Abstract
Background:
Prenatal exposure to persistent organic pollutants (POPs), widespread in North America, is associated with increased Attention Deficit/Hyperactivity Disorder (ADHD) symptoms and may be a modifiable risk for ADHD phenotypes. However, the effects of moderate exposure to POPs on task-based inhibitory control performance, related brain function, and ADHD-related symptoms remain unknown, limiting our ability to develop interventions targeting the neural impact of common levels of exposure.
Objectives:
The goal of this study was to examine the association between prenatal POP exposure and inhibitory control performance, neural correlates of inhibitory control and ADHD-related symptoms.
Methods:
Prospective data was gathered in an observational study of Canadian mother-child dyads, with moderate exposure to POPs, including polychlorinated biphenyls (PCBs) and polybrominated diphenyl ethers (PBDEs), as part of the GESTation and the Environment (GESTE) cohort in Sherbrooke, Quebec, Canada. The sample included 87 eligible children, 46 with maternal plasma samples, functional magnetic resonance imaging (fMRI) data of Simon task performance at 9–11 years, and parental report of clinical symptoms via the Behavioral Assessment System for Children 3 (BASC-3). Simon task performance was probed via drift diffusion modeling, and parameter estimates were related to POP exposure. Simon task-based fMRI data was modeled to examine the difference in incongruent vs congruent trials in regions of interest (ROIs) identified by meta analysis.
Results:
Of the 46 participants with complete data, 29 were male, and mean age was 10.42 ± 0.55 years. Increased POP exposure was associated with reduced accuracy (e.g. PCB molar sum rate ratio = 0.95; 95% CI [0.90, 0.99]), drift rate (e.g. for PCB molar sum β = −0.42; 95% CI [−0.77, −0.07]), and task-related brain activity (e.g. in inferior frontal cortex for PCB molar sum β = −0.35; 95% CI [−0.69, 0.02]), and increased ADHD symptoms (e.g. hyperactivity PCB molar sum β = 2.35; 95%CI [0.17, 4.53]), supporting the possibility that prenatal exposure to POPs is a modifiable risk for ADHD phenotypes.
Discussion:
We showed that exposure to POPs is related to task-based changes in neural activity in brain regions important for inhibitory control, suggesting a biological mechanism underlying previously documented associations between POPs and neurobehavioral deficits found in ADHD phenotypes.
Keywords: Attention deficit hyperactivity disorder (ADHD), Functional magnetic resonance imaging (fMRI), Inhibitory control, Polybrominated diphenyl ethers (PBDEs), Polychlorinated biphenyls (PCBs), Persistent organic pollutants (POPs)
1. Introduction
Attention Deficit/Hyperactivity Disorder (ADHD) is one of the most common childhood neurobehavioral disorders and is associated with a range of long-term negative outcomes, including increased risk for substance use, mood and anxiety disorders, motor vehicle accidents, difficulties with educational achievement and employment status (Sayal et al., 2018; Kupper et al., 2012; Geffen and Forster, 2018). Evidence of benefits of ADHD treatment are more often found in shorter-compared to longer-term follow ups (Arnold et al., 2015), suggesting that long-term sequelae of ADHD persist. During childhood, ADHD creates a significant economic burden for families via increased medical and indirect costs related to absenteeism and reduced productivity (Gupte--Singh et al., 2017). Improved understanding of modifiable risk factors of ADHD could elucidate paths to reducing both the personal and societal burden of this highly prevalent condition.
Studies examining the heritability of ADHD via family, twin and adoption studies, estimate a heritability rate at 74%–88% (Faraone and Larsson, 2019), however, heritability estimates from genome wide association studies (GWAS) are much lower, e.g. 22% (Demontis et al., 2019). The “heritability gap” between estimates from family compared to GWAS studies suggests that the environment may play an important role in ADHD risk. Observations of increasing prevalence rates of ADHD (Sayal et al., 2018; Vasiliadis et al., 2017; Visser et al., 2014) may also suggest that environmental exposures contribute to ADHD, although increased diagnosis and awareness of neurodevelopmental disorders contribute to these observations (Huffling et al., 2016; Lam et al., 2017; Landrigan et al., 2012).
Persistent organic pollutants (POPs), including polychlorinated biphenyls (PCBs) and polybrominated diphenyl ethers (PBDEs), are toxic chemicals once widely used in plastics, electrical equipment and flame retardants. Prenatal exposure to PCBs and PBDEs is associated with neurobehavioral deficits related to ADHD in observational studies, including reductions in inhibitory control and attention, and increased externalizing behaviors and ADHD symptoms based on teacher reports (Eubig et al., 2010; Verner et al., 2015; Sagiv et al., 2010, 2015; Stewart et al., 2003, 2005; Jacobson and Jacobson, 2003; Vreugdenhil et al., 2002; Vuong et al., 2018; Eskenazi et al., 2013; Herbstman et al., 2010; de Water et al., 2019). Therefore, prenatal exposure to POPs may be a modifiable risk for ADHD and related behavioral phenotypes.
In recognition of the toxicity of these chemicals, global treaties have banned or slowed the production of many POPs in over 90 countries (e.g. the Stockholm Convention (Stockholm Convention. In:, 2001)). However, POPs persist for long periods, bioaccumulate and move up the food chain (Guo et al., 2019; Norstrom et al., 2010). Due to the persistence of these chemicals, as well as the consumption of animal products in which POPs have bioaccumulated (e.g. meat, fish and dairy (Norstrom et al., 2010)) and the continued release of POPs from existing products (e.g. electronics and furniture (Peltier et al., 2012)), exposure continues to occur, despite the slowing production of POPs. Widespread exposure has been observed in North America, with recent studies finding the majority of the population exposed to PCBs (Singh et al., 2019; Xue et al., 2014) and near universal PBDE exposure (Harley et al., 2010; Rawn et al., 2014).
Prior task-based neuroimaging research documents POP exposure-related differences in neural activation between exposed and non-exposed groups in observational studies (Chu et al., 2019; White et al., 2011). However, only two such studies exist, and they have been limited by small sample sizes (12 and 21 participants, respectively), by restricting analyses to groups exposed to high levels of POPs (due to environmental disaster or high seafood diets), and by only including male participants. Thus, the effects of moderate POP exposure on brain function remain unknown, limiting our ability to develop interventions targeting the neural impact of common levels of POP exposure.
We addressed the limitations outlined above by evaluating the association between moderate prenatal exposure to POPs and neural activation during inhibitory control performance in a larger sample of 46 male and female children 9–11 years of age in the GESTation and the Environment (GESTE) cohort in Sherbrooke, Quebec, Canada, an ongoing prospective birth cohort of mother-child dyads, moderately exposed to POPs. Inhibitory control was measured via fMRI imaging of the Simon Task, a widely used task that has been found to engage brain regions important for inhibitory control (Hart et al., 2013; Norman et al., 2016), including the inferior frontal cortex (IFC), supplementary motor area (SMA) and anterior insula (AI) (McKenna et al., 2017). Meta-analyses have found decreased activity in IFC, SMA and AI in children with ADHD compared to typically developing children (TDC) (Hart et al., 2013; Norman et al., 2016).
To probe the relationship between POP exposure and inhibitory control, we modeled task performance using the hierarchical drift diffusion model (HDDM version 0.8.0) (Wiecki et al., 2013) estimating latent components of inhibitory control, including the rate at which information is extracted from stimuli and incorporated into decision-making (drift rate) and the level of response caution/impulsivity (boundary separation) (Ratcliff et al., 2016). In line with previous studies of ADHD, we hypothesized that higher levels of POP exposure would be related to reductions in drift rate (Karalunas et al., 2012, 2014; Metin et al., 2013) and that higher levels of POP exposure and deficits in performance would be associated with reduced brain activity in regions important for inhibitory control.
2. Methods
2.1. Study population
Participants were recruited as part of the GESTation and the Environment (GESTE) cohort in Sherbrooke, Quebec, Canada. Between 2007 and 2009, women were recruited during the first trimester of pregnancy and at delivery (n = 800). More specifically, 400 mothers were recruited during pregnancy (mean gestational week = 12 weeks, SD = 2.96 weeks), and 400 mothers were recruited at delivery. Maternal blood was collected at delivery to measure levels of POPs, including PCBs and PBDEs from plasma. Participants were followed up over 3 additional waves. Wave 4 is currently being collected, with children aged roughly between 9 and 11 years old. During Wave 4, children were assessed via behavioral measures including performance on the Simon task while undergoing functional magnetic resonance imaging (fMRI), and clinical measures, including parental report of clinical symptomology on the Behavioral Assessment System for Children 3 (BASC-3). Of the 800 mothers recruited during pregnancy and at delivery, 87 of their children provided Simon Task performance at Wave 4, 69 of these participants had analyzed maternal blood plasma samples, collected at delivery, 63 were assessed with the BASC-3, 51 had adequate Simon task performance (>50% accurate while responding to both congruent and incongruent trials), and 46 of these participants had MRI data without artifacts or excessive head motion (flow chart in Fig. S1 in supplement). All 46 participants were assessed with BASC-3 at the Wave 4 follow-up. Three of the 46 participants with adequate MRI data had taken ADHD medication within the last 3 months. Caregivers were asked to withhold this medication on the day of the MRI. Parents provided written informed consent at all waves, and children provided assent at Wave 4. Study protocols were approved by the institutional review boards of the University of Sherbrooke, Quebec, Canada, and Columbia University, New York, New York. This study followed the reporting guideline provided by Strengthening the Reporting of Observational Studies in Epidemiology (STROBE).
2.2. Exposure assessment
Maternal blood samples (10 mL) were collected at delivery in Sodium/Heparin Vacutainer Hemogard glass tubes (Becton-Dickinson, San Jose, CA). Whole blood was separated into components, and plasma was frozen at −20 °C in decontaminated Supelco glass storage tubes (Supelco, Inc, Bellefonte, Pennsylvania). Maternal plasma concentrations of four PBDE congeners (BDE-47, BDE-99, BDE-100, and BDE-153) and three PCB congeners (PCB-138, PCB-153, and PCB-180) were analyzed using solid-phase extraction and gas chromatography-electron capture negative ionization mass spectrometry (Serme-Gbedo et al., 2016; Abdelouahab et al., 2013). The limit of detection (LOD) was established at 0.1 pg/μL for PBDEs and at 0.02 pg/μL for PCBs. Values below the LOD were replaced by LOD divided by the square root of 2 (Baccarelli et al., 2005). Total plasma lipids were measured in maternal blood at delivery with the Phillips method (Phillips et al., 1989).
2.3. Image acquisition
Children ages 9–11 were scanned with a 3-T whole-body scanner using a 32-channel head coil (Ingenia; Philips Healthcare), providing T1-weighted structural and functional images (TR = 1075 ms, 290 vol per participant). Images were acquired on a Phillips Healthcare Ingenia 3 T whole-body scanner with a 32-channel head coil. For the T1-weighted structural scans, the imaging parameters were: T1 3D TFE (Turbo Field Echo) pulse sequence, 8° flip angle, FOV 240 mm, matrix size 240 × 240, slice thickness 1 mm. For task-based MRI, echoplanar images were collected with the following parameters: TR = 1075 ms, TE = 30 ms, 55° flip angle, single excitation per image, FOV 240 mm, matrix size 80 × 80, slice thickness 3 mm, 48 slices. Three runs of 290 vol were collected for each participant.
2.4. MRI preprocessing and approaches to head motion during scanning
To prepare fMRI data for first level analyses, the following steps were taken. First, a trained researcher visually inspected each structural image and the functional images from each run. For every functional task-based run, a separate 5 vol run was collected with reversed phase-encoded blips, providing a sequence of images with geometric distortions in the opposite direction. FSL’s topup function was used to estimate and correct the susceptibility-induced off-resonance field (Smith et al., 2004).
Following distortion correction, the following preprocessing steps were taken in SPM12 (http://www.fil.ion.ucl.ac.uk/spm/software/spm12/): slice timing correction, realignment, coregistration, segmentation, normalization and smoothing. We took several steps to limit the influence of in-scanner head motion on results. First, volumes with more than 0.5 mm framewise displacement were excluded from analyses. In addition, the six time-dependent motion parameter estimates output from registration, and the squares of the motion parameters and temporal derivatives were applied in multiple linear regression to remove the “nuisance” effects captured in these 24 parameters from the fMRI data (Friston et al., 1996; Power et al., 2012, 2014). Finally, subjects with more than 30% of volumes with more than 0.5 mm framewise displacement were excluded from analyses. The Statistical Parametric Mapping (SPM 12) software package was used for preprocessing as well as for first- and second-level analyses of task-based brain activity (see Statistical Analyses below) (Penny et al., 2011).
2.5. Task-based fMRI assessment
Inhibitory control was measured via the Simon spatial in-compatibility task, first developed in the 1960s, which has been widely used to examine inhibitory control, the neural correlates of inhibitory control, and neural and behavioral differences between children with and without an ADHD diagnoses (Hart et al., 2013; McKenna et al., 2017; LeMay and Simon, 1969; Simon and Reeve, 1990; Lu and Proctor, 1995; Mullane et al., 2009; Liu et al., 2004; Cespon et al., 2020). Stimuli were presented using EPRIME software (E-Prime 3, 2016) across three runs. White arrows pointing left or right were displayed against a black background to the left or right of a central white fixation cross. Participants were asked to report which direction the arrow was pointing while ignoring which side of the screen the arrow was presented on. Trials were either congruent: the stimulus pointed to the same side of the screen it was presented on, or incongruent: the stimuli pointed to the opposite side of the screen. Each run consisted of 22 congruent and 22 incongruent trials, counterbalanced to include equal numbers of trials presented on the left and right (Fig. S2 in the supplement).
2.6. Drift diffusion modeling of Simon Task performance
The drift diffusion model (DDM) uses the accuracy and the distribution of reaction times from task performance to model cognitive processes involved in decision making. The DDM assumes that on each trial of the Simon Task, perceptual evidence is accumulated until it reaches an evidence threshold and a response is made. The rate of evidence accumulation is modeled as the drift rate v, and the level of response caution is modeled by the boundary separation parameter, a. Parameters were estimated separately for congruent and incongruent trials. Model fits were validated by checking whether the posterior predictive distribution could reproduce key features of the subject- and group-level response distributions – e.g. mean reaction times and error rates.
2.7. Clinical outcomes
The BASC-3, collected via parent ratings (BASC-PRS), was used to assess clinical outcomes relevant to ADHD phenotypes during the same visit that task fMRI data were gathered (Reynolds CRK, 2015). T-scores from BASC-3 clinical indices, clinical scales, and content scales were used, including the ADHD Probability Index, Attention Problems, Hyperactivity, and Executive Functioning.
2.8. Covariates
All models were adjusted for covariates hypothesized to either confound the relationship between exposures and outcomes, or to add precision to effect estimation. The covariates used to adjust models include child age and estimated IQ percentile (assessed via the full scale Wechsler Intelligence Scale for Children V (Wechsler, 2014)), child sex, maternal age at birth, parity (first born vs not first born), breastfeeding (yes/no), maternal total plasma lipids at delivery, maternal education as a proxy measure of socioeconomic status (college vs no college), maternal BMI, maternal self-reported smoking during pregnancy (yes/no) and maternal alcohol use during pregnancy (yes/no). Missing covariate data were imputed with the median and mode for continuous and categorical variables, respectively. Differences in covariate distributions between our study sample (after imputing missing data) and the rest of the cohort were determined using chi-square goodness of fit tests for binary variables and two-sample t-tests for continuous variables (see Table S2 in the supplement). In a sensitivity analysis, all models were repeated with lipid standardization (congeners were expressed as nanogram of exposure per gram of lipid, before being scaled and centered) rather than including total lipids as a covariate.
3. Statistical analysis
3.1. Exposures and outcomes
In addition to modeling each PCB or PBDE congener individually, we created sum variables by adding the molar concentrations of all PCBs or PBDEs. Both molar sums and individual PCB or PBDE congeners were modeled in units of standard deviations from the mean (z-scores). We constructed a pairwise Pearson correlation matrix for all exposures (Fig. S3 in supplement). We estimated associations of each PCB and PBDE congener as well as their molar sums with Simon task accuracy using trials-based quasi-Poisson models. In separate models for congruent and incongruent trials, we set the total number of trials as the offset and modeled the number of correctly answered trials as the outcome. Using linear regressions, we modeled the associations of PCB and PBDE congeners as well as their molar sums with the a and v parameters from the DDM, MRI brain activity, and BASC-PRS scores. While parameter estimates derived from hierarchical models are not independent, recent work indicates that this is not a problem for subsequent correlation analyses (Katahira, 2016). We additionally investigated non-monotonic associations and mixture interactions using Bayesian kernel machine regression (BKMR) (Bobb et al., 2015). We report two outputs from BKMR. First, univariate associations of each congener with each BASC-PRS outcome modeled with penalized splines to assess non-monotonic associations. In univariate models, all other PCB and BDE congeners are fixed to the 50th percentile. Second, we report interactions of each congener with the rest of the mixture. In this method, for each congener, we modeled the associations of being at the 75th percentile compared to the 25th percentile of exposure with each BASC-PRS outcome. These models were repeated three times with all other congeners fixed to the 25th, 50th, and 75th percentiles. Any congener having different associations with the BASC-PRS outcomes depending on the fixed level of the other congeners would be interpreted as an interaction with the mixture. A sensitivity analysis accounting for ADHD medication found no meaningful differences (see supplement). Statistical analyses were conducted with R 4.1.0 (R: A language and environ, 2017).
3.2. fMRI analyses
Following distortion correction, standard preprocessing was performed in SPM12 (Penny et al., 2011). First-level analyses were performed for each participant using the general linear model function in SPM 12 (Penny et al., 2011). For correct trials only, BOLD contrast data were modeled separately for congruent and incongruent trials. To examine condition-related differences in neural activity, a contrast modeling incongruent minus congruent trials was created. Inhibitory control is associated with brain activity in the right IFC (rIFC), SMA and right AI (rAI), and differences in brain activity between TDC and children with ADHD performing inhibitory control tasks have been found in these regions (Hart et al., 2013; Norman et al., 2016; McKenna et al., 2017; Aron et al., 2014). Therefore, we created 5 mm ROI spheres of these regions based on coordinates (Table S1 in the supplement) from a meta-analysis of TDC aged 6–12 performing inhibitory control tasks using MarsBaR (Brett et al., 2002). Incongruent minus congruent activity was extracted from each of these three ROIs. To determine if significant inhibitory control-related activity was present within each ROI, one sample t-tests, two-sided, were performed with SPSS 26(IBM SPSS, 2019), with an FDR correction to account for multiple comparisons. The relationship between exposure and brain activity was examined in ROIs with significant inhibitory control-related activity using linear regressions controlling for the covariates described above.
4. Results
Characteristics of the study population and covariates are reported in Table 1. Compared to the rest of the cohort, our study sample had significantly reduced maternal smoking during pregnancy and increased breastfeeding (Table S2 in the supplement). The Simon effect was found among our sample of 46 children (29 male; mean age 10.42 ± 0.55); in other words, participants performed more accurately (t45 = 2.27 p = 0.03) and more quickly (t45 = 4.29, p < 0.01) on congruent compared to incongruent trials.
Table 1.
Characteristics of study population in the GESTE cohort.
| Overall (N = 63) | |
|---|---|
| Sex | |
| Female | 23 (36.5%) |
| Male | 40 (63.5%) |
| Child age at visit | |
| Mean (SD) | 10.65 (0.42) |
| Range | 9.61–11.45 |
| Maternal age at delivery | |
| Mean (SD) | 28.92 (4.78) |
| Range | 19–40 |
| Maternal education | |
| N-Miss | 1 |
| No college | 23 (36.5%) |
| Some College | 40 (63.5%) |
| Alcohol during pregnancy | |
| No | 44 (69.8%) |
| Yes | 19 (30.2%) |
| Smoked during pregnancy | |
| No | 61 (96.8%) |
| Yes | 2 (3.2%) |
| Parity | |
| First Born | 31 (49.2%) |
| Not First Born | 32 (50.8%) |
| Breastfeeding | |
| No | 30 (47.6%) |
| Yes | 33 (52.4%) |
| Maternal BMI | |
| N-Miss | 3 |
| Mean (SD) | 25.21 (5.23) |
| Range | 15.67–46.05 |
| IQ (percentile) a | |
| Mean (SD) | 60.03 (24.09) |
| Range | 9.00–99.00 |
A score in the 50 percentile = a standard score of 100, which is in the normal range.
Because POP exposures followed a lognormal distribution, we present the geometric mean (GM) and standard deviation (GSD) for each congener (Table 2). Pearson correlations POP congeners were observed, and are reported in Fig. S3 in the supplement. High correlations between PCB-138, PCB-153, and PCB molar sum (r2 > 0.95) were found, while correlations between PCB-180 and all other PCBs were substantially lower (r2 < 0.50).
Table 2.
Levels of POP Exposure in the GESTE Cohort (Exposures in ng/ml).
| Overall (N = 63) | N (%)<LOD | Geometric mean (GSD) | |
|---|---|---|---|
| Total lipids (g/L) | |||
| N-Miss | 1 | ||
| Mean (SD) | 6.70 (1.24) | ||
| Range | 4.30–11.43 | ||
| BDE-47 | |||
| Mean (SD) | 0.12 (0.16) | 0 (0%) | 0.06 (3.38) |
| Range | 0.01–0.85 | ||
| BDE-99 | |||
| Mean (SD) | 0.06 (0.11) | 7 (11%) | 0.02 (4.60) |
| Range | 0.00–0.59 | ||
| BDE-100 | |||
| Mean (SD) | 0.07 (0.11) | 7 (11%) | 0.03 (4.00) |
| Range | 0.00–0.54 | ||
| BDE-153 | |||
| Mean (SD) | 0.08 (0.10) | 8 (13%) | 0.04 (3.52) |
| Range | 0.00–0.49 | ||
| PCB-138 | |||
| Mean (SD) | 0.05 (0.08) | 13 (21%) | 0.02 (4.08) |
| Range | 0.00–0.58 | ||
| PCB-153 | |||
| Mean (SD) | 0.08 (0.12) | 5 (8%) | 0.05 (3.13) |
| Range | 0.00–0.92 | ||
| PCB-180 | |||
| Mean (SD) | 0.04 (0.04) | 8 (13%) | 0.02 (2.94) |
| Range | 0.00–0.21 |
4.1. Exposure and Simon Task performance
PCBs and PBDEs were associated with decreased Simon Task accuracy on congruent trials (Fig. 1; the numeric data used in the figures can be found in tables in the supplement). Standard deviation unit increases in the molar sums of PCB and BDE exposures were separately associated with approximately 5% and 6% decreased accuracy on congruent trials, respectively (PCB rate ratio = 0.95; 95% CI [0.90, 0.99]; BDE rate ratio = 0.94; 95% CI [0.90, 0.99]). Increases in PCB-138, PCB-153, BDE-99, BDE-100, and BDE-153 were similarly associated with decreased accuracy on congruent trials, while PCB-180 and BDE-47 were not.
Fig. 1.
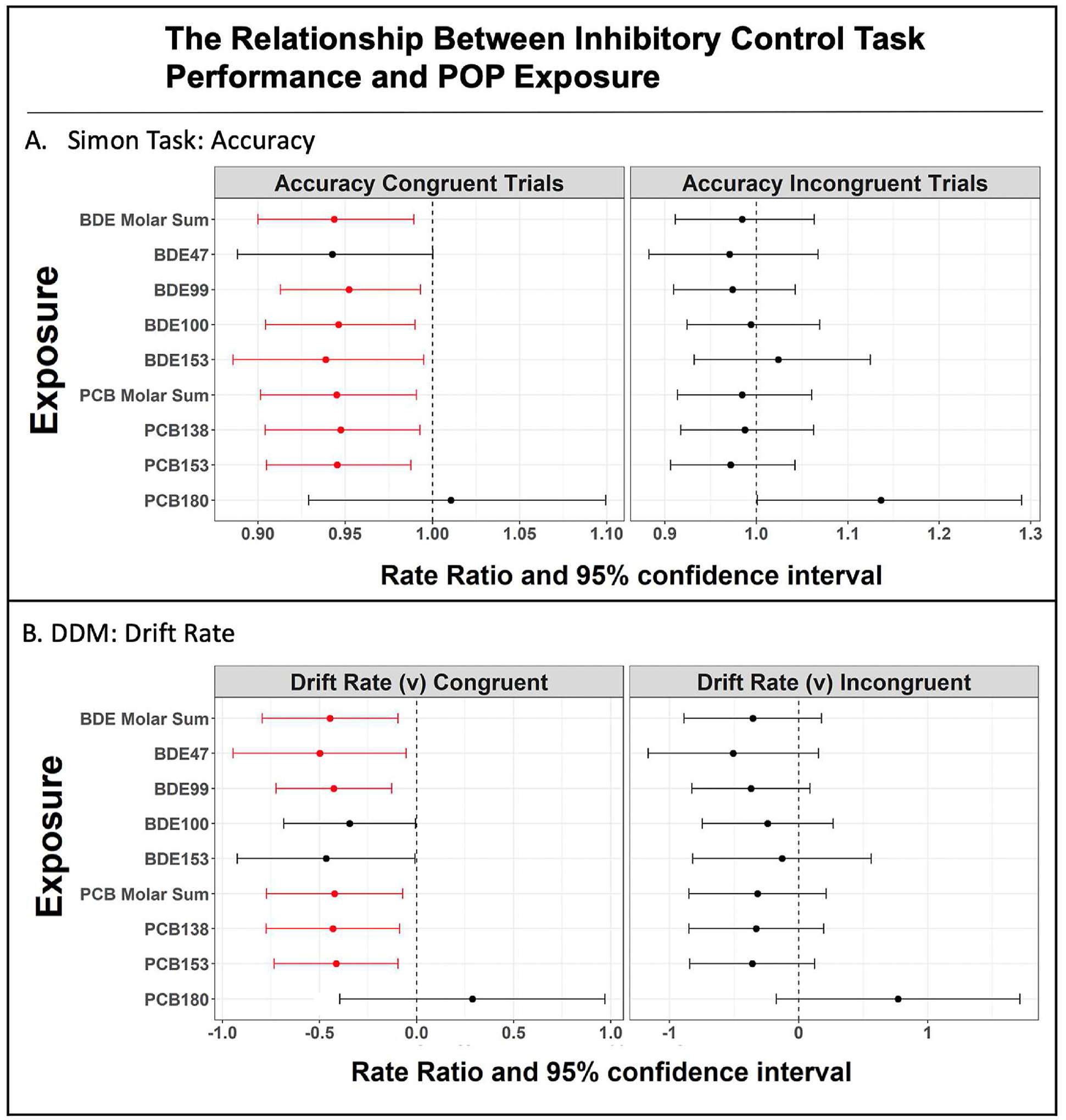
A) POP exposure was associated with accuracy on an inhibitory control (Simon) task, n = 46, according to quasi-Poisson models. B) Drift diffusion model parameters were estimated using the HDDM toolbox (n = 46). POP exposure was associated with drift rate on congruent, but not incongruent trials, according to linear regression analayses.
On incongruent trials, no exposures were associated with accuracy.
We observed similar patterns of association between exposures and DDM drift rate parameters (Fig. 1). On congruent trials, each one standard deviation unit increase in the molar sum of PCB exposure was associated with a 0.42-point decrease (β = −0.42; 95% CI [−0.77, −0.07]), and each standard deviation unit increase in the molar sum of BDE exposure was associated with a 0.45-point decrease (β = −0.45; 95% CI [−0.80, −0.10]) in the drift rate parameter. We observed similar trends with PCB-138, PCB-153, BDE-47, and BDE-99, while there were no associations of PCB-180, BDE-100 and BDE-153 with drift rate. Neither PCBs nor PBDEs were associated with drift rate on incongruent trials, or with boundary separation on congruent or incongruent trials.
4.2. Exposure and Simon Task-Related brain activity
Because PCBs and PBDEs were associated with Simon task performance, we hypothesized that they may also affect task-related brain activity in regions previously implicated in inhibitory control. Significant FDR corrected incongruent minus congruent activity was found in the rIFC (t45 = 2.57, p = 0.01), and the rAI (t45 = 2.40, p = 0.02), but not the SMA. Mirroring their associations with Simon task accuracy, PCB-138 (β =−0.38; 95% CI [−0.71, −0.06]), PCB-153 (β = −0.33; 95% CI [−0.63, −0.02]), and the molar sum of PCBs (β = −0.35; 95% CI [−0.69, −0.02]) were associated with decreased rIFC brain activity, while PCB-180 was not (Fig. 2). No PBDEs were associated with rIFC brain activity, but increased BDE-153 exposure was associated with decreased activity in the rAI (β = −0.41; 95% CI [−0.75, −0.07]). No other exposures were associated with brain activity in the rAI.
Fig. 2.
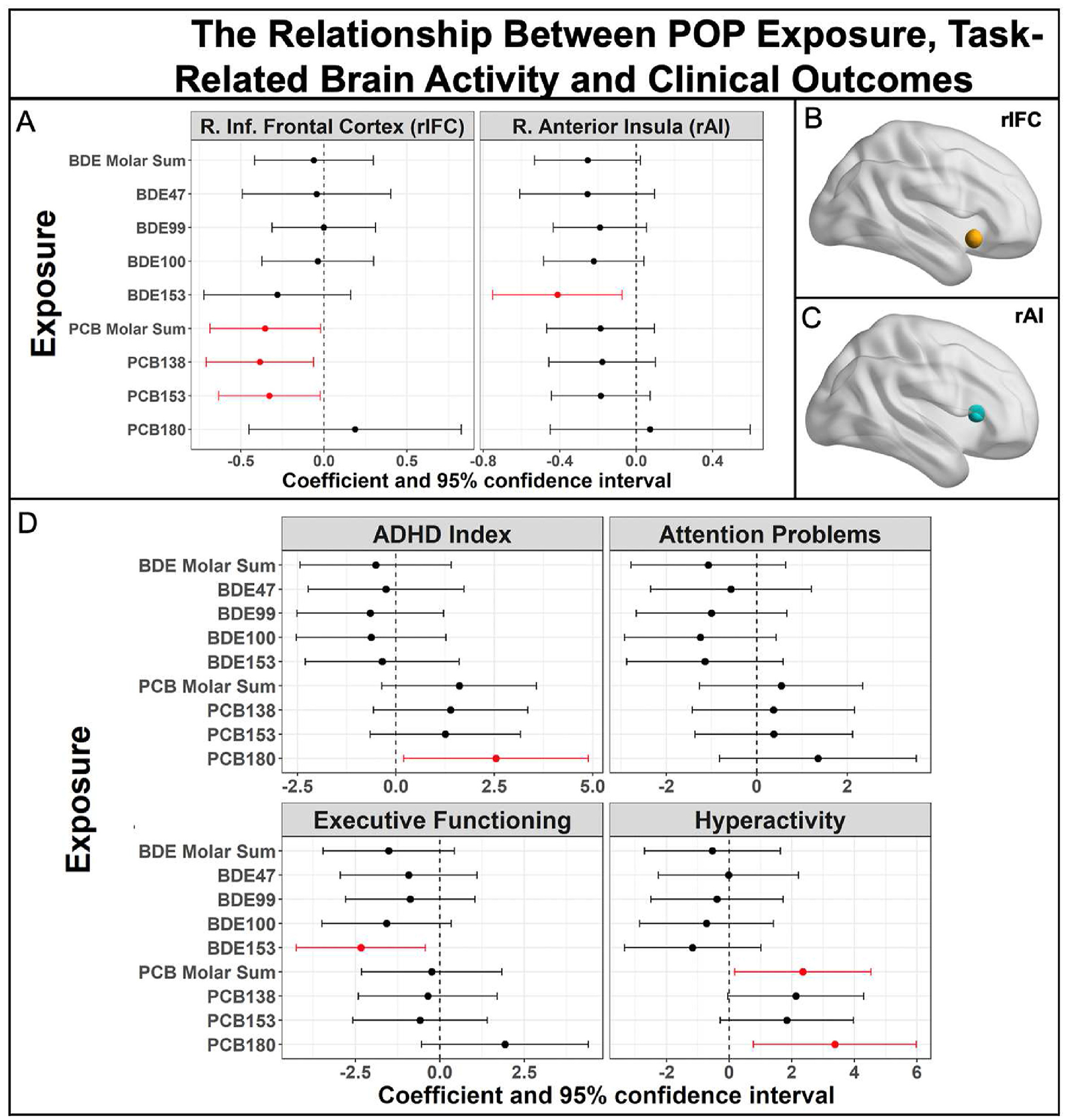
A) Inhibitory control task-based activity in right Inferior Frontal Cortex (rlFC) and right Anterior Insula (rAl) related to exposure to POPs, n = 46, according to linear regression analyses. B) A 5mm sphere in rlFC, based on a meta analysis of typically developing children performing inhibitory control tasks, from which task-based activity was extracted. C)A 5 mm sphere in rAl, based on the same meta analysis. D) Exposure to POPs were associated with ADHD related symptoms measured by the BASC-3,n = 63, according to linear regression analysis.
4.3. Exposure and clinical outcomes
Several PCBs and one PBDE were associated with BASC-PRS scores (Fig. 2). Each standard deviation unit increase in prenatal PCB-180 exposure was associated with higher ADHD Probability Index (β = 2.54; 95%CI [0.19, 4.89]) and Hyperactivity t-scores (β = 3.37; 95%CI [0.77, 5.97]), while PCB molar sum was only associated with hyperactivity (β = 2.35; 95%CI [0.18, 4.53]). Increased prenatal BDE-153 exposure was associated with lower executive functioning scores (β = −2.34; 95% CI [−4.24, −0.43]). Neither PCBs nor PBDEs were associated with Attention Problems. Models results were not substantially different in sensitivity analyses with lipid standardization (Figs. S4 and S5). Univariate BKMR models suggested a non-linear, “inverted-U” relationship of PCB-153 with ADHD Probability Index, Hyperactivity, and Attention Problems (Supplemental Fig. S6). Associations of the remaining congeners with BASC-PRS scores were mostly linear, although there were some “threshold effects” in which linear dose-responses flattened out at higher levels of exposure. The relationships of each congener with each BASC-PRS outcome did not substantially differ when the rest of the mixture was set to the 25th, 50th, or 75th percentile of exposure, suggesting no interactions (Supplemental Fig. S7).
5. Discussion
This observational study found that in a prospective, community sample of Canadian mother-child dyads moderately exposed to PCBs and PBDEs, increased exposure was associated with increases in neurobehavioral deficits found in ADHD (n = 46), providing support for the possibility that prenatal exposure to POPs is a modifiable risk for ADHD phenotypes. As hypothesized, we found that increases in prenatal POP exposure were associated with decrements in accuracy and drift rate, and reduced task-related brain activity during an inhibitory control task in children aged 9–11. In addition, increased levels of prenatal POPs were associated with increased clinical symptoms of ADHD, including increased Hyperactivity t-scores and increased scores on the ADHD Probability Index. Results presented here are also consistent with studies of other POPs, such as per-and polyfluoroalkyl substances (PFAS), which have been found to have an inverse relationship with neurodevelopmental measures and cognitive performance (Wang et al., 2015; Goudarzi et al., 2016; Spratlen et al., 2020; Park et al., 2021). To the best of our knowledge, this is the first study to show that POP exposure is related to task-based changes in neural activity in brain regions important for inhibitory control, suggesting a biological mechanism underlying previously documented associations between POP exposure and neurobehavioral deficits found in ADHD, including deficits in inhibitory control (Verner et al., 2015; Stewart et al., 2003, 2005; Jacobson and Jacobson, 2003).
Consistent with previous studies of ADHD (Karalunas et al., 2012, 2014; Metin et al., 2013), computational modeling revealed that POP-related decrements in accuracy were driven by slower drift rate, suggesting that POP exposure reduces the rate at which data can be extracted and incorporated into decision-making. Preclinical research in animal models has shown that POP exposure leads to impaired dopamine neurotransmission (Pessah et al., 2019; Seegal et al., 1991). In light of previous studies linking dopaminergic dysfunction to both ADHD and slowed drift rate, and ADHD medication (methylphenidate) with increased drift rate (Gold et al., 2014; Del Campo et al., 2011; Beste et al., 2018), these findings suggest a possible biological substrate for the observed POP-mediated decrements in task performance.
By examining the association between ADHD-related behaviors and symptoms with both the molar sums of PCBs and PBDEs and specific POP congeners, these results allow us to examine associations common across POP congeners as well as cases where a specific congener related to clinically relevant outcomes. Our data suggest that PCB and PBDE congeners have relatively uniform relationships with Simon Task behavoral indices, including accuracy and drift rate, but congener-specific relationships with ADHD-related clinical outcomes, such as the association between PCB-180 and the ADHD Probability Index and Hyperactivity t-scores on the BASC-3. However, differences between POP-Simon task performance associations and POP-ADHD symptom associations may be due to the Simon task measuring inhibitory control, and thus may track aspects of ADHD-risk more closely than the BASC-3, which measures clinical outcomes (Young et al., 2009). For example, inhibitory control, measured at age 5 has been shown to predict executive functioning in middle childhood (Berlin et al., 2003). Therefore, Simon task-based measures may reflect congener-specific associations with inhibitory control deficits more clearly than the BASC-3 scores we examined, which reflect ADHD symptomatology. In sum, congener specific results may reflect unique associations or the increased signal in the relationship between prenatal exposures and Simon Task performance compared to BASC-3 outcomes.
Prior studies have documented differential effects of specific POP congeners on neurobehavioral outcomes (Boix et al., 2011; Brun et al., 2021; Coburn et al., 2008; Usenko et al., 2015; Fernie et al., 2005). Specifically, prior work suggests that PCB-180 may relate to neural correlates and downstream outcomes differently than other PCB congeners. For example, pre-clinical work has shown that rats exposed prenatally to PCB-180 had increased extra-cellular dopamine in the nucleus accumbens, unlike rats who had been exposed to PCB-52 or PCB-138 (Boix et al., 2011). In addition, male rats prenatally exposed to PCB-180 also had reduced motor activity (Boix et al., 2011). In our study we found that, in contrast to other PCB congeners, prenatal exposure to PCB-180 was significantly associated with clinical outcomes measured on the BASC-3, but not with Simon task performance. Furthermore, it is possible that PCB-180 also had specific, unmeasured, neural effects in our sample that were associated with clinical problems indexed by BASC-3. Taken together, these findings suggest that the specific neural effects of PCB-180 may have unique effects on ADHD symptoms.
We similarly observed associations between specific PCB and PBDE congeners and neural activity in different brain ROIs: PCBs, but not PBDEs, contributed significantly to reductions in brain activity in rIFC, while only BDE-153 was significantly related to reduced brain activity in rAI. Again, these associations could reflect congener-specific relationships with brain activity, or alternatively, demonstrate that certain brain regions may be more sensitive indicators of neurobehavioral changes than others. While many associations did not reach significance, most congeners tended towards an association with reduced activity in both brain regions (except PCB-180), suggesting that with a larger sample, it is possible that other congeners would also be associated with reduced brain activity in IFC and AI. Lastly, univariate BKMR models suggested that PCB-153 had an inverted “u-shaped” non-linear relationship with ADHD clinical outcomes while most other congeners had linear associations (Supplemental Fig. S6), further underscoring how PCBs may have unique, congener-specific relationships with outcomes.
While, PCBs and PBDEs had an overall negative relationship with Simon task brain activity, only some congeners reached statistical significance. Furthermore, these were not generally the congeners that contributed significantly to clinical ADHD problems indexed by the BASC-3. Of the exposures that related to reduced inhibitory control-related brain activity, only the PCB molar sum was significantly related with the BASC-3 hyperactivity scale, but the association between hyperactivity and PCBs 138 and 153 was still in the expected direction. This discrepancy may be partially explained by a lack of power. For example, the PCB molar sum, PCB-138 and PCB-153 were all significantly associated with reduced activity in the rIFC, but not rAI, although they tended in the same direction. However, while the IFC and AI are both involved with inhibitory control, they also have dissociable roles: for example, activity in IFC, but not AI, has been found to predict individual differences in inhibitory control, while multivoxel patterns of activation in the AI, but not the IFC, can differentiate between correct and incorrect task performance (Cai et al., 2014). It is possible that these dissociable roles may help to explain why only PCBs were significantly associated with rIFC activity, and only one BDE congener was significantly associated with rAI activity.
We chose to allow our examination of brain activity to be hypothesis driven, and restricted analyses to ROIs defined by previous research in healthy children performing inhibitory control tasks. Therefore, it is possible that PCB-180 exposure related to neural activity in other brain regions not examined by this study, and that alterations in these unexamined brain regions during task performance relate to clinical symptoms. Future studies are required to determine if the differences between the effects of PCBs and PBDEs, and differences between congeners of PCBs and PBDEs observed here replicate in other samples. In addition, future work in non-human animals may be able to determine a more causal relationship between different POP types, and inhibitory control-related neural activity.
Replicating previous studies, this study found that POP exposure is linked with ADHD-related behaviors and symptoms (Verner et al., 2015; Sagiv et al., 2010; Stewart et al., 2005; Stewart et al., 2003; Jacobson and Jacobson, 2003; Vreugdenhil et al., 2002; Vuong et al., 2018; Eskenazi et al., 2013; Herbstman et al., 2010; Sagiv et al., 2015; de Water et al., 2019; Stockholm Convention. In:, 2001). Furthermore, results from this moderately exposed community sample may be more generalizable than previous studies, which were focused on highly exposed populations. Findings from this study may help future prevention, policy, and intervention efforts. More specifically, the following prevention efforts aimed at reducing the long-term negative effects of POP exposure could be explored in future research: targeting POPs in environmental cleanup efforts, altering eating guidelines during pregnancy to reduce POP exposure, and testing mothers at birth for POP levels to identify at-risk children.
Results from the current study are also consistent with research finding that exposure to other POPs, such as PFAS, has an inverse relationship with neurodevelopmental measures (Wang et al., 2015; Goudarzi et al., 2016; Spratlen et al., 2020). Pre-clinical research in rodents suggests that metabolic and elimination differences with POPs, including PCBs, PBDEs, and PFAS can be sex-dependent (Roth et al., 2021; Wu et al., 2013; Staskal et al., 2006). Therefore, examining if sex influences the relationship between POP exposure and ADHD phenotypes is an important goal for future research.
5.1. Strengths and limitations
Strengths of the present study include the measurement of POP exposure at birth in a cohort of prospectively followed children, in whom inhibitory control performance and related brain activity were measured at ages 9–11. While previous studies have documented a relationship between increased POP exposure and increased neurobehavioral deficits associated with ADHD, as well as POP-exposure related differences in brain activity, this study examined neural activity while performing a task used to assess inhibitory control in ADHD, allowing us to examine the relationship between prenatal POP exposure and childhood task-based brain activity. In addition, this study used computational modeling to elucidate the relationship between prenatal POP exposure and latent cognitive processes underlying inhibitory control during childhood.
While the present study accounted for key confounders, results could be influenced by unmeasured factors during the time between exposure and measurement of outcomes. It is possible that unknown genetic, social, cultural and familial factors could influence POP exposure. Finally, results could be prone to selection bias owing to loss to follow-up between recruitment and the Wave 4 visit, as well as differences in the rates of maternal smoking during pregnancy and breastfeeding between our study sample and the rest of the cohort.
6. Conclusions
By measuring the effects of prenatal POP exposure on inhibitory control task-related neural activity and performance, this study provides a plausible brain mechanism by which POP exposure may lead to neurobehavioral deficits found in ADHD. Computational modeling results revealed that, like ADHD, POP exposure slows drift rate on task performance. Together, these results support the possibility that prenatal POP-exposure could be a modifiable risk factor for ADHD phenotypes. Results from this study suggest novel approaches to prevention and intervention. Future research could determine if targeting POPs in environmental cleanup efforts could reduce the risk for ADHD phenotypes, if it is appropriate to change eating guidelines during pregnancy to reduce POP exposure, and if testing mothers at birth for POP levels could help identify at-risk children. Furthermore, future studies could examine the possibility that pharmacotherapies such as methylphenidate could treat POP-driven neurobehavioral deficits. Finally, future studies with larger sample sizes could help determine if the congener-specific results found here replicate reliably.
Supplementary Material
Acknowledgements
The authors would like to thank Dr. St Amant-Beaudouin for his generous help reviewing structural MRIs and providing families with clinical follow-ups regarding incidental findings.
Funding sources
This work was supported by the National Institute on Drug Abuse (DA049913), the National Institute of Environmental Health Sciences (ES027845), and the National Institute of Mental Health (MH119510). Dr. Posner has also received research support from Takeda (formerly Shire) and Aevi Genomics and consultancy fees from Innovative Science. No other disclosures were reported.
Footnotes
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.envres.2021.112593.
References
- Abdelouahab N, Langlois MF, Lavoie L, Corbin F, Pasquier JC, Takser L, 2013. Maternal and cord-blood thyroid hormone levels and exposure to polybrominated diphenyl ethers and polychlorinated biphenyls during early pregnancy. Am. J. Epidemiol 178 (5), 701–713. [DOI] [PubMed] [Google Scholar]
- Arnold LE, Hodgkins P, Caci H, Kahle J, Young S, 2015. Effect of treatment modality on long-term outcomes in attention-deficit/hyperactivity disorder: a systematic review. PLoS One 10 (2), e0116407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA, 2014. Inhibition and the right inferior frontal cortex: one decade on. Trends Cognit. Sci 18 (4), 177–185. [DOI] [PubMed] [Google Scholar]
- Baccarelli A, Pfeiffer R, Consonni D, et al. , 2005. Handling of dioxin measurement data in the presence of non-detectable values: overview of available methods and their application in the Seveso chloracne study. Chemosphere 60 (7), 898–906. [DOI] [PubMed] [Google Scholar]
- Berlin L, Bohlin G, Rydell AM, 2003. Relations between inhibition, executive functioning, and ADHD symptoms: a longitudinal study from age 5 to 8–1/2 years. Child Neuropsychol 9 (4), 255–266. [DOI] [PubMed] [Google Scholar]
- Beste C, Adelhofer N, Gohil K, Passow S, Roessner V, Li SC, 2018. Dopamine modulates the efficiency of sensory evidence accumulation during perceptual decision making. Int. J. Neuropsychopharmacol 21 (7), 649–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobb JF, Valeri L, Claus Henn B, et al. , 2015. Bayesian kernel machine regression for estimating the health effects of multi-pollutant mixtures. Biostatistics 16 (3), 493–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boix J, Cauli O, Leslie H, Felipo V, 2011. Differential long-term effects of developmental exposure to polychlorinated biphenyls 52, 138 or 180 on motor activity and neurotransmission. Gender dependence and mechanisms involved. Neurochem. Int 58 (1), 69–77. [DOI] [PubMed] [Google Scholar]
- Brett MA, J L, Valabregue R, Poline JB, 2002. Region of interest analysis using an SPM toolbox [abstract]. June 2–6. In: Presented at the 8th International Conference on Functional Mapping of the Human Brain (Sendai, Japan). [Google Scholar]
- Brun NR, Panlilio JM, Zhang K, et al. , 2021. Developmental exposure to non-dioxin-like polychlorinated biphenyls promotes sensory deficits and disrupts dopaminergic and GABAergic signaling in zebrafish. Commun Biol 4 (1), 1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai W, Ryali S, Chen T, Li CS, Menon V, 2014. Dissociable roles of right inferior frontal cortex and anterior insula in inhibitory control: evidence from intrinsic and task-related functional parcellation, connectivity, and response profile analyses across multiple datasets. J. Neurosci 34 (44), 14652–14667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cespon J, Hommel B, Korsch M, Galashan D, 2020. The neurocognitive underpinnings of the Simon effect: an integrative review of current research. Cognit. Affect Behav. Neurosci 20 (6), 1133–1172. [DOI] [PubMed] [Google Scholar]
- Chu CP, Wu SW, Huang YJ, Chiang MC, Hsieh ST, Guo YL, 2019. Neuroimaging signatures of brain plasticity in adults with prenatal exposure to polychlorinated biphenyls: altered functional connectivity on functional MRI. Environ. Pollut 250, 960–968. [DOI] [PubMed] [Google Scholar]
- Coburn CG, Curras-Collazo MC, Kodavanti PR, 2008. In vitro effects of environmentally relevant polybrominated diphenyl ether (PBDE) congeners on calcium buffering mechanisms in rat brain. Neurochem. Res 33 (2), 355–364. [DOI] [PubMed] [Google Scholar]
- de Water E, Curtin P, Zilverstand A, et al. , 2019. A preliminary study on prenatal polybrominated diphenyl ether serum concentrations and intrinsic functional network organization and executive functioning in childhood. JCPP (J. Child Psychol. Psychiatry) 60 (9), 1010–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Campo N, Chamberlain SR, Sahakian BJ, Robbins TW, 2011. The roles of dopamine and noradrenaline in the pathophysiology and treatment of attention-deficit/hyperactivity disorder. Biol. Psychiatr 69 (12), e145–157. [DOI] [PubMed] [Google Scholar]
- Demontis D, Walters RK, Martin J, et al. , 2019. Discovery of the first genome-wide significant risk loci for attention deficit/hyperactivity disorder. Nat. Genet 51 (1), 63–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E-Prime 3, 2016. Psychology Software Tools, Inc, 0 [computer program].
- Eskenazi B, Chevrier J, Rauch SA, et al. , 2013. In utero and childhood polybrominated diphenyl ether (PBDE) exposures and neurodevelopment in the CHAMACOS study. Environ. Health Perspect 121 (2), 257–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eubig PA, Aguiar A, Schantz SL, 2010. Lead and PCBs as risk factors for attention deficit/hyperactivity disorder. Environ. Health Perspect 118 (12), 1654–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraone SV, Larsson H, 2019. Genetics of attention deficit hyperactivity disorder. Mol. Psychiatr 24 (4), 562–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernie KJ, Shutt JL, Mayne G, et al. , 2005. Exposure to polybrominated diphenyl ethers (PBDEs): changes in thyroid, vitamin A, glutathione homeostasis, and oxidative stress in American kestrels (Falco sparverius). Toxicol. Sci 88 (2), 375–383. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Williams S, Howard R, Frackowiak RSJ, Turner R, 1996. Movement-related effects in fMRI time-series. Magn. Reson. Med 35 (3), 346–355. [DOI] [PubMed] [Google Scholar]
- Geffen J, Forster K, 2018. Treatment of adult ADHD: a clinical perspective. Ther Adv Psychopharmacol 8 (1), 25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold MS, Blum K, Oscar-Berman M, Braverman ER, 2014. Low dopamine function in attention deficit/hyperactivity disorder: should genotyping signify early diagnosis in children? Postgrad. Med 126 (1), 153–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudarzi H, Nakajima S, Ikeno T, et al. , 2016. Prenatal exposure to perfluorinated chemicals and neurodevelopment in early infancy: the Hokkaido Study. Sci. Total Environ 541, 1002–1010. [DOI] [PubMed] [Google Scholar]
- Guo WJ, Pan BH, Sakkiah S, et al. , 2019. Persistent organic pollutants in food: contamination sources, health effects and detection methods. Int. J. Environ. Res. Publ. Health 16 (22). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupte-Singh K, Singh RR, Lawson KA, 2017. Economic burden of attention-deficit/hyperactivity disorder among pediatric patients in the United States. Value Health 20 (4), 602–609. [DOI] [PubMed] [Google Scholar]
- Harley KG, Marks AR, Chevrier J, Bradman A, Sjodin A, Eskenazi B, 2010. PBDE concentrations in women’s serum and fecundability. Environ. Health Perspect 118 (5), 699–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart H, Radua J, Nakao T, Mataix-Cols D, Rubia K, 2013. Meta-analysis of functional magnetic resonance imaging studies of inhibition and attention in attention-deficit/hyperactivity disorder: exploring task-specific, stimulant medication, and age effects. JAMA Psychiatr 70 (2), 185–198. [DOI] [PubMed] [Google Scholar]
- Herbstman JB, Sjodin A, Kurzon M, et al. , 2010. Prenatal exposure to PBDEs and neurodevelopment. Environ. Health Perspect 118 (5), 712–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huffling K, Landrigan PJ, Lavin A, et al. , 2016. Project TENDR: targeting environmental neuro-developmental risks. The TENDR consensus statement. Environ. Health Perspect 124 (7), A118–A122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson JL, Jacobson SW, 2003. Prenatal exposure to polychlorinated biphenyls and attention at school age. J. Pediatr 143 (6), 780–788. [DOI] [PubMed] [Google Scholar]
- Karalunas SL, Huang-Pollock CL, Nigg JT, 2012. Decomposing attention-deficit/hyperactivity disorder (ADHD)-related effects in response speed and variability. Neuropsychology 26 (6), 684–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karalunas SL, Geurts HM, Konrad K, Bender S, Nigg JT, 2014. Annual research review: reaction time variability in ADHD and autism spectrum disorders: measurement and mechanisms of a proposed trans-diagnostic phenotype. JCPP (J. Child Psychol. Psychiatry) 55 (6), 685–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katahira K, 2016. How hierarchical models improve point estimates of model parameters at the individual level. J. Math. Psychol 73, 37–58. In this issue. [Google Scholar]
- Kupper T, Haavik J, Drexler H, et al. , 2012. The negative impact of attention-deficit/hyperactivity disorder on occupational health in adults and adolescents. Int. Arch. Occup. Environ. Health 85 (8), 837–847. [DOI] [PubMed] [Google Scholar]
- Lam J, Lanphear BP, Bellinger D, et al. , 2017. Developmental PBDE exposure and IQ/ADHD in childhood: a systematic review and meta-analysis. Environ. Health Perspect 125 (8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landrigan PJ, Lambertini L, Birnbaum LS, 2012. A research strategy to discover the environmental causes of autism and neurodevelopmental disabilities. Environ. Health Perspect 120 (7), a258–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeMay RP, Simon JR, 1969. Temporal and symbolic S-R compatibility in a sequential information processing task. J. Exp. Psychol 80, 558–560. [Google Scholar]
- Liu X, Banich MT, Jacobson BL, Tanabe JL, 2004. Common and distinct neural substrates of attentional control in an integrated Simon and spatial Stroop task as assessed by event-related fMRI. Neuroimage 22 (3), 1097–1106. [DOI] [PubMed] [Google Scholar]
- Lu CH, Proctor RW, 1995. The influence of irrelevant location information on performance - a review of the Simon and spatial stroop effects. Psychon. Bull. Rev 2 (2), 174–207. [DOI] [PubMed] [Google Scholar]
- McKenna R, Rushe T, Woodcock KA, 2017. Informing the structure of executive function in children: a meta-analysis of functional neuroimaging data. Front. Hum. Neurosci 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metin B, Roeyers H, Wiersema JR, van der Meere JJ, Thompson M, Sonuga-Barke E, 2013. ADHD performance reflects inefficient but not impulsive information processing: a diffusion model analysis. Neuropsychology 27 (2), 193–200. [DOI] [PubMed] [Google Scholar]
- Mullane JC, Corkum PV, Klein RM, McLaughlin E, 2009. Interference control in children with and without ADHD: a systematic review of Flanker and Simon task performance. Child Neuropsychol 15 (4), 321–342. [DOI] [PubMed] [Google Scholar]
- Norman LJ, Carlisi C, Lukito S, et al. , 2016. Structural and functional brain abnormalities in attention-deficit/hyperactivity disorder and obsessive-compulsive disorder A comparative meta-analysis. JAMA Psychiatr 73 (8), 815–825. [DOI] [PubMed] [Google Scholar]
- Norstrom K, Czub G, McLachlan MS, Hu D, Thorne PS, Hornbuckle KC, 2010. External exposure and bioaccumulation of PCBs in humans living in a contaminated urban environment. Environ. Int 36 (8), 855–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SK, Ding N, Han D, 2021. Perfluoroalkyl substances and cognitive function in older adults: should we consider non-monotonic dose-responses and chronic kidney disease? Environ. Res 192, 110346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltier MR, Klimova NG, Arita Y, et al. , 2012. Polybrominated diphenyl ethers enhance the production of proinflammatory cytokines by the placenta. Placenta 33 (9), 745–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penny WD, Friston KJ, Ashburner JT, Kiebel SJ, Nichols TE (Eds.), 2011. Statistical Parametric Mapping: the Analysis of Functional Brain Images. Elsevier. [Google Scholar]
- Pessah IN, Lein PJ, Seegal RF, Sagiv SK, 2019. Neurotoxicity of polychlorinated biphenyls and related organohalogens. Acta Neuropathol 138 (3), 363–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips DL, Pirkle JL, Burse VW, Bernert JT Jr., Henderson LO, Needham LL, 1989. Chlorinated hydrocarbon levels in human serum: effects of fasting and feeding. Arch. Environ. Contam. Toxicol 18 (4), 495–500. [DOI] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE, 2012. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion (vol 59, pg 2142, 2012). Neuroimage 63 (2), 999, 999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Mitra A, Laumann TO, Snyder AZ, Schlaggar BL, Petersen SE, 2014. Methods to detect, characterize, and remove motion artifact in resting state fMRI. Neuroimage 84, 320–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [computer program] R: A Language and Environment for Statistical Computing, 2017. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- Ratcliff R, Smith PL, Brown SD, McKoon G, 2016. Diffusion decision model: current issues and history. Trends Cognit. Sci 20 (4), 260–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawn DF, Ryan JJ, Sadler AR, et al. , 2014. Brominated flame retardant concentrations in sera from the Canadian Health Measures Survey (CHMS) from 2007 to 2009. Environ. Int 63, 26–34. [DOI] [PubMed] [Google Scholar]
- Reynolds Crk RW, 2015. Behavior Assessment System for Children, third ed. Pearson, Bloomington, MN. [Google Scholar]
- Roth K, Yang Z, Agarwal M, et al. , 2021. Exposure to a mixture of legacy, alternative, and replacement per- and polyfluoroalkyl substances (PFAS) results in sex-dependent modulation of cholesterol metabolism and liver injury. Environ. Int 157, 106843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagiv SK, Thurston SW, Bellinger DC, Tolbert PE, Altshul LM, Korrick SA, 2010. Prenatal organochlorine exposure and behaviors associated with attention deficit hyperactivity disorder in school-aged children. Am. J. Epidemiol 171 (5), 593–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagiv SK, Kogut K, Gaspar FW, et al. , 2015. Prenatal and childhood polybrominated diphenyl ether (PBDE) exposure and attention and executive function at 9–12 years of age. Neurotoxicol. Teratol 52 (Pt B), 151–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayal K, Prasad V, Daley D, Ford T, Coghill D, 2018. ADHD in children and young people: prevalence, care pathways, and service provision. Lancet Psychiatr 5 (2), 175–186. [DOI] [PubMed] [Google Scholar]
- Seegal RF, Bush B, Brosch KO, 1991. Comparison of effects of Aroclors 1016 and 1260 on non-human primate catecholamine function. Toxicology 66 (2), 145–163. [DOI] [PubMed] [Google Scholar]
- Serme-Gbedo YK, Abdelouahab N, Pasquier JC, Cohen AA, Takser L, 2016. Maternal levels of endocrine disruptors, polybrominated diphenyl ethers, in early pregnancy are not associated with lower birth weight in the Canadian birth cohort GESTE. Environ Health-Glob 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon JR, 1990. The effects of an irrelevant directional cue on humean information processing. In: Reeve RWPaTG. (Ed.), Stimulus-Response Compatibility. Elsevier Science Publishers B. V., North-Holland: ). [Google Scholar]
- Singh K, Karthikeyan S, Vladisavljevic D, St-Amand A, Chan HM, 2019. Factors associated with plasma concentrations of polychlorinated biphenyls (PCBs) and dichlorodiphenyldichloroethylene (p,p’-DDE) in the Canadian population. Int. J. Environ. Health Res 29 (3), 326–347. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, et al. , 2004. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23, S208–S219. [DOI] [PubMed] [Google Scholar]
- Spratlen MJ, Perera FP, Lederman SA, et al. , 2020. The association between prenatal exposure to perfluoroalkyl substances and childhood neurodevelopment. Environ. Pollut 263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staskal DF, Hakk H, Bauer D, Diliberto JJ, Birnbaum LS, 2006. Toxicokinetics of polybrominated diphenyl ether congeners 47, 99, 100, and 153 in mice. Toxicol. Sci 94 (1), 28–37. [DOI] [PubMed] [Google Scholar]
- Stewart P, Fitzgerald S, Reihman J, et al. , 2003. Prenatal PCB exposure, the corpus callosum, and response inhibition. Environ. Health Perspect 111 (13), 1670–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart P, Reihman J, Gump B, Lonky E, Darvill T, Pagano J, 2005. Response inhibition at 8 and 9 1/2 years of age in children prenatally exposed to PCBs. Neurotoxicol. Teratol 27 (6), 771–780. [DOI] [PubMed] [Google Scholar]
- Stockholm convention. In: Plenipotentiaries Cot, edMay, vol. 22, 2001. [Google Scholar]
- IBM SPSS, 2019. Statistics for Macintosh. IBM Corp, Armonk, NY. Version 26.0. [computer program]. [Google Scholar]
- Usenko CY, Abel EL, Kudela M, Janise A, Bruce ED, 2015. Comparison of PBDE congeners as inducers of oxidative stress in zebrafish. Environ. Toxicol. Chem 34 (5), 1154–1160. [DOI] [PubMed] [Google Scholar]
- Vasiliadis HM, Diallo FB, Rochette L, et al. , 2017. Temporal trends in the prevalence and incidence of diagnosed ADHD in children and young adults between 1999 and 2012 in Canada: a data linkage study. Can. J. Psychiatr 62 (12), 818–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verner MA, Hart JE, Sagiv SK, Bellinger DC, Altshul LM, Korrick SA, 2015. Measured prenatal and estimated postnatal levels of polychlorinated biphenyls (PCBs) and ADHD-related behaviors in 8-year-old children. Environ. Health Perspect 123 (9), 888–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser SN, Danielson ML, Bitsko RH, et al. , 2014. Trends in the parent-report of health care provider-diagnosed and medicated attention-deficit/hyperactivity disorder: United States, 2003–2011. J. Am. Acad. Child Psychiatr 53 (1), 34–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vreugdenhil HJ, Lanting CI, Mulder PG, Boersma ER, Weisglas-Kuperus N, 2002. Effects of prenatal PCB and dioxin background exposure on cognitive and motor abilities in Dutch children at school age. J. Pediatr 140 (1), 48–56. [DOI] [PubMed] [Google Scholar]
- Vuong AM, Yolton K, Dietrich KN, Braun JM, Lanphear BP, Chen A, 2018. Exposure to polybrominated diphenyl ethers (PBDEs) and child behavior: current findings and future directions. Horm. Behav 101, 94–104. [DOI] [PubMed] [Google Scholar]
- Wang Y, Rogan WJ, Chen HY, et al. , 2015. Prenatal exposure to perfluroalkyl substances and children’s IQ: the Taiwan maternal and infant cohort study. Int. J. Hyg Environ. Health 218 (7), 639–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D, 2014. WISC-V: Technical and Interpretive Manual. Pearson, Bloomington, MN. [Google Scholar]
- White RF, Palumbo CL, Yurgelun-Todd DA, et al. , 2011. Functional MRI approach to developmental methylmercury and polychlorinated biphenyl neurotoxicity. Neurotoxicology 32 (6), 975–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiecki TV, Sofer I, Frank MJ, 2013. HDDM: hierarchical bayesian estimation of the drift-diffusion model in Python. Front. Neuroinf 7, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Kania-Korwel I, Chen H, et al. , 2013. Metabolism of 2,2’,3,3’,6,6’-hexachlorobiphenyl (PCB 136) atropisomers in tissue slices from phenobarbital or dexamethasone-induced rats is sex-dependent. Xenobiotica 43 (11), 933–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue J, Liu SV, Zartarian VG, Geller AM, Schultz BD, 2014. Analysis of NHANES measured blood PCBs in the general US population and application of SHEDS model to identify key exposure factors. J. Expo. Sci. Environ. Epidemiol 24 (6), 615–621. [DOI] [PubMed] [Google Scholar]
- Young SE, Friedman NP, Miyake A, et al. , 2009. Behavioral disinhibition: liability for externalizing spectrum disorders and its genetic and environmental relation to response inhibition across adolescence. J. Abnorm. Psychol 118 (1), 117–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


