Abstract
Intraseason timing of influenza infection among persons of different ages could reflect relative contributions to propagation of seasonal epidemics and has not been examined among ambulatory patients. Using data from the US Influenza Vaccine Effectiveness Network, we calculated risk ratios derived from comparing weekly numbers of influenza cases prepeak with those postpeak during the 2010–2011 through 2018–2019 influenza seasons. We sought to determine age-specific differences during the ascent versus descent of an influenza season by influenza virus type and subtype. We estimated 95% credible intervals around the risk ratios using Bayesian joint posterior sampling of weekly cases. Our population consisted of ambulatory patients with laboratory-confirmed influenza who enrolled in an influenza vaccine effectiveness study at 5 US sites during 9 influenza seasons after the 2009 influenza A virus subtype H1N1 (H1N1) pandemic. We observed that young children aged <5 years tended to more often be infected with H1N1 during the prepeak period, while adults aged ≥65 years tended to more often be infected with H1N1 during the postpeak period. However, for influenza A virus subtype H3N2, children aged <5 years were more often infected during the postpeak period. These results may reflect a contribution of different age groups to seasonal spread, which may differ by influenza virus type and subtype.
Keywords: age distribution; disease transmission, infectious; epidemics; influenza, human; outpatients
Abbreviations
- CrI
credible interval
- H1N1
influenza A virus subtype H1N1
- H3N2
influenza A virus subtype H3N2
- IVB
influenza B virus
- RR
risk ratio
Influenza A virus subtypes H3N2 (H3N2) and H1N1 (H1N1) and influenza B virus (IVB) contribute to substantial morbidity and mortality during annual influenza epidemics (1, 2). An understanding of the dynamics of community influenza transmission is important, as increasing vaccination rates in groups that are affected early in an outbreak may change the seasonal course of the outbreak (1, 3, 4). Age has been used as a proxy for factors, such as contact patterns and susceptibility, that correlate with high transmission; school-aged children have been found to be key in early spread (3, 5). To investigate associations between age and timing of influenza cases, Worby et al. (4) proposed a ratio of incidence rates prepeak to those postpeak within age groups, which simplifies to a simple ratio (a risk ratio (RR)) comparing the number of cases before the maximum weekly cases in a season with the number observed after peak incidence within each age group. A larger proportion of cases occurring before peak incidence results in RR > 1 and is associated with epidemic propagation, while a greater proportion of cases occurring after peak incidence results in RR < 1. A higher RR may result from early depletion of susceptible individuals or particularly high virus transmission in that age group and the potential to drive epidemic spread and secondary infections to other age groups (4). We examined numbers of laboratory-confirmed influenza cases among outpatients enrolled in an influenza vaccine effectiveness study and calculated age-group–specific RRs to examine associations with differential timing of influenza infection across age groups and virus type or subtype.
METHODS
Study population
We analyzed data from the US Influenza Vaccine Effectiveness Study collected over 9 influenza seasons from 2010–2011 through 2018–2019 (6–9). Briefly, ambulatory patients aged ≥6 months with acute respiratory illness were enrolled in the study at health facilities associated with 4 network sites in Michigan, New York, Tennessee, and Wisconsin during 2010–2011 and 5 sites in Michigan, Pennsylvania, Texas, Washington, and Wisconsin during 2011–2012 through 2018–2019. Only participants enrolled during active influenza circulation in each geographic location who tested positive for influenza by means of reverse-transcription polymerase chain reaction assays developed by the Centers for Disease Control and Prevention (Atlanta, Georgia) were included in this analysis; influenza viruses were tested for type/subtype.
Statistical analysis
We determined the frequency of laboratory-confirmed influenza cases by season, influenza virus type/subtype, and age. We generated epidemic curves based on weekly case frequency across virus type/subtype and season and used G-tests to evaluate differences in the age distributions of cases by season (10). A Bonferroni-corrected P value was generated for each pairing of the 9 seasons; a P value less than 0.05 was considered statistically significant.
For each age group and season, risk ratios were calculated as numbers of cases with illness onset during prepeak weeks divided by numbers occurring postpeak, according to the method of Worby et al. (4). RR estimates were calculated separately by season, age group, and vaccination status. We defined age groups as 6 months–4 years (referred as <5 years), 5–17 years, 18–49 years, 50–64 years, and 65 years or older. Cases were aggregated into weekly case counts 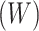 by week (t) of illness onset for each age group (g) by virus type/subtype
by week (t) of illness onset for each age group (g) by virus type/subtype 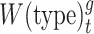 .
.
We defined the seasonal peak week of cases (tp) by influenza type/subtype as the week with the highest reported number of cases of that type/subtype. Cases occurring within the peak week and weeks tp ± 1 were excluded to reduce misclassification of cases as prepeak or postpeak. Thus, total prepeak cases were defined as the number of influenza cases from week 1 to week tp – 2 and total postpeak cases as the number of influenza cases starting from week tp + 2 to the end of seasonal enrollment. Prepeak and postpeak weekly case counts were summed and used to calculate an age-group–, type/subtype-, and season-specific RR:
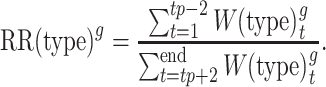 |
We compared RRs across age groups by calculating a type/subtype- and season-specific RR for all age groups combined and contrasted that with age-group–specific RR estimates:
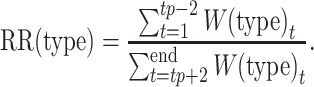 |
For seasons during which fewer than 30 virus type/subtype-specific cases were identified, RR estimates were not calculated. To estimate 95% credible intervals (CrIs), we adopted a Bayesian approach utilizing joint posterior sampling of our weekly case counts. As previously described (4), this method assumes that the number of detected outpatient cases is well approximated by a Poisson parameter, with mean proportional to the population incidence rate. Because the RR is a function of the parameter, one can generate samples of independent estimates for each quantity of interest from the corresponding posterior samples and generate CrIs. Statistical significance was defined as 95% CrIs that did not include 1.0.
RESULTS
During the 2010–2011 through 2018–2019 influenza seasons, 16,217 of 66,553 study participants had laboratory-confirmed influenza, including H3N2 (n = 8,615; 53%), H1N1 (n = 3,997; 25%), and IVB (n = 3,605; 22%) (Table 1). Of the 9 influenza seasons, H3N2 predominated during 5 seasons, H1N1 predominated during 2 seasons, and both H3N2 and H1N1 caused substantial illness during 2 seasons. IBVs occurred during seasons with coincident peaks (i.e., H1N1 and IVB in 2015–2016) and seasons with distinct peaks (i.e., H3N2 and IVB in 2016–2017). Within seasons, proportions of confirmed cases by virus type/subtype varied by time period (Table 1, Figure 1). Only 4 comparisons of seasonal age distributions reached statistical significance based on pairwise G-tests: age distributions of H1N1 cases in 2013–2014 and 2015–2016 and age distributions of H3N2 cases in several H3N2-predominant seasons (2011–2012 vs. 2018–2019; 2012–2013 vs. 2017–2018; and 2014–2015 vs. 2017–2018).
Table 1.
Numbers of Influenza Cases per Season Among Outpatients at US Influenza Vaccine Effectiveness Network Study Sites, by Influenza Season, Virus Type/Subtype, and Age, United States, 2010–2019
| Influenza Season | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | 2010–2011 | 2011–2012 | 2012–2013 | 2013–2014 | 2014–2015 | 2015–2016 | 2016–2017 | 2017–2018 | 2018–2019 | Total |
| Total | 666 | 706 | 2,275 | 1,179 | 2,258 | 1,306 | 2,029 | 3,053 | 2,745 | 16,217 |
| Virus type | ||||||||||
| H3N2 | 327 | 467 | 1,312 | 58 | 1,866 | 76 | 1,363 | 1,789 | 1,357 | 8,615 |
| H1N1 | 339 | 112 | 53 | 1,053 | 4 | 772 | 25 | 314 | 1,325 | 3,997 |
| IVB | 0 | 127 | 910 | 68 | 388 | 458 | 641 | 950 | 63 | 3,605 |
| Age group, years | ||||||||||
| <5 | 102 | 79 | 270 | 96 | 232 | 130 | 148 | 299 | 312 | 1,668 |
| 5–17 | 176 | 232 | 719 | 192 | 662 | 306 | 599 | 827 | 915 | 4,628 |
| 18–49 | 275 | 241 | 716 | 534 | 638 | 484 | 519 | 951 | 816 | 5,174 |
| 50–64 | 91 | 97 | 356 | 259 | 380 | 278 | 441 | 563 | 440 | 2,905 |
| ≥65 | 22 | 57 | 214 | 98 | 346 | 108 | 322 | 413 | 262 | 1,842 |
Abbreviations: H1N1, influenza A virus subtype H1N1; H3N2, influenza A virus subtype H3N2; IVB, influenza B virus.
Figure 1.
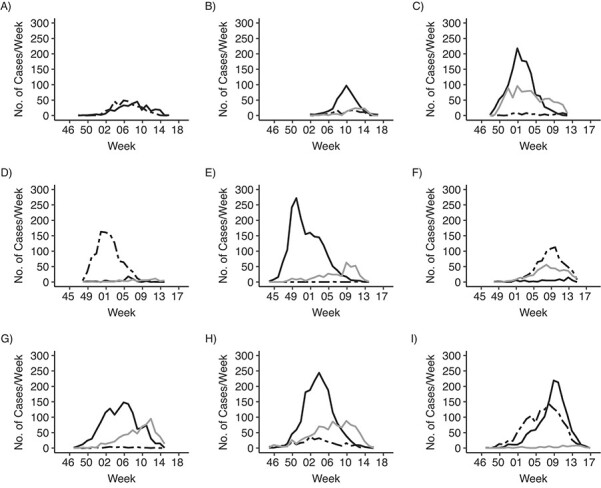
Weekly incidence of influenza among outpatients at US Influenza Vaccine Effectiveness Network study sites, by influenza season and virus type/subtype (solid black line, influenza A virus subtype H3N2; dashed line, influenza A virus subtype H1N1; gray line, influenza B virus), United States, 2010–2019. A) 2010–2011 influenza season; B) 2011–2012; C) 2012–2013; D) 2013–2014; E) 2014–2015; F) 2015–2016; G) 2016–2017; H) 2017–2018; I) 2018–2019.
When influenza cases due to all virus types/subtypes were combined, RR estimates derived from comparing incident cases prepeak with those postpeak showed no consistent age-dependent trends or patterns across seasons. When we stratified by virus type/subtype, RRs for children aged <5 years ranged from 1.05 (95% CrI: 0.69, 1.60) to 1.52 (95% CrI: 1.07, 2.19) during 3 years with substantial H1N1 circulation, indicating they were more likely to be infected prepeak than postpeak (Table 2). In contrast, RRs for adults aged ≥65 years were less than 1.0 in each season with substantial H1N1 circulation; however, the RR reached statistical significance only in the 2018–2019 season. For H3N2 seasons, RRs for children aged <5 years ranged from 0.61 (95% CrI: 0.41, 0.89) to 0.84 (0.61, 1.16), indicating that they were more likely to be infected with H3N2 postpeak than prepeak. Although the RR was statistically significant for only 1 of the 6 years, all 6 RRs were less than 1.0, and the RR was lower for children aged <5 years than for other age groups. For persons aged ≥5 years, findings were variable, with RRs greater than 1.0 and RRs less than 1.0 both being observed, including several seasons with statistically significant RRs. Cocirculation of H3N2 and H1N1 in the 2018–2019 season yielded similar results as single-influenza A–subtype seasons for children aged <5 years and adults aged ≥65 years. RRs for H1N1 and IVB cases among children aged <18 years during the 2015–2020 season tended to be greater than 1.0, indicating higher proportions of cases prepeak for both viruses that season. Trends in age-group–specific RRs for H3N2 and IVB viruses during the same seasons were inconsistent. We analyzed RR estimates for cases due to all virus types/subtypes by age group and vaccination status and found no consistent age-dependent trends or patterns across seasons (see Web Table 1, available at https://doi.org/10.1093/aje/kwab205).
Table 2.
Age-Group–Specific Risk Ratios Comparing Frequencies of Laboratory-Confirmed Influenza Cases Before Peak Influenza Incidencea With Those After Peak Incidence Among Outpatients at US Influenza Vaccine Effectiveness Network Study Sites, by Influenza Season and Virus Type/Subtype, United States, 2011–2019b
| Age Group, years | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| <5 | 5–17 | 18–49 | 50–64 | ≥65 | ||||||
|
Influenza Season |
RR | 95% CrI | RR | 95% CrI | RR | 95% CrI | RR | 95% CrI | RR | 95% CrI |
| Influenza A Virus Subtype H3N2 | ||||||||||
| 2011–2012 | 0.68 | 0.40, 1.11 | 1.17 | 0.89, 1.58 | 0.96 | 0.71, 1.28 | 1.08 | 0.68, 1.73 | 0.98 | 0.57, 1.69 |
| 2012–2013 | 0.61c | 0.41, 0.89 | 0.94 | 0.75, 1.18 | 0.96 | 0.82, 1.13 | 1.11 | 0.86, 1.42 | 1.52c | 1.11, 2.06 |
| 2014–2015 | 0.84 | 0.61, 1.16 | 1.80c | 1.44, 2.10 | 0.79c | 0.64, 0.96 | 0.92 | 0.71, 1.33 | 0.57c | 0.40, 0.76 |
| 2016–2017 | 0.74 | 0.51, 1.08 | 0.71c | 0.56, 0.97 | 1.21c | 1.00, 1.50 | 1.26 | 0.97, 1.60 | 1.06 | 0.77, 1.47 |
| 2017–2018 | 0.79 | 0.59, 1.06 | 0.81c | 0.58, 0.98 | 1.17c | 1.01, 1.37 | 1.17 | 0.93, 1.47 | 1.01 | 0.80, 1.29 |
| 2018–2019 | 0.79 | 0.56, 1.09 | 1.16 | 0.99, 1.42 | 0.95 | 0.78, 1.17 | 0.85 | 0.64, 1.15 | 1.03 | 0.74, 1.42 |
| Influenza A Virus Subtype H1N1 | ||||||||||
| 2013–2014 | 1.05 | 0.69, 1.60 | 0.86 | 0.56, 1.32 | 0.95 | 0.77, 1.12 | 1.27 | 0.93, 1.80 | 0.87 | 0.55, 1.42 |
| 2015–2016 | 1.16 | 0.79, 1.73 | 1.16 | 0.77, 1.78 | 0.98 | 0.81, 1.19 | 0.87 | 0.68, 1.14 | 0.98 | 0.59, 1.74 |
| 2018–2019 | 1.52c | 1.07, 2.19 | 0.97 | 0.79, 1.19 | 1.11 | 0.94, 1.33 | 0.86 | 0.69, 1.08 | 0.65c | 0.46, 0.99 |
| Influenza B Virus | ||||||||||
| 2012–2013 | 0.99 | 0.29, 1.48 | 1.10 | 0.91, 1.74 | 0.86 | 0.41, 1.16 | 0.86 | 0.45, 1.30 | 0.96 | 0.45, 1.73 |
| 2015–2016 | 1.31 | 0.71, 2.31 | 1.05 | 0.78, 1.52 | 0.73 | 0.53, 0.99 | 1.12 | 0.72, 1.82 | 1.29 | 0.74, 2.20 |
| 2016–2017 | 0.45c | 0.27, 0.79 | 0.92 | 0.71, 1.21 | 1.54c | 1.00, 2.55 | 1.27 | 0.86, 2.04 | 0.92 | 0.58, 1.54 |
| 2017–2018 | 1.57 | 0.91, 2.86 | 1.39c | 1.08, 1.92 | 1.03 | 0.82, 1.38 | 0.65c | 0.51, 0.82 | 0.92 | 0.64, 1.26 |
Abbreviations: CrI, credible interval; RR, risk ratio.
a Peak influenza incidence of each influenza virus type or subtype was defined separately for each season as the week with the largest number of laboratory-confirmed influenza cases, according to date of symptom onset.
b Influenza virus type- and subtype-specific RRs were not calculated for seasons that had fewer than 30 cases due to a specific virus type or subtype in every age group.
c Results reached statistical significance (P < 0.05).
DISCUSSION
For children aged <5 years, we found a trend toward a higher proportion of cases due to H1N1 before peak incidence during 3 seasons with substantial H1N1 circulation. We found that higher proportions of H3N2 cases in the <5-years age group occurred after peak incidence during seasons with substantial H3N2 circulation, but no age group consistently had more H3N2 cases before peak seasonal incidence. Among adults aged ≥65 years, higher proportions of H1N1 cases occurred after peak incidence during seasons with substantial H1N1 circulation. Our results show that timing of infections in the community vary by age during seasonal epidemics depending on the circulating influenza virus, and they add to the findings of previous studies that have explored associations between age-group–specific incidence and epidemic spread (4, 11–14). Factors other than age, including prior exposure to specific influenza viruses and subsequent immune responses, may affect preepidemic susceptibility and patterns of viral spread during a seasonal epidemic. This would result in differential depletion of susceptible populations during a season, with those affected during the ascent having lower incidence during the descent, potentially allowing for time-dependent interventions for certain populations.
Results from this analysis of outpatient data over 9 influenza seasons after the 2009 H1N1 pandemic contrast with age-specific patterns observed among hospitalized patients (4). Using data from laboratory-based surveillance for influenza-associated hospitalizations, Worby et al. (4) found that school-aged children were disproportionately represented in the prepeak period of influenza seasons. In our analyses using systematic testing of outpatient cases, patterns among children aged <5 years varied by influenza A virus subtype, tending to occur early in H1N1-predominant seasons but later in H3N2 seasons. Both our analyses and those of Worby et al. included the 2011–2012 and 2012–2013 H3N2-predominant seasons and the 2013–2014 H1N1-predominant season, allowing for some seasonal comparison. During these 3 seasons, RRs among outpatients aged <18 years ranged from 0.61 to 1.17, while RRs for inpatients ranged from 1.21 to 3.03 in H1N1 and H3N2 seasons; conversely, RRs among outpatients aged ≥65 years ranged from 0.87 to 1.52, while RRs for inpatients ranged from 0.39 to 0.94 (4).
Intraseason differences in timing of illness between age groups may be related to infections with influenza viruses early in life, known as immune imprinting or a birth cohort effect. With imprinting, an individual’s immune responses to influenza viruses encountered later in life are influenced by early infections, potentially affecting susceptibility to illness from homosubtypic or heterosubtypic viruses (11–13). Priming infection with H3N2 may relate to increased susceptibility to H1N1 and earlier infection during H1N1 seasons (14); conversely, persons aged ≥65 years were likely primed with H1N1 and would be more susceptible to H3N2 than other age groups, resulting in higher proportions of H1N1 cases postpeak among persons aged ≥65 years. Effects of imprinting may relate more to severe illness than to mild illness, if primary infections attenuate severe illness after secondary infections later in life to a greater extent than mild illness (14, 15). Additionally, this effect may not be limited to homosubtypic viruses but may also apply to drifted viruses within the same subtype (16–19). In addition, factors such as differential rates of antigenic change in H1N1 and H3N2 viruses may contribute to age-specific risk of influenza (20, 21).
This analysis had several limitations. Despite large numbers of laboratory-confirmed cases, stratification to discern age- and virus-specific associations yielded imprecise estimates with wide CrIs. Few estimates were statistically significant, and interpretations were based on consistency of trends within and across age groups. Second, peak influenza incidence for each season may have differed by study site. To account for geographic heterogeneity, we used a 3-week interval rather than a single week to define seasonal peak incidence. Third, epidemic curves were truncated at some study sites during prolonged influenza seasons due to enrollment’s ending before influenza virus circulation. Additionally, the study sites included in our analysis may not be representative of the US population as a whole, and population characteristics may influence the timing and age distribution of influenza cases during seasonal epidemics.
Our findings suggest that varying age group susceptibility by virus type/subtype contributes to early infections and that a single age group may not consistently drive the early spread of influenza to other age groups. Rather, drivers of transmission likely vary by season and circulating influenza viruses and may be affected by factors including prior infection and immunity. The complex role of these factors in the propagation of annual influenza epidemics warrants further investigation. Closer inspection of age distributions by virus type and potential effects of influenza vaccination on population susceptibility and disease transmission may require larger data sets with laboratory-confirmed outcomes. Greater understanding of susceptibility and its effects on epidemic propagation may help to predict the course of future epidemics.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Influenza Division, Centers for Disease Control and Prevention, Atlanta, Georgia, United States (Eric P. Griggs, Brendan Flannery, Ivo M. Foppa, Jessie R. Chung, Manish Patel); Baylor Scott & White Health, Temple, Texas, United States (Manjusha Gaglani, Kempapura Murthy); Department of Medical Education, College of Medicine, Texas A&M University, Temple, Texas, United States (Manjusha Gaglani); Kaiser Permanente Washington Health Research Institute, Seattle, Washington, United States (Michael L. Jackson, Lisa A. Jackson); Marshfield Clinic Research Institute, Marshfield, Wisconsin, United States (Edward A. Belongia, Huong Q. McLean); Department of Epidemiology, School of Public Health, University of Michigan, Ann Arbor, Michigan, United States (Emily T. Martin, Arnold S. Monto); Department of Family Medicine, School of Medicine, University of Pittsburgh, Pittsburgh, Pennsylvania, United States (Richard K. Zimmerman); and Department of Epidemiology, Graduate School of Public Health, University of Pittsburgh, Pittsburgh, Pennsylvania, United States (Goundappa K. Balasubramani).
This work was supported by the Centers for Disease Control and Prevention (CDC) through cooperative agreements U01IP001034–U01IP001039, along with cooperative agreements with the University of Michigan (U01 IP000474), Kaiser Permanente Washington Health Research Institute (U01 IP000466), the Marshfield Clinic Research Foundation (U01 IP000471), the University of Pittsburgh (U01 IP000467), and Baylor Scott & White Health (U01 IP000473), and by the National Institutes of Health (grants UL1RR024153 and UL1TR000005 to the University of Pittsburgh).
We thank Drs. Sarah Cobey and Phillip Arevalo of the University of Chicago Department of Ecology and Evolution for their critical review of an early version of this article and subsequent feedback. We also thank the individuals who participated in this study and the research staff at all study sites: Sarah Bauer, Kim Beney, Caroline K. Cheng, Amy Getz, Michelle Groesbeck, Emileigh Johnson, Anne Kaniclides, Armanda Kimberly, Lois E. Lamerato, Ryan E. Malosh, E. J. McSpadden, Joshua G. Petrie, Hannah Segaloff, and Rachel Truscon (University of Michigan, Ann Arbor, Michigan, and Henry Ford Health System, Detroit, Michigan); Jennifer P. King (Marshfield Clinic Research Institute, Marshfield, Wisconsin); Rose Azrak, Todd M. Bear, Duane Eisaman, Heather Eng, Andrew Fackler, Edward Garofolo, Robert Hickey, Philip Iozzi, Monika Johnson, Stephanie Kirk, Jason A. Lyons, Donald B. Middleton, Krissy K. Moehling, Mary P. Nowalk, Jonathan M. Raviotta, Evelyn C. Reis, Bret Rosenblum, Sean Saul, Theresa Sax, Michael Susick, Joe Suyama, Leonard F. Urbanski, Alexandra Weissman, and John V. Williams (University of Pittsburgh Schools of the Health Sciences and University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania); Alejandro Arroliga, Madhava Beeram, Wencong Chen, Lydia Clipper, Kayan Dunnigan, Eric Hoffman, Angela Kennedy, Mary Kylberg, Amanda McKillop, Manohar Mutnal, Teresa O’Quinn, Chandni Raiyani, Arundhati Rao, Michael Reis, Anne Robertson, Spencer Rose, Natalie Settele, Courtney Shaver, Michael Smith, Jennifer Thomas, Marcus Volz, Kimberly Walker, Jamie Walkowiak, Martha Zayed, and Tnelda Zunie (Baylor Scott and White Health, Temple, Texas); Rachael Burganowski, Erika Kiniry, Matt Nguyen, Suzie Park, C. Hallie Phillips, Stacie Wellwood, and Brianna Wickersham (Kaiser Permanente Washington Health Research Institute, Seattle, Washington); and research staff at Vanderbilt University School of Medicine and the University of Rochester Medical Center for participation in 2010–2011.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC. Vaccination data from Pennsylvania were supplied by the Bureau of Health Statistics and Registries, Pennsylvania Department of Health (Harrisburg, Pennsylvania). The Pennsylvania Department of Health specifically disclaims responsibility for any analyses, interpretations, or conclusions.
M.G. reports receiving grants from the CDC during the conduct of the study and grants from Janssen/Johnson & Johnson (New Brunswick, New Jersey) and Abt Associates Inc. (Rockville, Maryland) outside the scope of this work. M.L.J. reports receiving grants from the CDC during the conduct of the study and grants from Sanofi-Pasteur S.A. (Lyon, France) outside the scope of this work. L.A.J. reports receiving grants from the CDC during the conduct of the study and an organization research contract with Pfizer, Inc. (New York, New York) to fund clinical trials and studies outside the scope of this work. E.A.B. reports receiving grants from the CDC during the conduct of the study. H.Q.M. reports receiving grants from the CDC during the conduct of the study and grants from Seqirus (Maidenhead, United Kingdom) outside the scope of this work. E.T.M. reports receiving personal fees from Pfizer and grants from Merck & Co., Inc. (Kenilworth, New Jersey) outside the scope of this work. A.S.M. reports receiving consulting fees from Sanofi-Pasteur and Seqirus. R.K.Z. reports receiving grants from the CDC during the conduct of the study and grants from Merck and Sanofi-Pasteur outside the scope of this work. G.K.B. reports receiving grants from Merck outside the scope of this work and consulting fees from New World Medical Inc. (Cucamonga, California). All of the other authors report no potential conflicts of interest.
REFERENCES
- 1. Goldstein E, Apolloni A, Lewis B, et al. Distribution of vaccine/antivirals and the ‘least spread line’ in a stratified population. J R Soc Interface. 2010;7(46):755–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rolfes MA, Foppa IM, Garg S, et al. Annual estimates of the burden of seasonal influenza in the United States: a tool for strengthening influenza surveillance and preparedness. Influenza Other Respi Viruses. 2018;12(1):132–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wallinga J, van Boven M, Lipsitch M. Optimizing infectious disease interventions during an emerging epidemic. Proc Natl Acad Sci U S A. 2010;107(2):923–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Worby CJ, Chaves SS, Wallinga J, et al. On the relative role of different age groups in influenza epidemics. Epidemics. 2015;13:10–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schanzer D, Vachon J, Pelletier L. Age-specific differences in influenza A epidemic curves: do children drive the spread of influenza epidemics? Am J Epidemiol. 2011;174(1):109–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McLean HQ, Thompson MG, Sundaram ME, et al. Influenza vaccine effectiveness in the United States during 2012–2013: variable protection by age and virus type. J Infect Dis. 2015;211(10):1529–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ohmit SE, Thompson MG, Petrie JG, et al. Influenza vaccine effectiveness in the 2011–2012 season: protection against each circulating virus and the effect of prior vaccination on estimates. Clin Infect Dis. 2014;58(3):319–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kim SS, Flannery B, Foppa IM, et al. Effects of prior season vaccination on current season vaccine effectiveness in the United States Flu Vaccine Effectiveness Network, 2012–2013 through 2017–2018. Clin Infect Dis. 2021;73(3):497–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Treanor JJ, Talbot HK, Ohmit SE, et al. Effectiveness of seasonal influenza vaccines in the United States during a season with circulation of all three vaccine strains. Clin Infect Dis. 2012;55(7):951–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Arevalo P, McLean HQ, Belongia EA, et al. Earliest infections predict the age distribution of seasonal influenza A cases. eLife. 2020;9:e50060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cobey S, Hensley SE. Immune history and influenza virus susceptibility. Curr Opin Virol. 2017;22:105–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Monto AS, Malosh RE, Petrie JG, et al. The doctrine of original antigenic sin: separating good from evil. J Infect Dis. 2017;215(12):1782–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gostic KM, Bridge R, Brady S, et al. Childhood immune imprinting to influenza A shapes birth year-specific risk during seasonal H1N1 and H3N2 epidemics. PLoS Pathog. 2019;15(12):e1008109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Budd AP, Beacham L, Smith CB, et al. Birth cohort effects in influenza surveillance data: evidence that first influenza infection affects later influenza-associated illness. J Infect Dis. 2019;220(5):820–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gostic KM, Ambrose M, Worobey M, et al. Potent protection against H5N1 and H7N9 influenza via childhood hemagglutinin imprinting. Science. 2016;354(6313):722–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Belongia EA, McLean HQ. Influenza vaccine effectiveness: defining the H3N2 problem. Clin Infect Dis. 2019;69(10):1817–1823. [DOI] [PubMed] [Google Scholar]
- 17. Flannery B, Smith C, Garten RJ, et al. Influence of birth cohort on effectiveness of 2015–2016 influenza vaccine against medically attended illness due to 2009 pandemic influenza A(H1N1) virus in the United States. J Infect Dis. 2018;218(2):189–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Skowronski DM, Chambers C, Sabaiduc S, et al. Beyond antigenic match: possible agent-host and immuno-epidemiological influences on influenza vaccine effectiveness during the 2015–2016 season in Canada. J Infect Dis. 2017;216(12):1487–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Skowronski DM, Sabaiduc S, Leir S, et al. Paradoxical clade- and age-specific vaccine effectiveness during the 2018/19 influenza A(H3N2) epidemic in Canada: potential imprint-regulated effect of vaccine (I-REV). Euro Surveill. 2019;24(46):1900585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bedford T, Riley S, Barr IG, et al. Global circulation patterns of seasonal influenza viruses vary with antigenic drift. Nature. 2015;523(7559):217–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gouma S, Kim K, Weirick ME, et al. Middle-aged individuals may be in a perpetual state of H3N2 influenza virus susceptibility. Nat Commun. 2020;11(1):4566. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


