Abstract
Synucleinopathies are a subset of debilitating neurodegenerative disorders for which clinically approved therapeutic options to either halt or retard disease progression are currently unavailable. Multiple synergistic pathological mechanisms in combination with the characteristic misfolding of proteins are attributable to disease pathogenesis and progression. This complex interplay, as well as the difficult and multiscale nature of therapeutic delivery into the central nervous system, make finding effective treatments difficult. Nanocarriers (NCs) are a class of materials that can significantly improve therapeutic brain delivery and enable multifunctional therapies. In this review, an update on the known pathology of synucleinopathies is presented. Then, NC-enabled therapeutics designed to target the multiple mechanisms by combination therapies and multiscale targeting methods is reviewed. The implications of these strategies are synthesized and evaluated to suggest opportunities for the rational design of anti-neurodegenerative NC therapeutics.
Keywords: Neurodegeneration, nano, drug delivery
1. Introduction
Synucleinopathies are a subset of neurodegenerative diseases involving the pathogenic misfolding of alpha-synuclein (αSyn) that leads to a progressive loss of cognitive and motor functions. As the most prevalent example, over one million individuals in the U.S. have Parkinson’s Disease (PD) alone, which is associated with $26 billion in indirect and non-medical costs [1]. Synucleinopathies are difficult to diagnose early due to pathologic and symptomatic similarities between diseases and the lack of a clinically approved diagnostic tool, leading to a lower quality of life of afflicted individuals. There are also no approved treatments that slow disease progression, and supportive patient management measures only alleviate symptoms.
The hallmark pathology of synucleinopathies involves the buildup of misfolded protein aggregates like αSyn in and around affected neurons, which are known as Lewy Bodies (LBs) [2]. Disease-slowing therapeutics in development are designed to slow or prevent the buildup of aggregated αSyn (αSynagg) in the brain. However, due to the blood-brain barrier (BBB) protecting the brain from systemic circulation [3], effective delivery of therapeutics is also challenging.
A significant thrust in the field is dedicated towards developing nanoscale delivery platforms (e.g. nanocarriers, NCs) that can be targeted to specific areas of the brain to optimize therapeutic delivery. But the brain is highly susceptible to invasion of foreign substances, which can lead to toxic side-effects. Rational NC design strategies can enable both effective and safe delivery of new and novel therapeutics for synucleinopathies by minimizing dose and enhancing the ability to slow disease progression. This review evaluates underlying mechanisms and synucleinopathy-related pathology to provide insight into recent NC-based therapeutic strategies designed to address one or more of these mechanisms.
2. Synucleinopathy Pathology
2.1. Parkinsonian syndrome
Parkinsonian syndrome (PS) is a clinical syndrome that refers to a group of neurological disorders encompassing a spectrum of movement disabilities, including PD, Dementia with Lewy Bodies (DLB), Progressive Supranuclear Palsy (PSP) and Multiple System Atrophy (MSA) [4]. PSP and MSA belong to a class referred to as atypical parkinsonism [4]. Currently it is difficult to clinically diagnose the different PS due to overlapping motor symptoms, but studies are underway to determine misfolded αSyn and Tau aggregates as early stage biomarker(s) to differentiate PS [5–7].
2.2. CNS pathology
The characteristic pathology of PD is the loss of dopaminergic (DA) neurons in the substantia nigra of the basal ganglia (BG) and the presence of intraneuronal αSynagg LB inclusions [8]. MSA has glial αSyn cytoplasmic inclusions and PSP has glial and neuronal tau inclusions with loss of neurons in the BG, pons, cerebellum, and other similar components in the brain [8]. These intrinsically disordered proteins are correlated with disease state, with microscopically visible components appearing later in the disease process [9].
The appearance of LB and LB-like inclusions is initiated and propagated by a seeding process. This process involves the interaction between internalized exogenous αSyn aggregates with endogenous, intracellular αSyn via direct membrane penetration by fibrils or by encapsulation into endocytic vesicles or exosomal pathway [10**,11]. Microglia and neuron-secreted exosomes are integral to αSyn propagation [12]. In the case of endocytic encapsulation, Galectin-3 accumulation ruptures the endosome and allows fibrils to interact with cytoplasmic αSyn to seed aggregation [11].
The propagation of LBs throughout the central nervous system (CNS) leads to dysfunction of numerous important cellular functions. The ubiquitin-proteasome system (UPS) catabolic pathway and the autophagy-lysosome pathway (ALP) are both disrupted [13]. These pathways are important mediators for breaking down debris such as fibrils, but deregulation occurs when LB formation via the αSyn seeding process outpaces UPS and ALP function [13]. In PD, mutation of the gene LRRK2 additionally contributes to the disruption of normal endosomal function to allow for this buildup of debris [14]. The pro-inflammatory signaling cascade associated with LRRK2 mutations also involves activation of glial cells [14].
An excellent review explains how αSyn fibrillation is associated with the activation and brain-infiltration of T-effector (Teff) cells in the neurodegenerative pro-inflammatory cascade [15]. Microglia, which are analogous to macrophages of the peripheral immune system, interact with Teffs and are then activated to a pro-inflammatory M1 phenotype [15]. In response, they release TNF-α, IL-6 and IL-1β among other pro-inflammatory markers and contribute to the buildup of reactive oxygen species (ROS) [16]. Astroglia, which normally provide structural support for neurons and the BBB, filter toxins and fulfill other neuroprotective roles to address imbalances in homeostasis [17], also enter a pro-inflammatory state in response to LB formation, releasing more pro-inflammatory cytokines and contributing to mitochondrial dysfunction and oxidative stress through the release of excess glutamate [18]. T-regulatory (Treg) cells can indirectly counteract this pro-inflammatory response due to their anti-inflammatory phenotype, but Tregs are overwhelmed in disease due to low numbers and dysfunction [15].
Oligodendrocytes, which form protective sheaths around neuronal axons and enhance signal transmission, are also affected by LB-induced inflammation. Progressive degeneration of the oligodendrocyte-composed myelin sheaths and subsequent neurodegeneration results in a slow and inevitable loss of motor function within the body in MSA [19]. Additionally, in PD it was found that oligodendrocytes show varied genetic expression at even earlier stages of disease than DA neurons [19].
2.3. Gut-Brain Axis
The GI environment can widely influence and regulate CNS activity. The bidirectional signaling between the gut and the brain mediated through immune and/or nervous system regulates the homeostasis with reference to satiety and hunger and CNS inflammation. GI dysfunction in PD is manifested as mucosal inflammation of gut, constipation, decreased absorption of the nutrients, and delayed gastric emptying, enteric neuronal loss and enteric LB pathology [20]. GI dysfunction, a major non-motor symptom of PD could play a role as a potential early biomarker [21]. Braak’s hypothesis proposes misfolded αSyn aggregates from the enteric nervous system (ENS) propagate in a prion like manner to the CNS through the dorsal motor nucleus of the vagus leading to PD pathogenesis of DA degeneration and the loss of dopamine in the striatum [22]. Studies published this year have detailed that gut microbial metabolites produced by gut dysbiosis in animal models and in PD patients can promote αSyn aggregation, non-motor and motor impairment, which can be overcome by dietary intervention, truncal vagotomy or fecal microbiota transplantation [23,24].
3. Therapeutics and Nanomedicines strategies
3.1. NCs as a solution to therapeutic challenges
The multifaceted nature and complex interplay of these pathological mechanisms provide a broad range of therapeutic targets. Recent reviews detail treatments that differentially alleviate some of these mechanisms [15,25]. Having so many targets makes developing an all-encompassing, effective treatment difficult. In addition, poor pharmacokinetics and pharmacodynamics due to the stringent nature of the astroglia-supported BBB and the susceptibility of therapeutics in systemic circulation or other degradative environments in the body after administration limit the extent of therapeutic efficacy provided by any of these methods.
To improve therapeutic efficacy, NCs can encapsulate and protect the therapeutics from systemic degradation and improve local bioavailability. By releasing therapeutics over a longer time, the dosing frequency can be minimized. The versatility of NCs can help to optimize pharmacokinetic and pharmacodynamic profiles (Figure 1). NCs targeted towards attenuating αSyn fibril formation to alleviate pathology consist of a broad range of materials (Table 1).
Figure 1.
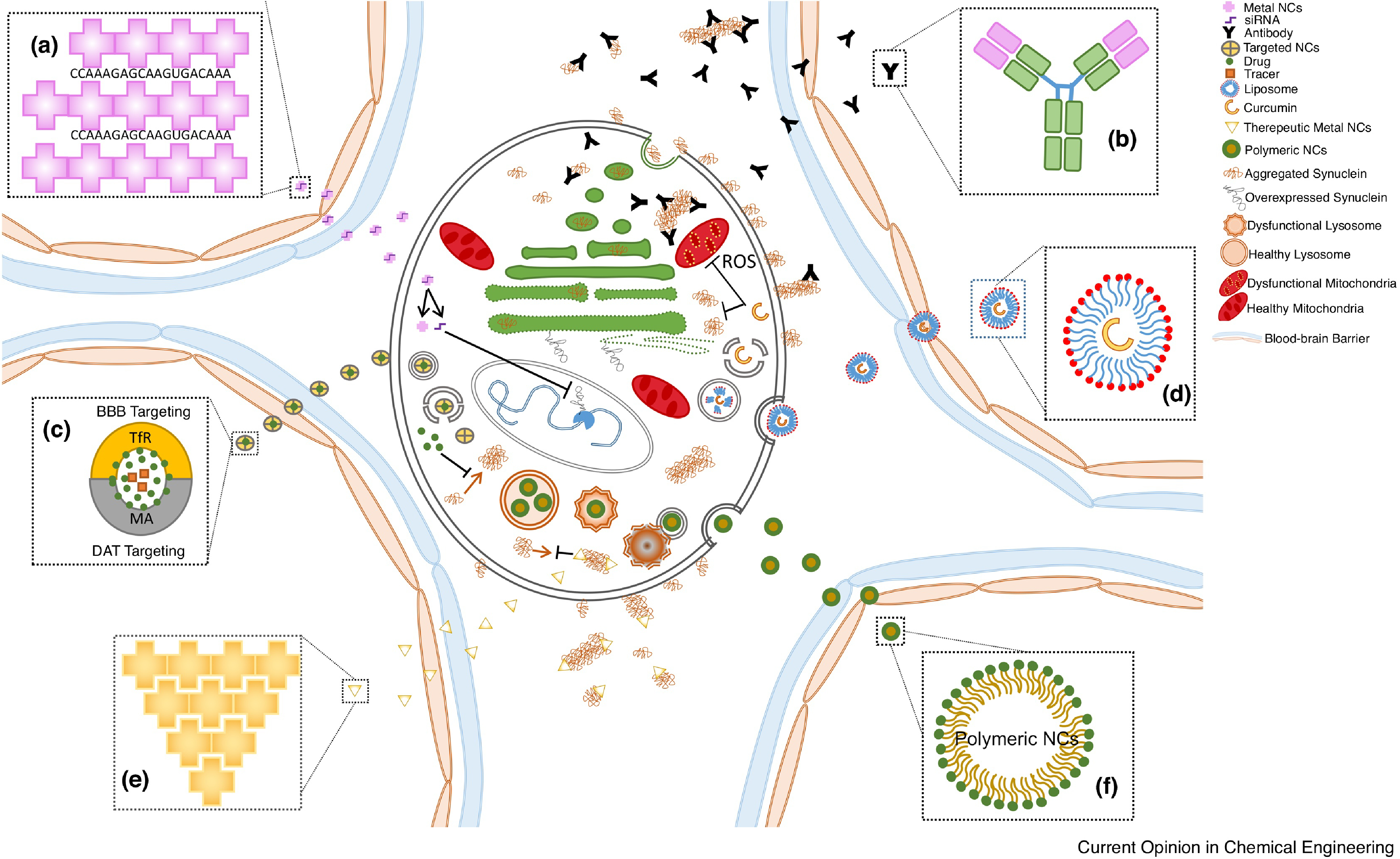
NC-based treatments for Synucleinopathies. A) Metal NCs are used as a carrier for therapeutic agents like siRNA to prevent overexpression and subsequent fibrillation B) Antibody strategies, both host and non-host derived, directly combating fibrillation C) Targeted NCs can further enhance delivery of therapeutics D) Lipid-based delivery of therapeutic compounds, including the ability to incoporate multiple therapeutics in the same formulation, e.g. multifunctional E) Metal NCs intrinsically act as therapeutics by directly interfering with fibril formation F) Polymeric NCs intrinsically act as therapeutics by restoring lysosome function
Table 1.
NCs for targeting different aspects of αSynagg-associated neuropathology.
| Class | Nanocarrier | Therapeutic | Therapeutic Purpose | Modifications | Model | Challenge | Admin | Ref |
|---|---|---|---|---|---|---|---|---|
| Synthetic | Silica NCs | Curcumin | Antioxidant, Antiamyloid | - | in vitro (PC-12) | αSyn | - | [42] |
| PLGA NCs | Dopamine | Neuro-protective | borneol (BBB) Lactoferrin (striatum) | in vivo (rats) | 6-OHDA | IN | [34**] | |
| - | restore lysosome acidification | - | in vitro (various) | Toxin and genetic models | - | [47] | ||
| in vivo (mice) | MPTP | Intra-cerebral | ||||||
| Polyethylenimine NCs | siRNA | aSyn gene silencing | - | in vivo (mice) | αSyn transgenic | ICV | [28] | |
| Lipid | Liposomes | - | in vitro (mouse primary neurons) | αSyn | - | [27] | ||
| Curcumin | Antioxidant, Antiamyloid | Ultrasound (BBB) Polysorbate 80 | in vivo (mice) | MPTP | IV | [37] | ||
| Curcumin, Piperine | Antioxidant, Antiamyloid, ALP repair | Glycerol monooleate (BBB) | in vitro (PC-12) | rotenone | - | [39] | ||
| in vivo (mice) | oral | |||||||
| - | αSyn fibrillation-inhibiting | Cholesterol, PEG | in vitro (PC-12) in vitro (SH-SY5Y) | αSyn transgenic | - | [46*] | ||
| Metal, metal oxide | DSPE-PEG-iron oxide NCs | EGCG | B6 (TfR -BBB) | in vitro (SH-SY5Y) | αSyn transgenic | - | [38**] | |
| in vivo (mice) | IV | |||||||
| Gold nanoclusters | - | N-isobutyryl-L-cysteine | in vitro | MPP+ | - | [43*] | ||
| in vivo (mice) | MPTP | Intra-peritoneal | ||||||
| Cerium Oxide nanoclusters | - | - | in vitro (SH-SY5Y) | αSynagg | - | [44] | ||
| in vivo (yeast cells) | αSyn transgenic | - | [45] | |||||
| Layered double hydroxide metal NCs | siRNA | αSyn gene silencing | - | in vitro (SH-SY5Y) | αSyn overexpressed | - | [26] | |
| Poly-saccharide | Glucan | αSyn (antigen) | Vaccine | rapamycin | in vivo (mice) | αSyn transgenic | Intra-peritoneal | [29] |
| Chitosan | Rotigotine | Dopamine agonist | - | in vitro (SH-SY5Y) in vivo (rats) | 6-OHDA | N/A | [40] | |
| haloperidol | IN | |||||||
| - | - | mAbs | anti-αSynagg | - | clinical trial | PD | IV | [30,31] |
3.2. Gene-silencing treatments
Several studies have used NCs to improve delivery of αSyn gene-silencing therapeutics to indirectly reduce the propensity for fibrillation (Figure 1A). Small interfering ribonucleic acids (siRNA) can interfere with αSyn fibrillation but exhibit poor brain and neuron-specific internalization. Acharya et al [26] encapsulated siRNA into layered double hydroxide NCs in vitro study to address this issue and effectively silence the αSyn gene in DA-like SH-SY5Y cells. Schlich et al [27] also saw an in vitro reduction in αSyn expression after treating mouse primary neurons with siRNA-encapsulated anionic liposomes. Helmschrodt et al [28] encapsulated siRNA in polyethylenimine NCs and administered via the intracerebroventricular (ICV) route to reduce αSyn gene expression in vivo. This was correlated with a reduced pro-inflammatory immune response [28].
3.3. Host and non-host derived antibodies against αSynagg
Anti-αSynagg antibodies can also be used to combat αSynagg-induced neuroinflammation (Figure 1B) [29–32]. Training the immune system towards an anti-inflammatory immune response, thereby generating host-derived anti-αSynagg antibodies, is one way to do this [15]. Rockenstein et al [29] immunized αSyn-transgenic mice with glucan microparticles with rapamycin and αSyn. They observed a reduction in αSyn-associated neuroinflammation, which was associated with induction of anti-αSynagg antibodies and a transition to CD4- (T-helper) and CD25-positive (Treg) cells for a more anti-inflammatory phenotype in the brain [29]. Such vaccine strategies may be beneficial for providing longer term protection against disease progression.
There are two non-host-derived anti-αSynagg therapeutic monoclonal antibodies (mAb) that have shown promising results in Phase I clinical trials [30,31]. Recent results with BIIB054, developed by Biogen [30], as well as Prasinezumab, developed by Hoffmann-La Roche and Prothena [31], have shown good pharmacokinetic and safety profiles in the brain after IV administration in patients. Both studies have progressed into ongoing Phase II clinical trials. Given the overall lack of effective and FDA-approved therapeutics for synucleinopathies, these are promising developments for PD treatment. FDA-approved NCs, a list of which are detailed in a recent review [33], could be used in mAb treatments to further optimize mAb bioavailability and therapeutic efficacy.
3.4. Targeting ligands for improved pharmacokinetics
Many treatment strategies for brain delivery have incorporated targeting ligands on, or integrated targeting strategies with, NCs to further improve therapeutic pharmacokinetics (Figure 1C) [34**–39]. Zhang et al [37] used ultrasound sonication to disrupt the BBB and found improved brain bioavailability in mice after intravenous (IV) administration of curcumin-encapsulated liposomes, which was correlated with an improvement of motor-function and restoration of an important DA-homestotatic molecule tyrosine hydroxylase (TH) after PS-inducing 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) challenge [37]. Recent research has shown that cationic ligands can improve both BBB-crossing and local mitochondrial delivery [35,36]. Some NCs can also intrinsically demonstrate brain-targeting characteristics, as shown by the chitosan NCs in the study by Bhattamisra et al [40].
Multiscale targeting techniques are growing in popularity due to the multiscale nature of brain delivery, as discussed in a recent review [41]. Tang et al [34**] encapsulated dopamine Printopoly(lactic-co-glycolic acid) (PLGA) NCs coated with borneol and lactoferrin to improve BBB-crossing and striatum-specific delivery, respectively. This promising multiscale NC-based treatment protected against the DA toxin oxidopamine (6-OHDA) in vivo [34**]. Additionally, Li et al [38**] incorporated the peptide B6 on polymeric-superparamagnetic iron oxide self-assembled NCs conjugated to the therapeutic epigallocatechin gallate (EGCG). The B6 ligand improved transport across an in vitro BBB model by targeting the transferrin receptor (TfR), and the mazindol modification on the NCs allowed for dopamine transporter targeting on neurons [38**]. The synergistic effects provided by this multicomponent NC improved efficacy in the treatment interfering with αSynagg fibrillation in vivo [38**].
3.5. Multifunctional and combination therapeutic treatments
Some neuroprotective drugs that suffer from poor pharmacokinetic profiles have multiple therapeutic effects. Taebnia et al [42] addressed poor pharmacokinetic properties of curcumin by encapsulating it in a mesoporous silica NC formulation to improve αSyn fibrillation-inhibiting, antioxidant and TH restoring properties in PC-12 cells. Kundu et al [39] co-encapsulated curcumin and piperine in a liposomal formulation coated with glycerol monooleate to improve brain delivery. Both curcumin and piperine have antioxidant properties, and the combination therapy significantly improved efficacy in protecting against αSynagg-driven PS in vitro and in vivo by restoring mitochondrial and ALP function and improving motor coordination [39]. Improving multifunctional drugs and enabling combination treatments is a significant benefit that NCs can provide for treatment regimen (Figure 1D).
3.6. Non-antibody based anti-αSynagg NCs
The most direct method to combat αSynagg pathology is by targeting αSyn fibrils for breakdown. Some NCs exhibit intrinsic targeting or therapeutic effects. For example, Bhattamisra et al [40] encapsulated Rotigotine into intrinsically brain-targeted chitosan NCs to protect rats against the PS-inducing agent Haloperidol [40]. Alternatively, some NCs intrinsically attenuate αSyn fibrillation (Figure 1E). Gao et al [43*] found that gold nanoclusters interact with and reduce αSyn in vitro and additionally protect against MPTP in vivo. Cerium oxide nanoclusters can also provide therapeutic effects ranging from reducing αSyn fibrillation via interaction kinetics to ameliorating oxidative stress and mitochondrial dysfunction, as demonstrated in vitro in SH-SY5Y cells after αSynagg challenge [44] and in vivo in αSyn-transgenic (e.g., αSyn-overexpressing) yeast cells [45]. Aliakbari et al [46*] found that zwitterionic, cholesterol-loaded liposomes interfered with fibrillation in vitro, and provided similar therapeutic effects in both SH-SY5Y and PC12 cells.
Most of the above therapeutics target αSynagg directly, but it is also crucial to evaluate therapeutic efficacy in specifically restoring ALP function (Figure 1F). Bourdenx et al [47] used a non-loaded, acidic PLGA formulation to restore lysosome function after testing against toxin- and genetic-based PS models by co-localizing with lysosomes and restoring lysosomal pH in vitro. This led to protection against MPTP-induced toxicity in vivo [47]. The multifunctional treatment by Kundu et al [39] described above also showed promising indications in the ability of their treatment to repair ALP function.
3.7. Gut-targeted treatment strategies
Due to evidence of the gut-brain connection in neurodegenerative disease, gut-targeted treatment is also necessary to slow disease progression. Since an unhealthy gut microbiota plays a key role in CNS pathology, repairing microbiota health is a primary focus in this thrust [48,49]. Many probiotics and prebiotics are being investigated to treat neurodegenerative disease in this way [49]. However, because probiotic treatments are usually delivered orally, there is often a potential for gastric degradation of these therapeutics, reducing therapeutic efficacy.
4. Perspectives
It’s possible that ENS and CNS pathology occur simultaneously [50]. The separate and significant pathology in both systems suggests the need for discovering synergistic CNS and ENS treatment paradigms. To appropriately screen for such treatment strategies, the screening process must include a variety of pathogenic models, including lysosomal impairment, MPTP/rotenone/6-OHDA challenge, and CNS and ENS-associated αSyn transgenic models, since each exacerbates different underlying mechanisms leading to disease. Additionally, the use of multiscale in vitro models like a transwell BBB model can be performed prior to in vivo experimentation to allow for more rapid screening of multiscale nanocarrier-based CNS treatments. Table 1 lists relevant studies covering these and other concepts.
There is an increased risk of steric hindrance that reduces functionality of the components in multifunctional schemes. NCs with intrinsic targeting or therapeutic properties could enable more facile multifunctional strategies because of the need for fewer components. Polymeric and liposomal NCs are typically larger in size than metallic NCs and are therefore more easily able to encapsulate therapeutics. They often also have many functional groups for facile incorporation of targeting ligands. Therefore, polymeric and liposomal NCs that intrinsically slow αSyn fibrillation have significant multifunctional potential.
The administration route will dictate many decisions about the NC chemistry. For example, ICV delivery may be a more direct route to the CNS but is highly invasive. Intranasal (IN) delivery will lead therapeutics to the nose-brain barrier, so IN-administered NCs would benefit from different targeting ligands than the BBB. Care must be taken with IV-administered NCs to avoid thrombosis after administration, reducing the applicability of flocculation-prone NCs. Orally administered probiotics or prebiotics pass through the highly acidic stomach and will degrade before reaching the target site, so encapsulation by low pH stable NCs can protect these therapeutics from this environment.
5. Conclusions
The complex nature of synucleinopathic disease progression necessitates the complex design of new and novel treatment strategies. By incorporating combination therapies in CNS-targeted NCs, the ability to alleviate or prevent αSyn fibrillation, oxidative stress, ALP and UPS impairment, and excessive brain inflammation could be significantly improved. Multiscale targeting can enable better bioavailability of such combination therapies. Additionally, due to the significance of the gut-brain axis in neurodegenerative disease, administration of such a CNS platform with an ENS-targeted NC treatment must be considered in all-encompassing treatment strategies. Multiscale, multifunctional, combination treatment paradigms like the ideas proposed herein have the potential to provide the road to a cure to diseases like PD, DLB and MSA.
Acknowledgments
Funding: The authors would like to thank the Iowa State University Nanovaccine Institute for financial support. AGK acknowledges in part funding from NIH grant ES026892 and U01 NS112008. BN acknowledges the Vlasta Klima Balloun Faculty Chair, SKM acknowledges the Carol Vohs Johnson Chair and AGK acknowledges the Lloyd Endowed Chair.
Abbreviations
- αSyn
Alpha-synuclein
- PD
Parkinson’s Disease
- LB
Lewy Bodies
- BBB
blood-brain barrier
- NCs
nanocarriers
- αSynagg
aggregated alpha-synuclein
- PS
Parkinsonian syndrome
- DLB
Dementia with Lewy Bodies
- PSP
Progressive Supranuclear Palsy
- MSA
Multiple System Atrophy
- DA
dopaminergic
- BG
basal ganglia
- UPS
ubiquitin-proteasome system
- ALP
autophagy-lysosome pathway
- Teff
T-effector
- ROS
reactive oxygen species
- Treg
T-regulatory
- siRNA
small interfering ribonucleic acids
- ICV
intracerebroventricular
- TfR
transferrin receptor
- TH
tyrosine hydroxylase
- IV
intravenous
- PLGA
poly(lactic-co-glycolic acid)
- 6-OHDA
oxidopamine
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- IN
intranasal
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
BN is a co-founder of ImmunoNanoMed Inc., a start-up in Ames, IA with business interests in the development of nano-based vaccines against infectious diseases. He also has a financial interest in Degimflex LLC (see below).
SKM is a co-founder of Degimflex LLC., a start-up in Ames, IA with business interests in the development of flexible degradable electronic films for biomedical applications. She also has a financial interest in ImmunoNanoMed Inc.
AGK and VA have an equity interest in PK Biosciences Corporation located in Ames, IA. AGK also has an equity interest in Probiome Therapeutics located in Ames, IA. The terms of this arrangement have been reviewed and approved by Iowa State University in accordance with its conflict of interest policies. Other authors declare no actual or potential competing financial interests.
References
- [1].Yang G, Schmiel L, Zhou M, Cintina I, Spencer D, Hogan P, Economic Burden and Future Impact of Parkinson’s Disease - Final Report, 2019.
- [2].Fonseca-Ornelas L, Eisbach SE, Paulat M, Giller K, Fernández CO, Outeiro TF, Becker S, Zweckstetter M, Small molecule-mediated stabilization of vesicle-associated helical α-synuclein inhibits pathogenic misfolding and aggregation, Nat. Commun. 5 (2014) 5857. [DOI] [PubMed] [Google Scholar]
- [3].Andreone BJ, Chow BW, Tata A, Lacoste B, Ben-Zvi A, Bullock K, Deik AA, Ginty DD, Clish CB, Gu C, Blood-Brain Barrier Permeability Is Regulated by Lipid Transport-Dependent Suppression of Caveolae-Mediated Transcytosis, Neuron. 94 (2017) 581–594.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Archer DB, Bricker JT, Chu WT, Burciu RG, McCracken JL, Lai S, Coombes SA, Fang R, Barmpoutis A, Corcos DM, Kurani AS, Mitchell T, Black ML, Herschel E, Simuni T, Parrish TB, Comella C, Xie T, Seppi K, Bohnen NI, Müller ML, Albin RL, Krismer F, Du G, Lewis MM, Huang X, Li H, Pasternak O, McFarland NR, Okun MS, Vaillancourt DE, Development and validation of the automated imaging differentiation in parkinsonism (AID-P): a multicentre machine learning study, Lancet Digit. Heal. 1 (2019) e222–e231. [DOI] [PubMed] [Google Scholar]
- [5].Singer W, Schmeichel AM, Shahnawaz M, Schmelzer JD, Boeve BF, Sletten DM, Gehrking TL, Gehrking JA, Olson AD, Savica R, Suarez MD, Soto C, Low PA, Alpha-Synuclein Oligomers and Neurofilament Light Chain in Spinal Fluid Differentiate Multiple System Atrophy from Lewy Body Synucleinopathies, Ann. Neurol. (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Sarkar S, Dammer EB, Malovic E, Olsen AL, Raza SA, Gao T, Xiao H, Oliver DL, Duong D, Joers V, Seyfried N, Huang M, Kukar T, Tansey MG, Kanthasamy AG, Rangaraju S, Molecular Signatures of Neuroinflammation Induced by αSynuclein Aggregates in Microglial Cells, Front. Immunol. 11 (2020) 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Manne S, Kondru N, Jin H, Anantharam V, Huang X, Kanthasamy A, Kanthasamy AG, α-Synuclein real-time quaking-induced conversion in the submandibular glands of Parkinson’s disease patients, Mov. Disord. 35 (2020) 268–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].De Luca CMG, Elia AE, Portaleone SM, Cazzaniga FA, Rossi M, Bistaffa E, De Cecco E, Narkiewicz J, Salzano G, Carletta O, Romito L, Devigili G, Soliveri P, Tiraboschi P, Legname G, Tagliavini F, Eleopra R, Giaccone G, Moda F, Efficient RT-QuIC seeding activity for α-synuclein in olfactory mucosa samples of patients with Parkinson’s disease and multiple system atrophy, Transl. Neurodegener. 8 (2019) 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ingelsson M, Alpha-synuclein oligomers-neurotoxic molecules in Parkinson’s disease and other lewy body disorders, Front. Neurosci. 10 (2016) 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].** Harischandra DS, Rokad D, Neal ML, Ghaisas S, Manne S, Sarkar S, Panicker N, Zenitsky G, Jin H, Lewis M, Huang X, Anantharam V, Kanthasamy A, Kanthasamy AG, Manganese promotes the aggregation and prion-like cell-to-cell exosomal transmission of α-synuclein, Sci. Signal. 12 (2019). Exosome mediated cell-to-cell transmission of alpha-synuclein is an emerging concept in PD pathology. Harischandra et al. provide in this paper further insight into the exosomal signaling mechanism of αSyn transmission after induction of toxic levels of Manganese. The implications of this research are far-reaching in that it can revolutionize the way researchers screen for effective anti-synucleinopathic therapeutics. [Google Scholar]
- [11].Jiang P, Gan M, Yen SH, McLean PJ, Dickson DW, Impaired endo-lysosomal membrane integrity accelerates the seeding progression of α-synuclein aggregates, Sci. Rep 7 (2017) 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Porro C, Panaro MA, Lofrumento DD, Hasalla E, Trotta T, The multiple roles of exosomes in Parkinson’s disease: an overview, Immunopharmacol. Immunotoxicol. 41 (2019) 469–476. [DOI] [PubMed] [Google Scholar]
- [13].Kim D, Hui Paik J, Shin D-W, Kim H-S, Park C-S, Kang J-H, What is the Clinical Significance of Cerebrospinal Fluid Biomarkers in Parkinson’s disease? Is the Significance Diagnostic or Prognostic?, Exp. Neurobiol. 23 (2014) 352–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Perrett RM, Alexopoulou Z, Tofaris GK, The endosomal pathway in Parkinson’s disease, Mol. Cell. Neurosci. 66 (2015) 21–28. [DOI] [PubMed] [Google Scholar]
- [15].Schwab AD, Thurston MJ, Machhi J, Olson KE, Namminga KL, Gendelman HE, Mosley RL, Immunotherapy for Parkinson’s disease, Neurobiol. Dis. 137 (2020) 104760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Gordon R, Singh N, Lawana V, Ghosh A, Harischandra DS, Jin H, Hogan C, Sarkar S, Rokad D, Panicker N, Anantharam V, Kanthasamy AG, Kanthasamy A, Protein kinase Cδ upregulation in microglia drives neuroinflammatory responses and dopaminergic neurodegeneration in experimental models of Parkinson’s disease, Neurobiol. Dis. 93 (2016) 96–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Sarkar S, Malovic E, Harischandra DS, Ngwa HA, Ghosh A, Hogan C, Rokad D, Zenitsky G, Jin H, Anantharam V, Kanthasamy AG, Kanthasamy A, Manganese exposure induces neuroinflammation by impairing mitochondrial dynamics in astrocytes, Neurotoxicology. 64 (2019) 204–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Yuste JE, Tarragon E, Campuzano CM, Ros-Bernal F, Implications of glial nitric oxide in neurodegenerative diseases., Front. Cell. Neurosci 9 (2015) 322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Bryois J, Skene NG, Hansen TF, Kogelman LJA, Watson HJ, Liu Z, Eating Disorders Working Group of the Psychiatric Genomics Consortium, International Headache Genetics Consortium, 23andMe Research Team, L. Brueggeman, G. Breen, C.M. Bulik, E. Arenas, J. Hjerling-Leffler, P.F. Sullivan, Genetic identification of cell types underlying brain complex traits yields insights into the etiology of Parkinson’s disease, Nat. Genet. 52 (2020) 482–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Houser MC, Tansey MG, The gut-brain axis: Is intestinal inflammation a silent driver of Parkinson’s disease pathogenesis?, Npj Park. Dis 3 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ghaisas S, Maher J, Kanthasamy A, Gut microbiome in health and disease: linking the microbiome- gut-brain axis and environmental factors in the pathogenesis of systemic and neurodegenerative diseases, Crime Justice Am 158 (2016) 52–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Braak H, De Vos RAI, Bohl J, Del Tredici K, Gastric α-synuclein immunoreactive inclusions in Meissner’s and Auerbach’s plexuses in cases staged for Parkinson’s disease-related brain pathology, Neurosci. Lett. 396 (2006) 67–72. [DOI] [PubMed] [Google Scholar]
- [23].Sampson TR, Challis C, Jain N, Moiseyenko A, Ladinsky MS, Shastri GG, Thron T, Needham BD, Horvath I, Debelius JW, Janssen S, Knight R, Wittung-Stafshede P, Gradinaru V, Chapman M, Mazmanian SK, A gut bacterial amyloid promotes a-synuclein aggregation and motor impairment in mice, Elife 9 (2020) 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hegelmaier T, Lebbing M, Duscha A, Tomaske L, Tönges L, Holm JB, Bjørn Nielsen H, Gatermann SG, Przuntek H, Haghikia A, Interventional Influence of the Intestinal Microbiome Through Dietary Intervention and Bowel Cleansing Might Improve Motor Symptoms in Parkinson’s Disease, Cells 9 (2020) 376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Assencio FR, Alpha-synuclein as therapeutic target in Parkinson’s disease, Neuroforum 25 (2019) 129–136. [Google Scholar]
- [26].Acharya R, Chakraborty M, Chakraborty J, Prospective treatment of Parkinson’s disease by a siRNA-LDH nanoconjugate, Medchemcomm 10 (2019) 227–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Schlich M, Longhena F, Faustini G, O’Driscoll CM, Sinico C, Fadda AM, Bellucci A, Lai F, Anionic liposomes for small interfering ribonucleic acid (siRNA) delivery to primary neuronal cells: Evaluation of alpha-synuclein knockdown efficacy, Nano Res 10 (2017) 3496–3508. [Google Scholar]
- [28].Helmschrodt C, Höbel S, Schöniger S, Bauer A, Bonicelli J, Gringmuth M, Fietz SA, Aigner A, Richter A, Richter F, Polyethylenimine Nanoparticle-Mediated siRNA Delivery to Reduce α-Synuclein Expression in a Model of Parkinson’s Disease, Mol. Ther. - Nucleic Acids. 9 (2017) 57–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Rockenstein E, Ostroff G, Dikengil F, Rus F, Mante M, Florio J, Adame A, Trinh I, Kim C, Overk C, Masliah E, Rissman RA, Combined active humoral and cellular immunization approaches for the treatment of synucleinopathies, J. Neurosci. 38 (2018) 1000–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Brys M, Fanning L, Hung S, Ellenbogen A, Penner N, Yang M, Welch M, Koenig E, David E, Fox T, Makh S, Aldred J, Goodman I, Pepinsky B, Liu YT, Graham D, Weihofen A, Cedarbaum JM, Randomized phase I clinical trial of anti-α-synuclein antibody BIIB054, Mov. Disord. 34 (2019) 1154–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Jankovic J, Goodman I, Safirstein B, Marmon TK, Schenk DB, Koller M, Zago W, Ness DK, Griffith SG, Grundman M, Soto J, Ostrowitzki S, Boess FG, Martin-Facklam M, Quinn JF, Isaacson SH, Omidvar O, Ellenbogen A, Kinney GG, Safety and Tolerability of Multiple Ascending Doses of PRX002/RG7935, an Anti-Synuclein Monoclonal Antibody, in Patients with Parkinson Disease: A Randomized Clinical Trial, JAMA Neurol. 75(2018)1206–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Schlichtmann BW, Kondru N, Hepker M, Kanthasamy AG, Anantharam V, John M, Ban B, Mallapragada SK, Narasimhan B, Enzyme Immunoassay-based Assay Platform for Accurate Detection of Serum Pathological Alpha Synuclein in Parkinson’s Disease Patients, Mol. Pharm. Submitted (2020). [DOI] [PubMed] [Google Scholar]
- [33].F. ud Din W, Aman A, Ullah I, Qureshi OS, Mustapha O, Shafique S, Zeb A, Effective use of nanocarriers as drug delivery systems for the treatment of selected tumors, Int. J. Nanomedicine 12 (2017) 7291–7309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].** Tang S, Wang A, Yan X, Chu L, Yang X, Song Y, Sun K, Yu X, Liu R, Wu Z, Xue P, Brain-targeted intranasal delivery of dopamine with borneol and lactoferrin co-modified nanoparticles for treating Parkinson’s disease, Drug Deliv 26 (2019) 700–707. The concept of multiscale targeting is a growing area of interest for brain-targeted, nanocarrier-based therapeutics. Tang et al. utilize the foundation of a well-studied and FDA-approved polymeric NC chemistry and apply multiple layers of targeting ligands that act synergistically to optimize therapeutic efficacy of dopamine in protecting against 6-OHDA challenge in rats in this influential study. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Dhar S, Baker EW, Marrache S, West FD, Wyatt E, Marrache S, West FD, Therapeutic Nanoparticles for Accumulation in the Brain. US Patent No. 20170216219, 20170216219, 2017.
- [36].Schlichtmann B, Kalyanaraman B, Nelson R, Panthani M, Anantharam V, Kanthasamy A, Mallapragada S, Narasimhan B, Neuronal Targeting of Triphenylphosphonium-functionalized Polyanhydride Nanoparticles to Combat Rotenone-induced Mitochondrial Dysfunction, Int. J. Nanomedicine. Submitted (2020). [Google Scholar]
- [37].Zhang N, Yan F, Liang X, Wu M, Shen Y, Chen M, Xu Y, Zou G, Jiang P, Tang C, Zheng H, Dai Z, Localized delivery of curcumin into brain with polysorbate 80-modified cerasomes by ultrasound-targeted microbubble destruction for improved Parkinson’s disease therapy, Theranostics 8 (2018) 2264–2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].** Hui Li Y, Chen ZX, Lu ZG, Yang QH, Liu LY, Jiang ZT, Zhang LQ, Zhang X, Qing H, “Cell-addictive” dual-target traceable nanodrug for Parkinson’s disease treatment via flotillins pathway, Theranostics 8 (2018) 5469–5481. The multi-component nature of the NC treatment used by Li et al alludes to the rational design concept focused on in this review. By synthesizing a BBB and dopamine transporter dual target scheme using B6 and mazindol, the multiscale targeting led to optimized therapeutic effect for the drug, EGCG by way of inhibiting aSyn fibrillation and restoring dopaminergic function. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Kundu P, Das M, Tripathy K, Sahoo SK, Delivery of Dual Drug Loaded Lipid Based Nanoparticles across the Blood-Brain Barrier Impart Enhanced Neuroprotection in a Rotenone Induced Mouse Model of Parkinson’s Disease, ACS Chem. Neurosci 7 (2016) 1658–1670. [DOI] [PubMed] [Google Scholar]
- [40].Bhattamisra SK, Shak AT, Xi LW, Safian NH, Choudhury H, Lim WM, Shahzad N, Alhakamy NA, K. M Anwer, Radhakrishnan AK, Md S, Nose to brain delivery of rotigotine loaded chitosan nanoparticles in human SH-SY5Y neuroblastoma cells and animal model of Parkinson’s disease, Int. J. Pharm. 579 (2020). [DOI] [PubMed] [Google Scholar]
- [41].Mullis AS, Schlichtmann BW, Narasimhan B, Cademartiri R, Mallapragada SK, Ligand-cascading nano-delivery devices to enable multiscale targeting of anti-neurodegenerative therapeutics, Biomed. Mater 13 (2018) 034102. [DOI] [PubMed] [Google Scholar]
- [42].Taebnia N, Morshedi D, Yaghmaei S, Aliakbari F, Rahimi F, Arpanaei A, Curcumin-Loaded Amine-Functionalized Mesoporous Silica Nanoparticles Inhibit α-Synuclein Fibrillation and Reduce Its Cytotoxicity-Associated Effects, Langmuir 32 (2016) 13394–13402. [DOI] [PubMed] [Google Scholar]
- [43].* Gao G, Chen R, He M, Li J, Wang L, Sun T, Gold nanoclusters for Parkinson’s disease treatment, Biomaterials 194(2019)36–46. Intrinsically therapeutic NCs are very intriguing for the rational design of all-encompassing anti-synucleinopathic treatments. Gao et al describe how metal NCs can provide therapeutic effect by alleviating fibril formulation and also protecting against MPTP challenge in mice. From this study, it is evident that Gold NCs should be considered when designing potential combination treatments for disease. [DOI] [PubMed] [Google Scholar]
- [44].Zand Z, Khaki PA, Salihi A, Sharifi M, Nanakali NMQ, Alasady AAB, Aziz FM, Shahpasand K, Hasan A, Falahati M, Cerium oxide NPs mitigate the amyloid formation of α-synuclein and associated cytotoxicity, Int. J. Nanomedicine 14 (2019) 6989–7000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Ruotolo R, De Giorgio G, Minato I, Bianchi MG, Bussolati O, Marmiroli N, Cerium oxide nanoparticles rescue α-synuclein-induced toxicity in a yeast model of parkinson’s disease, Nanomaterials 10 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].* Aliakbari F, Mohammad-Beigi H, Rezaei-Ghaleh N, Becker S, Dehghani Esmatabad F, Eslampanah Seyedi HA, Bardania H, Tayaranian Marvian A, Collingwood JF, Christiansen G, Zweckstetter M, Otzen DE, D. Morshedi, The potential of zwitterionic nanoliposomes against neurotoxic alpha-synuclein aggregates in Parkinson’s Disease, Nanoscale 10 (2018) 9174–9185. Aliakbari et al have evaluated a very interesting NC treatment, in that the studied liposomal formulation intrinsically interacts with aSyn to reduce the propensity for fibrillation. Incubating aSyn transgenic cells with these anionic NCs also reduced overall neurotoxic effects and reactive oxygen species in vitro. In an effort to discover promising multifunctional and/or multiscale targeting NC treatments, this study implicates that this and related chemistries could be a promising foundation for the rational design of anti-synucleinopathic treatments. [DOI] [PubMed] [Google Scholar]
- [47].Bourdenx M, Daniel J, Genin E, Soria FN, Blanchard-Desce M, Bezard E, Dehay B, Nanoparticles restore lysosomal acidification defects: Implications for Parkinson and other lysosomal-related diseases, Autophagy 12 (2016)472–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Parashar A, Udayabanu M, Gut microbiota: Implications in Parkinson’s disease, Park. Relat. Disord 38 (2017) 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Long-Smith C, O’Riordan KJ, Clarke G, Stanton C, Dinan TG, Cryan JF, Microbiota-Gut-Brain Axis: New Therapeutic Opportunities, Annu. Rev. Pharmacol. Toxicol. 60 (2020) 477–502. [DOI] [PubMed] [Google Scholar]
- [50].Leclair-Visonneau L, Neunlist M, Derkinderen P, Lebouvier T, The gut in Parkinson’s disease: Bottom-up, top-down, or neither?, Neurogastroenterol. Motil 32 (2020) 1–6. [DOI] [PubMed] [Google Scholar]


