Abstract
Background:
Data on drug-induced liver injury (DILI) and acute liver failure (ALF) on modern oncologic phase I trials are limited, specifically regarding the incidence and resolution of DILI and safety of drug rechallenge.
Methods:
We reviewed all patients who were recruited to oncologic phase I trials between 2013 and 2017 at Memorial Sloan Kettering Cancer Center. Clinicopathologic data were extracted to characterize DILI and attribution was assessed using prospectively generated data during the studies. Logistic regression models were used to explore factors related to DILI and DILI recurrence after drug rechallenge.
Results:
Among 1670 cases recruited to 85 phase I trials, 81 (4.9%) developed DILI. The rate of DILI occurrence was similar between patients in immune-based trials and targeted therapy trials (5.0% vs. 4.9%), as was the median time to DILI (5.5 vs. 6.5 weeks, respectively; P = 0.48). Two patients (0.12%) met the criteria of Hs’ law, although none developed ALF. DILI resolved in 96% of patients. Pretreatment factors did not predict for DILI development. Thirty-six of 81 patients underwent a drug rechallenge, of whom 28% developed DILI recurrence. Peak alanine aminotransferase during the initial DILI was associated with DILI recurrence (OR 1.04; 95% CI, 1.0 to 1.09; P = 0.035).
Conclusions:
On modern phase I oncology trials, DILI is uncommon, may occur at any time, and often resolves with supportive measures. Rechallenging after DILI is feasible; however, the high rate of DILI recurrence suggests that clinicians should consider the severity of the DILI episode and treatment alternatives.
Keywords: Advanced solid tumors, Phase I, Drug induced liver injury, Precision medicine, Immunotherapy
Precis for Use in the Table of Contents:
On modern phase I oncology trials, drug-induced liver injury (DILI) is uncommon and may occur at any time. Drug rechallenge after DILI is feasible; however, the high rate of DILI recurrence suggests that clinicians should consider the severity of the DILI episode and other treatment alternatives.
INTRODUCTION
Nearly half of all drugs entering early-phase clinical trials fail to advance to phase II/III studies due to intolerable toxicity, inactivity, and/or unfavorable pharmacokinetic properties.1 Hepatic function, a critical determinant of investigational drug metabolism, influences drug efficacy and adverse events (AEs). Given the paramount importance of liver function and hepatic metabolism, preclinical toxicology studies seek to extensively vet novel anticancer treatment candidates for their effects on the liver. Although rare, drug-induced liver injury (DILI) that progresses to acute liver failure (ALF) is a catastrophic event for patients with cancer, and may also delay or terminate oncologic drug development programs.2 Hence, predictive models designed to assess the potential for a drug to cause ALF, including Hs’ law, are often embedded into phase I studies.3–6 Data regarding the predictive features of such models are derived from studies enriched with patients who have idiosyncratic reactions to antibiotics and anti-epileptics drugs and have never been prospectively validated in oncology drug development.6, 7
Prior to the advent of both molecular-targeted and immune-based therapies, it was established that about 60% of patients experience some degree of liver test abnormalities in the context of investigational cytotoxic chemotherapy, and approximately 6% experience severe hepatic dysfunction as defined by the Organ Dysfunction Working Group.8 Phase I studies in oncology are now dominated by small molecule therapy and immunotherapies, for which there are limited data regarding the incidence, kinetics, treatment, and resolution of liver function abnormalities.9 As the pathophysiology of DILI is dependent on the mechanism of action of the proposed drug,10 a review of hepatic toxicity in contemporary early-phase trials is highly relevant. These findings have the potential to guide both the drug development and clinical management of this AE. Furthermore, it is unclear if rechallenge after DILI is a feasible strategy, which historically has been discouraged.11 In oncologic trials, rechallenge after DILI might be favored for patients without other treatment alternatives and a favorable perceived risk-benefit ratio. An adaptive response by the liver after exposure to the drug has been described, providing the rationale for rechallenge.12 Experts have advocated for this approach in specific circumstances,13 but data supporting the safety of rechallenge in patients with cancer are scarce.
Herein, we reviewed a large, prospectively-annotated, clinical database of solid tumor patients receiving contemporary investigational therapy. To define the DILI event, we used causality assessment generated during the prospective studies. Our goals were to describe the pattern and kinetics of DILI in patients enrolled in modern phase I trials; investigate factors that may predispose patients to DILI; and describe outcomes in those who developed DILI and later underwent rechallenge with the study drug.
MATERIALS AND METHODS
Study Design and Participants
All patients with advanced cancer recruited and treated for phase I trials between January 1, 2013 and December 31, 2017 on the Phase I Service at Memorial Sloan Kettering Cancer Center (MSK) were included for analysis. Patients were treated on a dedicated phase 1 service, which included board -certified medical oncologists, a dedicated research pharmacist, research nurses and research staff. Relevant clinical data and all AEs were recorded prospectively on the MSK Clinical Research Database (CRDB), an institutionally-supported, encrypted, electronic database.14 National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE) was used for prospective recording of all AEs, AE grade and severity, and AE attribution for all patients. Attribution/causality of AEs, including DILI, was assessed by the Principal Investigator of each clinical trial after review of real-time clinical data and medical work-up for alterative causes. Unrelated AEs were defined as those that were clearly not or doubtfully-related to the investigational product, while related AEs were at least possibly related to the investigational agent per the NCI criteria.
MSK-CRDB was queried for all patients treated during a 5-year period and all clinical data were extracted from the database. All patients who developed DILI during these trials were identified by performing a targeted electronic search of MSK-CRDB. The specific search terms used to identify toxicities included the following: “alanine aminotransferase increased”, “aspartate aminotransferase (AST) increased”, “alkaline phosphatase increased”, “blood bilirubin increased”, “INR increase”, ”encephalopathy,” and “hepatic failure.” All patient charts then underwent manual review by at least 2 adjudicators, and when required, a board-certified hepatologist, to assurance quality of the prospective data.
DILI was defined as alanine aminotransferase (ALT) > 5-fold the upper limit of normal (5× ULN), alkaline phosphatase (ALP) > 2× ULN, or ALT > 3× ULN and total bilirubin > 2× ULN.15 Hs’ law was defined as DILI resulting in increased ALT > 3× ULN and total bilirubin > 2× ULN after excluding other potential causes and without a significant cholestatic component (ALP > 2× ULN) according to the Food and Drug Administration guideline for DILI evaluation.16 ALF was defined as presence of hepatic encephalopathy assessed and allocated by the investigator during the study and coagulopathy (international normalized ratio > 1.5).17
Data Collection
Clinical data were extracted from the electronic medical records. Variables collected included the following: patient and tumor characteristics, treatments received, laboratory values, and clinical outcomes, including proportion of patients who experienced DILI, time to DILI onset, resolution of DILI, DILI recurrence after rechallenge, and prolonged treatment after rechallenge, which was defined as more than 3 months of treatment. This research was conducted under appropriate Institutional Review Board/Privacy Board protocols and waivers, and the study was conducted in accordance with recognized ethical guidelines.
Statistical Analyses
A descriptive analysis of the population was performed using central tendency and dispersion measures according to the nature of the variable. Fisher’s exact test was used to compare categorical variables and the Wilcoxon-Mann-Whitney test was used for continuous variables. Patterns of liver abnormalities were tabulated and summarized descriptively. Time to DILI among those with DILI was measured in weeks from treatment start to first documentation of DILI, according to abovementioned criteria. Associations between patient characteristics and liver toxicity were assessed univariately. Logistic regression models were used for the binary liver toxicity outcomes of DILI, rechallenge after DILI, and recurrence after rechallenge.
RESULTS
Patients
This study includes 1670 cases enrolled in a total of 85 phase I trials at MSK within the Phase I Service. This considers 1535 patients of whom 120 patients participated in more than one phase I trial. Patients received treatments with the following drug classes: immunotherapy (50%), targeted agents (42%), a combination of therapies (4%), cytotoxic agents (3%), and other types of drugs (1%). The most frequent immunotherapy strategy used for the management of these patients was combination immunotherapy (51%) followed by single-agent immune checkpoint modulators (41%). Patients were a median age of 60 years (range, 15 to 81 years), and 54% were female. In addition, gastrointestinal tract cancer was the most common type of malignancy (21.5%). A consort diagram of patients included in this study is depicted in Figure 1 and mechanism of action of study drugs in the DILI cohort is described in Supplementary Table 1.
Figure 1. Consort diagram.
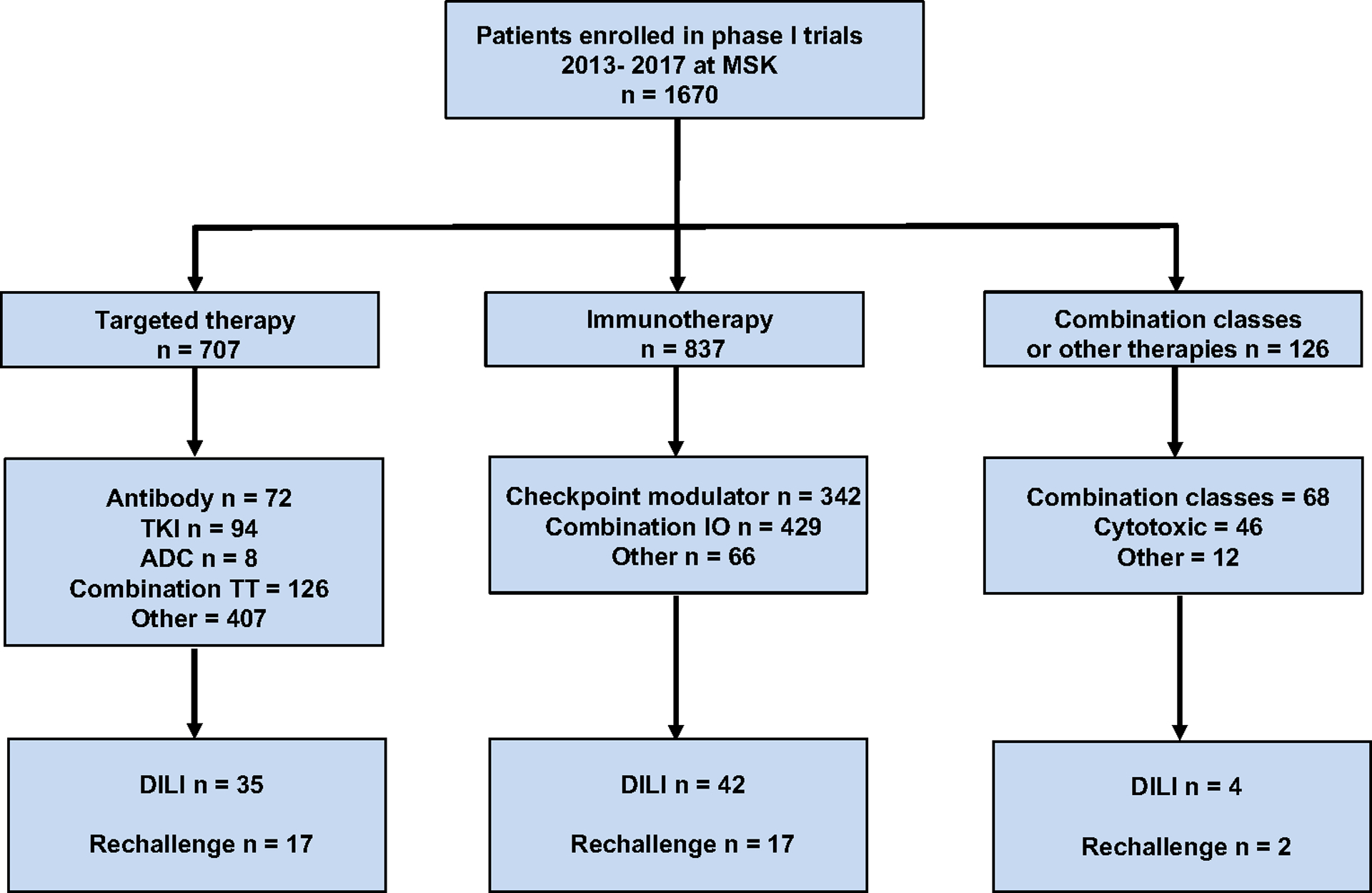
ADC = antibody-drug conjugate; DILI = drug-induced liver injury; IO = immunotherapy TKI = tyrosine kinase inhibitor; TT = targeted therapy.
Proportion of Patients with DILI and ALF
Among 1670 cases, 81 patients (4.9%) developed DILI during the study period. Baseline characteristics were similar among those who developed DILI and those who did not (Table 1). No patient with DILI had a previous diagnosis of chronic liver disease, including cirrhosis or a history of DILI. Sixty patients (74%) had baseline viral serology for hepatitis which was negative in all cases. The incidence of DILI was similar in immunotherapy trials (5%) compared with targeted therapy trials (4.9%) (P = 0.90). Seventy-five percent of patients had ALT > 5× ULN at diagnosis of DILI and 39% had ALP > 2× ULN. In 22% of patients, more than one criterion was met for the diagnosis of DILI. Four patients underwent liver biopsy as a result of hepatic test alterations; in 3 of these patients, the biopsy described changes associated with drug causality, and the remaining patient had non-specific pathologic findings.
Table 1.
Baseline characteristics
| Non-DILI cohort (n=1589) (%) |
DILI Cohort (n=81) (%) |
P value | |
|---|---|---|---|
| Median age, years (range) | 60.3 (15–90) | 59.8 (19–81) | 0.9 |
| Sex | 0.6 | ||
| Male | 725 (46) | 34 (42) | |
| Female | 864 (54) | 47 (58) | |
| Type of neoplasms | NA | ||
| Gastrointestinal | 343 (22) | 16 (20) | |
| Lung Cancer | 257 (16) | 12 (15) | |
| Gynecologic | 229 (14) | 18 (22) | |
| Breast Cancer | 157 (10) | 6 (7) | |
| Hematologic | 90 (6) | 4 (5) | |
| Genitourinary | 152 (10) | 11 (14) | |
| Other | 361 (23) | 14 (17) | |
| Type of treatment | 0.9 | ||
| Targeted therapy | 672 (42) | 35 (43) | |
| Immunotherapy | 795 (50) | 42 (52) | |
| Cytotoxic | 44 (3) | 2 (3) | |
| Other | 12 (1) | 0 | |
| Combination of classes | 66 (4) | 2 (3) |
DILI, drug-induced liver injury; NA, not applicable; y, years.
The median time to diagnosis of DILI was 6 weeks since the start of the study drug (range, 1 to 121 weeks) (Figure 2). In 50 patients (62%), the DILI event occurred after the first 4 weeks on study. Among those who developed DILI, the median time to onset was similar between patients receiving targeted therapy and immunotherapy (5.5 vs. 6.5 weeks, P= 0.48). During the evolution of DILI, the incidence of grade 4 AST, ALT, ALP, and bilirubin elevation was 5%, 11%, 1%, and 1%, respectively. During the study period, 2 patients (0.12%) met Hs’ law; no episodes of drug-related liver failure were identified. In most cases (96%), liver enzymes returned to grade 1 levels with supportive measures, study drug cessation, and immunosuppression in a median time of 13 days (range, 2 to 178 days). Two patients died due to disease progression before DILI resolution and 1 patient was lost to follow-up. In patients who developed DILI while receiving immunotherapy, 84% and 24% were treated with corticosteroids and combination of corticosteroids and mycophenolate, respectively. In patients enrolled into trials that included targeted therapies or a combination of different classes of agents, corticosteroids were used in 26%.
Figure 2. Time to drug-induced liver injury (n=81).
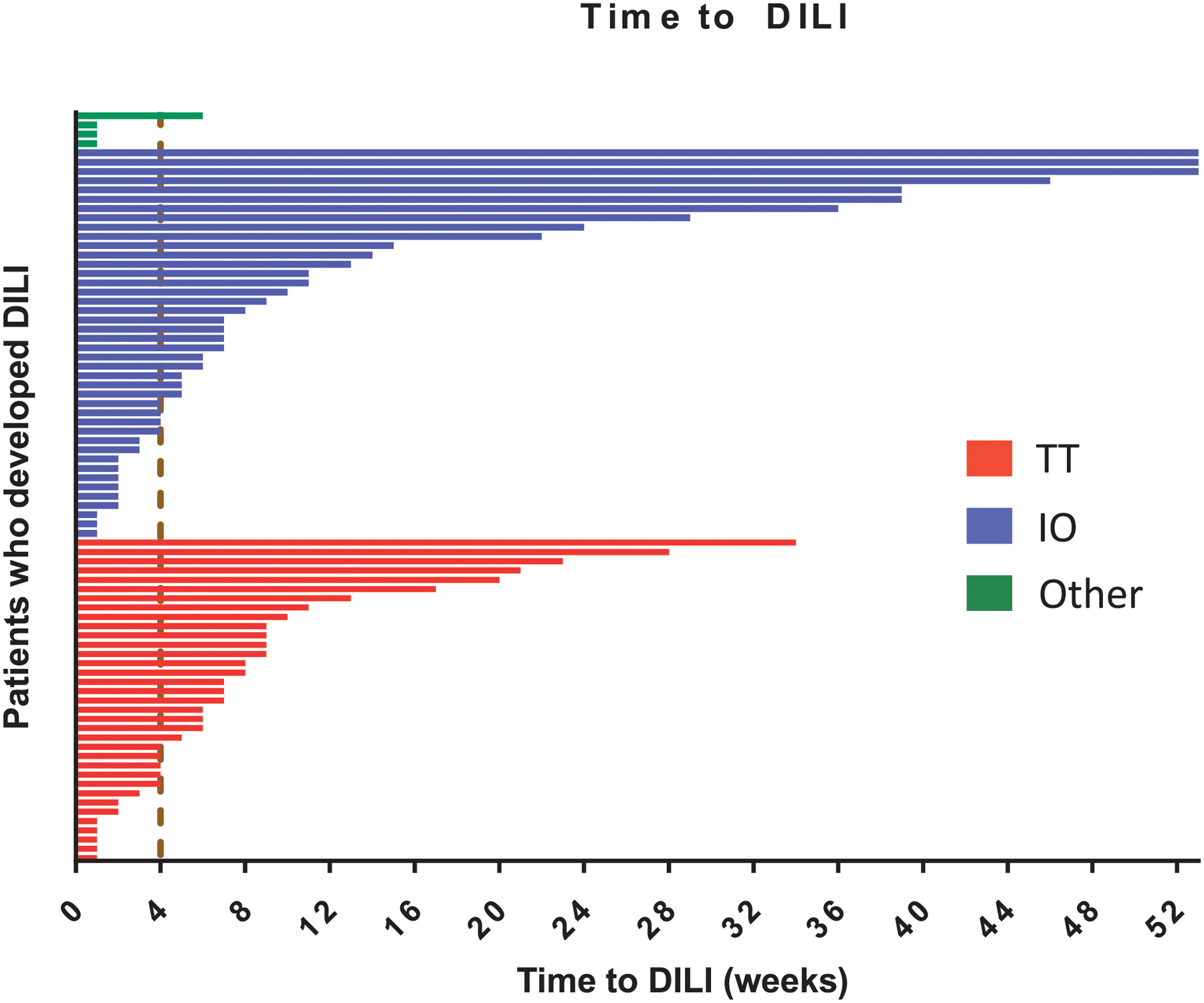
IO = immunotherapy; TT = targeted therapy.
a Dashed line represents 4-week mark, which is frequently considered the dose-limiting period in phase I trials.17
Rechallenge after DILI
In the group of patients with DILI, 36 (44%) underwent a drug rechallenge. In 21 patients (58%), the treatment was modified with a dose reduction or by stopping one of the drugs in combination regimens. In the rechallenge group, the incidence of grade 4 AST, ALT, ALP and bilirubin elevation was 0%, 6%, 0% and 0%, respectively. Characteristics of patients who underwent and did not undergo a rechallenge are described in Table 2. The previous use of mycophenolate was higher in patients without a rechallenge compared with those who underwent rechallenge (20% vs. 3%; P= 0.037). Subsequent DILI after rechallenge occurred in 28% of patients (Figure 3). Median time to DILI recurrence among those who underwent rechallenge and had a recurrence was 3 weeks (range, 1 to 10 weeks), and there were no cases of ALF. Fifty-six percent of patients received the study drug for more than 3 months after drug rechallenge. Fifteen patients (42%) received the study drug for more than 3 months without experiencing DILI recurrence.
Table 2.
Characteristics patients with and without drug rechallenge after DILI
| Rechallenge with study drug | |||
|---|---|---|---|
| Yes n=36 (%) |
No n=45 (%) |
P value | |
| Median age, years (range) | 60.1 (19–78) | 59.6 (31–81) | 0.8 |
| Sex | |||
| Male | 16 (44) | 18 (40) | 0.9 |
| Female | 20 (56) | 27 (60) | |
| Type of treatment | |||
| Targeted therapy | 17 (47) | 18 (40) | 0.9 |
| Immunotherapy | 17 (47) | 25 (56) | |
| Cytotoxic | 1 (3) | 1 (2) | |
| Combination of classes | 1 (3) | 1 (2) | |
| DILI criteria | |||
| ALT> 5x ULN | 29 (81) | 32 (71) | 0.5 |
| ALP> 2x ULN | 11 (31) | 21 (47) | 0.2 |
| ALT> 3x ULN/TBL> 2x ULN | 1 (3) | 3 (7) | 0.6 |
| Median LFTs at DILI diagnosis | |||
| AST | 147 | 185 | 0.5 |
| ALT | 245 | 214 | 0.5 |
| ALP | 146 | 210 | 0.2 |
| Total bilirubin | 0.65 | 0.6 | 0.4 |
| Median LFTs at peak | |||
| AST | 172 | 201 | 0.2 |
| ALT | 298 | 306 | 0.7 |
| ALP | 176 | 271 | 0.06 |
| Total bilirubin | 0.9 | 0.9 | 0.6 |
| Treatment for DILI | |||
| Corticosteroids | 22 (61) | 23 (51) | 0.5 |
| Mycophenolate | 1 (3) | 9 (20) | 0.037 |
ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; DILI, drug-induced liver injury; LFT, liver function test; ULN, upper limit of normal; y, years.
Figure 3. Characteristics and outcomes in patients who underwent a rechallenge with the study drug.
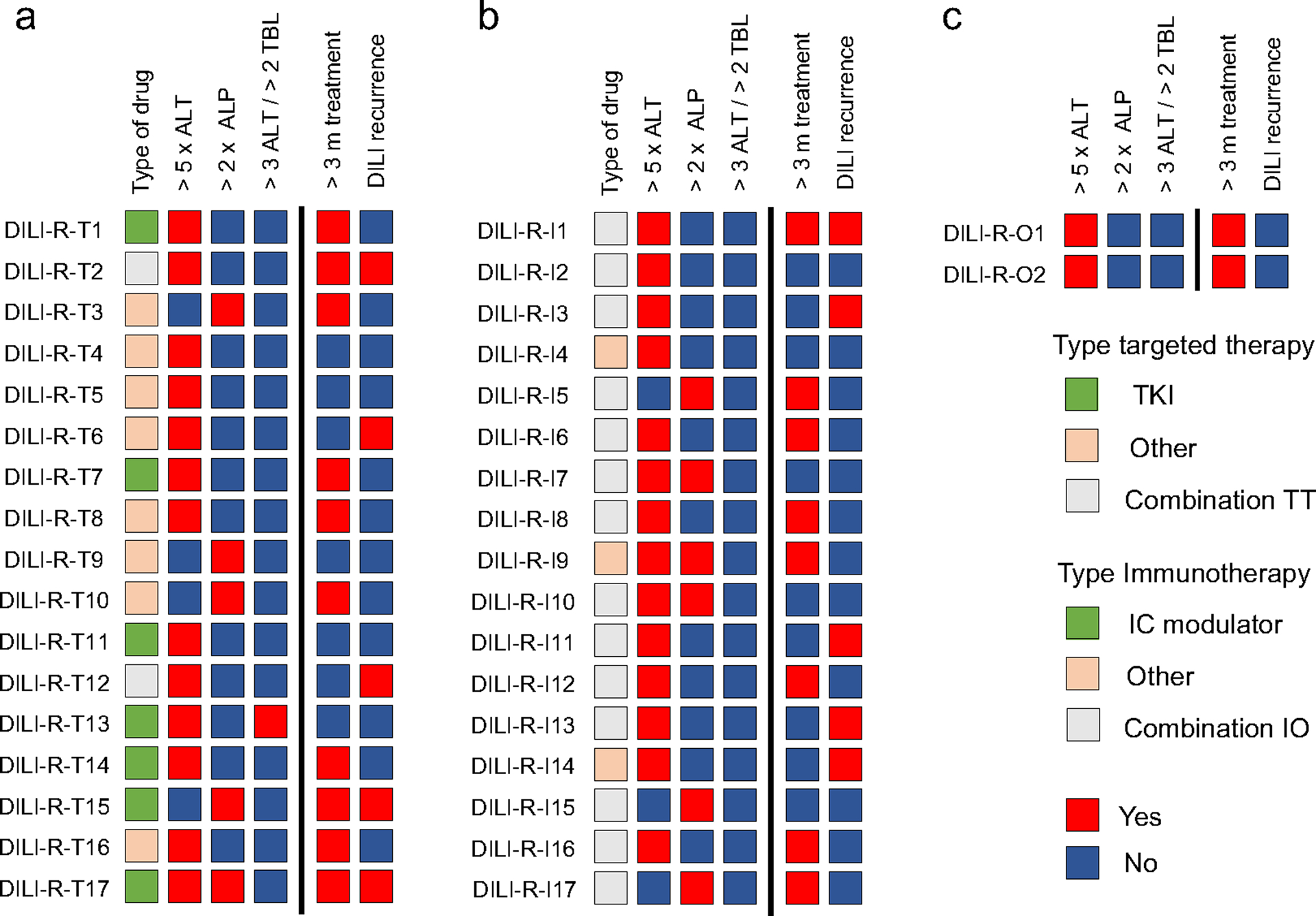
Outcomes include recurrence of DILI and continue study drug after rechallenge for more than 3 months. A) Targeted therapy cohort B) Immunotherapy cohort C) Combination of different classes cohort.
ALP = alkaline phosphatase; ALT= alanine aminotransferase; IC = immune checkpoint; IO = immunotherapy; m = months; DILI = drug-induced liver injury; TBL = total bilirubin; TKI= tyrosine kinase inhibitor; TT = targeted therapy.
Predictors of DILI and DILI recurrence
Age, sex, tumor type, and type of investigational treatment were not associated with DILI occurrence based on univariate analysis. Following a first episode of DILI and rechallenge, the only variable that was associated with recurrence of liver injury was the peak value of ALT at the time of the first episode of DILI (OR 1.04; 95% confidence interval, 1.0 to 1.09; P= 0.035) (Table 3). Multivariate analysis was not conducted given the limited number of DILI events and the observation that few patient characteristics were positive on univariate analysis.
Table 3.
Univariate logistic regression analysis of DILI development and DILI recurrence after rechallenge.
| DILI development | |||
|---|---|---|---|
| Covariate | OR | 95% CI | P value |
| Age | 1.00 | 0.98 to 1.01 | 0.7 |
| Female Sex | 1.16 | 0.74 to 1.84 | 0.5 |
| Type of treatment | 0.8 | ||
| Targeted therapy | Ref. | ||
| Immunotherapy | 1.01 | 0.64 to 1.61 | |
| Combination of classes | 0.58 | 0.09 to 1.97 | |
| Other | 0.69 | 0.11 to 2.33 | |
| DILI recurrence after Rechallenge | |||
| Covariate | OR | 95% CI | P value |
| Age | 1.01 | 0.96 to 1.07 | 0.6 |
| Female sex | 0.73 | 0.16 to 3.24 | 0.7 |
| ALT> 5x ULN | 2.70 | 0.38 to 54.9 | 0.3 |
| ALP> 2x ULN | 0.47 | 0.06 to 2.40 | 0.4 |
| ALT peak | 1.04 | 1.00 to 1.09 | 0.035 |
| ALP peak | 0.99 | 0.93 to 1.06 | 0.8 |
| Total bilirubin peak | 1.75 | 0.48 to 6.61 | 0.4 |
ALP, alkaline phosphatase; ALT, alanine aminotransferase; DILI, drug-induced liver injury; OR, odds ratio; ULN, upper limit of normal; Ref, reference.
DISCUSSION
In this large, single-center study, DILI occurred in approximately 5% of patients. Of note, ALF was not observed in our carefully monitored cohort, and most patients recovered with drug cessation with or without supportive medications. DILI incidence was similar in patients treated with targeted therapies and immunotherapy agents, and no baseline patient- or trial-specific factors appeared to predict for first DILI occurrence. The number of potential predictive factors built into the analysis was limited; however, the stringent inclusion criteria on phase I studies effectively harmonized factors of interest such as performance status, baseline organ function, alcohol use, herbal mediations, viral hepatitis, and disease stage.
Our report provides important insights into DILI in the context of modern drug development. First, our observation that more than half of all DILI events occur after 28 days (i.e., the dose-limiting toxicity window for many phase I studies) reflects the complex pathophysiology of DILI.18 Agents that cause direct hepatic toxicity do so with short latency, with DILI occurring on the order of days, while agents that result in idiosyncratic hepatotoxicity or indirect liver injury may have a long latency, with DILI occurring on the order of weeks to months from the initiation of therapy. Second, inferred from our data, clinicians often establish the diagnosis of DILI on clinical grounds alone, without the use of a liver biopsy, after alternative causes such as viral hepatitis have been excluded. Third, regarding treatment of DILI, patients undergoing immunotherapy received corticosteroids and in some cases mycophenolate, but interestingly, more than a quarter receiving other classes of agents were also treated with corticosteroids. Corticosteroids have been shown to decrease liver toxicity in some specific settings, such as in the prevention of trabectedin-associated liver toxicity.19, 20 However, their extended use in patients with DILI that is not associated with immunotherapeutics is investigational.
A critical aspect for clinicians, sponsors, and regulatory authorities is patient management after toxicity. Although our data indicate that drug rechallenge is feasible and that a proportion of patients will receive the investigational agent without DILI recurrence for a prolonged time, such an approach is certainly not without a risk of DILI recurrence. Indeed, this risk was approximately five times higher after rechallenge compared with the initial DILI risk in our cohort. Furthermore, as 56% of patients with an initial DILI event were not rechallenged with drug, it is possible that risk of DILI recurrence in our series is an underestimate.
In those patient who underwent rechallenge, the severity of initial DILI was associated with a higher risk of DILI recurrence. Rechallenge of cancer drugs after DILI has been studied in patients receiving FDA-approved drugs. In a review of nine prospective phase II and III trials that evaluated efficacy and safety of pazopanib in patients with advanced cancer, the incidence of ALT > 3× ULN among 2080 patients was 20% (11% ALT > 5× ULN).21 Rechallenge with pazopanib was attempted in 103 of these patients, of whom 60% did not develop recurrence of liver function alteration. In this study, like in ours, more severe first ALT elevation (ALT > 8–20× ULN) was associated with higher risk of recurrence. The safety of resuming immunotherapy agents after severe immune-related AEs (irAEs) was also evaluated in a retrospective study of 80 patients treated with combination immune checkpoint blockade.22 In this study, patients were rechallenged with anti-PD-1 therapy, with an irAE recurrence rate of 18%. Interestingly, the risk of recurrence was higher in patients with immune-related hepatitis (17%, 5/29 patients) compared with colitis (6%, 2/33 patients). Our findings are similar to these examples and highlight that therapeutic rechallenge is indeed possible.
This study has limitations. First, it is an analysis at a single site that includes patients treated with a variety of drugs in phase I trials. Thus, the clinical implications of our findings in a rapidly evolving landscape in drug development must be taken with precaution. Second, we did not apply scoring instruments for the causality of DILI, such as the Roussel Uclaf Causality Assessment Method. However, the causality assessment in our study was based on prospective NCI CTCAE grading, and there is consensus that expert opinion remains the gold standard over standardized scoring instruments for this purpose.23 Third, we could not evaluate the performance of predictive models such as Hs’ law due to insufficient events, although this argues in favor of the safety of modern phase I trials in terms of liver toxicity. Fourth, given the small sample size, we could not assess the contribution of other clinicopathologic factors in the model of DILI occurrence and recurrence after rechallenge. Finally, we did not include relevant pharmacogenomic factors, such as HLA haplotype in our analysis.24
In conclusion, DILI occurs in approximately 5% of patients included in modern phase I oncology trials, and the risk of drug-related liver failure appears low in this cohort. While our findings support the feasibility of drug rechallenge after DILI, the risk of its recurrence is high, and several unanswered questions remain on the optimal patient and situation to consider a rechallenge. Further collaborative research is needed on potential predictive models for DILI recurrence to better select rechallenge candidates. Meanwhile, a careful decision must be made prior to proceeding with rechallenge in this setting, with consideration of available toxicity data of the study drug, other treatment alternatives, and discussion with the patient about the risk/benefit ratio.
Supplementary Material
Acknowledgements:
The authors would like to acknowledge Eileen O’Reilly for helpful discussion on the final manuscript.
Funding:
This work was supported in part by the National Cancer Institute of the National Institutes of Health [P30 CA008748] and Nonna’s Garden Foundation.
Potential Conflicts of Interest:
Sebastian Mondaca is a consultant for Foundation Medicine and Roche. He also has received institutional research funding from BMS.
Dazhi Liu is a consultant for Pfizer and Heron Therapeutic.
Mrinal M. Gounder reports receiving speakers bureau honoraria from and is a consultant/advisory board member for Epizyme, Daiichi Sankyo Inc., Springworks, Bayer, Amgen, Karyopharm, and TRACON.
Danny N. Khalil is an inventor on patent applications related to CD40 and in situ vaccination (PCT/US2016/045970), filed by MSKCC.
Alexander E. Drilon received research funding from Pfizer, Exelixis, GlaxoSmithKlein, Teva, Taiho, PharmaMar and Foundation Medicine, and personal fees from Ignyta, Genentech/Roche, Loxo, Bayer, Lilly, Takeda, Ariad/Millenium, TP Therapeutics, AstraZeneca, Pfizer, Blueprint Medicines, Helsinn, Beigene, BergenBio, Hengrui Therapeutics, Exelixis, Tyra Biosciences, Verastem, MORE Health, Abbvie, Medscape, OncLive, PeerVoice, Physicians Education Resources, Targeted Oncology, Research to Practice, and Oncology. He also received royalties from Wolters Kluwer.
Bob T. Li has received institutional research funding from Genentech, Guardant Health, GRAIL, Resolution Bioscience, AstraZeneca, Hengrui Therapeutics, Amgen, Daiichi Sankyo, BioMed Valley and is a consultant for Genentech, Hengrui Therapeutics, Mersana Therapeutics, Guardant Health, ThermoFisher Scientific, Biosceptre Australia.
Komal L. Jhaveri has received institutional research funding from Genentech, Debio Pharmaceuticals, Novita Pharmaceuticals, ADC Therapeutics, Clovis Oncology, Lilly Pharmaceuticals, Immunomedics, Novartis, Zymeworks, Pfizer and is a consultant for Synthon, AstraZeneca, Genentech, Pfizer. She has received travel expenses from Taiho Pharmaceutical, Pfizer, Jounce Therapeutics, AstraZeneca.
Neil H. Segal is a consultant for PsiOxus, PureTech Ventures, Amgen, GSK, C Stone Pharmaceuticals, Synlogic, Pieris, MedImmune/AstraZeneca, Gritstone Oncology, TRM oncology, Roche/Genentech, BMS, Kyn Therapautics, Aduro, Boehringer Ingelheim.
Margaret K. Callahan reports institutional research support and employment of a family member by Bristol-Myers Squibb; personal fees for advisory/consulting role from AstraZeneca/MedImmune, Incyte, Moderna, and Merck.
David M. Hyman received research funding from AstraZeneca, Puma Biotechnology, Loxo, Bayer and is a consultant for Chugai Pharma, Boehringer Ingelheim, AstraZeneca, Pfizer, Bayer, Genentech, and Fount. He has received travel expenses from Genentech and Chugai Pharma and owes stocks in Fount.
James J. Harding has received research and personal fees from Bristol-Myers Squibb and personal fees from Eisai, Exelixis, CytomX, QED, and Elly Lilly.
All other authors have no relevant disclosures to report.
Footnotes
Data accessibility statement
All authors had full access to the raw data in the study. All data are stored on a secure server at Memorial Sloan Kettering Cancer Center. Data requests may be submitted to JJH, who will submit for ethical approval and data sharing (hardinJ1@mskcc.org).
REFERENCES
- 1.Paul SM, Mytelka DS, Dunwiddie CT, et al. How to improve R&D productivity: the pharmaceutical industry’s grand challenge. Nat Rev Drug Discov. 2010;9: 203–214. [DOI] [PubMed] [Google Scholar]
- 2.Ricart AD. Drug-induced liver injury in Oncology. Ann Oncol. 2017;28: 2013–2020. [DOI] [PubMed] [Google Scholar]
- 3.H Z. Hepatotoxicity: The Adverse Effects of Drugs and Other Chemicals on the Liver. 2nd edition ed. Philadelphia, PA: Lippincott, Williams and Wilkins, 1999. [Google Scholar]
- 4.Lo Re V 3rd, Haynes K, Forde KA, et al. Risk of Acute Liver Failure in Patients With Drug-Induced Liver Injury: Evaluation of Hy’s Law and a New Prognostic Model. Clin Gastroenterol Hepatol. 2015;13: 2360–2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reuben A Hy’s law. Hepatology. 2004;39: 574–578. [DOI] [PubMed] [Google Scholar]
- 6.Robles-Diaz M, Lucena MI, Kaplowitz N, et al. Use of Hy’s law and a new composite algorithm to predict acute liver failure in patients with drug-induced liver injury. Gastroenterology. 2014;147: 109–118 e105. [DOI] [PubMed] [Google Scholar]
- 7.Chalasani N, Bonkovsky HL, Fontana R, et al. Features and Outcomes of 899 Patients With Drug-Induced Liver Injury: The DILIN Prospective Study. Gastroenterology. 2015;148: 1340–1352 e1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mansfield AS, Rudek MA, Vulih D, et al. The Effect of Hepatic Impairment on Outcomes in Phase I Clinical Trials in Cancer Subjects. Clin Cancer Res. 2016;22: 5472–5479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hyman DM, Eaton AA, Gounder MM, et al. Nomogram to predict cycle-one serious drug-related toxicity in phase I oncology trials. J Clin Oncol. 2014;32: 519–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoofnagle JH, Bjornsson ES. Drug-Induced Liver Injury - Types and Phenotypes. N Engl J Med. 2019;381: 264–273. [DOI] [PubMed] [Google Scholar]
- 11.Lee WM, Senior JR. Recognizing drug-induced liver injury: current problems, possible solutions. Toxicol Pathol. 2005;33: 155–164. [DOI] [PubMed] [Google Scholar]
- 12.Lewis JH. The adaptive response (drug tolerance) helps to prevent drug-induced liver injury. Gastroenterol Hepatol (N Y). 2012;8: 333–336. [PMC free article] [PubMed] [Google Scholar]
- 13.Hunt CM, Papay JI, Stanulovic V, Regev A. Drug rechallenge following drug-induced liver injury. Hepatology. 2017;66: 646–654. [DOI] [PubMed] [Google Scholar]
- 14.Caron-Fabio D, Lin KH, Kaufman K, et al. Two-Team Approach to the Design and Maintenance of a Clinical Research Database. Clinical Trials. 2009;6: 493–524. [Google Scholar]
- 15.Aithal GP, Watkins PB, Andrade RJ, et al. Case definition and phenotype standardization in drug-induced liver injury. Clin Pharmacol Ther. 2011;89: 806–815. [DOI] [PubMed] [Google Scholar]
- 16.FDA. Guidance for Industry Drug-Induced Liver Injury: Premarketing Clinical Evaluation. [accessed May 26, 2019].
- 17.Ostapowicz G, Fontana RJ, Schiodt FV, et al. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med. 2002;137: 947–954. [DOI] [PubMed] [Google Scholar]
- 18.Le Tourneau C, Razak AR, Gan HK, et al. Heterogeneity in the definition of dose-limiting toxicity in phase I cancer clinical trials of molecularly targeted agents: a review of the literature. Eur J Cancer. 2011;47: 1468–1475. [DOI] [PubMed] [Google Scholar]
- 19.Donald S, Verschoyle RD, Greaves P, et al. Complete protection by high-dose dexamethasone against the hepatotoxicity of the novel antitumor drug yondelis (ET-743) in the rat. Cancer Research & Treatment. 2003;63: 5902–5908. [PubMed] [Google Scholar]
- 20.Grosso F, Dileo P, Sanfilippo R, et al. Steroid premedication markedly reduces liver and bone marrow toxicity of trabectedin in advanced sarcoma. Eur J Cancer. 2006;42: 1484–1490. [DOI] [PubMed] [Google Scholar]
- 21.Powles T, Bracarda S, Chen M, et al. Characterisation of liver chemistry abnormalities associated with pazopanib monotherapy: a systematic review and meta-analysis of clinical trials in advanced cancer patients. Eur J Cancer. 2015;51: 1293–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pollack MH, Betof A, Dearden H, et al. Safety of resuming anti-PD-1 in patients with immune-related adverse events (irAEs) during combined anti-CTLA-4 and anti-PD1 in metastatic melanoma. Ann Oncol. 2018;29: 250–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Regev A, Seeff LB, Merz M, et al. Causality assessment for suspected DILI during clinical phases of drug development. Drug Saf. 2014;37 Suppl 1: S47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Avigan MI, Bjornsson ES, Pasanen M, et al. Liver safety assessment: required data elements and best practices for data collection and standardization in clinical trials. Drug Saf. 2014;37 Suppl 1: S19–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


