Astroglia: Definition and Evolutionary Origins
Astroglia represents a class of neural cells of the ectodermal, neuroepithelial origin which are the principal homeostatic cells of the central nervous system (CNS) (Verkhratsky and Nedergaard 2016, 2018). The class of astroglia includes several cellular subtypes with distinct morphology and function (Fig. 1); astroglia includes parenchymal astrocytes of several types, radial astrocytes, tanycytes, pituicytes, ependymocytes, choroid plexus cells and retinal pigment epithelial cells. The term astrocyte (αστρον κψτοσ; astron, star and kytos, a hollow vessel, later cell that is a star-like cell) was introduced by Michael von Lenhossék in 1895 (Lenhossék 1895); for the history of glial research see also (Kettenmann and Verkhratsky 2008; Chvatal and Verkhratsky 2018). Lenhossék suggested naming all parenchymal neuroglia ‘spongiocytes’, while the term astrocyte he reserved to a sub-population of cells with star-like appearance when stained with the Golgi black reaction. Parenchymal (or protoplasmic) astrocytes in the nervous tissue indeed have complex spongioform morphology defined by extensive arborisation.
Fig. 1.
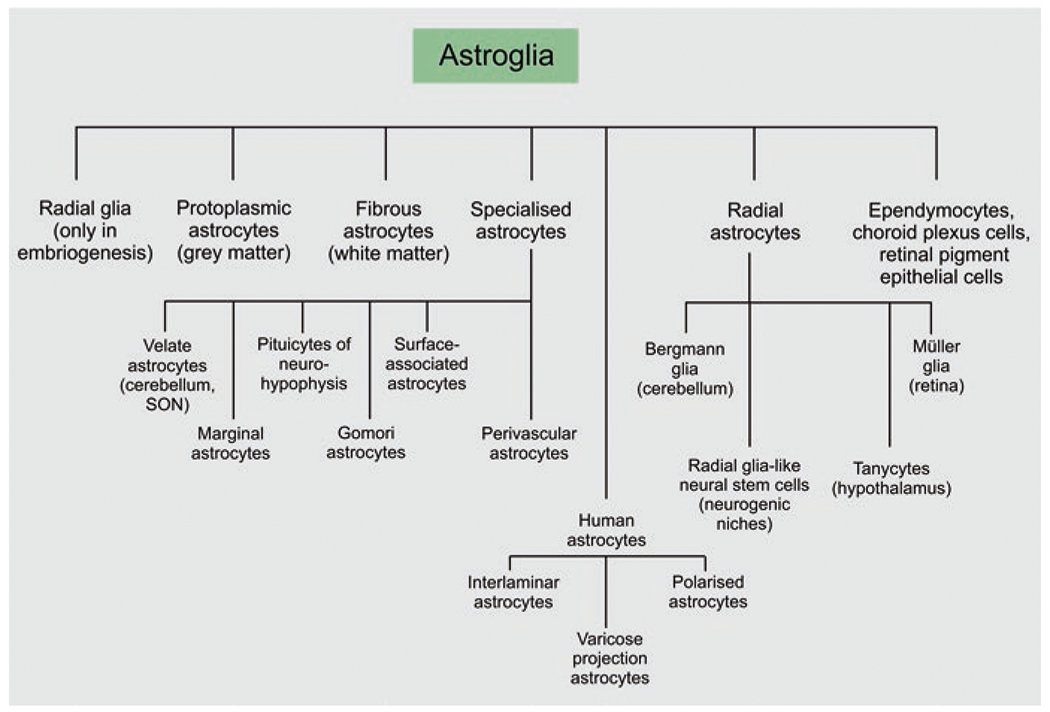
Classification of astroglia
Astroglial cells originate from radial glia that produce, through asymmetric division, astroglial progenitors. These progenitors populate the brain and, in the early postnatal period, propagate through symmetric division, thus producing the bulk of parenchymal astrocytes. After birth radial glial cells directly transform (transdifferentiate) into astrocytes; in neurogenic niches, these astrocytes can act as stem cells underlying adult neurogenesis.
Types of Astroglia
Protoplasmic Astrocytes
The terms of protoplasmic and fibrous glia, populating grey and white matter, respectively, have been introduced by Albert von Kölliker and William Lloyd Andriezen (Andriezen 1893; Kölliker 1896). Protoplasmic astrocytes are located in the grey matter of the brain and of the spinal cord; they have round somata of ~10μm in diameter and several primary processes. The primary processes divide into processes of higher order; these processes host tiny terminal processes, frequently referred to as peripheral astrocytic processes (PAPs); they are presented in a form of membranous leaflets, which give protoplasmic astrocyte distinctive spongioform appearance (Bushong et al. 2002; Popov et al. 2020). Processes of protoplasmic astrocytes can also be classified into (i) branches that represent processes containing organelles and (ii) leaflets which are thin membranous structures devoid of organelles (Fig. 2); protoplasmic astrocytes extend at least one process to the nearby blood vessel. These vascular processes end up with endfeet that cover blood vessels and form glia limitans vascularis (Khakh and Sofroniew 2015). Protoplasmic astrocytes occupy independent territorial domains; the neighbouring astrocytes overlap only with their distal processes and the degree of overlap does not exceed 5% (Bushong et al. 2002); this however may not occur in all species. Territories occupied by protoplasmic astrocytes define the confines of neurogliovascular units, which integrate parenchymal cellular elements with blood vessels (Iadecola 2017). Gap junctions, made from connexons and located at distal processes, integrate astrocytes into anatomically segregated syncytia (Giaume et al. 2021). The leaflets and peripheral branches contact synapses, creating a multipartite synapse or astroglial cradle that supports many aspects of synaptic function (Verkhratsky and Nedergaard 2014). Human protoplasmic astrocytes are substantially (10–20 times) larger and more complex than protoplasmic astrocytes in rodents (Oberheim et al. 2009).
Fig. 2.
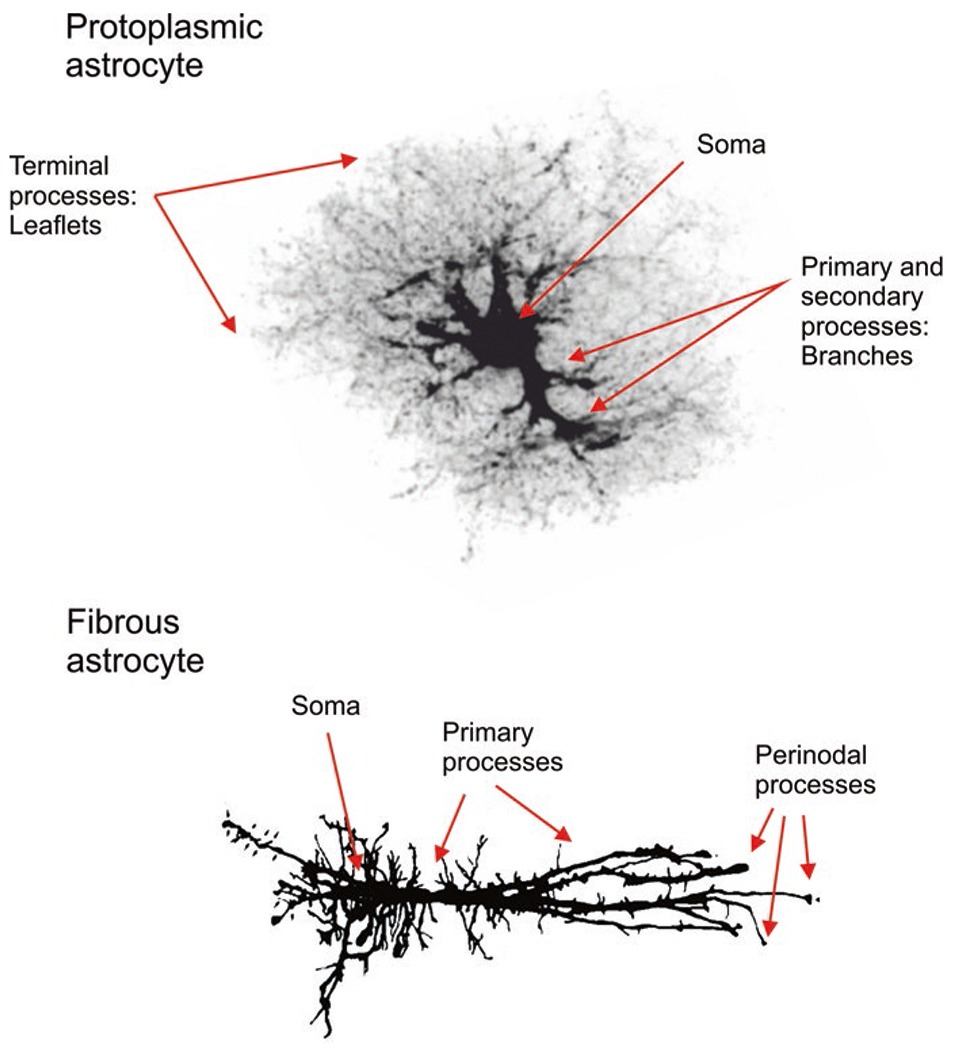
Protoplasmic and fibrous astrocytes. Protoplasmic astrocytes of the grey matter are composed of soma, primary processes or branches and organelle-free thin leaflets. Fibrous astrocytes of the white matter contain perinodal processes by which they contact oligodendrocytes and nodes of Ranvier. The protoplasmic astrocyte was traced using image provided by Milos Pekny (Gothenburg University); the camera lucida image for fibrous astrocyte was provided by Arthur Butt from University of Portsmouth
Fibrous Astrocytes
Fibrous astrocytes dwell in the white matter, in the optic nerve and in the nerve fibre layer of the retina. Fibrous astrocytes (Fig. 2) have small somata and straight nonbranched processes (up to 100μm in length) oriented in parallel to axonal bundles. These processes end up with multiple finger-like cytoplasmic protrusions (perinodal processes) that are establishing contacts with axonal perinodal spaces of the surrounding axons (Lundgaard et al. 2014). Fibrous astrocytes also contact blood vessels and create perivascular or subpial endfeet. Morphology of fibrous astrocytes is heterogeneous. In rodent optic nerve, fibrous astrocytes are classified (according to morphology) into transverse, random and longitudinal (Butt et al. 1994). Human fibrous astrocytes are approximately two times larger than the same cells in rodents (Oberheim et al. 2009).
Juxtavascular Astrocytes
A sub-population of protoplasmic astrocytes located in close proximity to the blood vessels is known as juxtavascular astrocytes. These cells have some distinct physiology and demonstrate proliferative potential in response to traumatic brain injury (Bardehle et al. 2013; Gotz et al. 2021).
Surface-Associated Astrocytes
Surface-associated astrocytes are the main cellular elements of the glia limitans externa in the posterior prefrontal and amygdaloid cortex. The cell bodies of surface-associated astrocytes are located at the cortical surface, and there are two types of processes: long parallel superficial process running beneath the pia mater vessels and shorter processes extending in all directions, with some of them projecting into the cortical layer 1 (Feig and Haberly 2011).
Velate Astrocytes
Velate astrocytes represent a special population of parenchymal astrocytes covering somata of densely packed neurones in the olfactory bulb or in the granular layer of the cerebellar cortex. Velate astrocytes are characterised by a small soma with several primary processes, which form large leaflets (with high surface-to-volume ratio of 20–30μm−1) enwrapping neuronal cell bodies. These perineuronal processes look like extended leaves, which defined their name derived from vellum (parchment in Latin) or velatus (which is Latin word for covered, wrapped, veiled). In the cerebellum these veil-like processes mainly cover the somata of granule cells/neurones (with a single astrocyte covering several neurones) as well as somata of Purkinje cells/neurones (Chan-Palay and Palay 1972; Buffo and Rossi 2013).
Radial Astrocytes
Radial astrocytes, named so because of their radially extended processes, are represented by (i) cerebellar Bergmann glial cells; (ii) retinal Müller glia; (iii) radial astrocytes of the supraoptic nucleus; (iv) radial glia-like neural stem cells, localised in neurogenic niches, and (v) tanycytes present in the periventricular organs, in the hypothalamus, in the hypophysis/pituitary gland and in the raphe part of the spinal cord (Reichenbach and Bringmann 2017; Verkhratsky and Nedergaard 2018).
Interlaminar and Varicose Projection Astrocytes in the Primate Brain
The brain of higher primates, and most notably of humans, contains several types of astrocytes which cannot be found in other species. These include (i) interlaminar astrocytes, with small somata located in the upper cortical layers and long processes penetrating through cortical layers; (ii) polarised astrocytes, with somata positioned in deep cortical layers, with several long processes penetrating into superficial cortical layers, and (iii) varicose projection astrocytes characterised by several very long (up to 1 mm) unbranched processes bearing varicosities and extending in all directions through the deep cortical layers (Oberheim et al. 2009; Sosunov et al. 2014; Colombo 2017).
Physiology of Astrocytes
Astrocytes control the homeostasis of the CNS at all levels of organisation. This includes homeostasis of ions, pH and neurotransmitters; supplying neurones with metabolic substrates; supporting oligodendrocytes and axons; regulating synaptogenesis, neurogenesis, and formation and maintenance of the blood-brain barrier (BBB); contributing to operation of the glymphatic system and regulating systemic homeostasis with some astrocytes being central chemosensors for oxygen, CO2 and Na+ (Verkhratsky and Nedergaard 2018).
Despite substantial morphological and functional heterogeneity, all astrocytes have common basic physiological features. First and foremost, all astrocytes are electrically non-excitable cells maintaining negative resting membrane potential (Vm) of about −80 mV. This is set up by transmembrane ionic gradients and very high membrane K+ permeability. Intracellular concentrations of major ions in astrocytes are somewhat different from neurones: in particular astrocytic cytoplasmic Na+ concentration is around 15–20 mM (which is twice as high as in neurones), and cytoplasmic concentration of Cl− is maintained at 30–50 mM (compare to ~5–10 mM in neurones). Intracellular concentration of Ca2+ in astrocytes is again higher than in the majority of neurones (~ 120–200 nM vs. 50–100 nM, respectively). Astrocytic intracellular concentration of K+ is ~120–140 mM, which is quite similar to neuronal cytosolic K+ (Verkhratsky and Nedergaard 2018). High membrane K+ permeability stipulates low input resistance (5–20 MΩ) of astrocytes (Fig. 3).
Fig. 3.
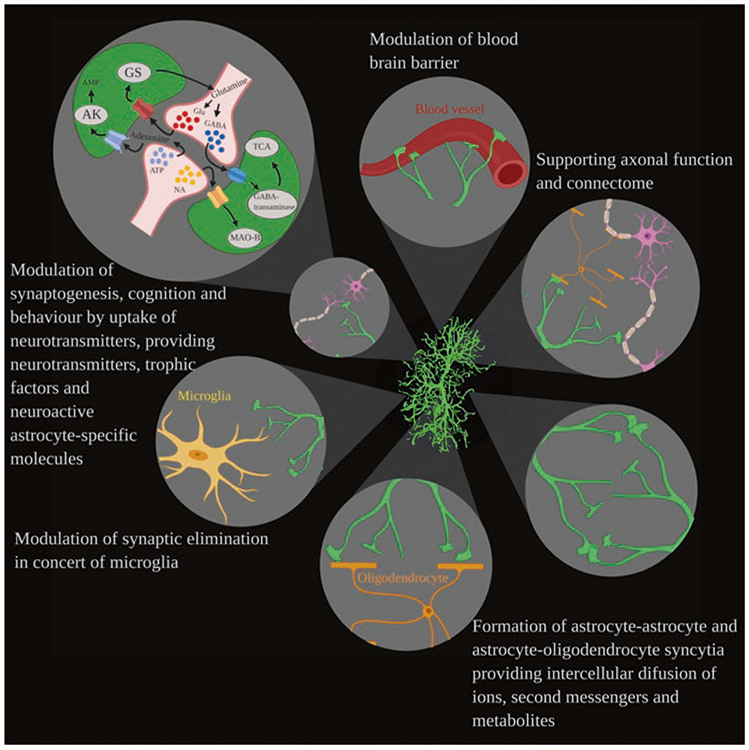
Functions of astrocytes. Astrocytes interact with diverse cellular structures including blood vessels, oligodendrocytes, neurones, microglia and other astrocytes, contributing to various functions (clockwise from the top) such as regulation of the blood-brain barrier, supporting axons, myelination and connectome, forming astrocyte-astrocyte and astrocyte-oligodendrocyte syncytia, controlling (in concert with microglia) synaptic elimination and modulating synaptogenesis, cognition and behaviour by the neurochemical dialogue with synapses. (Reproduced from Augusto-Oliveira et al. (2020))
Ion Channels
As mentioned above astroglial membrane permeability is dominated by K+ channels. Astrocytes express several types of K+ channels with distinct voltage dependence, which covers the whole range of physiological membrane potentials. As a result astrocytes demonstrate linear current-voltage relationship, which is their most characteristic electrophysiological signature maintained in vitro, in situ and in vivo (Fig. 4). This diverse complement of K+ channels allows maintenance of hyperpolarised membrane potential, which in turn provides the electrical gradient governing numerous membrane transporters and is thus critical for astroglial homeostatic function.
Fig. 4.
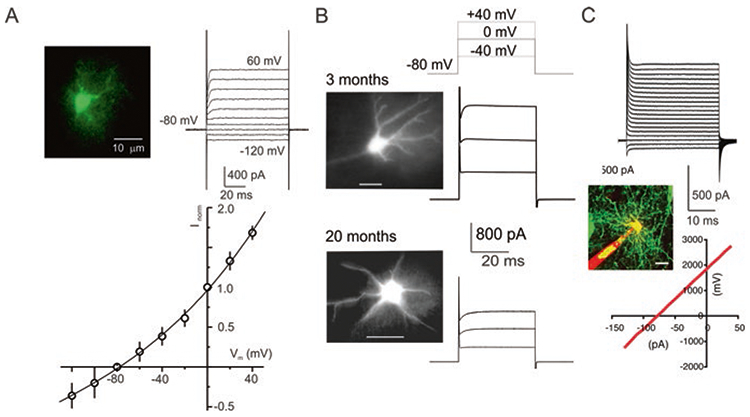
Passive membrane properties of astrocytes. (a) Voltage-clamp recordings from astrocytes freshly isolated from the cortex of transgenic mice expressing EGFP under control of the GFAP promoter. Astrocytes were identified by specific EGFP fluorescence; whole-cell currents were recorded in response to hyper- and depolarising steps from −120 to +60 mV (step interval 20 mV). To construct the I–V relationship, amplitudes of currents were normalised to the value measured at 0 mV; every point is mean ± SD for 20 cells. (b) Voltage-clamp recordings from astrocytes in acute slices obtained from 3-month-old and 20-month-old gfa::EGFP mice; astrocytes were identified by fluorescence. (c) Voltage-clamp recordings from human astrocytes grafted into mouse brain. (Reproduced with permission from Verkhratsky and Nedergaard (2018))
Inward rectifier potassium channels are most abundantly expressed in astrocytes. The main type of astrocytic inward rectifier channels is the Kir4.1 subtype (encoded by KCNJ10 gene). These Kir4.1 channels are expressed in astrocytes throughout the brain, in the spinal cord and in the retina (Kalsi et al. 2004; Butt and Kalsi 2006). K+ flux through Kir4.1 channels refines the resting membrane potential of astrocytes; pharmacological suppression or genetic deletion of these channels depolarises astrocytes by ~20 mV (Olsen et al. 2007; Seifert et al. 2009). Astrocytes also express Kir5.1 channels which may co-assemble with Kir4.1. channels; the resulting heteromeric channels are particularly densely expressed in perisynaptic and perivascular astroglial processes (Hibino et al. 2004; Brasko et al. 2017). The ATP-sensitive inward rectifying K+ channels (assembled from Kir6.x subunit and SUR1/2) have been also detected in astrocytes; these channels are activated in conditions of intracellular ATP depletion (Eaton et al. 2002; Skatchkov et al. 2002). Resting astroglial K+ permeability is also mediated by several voltage-independent channels of TREK and TWIK families including TREK1/K2P2.1, TREK2/K2P10.1 and TWIK1/K2P1.1 channels (Seifert et al. 2009; Zhou et al. 2009).
In addition to inward rectifying channels, astrocytes express voltage-dependent K+ channels, which include Kv1.5 (KCNA5), Kv1.4 (KCNA4) and Kv11.1/ERG1 (Bordey and Sontheimer 2000; Verkhratsky and Steinhauser 2000; Edwards et al. 2002) and Kv1, Kv3 and Kv4 mediating fast A-type K+ currents (Bekar et al. 2005). Finally, cortical astrocytes express SK (small conductance KCa 2.3/KCNN3) and IK (intermediate conductance KCa3.1/KCNN4) Ca2+-dependent K+ channels in their somata (Armstrong et al. 2005; Longden et al. 2011) and large-conductance (225 pS) BK (big conductance) channels in their endfeet (Filosa et al. 2006).
Several types of other voltage-dependent channels have been identified in astrocytes. These include tetrodotoxin (TTX)-sensitive and TTX-resistant Na+ channels which were mainly characterised in cultured astrocytes (Sontheimer et al. 1991; Sontheimer and Waxman 1992; Sontheimer et al. 1994). At transcriptional and protein levels, astrocytes were found to express mainly Nav1.5 subunit, with lower levels of Nav1.2, Nav1.3 and Nav1.6 channels (Pappalardo et al. 2014a, 2016; Zhu et al. 2016). Similarly, astrocytes were reported (at mRNA and sometimes at protein levels) to possess voltage-gated Ca2+ channels mainly of Cav1.2 and Cav1.3 types (Latour et al. 2003; Cahoy et al. 2008; Zhang et al. 2014). There are only few reports for functionally active Ca2+ channels in astrocytes in slice preparations (Parri et al. 2001; Letellier et al. 2016) with no evidence for these channels being operative in vivo.
Astrocytes in the subfornical organ express a specific type of Na+ channel known as Nax (encoded by SCN7A gene). This channel is activated by an increase in extracellular Na+ concentration above 140 mM and contribute to astroglia-dependent chemosensing of Na+ fluctuations in circulation (Hiyama et al. 2013; Noda and Sakuta 2013). Another channel type that may be involved in Na+ chemosensing is the epithelial Na+ channel (ENaC). Immunoreactivity for ENaC has been found in astrocytes in circumventricular organs, white matter and pia mater (Miller and Loewy 2013).
Astrocytes are in possession of several types of transient receptor potential channels (TRP). The TRPA1 (ankyrin-type) channel is operational in the somata and processes of astrocytes in the brain stem and hippocampus; these channels provide for background Ca2+ entry thus regulating resting Ca2+ concentration in the cytoplasm (Shigetomi et al. 2012, 2013). The TRPC1 (or canonical-type)-containing channels mediate the store-operated Ca2+ entry following depletion of Ca2+ stores (Malarkey et al. 2008; Reyes et al. 2013; Verkhratsky and Parpura 2014). The TRPV1 and TRPV4 (vanilloid-type) are identified in cortical, hippocampal and spinal cord astrocytes, where they are activated in response to hypo-osmotic stress and cell swelling (Benfenati et al. 2007; Butenko et al. 2012). The store-operated Ca2+ entry in astrocytes is also mediated by ORAI 1 and ORAI3 channels activated by the endoplasmic reticulum (ER) Ca2+ sensor STIM1 (Kwon et al. 2017; Toth et al. 2019).
Astrocytes are the main type of neural cells that express water channels or aquaporins (AQP). The most abundant is AQP4, with much lesser expression of AQP1 and AQP9. In healthy conditions, the AQP4 channels are predominantly concentrated in astrocytic perivascular and pial endfeet (Nagelhus and Ottersen 2013). These channels contribute to many functions from olfaction and K+ buffering to synaptic plasticity and memory (Lu et al. 2008; Skucas et al. 2011; Scharfman and Binder 2013; Lisjak et al. 2017). The endfeet AQP4 channels are particularly important for the function of the glymphatic system (Mestre et al. 2020).
Astrocytes are functionally integrated into syncytia by gap junctional plaques which are composed of several hundred of intercellular channels known as connexons, with each connexon being assembled from 12 subunits known as connexins (Giaume et al. 2021). Astrocytes express three types of connexins, namely, Cx26, Cx30 and Cx43, of which Cx43 is the most abundant and most widespread being expressed in all CNS regions (Nagy et al. 2004). Connexons assembled from Cx30 are mainly present in the thalamus and leptomeninges (Nagy et al. 2004; Sohl et al. 2004), whereas Cx26 is confined to the hypothalamus, reticular thalamic and subtalamic nuclei (Nagy et al. 2011). Astrocytes also establish syncytial connections with oligodendrocytes; the astrocyte-oligodendrocyte connexons are assembled from heterotypic channels composed of Cx47/Cx43, Cx32/Cx30, Cx47/Cx30 or Cx32/Cx26, of which the first two are predominant types in vivo (Orthmann-Murphy et al. 2007; Magnotti et al. 2011). Unpaired connexons, also known as hemichannels, have been identified in astrocytes in various brain regions, and they may mediate (together with Pannexin channels) diffusional secretion of various neuroactive substances (Orellana et al. 2009; Giaume et al. 2013; Verkhratsky et al. 2016).
Receptors of Neurotransmitters
Fundamentally, astrocytes are capable of expressing virtually every type of neurotransmitter/neuromodulator receptor existing in the CNS. At the same time this potential promiscuity is very much restricted by the neurochemical environment in different parts of the CNS. Generally, astrocytes express receptors of the same modality as their neuronal neighbours; these modalities are congruent to the neurotransmitters released in a given brain region (Verkhratsky et al. 1998; Verkhratsky 2010). For example, in the spinal cord, where glycine is the main inhibitory neurotransmitter, astrocytes specifically express glycine receptors; in the basal ganglia, which utilise dopamine, astrocytes are endowed with dopamine receptors. Thus, the expression of astroglial receptors in vivo is regulated by neurochemical input, which makes astrocytes perceptive to regional neurochemical environment.
Probably the most abundant astrocytic receptors are receptors of purines, as the purinergic signalling system is omnipresent in the CNS (Burnstock and Verkhratsky 2012). Metabotropic purinoceptors, represented by ATP/ADP P2Y1,2,4,6,12,13 and UDP-glucose P2Y14 receptor, predominate (Abbracchio and Ceruti 2006; Verkhratsky et al. 2009). Activation of metabotropic P2Y receptors is linked, through phospholipase C (PLC) and inositol-1,4,5-trisphophate (InsP3) receptors to Ca2+ signalling. Astrocytes also possess ionotropic purinoceptors, mainly of P2X7 type; murine cortical astrocytes also have P2X1/5 receptors (Lalo et al. 2008, 2011; Illes et al. 2012). Activation of ionotropic purinoceptors contributes to both Ca2+ and Na+ signalling in astrocytes.
The second class of astrocytic neurotransmitter receptors is represented by receptors of amino acid glutamate, GABA and glycine. Again astrocytes express several types of glutamate receptors. Ionotropic glutamate receptors functionally expressed in astrocytes are represented by AMPA receptors (with all four subunits being expressed, albeit in brain region-dependent fashion) and NMDA receptors. The latter are composed of two GluN1 subunits assembled with GluN2C or D and GluN3 subunits. Such composition infers weak Mg2+ block (which develops at ~ −120 mV) and relatively low Ca2+ permeability (PCa/Pmonovalent ~ 3), as well as sensitivity to memantine and GluN2C/D subunit-selective antagonist UBP141 (Lalo et al. 2006; Palygin et al. 2010; Verkhratsky and Chvatal 2020). Astrocytes also express metabotropic glutamate receptors, of which mGluR3 (connected to cAMP signalling cascade) and mGuR1/5 (connected to PLC-InsP3-Ca2+ signalling cascade) predominate. Astrocytes express ionotropic GABAA receptors, which mediate Cl− currents and, because of high [Cl−]i, depolarise the membrane (Kettenmann et al. 1987); astrocytic metabotropic GABAB receptors are linked to PLC-InsP3-Ca2+ signalling cascade (Nilsson et al. 1993). Spinal cord astrocytes express glycine receptors which mediate Cl− efflux and astrocytic depolarisation (Pastor et al. 1995).
The third class of receptors abundantly present in astrocytes is represented by receptors for monoamines. In particular, most of astrocytes express α- and β-adrenoceptors that were detected in culture, in slices and in vivo at transcriptional, protein and functional levels (Verkhratsky and Nedergaard 2018). Both α1 and α2 adrenoceptors are linked to Ca2+ signalling (Kirischuk et al. 1996; Ding et al. 2013); β1-adrenoceptors regulate glycogen synthesis, β2-adrenoceptors are linked to cAMP signalling and β3-adrenoceptors regulate glucose uptake by modulating GLUT1 plasmalemmal glucose transporter (Hutchinson et al. 2007; Dong et al. 2012). In addition, astrocytes express 5-HT1A, 5-HT2A, 5-HT2B and 5-HT5A metabotropic serotonin receptors, as well as D1, D2, D4 and D5 dopamine receptors, and H1, H2 and H3 histamine receptors (Verkhratsky and Nedergaard 2018).
Other receptors expressed in astrocytes include B2 bradykinin receptors, canna-binoid CB1 receptors, V1 vasopressin receptors, oxytocin receptors, ETA and ETB endothelin receptors, atrial natriuretic peptide receptors, receptors for leptin and insulin, platelet-activating factor receptors and protease-activated receptors, which fulfil various functions and are linked to different signalling cascades (Verkhratsky and Nedergaard 2018).
Astroglial Plasma Membrane Transporters
Plasmalemmal transporters are fundamentally important for astroglial homeostatic function because they accomplish transport of ions, neurotransmitters, scavengers of reactive oxygen species (ROS), metabolic substrates and various neuromodulators and hormones. All plasmalemmal transporters are classified into pumps (which utilise energy of ATP) and secondary plasmalemmal transporters or solute carriers (SLC) which use concentration gradients.
Astroglial Na+-K+ pump (NKA) has the idiosyncratic α2 catalytic subunit, which distinguishes it from the neuronal NKA, which contains α1 or α3 subunits. The affinity of the astroglial α2 subunit to extracellular K+ is much lower as compared to neuronal α1 and α3 subunits: [K+]0.5 for NKA composed from α2/β1 subunits is ~3.6 mM, whereas [K+]0.5 for neuronal NKAs assembled from α1/β1, α1/β2, α3/β1 or α3/β2 subunits lies between 0.25 and 0.65 mM (Larsen et al. 2014). As consequence, the activity of astroglial NKA is stimulated by physiological increases in the extracellular K+ concentration. To the contrary neuronal NKA is fully saturated and hence cannot respond to increases in extracellular K+ (Hertz et al. 2015). This stipulates the leading role of NKA in astrocytic K+ buffering (Verkhratsky and Nedergaard 2018).
Astrocytic SLC transporters are many and they perform numerous homeostatic functions. The largest group of homeostatic SLC transporters is represented by transporters for neurotransmitters; the majority of these transporters utilise transmembrane Na+ gradient to move neurotransmitters across the plasma membrane. Astrocytic transporters for glutamate are known as excitatory amino acid transporters 1 and 2 (EAAT1/SLC1A6 and EAAT2/SLC1A2 (Zhou and Danbolt 2013)), which in rodent experiments are referred to as glutamate-aspartate transporter (GLAST (Storck et al. 1992)) and glutamate transporter 1 (GLT-1 (Pines et al. 1992)). EAAT1 is mainly present in the cerebellum, in the retina and in circumventricular organs, whereas EAAT2 is expressed in all other parts of the brain; at the cellular level both transporters localise to perisynaptic astroglial processes (Zhou and Danbolt 2013; Verkhratsky and Nedergaard 2018). Glutamate transporters are Na+-dependent with stoichiometry 3 Na+, 1 H+ and 1 glutamate (in): 1 K+ (out) (Zerangue and Kavanaugh 1996). This transporter is electrogenic and generates transmembrane current carried mainly by Na+ ions (Kirischuk et al. 2007). In addition to EAATs, astrocytes express the cystine/glutamate antiporter Sxc− (SLC7A11) which is critical for cystine accumulation needed for the production of glutathione (Bridges et al. 2012). Glutamate homeostasis also depends on astrocytic glutamine transporters SNAT3/SLC38A3 and SNAT5/SLC38A5 which couple transport of glutamine with co-transport of 1 Na+ and counter-transport of 1 H+ (Todd et al. 2017). Astroglial GABA transporters are mainly represented by GAT3/SLC6A11 with stoichiometry of 1 GABA: 2 Na+: 1 Cl− (Kavanaugh et al. 1992); moderate increases in cytoplasmic Na+ may reverse this transporter (Unichenko et al. 2012). Astrocytes in the spinal cord possess glycine transporter GlyT1/SLC6A9 with stoichiometry of 1 glycine: 2 Na+: 1 Cl−; this transporter also can reverse in physiological conditions (Shibasaki et al. 2017). Monoamine accumulation into astrocytes is mediated by norepinephrine transporter NET/SLC6A2 that co-transports monoamines with 2 Na+ and 1 Cl− (Takeda et al. 2002). Astrocytes transport adenosine by equilibrative (i.e. controlled by adenosine transmembrane gradient (King et al. 2006)) plasmalemmal transporters ENT-1/SLC29A1, ENT-2/SLC29A2, ENT-3/SLC29A3 and ENT-4/SLC29A4, and Na+-dependent concentrative nucleoside plasmalemmal transporters CNT2/SLC28A2 and CNT3/SLC28A3 (King et al. 2006; Li et al. 2013).
The next group of SLC transporters is responsible for ion exchange. The most prominent member of this group is plasmalemmal sodium-calcium exchanger present in astrocytes in three isoforms NCX1/SLC8A1, NCX2/SLC8A2 and NCX3/SLC8A3 (Pappalardo et al. 2014b). Stoichiometry of astrocytic NCX is 3 Na+: 1 Ca2+ which sets the equilibrium potential at ~ −85 to −90 mV; this is very close to the resting membrane potential of astrocytes, and hence minor depolarisations or moderate increases in cytosolic Na+ readily reverse NCX operation from the forward mode (extrusion of Ca2+ in exchange for Na+) to the reverse mode (Ca2+ influx in exchange for Na+ exit) (Verkhratsky et al. 2018). Another transporter expressed in astrocytes is the NHE1/SLC9A1 Na+-H+ exchanger (Deitmer and Rose 1996; Chesler 2003) with electroneutral stoichiometry of 1 Na+ (in): 1 H+ (out) (Orlowski and Grinstein 1997); this transporter mediates efflux of protons from astrocytes (Rose and Ransom 1996; Chesler 2003). Additional transporter involved in pH homeostasis is the sodium-bicarbonate transporter NBCe1/SLC4A4 with stoichiometry of 1 Na+: 2 HCO3− or 1 Na+: 3 HCO3−, which allows its operation in both forward and reverse modes (Theparambil et al. 2015). The Na+-K+-Cl− co-transporter NKCC1/SLC12A2 with electroneutral stoichiometry of 1 Na+: 1 K+: 2 Cl− contributes to K+ and Cl− homeostasis especially in pathology (Macaulay and Zeuthen 2012).
Finally, astrocytes express several metabolic transporters responsible for transmembrane movements of energy substrates. In particular, astrocytes possess glucose transporter GLUT1/SLC2A1 predominantly localised in endfeet (Allen and Messier 2013). Monocarboxylate transporters 1 and 4 (MCT1/SLC16A1, MCT4/SLC16A3) are responsible for the export of lactate from astrocytes for local energy support of neurones; in certain conditions, however, these transporters can mediate lactate accumulation (Halestrap 2012).
Intracellular Excitability of Astrocytes
Astrocytes (similarly to other neuroglia) are electrically non-excitable cells of the CNS; in contrast to neurones astrocytes do not generate regenerative and propagating action potentials. At the same time astrocytes do generate active responses to all changes in nervous tissue environment; these responses are linked to transient fluctuations in cellular ionic content or generation/degradation of intracellular messengers. These molecular changes are the substrate for astrocytic excitability.
Astrocyte Ca2+ Excitability
Calcium ions are universal and evolutionary conserved intracellular messengers, involved in the regulation of numerous cellular processes, such as excitation-contraction coupling, secretion, gene expression, metabolism and cell death (Berridge et al. 2000; Carafoli 2002; Petersen et al. 2005; Plattner and Verkhratsky 2015). Cellular Ca2+ signalling and homeostatic control over cytosolic Ca2+ concentration ([Ca2+]i) rely on dedicated molecules, which include membrane channels and transporters, intracellular Ca2+-binding proteins and numerous Ca2+ sensors represented by Ca2+-regulated proteins.
Astrocytic Ca2+ excitability was discovered in experiments in vitro which demonstrated that challenging cultured astrocytes with neurotransmitters and neurohormones triggered [Ca2+]i transients and propagating Ca2+ waves (Enkvist et al. 1989; Cornell-Bell et al. 1990; McCarthy and Salm 1991; Verkhratsky and Kettenmann 1996; Petersen et al. 2005). Molecular pathways underlying astrocytic Ca2+ excitability rely on both intracellular and extracellular Ca2+ sources. Generation of Ca2+ signals in the soma and primary processes mainly depends on metabotropic receptors, which activate PLC that produces InsP3, which in turn activates InsP3 receptors residing in the membrane of the ER. This organelle is the main Ca2+ storage organelle in living cells; it is capable of accumulating, storing and releasing Ca2+ in physiological context. Concentration of ionised Ca2+ in the lumen of the ER is rather high, ranging between 200μM and 1 mM (Alonso et al. 1999; Alvarez and Montero 2002; Solovyova and Verkhratsky 2002; Solovyova et al. 2002). High intraluminal [Ca2+] creates a gradient aimed at the cellular cytosol; hence opening of Ca2+ release channels dwelling in the endomembrane generates Ca2+ efflux and thus generates cytosolic Ca2+ signals; in addition, intraluminal Ca2+ regulates both Ca2+ pump (SERCA, SarcoEndoplasmic Reticulum Ca2+-ATPase) and Ca2+ release channels (Burdakov et al. 2005). Besides being a dynamic Ca2+ store, the ER is responsible for protein synthesis, folding and haulage to their respective destinations, with all these processes being regulated by luminal [Ca2+] (Verkhratsky and Petersen 2002). Finally, the ER creates intracellular Ca2+ tunnels which allow long-distance diffusion of Ca2+, which is impossible in heavy Ca2+-buffered cytosol (Petersen and Verkhratsky 2007).
Both classes of intracellular Ca2+ release channels, the InsP3 receptors and ryanodine receptors (which are Ca2+-gated Ca2+channels) are expressed in glial cells (Verkhratsky et al. 1998). In astrocytes the type II InsP3 receptors predominate; these receptors are activated by InsP3 and positively modulated by [Ca2+]i; local increases in [Ca2+]i therefore can generate propagating wave of opening of InsP3 receptors along the ER membrane, making the latter an excitable media sustaining propagating Ca2+ waves (Verkhratsky et al. 2012).
As alluded before, release of Ca2+ from the ER, mediated by InsP3 receptors, represents the leading mechanism for Ca2+ signal generation in the soma and organelle-bearing processes; depletion of ER Ca2+ store activates secondary Ca2+ influx through plasmalemmal store-operated pathway mediated by TRP and ORAI channels (Burnstock and Verkhratsky 2012). |Geeneration of Ca2+ signals in the distal leaflets is different as it mainly relies on plasmalemmal Ca2+ entry (Fig. 5). The principal route for Ca2+ entry in response to neuronal activity is associated with the reversal of Na+-Ca2+ exchanger (NCX). The latter is controlled by transmembrane Na+ gradient; increase in [Na+]i associated with operation of glutamate transporters reverses the NCX (Reyes et al. 2012; Rose et al. 2020). In addition, Ca2+ entry into the leaflets is mediated by ionotropic receptors and possibly by TRP channels (Verkhratsky et al. 2012). Such a dichotomy is characteristic for protoplasmic astrocytes; in radial Bergmann glial cells, the main mechanism for Ca2+ signal generation is represented by PLC-InsP3 pathway (Kirischuk et al. 1999). The complex molecular machinery responsible for Ca2+ signalling in astrocytes results in emergence of spatially and temporally distinct Ca2+ signals in the form of microdomains or propagating Ca2+ waves of global Ca2+ signals (Grosche et al. 1999; Shigetomi et al. 2013; Khakh and Sofroniew 2015; Arizono et al. 2020).
Fig. 5.
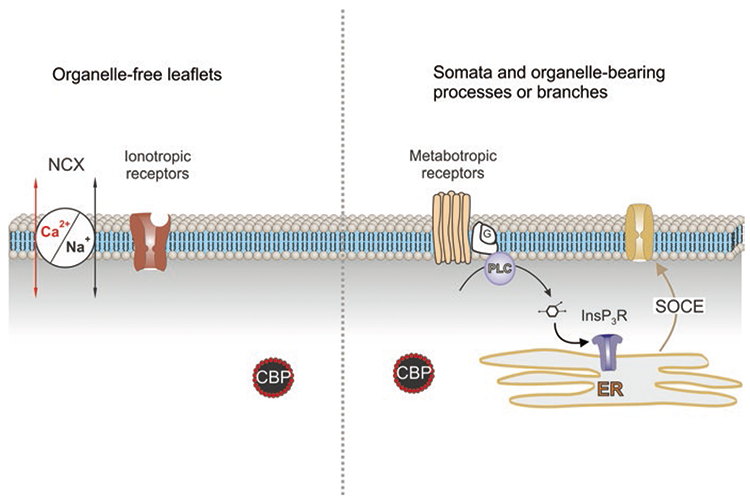
Ca2+ signalling in different compartments in protoplasmic astrocyte. Calcium signalling in the organelle-free leaflets is associated with Ca2+ entry through ionotropic receptors (NMDA glutamate receptors or P2X purinoceptors) or Ca2+-permeable channels (e.g. TRPA1 channels). Plasmalemmal Ca2+ influx can also be mediated by the Na+/Ca2+ exchanger (NCX) operating in the reverse mode. Calcium signalling in soma and branches is mainly associated with Ca2+ release from the endoplasmic reticulum (ER) with subsequent store-operated Ca2+ entry (SOCE). This Ca2+ release is mediated by InsP3 receptors (InsP3R); InsP3 is synthesised by phospholipase C (PLC) linked to G-protein metabotropic receptors. CBP, calcium binding proteins. (Reproduced with permission from Verkhratsky et al. (2020))
Sodium Signalling
The foundations for astrocytic Na+ signalling are associated solely with Na+ entry mediated by various proteins in the plasma membrane (Fig. 6). Cells have neither intracellular Na+ stores nor cytosolic Na+ buffers (Kirischuk et al. 2012; Rose and Verkhratsky 2016). Physiological stimulation triggers [Na+]i transients in astrocytes in vitro and in situ (Rose and Ransom 1996; Kirischuk et al. 1997; Langer and Rose 2009). The main source for generation of Na+ signals is mediated by Na+ entry through Na+-coupled SLC transporters, whereas extrusion of Na+ is primarily mediated by NKA (Rose and Verkhratsky 2016). In addition, Na+ influx can be mediated by ionotropic receptors and plasmalemmal channels, in particular TRPC channels (Reyes et al. 2013). Among Na+-dependent SLC transporters, glutamate and GABA transporters play the leading role in generation of Na+ signals. Increase in extracellular glutamate associated with neurotransmission is followed by rapid accumulation of glutamate into astrocytes; translocation of a single glutamate molecule is coupled with entry of 3 Na+. Increase in cytosolic Na+ concentration ([Na+]i) in response to glutamate may reach 10–20 mM (Kirischuk et al. 2007; Bennay et al. 2008). The main physiological target of astroglial Na+ signals is represented by SLC transporters sensitive to transmembrane Na+ gradients (Fig. 7); in addition [Na+]i regulates glutamine-glutamate (GABA) shuttle through direct action on glutamine synthetase and regulation of glutamine transporters (Benjamin 1987; Todd et al. 2017).
Fig. 6.
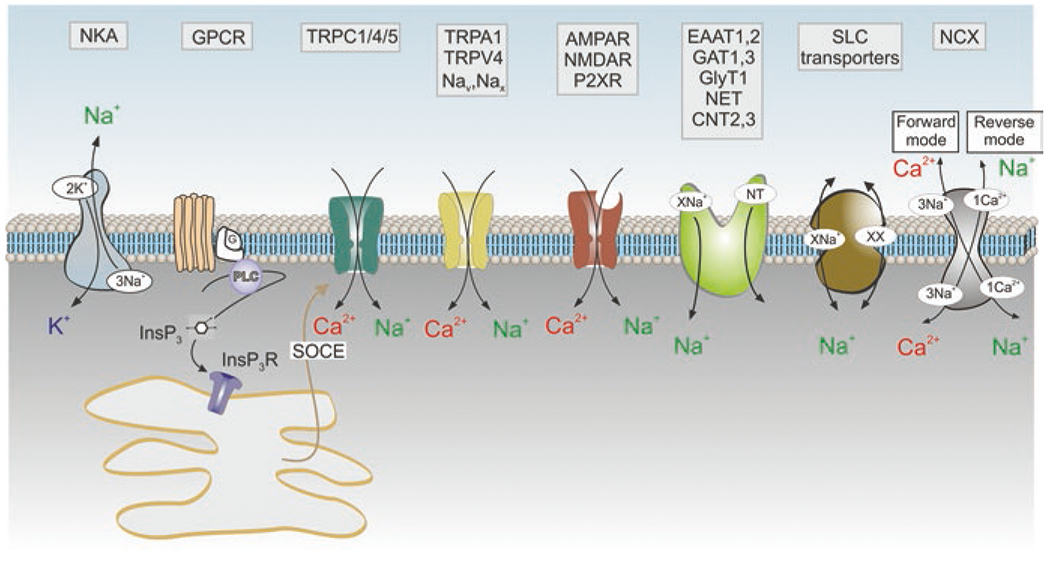
Membrane molecular pathways of Na+ signalling in astrocytes. Influx of Na+ occurs though (i) Na+-permeable channels which include ionotropic receptors (AMPAR, NMDAR, P2XR: AMPA, NMDA glutamate receptors and ionotropic purinoceptors, respectively); channels of the transient receptor potential (TRP) family (TRPC1/4/5 channels that operate as a part of store-operated Ca2+ entry and hence generate Na+ influx in response to the depletion of endoplasmic reticulum Ca2+ stores; as well as TRPA1 and TRPV4 channels); voltage-dependent Nav channels and [Na+]o-activated Nax channels; (ii) through Na+-dependent SLC transporters that include excitatory amino acid transporters EAAT1,2, GABA transporters GAT 1,3, glycine transporters GlyT, noradrenaline transporters NET and concentrative adenosine transporters CNT2,3. The main pathway for Na+ exit is provided by Na+-K+ pump, NKA. The Na+-Ca2+ exchanger NCX fluctuates between forward and reverse modes and couples Na+ and Ca2+ signalling. Other abbreivations as in Fig. 5. (Reproduced with permission from Verkhratsky et al. (2020))
Fig. 7.
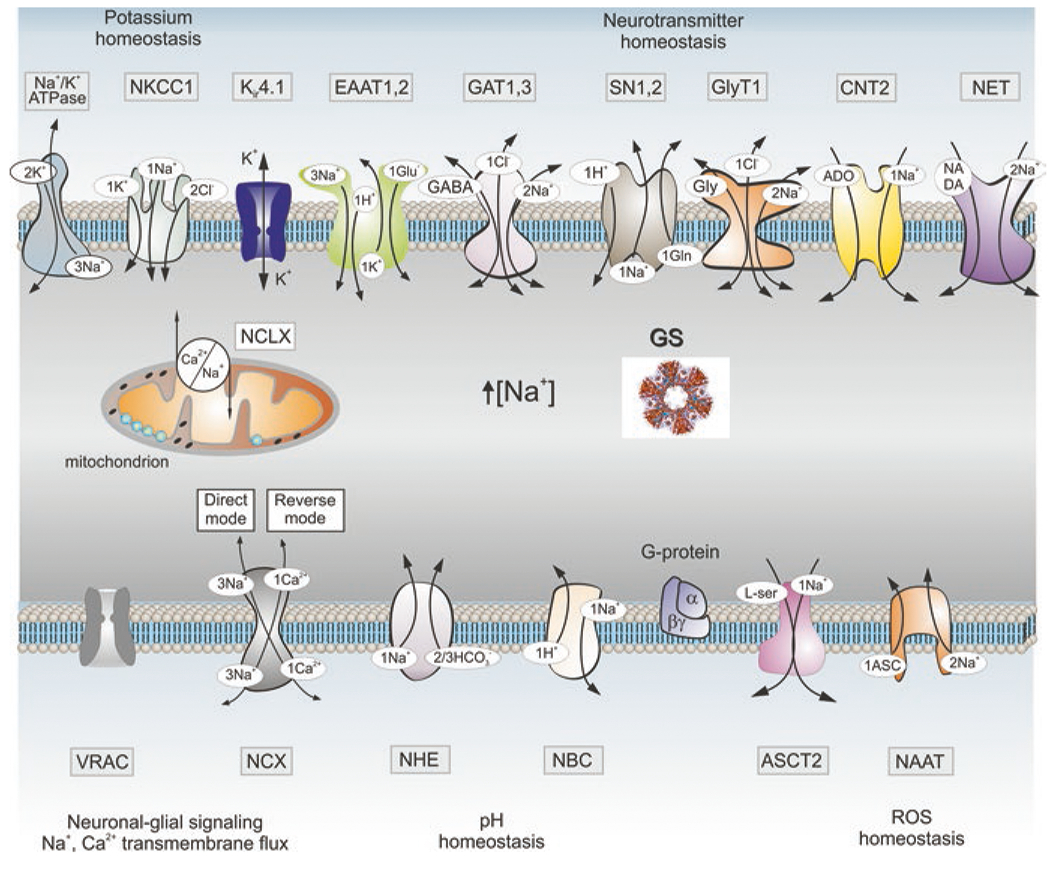
Molecular targets of Na+ signalling in astroglia. Abbreviations: ASCT2 alanine-serine-cysteine transporter 2, ASIC acid sensing ion channels, CNT2 concentrative nucleoside transporters, EAAT excitatory amino acid transporters, ENaC epithelial sodium channels, GAT GABA transporters, GS glutamine synthetase, GlyT1 glycine transporter, iGluRs ionotropic glutamate receptors, Nax Na+ channels activated by extracellular Na+, NAAT Na+-dependent ascorbic acid transporter, NBC Na+/HCO3− (sodium-bicarbonate) co-transporter, NCX Na+/Ca2+ exchanger, NCLX mitochondrial Na+/Ca2+ exchanger, NHE Na+/H+ exchanger, NKCC1 Na+/K+/Cl− co-transporter, NET norepinephrine transporter, MCT1 monocarboxylase transporter 1, P2XRs ionotropic purinoceptors, SN1/2 sodium-coupled neutral amino acid transporters which underlie exit of glutamine, TRP transient receptor potential channels, ROS reactive oxygen species, VRAC volume-regulated anion channels. Other abbreviations as in Fig. 5. See text for further explanation. (Modified and reproduced from Verkhratsky and Nedergaard (2018))
Astrocyte Functions
Astrocytes perform every known housekeeping and homeostatic function in the CNS from structural support and control over molecular homeostasis to regulation of blood flow, synaptogenesis, neurogenesis and development of the nervous system.
Ion Homeostasis in the CNS, or Ionostasis
Controlling ion concentrations in the interstitial fluids is of paramount importance for the functional activity of the nervous tissue, as even minor fluctuation in ionic composition may have profound influences on neuronal excitability and information processing in the neuronal networks. In particular, changes in ion concentrations are linked to changes in the brain state, such as sleep and arousal (Ding et al. 2016), while fluctuations in ion composition of interstitial fluids affect learning and memory (Hertz and Chen 2016). Control over ionostasis is one of the most fundamental functions of astrocytes.
Astrocytes are central for regulation of extracellular K+ concentration, the latter being directly linked to neuronal activity and neuronal excitability. Neuronal firing and synaptic transmission are associated with substantial K+ efflux, which may significantly change K+ concentration in the narrow and diffusion-restricted extracellular compartments. The leading role of astrocytes in the regulation of extracellular K+ homeostasis has been proposed in the mid-1960s by Leif Hertz, Steven Kuffler and Richard Orkand (Hertz 1965; Kuffler and Nicholls 1966; Orkand et al. 1966). Astrocytic K+ clearance from the interstitial fluid is mainly accomplished by active transport by NKA (Larsen et al. 2014), as astrocytic NKA is directly activated by K+ rise (see above). After being taken up by astrocytes, K+ is subsequently released by Kir4.1 channels back into the extracellular space, from which it is taken up by neurones to restore ionic gradients (Hertz and Chen 2016; Larsen et al. 2016). Such scenario implies the generation of K+ microdomains, which might be supported by physical properties of bilipid membranes trapping ions in narrow compartments of astrocytic leaflets (Breslin et al. 2018). In Müller glial cells in the retina, K+ buffering is mainly accomplished by K+ entry through Kir4.1 channels localised in perisynaptic processes in the inner plexiform layer of the retina. Subsequently, K+ is spred through the cell and is released (again through Kir4.1 channels) from the end-foot distantly to the site of accumulation; this process is known as K+ siphoning (Newman et al. 1984; Kofuji and Newman 2004).
Another important extracellular ion that may fluctuate during neuronal activity and synaptic transmission is Ca2+. Opening of plasmallemal Ca2+ channels with subsequent Ca2+ influx into presynaptic boutons may result in substantial drop in Ca2+ concentration in the narrow synaptic cleft, thus affecting neurotransmitter release (Rusakov and Fine 2003). Astrocytes seem to provide a pathway for replenishment of extracellular Ca2+ as decrease in [Ca2+]o below ~0.5 mM induces InsP3-induced Ca2+ release from the ER in astrocytes (Zanotti and Charles 1997). This Ca2+ then can be transported to extracellular space through plasmalemmal Ca2+ pumps or NCX.
A similar situation may also happen with extracellular Cl−. Overstimulation of neuronal ionotropic GABAA receptors may result in substantial Cl− accumulation into neurones; reduction in extracellular Cl− concentration may subsequently impair inhibitory transmission. As astrocytes have high cytoplasmic Cl− concentration, activation of astrocytic ionotropic GABAA receptors may initiate substantial Cl− efflux, thus restoring extracellular Cl− concentration (Kettenmann and Verkhratsky 2008). Indeed, manipulations with astrocytic intracellular Cl− were demonstrated to affect inhibitory transmission in hippocampal slices (Egawa et al. 2013). Astrocytes are also principal players in regulation of extracellular pH through transport of H+ and HCO3− (Deitmer and Rose 2010).
Neurotransmitter Homeostasis
Astrocytes control neurotransmitter homeostasis in the brain through removal (by dedicated transporters) and inactivation (by enzymatic conversion) of all four major neurotransmitters, namely, glutamate, GABA, adenosine and noradrenaline (Fig. 8).
Fig. 8.
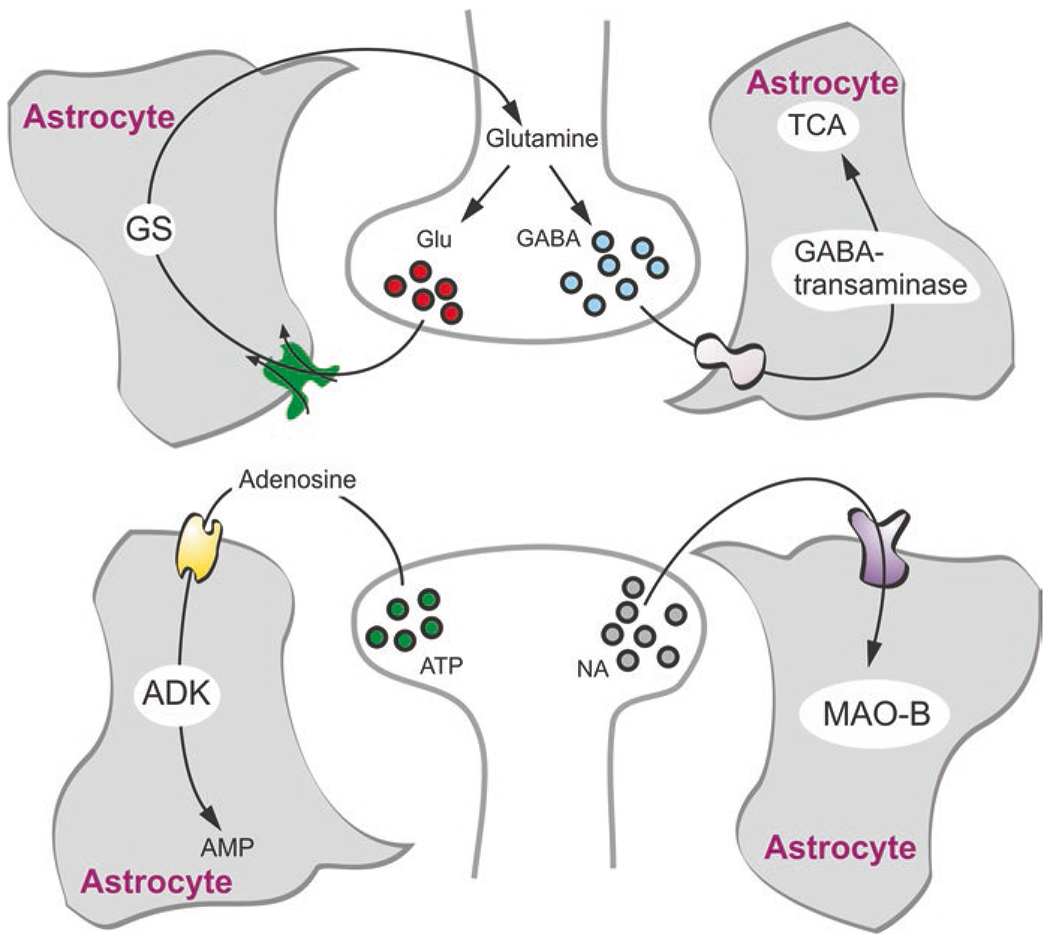
Astrocytes and neurotransmitter homeostasis. Astrocytes take up glutamate, GABA, adenosine and monoamines. Glutamate (Glu) is converted to glutamine by glutamine synthetase (GS) in astrocytes which also synthesise this transmitter de novo. In turn, glutamine is shuttled to neurones for subsequent conversion into glutamate and GABA. Astroglial accumulated GABA is mainly transaminated and consumed in tricarboxylic acid cycle (TCA). Adenosine is converted to AMP by adenosine kinase (ADK), while monoamines are degraded by astroglial monoamine oxidase type B (MAO-B). (Modified and reproduced from Verkhratsky and Nedergaard (2018))
Astrocytic glutamate homeostatic system is an indispensable element for glutamatergic and GABAergic transmission because it provides for (i) clearance of glutamate and GABA from the synaptic cleft, (ii) prevention of glutamate spillover and (iii) replenishment of the glutamate and GABA releasable pools of vesicles in the neuronal terminals by providing the obligatory precursor glutamine (Hertz et al. 1999; Verkhratsky and Nedergaard 2018). Astrocytic glutamate clearance also protects nervous tissue against glutamate excitotoxicity.
Neurones do not have enzymes for de novo synthesis of glutamate from pyruvate (generated from glucose or lactate); this synthesis occurs solely in astrocytes (Hertz et al. 1999). Glutamate produced in astrocytes is converted, with another astrocyte-specific enzyme, glutamine synthetase, into biologically inert glutamine, which is subsequently transported to neurones; when in neurones glutamine is converted into glutamate by glutaminase. In inhibitory terminals, glutamate is further converted into GABA.
Astrocytic glutamate transporters EAAT1/2 are responsible for the clearance of ~80% of all glutamate released during synaptic transmission (Danbolt 2001). Removal of glutamate by astrocytes dynamically modulates glutamate concentration in the synaptic cleft thus shaping kinetics of neuronal postsynaptic excitatory potentials (Marcaggi and Attwell 2004; Tzingounis and Wadiche 2007). Glutamate entering into astrocytes can undergo conversion to glutamine, which is subsequently shuttled to neurones through Na+-dependent glutamine transporters (described in a previous section). Increase in [Na+]i resulting from EAAT1/2-mediated uptake of glutamate stimulates glutamine efflux (Todd et al. 2017). This sequence of plasmalemmal transporting and enzymatic conversion is known as the glutamine-glutamate (GABA) shuttle that is fundamental for sustaining both excitatory and inhibitory neurotransmission in the CNS (Hertz 2013).
Astrocytes provide substantial contribution to the catabolism of monoamines in the CNS. The main converting enzyme that catabolises noradrenalin (norepinephrine), dopamine and serotonin, the monoamine oxidase B (MAO A/B), is preferentially expressed in astrocytes (Saura et al. 1992; Hertz et al. 2004). Monoamines are accumulated into astrocytes through plasmalemmal Na+-dependent norepinephrine transporter NET/SLC6A2 and possibly by dopamine transporter DAT/SLC6A3 (Verkhratsky and Nedergaard 2018). Finally, astrocytes express high levels of adenosine kinase which catalyses the bulk of adenosine conversion into AMP (Boison 2008); genetic deletion of adenosine kinase is incompatible with life (Boison et al. 2010).
Astroglial Cradle in Regulation of Synaptic Transmission
Astrocytic processes, including branches and leaflets, establish intimate dynamic contacts with synaptic structures; at least 50–60% of all synaptic contacts in the CNS are covered with astrocytic membranes (Witcher et al. 2007; Reichenbach et al. 2010). These astrocytic perisynaptic structures play most fundamental role in synaptic connectivity being involved in the regulation of synaptogenesis, synaptic maturation, synaptic maintenance, synaptic isolation and synaptic extinction; this multitude of functions stipulated the emergence of the concept of astroglial cradle (Nedergaard and Verkhratsky 2012; Verkhratsky and Nedergaard 2014) (Fig. 9). Astrocytes express numerous specific pathways through which they can regulate various aspects of synaptic transmission (Augusto-Oliveira et al. 2020).
Fig. 9.
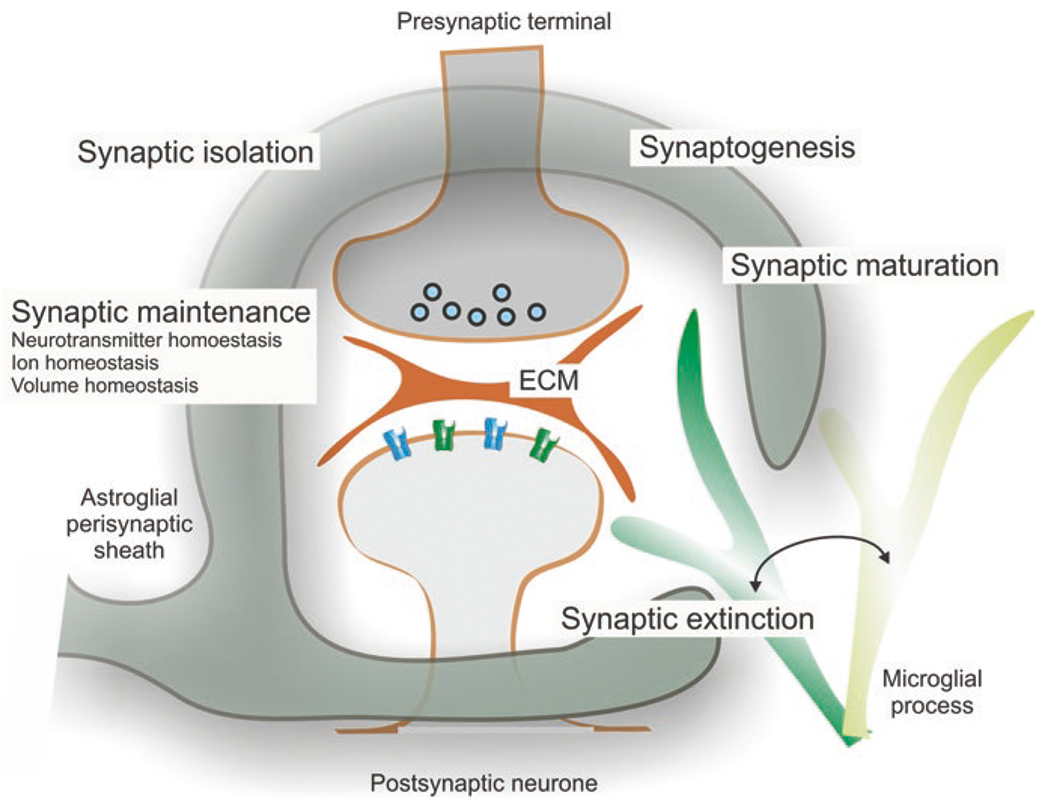
Astrocytic synaptic cradle. Astroglial cradle embraces and fosters multipartite synapse in the CNS. The majority of synapses in the brain and in the spinal cord is composed of several components that include the presynaptic terminal. These components are: the postsynaptic part, the perisynaptic process of the astrocyte, the process of neighbouring microglial cell that periodically contacts the synaptic structure and the extracellular matrix (ECM) present in the synaptic cleft and also extending extra-synaptically. Astroglial perisynaptic sheath enwraps synaptic structures; regulates, influences and assists synaptogenesis, synaptic maturation, synaptic maintenance and synaptic extinction; and also modulates synaptic transmission and plasticity. (Reproduced from Verkhratsky and Nedergaard (2014))
Synaptogenesis in particular requires astrocytes and astrocytic factors. The massive emergence of excitatory glutamatergic synapses occurs in the early postnatal period and coincides with massive wave of astrocytogenesis. Without astrocytes, synapses do not form in vitro, and similarly astrocytes are necessary for synapse formation in vivo (Pfrieger and Barres 1997; Eroglu and Barres 2010). Astrocytic support on synaptogenesis is mediated by the release of several factors, such as cholesterol, needed for membrane formation (Mauch et al. 2001), thrombospondins (Eroglu et al. 2009) estradiol, protocadherins or integrins (Pfrieger 2010). Astrocytes also produce and release hevin, which promotes the formation of excitatory synapses (Kucukdereli et al. 2011). Synaptic maturation also depends on astrocytic factors such as activity-dependent neurotrophic factor and tumour necrosis factor α, which regulate the trafficking of glutamate receptors to postsynaptic membranes (Eroglu and Barres 2010) and glypicans 4 and 6 that up-regulate the density of AMPA-type glutamate receptors at postsynaptic sites (Allen et al. 2012). Astrocytes maintain synaptic transmission through numerous homeostatic cascades and membrane transporters that control clearance of neurotransmitters and supply neuronal terminals with neurotransmitter precursors (such as glutamine or L-serine). Finally, astrocytes contribute to synaptic elimination by labelling terminals with complement factor C1q, which is recognised by microglia as an ‘eat-me’ signal that initiates synaptic pruning (Kettenmann et al. 2013).
Astrocytes Protect Nervous Tissue Against Reactive Oxygen Species
Nervous tissue has exceptional energy demands. The human brain, while representing only 2% of the body mass, consumes >20% of organism energy; most of this energy fuels NKA-dependent redressing of ionic gradients (Magistretti 2009). This high-energy consumption is associated with excessive production of ROS that need to be scavenged and neutralised. The anti-oxidant system of the nervous tissue mostly relies on glutathione and ascorbic acid (Makar et al. 1994). In the brain the bulk of glutathione is concentrated in astrocytes (Dringen et al. 2000), while neuronal synthesis of glutathione relies on astrocytes providing obligatory precursors cysteine or glutamylcysteine. Astrocytes accumulate cystine through the Sxc− glutamate/cystine exchanger, which is not expressed in neurones. Subsequently cystine is reduced to cysteine, which is further converted to glutamylcysteine (CysGlu) and glutathione. Astrocytes release cysteine which is shuttled to neurones (Chen and Swanson 2003). In the presence of astrocytes, in vitro neurones sustain high levels of glutathione, whereas removal of astrocytes instigates ROS neuronal damage (Dringen et al. 1999). Astrocytes also act as a reservoir for another anti-oxidant, ascorbic acid, which similarly protects neurones against oxidative stress (Wilson et al. 2000).
Neurogliovascular Unit and the Blood-Brain Barrier
The brain is one of the most vascularised organs in the human body. In addition, the brain vasculature possesses the BBB, which controls the nature of molecules entering the nervous tissue. Neuronal activity is functionally linked to the local circulation, and an increase in neuronal firing initiates focal vasodilatation of arterioles and capillaries, a phenomenon known as functional hyperemia (Mosso 1880; Roy and Sherrington 1890). Astrocytes are active contributors to both the barrier function and regulation of the local blood flow.
Protoplasmic astrocytes divide the grey matter into spatially segregated and relatively independent domains, within which astrocytes integrate neurones, synapses, microglial cells and neighbouring capillaries into the neurogliovascular (also known as neurovascular) unit (Iadecola 2017). The glia limitans perivascularis formed by astroglial endfeet cover the parenchymal part of brain vessels almost entirely (Mathiisen et al. 2010). The endfeet are functionally coupled to capillaries through the release of vasoconstrictors and vasodilatators, which include derivatives of arachidonic acid. In addition, the vascular tone can be regulated by local release of K+ (Zonta et al. 2003; Mulligan and MacVicar 2004; Filosa et al. 2006). Astrocytes can also function as intracranial baroreceptors contributing to the regulation of arterial blood pressure (Marina et al. 2020).
Astrocytes also form the parenchymal part of BBB. The barrier as such is created by the specialised brain capillary endothelial cells which are (together with pericytes and vascular smooth muscle cells) integrated into neurogliovascular unit. The contacts between brain endothelial cells are sealed with tight and adherent junctions, while several transporting systems ensure BBB permeability for selected molecules, which are allowed to enter the brain parenchyma (Sweeney et al. 2019). At the capillary level, astroglial endfeet, endothelial cells and pericytes share a common basement membrane. At the level of arteriolae, two basement membranes, the parenchymal (in contact with astroglial endfeet) and vascular (in contact with endothelial cells), create the perivascular space. Astrocytes are secreting numerous factors that control formation and maintenance of the BBB (Sweeney et al. 2019); in pathology, astroglia support dwindles which impairs the integrity of the barrier (Kriauciunaite et al. 2021).
Astrocytes and Gliotransmission
Astrocyte-neurone signalling, a.k.a. ‘gliotransmission’ (Santello et al. 2012; Verkhratsky et al. 2016), modulates synaptic transmission/plasticity at tripartite synapses as a part of the synaptic cradle (Perez-Alvarez and Araque 2013). Among the processes regulated by gliotransmission are sleep regulation (Haydon 2017), respiration (Sheikhbahaei et al. 2018) and learning/memory (Gibbs et al. 2011). Gliotransmission is a result of the astrocytic capacity to release several neurotransmitters and neuromodulators, which include ATP, glutamate, GABA, taurine and kynurenic acid. This release occurs through several pathways, which have been characterised in situ and in vitro astroglial preparations and include exocytotic (vesicular) release, diffusion through plasmalemmal channels (e.g. through unpaired connexons, i.e. hemichannels, or several types of large anion/cation channels), via reversed neurotransmitter transporters or previously mentioned cystine-glutamate exchangers (Verkhratsky et al. 2016).
Astrocytes and the Glymphatic System
The brain has a specialised system for the removal of soluble waste products known as a glymphatic system (Iliff et al. 2012; Nedergaard 2013). This system utilises perivascular space between parenchymal and vascular basement membranes and astrocytic AQP4 channels concentrated at the perivascular endfeet to create fluid flow through the brain parenchyma. This fluid flow is instrumental for interstitial solute clearance and is critical for the normal function of the brain. In pathology, under stress or in ageing, the glymphatic subsystem is often impaired; deficient glymphatic clearance contributes to the pathophysiology of several brain disorders including neurodegenerative and psychiatric diseases (Liang et al. 2020; Nedergaard and Goldman 2020).
Envoi
We have tersely summarised the morphology and function of astroglia. We presented them as a group of ectodermal cells with diverse morphology and specific functions, superordinate to the principle function of maintaining homeostasis of the central nervous system. We consider this summary as a necessity in order to better understand a possible role of these cells in pathophysiological condition and diseases.
Acknowledgements
BL’s work is supported by the National Natural Science Foundation of China (grant number 8187185), LiaoNing Revitalization Talents Program (grant number XLYC1807137), the Scientific Research Foundation for Returned Scholars of Education Ministry of China (grant number 20151098), LiaoNing Thousands Talents Program (grant number 202078) and ‘ChunHui’ Program of Education Ministry of China (grant number 2020703). CS’s work is supported by a grant from the Italian Ministry of Education, University and Research (2015KP7T2Y_002) and a grant from Sapienza University of Rome (RM11916B7A8D0225).
VP’s work is supported by a grant from the National Institute of General Medical Sciences of the National Institutes of Health (R01GM123971). VP is an Honorary Professor at University of Rijeka, Croatia.
Contributor Information
Alexei Verkhratsky, Faculty of Biology, Medicine and Health, The University of Manchester, Manchester, UK; Achucarro Center for Neuroscience, IKERBASQUE, Basque Foundation for Science, Bilbao, Spain.
Vladimir Parpura, Department of Neurobiology, The University of Alabama at Birmingham, Birmingham, AL, USA.
Baoman Li, Department of Forensic Analytical Toxicology, School of Forensic Medicine, China Medical University, Shenyang, China.
Caterina Scuderi, Department of Physiology and Pharmacology “Vittorio Erspamer”, SAPIENZA University of Rome, Rome, Italy.
References
- Abbracchio MP, Ceruti S (2006) Roles of P2 receptors in glial cells: focus on astrocytes. Purinergic Signal 2:595–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen A, Messier C (2013) Plastic changes in the astrocyte GLUT1 glucose transporter and beta-tubulin microtubule protein following voluntary exercise in mice. Behav Brain Res 240:95–102 [DOI] [PubMed] [Google Scholar]
- Allen NJ, Bennett ML, Foo LC, Wang GX, Chakraborty C, Smith SJ, Barres BA (2012) Astrocyte glypicans 4 and 6 promote formation of excitatory synapses via GluA1 AMPA receptors. Nature 486:410–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso MT, Barrero MJ, Michelena P, Carnicero E, Cuchillo I, Garcia AG, Garcia-Sancho J, Montero M, Alvarez J (1999) Ca2+-induced Ca2+ release in chromaffin cells seen from inside the ER with targeted aequorin. J Cell Biol 144:241–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez J, Montero M (2002) Measuring [Ca2+] in the endoplasmic reticulum with aequorin. Cell Calcium 32:251–260 [DOI] [PubMed] [Google Scholar]
- Andriezen WL (1893) The neuroglia elements of the brain. Br Med J 2:227–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arizono M, Inavalli V, Panatier A, Pfeiffer T, Angibaud J, Levet F, Ter Veer MJT, Stobart J, Bellocchio L, Mikoshiba K, Marsicano G, Weber B, Oliet SHR, Nagerl UV (2020) Structural basis of astrocytic Ca(2+) signals at tripartite synapses. Nat Commun 11:1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong WE, Rubrum A, Teruyama R, Bond CT, Adelman JP (2005) Immunocytochemical localization of small-conductance, calcium-dependent potassium channels in astrocytes of the rat supraoptic nucleus. J Comp Neurol 491:175–185 [DOI] [PubMed] [Google Scholar]
- Augusto-Oliveira M, Arrifano GP, Takeda PY, Lopes-Araujo A, Santos-Sacramento L, Anthony DC, Verkhratsky A, Crespo-Lopez ME (2020) Astroglia-specific contributions to the regulation of synapses, cognition and behaviour. Neurosci Biobehav Rev 118:331–357 [DOI] [PubMed] [Google Scholar]
- Bardehle S, Kruger M, Buggenthin F, Schwausch J, Ninkovic J, Clevers H, Snippert HJ, Theis FJ, Meyer-Luehmann M, Bechmann I, Dimou L, Gotz M (2013) Live imaging of astrocyte responses to acute injury reveals selective juxtavascular proliferation. Nat Neurosci 16:580–586 [DOI] [PubMed] [Google Scholar]
- Bekar LK, Loewen ME, Cao K, Sun X, Leis J, Wang R, Forsyth GW, Walz W (2005) Complex expression and localization of inactivating Kv channels in cultured hippocampal astrocytes. J Neurophysiol 93:1699–1709 [DOI] [PubMed] [Google Scholar]
- Benfenati V, Amiry-Moghaddam M, Caprini M, Mylonakou MN, Rapisarda C, Ottersen OP, Ferroni S (2007) Expression and functional characterization of transient receptor potential vanilloid-related channel 4 (TRPV4) in rat cortical astrocytes. Neuroscience 148:876–892 [DOI] [PubMed] [Google Scholar]
- Benjamin AM (1987) Influence of Na+, K+, and Ca2+ on glutamine synthesis and distribution in rat brain cortex slices: a possible linkage of glutamine synthetase with cerebral transport processes and energetics in the astrocytes. J Neurochem 48:1157–1164 [DOI] [PubMed] [Google Scholar]
- Bennay M, Langer J, Meier SD, Kafitz KW, Rose CR (2008) Sodium signals in cerebellar Purkinje neurons and Bergmann glial cells evoked by glutamatergic synaptic transmission. Glia 56:1138–1149 [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Lipp P, Bootman MD (2000) The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol 1:11–21 [DOI] [PubMed] [Google Scholar]
- Boison D (2008) Adenosine as a neuromodulator in neurological diseases. Curr Opin Pharmacol 8:2–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boison D, Chen JF, Fredholm BB (2010) Adenosine signaling and function in glial cells. Cell Death Differ 17:1071–1082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordey A, Sontheimer H (2000) Ion channel expression by astrocytes in situ: comparison of different CNS regions. Glia 30:27–38 [DOI] [PubMed] [Google Scholar]
- Brasko C, Hawkins V, De La Rocha IC, Butt AM (2017) Expression of Kir4.1 and Kir5.1 inwardly rectifying potassium channels in oligodendrocytes, the myelinating cells of the CNS. Brain Struct Funct 222:41–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslin K, Wade JJ, Wong-Lin K, Harkin J, Flanagan B, Van Zalinge H, Hall S, Walker M, Verkhratsky A, McDaid L (2018) Potassium and sodium microdomains in thin astroglial processes: a computational model study. PLoS Comput Biol 14:e1006151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges RJ, Natale NR, Patel SA (2012) System xc(−) cystine/glutamate antiporter: an update on molecular pharmacology and roles within the CNS. Br J Pharmacol 165:20–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffo A, Rossi F (2013) Origin, lineage and function of cerebellar glia. Prog Neurobiol 109:42–63 [DOI] [PubMed] [Google Scholar]
- Burdakov D, Petersen OH, Verkhratsky A (2005) Intraluminal calcium as a primary regulator of endoplasmic reticulum function. Cell Calcium 38:303–310 [DOI] [PubMed] [Google Scholar]
- Burnstock G, Verkhratsky A (2012) Purinergic signalling and the nervous system. Springer, Heidelberg [Google Scholar]
- Bushong EA, Martone ME, Jones YZ, Ellisman MH (2002) Protoplasmic astrocytes in CA1 stratum radiatum occupy separate anatomical domains. J Neurosci 22:183–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butenko O, Dzamba D, Benesova J, Honsa P, Benfenati V, Rusnakova V, Ferroni S, Anderova M (2012) The increased activity of TRPV4 channel in the astrocytes of the adult rat hippocampus after cerebral hypoxia/ischemia. PLoS One 7:e39959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt AM, Kalsi A (2006) Inwardly rectifying potassium channels (Kir) in central nervous system glia: a special role for Kir4.1 in glial functions. J Cell Mol Med 10:33–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt AM, Colquhoun K, Tutton M, Berry M (1994) Three-dimensional morphology of astrocytes and oligodendrocytes in the intact mouse optic nerve. J Neurocytol 23:469–485 [DOI] [PubMed] [Google Scholar]
- Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS, Xing Y, Lubischer JL, Krieg PA, Krupenko SA, Thompson WJ, Barres BA (2008) A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J Neurosci 28:264–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carafoli E (2002) Calcium signaling: a tale for all seasons. Proc Natl Acad Sci U S A 99:1115–1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan-Palay V, Palay SL (1972) The form of velate astrocytes in the cerebellar cortex of monkey and rat: high voltage electron microscopy of rapid Golgi preparations. Z Anat Entwicklungsgesch 138:1–19 [DOI] [PubMed] [Google Scholar]
- Chen Y, Swanson RA (2003) The glutamate transporters EAAT2 and EAAT3 mediate cysteine uptake in cortical neuron cultures. J Neurochem 84:1332–1339 [DOI] [PubMed] [Google Scholar]
- Chesler M (2003) Regulation and modulation of pH in the brain. Physiol Rev 83:1183–1221 [DOI] [PubMed] [Google Scholar]
- Chvatal A, Verkhratsky A (2018) Early history of neuroglial research: personalities. Neuroglia 1:245–281 [Google Scholar]
- Colombo JA (2017) The interlaminar glia: from serendipity to hypothesis. Brain Struct Funct 222:1109–1129 [DOI] [PubMed] [Google Scholar]
- Cornell-Bell AH, Finkbeiner SM, Cooper MS, Smith SJ (1990) Glutamate induces calcium waves in cultured astrocytes: long-range glial signaling. Science 247:470–473 [DOI] [PubMed] [Google Scholar]
- Danbolt NC (2001) Glutamate uptake. Prog Neurobiol 65:1–105 [DOI] [PubMed] [Google Scholar]
- Deitmer JW, Rose CR (1996) pH regulation and proton signalling by glial cells. Prog Neurobiol 48:73–103 [DOI] [PubMed] [Google Scholar]
- Deitmer JW, Rose CR (2010) Ion changes and signalling in perisynaptic glia. Brain Res Rev 63:113–129 [DOI] [PubMed] [Google Scholar]
- Ding F, O’Donnell J, Thrane AS, Zeppenfeld D, Kang H, Xie L, Wang F, Nedergaard M (2013) α1-adrenergic receptors mediate coordinated Ca2+ signaling of cortical astrocytes in awake, behaving mice. Cell Calcium 54:387–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding F, O’Donnell J, Xu Q, Kang N, Goldman N, Nedergaard M (2016) Changes in the composition of brain interstitial ions control the sleep-wake cycle. Science 352:550–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong JH, Chen X, Cui M, Yu X, Pang Q, Sun JP (2012) β2-adrenergic receptor and astrocyte glucose metabolism. J Mol Neurosci 48:456–463 [DOI] [PubMed] [Google Scholar]
- Dringen R, Pfeiffer B, Hamprecht B (1999) Synthesis of the antioxidant glutathione in neurons: supply by astrocytes of CysGly as precursor for neuronal glutathione. J Neurosci 19:562–569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dringen R, Gutterer JM, Hirrlinger J (2000) Glutathione metabolism in brain metabolic interaction between astrocytes and neurons in the defense against reactive oxygen species. Eur J Biochem 267:4912–4916 [DOI] [PubMed] [Google Scholar]
- Eaton MJ, Skatchkov SN, Brune A, Biedermann B, Veh RW, Reichenbach A (2002) SURI and Kir6.1 subunits of KATP-channels are co-localized in retinal glial (Muller) cells. Neuroreport 13:57–60 [DOI] [PubMed] [Google Scholar]
- Edwards L, Nashmi R, Jones O, Backx P, Ackerley C, Becker L, Fehlings MG (2002) Upregulation of Kv 1.4 protein and gene expression after chronic spinal cord injury. J Comp Neurol 443:154–167 [DOI] [PubMed] [Google Scholar]
- Egawa K, Yamada J, Furukawa T, Yanagawa Y, Fukuda A (2013) Cl− homeodynamics in gap junction-coupled astrocytic networks on activation of GABAergic synapses. J Physiol 591:3901–3917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enkvist MO, Holopainen I, Akerman KE (1989) Glutamate receptor-linked changes in membrane potential and intracellular Ca2+ in primary rat astrocytes. Glia 2:397–402 [DOI] [PubMed] [Google Scholar]
- Eroglu C, Barres BA (2010) Regulation of synaptic connectivity by glia. Nature 468:223–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eroglu C, Allen NJ, Susman MW, O’Rourke NA, Park CY, Ozkan E, Chakraborty C, Mulinyawe SB, Annis DS, Huberman AD, Green EM, Lawler J, Dolmetsch R, Garcia KC, Smith SJ, Luo ZD, Rosenthal A, Mosher DF, Barres BA (2009) Gabapentin receptor α2δ-1 is a neuronal thrombospondin receptor responsible for excitatory CNS synaptogenesis. Cell 139:380–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feig SL, Haberly LB (2011) Surface-associated astrocytes, not endfeet, form the glia limitans in posterior piriform cortex and have a spatially distributed, not a domain, organization. J Comp Neurol 519:1952–1969 [DOI] [PubMed] [Google Scholar]
- Filosa JA, Bonev AD, Straub SV, Meredith AL, Wilkerson MK, Aldrich RW, Nelson MT (2006) Local potassium signaling couples neuronal activity to vasodilation in the brain. Nat Neurosci 9:1397–1403 [DOI] [PubMed] [Google Scholar]
- Giaume C, Leybaert L, Naus CC, Saez JC (2013) Connexin and pannexin hemichannels in brain glial cells: properties, pharmacology, and roles. Front Pharmacol 4:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaume C, Naus CC, Saez JC, Leybaert L (2021) Glial connexins and pannexins in the healthy and diseased brain. Physiol Rev 101:93–145 [DOI] [PubMed] [Google Scholar]
- Gibbs ME, Shleper M, Mustafa T, Burnstock G, Bowser DN (2011) ATP derived from astrocytes modulates memory in the chick. Neuron Glia Biol 7:177–186 [DOI] [PubMed] [Google Scholar]
- Gotz S, Bribian A, Lopez-Mascaraque L, Gotz M, Grothe B, Kunz L (2021) Heterogeneity of astrocytes: electrophysiological properties of juxtavascular astrocytes before and after brain injury. Glia 69:346–361 [DOI] [PubMed] [Google Scholar]
- Grosche J, Matyash V, Moller T, Verkhratsky A, Reichenbach A, Kettenmann H (1999) Microdomains for neuron-glia interaction: parallel fiber signaling to Bergmann glial cells. Nat Neurosci 2:139–143 [DOI] [PubMed] [Google Scholar]
- Halestrap AP (2012) The monocarboxylate transporter family – structure and functional characterization. IUBMB Life 64:1–9 [DOI] [PubMed] [Google Scholar]
- Haydon PG (2017) Astrocytes and the modulation of sleep. Curr Opin Neurobiol 44:28–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz L (1965) Possible role of neuroglia: a potassium-mediated neuronal--neuroglial--neuronal impulse transmission system. Nature 206:1091–1094 [DOI] [PubMed] [Google Scholar]
- Hertz L (2013) The glutamate-glutamine (GABA) cycle: importance of late postnatal development and potential reciprocal interactions between biosynthesis and degradation. Front Endocrinol (Lausanne) 4:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz L, Chen Y (2016) Importance of astrocytes for potassium ion (K+) homeostasis in brain and glial effects of K+ and its transporters on learning. Neurosci Biobehav Rev 71:484–505 [DOI] [PubMed] [Google Scholar]
- Hertz L, Dringen R, Schousboe A, Robinson SR (1999) Astrocytes: glutamate producers for neurons. J Neurosci Res 57:417–428 [PubMed] [Google Scholar]
- Hertz L, Chen Y, Gibbs ME, Zang P, Peng L (2004) Astrocytic adrenoceptors: a major drug target in neurological and psychiatric disorders? Curr Drug Targets CNS Neurol Disord 3:239–267 [DOI] [PubMed] [Google Scholar]
- Hertz L, Gerkau NJ, Xu J, Durry S, Song D, Rose CR, Peng L (2015) Roles of astrocytic Na+, K+-ATPase and glycogenolysis for K+ homeostasis in mammalian brain. J Neurosci Res 93:1019–1030 [DOI] [PubMed] [Google Scholar]
- Hibino H, Fujita A, Iwai K, Yamada M, Kurachi Y (2004) Differential assembly of inwardly rectifying K+ channel subunits, Kir4.1 and Kir5.1, in brain astrocytes. J Biol Chem 279:44065–44073 [DOI] [PubMed] [Google Scholar]
- Hiyama TY, Yoshida M, Matsumoto M, Suzuki R, Matsuda T, Watanabe E, Noda M (2013) Endothelin-3 expression in the subfornical organ enhances the sensitivity of Nax, the brain sodium-level sensor, to suppress salt intake. Cell Metab 17:507–519 [DOI] [PubMed] [Google Scholar]
- Hutchinson DS, Summers RJ, Gibbs ME (2007) β2- and β3-adrenoceptors activate glucose uptake in chick astrocytes by distinct mechanisms: a mechanism for memory enhancement? J Neurochem 103:997–1008 [DOI] [PubMed] [Google Scholar]
- Iadecola C (2017) The neurovascular unit coming of age: a journey through neurovascular coupling in health and disease. Neuron 96:17–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, Benveniste H, Vates GE, Deane R, Goldman SA, Nagelhus EA, Nedergaard M (2012) A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci Transl Med 4:147ra111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illes P, Verkhratsky A, Burnstock G, Franke H (2012) P2X receptors and their roles in astroglia in the central and peripheral nervous system. Neuroscientist 18:422–438 [DOI] [PubMed] [Google Scholar]
- Kalsi AS, Greenwood K, Wilkin G, Butt AM (2004) Kir4.1 expression by astrocytes and oligodendrocytes in CNS white matter: a developmental study in the rat optic nerve. J Anat 204:475–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavanaugh MP, Arriza JL, North RA, Amara SG (1992) Electrogenic uptake of γ-aminobutyric acid by a cloned transporter expressed in Xenopus oocytes. J Biol Chem 267:22007–22009 [PubMed] [Google Scholar]
- Kettenmann H, Verkhratsky A (2008) Neuroglia: the 150 years after. Trends Neurosci 31:653–659 [DOI] [PubMed] [Google Scholar]
- Kettenmann H, Backus KH, Schachner M (1987) γ-aminobutyric acid opens Cl− channels in cultured astrocytes. Brain Res 404:1–9 [DOI] [PubMed] [Google Scholar]
- Kettenmann H, Kirchhoff F, Verkhratsky A (2013) Microglia: new roles for the synaptic stripper. Neuron 77:10–18 [DOI] [PubMed] [Google Scholar]
- Khakh BS, Sofroniew MV (2015) Diversity of astrocyte functions and phenotypes in neural circuits. Nat Neurosci 18:942–952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AE, Ackley MA, Cass CE, Young JD, Baldwin SA (2006) Nucleoside transporters: from scavengers to novel therapeutic targets. Trends Pharmacol Sci 27:416–425 [DOI] [PubMed] [Google Scholar]
- Kirischuk S, Tuschick S, Verkhratsky A, Kettenmann H (1996) Calcium signalling in mouse Bergmann glial cells mediated by α1-adrenoreceptors and H1 histamine receptors. Eur J Neurosci 8:1198–1208 [DOI] [PubMed] [Google Scholar]
- Kirischuk S, Kettenmann H, Verkhratsky A (1997) Na+/Ca2+ exchanger modulates kainate-triggered Ca2+ signaling in Bergmann glial cells in situ. FASEB J 11:566–572 [DOI] [PubMed] [Google Scholar]
- Kirischuk S, Kirchhoff F, Matyash V, Kettenmann H, Verkhratsky A (1999) Glutamate-triggered calcium signalling in mouse bergmann glial cells in situ: role of inositol-1,4,5-trisphosphate-mediated intracellular calcium release. Neuroscience 92:1051–1059 [DOI] [PubMed] [Google Scholar]
- Kirischuk S, Kettenmann H, Verkhratsky A (2007) Membrane currents and cytoplasmic sodium transients generated by glutamate transport in Bergmann glial cells. Pflugers Arch 454:245–252 [DOI] [PubMed] [Google Scholar]
- Kirischuk S, Parpura V, Verkhratsky A (2012) Sodium dynamics: another key to astroglial excitability? Trends Neurosci 35:497–506 [DOI] [PubMed] [Google Scholar]
- Kofuji P, Newman EA (2004) Potassium buffering in the central nervous system. Neuroscience 129:1045–1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kölliker AV (1896) Handbuch der Gewebelehre des Menschen. 6 Aufl. Engelmann, Leipzig [Google Scholar]
- Kriauciunaite K, Kausyle A, Pajarskiene J, Tunaitis V, Lim D, Verkhratsky A, Pivoriunas A (2021) Immortalised hippocampal astrocytes from 3xTG-AD mice fail to support BBB integrity in vitro: role of extracellular vesicles in glial-endothelial communication. Cell Mol Neurobiol 41:551–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucukdereli H, Allen NJ, Lee AT, Feng A, Ozlu MI, Conatser LM, Chakraborty C, Workman G, Weaver M, Sage EH, Barres BA, Eroglu C (2011) Control of excitatory CNS synaptogenesis by astrocyte-secreted proteins Hevin and SPARC. Proc Natl Acad Sci U S A 108:E440–E449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuffler SW, Nicholls JG (1966) The physiology of neuroglial cells. Ergeb Physiol 57:1–90 [PubMed] [Google Scholar]
- Kwon J, An H, Sa M, Won J, Shin JI, Lee CJ (2017) Orai1 and Orai3 in combination with Stim1 mediate the majority of store-operated calcium entry in astrocytes. Exp Neurobiol 26:42–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalo U, Pankratov Y, Kirchhoff F, North RA, Verkhratsky A (2006) NMDA receptors mediate neuron-to-glia signaling in mouse cortical astrocytes. J Neurosci 26:2673–2683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalo U, Pankratov Y, Wichert SP, Rossner MJ, North RA, Kirchhoff F, Verkhratsky A (2008) P2X1 and P2X5 subunits form the functional P2X receptor in mouse cortical astrocytes. J Neurosci 28:5473–5480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalo U, Pankratov Y, Parpura V, Verkhratsky A (2011) Ionotropic receptors in neuronal-astroglial signalling: what is the role of “excitable” molecules in non-excitable cells. Biochim Biophys Acta 1813:992–1002 [DOI] [PubMed] [Google Scholar]
- Langer J, Rose CR (2009) Synaptically induced sodium signals in hippocampal astrocytes in situ. J Physiol 587:5859–5877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen BR, Assentoft M, Cotrina ML, Hua SZ, Nedergaard M, Kaila K, Voipio J, MacAulay N (2014) Contributions of the Na+/K+-ATPase, NKCC1, and Kir4.1 to hippocampal K+ clearance and volume responses. Glia 62:608–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen BR, Stoica A, MacAulay N (2016) Managing brain extracellular K+ during neuronal activity: the physiological role of the Na+/K+-ATPase subunit isoforms. Front Physiol 7:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latour I, Hamid J, Beedle AM, Zamponi GW, Macvicar BA (2003) Expression of voltage-gated Ca2+ channel subtypes in cultured astrocytes. Glia 41:347–353 [DOI] [PubMed] [Google Scholar]
- Lenhossék MV (1895) Der feinere Bau des Nervensystems im Lichte neuester Forschung, 2nd edn. Fischer’s Medicinische Buchhandlung H. Kornfield, Berlin [Google Scholar]
- Letellier M, Park YK, Chater TE, Chipman PH, Gautam SG, Oshima-Takago T, Goda Y (2016) Astrocytes regulate heterogeneity of presynaptic strengths in hippocampal networks. Proc Natl Acad Sci U S A 113:E2685–E2694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Gu L, Hertz L, Peng L (2013) Expression of nucleoside transporter in freshly isolated neurons and astrocytes from mouse brain. Neurochem Res 38:2351–2358 [DOI] [PubMed] [Google Scholar]
- Liang S, Lu Y, Li Z, Li S, Chen B, Zhang M, Chen B, Ji M, Gong W, Xia M, Verkhratsky A, Wu X, Li B (2020) Iron aggravates the depressive phenotype of stressed mice by compromising the glymphatic system. Neurosci Bull 36:1542–1546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisjak M, Potokar M, Rituper B, Jorgacevski J, Zorec R (2017) AQP4e-based orthogonal arrays regulate rapid cell volume changes in astrocytes. J Neurosci 37:10748–10756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longden TA, Dunn KM, Draheim HJ, Nelson MT, Weston AH, Edwards G (2011) Intermediate-conductance calcium-activated potassium channels participate in neurovascular coupling. Br J Pharmacol 164:922–933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu DC, Zhang H, Zador Z, Verkman AS (2008) Impaired olfaction in mice lacking aquaporin-4 water channels. FASEB J 22:3216–3223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundgaard I, Osorio MJ, Kress BT, Sanggaard S, Nedergaard M (2014) White matter astrocytes in health and disease. Neuroscience 276:161–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macaulay N, Zeuthen T (2012) Glial K+ clearance and cell swelling: key roles for cotransporters and pumps. Neurochem Res 37:2299–2309 [DOI] [PubMed] [Google Scholar]
- Magistretti PJ (2009) Neuroscience. Low-cost travel in neurons. Science 325:1349–1351 [DOI] [PubMed] [Google Scholar]
- Magnotti LM, Goodenough DA, Paul DL (2011) Functional heterotypic interactions between astrocyte and oligodendrocyte connexins. Glia 59:26–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makar TK, Nedergaard M, Preuss A, Gelbard AS, Perumal AS, Cooper AJ (1994) Vitamin E, ascorbate, glutathione, glutathione disulfide, and enzymes of glutathione metabolism in cultures of chick astrocytes and neurons: evidence that astrocytes play an important role in anti-oxidative processes in the brain. J Neurochem 62:45–53 [DOI] [PubMed] [Google Scholar]
- Malarkey EB, Ni Y, Parpura V (2008) Ca2+ entry through TRPC1 channels contributes to intracellular Ca2+ dynamics and consequent glutamate release from rat astrocytes. Glia 56:821–835 [DOI] [PubMed] [Google Scholar]
- Marcaggi P, Attwell D (2004) Role of glial amino acid transporters in synaptic transmission and brain energetics. Glia 47:217–225 [DOI] [PubMed] [Google Scholar]
- Marina N, Christie IN, Korsak A, Doronin M, Brazhe A, Hosford PS, Wells JA, Sheikhbahaei S, Humoud I, Paton JFR, Lythgoe MF, Semyanov A, Kasparov S, Gourine AV (2020) Astrocytes monitor cerebral perfusion and control systemic circulation to maintain brain blood flow. Nat Commun 11:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathiisen TM, Lehre KP, Danbolt NC, Ottersen OP (2010) The perivascular astroglial sheath provides a complete covering of the brain microvessels: an electron microscopic 3D reconstruction. Glia 58:1094–1103 [DOI] [PubMed] [Google Scholar]
- Mauch DH, Nagler K, Schumacher S, Goritz C, Muller EC, Otto A, Pfrieger FW (2001) CNS synaptogenesis promoted by glia-derived cholesterol. Science 294:1354–1357 [DOI] [PubMed] [Google Scholar]
- McCarthy KD, Salm AK (1991) Pharmacologically-distinct subsets of astroglia can be identified by their calcium response to neuroligands. Neuroscience 41:325–333 [DOI] [PubMed] [Google Scholar]
- Mestre H, Mori Y, Nedergaard M (2020) The Brain’s glymphatic system: current controversies. Trends Neurosci 43:458–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RL, Loewy AD (2013) ENaC γ-expressing astrocytes in the circumventricular organs, white matter, and ventral medullary surface: sites for Na+ regulation by glial cells. J Chem Neuroanat 53:72–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosso A (1880) Sulla circolazione del sangue nel cervello dell’uomo. Mem Real Acc Lincei 5:237–358 [Google Scholar]
- Mulligan SJ, MacVicar BA (2004) Calcium transients in astrocyte endfeet cause cerebrovascular constrictions. Nature 431:195–199 [DOI] [PubMed] [Google Scholar]
- Nagelhus EA, Ottersen OP (2013) Physiological roles of aquaporin-4 in brain. Physiol Rev 93:1543–1562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy JI, Dudek FE, Rash JE (2004) Update on connexins and gap junctions in neurons and glia in the mammalian nervous system. Brain Res Brain Res Rev 47:191–215 [DOI] [PubMed] [Google Scholar]
- Nagy JI, Lynn BD, Tress O, Willecke K, Rash JE (2011) Connexin26 expression in brain parenchymal cells demonstrated by targeted connexin ablation in transgenic mice. Eur J Neurosci 34:263–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedergaard M (2013) Neuroscience. Garbage truck of the brain. Science 340:1529–1530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedergaard M, Goldman SA (2020) Glymphatic failure as a final common pathway to dementia. Science 370:50–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedergaard M, Verkhratsky A (2012) Artifact versus reality – how astrocytes contribute to synaptic events. Glia 60:1013–1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman EA, Frambach DA, Odette LL (1984) Control of extracellular potassium levels by retinal glial cell K+ siphoning. Science 225:1174–1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson M, Eriksson PS, Ronnback L, Hansson E (1993) GABA induces Ca2+ transients in astrocytes. Neuroscience 54:605–614 [DOI] [PubMed] [Google Scholar]
- Noda M, Sakuta H (2013) Central regulation of body-fluid homeostasis. Trends Neurosci 36:661–673 [DOI] [PubMed] [Google Scholar]
- Oberheim NA, Takano T, Han X, He W, Lin JH, Wang F, Xu Q, Wyatt JD, Pilcher W, Ojemann JG, Ransom BR, Goldman SA, Nedergaard M (2009) Uniquely hominid features of adult human astrocytes. J Neurosci 29:3276–3287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen ML, Campbell SL, Sontheimer H (2007) Differential distribution of Kir4.1 in spinal cord astrocytes suggests regional differences in K+ homeostasis. J Neurophysiol 98:786–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orellana JA, Saez PJ, Shoji KF, Schalper KA, Palacios-Prado N, Velarde V, Giaume C, Bennett MV, Saez JC (2009) Modulation of brain hemichannels and gap junction channels by pro-inflammatory agents and their possible role in neurodegeneration. Antioxid Redox Signal 11:369–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orkand RK, Nicholls JG, Kuffler SW (1966) Effect of nerve impulses on the membrane potential of glial cells in the central nervous system of amphibia. J Neurophysiol 29:788–806 [DOI] [PubMed] [Google Scholar]
- Orlowski J, Grinstein S (1997) Na+/H+ exchangers of mammalian cells. J Biol Chem 272:22373–22376 [DOI] [PubMed] [Google Scholar]
- Orthmann-Murphy JL, Freidin M, Fischer E, Scherer SS, Abrams CK (2007) Two distinct heterotypic channels mediate gap junction coupling between astrocyte and oligodendrocyte connexins. J Neurosci 27:13949–13957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palygin O, Lalo U, Verkhratsky A, Pankratov Y (2010) Ionotropic NMDA and P2X1/5 receptors mediate synaptically induced Ca2+ signalling in cortical astrocytes. Cell Calcium 48:225–231 [DOI] [PubMed] [Google Scholar]
- Pappalardo LW, Liu S, Black JA, Waxman SG (2014a) Dynamics of sodium channel Nav1.5 expression in astrocytes in mouse models of multiple sclerosis. Neuroreport 25:1208–1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappalardo LW, Samad OA, Black JA, Waxman SG (2014b) Voltage-gated sodium channel Nav 1.5 contributes to astrogliosis in an in vitro model of glial injury via reverse Na+/Ca2+ exchange. Glia 62:1162–1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappalardo LW, Black JA, Waxman SG (2016) Sodium channels in astroglia and microglia. Glia 64:1628–1645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parri HR, Gould TM, Crunelli V (2001) Spontaneous astrocytic Ca2+ oscillations in situ drive NMDAR-mediated neuronal excitation. Nat Neurosci 4:803–812 [DOI] [PubMed] [Google Scholar]
- Pastor A, Chvatal A, Sykova E, Kettenmann H (1995) Glycine- and GABA-activated currents in identified glial cells of the developing rat spinal cord slice. Eur J Neurosci 7:1188–1198 [DOI] [PubMed] [Google Scholar]
- Perez-Alvarez A, Araque A (2013) Astrocyte-neuron interaction at tripartite synapses. Curr Drug Targets 14:1220–1224 [DOI] [PubMed] [Google Scholar]
- Petersen OH, Verkhratsky A (2007) Endoplasmic reticulum calcium tunnels integrate signalling in polarised cells. Cell Calcium 42:373–378 [DOI] [PubMed] [Google Scholar]
- Petersen OH, Michalak M, Verkhratsky A (2005) Calcium signalling: past, present and future. Cell Calcium 38:161–169 [DOI] [PubMed] [Google Scholar]
- Pfrieger FW (2010) Role of glial cells in the formation and maintenance of synapses. Brain Res Rev 63:39–46 [DOI] [PubMed] [Google Scholar]
- Pfrieger FW, Barres BA (1997) Synaptic efficacy enhanced by glial cells in vitro. Science 277:1684–1687 [DOI] [PubMed] [Google Scholar]
- Pines G, Danbolt NC, Bjoras M, Zhang Y, Bendahan A, Eide L, Koepsell H, Storm-Mathisen J, Seeberg E, Kanner BI (1992) Cloning and expression of a rat brain L-glutamate transporter. Nature 360:464–467 [DOI] [PubMed] [Google Scholar]
- Plattner H, Verkhratsky A (2015) The ancient roots of calcium signalling evolutionary tree. Cell Calcium 57:123–132 [DOI] [PubMed] [Google Scholar]
- Popov A, Brazhe A, Denisov P, Sutyagina O, Lazareva N, Verkhratsky A, Semyanov A (2020) Astrocytes dystrophy in ageing brain parallels impaired synaptic plasticity. bioRxiv [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichenbach A, Bringmann A (2017) Comparative anatomy of glial cells in mammals. In: Kaas J (ed) Evolution of nervous systems, 2nd edn. Academic Press/Elsevier, New York, pp 309–348 [Google Scholar]
- Reichenbach A, Derouiche A, Kirchhoff F (2010) Morphology and dynamics of perisynaptic glia. Brain Res Rev 63:11–25 [DOI] [PubMed] [Google Scholar]
- Reyes RC, Verkhratsky A, Parpura V (2012) Plasmalemmal Na+/Ca2+ exchanger modulates Ca2+-dependent exocytotic release of glutamate from rat cortical astrocytes. ASN Neuro 4:00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes RC, Verkhratsky A, Parpura V (2013) TRPC1-mediated Ca2+ and Na+ signalling in astroglia: differential filtering of extracellular cations. Cell Calcium 54:120–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose CR, Ransom BR (1996) Intracellular sodium homeostasis in rat hippocampal astrocytes. J Physiol 491(Pt 2):291–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose CR, Verkhratsky A (2016) Principles of sodium homeostasis and sodium signalling in astroglia. Glia 64:1611–1627 [DOI] [PubMed] [Google Scholar]
- Rose CR, Ziemens D, Verkhratsky A (2020) On the special role of NCX in astrocytes: translating Na+-transients into intracellular Ca2+ signals. Cell Calcium 86:102154. [DOI] [PubMed] [Google Scholar]
- Roy CS, Sherrington CS (1890) On the regulation of the blood-supply of the brain. J Physiol (Lond) 11:85–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusakov DA, Fine A (2003) Extracellular Ca2+ depletion contributes to fast activity-dependent modulation of synaptic transmission in the brain. Neuron 37:287–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santello M, Cali C, Bezzi P (2012) Gliotransmission and the tripartite synapse. Adv Exp Med Biol 970:307–331 [DOI] [PubMed] [Google Scholar]
- Saura J, Kettler R, Da Prada M, Richards JG (1992) Quantitative enzyme radioautography with 3H-Ro 41-1049 and 3H-Ro 19-6327 in vitro: localization and abundance of MAO-A and MAO-B in rat CNS, peripheral organs, and human brain. J Neurosci 12:1977–1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, Binder DK (2013) Aquaporin-4 water channels and synaptic plasticity in the hippocampus. Neurochem Int 63:702–711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert G, Huttmann K, Binder DK, Hartmann C, Wyczynski A, Neusch C, Steinhauser C (2009) Analysis of astroglial K+ channel expression in the developing hippocampus reveals a predominant role of the Kir4.1 subunit. J Neurosci 29:7474–7488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheikhbahaei S, Turovsky EA, Hosford PS, Hadjihambi A, Theparambil SM, Liu B, Marina N, Teschemacher AG, Kasparov S, Smith JC, Gourine AV (2018) Astrocytes modulate brainstem respiratory rhythm-generating circuits and determine exercise capacity. Nat Commun 9:370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibasaki K, Hosoi N, Kaneko R, Tominaga M, Yamada K (2017) Glycine release from astrocytes via functional reversal of GlyT1. J Neurochem 140:395–403 [DOI] [PubMed] [Google Scholar]
- Shigetomi E, Tong X, Kwan KY, Corey DP, Khakh BS (2012) TRPA1 channels regulate astrocyte resting calcium and inhibitory synapse efficacy through GAT-3. Nat Neurosci 15:70–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigetomi E, Jackson-Weaver O, Huckstepp RT, O’Dell TJ, Khakh BS (2013) TRPA1 channels are regulators of astrocyte basal calcium levels and long-term potentiation via constitutive D-serine release. J Neurosci 33:10143–10153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skatchkov SN, Rojas L, Eaton MJ, Orkand RK, Biedermann B, Bringmann A, Pannicke T, Veh RW, Reichenbach A (2002) Functional expression of Kir 6.1/SUR1-KATP channels in frog retinal Muller glial cells. Glia 38:256–267 [DOI] [PubMed] [Google Scholar]
- Skucas VA, Mathews IB, Yang J, Cheng Q, Treister A, Duffy AM, Verkman AS, Hempstead BL, Wood MA, Binder DK, Scharfman HE (2011) Impairment of select forms of spatial memory and neurotrophin-dependent synaptic plasticity by deletion of glial aquaporin-4. J Neurosci 31:6392–6397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohl G, Odermatt B, Maxeiner S, Degen J, Willecke K (2004) New insights into the expression and function of neural connexins with transgenic mouse mutants. Brain Res Brain Res Rev 47:245–259 [DOI] [PubMed] [Google Scholar]
- Solovyova N, Verkhratsky A (2002) Monitoring of free calcium in the neuronal endoplasmic reticulum: an overview of modern approaches. J Neurosci Methods 122:1–12 [DOI] [PubMed] [Google Scholar]
- Solovyova N, Veselovsky N, Toescu EC, Verkhratsky A (2002) Ca2+ dynamics in the lumen of the endoplasmic reticulum in sensory neurons: direct visualization of Ca2+-induced Ca2+ release triggered by physiological Ca2+ entry. EMBO J 21:622–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sontheimer H, Waxman SG (1992) Ion channels in spinal cord astrocytes in vitro. II. Biophysical and pharmacological analysis of two Na+ current types. J Neurophysiol 68:1001–1011 [DOI] [PubMed] [Google Scholar]
- Sontheimer H, Minturn JE, Black JA, Ransom BR, Waxman SG (1991) Two types of Na+-currents in cultured rat optic nerve astrocytes: changes with time in culture and with age of culture derivation. J Neurosci Res 30:275–287 [DOI] [PubMed] [Google Scholar]
- Sontheimer H, Fernandez-Marques E, Ullrich N, Pappas CA, Waxman SG (1994) Astrocyte Na+ channels are required for maintenance of Na+/K+-ATPase activity. J Neurosci 14:2464–2475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosunov AA, Wu X, Tsankova NM, Guilfoyle E, McKhann GM 2nd, Goldman JE (2014) Phenotypic heterogeneity and plasticity of isocortical and hippocampal astrocytes in the human brain. J Neurosci 34:2285–2298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storck T, Schulte S, Hofmann K, Stoffel W (1992) Structure, expression, and functional analysis of a Na+-dependent glutamate/aspartate transporter from rat brain. Proc Natl Acad Sci U S A 89:10955–10959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney MD, Zhao Z, Montagne A, Nelson AR, Zlokovic BV (2019) Blood-brain barrier: from physiology to disease and back. Physiol Rev 99:21–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda H, Inazu M, Matsumiya T (2002) Astroglial dopamine transport is mediated by norepinephrine transporter. Naunyn Schmiedeberg’s Arch Pharmacol 366:620–623 [DOI] [PubMed] [Google Scholar]
- Theparambil SM, Naoshin Z, Thyssen A, Deitmer JW (2015) Reversed electrogenic sodium bicarbonate cotransporter 1 is the major acid loader during recovery from cytosolic alkalosis in mouse cortical astrocytes. J Physiol 593:3533–3547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd AC, Marx MC, Hulme SR, Broer S, Billups B (2017) SNAT3-mediated glutamine transport in perisynaptic astrocytes in situ is regulated by intracellular sodium. Glia 65:900–916 [DOI] [PubMed] [Google Scholar]
- Toth AB, Hori K, Novakovic MM, Bernstein NG, Lambot L, Prakriya M (2019) CRAC channels regulate astrocyte Ca2+ signaling and gliotransmitter release to modulate hippocampal GABAergic transmission. Sci Signal 12:5450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzingounis AV, Wadiche JI (2007) Glutamate transporters: confining runaway excitation by shaping synaptic transmission. Nat Rev Neurosci 8:935–947 [DOI] [PubMed] [Google Scholar]
- Unichenko P, Myakhar O, Kirischuk S (2012) Intracellular Na+ concentration influences short-term plasticity of glutamate transporter-mediated currents in neocortical astrocytes. Glia 60:605–614 [DOI] [PubMed] [Google Scholar]
- Verkhratsky A (2010) Physiology of neuronal-glial networking. Neurochem Int 57:332–343 [DOI] [PubMed] [Google Scholar]
- Verkhratsky A, Chvatal A (2020) NMDA receptors in astrocytes. Neurochem Res 45:122–133 [DOI] [PubMed] [Google Scholar]
- Verkhratsky A, Kettenmann H (1996) Calcium signalling in glial cells. Trends Neurosci 19:346–352 [DOI] [PubMed] [Google Scholar]
- Verkhratsky A, Nedergaard M (2014) Astroglial cradle in the life of the synapse. Philos Trans R Soc Lond Ser B Biol Sci 369:20130595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkhratsky A, Nedergaard M (2016) The homeostatic astroglia emerges from evolutionary specialization of neural cells. Philos Trans R Soc Lond Ser B Biol Sci 371:20150428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkhratsky A, Nedergaard M (2018) Physiology of astroglia. Physiol Rev 98:239–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkhratsky A, Parpura V (2014) Store-operated calcium entry in neuroglia. Neurosci Bull 30:125–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkhratsky A, Petersen OH (2002) The endoplasmic reticulum as an integrating signalling organelle: from neuronal signalling to neuronal death. Eur J Pharmacol 447:141–154 [DOI] [PubMed] [Google Scholar]
- Verkhratsky A, Steinhauser C (2000) Ion channels in glial cells. Brain Res Brain Res Rev 32:380–412 [DOI] [PubMed] [Google Scholar]
- Verkhratsky A, Orkand RK, Kettenmann H (1998) Glial calcium: homeostasis and signaling function. Physiol Rev 78:99–141 [DOI] [PubMed] [Google Scholar]
- Verkhratsky A, Krishtal OA, Burnstock G (2009) Purinoceptors on neuroglia. Mol Neurobiol 39:190–208 [DOI] [PubMed] [Google Scholar]
- Verkhratsky A, Rodriguez JJ, Parpura V (2012) Calcium signalling in astroglia. Mol Cell Endocrinol 353:45–56 [DOI] [PubMed] [Google Scholar]
- Verkhratsky A, Matteoli M, Parpura V, Mothet JP, Zorec R (2016) Astrocytes as secretory cells of the central nervous system: idiosyncrasies of vesicular secretion. EMBO J 35:239–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkhratsky A, Trebak M, Perocchi F, Khananshvili D, Sekler I (2018) Crosslink between calcium and sodium signalling. Exp Physiol 103:157–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkhratsky A, Semyanov A, Zorec R (2020) Physiology of astroglial excitability. Function 1:zqaa016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JX, Peters CE, Sitar SM, Daoust P, Gelb AW (2000) Glutamate stimulates ascorbate transport by astrocytes. Brain Res 858:61–66 [DOI] [PubMed] [Google Scholar]
- Witcher MR, Kirov SA, Harris KM (2007) Plasticity of perisynaptic astroglia during synaptogenesis in the mature rat hippocampus. Glia 55:13–23 [DOI] [PubMed] [Google Scholar]
- Zanotti S, Charles A (1997) Extracellular calcium sensing by glial cells: low extracellular calcium induces intracellular calcium release and intercellular signaling. J Neurochem 69:594–602 [DOI] [PubMed] [Google Scholar]
- Zerangue N, Kavanaugh MP (1996) Flux coupling in a neuronal glutamate transporter. Nature 383:634–637 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Chen K, Sloan SA, Bennett ML, Scholze AR, O’Keeffe S, Phatnani HP, Guarnieri P, Caneda C, Ruderisch N, Deng S, Liddelow SA, Zhang C, Daneman R, Maniatis T, Barres BA, Wu JQ (2014) An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J Neurosci 34:11929–11947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Danbolt NC (2013) GABA and glutamate transporters in brain. Front Endocrinol (Lausanne) 4:165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M, Xu G, Xie M, Zhang X, Schools GP, Ma L, Kimelberg HK, Chen H (2009) TWIK-1 and TREK-1 are potassium channels contributing significantly to astrocyte passive conductance in rat hippocampal slices. J Neurosci 29:8551–8564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Zhao Y, Wu H, Jiang N, Wang Z, Lin W, Jin J, Ji Y (2016) Remarkable alterations of Nav1.6 in reactive astrogliosis during epileptogenesis. Sci Rep 6:38108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zonta M, Angulo MC, Gobbo S, Rosengarten B, Hossmann KA, Pozzan T, Carmignoto G (2003) Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nat Neurosci 6:43–50 [DOI] [PubMed] [Google Scholar]


