Abstract
Background
Alterations of heart rate variability (HRV) in epileptic patients were the field of interest of several studies for many reasons, particularly the contribution toward sudden unexpected death in epilepsy (SUDEP).
Aim
We aimed at evaluation of autonomic dysfunction in epileptic patients during awake and sleep in addition to studying the association between SUDEP risk with different Holter parameters.
Patients and Methods
The study included eighty epileptic patients (40 controlled epileptic patients and 40 refractory epileptic patients) compared to 30 volunteers as control group. They underwent detailed epileptic history, Chalfont seizure severity scale, sudden unexpected death in epilepsy (SUDEP)-7 risk score and 24 hour Holter monitoring to assess HRV parameters.
Results
Patients with refractory epilepsy had longer duration of epilepsy with increased number of used AEDs compared to controlled epileptic group. Both controlled and refractory epileptic patients had significantly higher average heart rate (AV.HR), sympatho-vagal ratio (low-frequency/high-frequency (LF/HF) ratio in 24 hours, daytime, and nighttime), and LF and HF values compared to controls. The rMSSD (the root mean square of difference between successive normal intervals), Tri.Index (triangular index), and pNN50 (percentage of the number of pairs of consecutive beat-to-beat intervals that varied by 50 ms) were significantly reduced in both epileptic groups compared to controls. Among refractory epileptic patients, patients with generalized epilepsy had significantly higher severity epileptic scale, average heart rate, minimum heart rate, and LF/HF night, in addition to lower rMSSD and pNN50 compared to patients with focal epilepsy. We found positive correlation between the following Holter indices (LF/HF 24, LF/HF day, and LF/HF night) and the duration of the epilepsy, while negative correlations between Tri.Index, LF, and HF and the epileptic duration were detected. SUDEP-7 risk was negatively correlated with pNN50 and rMSSD; meanwhile, it was positively correlated with LF/HF 24. The severity of epilepsy among refractory epileptic patients was positively correlated with average heart rate but negatively correlated with pNN50 and rMSSD. Using linear regression analysis, we found that pNN50 and rMSSD could predict SUDEP-7 risk and severity of epilepsy in refractory epileptic patients.
Conclusion
Epileptic patients (particularly refractory patients with generalized EEG findings and long duration) had reduced heart rate variability and hence impairment of parasympathetic activity with increased susceptibility for adverse outcomes. Moreover, pNN50 and rMSSD could be used as predictors for SUDEP-7 risk as well as severity of epilepsy in refractory epileptic patients.
Keywords: epilepsy, HRV, ANS, pNN50, rMSSD, SUDEP
Introduction
Epilepsy is a neurological disorder characterized by repeated epileptic seizures with considerable social impact due to high incidence and chronicity.1 Two-thirds of epileptic patients are controlled with drug therapy, whereas the remaining cases suffer from drug-resistant epilepsy.2
Epileptic patients are thought to be at particular risk of sudden unexpected death in epilepsy (SUDEP), especially if their seizures are unnoticed. The risk of sudden accidental death in epileptic patients is 24 times higher than the overall population, contributing an average of 17% of deaths.3 Patients with refractory epilepsy have high risk of SUDEP. SUDEP may be caused by a number of pathophysiological causes, including central apnea, autonomic dysregulation of cerebral and cardiac blood flow, decreased heart rate variability (HRV), and neurogenic pulmonary edema.4 SUDEP can arise as a consequence of a terminal cardiac arrhythmia caused by highly frequent seizure-triggered hypoxic stress, as well as excessive sympathetic overstimulation, which provokes neurocardiogenic injury and affects electrical properties of the myocardium, resulting in a fatal cardiac arrhythmia.5 Hence, the concept of “Epileptic Heart” emerged and was defined as a heart and coronary vasculature damaged by chronic epilepsy.6
Previous studies showed that many seizures are associated with alterations in the autonomic nervous system (ANS) activity that could be evaluated using HRV parameters.7 In addition to the alterations of the ANS, one-third of epileptic patients had cardiac modulation presented by tachycardia, bradycardia, asystole, or modification of ventricular repolarization.8
HRV is a marker of ANS activity in which increased HRV reflects parasympathetic predominance, whereas decreased HRV suggests sympathetic predominance.9 HRV parameters showed increases during sleep and decreases during awaking. Interictal HRV was found to be impaired in individuals with epilepsy, including those who are newly diagnosed and drug-naïve.10,11 However, dysregulation is neither simple nor uniform impairment during sleep or awaking.12
Delineation of HRV differences during sleep and awake may be necessary, as autonomic dysregulation has been suggested as risk factor in SUDEP.13
There is great interest for using HRV analyses to provide indirect features for epileptic severity and subsequent adverse outcomes such as SUDEP risk specifically in refractory epileptic patients. Thus, our objectives were to evaluate autonomic dysfunction by assessing HRV parameters in epileptic patients (both refractory and well-controlled) during awake and sleep. In addition, we investigated the association between the epileptic severity and SUDEP risk with different Holter parameters.
Patients and Methods
This case-controlled study was conducted at Al Zahraa University Hospital, Al- Azhar University, Cairo, Egypt. The study was done by collaboration of neurology, cardiology, and internal medicine consultants who evaluated the subjects before enrollment in the study.
The study enrolled eighty epileptic patients who were diagnosed according to the criteria established in the 2017 International Classification of Epileptic Syndromes.14 They were selected from the outpatients neurology clinic at Al-Zahraa University Hospital between October 2019 and March 2020. It also included thirty apparently healthy subjects selected as a control group.
Any patient with history of cardiac diseases such as hypertension, DM, preexisting serious cardiac arrhythmia (eg, atrial fibrillation, ventricular arrhythmia, or heart block) or any other neurological or psychiatric comorbidities was excluded.
Inclusion criteria for epileptic patients were: confirmed diagnosis of idiopathic epilepsy, and their ages between 18 and 40 years.
The study was carried out following the ethical guidelines for human experimentation. Before enrolling in this study, informed written consent was obtained from all cases after describing the purpose of the study and the need for the appropriate investigation.
Neurological Evaluation
All patients were subjected to full epilepsy history taking including age of onset, seizure semiology, seizure types, duration of epilepsy, as well as last attack and types of antiepileptic drugs (AEDs) with complete systemic, cardiac, and neurological examination. Defining the type of epilepsy was done based on the International League Against Epilepsy (ILAE) committee on diagnosis, classification, and terminology.14
The severity of seizures was estimated using the Chalfont seizure severity scale, which incorporated aspects of both a patient-based and an observer-based scale. The factors to be included were identified through an open interview with caregivers, and factor weightings were influenced by patients, caregivers, and neurology specialist opinions. An observer sets the scale, but the responses were decided by the patient. Scores for the following variables were divided into fractions: we graded what typically happened in that seizure form (drop/spill a held object, etc.), tongue-biting, swelling, and lacerations were examples of injuries. Urine and/or feces were examples of incontinence, automatism “convulsion” referred to clonic jerking of the limbs, seizure period, and time to return to normal.15
The estimation of potential SUDEP risk was performed using an inventory of seven validated SUDEP risk factors (SUDEP-7) based on a large prospective cohort study.16 It is formed of seven items with total score of 10 depending mainly on the type of seizure (mainly generalized tonic-clonic seizures given highest score), the number of seizures in the last year, duration of the epilepsy, the patient’s IQ, and the number(s) of AED of concomitant use of ≥3 drugs reflecting the severity of epilepsy, increasing the total score with more risk for SUDEP.13
Cardiac Evaluation
All the study population underwent echocardiographic examination for exclusion of any significant cardiac diseases.
24-Hour Holter Monitoring Acquisition and Analysis
Holter Data acquisition and analysis were performed using Windows version 5.1 with software Version 1 (SEER Light, GE Healthcare, Milwaukee, WI, US). A 3-channel ECG was obtained and analyzed for the whole period of 24 hours. Surface electrodes on the chest generated the processed signal. The system automatically detected and labeled QRS complexes, which were then manually checked to exclude any possible artifacts. Assessment of HRV included time domain and frequency domain parameters.
The following time domain parameters were measured:17
Mean RR (ms): the mean of the RR interval.
pNN50 (%): the percentage of differences greater than 50 ms between successive normal RR intervals in a 24-hour ECG record. It predominantly reflects the parasympathetic activity.
SDNN (ms): the standard deviation of the RR interval. SDNN reflects the parasympathetic component of the autonomic function.
SDANN (ms): the standard deviation of the average NN intervals for each 5 min segment of 24 h HRV recording.
rMSSD (ms): the root mean square of difference between successive normal intervals. It is an important indicator of parasympathetic activity.
Triangular index: the integral of the density of the RR interval histogram divided by its height.
The following frequency domain parameters were measured:17
LF (ms2): it includes the absolute power of low-frequency band range between 0.04 Hz and 0.15 Hz and consists of a combination of sympathetic and parasympathetic effects.
HF (ms2): it includes the absolute power of high-frequency band range between 0.16 Hz and 0.4 Hz. It is considered that it is modulated by the parasympathetic activity of ANS.
The LF/HF ratio: the ratio of LF-to-HF power. It reflects the sympathovagal balance and can be used to estimate HRV in general.
The HRV parameter was evaluated for 24 hours. Parameters were also investigated in two separate time periods: daytime (8:00–12:00 a.m.) and nighttime (12:00–8:00 a.m.).
The enrolled participants were classified into three groups:
Group 1 included 40 patients with controlled epilepsy with no history of seizures for at least 1 year and all included patients on same AED (levetiracetam to exclude effects of different AEDs on HRV indices).
Group 2 included 40 patients with refractory epilepsy with recurrent seizures (patients were maintained on ≥2 AEDs, one of them levetiracetam).
Group 3 included 30 healthy participants with no cardiac, neurological, or psychiatric complaint.
Statistical Analysis
The normality of distribution of all metric data was tested with the Shapiro–Wilk test and significance set at p < 0.05 for non-normal distribution. For parametric variables (age, number of AEDs, AV.HR, max.HR, min.HR, LF/HF 24, LF/HF day, LF/HF night, rMSSD, pNN50, SDNN, SDANN, Tri.Index, LF, HF, severity scale, and SUDEP-7 risk), data are expressed as means with standard deviations, and for non-parametric variables (duration of epilepsy), median (IQR). For the estimation of continuous variables, Student’s t-tests were used. In univariate analyses, chi-square analysis, Student’s unpaired t-test, one-way ANOVA, and Mann–Whitney tests were used to compare variables between groups. To assess the association between variables, Pearson and Spearman correlation analyses were used. Linear regression analysis was used to predict the severity of epilepsy and SUDEP-7 risk in refractory epileptic patients using different Holter parameters. P-values <0.05 were considered statistically significant. The SPSS 16.0 software (SPSS Inc., Chicago, IL, USA) was used to measure all statistics.
Results
Demographic and Neurological Data
All groups were age- and sex-matched (Table 1). Group 2 had significantly longer duration of epilepsy with increased number of used AEDs compared to group 1 (Table 1).
Table 1.
Comparison Among the 3 Studied Groups Regarding Demographic and Neurological Data
| Group 1 N=40 | Group 2 N=40 | Group 3 N=30 | p value | |
|---|---|---|---|---|
| Age (years) (mean ±SD) | 28.4±8.2 | 28.7±7 | 29.1±7 | 0.8 (NS) |
| Sex | ||||
| ● Male | 18 | 19 | 7 | 0.9 (NS) |
| ● Female | 22 | 21 | 13 | |
| EEG findings | ||||
| ● Focal | 22 | 21 | No seizures | 0.1 (NS) |
| ● Generalized | 18 | 19 | ||
| Types of seizures | ||||
| ● Focal aware tonic | 10 | 3 | ||
| ● Focal to bilateral tonic clonic | 12 | 6 | No seizures | |
| ● Generalized motor tonic clonic | 18 | 26 | ||
| ● Unknown onset motor | 5 | |||
| Duration of epilepsy (months), Median (IQR) | 72±108 | 138±84 | No seizures | 0.03 |
| Number of used AEDs (mean ±SD) | 1±0.0 | 2.2±0.4 | No AEDs | 0.001 |
Abbreviations: AEDs=anti-epileptic drugs, NS=non significant, SD=standard deviation
Types of seizures in epileptic groups were distributed as the following (Table 1):
Group 1: 10 patients had focal aware tonic seizures, 12 patients had focal to bilateral tonic clonic seizures, and 18 patients had generalized motor tonic-clonic seizures.
Group 2: 3 patients had focal aware tonic seizures, 6 patients had focal to bilateral tonic clonic seizures, 26 patients had generalized motor tonic-clonic seizures, and 5 patients had unknown onset motor seizures.
The combinations of AEDs with levetiracetam in refractory epileptic patients were distributed as follows: eslicarbazepine used in 6 patients, valproate used in 11 patients, lacosamide used in 5 patients, eslicarbazepine with valproate used in 10 patients, carbamazepine with lamotrigine used in 5 patients, and valproate with lamotrigine used in 3 patients.
(24)-Hours Holter Parameters
When compared to controls, epileptic patients (both controlled and refractory) had substantially higher average heart rate as well as significantly lower rMSSD, Tri.Index, and pNN50 values. Also, group 2 patients had significantly increased LF/HF 24, LF/HF day, and LF/HF night, while LF and HF values were significantly reduced when compared to group 3 (Table 2).
Table 2.
Comparison Among the Studied 3 Groups Regarding Holter Monitoring Parameters
| Parameters | Group 1 N=40 | Group 2 N=40 | Group 3 N=30 | Pa | Pb | Pc |
|---|---|---|---|---|---|---|
| AV.HR (b/m) (mean±SD) | 80.5±8.9 | 82.3±10.2 | 70.7±5.5 | 0.8 | 0.001 | 0.0001 |
| Max.HR (b/m) (mean±SD) | 144.7±12.1 | 149.3±15.5 | 140.6±7.6 | 0.4 | 0.5 | 0.06 |
| Min.HR (b/m) (mean±SD) | 53.1±7.6 | 54.7±7.4 | 50.2±4.9 | 0.6 | 0.09 | 0.5 |
| LF/HF24 (mean±SD) | 1.6±0.5 | 2.1±0.8 | 1.5±0.2 | 0.06 | 0.9 | 0.02 |
| LF/HF day (mean±SD) | 2.2±1.1 | 2.5±1.1 | 1.6±0.4 | 0.8 | 0.1 | 0.03 |
| LF/HF night (mean±SD) | 1.6±0.9 | 2.0±0.9 | 1.3±0.3 | 0.8 | 0.6 | 0.01 |
| rMSSD (ms) (mean±SD) | 22±9.6 | 22.2±7.9 | 32.7±11.7 | 0.8 | 0.0001 | 0.0001 |
| pNN50 (%) (mean ±SD) | 5.3±2.8 | 5.6±4.0 | 11.9±5.7 | 0.9 | 0.0001 | 0.001 |
| SDNN (ms) (mean±SD) | 125.2±39.8 | 121.4±37.7 | 125.3±16.3 | 0.9 | 0.8 | 0.9 |
| SDANN (ms) (mean±SD) | 101.7±40.3 | 90.9±36.7 | 106.2±20.8 | 0.57 | 0.9 | 0.33 |
| Tri.Index (mean±SD) | 23.7±5.2 | 17.4±4.6 | 33.3±6.0 | 0.001 | 0.0001 | 0.0001 |
| LF (ms2) (mean±SD) | 1147.9±242.8 | 841.6±279.6 | 1274.7±220.9 | 0.0001 | 0.3 | 0.0001 |
| HF (ms2) (mean±SD) | 595.6±241.4 | 495.1±301.9 | 762.9±252.2 | 0.4 | 0.1 | 0.004 |
Notes: Pa= comparison between controlled epileptic and refractory epileptic patients. Pb=comparison between controlled epileptic patients and control group. Pc=comparison between refractory epileptic patients and control group.
Abbreviations: AV.HR, average heart rate; Max., maximum; Min., minimum; LF/HF, the ratio of low-frequency/high-frequency power; rMSSD, root mean square of the difference between successive normal intervals; pNN50, the percentage of the number of pairs of consecutive beat-to-beat intervals that differed by 50 ms; SDNN, the standard deviation of the RR interval; SDANN, the standard deviation of the average NN intervals; Tri.Index, Triangular index; LF, low frequency; HF, high frequency; SD, standard deviation.
Comparison Regarding Holter Parameters Between Focal and Generalized EEG Findings in Refractory Epileptic Patients
Patients with generalized epilepsy had significantly higher severity scale, frequency of epilepsy/year, average heart rate, minimum heart rate, LF/HF night in addition to reduced rMSSD and pNN50 compared to patients with focal epilepsy in refractory epileptic patients. Meanwhile, no significant difference was found between both groups regarding SUDEP-7 risk, latency between last seizure and Holter test, maximum heart rate, LF/HF 24, LF/HF day, SDNN, Tri.Index, LF, and HF (Table 3).
Table 3.
Comparison of Severity Scale, SUDEP-7 Risk, and Holter Parameters Between Focal and Generalized EEG Findings in Refractory Epileptic Patients
| Parameters | Group 2a (Focal) N=21 | Group 2b (Generalized) N=19 | p |
|---|---|---|---|
| Severity scale (mean±SD) | 429.1±180.4 | 1072.7±722.7 | 0.03 |
| SUDEP-7 risk (mean±SD) | 4.3±0.8 | 3.7±1.0 | 0.16 |
| Frequency of epilepsy/year (mean±SD) | 4.7±1.9 | 6.7±3.8 | 0.04 |
| Latency between last seizure and Holter test | 4.1±1.0 | 3.5±2.0 | 0.22 |
| AV.HR (b/m) (mean±SD) | 77.6±10.45 | 86.2±6.9 | 0.04 |
| Max.HR (b/m) (mean±SD) | 152.8±9.4 | 145.3±19.9 | 0.32 |
| Min.HR (b/m) (mean±SD) | 49.0±7.3 | 57.1±5.6 | 0.01 |
| LF/HF 24 (mean±SD) | 2.0±0.8 | 2.1±0.8 | 0.72 |
| LF/HF day (mean±SD) | 2.0±0.8 | 2.9±1.2 | 0.09 |
| LF/HF night (mean±SD) | 1.3±0.8 | 2.3±1.1 | 0.04 |
| rMSSD (ms) (mean±SD) | 26.4±7.9 | 17.11±4.6 | 0.004 |
| pNN50 (%) (mean±SD) | 7.8±4.1 | 2.9±1.8 | 0.003 |
| SDNN (ms) (mean±SD) | 135.1±42.0 | 105.1±20.8 | 0.06 |
| Tri.Index (mean±SD) | 15.8±4.4 | 19.3±4.5 | 0.1 |
| LF (ms2) (mean±SD) | 741.5±263.5 | 919.1±298.3 | 0.81 |
| HF (ms2) (mean±SD) | 472.7±296.9 | 494.6±319.7 | 0.87 |
Abbreviations: LF/HF, the ratio of low-frequency/high-frequency power; rMSSD, root mean square of the difference between successive normal intervals; pNN50, the percentage of the number of pairs of consecutive beat-to-beat intervals that differed by 50 ms; SDNN, the standard deviation of the RR interval; Tri.Index, Triangular index; LF, low frequency; HF, high frequency; SUDEP, sudden unexpected death in epilepsy; SD, standard deviation.
Correlation Between Duration of Epilepsy and Different Holter Parameters
The duration of epilepsy was positively correlated with LF/HF 24, LF/HF day, and LF/HF night, while the epileptic duration had significant negative correlation with Tri.Index, LF, and HF in all studied epileptic patients (Table 4).
Table 4.
Correlation Between Duration of Epilepsy, Severity of Epilepsy, and SUDEP-7 Risk with Different Holter Parameters
| Parameters | R | P |
|---|---|---|
| Duration of epilepsy and different Holter parameters | ||
| LF/HF 24 | 0.392 | 0.02 |
| LF/HF day | 0.394 | 0.01 |
| LF/HF night | 0.461 | 0.004 |
| Tri.Index | −0.371 | 0.05 |
| LF (ms2) | −0.448 | 0.005 |
| HF (ms2) | −0.355 | 0.04 |
| SUDEP-7 risk and different Holter parameters | ||
| LF/HF 24 | 0.328 | 0.04 |
| pNN50 (%) | −0.510 | 0.001 |
| rMSSD (ms) | −0.790 | 0.0001 |
| Severity of epilepsy and different Holter parameters | ||
| AV.HR | 0.575 | 0.01 |
| pNN50 (%) | −0.521 | 0.02 |
| rMSSD (ms) | −0.477 | 0.03 |
Abbreviations: AV.HR, average heart rate; LF/HF, the ratio of low-frequency/high-frequency power; rMSSD, root mean square of the difference between successive normal intervals; pNN50, the percentage of the number of pairs of consecutive beat-to-beat intervals that differed by 50 ms; SDNN, the standard deviation of the RR interval; Tri.Index, Triangular index; LF, low frequency; HF, high frequency.
Correlation and Prediction of SUDEP-7 Risk Using Different Holter Parameters
SUDEP-7 risk was negatively correlated with pNN50 and rMSSD, while it was positively correlated with LF/HF 24 (Table 4).
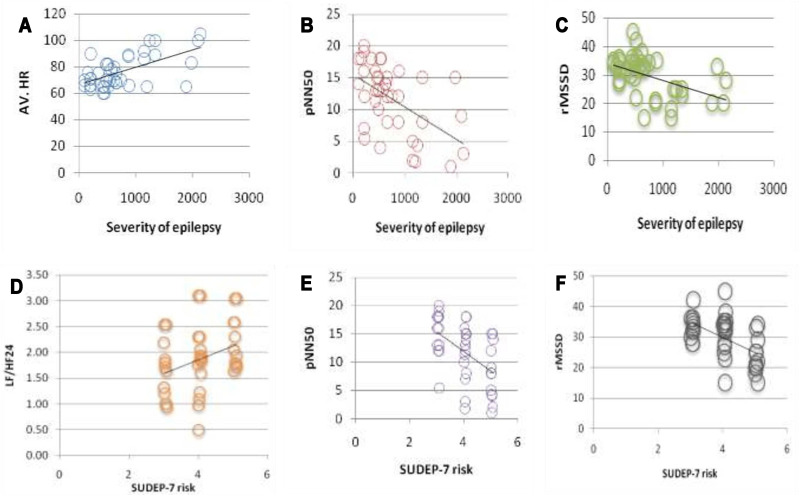
Using linear regression, we found that LF/HF 24, pNN50, and rMSSD could predict SUDEP-7 risk among refractory epileptic patients (r2=0.108, p=0.04; r2=0.510, p=0.001; r2=0.623, p=0.0001, respectively) (Figure 1).
Figure 1.
Upper row shows correlations between severity of epilepsy with (A) AV.HR, (B) pNN50, and (C) rMSSD. Lower row shows correlations between SUDEP-7 risk with (D) LF/HF24, (E) pNN50, and (F) rMSSD.
Abbreviations: SUDEP, sudden unexpected death in epilepsy; LF/HF, the ratio of low-frequency/high-frequency power; pNN50, the percent of the number of pairs of consecutive beat-to-beat intervals that differed by 50 ms; rMSSD, root mean square of the difference between successive normal intervals.
Correlation and Prediction of Epileptic Severity Using Different Holter Parameters Among Refractory Epileptic Patients
The severity of epilepsy was positively correlated with average heart rate but negatively correlated with pNN50 and rMSSD in all studied epileptic patients (Table 4).
Using linear regression, we found that average heart rate, pNN50, and rMSSD could predict severity of epilepsy among refractory epileptic patients (r2=0.237, p=0.03; r2=0.245, p=0.03; r2=0.233, p=0.03, respectively) (Figure 1).
Comparison Between Control Group and Refractory Epileptic Patients with Focal EEG Findings Regarding Holter Parameters
Refractory epileptic patients with focal EEG findings demonstrated significantly higher AV.HR, max.HR, LF/HF 24, and LF/HF day, in addition to significantly lower rMSSD, pNN50, Tri.index, LF, and HF compared to control group (Table 5).
Table 5.
Comparison Between Control Group and Refractory Epileptic Patients with Focal EEG Findings Regarding Holter Parameters
| Parameters | Group 2a (Focal) N=21 | Group 3 (Control) N=30 | p |
|---|---|---|---|
| AV.HR (b/m) (mean±SD) | 77.6±10.45 | 70.7±5.5 | 0.001 |
| Max.HR (b/m) (mean±SD) | 152.8±9.4 | 140.6±7.6 | 0.0001 |
| Min.HR (b/m) (mean±SD) | 49.0±7.3 | 50.2±4.9 | 0.79 |
| LF/HF 24 (mean±SD) | 2.0±0.8 | 1.5±0.2 | 0.001 |
| LF/HF day (mean±SD) | 2.0±0.8 | 1.6±0.4 | 0.01 |
| LF/HF night (mean±SD) | 1.3±0.8 | 1.3±0.3 | 0.98 |
| rMSSD (ms) (mean±SD) | 26.4±7.9 | 32.7±11.7 | 0.04 |
| pNN50 (%) (mean±SD) | 7.8±4.1 | 11.9±5.7 | 0.02 |
| SDNN (ms) (mean±SD) | 135.1±42.0 | 125.3±16.3 | 0.22 |
| Tri.Index (mean±SD) | 15.8±4.4 | 33.3±6.0 | 0.0001 |
| LF (ms2) (mean±SD) | 741.5±263.5 | 1274.7±220.9 | 0.0001 |
| HF (ms2) (mean±SD) | 472.7±296.9 | 762.9±252.2 | 0.0001 |
Abbreviations: LF/HF, the ratio of low-frequency/high-frequency power; rMSSD, root mean square of the difference between successive normal intervals; pNN50, the percentage of the number of pairs of consecutive beat-to-beat intervals that differed by 50 ms; SDNN, the standard deviation of the RR interval; Tri.Index, Triangular index; LF, low frequency; HF, high frequency; SD, standard deviation.
Discussion
Autonomic imbalance and HRV alteration are crucial cardiovascular risk factors in epileptic patients. These disturbances implicate the adverse effect of reduced parasympathetic tone that predisposes to a “proarrhythmic” status.18 Assessment of ANS activity (both sympathetic and parasympathetic) had been established using HRV quantification.19
Interictal autonomic modulations increase susceptibility for SUDEP.20 A seizure’s abnormal cortical activity may include central regions that control autonomic activity, resulting in autonomic symptoms, either immediately or later in the seizure’s progression. The cardiovascular system is altered by seizure-related hypo- or hyperactivity.21 Heart rhythm is affected by epilepsy-related autonomic dysfunctions.22
In epileptic patients, the role of ANS function and its diurnal variations were highlighted in this study. The goal of our study was to evaluate autonomic dysfunction in patients with refractory and controlled epilepsy during awake and sleep using heart rate variability indices in addition to studying the association between SUDEP risk with different Holter parameters.
The current study demonstrated that both refractory and controlled epileptic patients had lower time domain parameters of HRV (pNN50, rMSSD, and Tri.Index) in addition to higher average heart rate compared to the control group. Also, those patients with refractory epilepsy had lower Tri.Index and LF compared to patients with controlled epilepsy.
Our findings suggested there is evidence of reduced parasympathetic system activity in epileptic patients which was consistent with the previous studies by Mukherjee et al,21 Lotufo et al,23 and Yilmaz et al.17
According to the results from Kolsal et al,22 patients with refractory epilepsy exhibited severe deterioration in HRV due to parasympathetic dysfunction, whereas patients with controlled epilepsy had moderate deterioration of HRV.
Our results were partially comparable with findings from Baysal-Kirac et al24 who found that time domain measures (SDNN, SDANN, rMSSD, pNN50) were markedly suppressed in patients with drug-resistant seizures relative to healthy controls. SDNN and SDANN did not vary between the two groups in our research. The reason for these heterogeneous results might be attributed to longer duration of epilepsy among patients included in the study of Baysal-Kirac et al24 (20.5±10.3 years) compared to our study (72±108 months for refractory epileptic cases and 138±84 months for controlled epileptic cases).
Repeated seizures had an impact on the myocardium and cardiac conducting system, as evidenced by raised average heart rates and decreased time domain HRV parameters. Baysal-Kirac et al24 included patients without overt cardiovascular disease, which was excluded before inclusion. They reported that seizures resulted in frequent hypoxemia and/or increased catecholamine levels that could lead to subclinical heart damage and fibrosis.
Excessive autonomic changes during seizures typically result in sinus tachycardia or tachyarrhythmia, and thereby the reduced vagal activity would be unable to restore normal heart rhythm, potentially facilitating the development of ventricular arrhythmia.23
Few researches have studied the link between HRV during sleep and wakefulness in epilepsy, which is surprising given that the autonomic balance varies throughout the day. With deeper stages of sleep, parasympathetic activity rises, with a transition back to sympathetic dominance during rapid eye movement sleep.25 There is substantial evidence that epileptic patients’ diurnal HRV patterns are disrupted; nevertheless, the exact nature of the disruption is unknown.12
We demonstrated that frequency domain parameters of HRV defined by ratios of LF/HF in 24 hours, daytime, and nighttime were substantially higher in refractory epileptic patients relative to the control group, but these parameters did not vary between the two epileptic groups. Also, LF and HF values were significantly lower in the refractory epileptic population compared to healthy participants, which was consistent with previous researches by Ronkainen et al26 and Ferri et al.27
In contrast to our findings, Persson et al28 observed that epilepsy had no significant impact on frequency domain of HRV in patients with untreated epilepsy. Daytime and nighttime recordings showed very similar results. They stated that no statistically significant differences were found in any of the examined parameters, including LF, HF, or LF/HF, as compared to age- and sex-matched healthy controls. This may be explained by the fact that the study only focused on newly diagnosed patients, and the findings did not extend to chronic epilepsy patients as included in our study.
Our results demonstrated that autonomic regulation determined by both time and frequency domains of HRV in epileptic patients is significantly impaired and showed differential patterns during wakefulness and sleep. In general, HRV decreases during the ictal phase, gradually returning to baseline in the postictal period.29–31
The cortical epileptic area, the medullary cardiac regulatory centers, and the sinoatrial node in the heart are all linked, as was suggested by Lathers et al.32 The cardiac electrical substrate for SUDEP32 is provided by seizure-induced activation of the central ANS, which can directly alter postganglionic discharges on the heart. This mechanism could be exacerbated during non-rapid eye movement (NREM) sleep, when interictal epileptic activity and subclinical epileptic discharges are accompanied by periodic cortical and autonomic arousal fluctuations. Seizures during sleep may be also responsible for the shift from dominant vagal tone to sympathetic hyperactivity.33 Patients who die from SUDEP are nearly twice as likely as those who do not die from SUDEP to have nocturnal seizures.34 It is widely accepted that NREM sleep enhances the incidence of seizures and that seizures occur infrequently during rapid eye movement (REM) sleep.35 Seizures that occur while sleeping are longer and are more likely to progress from localized to bilateral tonic-clonic seizures.36 There are day-night variances in seizure severity, and it is hypothesized that these differences are caused by fluctuations in serotonin and norepinephrine levels in various brain locations.37
There are different types of seizures, which are associated with various risk factors for the patients depending on type and severity of epilepsy. Partial epilepsy is caused by damage to one side of the brain, while generalized epilepsy affects both sides of the brain and the affected individual suddenly becomes unconscious.38
The current study revealed that refractory epileptic patients with a generalized form of EEG findings had a more advanced form on the severity scale and preferential HRV dysfunction (detected by higher average HR, minimum HR, LF/HF night, pNN50, and rMSSD) compared to those with focal EEG findings. Moreover, refractory patients with focal EEG findings showed significant increase of AV.HR, max.HR, LF/HF 24, and LF/HF day, besides significant reduction of rMSSD, pNN50, Tri.Index, LF, and HF compared to the control group. According to these results, we suggested that patients with generalized type are more prone to detrimental outcomes followed by patients with focal type compared to non-epileptic group.
Myers et al12 reported that the overall evidence of HRV is abnormal in adults with generalized epilepsy indicating an interictal shift toward sympathetic dominance.
HRV was evaluated in partial and generalized epilepsy patients as well as healthy controls by Yildiz et al.39 They demonstrated sympathetic dominance in the time and frequency domain variables, as well as differences between epilepsy groups.39
Abnormal autonomic regulatory functions imply that epilepsy may change autonomic function, and this is true not only of temporal lobe epilepsy but also of idiopathic generalized epilepsy. The changes in the ANS in epileptic patients confirm that electrophysiologic measurements of autonomic function may be useful in preventing sudden unexpected death related to epilepsy.40
The duration of epilepsy was significantly correlated with HRV frequency domain parameters in our research. In accordance with our results, Sathyaprabha et al41 found that refractory epilepsy patients with longer epileptic duration had more serious autonomic dysfunction than those with shorter durations.
In addition, Yildiz et al.39 reported that epilepsy period of greater than 10 years was associated with higher LF values and LF/HF ratios as well as lower HF values when compared to patients with epilepsy of less than 10 years in their research.
Moreover, we found that not only were pNN50 and rMSSD correlated to SUDEP-7 risk and epileptic severity, but also that they could predict SUDEP risk and epileptic severity in refractory epileptic patients. Our findings were in accordance with DeGiorgio et al13 who stated that they supposed rMSSD to be a biomarker for severity of epilepsy, and that subjects with low rMSSD values have higher SUDEP scores. Our findings supported the results of previous studies which concluded that the severity of autonomic disturbances was related to seizure severity as reported by Myers et al12 and Mukherjee et al.21.
Kolsal et al22 suggested that deterioration of time domain parameters of HRV (pNN50 and rMSSD) increases with increasing disease severity.
Lower HRV measurements in patients with poorly controlled epilepsy were associated with a higher risk of SUDEP in previous studies.39 Long-standing disease, poor seizure control, and multiple antiepileptic therapies are currently known risk factors for SUDEP risk.42
Prediction of epileptic severity is of great importance for patient’s safety as it provides sufficient time for therapeutic interventions that can minimize severity of seizures.43 Several studies have suggested that HRV can be used as a predictor of seizures. Results from Kolsal et al22 revealed that the sympathetic system began to dominate before the seizure, and as a result, epilepsy-induced autonomic dysfunction and deterioration became more obvious during seizures.
Pre-ictal assessment of HRV for patients needed be studied in larger groups as HRV may be a useful indicator of seizures in the future.22
Clinical management implications from the correlation of altered HRV indices with severity of epilepsy and SUDEP may be present, although further studies are needed. Evaluation of HRV could be incorporated into the protocols to assess both epileptic severity and SUDEP risk. This could raise the awareness of patients and families for SUDEP and highlight specific interventions or precautions. In addition, there are therapeutic implications of HRV if it is confirmed to be a reliable biomarker of SUDEP. Therapeutic application of vagal nerve stimulation (VNS) revealed reduction of T-wave alternans, a marker of cardiac electrical abnormality, suggesting that VNS could be used to minimize SUDEP risk. However, the impact of VNS on HRV has not been well clarified, and further studies are required. Future researches on readjustment of HRV indices to evaluate the efficacy of emerging therapies are also required.44
Limitations
A limited number of patients were included in this research. More study is warranted to validate these preliminary findings in a larger number of subjects.
The lack of a standardized approach for measuring and reporting HRV in epileptic patients is currently affecting HRV’s utilization as a clinical biomarker for epilepsy patients.
Our data should be interpreted cautiously as confounding factors might affect HRV including AEDs and seizure frequency.
Conclusions
We concluded that epileptic patients (both refractory and managed) have altered time and frequency domain HRV indices, reflecting decreased parasympathetic activity with sympathetic hyperactivity during awake or sleep. The impairment of the autonomic nervous system (ANS) was more prominent in refractory epileptic patients, as evidenced by higher frequency domain measures during waking and sleep. Refractory epileptic patients with generalized EEG findings had more severe scale of seizures and significant HRV dysfunction than did individuals with focal EEG findings, indicating that such patients were more vulnerable to unfavorable outcomes. The duration of epilepsy was significantly correlated with frequency domain parameters of HRV. Furthermore, pNN50 and rMSSD could be employed as predictors of SUDEP risk and epileptic severity in refractory patients.
Funding Statement
There is no funding to report.
Ethical Statement
This study was conducted after approval of the institutional review boards of the Faculty of Medicine, Al-Azhar University, IRB (RHBIRB2018122001), Registration date: Dec 20, 2018 in line with the Helsinki Declaration on clinical research involving humans. All participants gave informed consent.
Disclosure
There are no conflicts of interest related to this publication that the authors are aware of.
References
- 1.Engel JJr, Pedley TA. Epilepsy: A Comprehensive Textbook. Vol 3. 2nd ed. Wolters Kluwer Health/Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 2.Jacoby A, Austin JK. Social stigma for adults and children with epilepsy. Epilepsia. 2007;48(SUPPL. 9):6–9. doi: 10.1111/J.1528-1167.2007.01391.X [DOI] [PubMed] [Google Scholar]
- 3.Barot N, Nei M. Autonomic aspects of sudden unexpected death in epilepsy (SUDEP). Clin Auton Res. 2019;29(2):151–160. doi: 10.1007/s10286-018-0576-1 [DOI] [PubMed] [Google Scholar]
- 4.Jansen K, Lagae L. Cardiac changes in epilepsy. Seizure. 2010;19(8):455–460. doi: 10.1016/j.seizure.2010.07.008 [DOI] [PubMed] [Google Scholar]
- 5.Nashef L, So EL, Ryvlin P, Tomson T. Unifying the definitions of sudden unexpected death in epilepsy. Epilepsia. 2012;53(2):227–233. doi: 10.1111/J.1528-1167.2011.03358.X [DOI] [PubMed] [Google Scholar]
- 6.Verrier RL, Pang TD, Nearing BD, Schachter SC. Epileptic heart: a clinical syndromic approach. Epilepsia. 2021;62(8):1780–1789. doi: 10.1111/epi.16966 [DOI] [PubMed] [Google Scholar]
- 7.Heart rate variability (HRV) Analysis in patients with epilepsy - Health Research Authority. Available from: https://www.hra.nhs.uk/planning-and-improving-research/application-summaries/research-summaries/heart-rate-variability-hrv-analysis-in-patients-with-epilepsy/. Accessed September 23, 2021.
- 8.Moghimi N, Lhatoo SD. Sudden unexpected death in epilepsy or voodoo heart: analysis of heart/brain connections. Curr Cardiol Rep. 2013;15(12). doi: 10.1007/S11886-013-0424-9 [DOI] [PubMed] [Google Scholar]
- 9.Katona PG, Jih F. Respiratory sinus arrhythmia: noninvasive measure of parasympathetic cardiac control. J Appl Physiol. 1975;39(5):801–805. doi: 10.1152/JAPPL.1975.39.5.801 [DOI] [PubMed] [Google Scholar]
- 10.Goit RK, Jha SK, Pant BN. Alteration of cardiac autonomic function in patients with newly diagnosed epilepsy. Physiol Rep. 2016;4(11):12826. doi: 10.14814/PHY2.12826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meghana A, Sriranjini SJ, Sathyaprabha T, Sanjib S, Prathyusha V, Satishchandra P. Autonomic function in reflex and non-reflex epilepsy–an exploratory study. Acta Neurol Scand. 2016;133(6):459–465. doi: 10.1111/ANE.12486 [DOI] [PubMed] [Google Scholar]
- 12.Myers KA, Sivathamboo S, Perucca P. Heart rate variability measurement in epilepsy: how can we move from research to clinical practice? Epilepsia. 2018;59(12):2169–2178. doi: 10.1111/epi.14587 [DOI] [PubMed] [Google Scholar]
- 13.DeGiorgio CM, Miller P, Meymandi S, et al. RMSSD, a measure of vagus-mediated heart rate variability, is associated with risk factors for SUDEP: the SUDEP-7 Inventory. Epilepsy Behav. 2010;19(1):78–81. doi: 10.1016/j.yebeh.2010.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scheffer IE, Berkovic S, Capovilla G, et al. ILAE classification of the epilepsies: position paper of the ILAE commission for classification and terminology. Epilepsia. 2017;58(4):512–521. doi: 10.1111/EPI.13709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duncan JS, Sander JWAS. The Chalfont seizure severity scale. J Neurol Neurosurg Psychiatry. 1991;54(10):873–876. doi: 10.1136/jnnp.54.10.873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walczak TS, Leppik IE, D’Amelio M, et al. Incidence and risk factors in sudden unexpected death in epilepsy. Neurology. 2001;56(4):519–525. doi: 10.1212/WNL.56.4.519 [DOI] [PubMed] [Google Scholar]
- 17.Yilmaz M, Kayancicek H, Cekici Y. Heart rate variability: highlights from hidden signals. J Integr Cardiol. 2018;4(5):1–8. doi: 10.15761/jic.1000258 [DOI] [Google Scholar]
- 18.Hou Y, Zhou Q, Po SS. Neuromodulation for cardiac arrhythmia. Heart Rhythm. 2016;13(2):584–592. doi: 10.1016/J.HRTHM.2015.10.001 [DOI] [PubMed] [Google Scholar]
- 19.Laborde S, Schiecke K, Mosley E, et al. Interictal heart rate variability analysis reveals lateralization of cardiac autonomic control in temporal lobe epilepsy. Front Neurol. 2020;1:842. doi: 10.3389/fneur.2020.00842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Atalar AÇ, Savrun FK, Yeni SN. Autonomic dysfunction during the interictal period: an electrophysiologic study. Neurol Sci Neurophysiol. 2019;36(1):9–15. doi: 10.5152/NSN.2019.10702 [DOI] [Google Scholar]
- 21.Mukherjee S, Tripathi M, Chandra PS, et al. Cardiovascular autonomic functions in well-controlled and intractable partial epilepsies. Epilepsy Res. 2009;85(2–3):261–269. doi: 10.1016/j.eplepsyres.2009.03.021 [DOI] [PubMed] [Google Scholar]
- 22.Kolsal E, Serdaroǧlu A, Çilsal E, et al. Can heart rate variability in children with epilepsy be used to predict Seizures?. Seizure. 2014;23(5):357–362. doi: 10.1016/j.seizure.2014.01.025 [DOI] [PubMed] [Google Scholar]
- 23.Lotufo PA, Valiengo L, Benseñor IM, Brunoni AR. A systematic review and meta-analysis of heart rate variability in epilepsy and antiepileptic drugs. Epilepsia. 2012;53(2):272–282. doi: 10.1111/j.1528-1167.2011.03361.x [DOI] [PubMed] [Google Scholar]
- 24.Baysal-Kirac L, Serbest NG, Şahin E, et al. Analysis of heart rate variability and risk factors for SUDEP in patients with drug-resistant epilepsy. Epilepsy Behav. 2017;71(Pt A):60–64. doi: 10.1016/j.yebeh.2017.04.018 [DOI] [PubMed] [Google Scholar]
- 25.Boudreau P, Yeh WH, Dumont GA, Boivin DB. Circadian variation of heart rate variability across sleep stages. Sleep. 2013;36(12):1919–1928. doi: 10.5665/sleep.3230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ronkainen E, Korpelainen JT, Heikkinen E, Myllylä VV, Huikuri HV, Isojärvi JIT. Cardiac autonomic control in patients with refractory epilepsy before and during vagus nerve stimulation treatment: a one-year follow-up study. Epilepsia. 2006;47(3):556–562. doi: 10.1111/j.1528-1167.2006.00467.x [DOI] [PubMed] [Google Scholar]
- 27.Ferri R, Curzi-Dascalova L, Arzimanoglou A, et al. Heart rate variability during sleep in children with partial epilepsy. J Sleep Res. 2002;11(2):153–160. doi: 10.1046/j.1365-2869.2002.00283.x [DOI] [PubMed] [Google Scholar]
- 28.Persson H, Ericson M, Tomson T. Heart rate variability in patients with untreated epilepsy. Seizure. 2007;16(6):504–508. doi: 10.1016/j.seizure.2007.03.010 [DOI] [PubMed] [Google Scholar]
- 29.Jaychandran R, Chaitanya G, Satishchandra P, et al. Monitoring peri-ictal changes in heart rate variability, oxygen saturation and blood pressure in epilepsy monitoring unit. Epilepsy Res. 2016;125:10–18. doi: 10.1016/J.EPLEPSYRES.2016.05.013 [DOI] [PubMed] [Google Scholar]
- 30.Gong X, Mao X, Chen Y, et al. The changes of HRV in refractory epilepsy: the potential index to predict the onset of epilepsy in children. J Xray Sci Technol. 2016;24(2):309–317. doi: 10.3233/XST-160558 [DOI] [PubMed] [Google Scholar]
- 31.Romigi A, Albanese M, Placidi F. Heart rate variability in untreated newly diagnosed temporal lobe epilepsy: evidence for ictal sympathetic dysregulation. Epilepsia. 2016;57(3):418–426. doi: 10.1111/EPI.13309 [DOI] [PubMed] [Google Scholar]
- 32.Lathers CM, Schraeder PL, Weiner FL. Synchronization of cardiac autonomic neural discharge with epileptogenic activity: the lockstep phenomenon. Electroencephalogr Clin Neurophysiol. 1987;67(3):247–259. doi: 10.1016/0013-4694(87)90023-X [DOI] [PubMed] [Google Scholar]
- 33.Nobili L, Proserpio P, Rubboli G, Montano N, Didato G, Tassinari CA. Sudden unexpected death in epilepsy (SUDEP) and sleep. Sleep Med Rev. 2011;15(4):237–246. doi: 10.1016/J.SMRV.2010.07.006 [DOI] [PubMed] [Google Scholar]
- 34.Van Der Lende M, Hesdorffer DC, Sander JW, Thijs RD. Nocturnal supervision and SUDEP risk at different epilepsy care settings. Neurology. 2018;91(16):e1508–e1518. doi: 10.1212/WNL.0000000000006356 [DOI] [PubMed] [Google Scholar]
- 35.Ng M, Pavlova M. Why are seizures rare in rapid eye movement sleep? Review of the frequency of seizures in different sleep stages. Epilepsy Res Treat. 2013;2013:1–10. doi: 10.1155/2013/932790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bazil CW, Walczak TS. Effects of sleep and sleep stage on epileptic and nonepileptic seizures. Epilepsia. 1997;38(1):56–62. doi: 10.1111/J.1528-1157.1997.TB01077.X [DOI] [PubMed] [Google Scholar]
- 37.Ågren H, Koulu M, Saavedra JM, Potter WZ, Linnoila M. Circadian covariation of norepinephrine and serotonin in the locus coeruleus and dorsal raphe nucleus in the rat. Brain Res. 1986;397(2):353–358. doi: 10.1016/0006-8993(86)90638-4 [DOI] [PubMed] [Google Scholar]
- 38.Moridani MK, Farhadi H. Heart rate variability as a biomarker for epilepsy seizure prediction. Bratisl Med J. 2017;18(1):3–8. doi: 10.4149/BLL_2017_001 [DOI] [PubMed] [Google Scholar]
- 39.Yildiz GU, Dogan EA, Dogan U, et al. Analysis of 24-hour heart rate variations in patients with epilepsy receiving antiepileptic drugs. Epilepsy Behav. 2011;20(2):349–354. doi: 10.1016/j.yebeh.2010.12.001 [DOI] [PubMed] [Google Scholar]
- 40.Shaker KK, Al Mahdawi AM, Hamdan FB. Interictal autonomic dysfunction in patients with epilepsy. Egypt J Neurol Psychiatry Neurosurg. 2021;57(1):1–7. doi: 10.1186/S41983-021-00422-0 [DOI] [Google Scholar]
- 41.Sathyaprabha TN, Satishchandra P, Netravathi K, Sinha S, Thennarasu K, Raju TR. Cardiac autonomic dysfunctions in chronic refractory epilepsy. Epilepsy Res. 2006;72(1):49–56. doi: 10.1016/j.eplepsyres.2006.07.011 [DOI] [PubMed] [Google Scholar]
- 42.Hitiris N, Suratman S, Kelly K, Stephen LJ, Sills GJ, Brodie MJ. Sudden unexpected death in epilepsy: a search for risk factors. Epilepsy Behav. 2007;10(1):138–141. doi: 10.1016/j.yebeh.2006.11.010 [DOI] [PubMed] [Google Scholar]
- 43.Behbahani S, Dabanloo NJ, Nasrabadi AM, Dourado A. Prediction of epileptic seizures based on heart rate variability. Technol Health Care. 2016;24(6):795–810. doi: 10.3233/THC-161225 [DOI] [PubMed] [Google Scholar]
- 44.Myers KA, Bello-Espinosa LE, Symonds JD, et al. Heart rate variability in epilepsy: a potential biomarker of sudden unexpected death in epilepsy risk. Epilepsia. 2018;59(7):1372–1380. doi: 10.1111/EPI.14438 [DOI] [PubMed] [Google Scholar]