-π interactions.70 This assay allows the use of a monosaccharide and an aromatic compound in aqueous solution,70 isolating the CH-π interaction while accounting for solvent effects. Hudson et al. used this system to investigate the role of electronics. Using indoles with a range of electron-donating and electron-withdrawing groups, the authors demonstrated that an electron-rich π system promotes this interaction (Figure 3).40 These findings indicate that optimizing electronic interactions between the partially positive aliphatic hydrogens on the carbohydrate and the partially negative face of the aromatic can promote CH-π interactions in water. This assay also revealed differences in the stacking ability of different glycans (vide infra).
Figure 3. Linear free energy relationship analyzing chemical shift perturbation of β-galactoside protons in the presence of 5-substituted indoles.
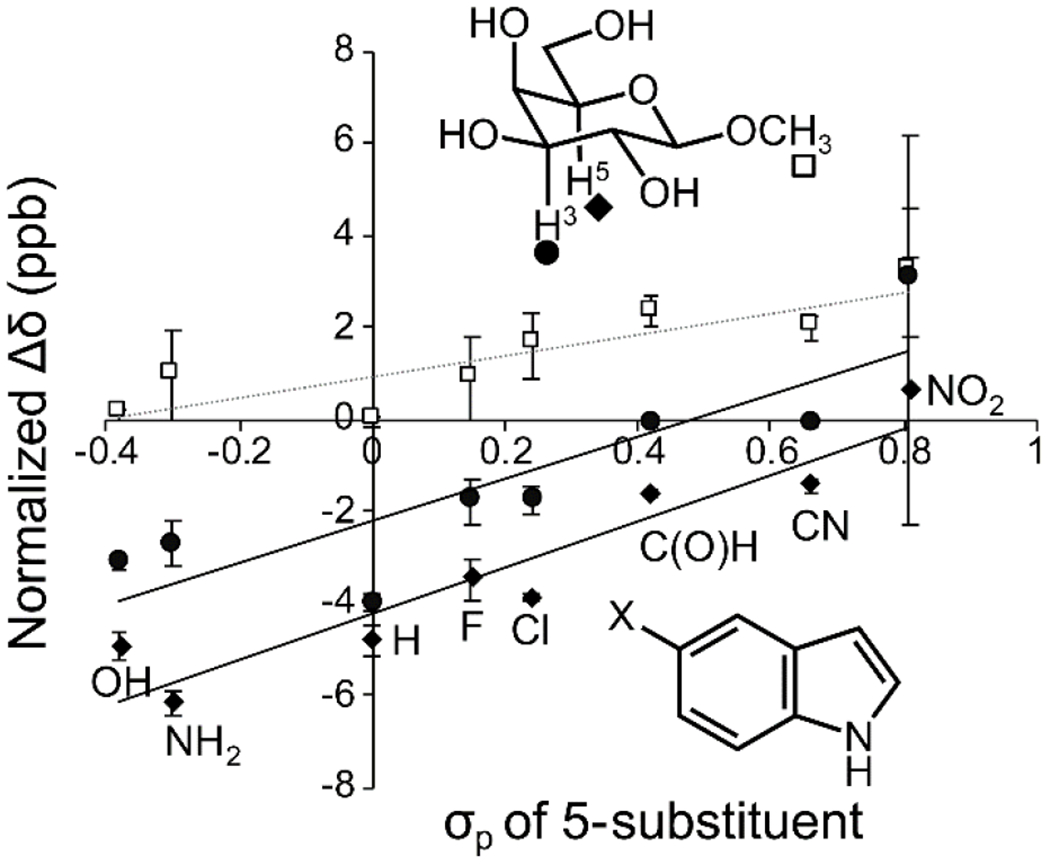
Indoles substituted with electron-donating groups have little effect on the methyl proteins (square) but induce higher upfield shifts of the interacting H3 (circle) and H5 (diamond) protons. The shifts are normalized to 10 mM indole using an observed linear relationship between concentration and chemical shift perturbation. Reproduced from Hudson, K. L.; Bartlett, G. J.; Diehl, R. C.; Agirre, J.; Gallagher, T.; Kiessling, L. L.; Woolfson, D. N., Carbohydrate-Aromatic Interactions in Proteins. J. Am. Chem. Soc. 2015, 137, 15152-15160. Copyright 2015 American Chemical Society.
One unresolved question is the difference between tyrosine and phenylalanine in glycan-binding sites. Phenylalanine is present at a similar proportion as in the UniProt database as a whole, but tyrosine is overrepresented.40 This finding is surprising because assessments of CH-π interaction strength outside of proteins suggest little difference between benzene and phenol. The minor discrepancy observed is far too small to explain the disparity in glycan-binding sites. The greater hydrophilicity of tyrosine could contribute to its increased presence in solvent-exposed carbohydrate-binding sites;70 however, we postulate that the nature of the residue may be beneficial for CH-π interactions. For example, the ability of the tyrosine phenol group to engage in hydrogen bonding could preorganize the system by orienting the aromatic ring in a favorable geometric arrangement for stacking with the glycan. Additionally, hydrogen bonding within the active site could augment the electron density of the tyrosine π-system, strengthening the electronics of a CH-π interaction beyond that provided by a phenol group. Elucidating the factors responsible for the distinct preference for tyrosine will be valuable for understanding and predicting protein–glycan interactions.
ELECTRONIC NATURE OF CH-π INTERACTIONS
The forces underlying CH-π interactions have been controversial. In the 1980s, aliphatic and aromatic CH protons were proposed to act as hydrogen bond donors to oxygen or nitrogen-based hydrogen bond acceptors.72 In keeping with this analogy, the CH bond serves as a donor and the π system as the acceptor. We note, however, that the interaction involves C-H anti-bonds accepting electron density from the aromatic π system.
A key question regarding CH-π interactions is which intermolecular forces are most important in determining binding affinity. Hydrophobicity was the force initially credited with mediating carbohydrate-aromatic interactions; however, the features of the aromatic residue and the enthalpic nature of the interaction both indicate that the hydrophobic effect is not the dominant force.28, 40 The other two forces commonly cited are dispersion and electronics.60, 65, 73 Dispersive interactions involve attraction between oscillating partial charges on the C–H proton and the face of the aromatic ring; these effects are amplified by the delocalization and polarizability of the π-system. These properties are relatively insensitive to the ground-state partial charges of the interaction partners. By contrast, electronic interactions are sensitive to charge density. In the case of CH-π interactions, the relevant contributors would be the electron density of the aromatic quadrupole and the partial positive charge of interacting CH protons. Earlier ab initio calculations generally attribute the majority of the interaction energy to dispersive forces.74 However, methane CH bonds interact weakly with an aromatic π system such as benzene.75, 76 Partial substitution of methane protons with chlorine and fluorine, by contrast, results in a considerably stronger interaction between the remaining hydrogens and the benzene π system,76–79 suggesting that the interaction is more favorable when more electron-poor C–H bonds are involved.80
Most quantum mechanical calculations of carbohydrate-aromatic CH-π interactions are conducted on systems in the gas phase with simplified components (e.g., methane and benzene). In these studies, the choice of the method is vital; the ab initio MP2 method accounts for the large dispersion terms involved in the interaction, as do dispersion-corrected density functional theory (DFT) methods such as M06-2X.81 Simpler DFT methods such as B3LYP fail to account for large portions of the interaction energy.72 Quantum mechanical calculations of complexes between monosaccharides and simple aromatics, such as fucose and benzene, have suggested that the interaction energy is around 3-7 kcal/mol with large electronic and dispersive components.82, 83 These studies, however, did not predict the relative propensities of the different saccharides to engage in CH-π interactions in solution. Other computational studies suggest that the indole group of tryptophan should be a better CH-π acceptor than phenylalanine due to its greater π-electron density.73, 84 Gas-phase calculations, however, tend to favor OH-π interactions that cannot compete with canonical hydrogen bonds in water. 82, 83 Similarly, the NH bonds of GlcNAc, GalNAc, and ManNAc preferentially form canonical hydrogen-bonds rather than CH-π interactions.
The CH-π interaction also has been explained in terms of hard/soft acid-base theory, where it has been described as a weak hydrogen bond between a soft acid (the CH group) and a soft base (the π system).60 Lisbjerg et al. have suggested that CH bonds can act as soft acids based upon the preference of a biotin-derived synthetic anionophore for softer chloride over harder oxyanions.85
Still, most C-H bonds are not highly polarizable (i.e., they are not very good soft acids); those with increased polarization should engage in more favorable CH-π interactions. Thus, both electronic forces and the hydrophobic effect can promote these interactions in water. 68 Another feature of these interaction is that they are solvent-dependent86, 87 and often cooperative.88, 89 For nonpolar CH groups89, 90 and weakly polarized carbohydrates such as β-GlcNAc,65 the dispersive component dominates. Interestingly, these glycan residues do not frequently stack on aromatic residues in protein-glycan complexes. In contrast, strongly polarized carbohydrates such as β-Gal are more likely to be proximal to an aromatic group;40 in these cases, the electronic component tends to be large. While the dispersive contribution varies little between different saccharides and aromatic groups,91 the electronic component changes. Thus, the latter is far more influential in determining the relative strengths of CH-π interactions.40
CARBOHYDRATES WITH POLARIZED C–H BONDS FORM FAVORABLE CH-π INTERACTIONS
The importance of the partial negative charge on the face of an electron-rich π system suggests that monosaccharide protons that present a partial positive charge should be excellent CH-π donors. Although the field lacks a quantitative measurement of CH-π interactions in a protein, several lines of evidence support the importance of electron deficient CH bonds. First, some monosaccharides are found stacking over aromatic residues far more often than others. Specifically, β-galactose (β-Gal), β-N-acetylgalactosamine (β-GalNAc), and β-mannose (β-Man) are strong CH-π donors. In the case of β-galactose, an aromatic residue is nearly always present. The interaction occurs with the aliphatic protons of the α-face, either the H1, H3, and H5 protons or the H3, H4, and H5 protons (Figure 4).40 Another favorable residue for CH-π interactions is β-mannose. Like β-galactose, it presents an arrangement of electropositive C-H bonds on a single face that can engage with an aromatic residue. An example is the complex of Oscillatoria agardhii agglutinin with oligomannose N-glycans.92 Data from 1H NMR experiments also support the strength of β-galactose and β-mannose as CH-π donors; both complex strongly with benzene and indole in solution.40, 70, 93 Similarly, aromatic residues are prevalent in β-N-acetylgalactosamine (GalNAc) complexes.94 Thus, β-Gal, β-GalNAc, and β-Man are the strongest CH-π donors among mammalian glycans. They share two features: all present a continuous patch of four aliphatic hydrogens allowing for two geometries where three CH bonds interact with the π system, and all bear a hydroxyl group antiperiplanar to two of those aliphatic hydrogens. In this arrangement, the CH bonds are polarized by overlap with the CO antibonding orbital, strengthening the partial positive charge of the interacting proton and thereby enhancing the CH-π interaction (Figure 5). 95, 96
Figure 4. CH-π stacking in solution reflects patterns found in carbohydrate binding sites.
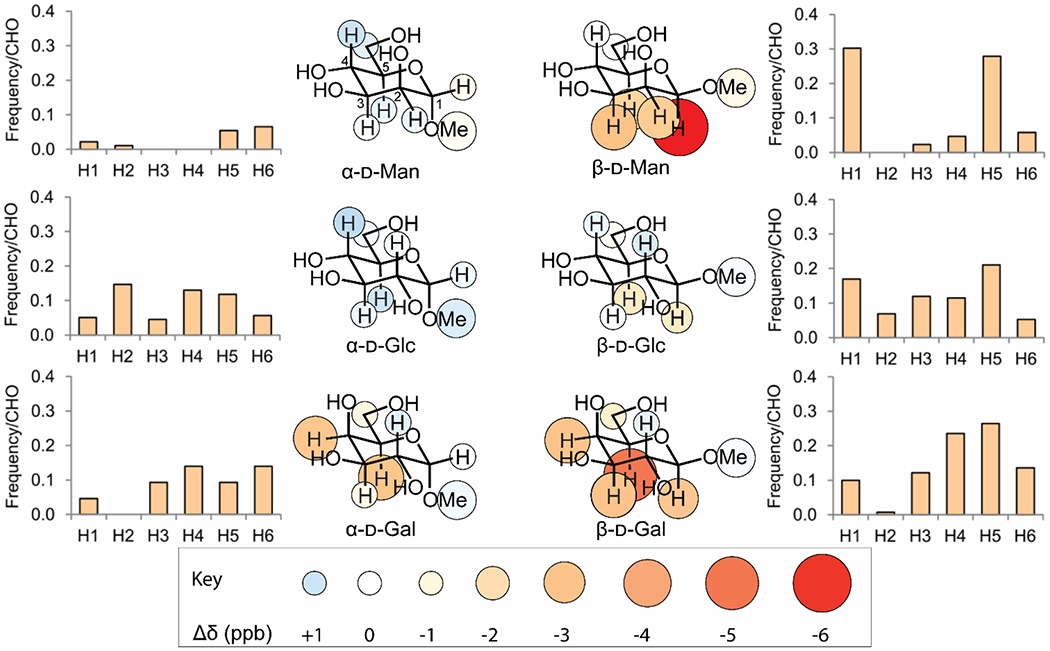
Inner panels show the change in chemical shift for each proton when 10 mM indole is added to a 0.5 mM solution of the indicated glycoside. Larger and darker red circles indicate stronger shielding from CH-π stacking. Outer panels show the probability of a binding site for the indicated monosaccharide having an aromatic group positioned closest to the indicated carbon. Data reproduced from Hudson, K. L.; Bartlett, G. J.; Diehl, R. C.; Agirre, J.; Gallagher, T.; Kiessling, L. L.; Woolfson, D. N., Carbohydrate-Aromatic Interactions in Proteins. J. Am. Chem. Soc. 2015, 137, 15152-15160. Copyright 2015 American Chemical Society. Figure adapted from Diehl 2021.95
Figure 5. The CH3 bond of β-galactose is polarized by overlap with the antiperiplanar CO4 hydroxyl group.
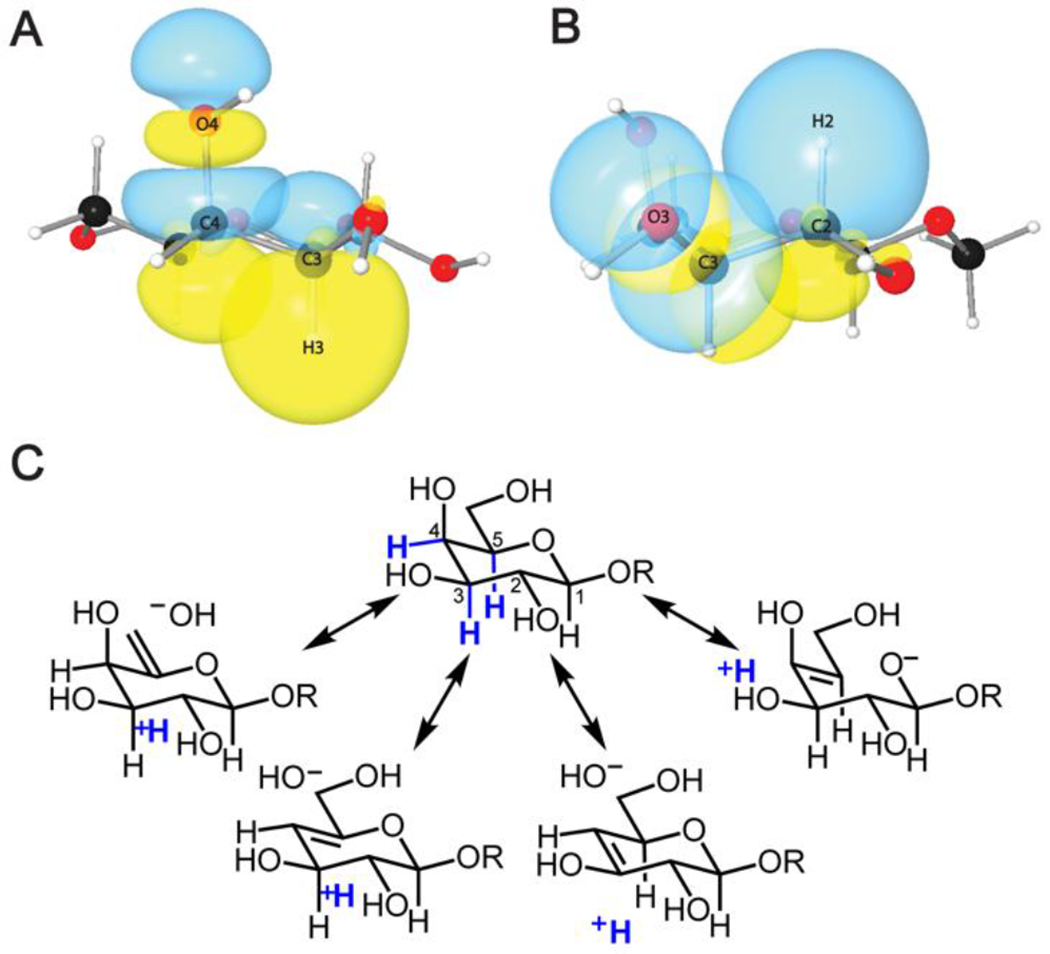
3D natural bond orbital (NBO) diagrams are depicted in panels A and B. A. Overlap between C–H3 bonding orbital and C–O4 antibonding orbital of β-D-galactose. B. Non-overlap between the C–H2 bonding orbital and C–O3 antibonding orbital of β-D-galactose. Notably, these orbitals are antiperiplanar in A and gauche in B.95 C. Resonance structures depicting polarization of the relevant C-H bonds rendering some protons with more electropositive (blue) depending on hydroxyl group stereochemistry.
Several other monosaccharides engage in CH-π interactions in biological systems. Due to their orbital overlap and ability to achieve the H3-H4-H5 stacking geometry that β-galactose prefers, α-galactose and α-fucose appear to make CH-π interactions that are only slightly weaker than those of β-galactose.93 However, these data are from studies that were conducted with protected glycans using benzene. In cyclohexane solution, the permethyl α-galactoside-benzene complex is strong enough to resolve the glycoside from the poor CH-π donor α-mannoside, which is impossible in the absence of benzene.97 Because the aromatic π cloud is electron-rich, CH-π interactions rarely involve anionic carbohydrate residues such as sialic acid.40 As sugars capable of presenting a trivalent interaction, β-glucose is capable of CH-π interactions under especially favorable conditions as in cellulases, where the highly ordered β-glucosides can be sandwiched by tyrosine and tryptophan residues to separate them from water or other polysaccharide chains.98 A similar situation for β-N-acetylglucosamine (β-GlcNAc) is present in hevein, where two tryptophan residues engage in CH-π interactions with adjacent β-N-acetylglucosamine residues.99 Even in this case, the CH-π bonds provide a roughly equivalent amount of binding energy as the OH-O hydrogen bonds; they are not strong enough to account for a majority of the binding energy.100 The C6 methyl group of fucose and the acetyl group of β-N-acetylglucosamine are not strong CH-π donors, a finding that agrees with the preference for polarized CH bonds (Figure 6). Thus, N-glycosylation may be enhanced by CH-π interactions,65, 66 but to gain the full benefit, three CH bonds must interact with the π-system.101 This importance of geometry is highlighted by Kobayashi et al. in their purification of glucose-based disaccharides on fullerene-based columns. The dipole moment of the disaccharide had a dramatic influence on retention times, highlighting the electronic nature of CH-π interactions. 102
Figure 6. CH-π interactions involving fucose and N-acetylglucosamine resemble those involving galactose and glucose respectively.
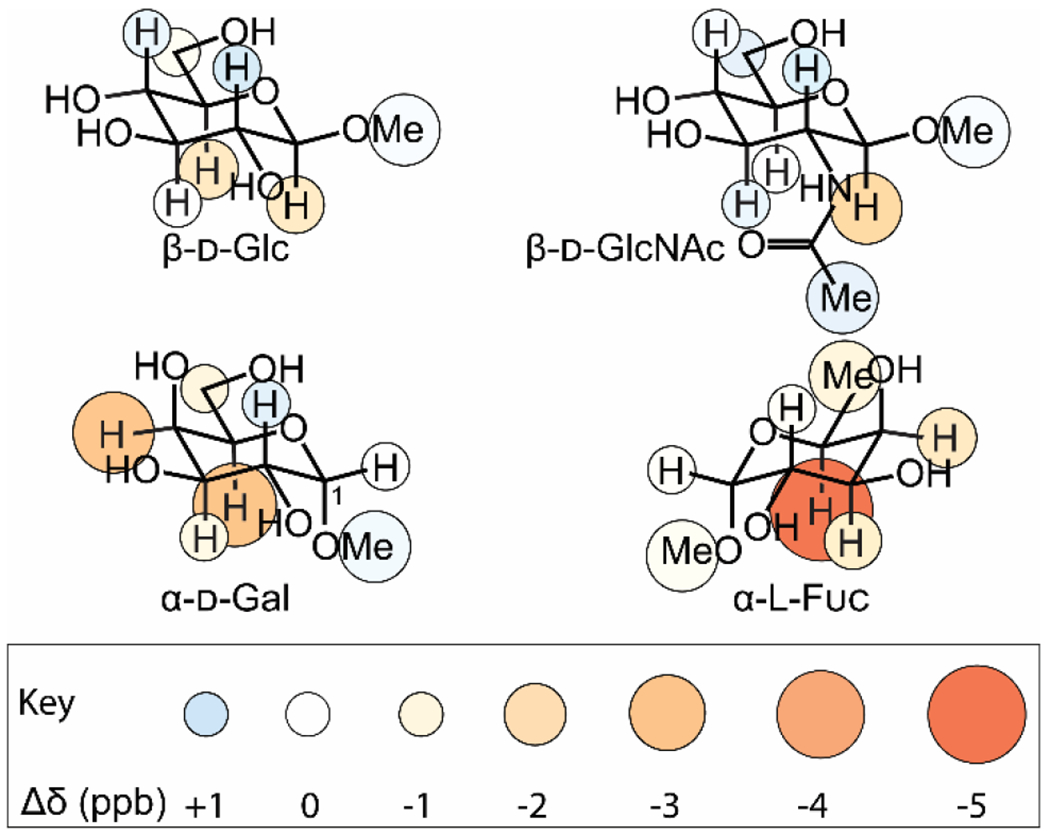
As in Figure 4, circles show change in chemical shift upon addition of 10 mM indole, and larger and redder circles indicate stronger shielding and therefore higher propensity to stack on the aromatic ring. 95
Monosaccharides unable to place a continuous patch of three electropositive protons in contact with an aromatic ring, such as α-mannose or α-glucose, are very poor CH-π donors.40, 93, 97 Likewise, many furanosides appear to be weak CH-π donors due to their flexibility and poor geometric complementarity with aromatic groups.61 Still, there are exceptions, as illustrated by the complex of human intelectin-1 with galactofuranose, in which an aromatic box is critical for selective glycan recognition.46, 103 Those saccharides that do not derive sufficient benefit from CH-π interactions use other means of recognition and binding, such as bifurcated hydrogen bonds and calcium coordination.103 CH-π interactions are a prominent and crucial feature of protein-carbohydrate interactions, but they are not universal. Moreover, as described above, some CH-π interactions are far stronger than others.
In complexes driven by CH-π interactions, the monosaccharide residue and aromatic group must adopt relevant geometry. While CH-π interactions have less strict orientation requirements than hydrogen bonds,60, 61 there is a preference for aligning multiple CH bonds with a given π-system. In most biologically relevant glycan interactions, three CH protons on the same monosaccharide are positioned over an aromatic ring. Neither α-glucose nor α-mannose can present three CH protons on the same face; consequently, they are rarely found in CH-π interactions. Because the aromatic π cloud is electron-rich, CH-π interactions are not typically found in complexes with anionic carbohydrate residues such as sialic acid. 40
FAVORABLE CH-π INTERACTIONS INFLUENCE GLYCAN REACTIVITY
Although the findings in this review primarily derive from studies of lectins, they also have implications for catalytic processes. The reactions catalyzed by glycosyltransferases and glycosyl hydrolases proceed through transition states stabilized by CH-π interactions. For example, glycosylation reactions proceed through a transition state where the anomeric carbon is cationic or has cationic character. Often, a cation-π interaction occurs in the transition state that increases the catalytic rate.69 This cation-π interaction involves stronger CH-π interactions, which are present in the starting enzyme-substrate complex and increase in strength at the transition state. Thus, they not only stabilize the intermediate but also aid in substrate binding. In glycosyl hydrolases, CH-π interactions can position the substrate for hydrolysis and similarly stabilize the intermediate. For example, cellulases sandwich β-glucose residues between two aromatic residues to hold the polysaccharide in place for cleavage.98 CH-π interactions also play critical roles in many carbohydrate-binding modules, which have non-catalytic domains that tether the catalytic domain to a polysaccharide substrate, increasing its local concentration and enhancing the hydrolytic rate.
Chemical reactions can also exploit CH-π interactions to enhance reactivity or selectivity. For example, an aromatic group was used to stabilize the transition state in the aminolysis of acetyl β-galactoside.104 In an elegant use of CH-π interactions in catalysis, the Jacobsen group carried out rhamnosylations and mannosylations of a broad range of substrates to achieve high β-selectivity. Aromatic residues were appended to a thiourea group that could engage in CH-π interactions in the glycosidic bond-forming transition state (Figure 7).105 These findings highlight how CH-π interactions can be exploited in chemical catalysis.
Figure 7. Stabilization of β-mannosylation product through a CH-π interaction.
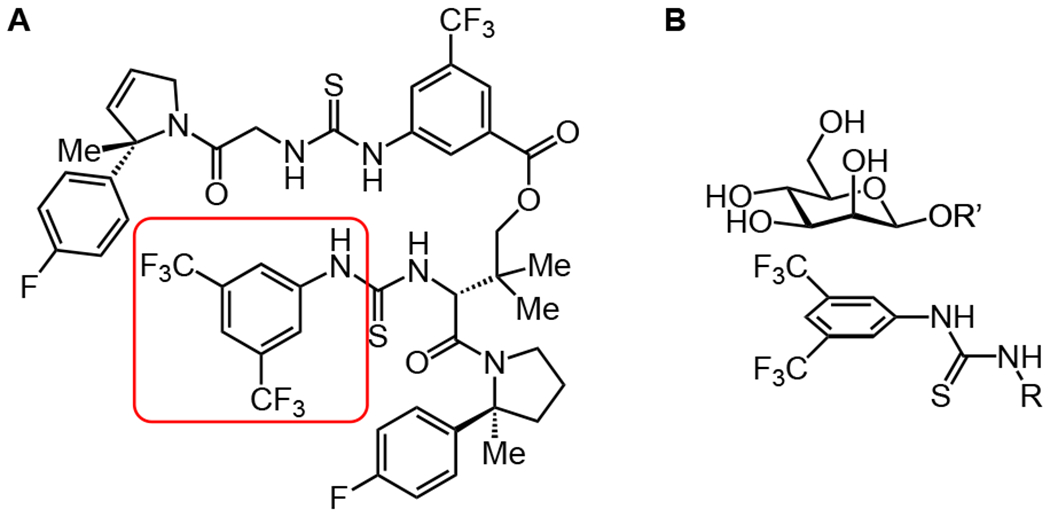
A. Structure of the bis-thiourea catalyst developed by Jacobsen Group for specific β-mannosylations and β-rhamnosylations, with the portion of the molecule that could interact with the α-face of mannose circled in red. B. Potential stacking interaction of aryl group on catalyst with the strongly CH-π donating α-face of the β-mannoside product. This interaction does not occur in the α-linked starting material. Adapted from Li et al. 2020.105
CONCLUSION
To predict, probe, and exploit CH-π interactions, it is crucial to understand what factors determine their strength. While computational studies show a large dispersive term in the energy of CH-π interactions in the gas phase, in solution, the electronic term is large and variable. The most favorable CH-π interactions for carbohydrate binding, involve those in which an electropositive CH-bond interacts with an electron-rich aromatic ring. Thus, factors that enhance the electopositivity of a ligand can be used to stabilize the complex. Similarly, enhancing the electron density of the aromatic ring will also yield a more stable complex. Such information can be used in inhibitor design or to engineer and generate glycan-binding antibodies, synthetic receptors, or proteins.
ACKNOWLEDGMENT
Our research in this area is supported by the National Institute of Allergy and Infectious Diseases under grant number R01 AI055258 (LLK) and the National Cancer Institute U01CA231079 (LLK).
Glossary
- CH-π interaction
an attractive interaction between a C-H antibonding orbital and a π orbital
- carbohydrates
monosaccharides or larger molecules consisting of monosaccharide residues
- glycans
biomolecules consisting of a large number of monosaccharide residues or any number of such residues attached to a lipid, protein, or other large biomolecule
- aromatic compounds
a compound where π electrons are delocalized around the entirety of a ring
- lectins
non-antibody proteins that recognizes glycans
- hydrogen bonding
a noncovalent bond between a lone pair or π system and a hydrogen atom bonded to a third atom
- dispersion forces
interactions between mutually induced dipoles
- orbital overlap
a situation where two orbitals are sufficiently present in the same area for electrons to be donated between them
Footnotes
The authors declare no competing financial interest.
REFERENCES
- 1.Varki A, Evolutionary forces shaping the Golgi glycosylation machinery: Why cell surface glycans are universal to living cells. Cold Spring Harb. Perspect. Biol 2011, 3 (6), 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhuo L; Kanamori A; Kannagi R; Itano N; Wu J; Hamaguchi M; Ishiguro N; Kimata K, SHAP potentiates the CD44-mediated leukocyte adhesion to the hyaluronan substratum. J. Biol. Chem 2006, 281 (29), 20303–20314. [DOI] [PubMed] [Google Scholar]
- 3.Pipirou Z; Powlesland AS; Steffen I; Pöhlmann S; Taylor ME; Drickamer K, Mouse LSECtin as a model for a human Ebola virus receptor. Glycobiology 2011, 21 (6), 806–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang SF; Tsao CH; Lin YT; Hsu DK; Chiang ML; Lo CH; Chien FC; Chen P; Arthur Chen YM; Chen HY; Liu FT, Galectin-3 promotes HIV-1 budding via association with Alix and Gag p6. Glycobiology 2014, 24 (11), 1022–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pang P.-c.; Chiu PCN; Lee C.-l.; Chang L.-y.; Panico M; Morris HR; Haslam SM; Khoo K.-h.; Clark GF; Yeung WSB; Dell A, Human Sperm Binding Is Mediated by the Sialyl-Lewis x Oligosaccharide on the Zona Pellucida. Science 2011, 333 (September), 1761–1765. [DOI] [PubMed] [Google Scholar]
- 6.Branderhorst HM; Liskamp RMJ; Visser M; Pieters RJ, Strong inhibition of cholera toxin binding by galactose dendrimers. Chem. Commun 2007, (i), 5043–5045. [DOI] [PubMed] [Google Scholar]
- 7.Lerrer B; Lesman-Movshovich E; Gilboa-Garber N, Blocking of Pseudomonas aeruginosa lectins by human milk glycans. Can. J. Microbiol 2003, 49 (2), 230–235. [DOI] [PubMed] [Google Scholar]
- 8.Lis H; Sharon N, Lectins: Carbohydrate-specific proteins that mediate cellular recognition. Chem. Rev 1998, 98 (2), 637–674. [DOI] [PubMed] [Google Scholar]
- 9.Tsaneva M; Van Damme EJM, 130 years of Plant Lectin Research. Glycoconj. J 2020, 37 (5), 533–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.MacKenzie CR; Hirama T; Lee KK, Quantitative Analysis of Bacterial Toxin Affinity and Specificity for Glycolipid Receptors by Surface Plasmon Resonance. J. Biol. Chem 1997, 272 (9), 5533–5538. [DOI] [PubMed] [Google Scholar]
- 11.Polgár J; Clemetson JM; Kehrel BE; Wiedemann M; Magnenat EM; Wells TNC; Clemetson KJ, Platelet activation and signal transduction by convulxin, a C-type lectin from Crotalus durissus terrificus (Tropical rattlesnake) venom via the p62/GPVI collagen receptor. J. Biol. Chem 1997, 272 (21), 13576–13583. [DOI] [PubMed] [Google Scholar]
- 12.Wangkanont K; Wesener DA; Vidani JA; Kiessling LL; Forest KT, Structures of Xenopus embryonic epidermal lectin reveal a conserved mechanism of microbial glycan recognition. J. Biol. Chem 2016, 291 (11), 5596–5610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharon N; Lis H, History of lectins : from hemagglutinins to biological recognition molecules. Glycobiology 2004, 14 (11), 53–62. [DOI] [PubMed] [Google Scholar]
- 14.Sumner JB; Howell SF, Identification of Hemagglutinin of Jack Bean with Concanavalin A. J. Bacteriol 1936, 32 (2), 227–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Watkins WM; Morgan WTJ, Neutralization of the Anti-H Agglutinin in Eel Serum by Simple Sugars. Nature 1952, 169, 825–825. [DOI] [PubMed] [Google Scholar]
- 16.Taylor ME; Drickamer K; Schnaar RL; Etzler ME; Varki A, Discovery and Classification of Glycan-Binding Proteins. 3rd ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, 2017. [Google Scholar]
- 17.Ashwell G; Harford J, Carbohydrate-specific receptors of the liver. Annu. Rev. Biochem 1982, 51, 531–531. [DOI] [PubMed] [Google Scholar]
- 18.Edelman GM; Cunningham BA; Reeke GN; Becker JW; Waxdal MJ; Wang JL, The covalent and three-dimensional structure of concanavalin A. Proc. Natl. Acad. Sci. U. S. A 1972, 69 (9), 2580–2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weis WI; Crichlow GV; Murthy HMK; Hendrickson WA; Drickamer K, Physical characterization and crystallization of the carbohydrate-recognition domain of a mannose-binding protein from rat. J. Biol. Chem 1991, 266 (31), 20678–20686. [PubMed] [Google Scholar]
- 20.Drickamer K, Two distinct classes of carbohydrate-recognition domains in animal lectins. J. Biol. Chem 1988, 263 (20), 9557–9560. [PubMed] [Google Scholar]
- 21.Rillahan CD; Paulson JC, Glycan microarrays for decoding the glycome. Annu. Rev. Biochem 2011, 80, 797–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park S; Gildersleeve JC; Blixt O; Shin I, Carbohydrate microarrays. Chem. Soc. Rev 2013, 42 (10), 4310–4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Showalter AM, Structure and Function of Plant Cell Wall Proteins. The Plant Cell 1993, 5 (1), 9–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bray M.-a.; Singh S; Han H; Davis CT; Borgeson B; Hartland C; Kost-alimova M; Gustafsdottir SM; Gibson CC; Carpenter AE, Cell Painting, a high-content image-based assay for morphological profiling using multiplexed fluorescent dyes. Nat. Protoc 2016, 11 (9), 1757–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hebert DN; Lamriben L; Powers ET; Kelly JW, The intrinsic and extrinsic effects of N-linked glycans on glycoproteostasis. Nat. Chem. Biol 2014, 10 (11), 902–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee RT; Hsu TL; Huang SK; Hsieh SL; Wong CH; Lee YC, Survey of immune-related, mannose/fucose-binding C-type lectin receptors reveals widely divergent sugar-binding specificities. Glycobiology 2011, 21 (4), 512–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jayaraman N, Multivalent ligand presentation as a central concept to study intricate carbohydrate-protein interactions. Chem. Soc. Rev 2009, 38 (12), 3463–3483. [DOI] [PubMed] [Google Scholar]
- 28.Weis WI; Drickamer K, Structural Basis of Lectin-Carbohydrate Recognition. Annu. Rev. Biochem 1996, 65, 441–473. [DOI] [PubMed] [Google Scholar]
- 29.Cumpstey I; Salomonsson E; Sundin A; Leffler H; Nilsson UJ, Double affinity amplification of galectin-ligand interactions through arginine-arene interactions: synthetic, thermodynamic, and computational studies with aromatic diamido thiodigalactosides. Chem. - Eur. J 2008, 14 (14), 4233–45. [DOI] [PubMed] [Google Scholar]
- 30.Saraboji K; Håkansson M; Genheden S, The carbohydrate-binding site in galectin-3 is preorganized to recognize a sugarlike framework of oxygens: ultra-high-resolution structures and water dynamics. Biochemistry 2012, 51, 296–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schwefel D; Maierhofer C; Beck JG; Seeberger S; Diederichs K; Möller HM; Welte W; Wittmann V, Structural basis of multivalent binding to wheat germ agglutinin. J. Am. Chem. Soc 2010, 132 (25), 8704–8719. [DOI] [PubMed] [Google Scholar]
- 32.Li Z; Lazaridis T, The effect of water displacement on binding thermodynamics: Concanavalin A. J. Phys. Chem. B 2005, 109 (1), 662–670. [DOI] [PubMed] [Google Scholar]
- 33.Loris R; Maes D; Poortmans F; Wyns L; Bouckaert J, A structure of the complex between concanavalin A and methyl-3,6-di-O- (α-D-mannopyranosyl)-α-D-mannopyranoside reveals two binding modes. J. Biol. Chem 1996, 271 (48), 30614–30618. [DOI] [PubMed] [Google Scholar]
- 34.Naismith JH; Field RA, Structural basis of trimannoside recognition by concanavalin A. J. Biol. Chem 1996, 271 (2), 972–976. [DOI] [PubMed] [Google Scholar]
- 35.Dam TK; Brewer CF, Thermodynamic studies of lectin-carbohydrate interactions by isothermal titration calorimetry. Chem. Rev 2002, 102 (2), 387–429. [DOI] [PubMed] [Google Scholar]
- 36.Collins PM; Hidari KIPJ; Blanchard H, Slow diffusion of lactose out of galectin-3 crystals monitored by X-ray crystallography: possible implications for ligand-exchange protocols. Acta Crystallogr., Sect. D: Biol. Crystallogr 2007, 63 (Pt 3), 415–9. [DOI] [PubMed] [Google Scholar]
- 37.Biedermann F; Schneider HJ, Experimental Binding Energies in Supramolecular Complexes. Chem. Rev 2016, 116 (9), 5216–5300. [DOI] [PubMed] [Google Scholar]
- 38.Boraston AB; Nurizzo D; Notenboom V; Ducros V; Rose DR; Kilburn DG; Davies GJ, Differential oligosaccharide recognition by evolutionarily-related β-1,4 and β-1,3 glucan-binding modules. J. Mol. Biol 2002, 319 (5), 1143–1156. [DOI] [PubMed] [Google Scholar]
- 39.Sun G; Zhao H; Kalyanaraman B; Dahms NM, Identification of residues essential for carbohydrate recognition and cation dependence of the 46-kDa mannose 6-phosphate receptor. Glycobiology 2005, 15 (11), 1136–1149. [DOI] [PubMed] [Google Scholar]
- 40.Hudson KL; Bartlett GJ; Diehl RC; Agirre J; Gallagher T; Kiessling LL; Woolfson DN, Carbohydrate-Aromatic Interactions in Proteins. J. Am. Chem. Soc 2015, 137 (48), 15152–15160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xie H; Bolam DN; Nagy T; Szabó L; Cooper A; Simpson PJ; Lakey JH; Williamson MP; Gilbert HJ, Role of hydrogen bonding in the interaction between a xylan binding module and xylan. Biochemistry 2001, 40 (19), 5700–5707. [DOI] [PubMed] [Google Scholar]
- 42.Vennelakanti V; Qi HW; Mehmood R; Kulik HJ, When are two hydrogen bonds better than one? Accurate first-principles models explain the balance of hydrogen bond donors and acceptors found in proteins. Chem. Sci 2021, 12 (3), 1147–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Habash J; Raftery J; Nuttall R; Price HJ; Wilkinson C; Kalb AJ; Helliwell JR, Direct determination of the positions of the deuterium atoms of the bound water in concanavalin A by neutron Laue crystallography. Acta Crystallogr., Sect. D: Biol. Crystallogr 2000, 56 (5), 541–550. [DOI] [PubMed] [Google Scholar]
- 44.Botos I; Wlodawer A, Proteins that bind high-mannose sugars of the HIV envelope. Prog. Biophys. Mol. Biol 2005, 88, 233–282. [DOI] [PubMed] [Google Scholar]
- 45.Holla A; Skerra A, Comparative analysis reveals selective recognition of glycans by the dendritic cell receptors DC-SIGN and Langerin. Protein Eng. Des. Sel 2011, 24 (9), 659–669. [DOI] [PubMed] [Google Scholar]
- 46.Wesener DA; Wangkanont K; McBride R; Song X; Kraft MB; Hodges HL; Zarling LC; Splain RA; Smith DF; Cummings RD; Paulson JC; Forest KT; Kiessling LL, Recognition of microbial glycans by human intelectin-1. Nat. Struct. Mol. Biol 2015, 22 (8), 603–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Houser J; Kozmon S; Mishra D; Hammerová Z; Wimmerová M; Koča J, The CH–π Interaction in Protein–Carbohydrate Binding: Bioinformatics and In Vitro Quantification. Chem. - Eur. J 2020, 26 (47), 10769–10780. [DOI] [PubMed] [Google Scholar]
- 48.Diehl C; Genheden S; Modig K; Ryde U; Akke M, Conformational entropy changes upon lactose binding to the carbohydrate recognition domain of galectin-3. J. Biomol. NMR 2009, 45 (1-2), 157–69. [DOI] [PubMed] [Google Scholar]
- 49.Johannes L; Jacob R; Leffler H, Galectins at a glance. J. Cell Sci 2018, 131 (9), 1–9. [DOI] [PubMed] [Google Scholar]
- 50.Vyas NK, Atomic features of protein-carbohydrate interactions. Curr. Opin. Struct. Biol 1991, 1 (5), 732–740. [Google Scholar]
- 51.Blake CCF; Johnson LN; Mair GA; North ACT; Phillips DC; Sarma VR, Crystallograhic Studies of Activity of Hen Egg-White Lysozyme. Proc. Royal Soc. B Biol Sci 1967, 167 (1009), 378–88. [DOI] [PubMed] [Google Scholar]
- 52.Quiocho FA, Carbohydrate-Binding Proteins - Tertiary Structures and Protein-Sugar Interactions. Annu. Rev. Biochem 1986, 55, 287–315. [DOI] [PubMed] [Google Scholar]
- 53.Maenaka K; Kawai G; Watanabe K; Sunada F; Kumagai I, Functional and Structural Role of a Tryptophan Generally Observed In Protein-Carbohydrate Interaction - Trp-62 of Hen Egg-White Lysozyme. J. Biol. Chem 1994, 269 (10), 7070–7075. [PubMed] [Google Scholar]
- 54.Muraki M; Harata K; Sugita N; Sato K, Protein-carbohydrate interactions in human lysozyme probed by combining site-directed mutagenesis and affinity labeling. Biochemistry 2000, 39 (2), 292–299. [DOI] [PubMed] [Google Scholar]
- 55.Miller DM; Olson JS; Pflugrath JW; Quiocho FA, Rates of Ligand-Binding to Periplasmic Proteins Involved in Bacterial Transport and Chemotaxis. J. Biol. Chem 1983, 258 (22), 3665–3672. [PubMed] [Google Scholar]
- 56.Spurlino JC; Lu GY; Quiocho FA, THE 2.3-A Resolution Structure of the Maltose-Binding or Maltodextrin-Binding Protein, a Primary Receptor of Bacterial Active-Transport and Chemotaxis. J. Biol. Chem 1991, 266 (8), 5202–5219. [DOI] [PubMed] [Google Scholar]
- 57.Vyas NK; Vyas MN; Quiocho FA, Comparison of the Periplasmic Receptors for L-arabinose, Dglucose, D-galactose, and D-ribose - Structural and Functional Similarity. J. Biol. Chem 1991, 266 (8), 5226–5237. [PubMed] [Google Scholar]
- 58.Borrok MJ; Kiessling LL; Forest KT, Conformational changes of glucose/galactose-binding protein illuminated by open, unliganded, and ultra-high-resolution ligand-bound structures. Protein Sci. 2007, 16 (6), 1032–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cygler M; Rose DR; Bundle DR, Recognition of a Cell-Surface Oligosaccharide of Pathogenic Salmonella by an Antibody Fab Fragment. Science 1991, 253 (5018), 442–445. [DOI] [PubMed] [Google Scholar]
- 60.Nishio M, The CH/π hydrogen bond in chemistry. Conformation, supramolecules, optical resolution and interactions involving carbohydrates. Phys. Chem. Chem. Phys 2011, 13 (31), 13873–900. [DOI] [PubMed] [Google Scholar]
- 61.Asensio JL; Arda A; Canada FJ; Jiménez-Barbero J, Carbohydrate-Aromatic Interactions. Acc. Chem. Res 2013, 46 (4), 946–954. [DOI] [PubMed] [Google Scholar]
- 62.Gallivan JP; Dougherty DA, Cation-π interactions in structural biology. Proc. Natl. Acad. Sci. U. S. A 1999, 96 (August), 9459–9464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dougherty DA, Cation-π interactions in chemistry and biology: A new view of benzene, Phe, Tyr, and Trp. Science 1996, 271 (5246), 163–168. [DOI] [PubMed] [Google Scholar]
- 64.Kiehna SE; Laughrey ZR; Waters ML, Evaluation of a carbohydrate-π interaction in a peptide model system. Chem. Commun 2007, 2 (39), 4026–4028. [DOI] [PubMed] [Google Scholar]
- 65.Chen W; Enck S; Price JL; Powers DL; Powers ET; Wong C.-h.; Dyson HJ; Kelly JW, Structural and Energetic Basis of Carbohydrate–Aromatic Packing Interactions in Proteins. J. Am. Chem. Soc 2013, 135, 9877–9884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hsu CH; Park S; Mortenson DE; Foley BL; Wang X; Woods RJ; Case DA; Powers ET; Wong CH; Dyson HJ; Kelly JW, The Dependence of Carbohydrate-Aromatic Interaction Strengths on the Structure of the Carbohydrate. J. Am. Chem. Soc 2016, 138 (24), 7636–7648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Laughrey ZR; Kiehna SE; Riemen AJ; Waters ML, Carbohydrate-π interactions: What are they worth? J. Am. Chem. Soc 2008, 130 (44), 14625–14633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jiménez-Moreno E; Jiménez-Osés G; Gómez AM; Santana AG; Corzana F; Bastida A; Jiménez-Barbero J; Asensio JL, A thorough experimental study of CH/π interactions in water: quantitative structure-stability relationships for carbohydrate/aromatic complexes. Chem. Sci 2015, 6 (11), 6076–6085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Montalvillo-Jiménez L; Santana A. s. G.; Corzana F; Jiménez-Osés G; Jiménez-Barbero J. s.; Gómez AM; Asensio JL, Impact of Aromatic Stacking on Glycoside Reactivity: Balancing CH/π and Cation/π Interactions for the Stabilization of Glycosyl- Oxocarbenium Ions. J. Am. Chem. Soc 2019, 13372–13372. [DOI] [PubMed] [Google Scholar]
- 70.Vandenbussche S; Díaz D; Fernández-Alonso MC; Pan W; Vincent SP; Cuevas G; Cañada FJ; Jiménez-Barbero J; Bartik K, Aromatic-carbohydrate interactions: an NMR and computational study of model systems. Chem. - Eur. J 2008, 14 (25), 7570–8. [DOI] [PubMed] [Google Scholar]
- 71.Fauchere JL; Pliska V, Hydrophobic parameters II of amino acid side-chains from the partitioning of N-acetyl-amino acid amides. E.Eur. J. Med. Chem 1983, 18, 369–369. [Google Scholar]
- 72.Taylor R; Kennard O, Crystallographic Evidence for the Existence of C-H⋯O, C-H⋯N, and C-H⋯Cl Hydrogen Bonds. J. Am. Chem. Soc 1982, 104 (19), 5063–5070. [Google Scholar]
- 73.Nishio M; Umezawa Y; Fantini J; Weiss MS; Chakrabarti P, CH-π hydrogen bonds in biological macromolecules. Phys. Chem. Chem. Phys 2014, 16 (25), 12648–12683. [DOI] [PubMed] [Google Scholar]
- 74.Oki M; Takano S; Toyota S, Benzene-Ethene Interactions as Studied by ab initio Calculations. Bull. Chem. Soc. Jpn 2000, 73, 2221–2230. [Google Scholar]
- 75.Samanta U; Chakrabarti P; Chandrasekhar J, Ab Initio Study of Energetics of X-H … π ( X ) N , O , and C ) Interactions Involving a Heteroaromatic Ring. J. Phys. Chem. A 1998, 8964–8969. [Google Scholar]
- 76.Tsuzuki S; Honda K; Uchimaru T; Mikami M; Tanabe K, The interaction of Benzene with Chloro- and Fluoromethanes: Effects of Halogenation on CH/π interaction. J. Phys. Chem. A 2002, 106 (17), 4423–4428. [Google Scholar]
- 77.Ams MR; Fields M; Grabnic T; Janesko BG; Zeller M; Sheridan R; Shay A, Unraveling the Role of Alkyl F on CH-π Interactions and Uncovering the Tipping Point for Fluorophobicity. J. Org. Chem 2015, 80 (15), 7764–7769. [DOI] [PubMed] [Google Scholar]
- 78.Dey RC; Seal P; Chakrabarti S, CH/π interaction in benzene and substituted derivatives with halomethane: A combined density functional and dispersion-corrected density functional study. J. Phys. Chem. A 2009, 113 (37), 10113–10118. [DOI] [PubMed] [Google Scholar]
- 79.Platzer G; Mayer M; Beier A; Br S; Fuchs JE; Engelhardt H; Geist L; Bader G; Schçrghuber J; Lichtenecker R; Wolkerstorfer B; Kessler D; McConnell DB; Konrat R, PI by NMR : Probing CH–pi Interactions in Protein – Ligand Complexes by NMR Spectroscopy Research Articles. Angew. Chem., Int. Ed 2020, 14861–14868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ugozzoli F; Arduini A; Massera C; Pochini A; Secchi A, CH/π interaction between benzene and model neutral organic molecules bearing acid CH groups. New J. Chem 2002, 26 (12), 1718–1723. [Google Scholar]
- 81.Zhao Y; Truhlar DG, The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other function. Theor. Chem. Acc 2008, 120 (1-3), 215–241. [Google Scholar]
- 82.del Carmen Fernández-Alonso M; Cañada FJ; Jiménez-Barbero J; Cuevas G, Molecular recognition of saccharides by proteins. Insights on the origin of the carbohydrate-aromatic interactions. J. Am. Chem. Soc 2005, 127 (20), 7379–86. [DOI] [PubMed] [Google Scholar]
- 83.Kozmon S; Matuška R; Spiwok V; Koča J, Three-dimensional potential energy surface of selected carbohydrates’ ch/π dispersion interactions calculated by high-level quantum mechanical methods. Chem. - Eur. J 2011, 17 (20), 5680–5690. [DOI] [PubMed] [Google Scholar]
- 84.Tsuzuki S; Uchimaru T; Mikami M, Magnitude and nature of carbohydrate-aromatic interactions in fucose-phenol and fucose-indole complexes: CCSD(T) level interaction energy calculations. J. Phys. Chem. A 2011, 115 (41), 11256–11262. [DOI] [PubMed] [Google Scholar]
- 85.Lisbjerg M; Valkenier H; Jessen BM; Al-Kerdi H; Davis AP; Pittelkow M, Biotin[6]uril esters: Chloride-selective transmembrane anion carriers employing C-H⋯anion interactions. J. Am. Chem. Soc 2015, 137 (15), 4948–4951. [DOI] [PubMed] [Google Scholar]
- 86.Stanca-Kaposta EC; Çarçabal P; Cocinero EJ; Hurtado P; Simons JP, Carbohydrate-aromatic interactions: Vibrational spectroscopy and structural assignment of isolated monosaccharide complexes with p-hydroxy toluene and N-acetyl L-tyrosine methylamide. J. Phys. Chem. B 2013, 117 (27), 8135–8142. [DOI] [PubMed] [Google Scholar]
- 87.Salonen LM; Ellermann M; Diederich F, Aromatic rings in chemical and biological recognition: Energetics and structures. Angew. Chem., Int. Ed 2011, 50 (21), 4808–4842. [DOI] [PubMed] [Google Scholar]
- 88.Zhao C; Li P; Smith MD; Pellechia PJ; Shimizu KD, Experimental study of the cooperativity of CH-π interactions. Org. Lett 2014, 16 (13), 3520–3523. [DOI] [PubMed] [Google Scholar]
- 89.Baggioli A; Meille SV; Raos G; Po R; Brinkmann M; Famulari A, Intramolecular CH/π interactions in alkylaromatics: Monomer conformations for poly(3-alkylthiophene) atomistic models. Int. J. Quantum Chem 2013, 113 (18), 2154–2162. [Google Scholar]
- 90.Ninković DB; Vojislavljević-Vasilev DZ; Medaković VB; Hall MB; Brothers EN; Zarić SD, Aliphatic-aromatic stacking interactions in cyclohexane-benzene are stronger than aromatic-aromatic interaction in the benzene dimer. Phys. Chem. Chem. Phys 2016, 18 (37), 25791–25795. [DOI] [PubMed] [Google Scholar]
- 91.Spiwok V, CH/ π Interactions in Carbohydrate Recognition. Molecules 2017, (April), 1038–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Koharudin LM; Gronenborn AM, Antiviral lectins as potential HIV microbicides. Curr. Opin. Virol 2014, 7 (1), 95–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ramírez-Gualito K; Alonso-Ríos R; Quiroz-García B; Rojas-Aguilar A; Díaz D; Jiménez-Barbero J; Cuevas G, Enthalpic nature of the CH/pi interaction involved in the recognition of carbohydrates by aromatic compounds, confirmed by a novel interplay of NMR, calorimetry, and theoretical calculations. J. Am. Chem. Soc 2009, 131 (50), 18129–38. [DOI] [PubMed] [Google Scholar]
- 94.Bernardi A; Arosio D; Potenza D; Sánchez-Medina I; Mari S; Cañada FJ; Jiménez-Barbero J, Intramolecular carbohydrate-aromatic interaction and intermolecular van der Waals interactions enhance the molecular recognition ability of GM1 glycomimetics for cholera toxin. Chem. - Eur. J.l 2004, 10 (18), 4395–4406. [DOI] [PubMed] [Google Scholar]
- 95.Diehl RC CH-π interactions play a central role in protein recognition of carbohydrates. Ph.D. Dissertation, Massachusetts Institute of Technology, Cambridge, MA, 2021. [Google Scholar]
- 96.Kiessling LL, Chemistry-driven glycoscience. Bioorg. Med. Chem 2018, 26 (September), 5229–5238. [DOI] [PubMed] [Google Scholar]
- 97.Jutten L; Ramirez-Gualito K; Weilhard A; Albrecht B; Cuevas G; Ferna C; Luis A; Erro E, Exploring the Role of Solvent on Carbohydrate–Aryl Interactions by Diffusion NMR-Based Studies. ACS Omega 2018, 3, 536–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Payne CM; Bomble YJ; Taylor CB; McCabe C; Himmel ME; Crowley MF; Beckham GT, Multiple functions of aromatic-carbohydrate interactions in a processive cellulase examined with molecular simulation. J. Biol. Chem 2011, 286 (47), 41028–41035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Itakura Y; Nakamura-Tsuruta S; Kominami J; Tateno H; Hirabayashi J, Sugar-binding profiles of chitin-binding lectins from the hevein family: A comprehensive study. Int. J. Mol. Sci 2017, 18 (6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mareska V; Tvaroska I; Kralova B; Spiwok V, Molecular simulations of hevein/(GlcNAc)(3) complex with weakened OH/O and CH/pi hydrogen bonds: implications for their role in complex stabilization. Carbohydr. Res 2015, 408, 1–7. [DOI] [PubMed] [Google Scholar]
- 101.Bautista-Ibañez L; Ramírez-Gualito K; Quiroz-García B; Rojas-Aguilar A; Cuevas G, Calorimetric measurement of the CH/pi interaction involved in the molecular recognition of saccharides by aromatic compounds. J. Org. Chem 2008, 73 (3), 849–57. [DOI] [PubMed] [Google Scholar]
- 102.Kobayashi H; Okada K; Tokuda S; Kanao E; Masuda Y, Separation of saccharides using fullerene - bonded silica monolithic columns via π interactions in liquid chromatography. Sci. Rep 2020, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.McMahon CM; Isabella CR; Windsor IW; Kosma P; Raines RT; Kiessling LL, Stereoelectronic Effects Impact Glycan Recognition. J. Am. Chem. Soc 2020, 142 (5), 2386–2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cuétara-Guadarrama F; Hernández-Huerta E; Rojo-Portillo T; Reyes-López E; Jiménez-Barbero J; Cuevas G, Experimental and theoretical study of the role of CH / π interactions in the aminolysis reaction of acetyl galactoside. Carbohydr. Res 2019, 486 (May), 107821–107821. [DOI] [PubMed] [Google Scholar]
- 105.Li Q; Levi SM; Jacobsen EN, Highly Selective β-Mannosylations and β-Rhamnosylations Catalyzed by Bis-thiourea. J. Am. Chem. Soc 2020, 142 (27), 11865–11872. [DOI] [PMC free article] [PubMed] [Google Scholar]


