Abstract
In vitro follicle development (IVFD) is an adequate model to obtain basic knowledge of folliculogenesis and provides a tool for ovarian toxicity screening. IVFD yielding competent oocytes may also offer an option for fertility and species preservation. To promote follicle growth and oocyte maturation in vitro, various culture systems are utilized for IVFD in rodents, domestic animals, wild animals, nonhuman primates, and humans. Follicle culture conditions have been improved by optimizing gonadotropin levels, regulatory factors, nutrient supplements, oxygen concentration, and culture matrices. This review summarizes quality assessment of oocytes generated from in vitro-developed antral follicles from the preantral stage, including oocyte epigenetic and genetic profile, cytoplasmic and nuclear maturation, preimplantation embryonic development following in vitro fertilization, as well as pregnancy and live offspring after embryo transfer. The limitations of oocyte quality evaluation following IVFD and the gaps in our knowledge of IVFD to support proper oocyte development are also discussed. The information may advance our understanding of the requirements for IVFD, with a goal of producing competent oocytes with genetic integrity to sustain embryonic development resulting in healthy offspring.
Keywords: oocyte quality, oocyte maturation, oocyte competence, in vitro follicle development, follicle culture
Although advances in current IVFD systems have greatly enhanced oocyte growth and maturation, further optimization is required to improve oocyte competence with genetic integrity for proper embryonic development.
Introduction
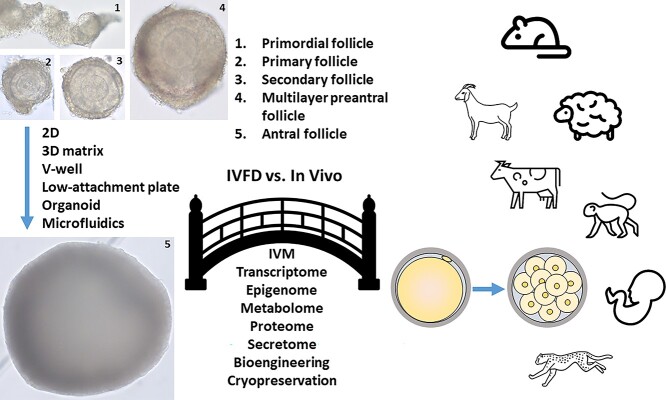
In vitro follicle development (IVFD) yielding a mature oocyte is a complex process worth pursuing for many applications. Because individual or groups of follicles can be monitored and manipulated at specific developmental stages in controlled environments, follicle culture is an adequate model to obtain basic knowledge of folliculogenesis and oogenesis [1]. It also provides a tractable system for screening potential ovarian toxic compounds or endocrine disruptors [2, 3]. If mature oocytes derived from in vitro-developed follicles are capable of acquiring meiotic and developmental competence, as well as maintaining genetic integrity, follicle culture may offer an option for fertility preservation in women [4, 5], for production of genetically important livestock breeds [6], and for propagation of endangered species [7].
There are many excellent reviews on IVFD in mice [8–11], domestic animals [9, 12], wild animals [7], nonhuman primates, and women [1, 4, 13]. Recently, the numerous experimental systems (media, matrices, hormone supplementation, etc.) used to support ovarian follicle growth in vitro from a variety of mammalian species has been thoroughly reviewed [14, 15]. The present review will not describe primordial follicle culture in-depth because the complete complement of factors that activate and then propel the progression of primordial follicles to the primary stage are not entirely known, and the majority of studies only conduct culture for short intervals that do not culminate in a mature oocyte [16–19]. In vitro gametogenesis from induced pluripotent stem cells is also being pursued as another avenue to obtain competent oocytes; the current state of this rapidly developing field has been recently reviewed [20] and will not be discussed further herein. Organ culture followed by follicle isolation in neonatal mice and culture of ovarian cortical tissue prior to removal of primary or secondary follicles for further IVFD are presented.
Oocyte developmental competence is typically defined as the ability of an oocyte (1) to resume and complete nuclear maturation with correct formation of the meiotic spindle and chromosome trafficking that are necessary for normal fertilization; (2) to undergo cytoplasmic maturation that includes extensive remodeling of intracellular organelles, biogenesis of maternal RNA, and establishment of epigenetic profile necessary for supporting early embryonic events prior to activation of the embryonic genome, as well as organization of a specialized cytoplasmic lattice that assists with storage of maternal RNA and important RNA translation events needed for protein synthesis; and (3) to support embryonic development after fertilization, establishment of pregnancy, and birth of live offspring [21]. Many of the paracrine mechanisms underlying the continuous dialog between the gamete and somatic cells are known [22], but there are equally important signaling mechanisms involving cell–cell interaction via gap junctions, intercellular contact via transzonal projections (TZPs) and oocyte-derived microvilli, and remodeling of the oocyte cortical cytoplasm by receptor tyrosine kinases [23, 24]. The follicle with its enclosed oocyte is characterized by highly specialized, adjacent cellular compartments that are exquisitely metabolically coupled in ways that are absolutely requisite for establishing developmental competence. Collectively, these processes are extremely complex and our understanding of the biological function of many recently identified factors required for coordinated development of the oocyte and somatic cells within the ovarian follicle is incomplete [22]. For the purposes of IVFD, maintaining this coordinated signaling in a temporal fashion during the progression of transitions from one stage of follicular development to another is paramount for supporting oocyte competence, and is challenging to achieve.
This review focuses primarily on the literature that describes (1) the ability of oocytes enclosed in preantral follicles grown in vitro to acquire meiotic and/or developmental competence resulting in preimplantation embryos or live offspring; (2) the limitations inherent in reprising the various mechanisms underlying the coordinated interactions between the oocyte and somatic cells within their given in vitro environment to allow for production of competent oocytes; and (3) the gaps in our knowledge of IVFD that, when eventually filled, will have a great potential to advance our understanding of the requirements for achieving oocyte developmental competence to improve fertility in a number of species, including humans.
MII oocytes derived from in vitro-developed rodent follicles are competent for producing live offspring
Multiple culture systems support growth of immature murine follicles in vitro that have produced oocytes capable of maturation and fertilization with subsequent development into live offspring. Two major systems have been employed. With two-dimensional (2D) culture, follicle integrity is not preserved; granulosa cell (GC)–oocyte complexes are grown on a flat surface till oocytes are released for maturation in vitro. In contrast, three-dimensional (3D) culture preserves follicle architecture with resultant antrum formation. The follicle-enclosed cumulus–oocyte complex (COC) is exposed to ovulatory stimuli in vitro to induce oocyte meiosis resumption. In both systems, the close contact between the oocyte and its surrounding somatic cells is kept intact as this is absolutely necessary for folliculogenesis to proceed [25, 26]. Conventional in vitro fertilization (IVF) is typically used to assess embryonic development and in some cases, developmental competence in vivo to live births. Table 1 depicts the development of 2D and 3D systems for IVFD of preantral follicles from mice that yielded competent oocytes that matured, fertilized, and yielded embryos and/or live offspring. The efficacy of offspring production is indicated when available in the literature.
Table 1.
Maturation and competence of oocytes derived from cultured follicles in mice
| Strain | Age | Initial follicle stage and isolation method | Culture system and interval | Basal media, supplement and O2 level | Oocyte assessment and outcomes | References |
|---|---|---|---|---|---|---|
| B6SJLF1 | Postnatal Day 0 |
Ovarian organ culture followed by collagenase/DNase isolation of oocyte-granulosa cell complexes from primordial follicles; |
Original protocol
1. Ovarian tissue culture for 8 days; 8 ovaries per membrane insert for 8 days 2. Granulosa cell-oocyte complexes individual culture on transwell membranes in wells for 8 days; 3. Granulosa cell-oocyte complexes individual culture on membranes in wells for 6 days 4. Granulosa cell–oocyte complexes individual culture on membranes for 15 h–17 h |
Original protocol
1. Waymouth + pyruvic acid, 3 mg/ml BSA, 1 mg/ml fetuin, 5 μg/ml insulin, 0.5 ng/ml rhFSH, 1 ng/ml EGF 2. Waymouth + pyruvic, 3 mg/ml BSA, fetuin, 100 ng/ml rhFSH Revised protocol 3. αMEM, 3 mg/ml BSA, 1 mg/ml fetuin, 5 μg/ml insulin 4. αMEM, 3 mg/ml BSA, 1 mg/ml fetuin, 5 μg/ml insulin,100 ng/ml rhFSH, 1 ng/ml EGF 1–4: 5% O2, 5% CO2, 90% N2 |
Original protocol 17% MII 33% cleaved 2-cell 15% blastocyst; n = 20 Hatching blastocysts rarely seen Revised protocol 44% MII 53% cleaved 2-cell 23% blastocyst; n = 75 Hatching blastocysts observed 59/1160 (5.7%) live pups |
[30] |
| C57BL/6 × CBA/Ca | 32-day old | Primary follicles Mechanical |
1. Individual culture in 96-well V- or U-well plates for 5–6 days 2. Individual culture with or without rhLH on final day of culture 3. COCs removed, IVM with or without EGF |
1. αMEM, 5% serum from 3–4 week old mice, 5 μg/ml insulin, 1 IU/ml rhFSH, 2. 0 or 1 IU rhLH added on Day 5 3. Leibovitz, 5% mouse serum, 15 mg/ml BSA or M16, 5% FCS, 1 IU/ml rhFSH, 10 ng/ml EGF 5% CO2 in air |
0% 2-cell or blastocyst without EGF 9% 2-cell, 100% blastocyst with EGF Day 6 41% 2-cell, 100% blastocyst with EGF Days 5–6 1 live pup from IVM + EGF |
[31] |
| C57BL/6 × CBA/Ca | 14-day old | Preantral – primary/ early secondary Pederson type 3b and 4 (100–130 μm diameter) Mechanical |
Individual culture in droplets under oil for up to 14 days; 12 days optimal 20 follicles/60 mm Petri dish |
1. αMEM glutamax, 5% heat-inactivated FBS, 5 μg/ml insulin; with 100 mIU/ml rhFSH or 100 mIU/m rhFSH +10 mIU/ml rhLH or100 mIU/ml rhFSH +100 mIU rhLH 2. IVM or ovulatory stimulus of 5 ng/ml hCG + 5–50 ng/ml EGF 5% CO2 in air |
23–30% MII on Day 9 without ovulatory stimulus 60% MII with ovulatory stimulus regardless of rhFSH alone or rhFSH + rhLH treatment 50% fertilization in vitro 50% hatched blastocysts Live offspring obtained (efficiency unknown) |
[32, 33] |
| C57BL/6 × CBA/Ca | 16-day old | Multi-layer secondary Pederson Type 5b (150–180 μm diameter) Two-layer secondary Mechanical |
1. 0.25–1.5% (w/v) alginate-embedded individual culture in 96-well plates for 8 days; followed by maturation 2. Alginate lyase to recover follicles for ovulatory maturation |
1. αMEM, 3 mg/ml BSA, 5 μg/ml insulin, fetuin,10 mIU/ml rhFSH 2. αMEM, 10% FCS, 1.5 mIU/ml hCG, 5 ng/ml EGF COC removed, 5% CO2 in air |
Multi-layer secondary in 1.5% alginate: 70/99 (71%) MII 60/86 (68%) fertilized 20 zygotes transferred to recipients 4 normal, fertile offspring (20%) Two-layer secondary in 0.25% alginate: 32/76 (42%) 2-cell embryos 9/32 (29%) blastocysts |
[35, 36] |
| CF1 B6SJLF1 |
12-day old 12-day old |
Secondary; mechanical Early secondary; collagenase digestion |
1. 0.25% (w/v) alginate-embedded individual culture in 96-well plates for 10 or 12 days 2. Alginate lyase to recover follicles for ovulatory maturation 1a. Collagen-impregnated membranes for 10 days 1b. Oocyte maturation of COCs |
1. αMEM, 3 mg/ml BSA, 5 μg/ml insulin, 1 mg/ml fetuin, 10 mIU/ml rhFSH 2. αMEM, 10% FCS, 1.5 IU/ml hCG, 5 ng/ml EGF 1a. αMEM, 3 mg/ml BSA, 1 mg/ml fetuin, 5 μg/ml insulin 1b. αMEM, 3 mg/ml BSA, 1 mg/ml fetuin, 10 ng/ml EGF 5% CO2 in air |
Secondary 3D culture: 100/134 (75%) MII 19/100 (19%) fertilization 1/19 (5%) blastocyst rate Early secondary 2D culture: 64% MII 81% fertilization |
[37] |
| C57BL/6 × CBA/Ca | 8-day old | Ovarian organ culture followed by dissection of individual secondary follicles Mechanical |
1. Ovaries placed on well inserts in 24-well plates, 6 ovaries per well, for 4 days 2. 0.25% (w/v) alginate or fibrin-0.25% (w/v) alginate-embedded culture in 96-well plates for 12 days 3. Cumulus-enclosed oocytes removed from antral follicles for maturation |
1–2. αMEM, 3 mg/ml BSA, 1 mg/ml fetuin, 5 ng/ml insulin, 10 mIU/ml rhFSH, 1. αMEM, 10% FCS, 1.5 mIU/ml rhFSH, 5 ng/ml EGF 5% CO2 |
Alginate 59/96 (61%) MII 19/59 (33%) 2-cell embryos Fibrin-alginate 44/50 (88%) MII 24/44 (54%) 2-cell embryos |
[38] |
| BDF1 from C57BL/6 females × DBA/2 males | 16-day old 6-day old |
Preantral follicles (125–140 μm diameter) Primary/early secondary Pederson type 3a and 3b (<100 μm diameter) Mechanical |
1. Embedded in collagen gel for 9 days 2. Removed from gel with collagenase, transferred to 12-well plates with collagen-coated membranes for 8 days 3. Follicles removed from membrane, induce oocyte maturation |
1. αMEM, 5% FCS, 10 μg/ml insulin, 1 mIU/ml rhFSH, 1 ng/ml EGF 2. αMEM, 5% FCS, 10 μg/ml insulin, 100 mIU/ml rhFSH 3. Ovulatory stimulus of 2.5 mIU/ml hCG, 10 ng/ml EGF 1–2: 5% O2, 5% CO2, 90% N2 |
Preantrals 80/134 (60%) MII 47/80 (59%) fertilized 42/47 (89%) 2-cell 130 two-cell embryos cryopreserved; 53 transferred to 4 recipients; 3 pregnant; 2 live fertile offspring (4%) Early Secondary – no MII, no embryo/ET data |
[39] |
| B6D2F1 (C57BL/6 N × DBA/2) | 10-day old | Secondary follicles Mechanical |
1. 0.1% collagenase after isolation, placed on membrane in well plates for 12 days 2. COC removed from antral follicles for maturation |
1. αMEM, 2% PVP, 5% FBS, 0.1 IU/ml rhFSH; tested optimal MW for PVP and optimal O2 2. αMEM, 5% FBS, 0.1 IU/ml rhFSH, 1.2 IU/ml hCG, 4 ng/ml EGF 5% CO2 in air for PVP experiments 5, 7, 10 and 20% O2 for oxygen experiments |
PVP 360 K 164/230 (71%) MII 49/82 (60%) blastocyst PVP 1300 K 179/238 (75%) MII 34/54 (63%) blastocyst 7% O2 in PVP 360 K 277/366 (76%) MII 58/69 (84%) blastocyst 20% O2 in PVP 360 K 273/294 (93%) MII 70/117 (60%) blastocyst |
[40] |
| CBA × C57BL/6 | 12 days | Secondary (type 4) Mechanical |
1. 0.15% (w/v) alginate-encapsulation 2. COC removed for IVM 12 days |
1. αMEM, 5% FCS, 10 μg/ml insulin, 100 mIU/ml rhFSH, 10 mIU/ml rhLH, 1 mM L-carnitine 2. αMEM, 10% FCS, 1.5 mIU/ml hCG 6% CO2 in 94% air |
~50% MII ~40% 2-cell 2.3-fold increase in fertilization over control 40% blastocyst from surviving follicles 53% blastocyst from antral follicles 3.1-fold increase in blastocyst over control |
[98] |
2D, 2-dimensional; 3D, 3-dimensional; BSA, bovine serum albumin; CO2, carbon dioxide; COC, cumulus–oocyte complex; DNase, deoxyribonuclease; EGF, epidermal growth factor; ET, embryo transfer; FBS, fetal bovine serum; FCS, fetal calf serum; hCG, human chorionic gonadotropin; IVM, in vitro maturation; rhFSH, recombinant human follicle-stimulating hormone; rhLH, recombinant human luteinizing hormone; M16, media 16; αMEM, alpha minimum essential medium; MII, metaphase II; MW, molecular weight; N2, nitrogen; O2, oxygen; PVP, polyvinylpyrrolidone.
Early methods for derivation of oocytes from in vitro-developed follicles included culture of GC–oocyte complexes on collagen-coated membranes wherein 50% of two-cell embryos developed to the blastocyst stage [27, 28]. In the protocol used to produce Eggbert, the first mouse born from 2D primordial follicle culture in the U.S. [29], less than 2% of two-cell stage embryos developed to the blastocysts. Furthermore, oocyte competence from these early endeavors was compromised since Eggbert developed late onset obesity and neurological anomalies. The animal was later joined by 59 murine offspring derived from oocytes grown in vitro from primordial follicles in a two-step protocol (Table 1) [30]. Improvements to the protocol included the use of alpha minimum essential medium (αMEM) as well as omission of follicle stimulating hormone (FSH) during the final 6 days of culture and during the interval for oocyte maturation that resulted in higher quality oocytes capable of nuclear, cytoplasmic, and epigenetic maturation. Although the above hallmarks of oocyte competence were lower in oocytes from IVFD than those derived in vivo, these experiments provided the earliest proof that oocytes with full developmental potential can be produced in vitro from primordial follicles.
Spears et al. obtained the first murine offspring from an intact primary follicle grown in vitro in a low attachment environment (Table 1) [31]. Despite the low birth rate after embryo transfer, all of the two-cell embryos produced under the tested experimental conditions developed to blastocysts, indicating that high-quality oocytes were generated from primary follicles grown in vitro. However, the efficacy of this system for consistent production of offspring had not been rigorously tested. Optimal oocyte survival in preantral follicles (presumably secondary follicles) grown in vitro under 2D conditions was sustained for 12 days. Oocyte meiotic capacity on Days 6 and 8 of culture was enhanced with the addition of luteinizing hormone (LH) to FSH throughout the culture interval [32]. Exposure to an ovulatory stimulus of human chorionic gonadotropin (hCG) in combination with epidermal growth factor (EGF) increased fertilization rates and blastocyst formation rates in vitro [33]. Live offspring were reported, although the efficiency of offspring production is unknown [34].
IVFD then involved analyzing encapsulation matrices that maintained the 3D follicle structure while allowing follicles to grow. Multilayer secondary follicles from mice that were encapsulated in an alginate hydrogel matrix for culture displayed morphological and functional characteristics that mimicked in vivo-derived follicles. Importantly, secondary follicles embedded in alginate could produce competent oocytes that underwent nuclear maturation, fertilization via IVF and subsequent development to yield normal, viable offspring after embryo transfer (Table 1) [35]. Furthermore, this early report of live birth rates from alginate-encapsulated 3D follicle culture was similar (20%) to those reported for 2D culture systems. Extending these observations to two-layer secondary follicles helped to identify the optimal and permissive concentration of alginate for IVFD to support oocyte competence [36]. Less rigid environments, while having no effect on follicle survival per se, improved GC proliferation, antrum formation, and steroid production. Importantly, 0.25% alginate supported the accelerated follicle growth from the preantral to antral stage, a critical time for RNA and protein accumulation necessary for subsequent oocyte maturation and embryonic development. Oocytes obtained from secondary follicles enclosed in 0.25% alginate retained the highest developmental competence that led to the highest two-cell embryo and blastocyst formation rates (29%), though the capacity for offspring production was not assessed in this study.
In a robust experiment, Mainigi et al. compared maturation, fertilization, and blastocyst formation rates in murine oocytes derived from alginate-encapsulated and 2D secondary follicle culture systems to those obtained in vivo via superovulation (Table 1) [37]. Although nuclear maturation rates were similar, oocytes obtained from alginate-encapsulated culture had lower fertilization rates compared with those from 2D culture. It could be due to differences in the final oocyte maturation protocols. For example, oocytes were matured within the follicle during the 3D culture, whereas COCs were obtained for in vitro maturation (IVM) of oocytes after the 2D culture. There were also differences in serum supplements and concentrations of EGF in the maturation media. Nonetheless, the blastocyst formation rate after 3D culture was similar to that observed in the original experiments in mice [36]. No differences were noted between the 2D and 3D culture systems with regard to the oocyte transcriptome. However, when compared with oocytes developed in vivo, oocytes derived from follicles grown in either 3D or 2D culture exhibited an increased incidence of spindle defects and chromosomal misalignments that likely contributed to the subpar developmental competence. The authors conclude that while alginate-based systems are not efficient for maintaining oocyte competence, they are still useful for investigating coordinated interactions between GC and theca cells.
Organ culture of neonatal mouse ovaries followed by isolation of secondary follicles was used to compare IVFD outcomes after encapsulation in alginate alone or fibrin-alginate (Table 1) [38]. As in prior studies tracking alginate-enclosed secondary follicles, follicle growth in vitro was accompanied by oocyte growth and steroid production, with oocytes derived from follicles grown in fibrin-alginate exhibiting a greater propensity for fertilization and development to two-cell embryos, albeit at rates similar to previous reports using alginate alone. This two-step system harkened back to the earlier studies by Eppig and colleagues with the advantage of starting from primordial follicles followed by subsequent development of intact follicles to the antral stage [30], but did not improve oocyte competence overall.
Using collagen gel as an extracellular matrix for IVFD of murine secondary follicles, Mochida et al. reported that a two-step system was necessary to support normal follicle growth (Table 1) [39]. Follicles initially embedded in collagen required to be released and cultured on a collagen layer for antrum formation to support oocyte nuclear maturation, fertilization, and developmental competence. Two live, fertile offspring were obtained from this system. Recently, polyvinylpyrrolidone (PVP) of differing molecular weights was included in media for individual secondary follicle development in vitro [40]. PVP of molecular weights less than 40 K significantly inhibited blastocyst formation of fertilized oocytes derived from cultured follicles, while oocytes exposed to PVP of higher molecular weights yielded blastocyst rates (56–63%) comparable to those cultured without PVP.
Oocyte competence to support early cleavage or blastocyst formation in mice was enhanced in 3D culture of secondary follicles (with two layers of GCs), either isolated directly from the ovary or from cultured ovaries, encapsulated in alginate, with evidence of four live offspring. However, the highest two-cell embryo formation rate to date was noted in secondary follicles embedded in collagen followed by culture on a collagen membrane that resulted in a 4% live birth rate [39], similar to that achieved in 2D culture [30]. Isolated secondary follicles cultured in the presence of PVP and low oxygen (O2) yielded the highest blastocysts rates (60%) reported to date [40], but offspring production was not attempted. Regardless of the culture system used, clearly, the rates of embryo and live offspring produced from oocytes that developed from primordial follicles in organ culture or cultured secondary follicles isolated from the ovary are meager at best when compared with those of in vivo-produced oocytes. Understanding why oocyte developmental competence is compromised is paramount to establishing robust methods for IVFD and to assurance that the basic follicle biology being studied can lead to production of a competent oocyte.
MII oocytes derived from in vitro-developed domestic animal follicles are competent for generating embryos capable of implantation
IVFD of preantral and early antral follicles from cattle, goats and sheep over the past 2 decades has been extensively reviewed by Figueiredo and colleagues [6, 12]. Table 2 summarizes the current literature on oocyte competence under various culture conditions in these species.
Table 2.
Maturation and competence of oocytes derived from cultured follicles in domestic animals
| Species | Age | Initial follicle stage and isolation method | Culture system and interval | Basal media, supplement and O2 level | Oocyte assessment and outcomes | References |
|---|---|---|---|---|---|---|
| Cattle | Unknown | Early antral 0.5–0.7 mm Mechanical |
1. COCs with partial presence of granulosa cells embedded in collagen gels, 4–6 per gel, 14 days until antral follicle-like structure formed 2. COCs extracted mechanically and cultured with additional cumulus cells and inseminated 14 days |
1–2. TCM199, 10% FCS, 4 mM hypoxanthine, 5% CO2 in air | 6/22 (27%) MII 10/24 (42%) fertilization 21/135 (18%) 2-cell 6/135 (4%) blastocyst 3 blastocysts/3 recipients 1 live offspring | [41] |
| Cattle | Unknown | Early antral 0.4–0.7 mm Mechanical |
1. COCs with partial presence of granulosa cells, 3–4 placed onto collagen-coated membrane inserts 2. COCs with partial presence of granulosa cells individually cultured in collagen-coated 96-well plates, 14 days until antral follicle-like structure formed 3. COCs removed mechanically, 6–10 per drop maturation media 14 days |
1–2. TCM199, 5% FBS, 4 mM hypoxanthine, 0.1 mg/ml estradiol, 4% PVP (MW 360 K) 3. TCM199, 5% FBS, 0.1 mg/ml sodium pyruvate, 20 mIU/ml rbFSH 5% CO2 in air |
51/58 (88%) MII 48/51 (94%) fertilization 20/48 (27%) cleavage 7/48 (17%) blastocyst 10/123 (8%) blastocyst per total number oocytes recovered 4 blastocysts/4 recipients 1 live offspring | [42] |
| Goat | Adult, 1–3 year-old | Preantral (secondary) ≥ 150 μm Mechanical |
1. Individual culture in drops on Petri dish 2. COCs removed for IVM/IVF 18 days |
1. α-MEM, 3 mg/ml BSA, 1 mg/ml fetuin, 10 μg/ml insulin, 2 mM glutamine, 2 mM hypoxanthine, 50–100 ng/ml rbLH, 50–100 ng/ml EGF, 100 ng/ml rbFSH Days 0–6, 500 ng/ml rbFSH Days 6–12, 1000 ng/ml rbFSH Days 12–18 2. TCM199, 1 mg/ml BSA, 22 μg/ml pyruvate, 100 μM cysteamine, 1 μg/ml estradiol, 0.5 μg/ml rbFSH, 5 μg/ml rbLH, 10 ng/ml EGF, 50 ng/ml IGF1 5% CO2 in air |
2/32 (6%) MII One 8-cell One 16-cell |
[43] |
| Goat | Adult, 1–3 year-old | Preantral (secondary) follicles, 200 μm Mechanical |
1. Individual culture in drops on Petri dish 2. COCs removed for IVM/IVF 18 days |
1. α-MEM, 3 mg/ml BSA, 1 mg/ml fetuin, 10 μg/ml insulin, 2 mM glutamine, 2 mM hypoxanthine, 50 ng/ml GH, 100 ng/ml rbFSH Days 0–6, 500 ng/ml rbFSH Days 6–12, 1000 ng/ml rbFSH Days 12–18 2. TCM199, 1 mg/ml BSA, 22 μg/ml pyruvate, 100 μM cysteamine, 1 μg/ml estradiol, 0.5 μg/ml rbFSH, 5 μg/ml rbLH, 10 ng/ml EGF, 50 ng/ml IGF1 5% CO2 in air |
10/20 (50%) MII 1/35 (3%) 2-cell embryo, progressed to compact morula |
[44] |
| Goat | Adult and prepubertal | Secondary Mechanical |
Single follicle 2D 18 days |
α-MEM, 3 mg/ml BSA, 1 mg/ml fetuin, 10 μg/ml insulin, 2 mM glutamine, 2 mM hypoxanthine, 100 ng/ml VEGF, 100 ng/ml rbFSH Days 0–6, 500 ng/ml rbFSH Days 6–12, 1000 ng/ml rbFSH Days 12–18 5% CO2 in air |
2D: four 2-cell embryos 3D: one 8-cell embryo |
[45] |
| Goat | Adult, 1–3 year-old | Multi-layer secondary follicles (≥200 μm) Mechanical |
1. Individual follicle embedded in 0.5% (w/v) alginate, 48-well plates, 18 days 2. Group of 5 follicles embedded in 0.5% (w/v) alginate, fibrin-alginate or hyaluronate, 18 days 3. 5 follicles/bead in fibrin-alginate, 30 days 4. COCs removed mechanically for maturation |
1–3. α-MEM, 3 mg/ml BSA, 10 ng/ml insulin, 2 mM glutamine, 2 mM hypoxanthine, 0.9 mM pyruvate, 100 ng/ml rbFSH Days 0–6, 500 ng/ml rbFSH Days 6–12, 1000 ng/ml rbFSH Days 12–18 2–3. same as 1 with 100 ng/ml VEGF 4. TCM199, 0.4% BSA, 5 mM ionomycin, 1 mM 60DMAP, 10% FCS 5% CO2 in air |
1. Single follicle in Alginate 2/14 (14%) MII 2. 5 follicles/bead Alginate: no oocytes matured Fibrin-alginate: 5/17 (29%) MII Hyaluronate: none matured 3. Fibrin alginate 30 days 7/12 (58%) MII 3/4 parthenotes cleaved and developed to 8-cell |
[46] |
| Goat | Adult, 1–3 year-old | Early antral (350 μm) Mechanical |
1. Individual culture in drops on Petri dish 2. COCs removed for IVM/IVF 18 days |
1. α-MEM, 3 mg/ml BSA, 10 μg/ml insulin, 2 mM glutamine, 2 mM hypoxanthine, 0.9 mM pyruvate, 50 ng/ml GH, 300 μg/ml anethole 2. TCM199, 1 mg/ml BSA, 1 mM pyruvate, 10 μM cysteamine, 1 μg/ml estradiol, 0.5 μg/ml rbFSH, 5 μg/ml rbLH, 10 ng/ml EGF, 50 ng/ml IGF1 5% CO2 in air |
56/97 (58%) MII 29/79 (37%) cleavage 10/77 (13%) parthenotes 14/60 (23%) cleavage IVF 6/60 (10%) embryos IVF 6 cleaved embryos/3 recipients 1 pregnancy to Day 30 gestation |
[47] |
| Sheep | Preantral follicles 250–400 μm Mechanical |
1. Microdrops or agar gel 6 days 2. COCs removed for IVM/IVF |
1. TCM 199, serum-free, 2 μg/ml T4, 2 μg/ml ovine FSH, insulin (2.5–15 ng/ml), IGF1 (5–20 ng/ml), GH (0.5–2 mIU/ml), TGF-β (1–15 ng/ml) 2. 10 μg/mL each of ovine FSH, Ovine LH, 1 mg/ml BSA, 1 μg/mL estradiol 5% CO2 in air |
T4+ FSH + ITS+IGF1 + GH 159/250 (64%) MII 35/159 (22%) 2-cell 3/36 (1%) morula T4 + FSH + IGF1 + GH 170/250 (68%) MII 43/170 (26%) 2-cell 7/43 (1%) morula |
[48] | |
| Sheep | Prepubertal | Preantral with evident theca layer (secondary) 170 ± 30 μm Early antral Mechanical |
Individual follicles placed in 96-V-well plates 14 days |
1. α-MEM, 2% FCS, 1% insulin, 1 μg/ml ovine FSH 5% CO2 in air |
Preantral – no data Early Antral: 47/110 (43%) MII Parthenotes 51/150 (34%) <16-cell 8/150 (5%) >16-cell IVM/IVF 107/336 (32%) fertilized 97/107 (91%) <16-cell 11/107 (10%) >16-cell |
[49] |
| Sheep | Adult (1–3 years) |
Preantral (secondary) >200 μm Mechanical |
Individual follicles in drops in Petri dishes 18 days |
α-MEM, 3 mg/ml BSA, 10 μg/ml insulin, 2 mM glutamine, 2 mM hypoxanthine, 50 ng/ml IGF1 or LIF or KL, 100 ng/ml ovine FSH Days 0–6, 1000 ng/ml ovine FSH Days 6–12, 200 ng/ml ovine FSH Days 12–18 5% CO2 in air |
50 ng/ml LIF 8/27 (29%) MII 7/12 (58%) 8-cell parthenotes 50 ng/ml KL 32% fertilization 1 morula after IVF |
[50, 52] |
| Sheep | Prepubertal to 10 month-old | Preantral 160–240 μm 0.1% Collagenase IA and 0.01% DNase I, followed by Mechanical |
1. Individual follicles in drops in Petri dishes 2. COCs removed mechanically for maturation 20 days |
1. α-MEM, 1.25 mg/ml BSA, 6.25 μg/mL insulin, 2 mM glutamine, 2 mM hypoxanthine,100 ng/ml ovine FSH alone or with 50 ng/mL LIF 2. TCM199, 10 ng/ml EGF,100 μM cysteamine |
LIF + ovine FSH 56% MII |
[51] |
| Pig | Prepubertal | Preantral (multi-layer) 200–310 μm; 296 ± 9 μm Mechanical |
1. 24-well dishes, 3 follicles per well 2. COCs removed mechanically for maturation and IVF 4 days |
1. NCSU23, 7.5% porcine serum, 3.5 μg/ml insulin, 1.5 ng/ml ovine FSH 2.NCSU23, 0.12 μg/ml ovine FSH, 2.5 μg/ml ovine LH, 20 ng/ml EGF 5% CO2 in air |
IVF 124/244 (51%) MII 60/114 (53%) fertilization 26/60 (43%) 2-cell 8/60 (13%) blastocyst ICSI 57/89 (64%) fertilization 29/57 (51%) 2-cell 12/57 (21%) blastocyst |
[53, 54] |
| Buffalo | Mature | Preantral, (multi-layer) 265–295 μm 1% trypsin, then mechanical |
1. 2–3 follicles per well; supplemented with ovarian mesenchymal cells or cumulus cells 2. COCs removed mechanically for maturation and IVF 100 days |
1. α-MEM, 10% steer serum, 6.25 μg/mL insulin, 0.23 mM pyruvate, 2 mM glutamine, 2 mM hypoxanthine, 3 μg/ml rbFSH 2. TCM199, 10% steer serum, 0.05 IU/ml rbFSH 5% CO2 in air |
82–100% oocyte recovery rate 38–46% cleavage 11–12% morulae/blastocyst |
[55] |
2D, 2-dimensional; 3D, 3-dimensional; BSA, bovine serum albumin; CO2, carbon dioxide; COC, cumulus–oocyte complex; DNase, deoxyribonuclease; DMAP, dimethylaminopyridine; EGF, epidermal growth factor; FBS, fetal bovine serum; FCS, fetal calf serum; GH, growth hormone; ICSI, intracytoplasmic sperm injection; IGF1, insulin-like growth factor 1; ITS, insulin–transferrin–selenium; IVF, in vitro fertilization; IVM, in vitro maturation; KL, kit ligand; LIF, leukemia inhibitory factor; αMEM, alpha minimum essential medium; MII, metaphase II; MW, molecular weight; NCSU, North Carolina State University; O2, oxygen; PVP, polyvinylpyrrolidone; rb-FSH, recombinant bovine follicle stimulating hormone; rbLH, recombinant bovine luteinizing hormone; T4, thyroxine; TCM199, tissue culture media 199; TGF-β, transforming growth factor-β; VEGF, vascular endothelial growth factor.
The majority of published studies on culture of bovine preantral follicles do not include information on oocyte competence [12]. However, an early report demonstrated that COCs with parietal GCs from early antral follicles could re-assemble into antral follicle-like structures when embedded in collagen for culture (Table 2) [41]. Oocytes obtained from these structures acquired full developmental competence in the absence of gonadotropins in the culture media; one live offspring was obtained after blastocyst transfer. A later study also showed that bovine COCs derived from early antral follicles could re-form an antrum when cultured on collagen-coated membranes or wells [42]. The addition of PVP to the culture media and IVM in the presence of FSH improved oocyte competence to support blastocyst development, and one live calf was born.
A novel feature of preantral follicle development in vitro in goats and sheep employs a sequential exposure to increasing concentrations of FSH during the 18-day culture interval. A 2D culture of caprine secondary follicles using media that contained a combination of FSH, LH, and EGF resulted in the first embryos reported from this species (Table 2) [43]. Gonadotropin-containing media that also included growth hormone (GH) or vascular endothelial growth factor (VEGF) improved nuclear maturation of oocytes derived from caprine secondary follicles grown in either a 2D or a 3D culture system [12]. Inclusion of GH or VEGF in subsequent experiments was associated with embryo production from these follicles; however, it is unknown whether they are the major factors driving the improvement in oocyte competence. GH supplementation improved oocyte meiotic resumption following both 2D and 3D (alginate-encapsulated) culture of caprine secondary follicles, resulting in oocytes capable of nuclear maturation as well as early embryogenesis of a few embryos in each condition [44, 45]. When multilayered preantral follicles isolated from caprine ovaries were cultured individually in alginate and in groups of five in alginate, fibrin-alginate or hyaluronate for 18 days, they developed to the antral stage containing fully grown oocytes, but only those grown in fibrin-alginate achieved oocyte nuclear maturation [46]. Group culture in fibrin-alginate for 30 days increased oocyte competence for both nuclear maturation and embryo development, and resulted in three parthenotes that developed to the eight-cell stage. The addition of anethole, an antioxidant, to caprine early antral follicles cultured in 2D system improved oocyte nuclear maturation and embryonic development, resulting in the first pregnancy in goats after embryo transfer [47].
Anakumari et al. exposed ovine secondary follicles to various concentrations of insulin, insulin-like growth factor 1 (IGF1), and GH in the presence of FSH (Table 2) [48]. A synergistic effect of IGF1 and GH increased preantral follicle growth, and thyroxine + FSH + IGF1 + GH supported both oocyte nuclear maturation and early embryonic development. The addition of transforming growth factor beta (TGF-β) suppressed oocyte nuclear maturation. Oocytes that developed within ovine secondary follicles grown in vitro under ideal hormone and growth factor conditions produced the first reported morulae, but the rate (1%) was modest relative to that of oocytes from antral follicles grown in vivo (50%). Oocytes from ovine early antral follicles developed in vitro-produced embryos with less than 16-cells after parthenogenesis or IVM/IVF, but very few embryos with greater than 16-cells were obtained [49]. In this study, secondary follicles progressed to the early antral stage during 14 days of culture in the presence of FSH. Ovine secondary follicles grown in vitro under 2D conditions in the presence of FSH and leukemia inhibitory factor (LIF) developed to antral follicles capable of producing oocytes competent for nuclear maturation and parthenogenetic activation [50]. Attributes of LIF included maintenance of follicular viability, prevention of apoptosis, as well as enhancement of antrum formation and GC/theca cell proliferation. Using a similar culture system for ovine secondary follicles, Cadoret et al. reported that LIF did not alter follicle growth or antrum formation, but modulated the differentiation of GCs in the absence of FSH [51]. The presence of both LIF and FSH during follicle growth improved oocyte meiotic competence (56%) compared with in vitro-derived antral follicles of similar size cultured with FSH alone (28%) or in vivo-developed follicles (9%). Embryonic development was not assessed in this study. However, LIF maintained or stimulated expression of genes necessary for oocyte-somatic cell paracrine regulation (discussed below). Lastly, one morula resulted from the addition of kit ligand to the same culture system for ovine secondary follicles [52].
Rapid growth of porcine multilayer preantral follicles to the antral stage (~500 μm diameter) in media containing FSH and serum was observed in 3–4 days. These 2D culture conditions supported oocyte maturation and fertilization via IVF or intracytoplasmic sperm injection (ICSI) with 13–21% of zygotes reaching the blastocyst stage (Table 2) [53, 54]. Blastocysts maintained normal actin filament distribution during fertilization and embryonic development.
When co-cultured with ovarian mesenchymal cells or cumulus cells, multilayer preantral follicles from buffalo ovaries grown in 2D system with FSH and serum produced antral follicles in 100 days (Table 2) [55]. Oocytes were recovered for IVF, and an 11–12% morula/blastocyst rate was reported, but embryo transfer was not performed. Although 76–87% of follicles survived this very prolonged culture, a low level of oocyte competence was observed.
In contrast to mice, preantral follicles from cattle, goats and sheep developed in vitro in either 2D or 3D culture systems require a longer interval (18–30 days) to support adequate growth and nuclear maturation of oocytes, but do not robustly improve oocyte competence to produce embryos. Although embryos were derived from parthenogenetically activated mature oocytes in goats and sheep, a single pregnancy in the goat provides some promise that oocyte competence can be supported, at least from the early antral follicle developed further in vitro. Porcine preantral follicles achieved maximal antral size and oocyte competence within 4 days, perhaps due to the culture of multilayer follicles. While blastocyst rates were highest in pigs relative to the other species, embryo transfer was not attempted. None of the current culture systems employing preantral follicles has reported embryo production in cattle, or live offspring in cattle or buffalo.
MII oocytes derived from in vitro-developed nonhuman primate follicles are competent for preimplantation embryonic development
IVFD has been performed in nonhuman primates, particularly in Old World female monkeys that experience ~28-day menstrual cycles like women, and the studies are summarized in Table 3. Early stage nonhuman primate follicles often require a 3D culture system to support their survival and growth in vitro, maintaining the architecture similar to in vivo-developed follicles. In an early study using olive baboons (Papio anubis), multilayer preantral follicles (diameter = 260–300 μm) were isolated following Liberase-DNase treatment and embedded in a specialized fibrin–alginate–matrigel matrix for individual culture [56]. Follicles grew to the small antral stage (diameter = 400–500 μm) in 10–14 days in αMEM-based media supplemented with insulin and various doses of recombinant human FSH (rhFSH). FSH supplementation appeared to promote the growth of follicles, but not the enclosed oocytes. When COCs were collected from in vitro-developed antral follicles in the absence of rhFSH and underwent 48 h of IVM, 2 out of 16 oocytes matured to the metaphase II (MII) stage with typical meiotic spindle structure and chromosome alignment as determined by immunofluorescence staining and confocal microscopy. Because oocytes were not inseminated, their developmental competence was unknown.
Table 3.
Maturation and competence of oocytes derived from cultured follicles in nonhuman primates
| Species | Age | Initial follicle stage and isolation method | Culture system and interval | Basal media, supplement and O2 level | Oocyte assessment and outcomes | References |
|---|---|---|---|---|---|---|
| Olive baboon | 9–16 years | Multilayer preantral; Liberase and DNase |
Fibrin-0.25% (w/v) alginate-matrigel embedded individual culture for 14 days followed by 48-h IVM | αMEM, 5 μg/ml insulin; No rhFSH; No O2 control |
2/16 MIIs with typical spindle structure by immunofluorescence | [56] |
| Rhesus macaque | 6.4 years in average | Small antral; Mechanical |
Individual culture without or with 0.25% (w/v) alginate-embedding for 34 h | αMEM, 5 μg/ml insulin; 220 mIU/ml rhFSH, without or with 200 IU/ml hCG; No O2 control |
-Alginate/-hCG: 4/27 MIIs, −alginate/+hCG: 14/41 MIIs, and + alginate/+hCG: 4/11 MIIs by light microscopy | [57] |
| 4–9 years | Multilayer preantral; Mechanical |
Individual culture in media droplets covered with oil for 14 days followed by IVM | αMEM, 5 μg/ml insulin; 200 mIU/ml rhFSH, 100 or 200 mIU/ml rhLH; No O2 control |
2/24 MIIs by light microscopy | [58] | |
| 5–10 years | Secondary; Collagenase and DNase |
0.25% (w/v) alginate embedded individual culture for 40 days followed by 34-h 100 ng/ml hCG treatment | αMEM, 5 μg/ml insulin; 0.3 (low) or 15 (high) ng/ml rhFSH; No O2 control or 5% O2 |
High rhFSH/5% O2: 1/25 MII with typical spindle structure by immunofluorescence; Low rhFSH/5% O2: 1/8 MII developed to a 2-cell embryo after ICSI; Low rhFSH/no O2 control: 1 MII by light microscopy |
[59] | |
| 7–14 years | Secondary and primary; Mechanical |
Secondary: 0.25% (w/v) alginate embedded individual culture for 5 weeks, and primary: fibrin-0.25% (w/v) alginate embedded individual culture for 13 weeks followed by 34-h 100 ng/ml hCG treatment | αMEM, 5 μg/ml insulin; 3 ng/ml rhFSH; 5% O2 |
Secondary: 1/15 MII developed to the morula stage after ICSI; Primary: 1/5 MII formed a zygote after ICSI |
[60] | |
| 7 years | Secondary; Mechanical |
0.25% (w/v) alginate embedded individual culture for 5 weeks followed by 34-h 100 ng/ml hCG treatment | αMEM, 5 μg/ml insulin; 3 ng/ml rhFSH in week 0–3 followed by 0.3 ng/ml in week 4–5; 5% O2 |
2/22 MIIs developed to the morula stage after IVF | [61] | |
| Reproductive age | Secondary; Mechanical |
0.25% (w/v) alginate embedded individual culture for 40 days followed by 34-h 100 ng/ml hCG treatment | αMEM, 5 μg/ml insulin; 3 ng/ml rhFSH in Days 0–20 followed by 0.3 ng/ml in Days 21–40, without or without androgen modulation; 5% O2 |
-Androgen modulation: 4/49 MIIs with 2 formed zygotes and 1 developed to the morula stage after IVF; TRL steroid depletion+50 ng/ml T: 1/13 MII, and TRL steroid depletion+50 ng/ml DHT: 1/13 MII formed a zygote after IVF |
[62] | |
| 9.5 ± 2.1 years | Secondary; Mechanical |
0.25% (w/v) alginate embedded individual culture for 5 weeks followed by 34-h 100 ng/ml hCG treatment | αMEM, 0.5 μg/ml insulin; 3 ng/ml/100 mIU rhFSH in week 0–3 followed by 0.3 ng/ml in week 4–5, without or without estrogen modulation; 5% O2 |
-Estrogen modulation: 1/13 MII, and TRL steroid depletion+100 ng/ml E2: 1/14 MII developed to the morula stage after IVF | [63] | |
| 6–14 years | Secondary; Mechanical |
0.15% (w/v) alginate embedded individual culture for 5 weeks followed by 34-h 100 ng/ml hCG treatment | αMEM, 5 μg/ml insulin; 1 ng/ml rhFSH, 25 pg/ml VD3 in weeks 3–5; 5% O2 |
1/22 MII by light microscopy | [64] | |
| 7–13 years | Secondary; Mechanical |
Individual culture in 96-well round-bottom ultra-low attachment plate for 5 weeks followed by 34-h 100 ng/ml hCG treatment | αMEM, 5 μg/ml insulin; 1 ng/ml rhFSH, without or with 100 ng/ml rhAMH in week 0–2; No O2 control |
-rhAMH: 1/14 MII, and + rhAMH: 2/19 MIIs developed to the morula stage after IVF | [65] | |
| Reproductive age | Secondary; Mechanical |
0.25% (w/v) alginate embedded individual culture for 40 days followed by 34-h 100 ng/ml hCG treatment | αMEM, 5 μg/ml insulin; 1 ng/ml rhFSH, 120 ng/ml AMH-Ab in week 3–5; 5% O2 |
1/10 MII developed to an 8-cell embryo after IVF | [68] | |
| 6–10 years | Secondary and primary; Mechanical |
Secondary: individual culture in 96-well round-bottom ultra-low attachment plate for 5 weeks, and primary: group culture in 96-well round-bottom ultra-low attachment plate for 7 weeks followed by 34-h 100 ng/ml hCG treatment | αMEM, 5 μg/ml insulin; 1 ng/ml rhFSH, primary: 100 ng/ml rhAMH in week 0–5 and secondary: without or with AMH modulation; No O2 control |
Secondary/-rhAMH modulation: 2/16 MIIs developed to the morula stage after ICSI; Secondary/+rhAMH in week 0–3 + AMH-Ab in week 4–5: 10/36 MIIs with 9 developed to the morula stage and 1 developed to the blastocyst stage after ICSI; Primary: 2/34 MII developed to the morula stage after IVF |
[66] |
AMH, anti-Müllerian hormone; AMH-Ab, neutralizing anti-human anti-Müllerian hormone antibody; DHT, dihydrotestosterone; DNase, deoxyribonuclease; E2, estradiol; hCG, human chorionic gonadotropin; ICSI, intracytoplasmic sperm injection; IVF, in vitro fertilization; IVM, in vitro maturation; αMEM, alpha minimum essential medium; MII, metaphase II; O2, oxygen; rhAMH, anti-Müllerian hormone; rhFSH, human recombinant follicle-stimulating hormone; rhLH, human recombinant luteinizing hormone; T, testosterone; TRL, trilostane; VD3, 1α,25-dihydroxyvitamin D3.
The IVFD protocols in nonhuman primates are further developed in rhesus macaques (Macaca mulatta) (Table 3). The αMEM-based media supplemented with insulin are generally used to support follicle survival and growth in culture. The effects of gonadotropin supplementation, such as rhFSH, recombinant human LH (rhLH) and hCG, on follicular growth and oocyte maturation were evaluated. Mechanically isolated from the ovarian medulla, small antral follicles (diameter > 0.5 mm) were cultured individually for 34 h [57]. While ~15% oocytes from follicles cultured in the absence of hCG resumed meiosis, ~35% oocytes from follicles exposed to hCG matured to the MII stage regardless of alginate embedding. Following mechanical isolation, multilayer preantral follicles (diameter = 220–380 μm) survived 14 days of individual 2D culture [58]. The gonadotropin supplementation at a ratio of rhFSH: rhLH = 2:1 enhanced follicle growth and supported oocyte maturation. After IVM, 2 MII oocytes were obtained from COCs of 24 in vitro-developed antral follicles. However, only polar body extrusion was observed in MII oocytes from these studies by light microscopy. Data on oocyte spindle configuration or fertilization outcomes were not available.
During the past 10 years, efforts have been made in rhesus macaques to extend the initial developmental stages of cultured follicles to the secondary and primary stages (Table 3). Enzymatic methods were used to isolate follicles in early studies, whereas mechanical follicle isolation is applied to recent studies to minimize damages of the extracellular matrix and cell membrane. Media supplements, such as gonadotropins, steroid hormones, and environmental O2 levels were modulated with a goal of improving follicular development in vitro to generate mature oocytes competent for preimplantation embryonic development. Secondary follicles (diameter = 125–225 μm) could be isolated from the ovarian cortex after collagenase and DNase treatment [59]. A relatively long culture period (40 days) was required for these early stage follicles to reach the small antral stage (diameter = 400–500 μm) when embedded in alginate for individual culture. Low levels of rhFSH (0.3 ng/ml) and O2 (5%) appeared to promote follicle growth, whereas rhFSH concentrations or O2 tension had minimal effects on oocyte maturation. When in vitro-developed antral follicles were treated with hCG for 34 h, 3 MII oocytes were obtained from dozens of follicles cultured under various combinations of rhFSH and O2. The MII oocytes exhibited typical meiotic spindle structure as shown by immunofluorescence staining and confocal microscopy. After insemination by ICSI, 1 oocyte formed a two-cell embryo. In several subsequent studies, the similar protocol was used to culture mechanically isolated rhesus macaque secondary follicles (diameter = 125–250 μm), except that a medium dose (3 ng/ml) of rhFSH was supplemented which was reduced to a lower dose (0.3 ng/ml) after antrum formation in some cases [60–63]. In vitro-developed small antral follicles acquired larger diameters (500–800 μm). Although the maturation rates were relatively low (<10%), MII oocytes were consistently obtained from cultured follicles, which were capable of zygote formation and preimplantation embryonic development to the morula stage after IVF or ICSI. Androgen and estrogen appear to be essential for proper follicular development in vitro. The detrimental effects of steroid depletion on follicle growth and oocyte maturation could be limited or prevented by androgen (testosterone and dihydrotestosterone) or estrogen (estradiol) replacement [62, 63]. The metaphase stage oocytes were also derived from in vitro-developed follicles exposed to bioactive vitamin D after antrum formation [64]. However, vitamin D supplementation did not seem to significantly increase oocyte maturation rate, and oocyte competence was not assessed. Mechanically isolated rhesus macaque primary follicles (diameter = 80–120 μm) could grow to the small antral stage (diameter = 600–700 μm) when embedded in a fibrin–alginate matrix for prolonged (13 weeks) individual culture [60]. To date, only 1 MII oocyte was obtained from individual culture of nonhuman primate primary follicles, which formed a zygote after ICSI without further embryonic cleavage.
The culture system has been further modified in order to increase the maturation rates and developmental competence of oocytes derived from in vitro-developed rhesus macaque follicles (Table 3). A matrix-free 3D system was adapted to facilitate efficient diffusion of macronutrients and to prevent physical restraint from embedding matrix, especially during rapid growth of primate follicles after antrum formation. Secondary follicles were mechanically isolated and cultured individually in the 96-well round-bottom ultra-low attachment plate [65, 66]. Within the 5-week culture period, cultured follicles developed to the small antral stage with average diameters similar to those of alginate-embedded follicles [65]. Notably, greater than 10% of the in vitro-developed antral follicles had a diameter over 1000 μm [66]. After 34 h of hCG treatment, ~10% oocytes harvested matured to the MII stage, which developed to the morula stage after IVF or ICSI. Furthermore, paracrine factor modulation has been considered to promote follicle growth and oocyte maturation in vitro. For example, evidence suggests that anti-Müllerian hormone (AMH) is highly produced by rhesus macaque preantral follicles with a greater potential of growth in vitro to yield mature oocytes [67]. AMH promotes preantral follicle growth, but inhibits antral follicle maturation in rhesus macaques [65, 68]. MII oocytes were obtained from in vitro-developed follicles with AMH supplementation at the preantral stage or AMH depletion after antrum formation, which were competent for fertilization and preimplantation embryonic development in vitro. When secondary follicles (diameter = 150–225 μm) were cultured in the matrix-free 3D system with stage-specific AMH modulation, follicle growth was significantly increased with 8% of the in vitro-developed antral follicles having a diameter greater than 2000 μm [66]. More than 25% oocytes derived from cultured follicles matured to the MII stage and developed to morula stage after ICSI. One embryo further developed to the blastocyst stage. In addition, matrix-free group culture, in combination with AMH modulation, improved primary follicle (diameter = 110–120 μm) growth to the small antral stage within 7 week in vitro [66]. Although the maturation rates remained low (<6%), 2 MII oocytes developed to the morula stage after IVF indicating the increased developmental competence. With increased MII oocyte yield and quality, embryo transfer may be attempted in future studies to generate offspring.
MII oocytes derived from in vitro-developed human follicles have typical meiotic spindle configuration and ultrastructure
Knowledge gained from animal studies facilitates the protocol development for human IVFD. Multistep culture systems have been used to support human follicle growth in vitro. To date, three research groups reported maturation of oocytes derived from in vitro-developed human follicles, as summarized in Table 4. Based on ethical principles, human oocytes cannot be fertilized to determine their developmental competence. Instead, oocyte morphology, spindle configuration, and ultrastructure are usually assessed to evaluate oocyte quality.
Table 4.
Maturation and morphology of oocytes derived from cultured follicles in humans
| Patient | Age | Initial follicle stage and isolation method | Culture system and interval | Basal media, supplement and O2 level | Oocyte assessment and outcomes | References |
|---|---|---|---|---|---|---|
| Cancer, aplastic anemia, beta-thalassemia | 5–33 years | Secondary; Mechanical |
1. 0.5% (w/v) alginate-embedded individual follicle culture till follicle diameter = 400–500 μm with antrum formation; 2. Individual follicle culture in low attachment plate till 30–40 days; 3. Individual follicle incubation with 1.5 IU/ml hCG and 10 ng/ml EGF for 16 h |
1/2. αMEM Glutamax and F-12 Glutamax at 1:1, 5 μg/ml insulin, 10 mIU/ml rhFSH; 3. αMEM, 10% FBS, 10 mIU/ml rhFSH; 1–3: no O2 control |
4/20 MIIs with typical spindle structure by immunofluorescence, polar body fragmentation by light microscopy | [69] |
| Elective cesarean section | 25–39 years | Unilaminar (primordial, intermediary or primary); Mechanical |
1. Ovarian tissue culture for 8 days; 2. Secondary follicle individual culture in 96-well V-bottom plate for 8 days; 3. COC group culture on track-etched nucleopore membrane for 4–6 days; 4. 24 h of IVM |
1. McCoy’s 5A, 10 ng/ml insulin, 50 μg/ml ascorbic acid, 1 ng/ml rhFSH; 2/3. McCoy’s 5A, 10 ng/ml insulin, 50 μg/ml ascorbic acid, 1 ng/ml rhFSH, 100 ng/ml rhACVA; 4. SAGE IVM media, 75 mIU/ml rhFSH, 75 mIU/ml rhLH; 1–4: no O2 control |
9/32 MIIs with typical spindle structure by immunofluorescence, large polar body by light microscopy | [71] |
| Benign or malignant gynecologic conditions | 22–45 years | Unilaminar (primordial or intermediary); Mechanical |
1. Ovarian tissue culture for 3 weeks; 2. Secondary follicle group culture in 96-well round-bottom ultra-low attachment plate for 6 weeks; 3. 48 h of IVM |
1. αMEM, 5 μg/ml insulin, 15 mIU/ml rhFSH; 2. αMEM, 5 μg/ml insulin, 15 mIU/ml rhFSH, 100 ng/ml rhAMH in week 0–3 and 100 ng/ml AMH-Ab in week 4–6; 3. SAGE IVM media, 75 mIU/ml rhFSH, 75 mIU/ml rhLH; 1/2: no O2 control; 3: 5% O2 |
3/14 MIIs with normal spindle/polar body size/position and typical ultrastructure by polarized microscopy and electron microscopy | [73] |
AMH, anti-Müllerian hormone; AMH-Ab, neutralizing anti-human anti-Müllerian hormone antibody; COC, cumulus–oocyte complex; EGF, epidermal growth factor; FBS, fetal bovine serum; hCG, human chorionic gonadotropin; IVM, in vitro maturation; αMEM, alpha minimum essential medium; MII, metaphase II; rhACVA, human recombinant activin A; rhAMH, recombinant human anti-Müllerian hormone; rhFSH, recombinant human follicle-stimulating hormone; rhLH, recombinant human luteinizing hormone.
The first cohorts of MII oocytes were generated from individually cultured follicles starting at the secondary stage (diameter = ~200 μm in average) (Table 4) [69]. Ovarian tissues were collected from patients with a wide age range (5–33 years old) who were diagnosed with cancer, aplastic anemia, or beta-thalassemia. Mechanically isolated follicles were first embedded in alginate and cultured in αMEM Glutamax and F-12 Glutamax (1:1)-based media supplemented with 5 μg/ml insulin and 10 mIU/ml rhFSH. Once follicle diameters reached 400–500 μm after antrum formation, follicles were released from alginate for matrix-free culture in the low attachment plate till 30–40 days. After 16 h of hCG and EGF treatment, 4 out of 20 oocytes matured to the MII stage with typical meiotic spindle structure and chromosome alignment as indicated by immunofluorescence staining and confocal microscopy. Polar body fragmentation was observed, which could potentially impair fertilization in vitro and subsequent embryonic development [70]. In addition, fetal bovine serum was used in the final step of culture, which limits the application of this protocol in clinical settings.
In order to support survival and growth of human follicles in the early developmental stages, such as primordial, intermediary and primary follicles, ovarian cortical tissue culture step was added to the protocol (Table 4) [71]. Ovarian cortical biopsies from patients undergoing cesarean section were cut into ~1 × 1 × 0.5 mm3 fragments containing early stage follicles (diameter ≤ 40 μm) and cultured for 8 days in McCoy’s 5A media supplemented with 10 ng/ml insulin, 50 μg/ml ascorbic acid, and 1 ng/ml rhFSH. Secondary follicles (diameter = 100–150 μm) developed in cultured tissue were mechanically dissected for individual matrix-free culture in the 96-well V-bottom plate with additional activin A supplementation for 8 days. COCs were then obtained from in vitro-developed small antral follicles and group cultured on the track-etched nucleopore membrane for 4–6 days in the same follicle culture media. After IVM, 9 out of 32 oocytes matured to the MII stage with typical meiotic spindle structure and chromosome alignment as shown by immunofluorescence staining and confocal microscopy. Unusually large polar bodies were observed, indicating possible abnormal spindle position and/or inter-chromosomal spacing [72].
In a recent study, the optimized follicle culture protocol in nonhuman primates was used to facilitate human follicular development and oocyte maturation in vitro (Table 4) [73]. Small ovarian cortical tissue fragments (∼1.4 × 10−2 mm3) from patients with benign or malignant gynecologic conditions were cultured in αMEM media supplemented with 5 μg/ml insulin and 15 mIU/ml rhFSH. Secondary follicles (diameter = 125.0–198.4 μm) developed in cultured tissue were mechanically dissected for 6 weeks of matrix-free group culture in the low-attachment round-bottom plate with AMH modulation, i.e., AMH supplementation at the preantral stage followed by AMH depletion after antrum formation. COCs were then obtained from in vitro-developed small antral follicles for 48 h of IVM at 5% O2. Three out of 14 oocytes matured to the MII stage including the polar body and meiotic spindle with normal size and position, as determined by polarized microscopy and electron microscopy. The typical chromosome alignment and ultrastructure were observed by electron microscopy, including microvilli on the plasma membrane, cortical granules below the plasma membrane, as well as mitochondria associated with the smooth endoplasmic reticulum and small vesicles. Because only five patients contributed ovarian tissues to this research, future studies with increased sample size are needed to investigate the efficacy of yielding normal mature oocytes from in vitro-developed human follicles using this protocol, and to improve oocyte maturation rate.
Epigenetic and genetic assessment of MII oocytes and resulting embryos following IVFD
Currently, the quality of oocytes derived from in vitro-developed follicles is generally evaluated by morphology, fertilization capacity, preimplantation embryonic development, and live offspring. In addition, epigenetic and genetic assessment of oocytes and resulting preimplantation embryos has been attempted, particularly in mice, to provide deeper analyses on possible alterations in genomic imprinting and gene expression following prolonged in-vitro manipulation of follicles and oocytes.
The majority of imprinted genes are epigenetically modified during oogenesis, which is vital through both development and adult life. In an early study, secondary follicles (diameter = 100–130 μm) from mouse pups (13–14 days old) were cultured in a 2D system for 12 days followed by 18 h of hCG and EGF treatment to generate MII oocytes [74]. MII oocytes were also retrieved from adult mice (8 weeks old) after superovulation. The methylation status of key imprinted genes, including small nuclear ribonucleoprotein N (Snrpn), insulin-like growth factor 2 receptor (Igf2r), paternally expressed gene 3 (Peg3), and H19 imprinted maternally expressed transcript (H19), were compared between in vitro- and in vivo-derived oocytes by limiting-dilution bisulphite sequencing. The comparable DNA methylation patterns at the studied regulatory sequences of genes suggested that the 12-day follicle culture did not modify the establishment of imprinting in the mouse oocytes. A similar study assessed quality of MII oocytes obtained from in vitro-developed mouse follicles after 8 or 14 days of culture, except that prepubertal mice (25 days old) were used for superovulation to obtain in vivo-derived oocytes [75]. DNA methylation was analyzed for Snrpn, octamer-binding transcription factor 4 (Oct4), and mesoderm-specific transcript (Mest). Data showed that the shortened culture period did not induce aberrant DNA methylation in genes studied, whereas the prolonged culture period was associated with a low level (1 of 54 alleles) oocyte epimutation in the imprinted Mest gene. Therefore, oocyte meiotic maturation need to be induced timely when in vitro-developed follicles reach the preovulatory stage to avoid preovulatory oocyte aging [76]. The selected imprinted genes (Snrpn, Mest, and H19) were also evaluated in preimplantation mouse embryos to examine the impact of IVFD on the maintenance of DNA methylation [77]. Although the embryo culture process resulted in a loss of imprinted DNA methylation compared with embryos derived from natural pregnancy, differences in methylation profiles of studied genes were not identified between blastocysts derived from oocytes following follicle culture and superovulation.
The genome-wide analysis of DNA methylation by bisulphite sequencing was conducted recently in mouse MII oocytes obtained in vitro and in vivo [78]. Secondary follicles (diameter = 110–130 μm) from mouse pups (13 days old) were cultured in a 2D system for 9 days followed by 18 h of hCG and EGF treatment to generate MII oocytes. In vivo-derived MII oocytes were from prepubertal (23 days old) and adult (10 weeks old) mice after superovulation, as well as from adult (10 weeks old) mice after natural ovulation. Data suggested that DNA methylation profiles were globally similar between oocytes developed in vitro and in vivo, though specific methylation differences existed. For example, 17 genes were identified in in vitro-derived oocytes to contain multiple hypomethylated tiles. Most of these genes were associated with nervous system development and/or neuron differentiation, such as SRY-box containing gene 5 (Sox5) and myosin XVI (Myo16).
In addition to DNA methylation analysis, the expression of imprinting maintenance genes have been assessed by real-time PCR in in vitro- and in vivo-derived MII oocytes in mice. Secondary follicles (diameter = 110–130 μm) from mouse pups (13–14 days old) were cultured in a 2D system for 12 days followed by 18 h of hCG and EGF treatment to generate MII oocytes [75, 77]. MII oocytes were also retrieved from prepubertal mice (25 days old) after superovulation. The expression levels of genes that are essential for imprinting establishment were comparable between in vitro- and in vivo-derived oocytes, including DNA methyltransferase 1 oocyte-specific form (Dnmt1o), 3 alpha (Dnmt3a), and 3 like (Dnmt3l), as well as zinc finger protein 57 homolog (Zfp57), methyl-CpG binding domain protein 3 (Mbd3), and developmental pluripotency-associated 3 (Dppa3). It appears that IVFD in a relatively short period of time does not lead to significant alterations in DNA methylation landscape, at least in mice. However, information is lacking in other species in terms of epigenetic status of oocytes or embryos generated from in vitro-developed follicles, especially follicles at early developmental stages which require prolonged culture interval to reach the antral stage.
Genetic assessment of in vitro-developed follicles and enclosed germinal vesicle oocytes
Oocyte transcriptome profile changes dynamically during follicular development from the primordial to preovulatory stage. When the oocyte starts to grow upon primordial follicle activation, RNA is actively transcribed for protein synthesis and accumulation to achieve oocyte cytoplasmic maturation. Concurrently, oocyte-originated factors control gene expression in follicular cells for proper proliferation and differentiation to support continuous development of the follicle and enclosed oocyte. Therefore, studies have attempted to determine whether transcriptomes are comparable between in vitro- and in vivo-developed follicles and their enclosed germinal vesicle (GV) oocytes, which can have impact on the final oocyte nuclear maturation and developmental competence.
Gene expression profiles of in vitro-developed follicles were analyzed in multiple species. When secondary follicles (diameter = 150–180 μm) from prepubertal mice (16 days old) were cultured in an alginate-encapsulated system for 8 days, the in vitro-developed preovulatory follicles expressed proper antral follicle markers, such as inhibin beta A (Inhba) and vascular endothelial growth factor (Vegf), as determined by microarray and real-time PCR [79]. The expression of a particular gene cartilage oligomeric matrix protein (Comp) was upregulated, which was consistent with elevated COMP protein levels in antral follicles preceding ovulation in cycling (>6 weeks old) and equine chorionic gonadotropin (eCG)-primed prepubertal (18 days old) mice. Under a similar experimental setting, comparable expression profiles of genes involved in cell cycle and various metabolic processes were also identified in somatic cells isolated from in vitro- and in vivo-developed mouse follicles [80]. In a study using prepubertal sheep (6–8 months old), size-matched small antral follicles were isolated from the ovary and were obtained from 20-day culture of secondary follicles (diameter = 160–240 μm) without matrix encapsulation [81]. The expression of 40 genes critical for the preantral-to-antral follicular transition was examined using the dynamic array integrated fluidic circuits. The altered expression levels were identified for genes involved in GC proliferation, estradiol biosynthesis and signaling, and oocyte maturation (e.g., KIT proto-oncogene receptor tyrosine kinase/KIT and bone morphogenetic protein 15/BMP15), suggesting the accelerated follicular development in vitro. Gene expression profiles were also compared between in vitro- and in vivo-developed small antral follicles in rhesus macaques by microarray [1]. While similarities were identified between the two follicle cohorts, expression levels of certain genes increased in cultured follicles due to prolonged FSH exposure over 5 weeks, e.g., low-density lipoprotein receptor (LDLR), EGF receptor (EGFR), and glutamate-cysteine ligase catalytic subunit (GCLC). Therefore, extended time interval of in vitro manipulation may increase the chance of gene expression alterations in cultured follicles including the enclosed oocyte.
To better elucidate oocyte transcriptome profiles following IVFD, GV oocytes are isolated from cultured follicles for gene expression analysis. To date, researchers have obtained secondary follicles (diameter = 100–130 μm) from prepubertal mice (10–13 days old) for 8–12 days of culture to the preovulatory stage. Gene expression levels of GV oocytes from cultured follicles were compared with those of in vivo-developed GV oocytes from eCG-primed age-matched prepubertal mice or adult (8 weeks old) animals. Real-time PCR data from studies of the same research group suggested that, under optimized culture conditions, mRNA levels of oocyte-specific genes were comparable between GV oocytes derived in vitro from a 2D follicle culture and in vivo, including Bmp15, growth differentiation factor 9 (Gdf9), mater (Nlrp5), nucleoplasmin 2 (Npm2), and fibroblast growth factor 8 (Fgf8) [82–84]. Similar gene expression patterns between GV oocytes from preovulatory follicles developed in vitro on a membrane insert and in vivo were also demonstrated by single-cell RNA sequencing, including the expression of transcription factors, e.g., NOBOX oogenesis homeobox (Nobox) [85]. Consistently, microarray analysis showed minimal effects of alginate-encapsulated follicle culture on GV oocyte transcriptome, with only 50 genes exhibiting a greater than 2-dold difference in mRNA levels when compared with in vivo-developed GV oocytes [37]. However, the transcript integrity in GV oocytes may not always correlate with cytoplasmic maturation status after the resumption of meiosis or developmental competence of the resulting MII oocytes. Differences in gene expression levels were identified by real-time PCR between MII oocytes matured in vitro following follicle culture and those matured in vivo after superovulation [82]. It was also reported that in vitro-derived MII oocytes had increased incidence of defects in spindle formation, chromosome alignment, and cortical granule biogenesis [37]. Thus, transcriptomics of MII oocytes from in vitro-developed follicles and its association with the subsequent embryonic development are warranted in various species.
Folliculogenesis is a dynamic process with differential gene expression patterns at specific developmental stages. Microarray database search followed by computational analysis provided a landscape of inter- and intra-cellular signaling pathways with related ligands and receptors in mouse folliculogenesis in vivo from primordial to antral stage [86]. Gene expression patterns in follicular cells were also studied recently in in vivo-developed mouse follicles from the secondary to ovulatory stage and human antral follicles using single-cell RNA sequencing [87–89]. Data provide a basis for continued analysis of follicular remodeling that includes GC differentiation, theca cell differentiation, and cumulus cell formation during the transition between follicular developmental stages. In addition, RNA profiling was conducted in in vivo-derived bovine GV oocytes and MII oocytes with normal or poor developmental competence, and suggested that developmental competence is acquired by the oocyte throughout folliculogenesis via fine-tuned gene regulation [90]. Single-cell transcriptome profiling was also performed in cynomolgus macaque (Macaca fascicularis) and human GV and MII oocytes obtained from the ovary, though oocyte competence was unknown [91–93]. Databases generated from these studies provide references to follicular development regulation and oocyte quality improvement. Comparing gene expression of follicles developed in vitro and in vivo may facilitate identifying regulatory factors needed for supporting proper follicular development in vitro to achieve oocyte cytoplasmic and nuclear maturation.
Metabolic evaluation of preantral follicles developed in vitro
Because IVFD occurs in the absence of a circulating blood supply, careful consideration of energy sources in the media has led to the well-known fact that one of the major sources for energy consumption by follicular somatic cells is glucose, whereas pyruvate is required for the cumulus-enclosed oocyte [9]. In addition to glucose, follicles utilize amino acids, fatty acids and glycerol as other sources for adenosine triphosphate (ATP) production via the tricarboxylic acid cycle (TCA) and oxidative phosphorylation. The first detailed investigation of carbohydrate metabolism in individual murine late primary/early secondary follicles, as well as matured and ovulated oocytes, over a 13-day interval in 2D culture was reported by Harris et al. who measured glucose, lactate, and pyruvate production in the media [94]. Both glucose consumption and lactate production increased in intact follicles throughout culture. Likewise, glycolytic ATP production increased throughout culture with a marked rise in energy production after ovulation induction in vitro. A low level of non-oxidative glucose consumption was noted early in the culture interval, suggesting that glycolysis may not be a major source of energy production in preantral follicles in vitro, but used for other cellular processes. As follicle growth progressed in vitro to the antral stage, glycolysis accounted for a greater proportion of glucose consumption. Pyruvate production by intact follicles was below the limit of detection. In vitro-developed follicles with fewer theca cells produced COCs with higher lactate formation, an indicator of cellular stress. In both in vitro- and in vivo-derived COCs, glucose was consumed, and pyruvate and lactate were produced, but in vitro-derived COCs consumed more glucose. Differences in rates of nutrient turnover between in vitro- and in vivo-derived COCs may be one important factor that contributes to the low oocyte competence observed in IVFD, and deserves further investigation.
Seven years later, the metabolite profile of murine primary and secondary follicles in serum-free 2D culture in different O2 environments was assessed using proton nuclear magnetic resonance [95]. A high O2 environment accelerated glucose utilization of early preantral stage follicles, showing evidence for up-regulation of the TCA cycle and oxidative phosphorylation. By Day 8, follicles in 20% O2 exhibited a slower glucose utilization, while maintaining high ATP production and oxidative phosphorylation. In contrast, the follicle was relatively inactive with respect to glucose consumption on Days 1–4 in the presence of 5% O2 which agreed with earlier observations [94]. Production of lactate on Day 8 via glycolysis was observed in healthy follicles in 5% O2, with half of the glucose directed to lactate and the other half to either the TCA cycle or the pentose phosphate pathway (PPP). Directing metabolism to the PPP results in the generation of nucleotide precursors and NADPH (nicotinamide adenine dinucleotide phosphate) for biosynthesis, as well as provision of reduced glutathione, a key intracellular anti-oxidant. The consumption and production of key amino acids indicated that preantral follicles in 5% O2 were metabolically active during early culture, and the metabolic rate decreased in antral follicles.
Lipid metabolism also contributes to the generation of ATP; adequate intracellular ATP is required for optimal oocyte developmental competence [96]. Fatty acids could be delivered to follicles in vitro through the media environment in the absence of an in vivo circulation, or generated intracellularly from lipid droplet stores [97]; the relative importance of each has not been thoroughly examined in the oocyte or follicular cells during IVFD. Regardless, fatty acid consumption to yield ATP occurs in the mitochondrial matrix through the process of β-oxidation. In COCs that mature in vitro compared with those matured in vivo, β-oxidation reduced in many species and was associated with dysregulation of genes involved in the process [96]. The entry of long-chain fatty acids into mitochondria for β-oxidation and ATP production requires a co-factor, carnitine. While carnitine is also an anti-oxidant, its beneficial effects on oocyte maturation are most likely due to the support of lipid metabolism. Supplementation of culture media with L-carnitine significantly enhanced both embryo cleavage rates and the number of blastocysts generated from oocytes derived from 2D culture of murine secondary follicles (Table 1) [98]. Of all the media supplements evaluated in the literature for all species, L-carnitine has had the most notable effect on improving oocyte developmental competence during IVFD; whether this translates to more offspring awaits investigation.
During preantral development, follicles utilize a combination of glycolysis and aerobic glucose metabolism, but during antrum formation glucose consumption is predominantly driven by glycolysis. Metabolism differed between in vitro- and in vivo-derived oocytes. Both GCs and theca cells, as well as cross-talk between the somatic compartments and the oocyte, are critical for normal folliculogenesis and oogenesis. The structural and functional interactions between oocytes and GCs/cumulus cells are essential for metabolic stability within the follicle. Thus, the culture environment can contribute significantly to altered metabolic profiles that will impact oocyte quality. Therefore, a more careful, detailed analysis of metabolism and nutrient availability during IVFD, as has been studied in COCs during IVM [99], is greatly needed in order to identify factors that promote oocyte developmental competence. Carnitine was beneficial to developmental competence during IVFD of murine preantral follicles [98]. Further studies are thus warranted to determine whether the favorable effects of L-carnitine on oocyte competence in IVFD can be observed in other species.
Future investigative frontiers for IVFD
Efficiency of embryo production from cultured follicles is meager, but innovative culture systems hold promise for improvement
Clearly, preantral follicles from many species, including laboratory rodents, domestic animals, nonhuman primates, and humans, can grow in vitro, produce a variety of hormones, and yield mature oocytes. A plethora of studies conducted over the past decades have investigated different culture conditions and media supplementations in order to optimize IVFD [12, 14]. The question still remains as to identification of the specific culture condition for a given species that permits both efficient and consistent production of competent oocytes during IVFD that can eventually yield healthy offspring. While a few offspring have been reported to show proof-of-concept of the ability of IVFD to support oocyte competency, studies describing the repeatability of this critical event are lacking in the literature, most likely due to the inability of most current culture systems and/or quality of the individual follicle to sustain the proper coordination of both somatic and gametogenic events in vitro. Mouse IVFD probably comes the closest at the current time, but considerable room for improvement remains, particularly for other mammalian species that require longer intervals for folliculogenesis leading to competent oocytes. Of interest is the potential for microfluidic systems to more closely mimic in vivo conditions during preantral follicular development in vitro [15, 100–102]. By controlling the rate of media perfusion, autocrine/paracrine factors could be maintained in the follicular environment like in static culture, but with more dynamic exchange of nutrients and oxygen during development of individual follicles, or co-culture using groups of follicles. Indeed, solo, dual and multi-platform microfluidic devices supported the growth and maturation of murine primary follicles with physiological steroid production [103]; however, oocyte harvest post-culture for fertilization has not yet been reported. Whether microfluidic follicle culture will be advantageous for enhancing oocyte competence to allow increased efficiency of embryo production leading to live offspring remains of considerable interest in the field.
Selection of follicles for culture and their inherent heterogeneity affect oocyte competence
In the mammalian ovary, a cohort of primordial follicles and their enclosed oocytes are activated to periodically enter the growth phase, but fewer undergo subsequent growth to the preovulatory stage that precedes ovulation of a mature, competent oocyte. The vast majority of follicles/oocytes undergo atresia and degenerate at various times across the follicle growth trajectory. Preantral follicle development in vitro has been touted as a way to remove as many follicles as possible from the ovary that would otherwise naturally degenerate. An alternate view is that by starting with follicles at the primary or secondary stages, rather than the primordial stage, may be more advantageous in that these follicles have already passed through one or two stages of selection in vivo, and as such will increase the odds of successful IVFD thereby increasing competent oocyte yield.
That preantral follicles of a given class with similar morphology and survivability in culture are not equal with respect to their developmental progression is an emerging theme that must be considered, particularly in species wherein follicle selection in vivo takes place over years or decades. Evidence of differing rates of follicle growth in vitro suggests some inherent selection for the “best” follicles may already be in play at the initiation of follicle culture. Variations in growth rates of preantral follicles that survive during IVFD have been noted in mice [31, 34, 104], goats [45, 105], cattle [106], and macaques [59, 60, 107]. For example, macaque secondary follicles encapsulated in alginate and cultured in the presence of FSH revealed three distinct cohorts based on follicle diameters at week 5 [59, 107]; “no-grow” follicles remained similar in size to secondary follicles with no change in diameter (<250 μm); “slow-grow” follicles doubled their diameters (250–500 μm); and “fast-grow” follicles increased their diameters >3-fold (>500 μm, in some instances >1000 μm). Both slow- and fast-grow follicles developed an antral cavity within 3–4 weeks of culture. The first report of human secondary follicles in 3D culture did not observe these differences in growth rates, but it was noted that the majority of surviving follicles developed antrum while a lesser cohort remained at the multilayer stage over 30 days of culture [108]. Likewise, no-grow and fast-grow follicle cohorts were observed during months of culture when macaque primary follicles were encapsulated in fibrin-alginate [60]. Most notably, in macaques, fast-grow follicles are the only cohort to yield mature oocytes and/or oocytes capable of embryonic development (see Table 3). Faster growing murine follicles also displayed increased fertilization rates and a live birth [31]. In contrast, accelerated growth of caprine or bovine secondary follicles inhibited survival and suppressed oocyte meiotic resumption [105, 106]. Differences in growth rate may reflect variable responses to FSH in vitro or the ability to synthesize and respond to autocrine/paracrine factors that modulate follicular growth. In this regard, AMH measured in media was significantly elevated by week 1 of culture in fast-grow compared to no-grow follicles [59, 60, 107]. This inherent heterogeneity is also evident in human early antral follicles and is associated with the capacity for oocyte maturation in vitro [109].
It may be impossible to “correct” a follicle in vitro that might normally be passed up for future selection in vivo due to inherent abnormalities that would normally defer it down the pathway of atresia. Thus, noninvasive biomarkers of follicle health and competency at the beginning of culture, or shortly after culture begins, like AMH in primates [67], COMP in mice [79] or perhaps GDF9 and/or BMP15 [110] will be very useful for selecting a priori not only those that are most likely to continue normal development, but also to produce a competent oocyte. This will assist in determining the maximum limit of efficient embryo production in a specific culture system given the inherent heterogeneity of follicles that are chosen for culture.
Antral follicles developed from the preantral stage in vitro need to attain adequate diameters in order to support oocyte competence
While this does not appear to be an issue for mice that attain mature follicles with competent oocytes at diameters of ~350 μm, determining the largest follicle diameter necessary to support oocyte competence is critical for species that develop considerably larger preovulatory follicles during normal reproductive cycles (i.e., macaques: 6 mm, cattle: ≥12 mm, humans: ≥15 mm). These larger diameters may never be achievable in vitro, and perhaps they are not necessary. Peluffo and colleagues determined that, for antral follicles isolated from the ovarian medulla of adult macaques during the early follicular phase of normal menstrual cycles, the minimal diameter was 500 μm to support oocyte developmental competence [111]. This diameter is easily attained in the current culture systems; macaque follicles >1000 μm derived from matrix-free primary and/or secondary follicle culture can yield mature oocytes capable of embryonic development (Table 3) [66]. Human secondary follicles developed in vitro to diameters of 500–1000 μm [69, 73, 108]; however, their ability to support embryonic development could not be assessed. Nonetheless, aspiration of in vivo-developed antral follicles with diameters ≤6 mm yielded COCs for IVM to generate mature oocytes that resulted in births via IVF [112]. Continued improvements in IVM methods for human oocytes to acquire developmental potential preclude the necessity for completing all steps of final oocyte development during IVFD [113, 114]. Comparisons are needed between oocytes matured within follicles grown in vitro and COCs extracted from in vitro-developed follicles for more up-to-date IVM to determine whether in follicle maturation or IVM can contribute to enhancement of oocyte competence. Thus, optimizing preantral follicle development in vitro is paramount to set the stage for coordinated oocyte development that will also support subsequent IVM as a means for obtaining offspring.
Comparative “omics” of follicles developed in vitro to in vivo as tools to aid acquisition of oocyte competence
Current culture systems robustly support follicle growth to a diameter necessary for oocyte nuclear maturation, steroidogenesis, and production of paracrine factors, but fall far short of providing the penultimate prize: a fully competent oocyte. Some guidelines to establish “quality control” endpoints for developing the best follicle and its enclosed oocyte are needed for each step of the trajectory of a preantral follicle to an antral follicle capable of generating a competent oocyte. Comparisons between antral follicles obtained from IVFD and the “gold standard” of those grown in vivo with respect to transcriptomics [86, 87], epigenetic profiles [75], metabolomics [99, 115], proteomics [116, 117], and secretomics [118–120] are emerging areas of future focus for unraveling the mechanisms necessary for establishment of normal follicular development in vitro. “Omics” approaches may provide some clues as to what may be missing in current culture systems, what may be aberrantly produced to negatively impact oocyte competence, or what mechanisms may remain intact to allow the coordination of oogenesis with folliculogenesis in vitro. Approaches used for detailed characterization of the critical communication between the oocyte and its surrounding cumulus cells for purposes of improving IVM, including transcriptomics to identify functional gene regulatory circuits [121] and metabolomics to understand the co-dependent metabolic processes [99, 122, 123] that are associated with oocyte developmental competence, can serve as strategies in future follicle culture experiments. Likewise, single-cell transcriptomic analysis of human antral follicle maturation [88, 89] provides a basis for continued analysis of in vitro follicular remodeling that includes GC and theca cell differentiation, and may identify novel factors essential for oocyte-driven functions, such as cumulus differentiation [124], and establishment of oocyte-somatic cell communication [125, 126]. A resident population of immune cells was recently identified in murine follicles prior to the preovulatory stage using single-cell transcriptomics [87], which raises questions as to their presence and/or absence in follicles developed in vitro without a circulating blood supply. Age-related changes in single oocyte gene expression from preovulatory human [92, 93] and nonhuman primate follicles [91] are associated with decreased oocyte competence and can be compared with that of oocytes derived from IVFD to look for ways to improve follicle culture. Further studies in the cellular compartmentalization of these molecular events at each stage of follicular development in vitro, as has been performed in murine ovaries ex vivo [127], will be an important feature to include and critical to guide optimization of IVFD methods. In the context of IVM, Richani et al. reviewed in detail some new technologies of non-invasive imaging to evaluate co-dependent metabolic processes in COCs that could be considered for implementation in follicle culture systems [99]. Proteomic analyses using matrix-assisted laser desorption/ionization-mass spectrometry (MALDI-MS) and mass spectrometry imaging (MSI) in murine follicles from IVFD, as well as single bovine primordial, primary and secondary follicles derived in vivo, identified, for the first time, unique patterns of differential molecular profiles of preantral follicles [116, 117]; comparisons between in vivo- and in vitro-derived follicles within a species are warranted. Innovative methods for mapping the secretome, such as TRanscriptional Activity CEllular aRray (TRACER) combined with data-driven multivariate modeling, were used for the first time to chart paracrine signaling across the trajectory of murine folliculogenesis in vitro [118], which could be applied for IVFD in other species and correlated to oocyte competency.
Dynamic oxygen levels during IVFD will better mimic the ovarian microenvironment in vivo
Preantral follicles are located in the ovarian cortex, an avascular region without ready access to the circulation [128, 129]. The expression of minichromosome maintenance proteins by preantral follicles is important for preventing cell cycle inhibition by physiological hypoxia, and thus promoting GC proliferation and follicle growth [130, 131]. Additionally, creatine metabolism catalyzed by creatine kinases is essential for energy production to support cell cycle progression of GCs in preantral follicles [132], when ATP generated by mitochondrial respiration is limited under hypoxic conditions. In contrast, antral follicles reside in a highly vascularized medulla region of the ovary and are surrounded by capillary network [128]. The increased ambient oxygen levels in this microenvironment and mitochondrial respiratory capacity in GCs are important for active GC proliferation, steroid hormone production, and oocyte development during antral follicle maturation [128, 133, 134]. Therefore, maintaining cell cycle progression and cellular energy homeostasis is a fundamental process to match demands of follicles at different developmental stages in their particular microenvironment.
For in vitro development of preantral follicles, the hypoxic conditions could be mimicked using a low O2 culture [59]. O2-dependent cellular adaptation for energy production was demonstrated by a study, in which mouse preantral follicles cultured at 5% O2 had increased utilization of arginine and glycine (substrates for creatine biosynthesis) and decreased utilization of branched-chain amino acids (substrates for mitochondrial oxidative metabolism) [95]. Therefore, the IVFD protocol can be improved by controlling cell cycle progression and cellular energy production to support stage-specific follicular development with differential oxygenation, which affects developmental competence of the enclosed oocyte [135].
Cryopreservation of individual preantral follicles with subsequent development in vitro will be important for fertility preservation
In order to fulfill the promise of fertility preservation for human patients and species propagation, a method for cryopreserving individual follicles will require development in tandem with optimizing culture conditions as well as complimenting emerging technologies that utilize bioengineered matrices intricately designed to support the growth and maturation of follicles and their enclosed oocytes [136–138]. Live offspring in mice [139, 140] and embryos in cattle [141] have been reported after slow-freezing and thawing of individual preantral follicles subsequently cultured in vitro. Undoubtedly, this adds another layer of complexity to the entire process of IVFD as cryodamage to TZPs would hinder subsequent oocyte competence [142, 143]. However, the ability of cryopreserved follicles to resume growth and oocyte development in vitro in mice [144–146], goats [147, 148], cattle [143], and macaques [142] suggests that continued development of this technology may be useful both experimentally and clinically, contingent upon whether cryopreserved follicles can support oocyte competence for pregnancy. For example, successful cryopreservation of murine follicles exhibiting subsequent growth and oocyte maturation during culture can provide a basis for high-throughput testing of environmental toxicants or screening of novel anti-cancer therapies with unknown effects on ovarian function [145]. Importantly, cryo-storage of a patient’s own follicles for subsequent use to restore fertility after surviving cancers that are contraindicated for ovarian tissue transplantation due to the risk of transmitting malignant cells is one of the very few options available for young patients. Likewise, preservation of endangered species can benefit from the availability of cryopreserved follicles for subsequent IVFD with the purpose of species propagation.
Conclusion
The advent of IVFD decades ago heralded a paradigm shift in the ability to study discrete events of folliculogenesis in a controlled environment. The lofty goal of using IVFD for production of healthy offspring for a number of conditions and uses has yet to be achieved in a consistent manner for most species. Placing more emphasis on understanding the basic physiological processes necessary for oogenesis and folliculogenesis to proceed in a coordinated manner in vitro toward production of competent oocytes will allow more advancement in the field, as will interrogating the bioinformation arising from “omic” technologies into testable hypotheses using intact follicles in vitro. Comparisons between antral follicles and their enclosed oocytes produced via IVFD and those derived in vivo will provide critical information as to which pathways are critical for cross-talk between somatic cells and the oocyte and to identify those that may be deficient in vitro, and importantly, those that are maintained (Figure 1). Optimization of IVFD for the improvement of oocyte competence to maximum efficiency will reflect more “authentic” follicle biology. As with every technology that aspires to mimic events in nature, continued investigations will eventually give way to breakthroughs for improving oocyte competency during IVFD, leading to increased production of desired offspring as well as optimization of IVFD systems for screening of agents that may harm female reproduction. How exciting, albeit challenging, it will eventually be to integrate findings from modern “omics” and bioengineering approaches to create a comparative ‘map’ of folliculogenesis as it occurs in vitro and in vivo, and to chart the routes that will lead toward optimal oocyte competence.
Figure 1.
Bridging the gap to improving oocyte competence in IVFD. Follicles from early developmental stages can develop to the antral stage in vitro in the optimized culture systems. Comparing follicles developed in vitro and in vivo will identify critical factors that can improve IVFD to produce mature oocytes competent for embryonic development resulting in live offspring in various species. 2D, two-dimensional; 3D, three-dimensional; IVM, in vitro maturation.
Footnotes
† Grant Support: This work was supported by the National Institutes of Health (NIH) Eunice Kennedy Shriver National Institute of Child Health & Human Development 2R01HD082208 (JX), R01HD45787 (MBZ) and P51OD011092 DPCPSI, ORIP, NIH (Oregon National Primate Research Center). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Contributor Information
Jing Xu, Division of Reproductive & Developmental Sciences, Oregon National Primate Research Center, Oregon Health & Science University, Beaverton, OR, USA; Department of Obstetrics and Gynecology, School of Medicine, Oregon Health & Science University, Portland, OR, USA.
Mary B Zelinski, Division of Reproductive & Developmental Sciences, Oregon National Primate Research Center, Oregon Health & Science University, Beaverton, OR, USA; Department of Obstetrics and Gynecology, School of Medicine, Oregon Health & Science University, Portland, OR, USA.
Authors’ contributions
MBZ conceived the topic. JX and MBZ reviewed the literature and wrote the manuscript. All authors contributed to critical manuscript revising for important intellectual content and approved the final version of the manuscript to be submitted for publication.
Conflict of interest
The authors do not have any potential or actual conflicts of interest with respect to the work reported in the article.
Data availability
The data underlying this article are available in the article.
References
- 1. Xu J, Xu M, Bernuci MP, Fisher TE, Shea LD, Woodruff TK, Zelinski MB, Stouffer RL. Primate follicular development and oocyte maturation in vitro. Adv Exp Med Biol 2013; 761:43–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cortvrindt RG, Smitz JEJ. Follicle culture in reproductive toxicology: a tool for in-vitro testing of ovarian function? Hum Reprod Update 2002; 8:243–254. [DOI] [PubMed] [Google Scholar]
- 3. Zhou H, Shikanov A. Three-dimensional hydrogel-based culture to study the effects of toxicants on ovarian follicles. Methods Mol Biol 2018; 758:55–72. [DOI] [PubMed] [Google Scholar]
- 4. Telfer EE, Zelinski MB. Ovarian follicle culture: advances and challenges for human and nonhuman primates. Fertil Steril 2013; 99:1523–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Duncan FE, Feinberg E, Brannigan RE, Edmonds M, Ataman L, Woodruff TK. Fertility preservation. Chapter 33. In: Strauss J, Barbieri R (eds.), Yen & Jaffe's Reproductive Endocrinology, 8th ed. Elsevier Inc., Philadelphia, PA. 2017: 857–886.
- 6. Silva JRV, van den Hurk R, Figueiredo JR. Ovarian follicle development in vitro and oocyte competence: advances and challenges for farm animals. Domestic Anim Endocrinol 2016; 55:123–135. [DOI] [PubMed] [Google Scholar]
- 7. Campos LB, Praxeded ECG, Saraiva MVA, Comizzoli P, Silva AR. Advances and challenges of using ovarian preantral follicles to develop biobanks of wild mammals. Biopres Biobanking 2019; 4:334–341. [DOI] [PubMed] [Google Scholar]
- 8. Cortvrindt R, Smitz J. In vitro follicle growth: achievements in mammalian species. Reprod Dom Anim 2001; 36:3–9. [DOI] [PubMed] [Google Scholar]
- 9. Picton HM, Harris SE, Muruvi W, Chamgers EL. The in vitro growth and maturation of follicles. Reproduction 2008; 136:703–715. [DOI] [PubMed] [Google Scholar]
- 10. Albertini DF, Akkoyunlu G. Ovarian follicle culture systems for mammals. Meth Enzymol 2010; 476:107–121. [DOI] [PubMed] [Google Scholar]
- 11. Green LJ, Shikanov A. In vitro culture methods of preantral follicles. Theriogenology 2016; 86:229–238. [DOI] [PubMed] [Google Scholar]
- 12. Figueiredo JR, Cadenas J, Lima LF, Santos RR. Advances in in vitro folliculogenesis in domestic ruminants. Anim Reprod 2019; 16:52–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Telfer EE, Andersen CY. In vitro growth and maturation of primordial follicles and immature oocytes. Fertil Steril 2021; 115:1116–1125. [DOI] [PubMed] [Google Scholar]
- 14. Simon LE, Kumar TR, Duncan FE. In vitro ovarian follicle growth: a comprehensive analysis of key protocol variables. Biol Reprod 2020; 103:455–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Desai N, Alex A, Calabro AHF, A, Goldfarb J, Fleischman A, Falcone T. Three-dimensional in vitro follicle growth: overview of culture models, biomaterials, design parameters and future directions. Reprod Biol Endocrinol 2010; 8:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Grosbois J, Devos M, Demeestere I. Implications of nonphysiological ovarian primordial follicle activation for fertility preservation. Endocrine Rev 2020; 41:1–26. [DOI] [PubMed] [Google Scholar]
- 17. Ford EA, Beckett EL, Roman SD, McLaughlin EA, Sutherland JM. Advances in human primordial follicle activation and premature ovarian insufficiency. Reproduction 2020; 159:R15–R29. [DOI] [PubMed] [Google Scholar]
- 18. Yang Q, Zhu L, Jin L. Human follicle in vitro culture including activation, growth, and maturation: a review of research progress. Front Endocrinol 2020; 11:548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhao Y, Feng H, Zhang Y, Zhang JV, Wang X, Liu D, Wang T, Li RHW, Ng EHY, Yeung WSB, Rodriguez-Wallberg KA, Liu K. Current understandings of core pathways for the activation of mammalian primordial follicles. Cells 2021; 10:1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Saitou M, Hayashi K. Mammalian in vitro gametogenesis. Science 2021; 374:eaaz6830. [DOI] [PubMed] [Google Scholar]
- 21. Conti M, Franciosi F. Acquisition of oocyte competence to develop as an embryo: integrated nuclear and cytoplasmic events. Hum Reprod Update 2018; 24:245–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zuccotti M, Merico V, Ceccfoni S, Redi CA, Garagna S. What does it take to make a developmentally competent mammalian egg? Hum Reprod Update 2011; 17:525–540. [DOI] [PubMed] [Google Scholar]
- 23. McGinnis LK, Limback SD, Albertini DF. Signaling modalities during oogenesis in mammals. Curr Top Dev Biol 2013; 102:227–242. [DOI] [PubMed] [Google Scholar]
- 24. Zhang Y, Wang Y, Feng X, Zhang S, Xu X, Li L, Niu S, Bo Y, Wang C, Li Z, Xia G, Zhang H. Oocyte-derived microvilli control female fertility by optimizing ovarian follicle selection in mice. Nat Commun 2021; 12:2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Eppig JJ. Oocyte control of ovarian follicular development and function in mammals. Reproduction 2001; 122:829–838. [DOI] [PubMed] [Google Scholar]
- 26. Hutt KJ, Albertini DF. An oocentric view of folliculogenesis and embryogenesis. Reprod Biomed Online 2007; 14:758–764. [DOI] [PubMed] [Google Scholar]
- 27. Eppig JJ, Schroeder AC. Capacity of mouse oocytes from preantral follicles to undergo embryogenesis and development to live young after growth, maturation, and fertilization in vitro. Biol Reprod 1989; 41:268–276. [DOI] [PubMed] [Google Scholar]
- 28. Eppig JJ, Wigglesworth K, O’Brien MJ. Comparison of embryonic developmental competence of mouse oocyes grown with and without serum. Mol Reprod Dev 1992; 32:3–40. [DOI] [PubMed] [Google Scholar]
- 29. Eppig JJ, O’Brien MJ. Development in vitro of mouse oocytes from primordial follicles. Biol Reprod 1996; 54:197–207. [DOI] [PubMed] [Google Scholar]
- 30. O’Brien MJ, Pendola JK, Eppig JJ. A revised protocol for in vitro development of mouse oocytes from primordial follicles dramatically improves their developmental competence. Biol Reprod 2003; 68:1682–1686. [DOI] [PubMed] [Google Scholar]
- 31. Spears N, Boland NI, Murray AA, Godsen RG. Mouse oocytes derived from in vitro grown primary ovarian follicles are fertile. Hum Reprod 1994; 9:527–532. [DOI] [PubMed] [Google Scholar]
- 32. Cortvrindt RG, Hu Y, Liu J, Smitz JE. Timed analysis of the nuclear maturation of oocytes in early preantral mouse follicle culture supplemented with recombinant gonadotropin. Fertil Steril 1998; 70:1114–1125. [DOI] [PubMed] [Google Scholar]
- 33. Cortvrindt R, Smitz J, Van Sterteghem AC. In-vitro maturation, fertilization and embryo development of immature oocytes from early preantral follicles from perpubertal mice in a simplified culture system. Hum Reprod 1996; 11:2656–2666. [DOI] [PubMed] [Google Scholar]
- 34. Smitz JEJ, Cortvrindt RG. The earliest stages of folliculogenesis in vitro. Reproduction 2002; 123:185–202. [DOI] [PubMed] [Google Scholar]
- 35. Xu M, Kreeger PK, Shea LD, Woodruff TK. Tissue-engineered follicles produce live, fertile offspring. Tiss Eng 2006; 12:2739–2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Xu M, West E, Shea LD, Woodruff TK. Identification of a stage-specific permissive in vitro culture environment for follicle growth and oocyte development. Biol Reprod 2006; 75:916–923. [DOI] [PubMed] [Google Scholar]
- 37. Mainigi MA, Ord T, Schultz RM. Meiotic and developmental competence in mice are compromised following follicle development in vitro using an alginate-based culture system. Biol Reprod 2011; 85:269–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jin SY, Lei L, Shikanov A, Shea LD, Woodruff TK. A novel two-step strategy for in vitro culture of early-stage ovarian follicles in the mouse. Fertil Steril 2010; 93:2633–2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mochida N, Akatani-Hasegawa A, Saka K, Ogino M, Hosoda Y, Wada R, Sawai H, Shibahara H. Live births from isolated primary/early secondary follicles following a multistep culture without organ culture in mice. Reproduction 2013; 146:37–47. [DOI] [PubMed] [Google Scholar]
- 40. Ota S, Ikeda S, Takashima T, Obata Y. Optimal conditions for mouse follicle culture. J Reprod Devel 2021; 67:327–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yamomoto K, Otoi T, Koyama N, Horikita N, Tachikawa S, Miyano T. Development of live young from bovine small oocytes after growth, maturation and fertilization in vitro. Theriogenology 1999; 52:81–89. [DOI] [PubMed] [Google Scholar]
- 42. Hirao Y, Itoh T, Shimizu M, Iga K, Aoyagi K, Kobayashi M, Kacchi M, Hoshi H, Takenouchi N. In vitro growth and development of bovine oocyte-granulosa cell complexes on the flat substratum: effects of high polyvinylpyrrolidone concentration in culture medium. Biol Reprod 2004; 70:83–91. [DOI] [PubMed] [Google Scholar]
- 43. Saraiva MVA, Rossetto R, Brito IR, JJH C, CMG S, Faustino LR, Almeida AP, Bruno JB, Magalhaes DM, MHT M, Campello CC, Figueiredo JR. Dynamic medium produces caprine embryo from preantral follicles grown in vitro. Repro Sci 2010; 17:1135–1143. [DOI] [PubMed] [Google Scholar]
- 44. Malgahaes DM, Duarte ABG, Araujo VR, Vrito IR, Soares TG, Lima IMT, Lopes CAP, Campello CC, Rodrigues APR, Figueiredo JR. In vitro production of a caprine embryo from a preantral follicle cultures in media supplemented with growth hormone. Theriogenology 2011; 75:182–188. [DOI] [PubMed] [Google Scholar]
- 45. Silva GM, Rossetto R, Salles MGF, Sa MFS, Silva SCJS RE, APR R, Campello CC, Figueiredo JR. Survival, growth, and hormone production in vitro from preantral follicles of crossbred female goats (Saanen × Anglo-Nubian). Anim Reprod 2014; 11:56–60. [Google Scholar]
- 46. Brito IR, Silva GM, Slaes AD, Lobo CH, Rodrigues GQ, Sousa RF, Moura AAA, Calderon CEM, Bertolini M, Campello CC, Smitz J, Figueiredo JR. Fibrin-alginate hydrogel supports steroidogenesis, in vitro maturation of oocytes and parthenotes production from caprine preantral follicles cultured in group. Reprod Dom Anim 2016; 51:997–1009. [DOI] [PubMed] [Google Scholar]
- 47. de Sa NAR, Ferreira ACA, Sousa FGC, Duarte ABG, Paes VM, Cadenas J, Anjos JC, Fernandes CCL, Roseseto R, Cibin FWS, Alves BG, Rodrigues APR et al. First pregnancy after in vitro culture of early antral follicles in goats: positive effects of anethole on follicle development and steroidogenesis. Mol Reprod Devel 2020; 87:966–977. [DOI] [PubMed] [Google Scholar]
- 48. Arunakumari G, Shanmugasundaram N, Rao VH. Development of morulae from the oocytes of culture sheep preantral follicles. Theriogenology 2010; 74:884–894. [DOI] [PubMed] [Google Scholar]
- 49. Barboni B, Russo V, Cecconi S, Curini V, Colosimo A, Garofalo MLA, Capacchietti G, Di Giacinto O, Mattioli M. In vitro grown sheep preantral follicles yield oocyes with normal nuclear-epigenetic maturation. PLoS One 2011; 6:e27550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Luz VB, Araujo VR, Duarte ABG, Celestino JJH, Silva TFP, Magalhaes-Padilha DM, Chaves RN, Brito IR, Almeida AP, Campello CC, Feltrin C, Bertolini M et al. Eight-cell parthenotes originated from in vitro grown sheep preantral follicles. Reprod Sci 2012; 19:1219–1225. [DOI] [PubMed] [Google Scholar]
- 51. Cadoret V, Jarrier-Gaillared P, Papillier P, Monniaux D, Fuerif F, Dalbeis-Tran R. Leukemia-inhibitory factor modulates the differentiation of granulosa cells during sheep in vitro preantral to antral follicle development and improves oocyte meiotic competence. Mol Hum Reprod 2021; 27:gaab051. [DOI] [PubMed] [Google Scholar]
- 52. Luz VB, Araujo VR, Duarte ABG, Silva GM, Chaves RN, Brito IR, Srafim MKB, Campello CC, Feltrin C, Bertolini M, Almeida AP, Santos RR et al. Kit ligand and insulin-like growth factor I affect the in vitro development of ovine preantral follicles. Small Rumin Res 2013; 115:99–102. [Google Scholar]
- 53. Wu J, Emery BR, Carrell DT. In vitro growth, maturation, fertilization, and embryonic development of oocytes from porcine preantral follicles. Biol Reprod 2001; 64:375–381. [DOI] [PubMed] [Google Scholar]
- 54. Wu J, Carrell DT, Wilcox AL. Development of in vitro-matured oocytes from porcine preantral follicles following intracytoplasmic sperm injection. Biol Reprod 2001; 65:1579–1585. [DOI] [PubMed] [Google Scholar]
- 55. Gupta PSP, Ramesh HS, Manjunatha BM, Nandi S, Ravindra JP. Production of buffalo embryos using oocytes from in vitro grown preantral follicles. Zygote 2008; 16:57–63. [DOI] [PubMed] [Google Scholar]
- 56. Xu M, Fazleabas AT, Shikanov A, Jackson E, Barrett SL, Hirshfeld-Cytron J, Kiesewetter SE, Shea LD, Woodruff TK. In vitro oocyte maturation and preantral follicle culture from the luteal-phase baboon ovary produce mature oocytes. Biol Reprod 2011; 84:689–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Peluffo MC, Hennebold JD, Stouffer RL, Zelinski MB. Oocyte maturation and in vitro hormone production in small antral follicles (SAFs) isolated from rhesus monkeys. J Assist Reprod Genet 2013; 30:353–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kim YY, Yun JW, Kim JM, Park CG, Rosenwaks Z, Liu HC, Kang BC, Ku SY. Gonadotropin ratio affects the in vitro growth of rhesus ovarian preantral follicles. J Investig Med 2016; 64:888–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Xu J, Lawson MS, Yeoman RR, Pau KY, Barrett SL, Zelinski MB, Stouffer RL. Secondary follicle growth and oocyte maturation during encapsulated three-dimensional culture in rhesus monkeys: effects of gonadotrophins, oxygen and fetuin. Hum Reprod 2011; 26:1061–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Xu J, Lawson MS, Yeoman RR, Molskness TA, Ting AY, Stouffer RL, Zelinski MB. Fibrin promotes development and function of macaque primary follicles during encapsulated three-dimensional culture. Hum Reprod 2013; 28:2187–2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Xu J, McGee WK, Bishop CV, Park BS, Cameron JL, Zelinski MB, Stouffer RL. Exposure of female macaques to western-style diet with or without chronic T in vivo alters secondary follicle function during encapsulated 3-dimensional culture. Endocrinology 2015; 156:1133–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Rodrigues JK, Navarro PA, Zelinski MB, Stouffer RL, Xu J. Direct actions of androgens on the survival, growth and secretion of steroids and anti-Mullerian hormone by individual macaque follicles during three-dimensional culture. Hum Reprod 2015; 30:664–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ting AY, Xu J, Stouffer RL. Differential effects of estrogen and progesterone on development of primate secondary follicles in a steroid-depleted milieu in vitro. Hum Reprod 2015; 30:1907–1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Xu J, Lawson MS, Xu F, Du Y, Tkachenko OY, Bishop CV, Pejovic-Nezhat L, Seifer DB, Hennebold JD. Vitamin D3 regulates gollicular development and intrafollicular vitamin D biosynthesis and signaling in the primate ovary. Front Physiol 2018; 9:1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Xu J, Xu F, Lawson MS, Tkachenko OY, Ting AY, Kahl CA, Park BS, Stouffer RR, Bishop CV. Anti-Mullerian hormone is a survival factor and promotes the growth of rhesus macaque preantral follicles during matrix-free culture. Biol Reprod 2018; 98:197–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Xu J, Lawson MS, Mitalipov SM, Park BS, Xu F. Stage-specific modulation of antimullerian hormone promotes primate follicular development and oocyte maturation in the matrix-free three-dimensional culture. Fertil Steril 2018; 110:1162–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Xu J, Xu F, Letaw JH, Park BS, Searles RP, Ferguson BM. Anti-Mullerian hormone is produced heterogeneously in primate preantral follicles and is a potential biomarker for follicle growth and oocyte maturation in vitro. J Assist Reprod Genet 2016; 33:1665–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Xu J, Bishop CV, Lawson MS, Park BS, Xu F. Anti-Mullerian hormone promotes pre-antral follicle growth, but inhibits antral follicle maturation and dominant follicle selection in primates. Hum Reprod 2016; 31:1522–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Xiao S, Zhang J, Romero MM, Smith KN, Shea LD, Woodruff TK. In vitro follicle growth supports human oocyte meiotic maturation. Sci Rep 2015; 5:17323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Rose BI, Laky D. Polar body fragmentation in IVM oocytes is associated with impaired fertilization and embryo development. J Assist Reprod Genet 2013; 30:679–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. McLaughlin M, Albertini DF, Wallace WHB, Anderson RA, Telfer EE. Metaphase II oocytes from human unilaminar follicles grown in a multi-step culture system. Mol Hum Reprod 2018; 24:135–142. [DOI] [PubMed] [Google Scholar]
- 72. Coticchio G, Guglielmo MC, Dal Canto M, Fadini R, Mignini Renzini M, De Ponti E, Brambillasca F, Albertini DF. Mechanistic foundations of the metaphase II spindle of human oocytes matured in vivo and in vitro. Hum Reprod 2013; 28:3271–3282. [DOI] [PubMed] [Google Scholar]
- 73. Xu F, Lawson MS, Bean Y, Ting AY, Pejovic T, De Geest K, Moffitt M, Mitalipov SM, Xu J. Matrix-free 3D culture supports human follicular development from the unilaminar to the antral stage in vitro yielding morphologically normal metaphase II oocytes. Hum Reprod 2021; 36:1326–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Anckaert E, Adriaenssens T, Romero S, Dremier S, Smitz J. Unaltered imprinting establishment of key imprinted genes in mouse oocytes after in vitro follicle culture under variable follicle-stimulating hormone exposure. Int J Dev Biol 2009; 53:541–548. [DOI] [PubMed] [Google Scholar]
- 75. Anckaert E, Sánchez F, Billooye K, Smitz J. Dynamics of imprinted DNA methylation and gene transcription for imprinting establishment in mouse oocytes in relation to culture duration variability. Biol Reprod 2013; 89:130. [DOI] [PubMed] [Google Scholar]
- 76. Demond H, Trapphoff T, Dankert D, Heiligentag M, Grümmer R, Horsthemke B, Eichenlaub-Ritter U. Preovulatory aging in vivo and in vitro affects maturation rates, abundance of selected proteins, histone methylation pattern and spindle integrity in murine oocytes. PLoS One 2016; 11:e0162722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Saenz-de-Juano MD, Billooye K, Smitz J, Anckaert E. The loss of imprinted DNA methylation in mouse blastocysts is inflicted to a similar extent by in vitro follicle culture and ovulation induction. Mol Hum Reprod 2016; 22:427–441. [DOI] [PubMed] [Google Scholar]
- 78. Saenz-de-Juano MD, Ivanova E, Billooye K, Herta AC, Smitz J, Kelsey G, Anckaert E. Genome-wide assessment of DNA methylation in mouse oocytes reveals effects associated with in vitro growth, superovulation, and sexual maturity. Clin Epigenetics 2019; 11:197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Skory RM, Bernabé BP, Galdones E, Broadbelt LJ, Shea LD, Woodruff TK. Microarray analysis identifies COMP as the most differentially regulated transcript throughout in vitro follicle growth. Mol Reprod Dev 2013; 80:132–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Jones A, Bernabé BP, Padmanabhan V, Li J, Shikanov A. Capitalizing on transcriptome profiling to optimize and identify targets for promoting early murine folliculogenesis in vitro. Sci Rep 2021; 11:12517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Cadoret V, Frapsauce C, Jarrier P, Maillard V, Bonnet A, Locatelli Y, Royère D, Monniaux D, Guérif F, Monget P. Molecular evidence that follicle development is accelerated in vitro compared to in vivo. Reproduction 2017; 153:493–508. [DOI] [PubMed] [Google Scholar]
- 82. Sánchez F, Adriaenssens T, Romero S, Smitz J. Quantification of oocyte-specific transcripts in follicle-enclosed oocytes during antral development and maturation in vitro. Mol Hum Reprod 2009; 15:539–550. [DOI] [PubMed] [Google Scholar]
- 83. Sánchez F, Romero S, Smitz J. Oocyte and cumulus cell transcripts from cultured mouse follicles are induced to deviate from normal in vivo conditions by combinations of insulin, follicle-stimulating hormone, and human chorionic gonadotropin. Biol Reprod 2011; 85:565–574. [DOI] [PubMed] [Google Scholar]
- 84. Sánchez F, Romero S, Albuz FK, Smitz J. In vitro follicle growth under non-attachment conditions and decreased FSH levels reduces Lhcgr expression in cumulus cells and promotes oocyte developmental competence. J Assist Reprod Genet 2012; 29:141–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Takashima T, Fujimaru T, Obata Y. Effect of in vitro growth on mouse oocyte competency, mitochondria and transcriptome. Reproduction 2021; 162:307–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Bernabé BP, Woodruff T, Broadbelt LJ, Shea LD. Ligands, receptors, and transcription factors that mediate inter-cellular and intra-cellular communication during ovarian follicle development. Reprod Sci 2020; 27:690–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Li S, Chen L, Zhu H, Feng X, Xie F, Luo S, Ou X, Ma J. Single-cell RNA sequencing analysis of mouse follicular somatic cells. Biol Reprod 2021; 105:1234–1245. [DOI] [PubMed] [Google Scholar]
- 88. Fan X, Bialecka M, Moustakas I, Lam E, Torrnes-Juanda V, Borggreven NV, Trouw L, Louwe LA, GSK P, Mei H, van der Westerlaken L, De Sousa Lopes SM. Single-cell reconstruction of follicular remodeling in the human adult ovary. Nat Commun 2019; 10:3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Man L, Lustgarten-Guahmich N, Kallinos E, Redhead-Laconte Z, Liu S, Schattman B, Redmond D, Hancock K, Zaninovic N, Schattman G, Rosenwaks Z, James D. Comparison of human antral follicles of xenograft versus ovarian origin reveals disparate molecular signatures. Cell Rep 2020; 32:108027. [DOI] [PubMed] [Google Scholar]
- 90. Walker BN, Biase FH. The blueprint of RNA storages relative to oocyte developmental competence in cattle (Bos taurus). Biol Reprod 2020; 102:784–794. [DOI] [PubMed] [Google Scholar]
- 91. Wang S, Zheng Y, Li J, Yu Y, Zhang W, Song M, Liu Z, Min Z, Hu H, Jing Y, He X, Sun L et al. Single-cell transcriptomic atlas of primate ovarian aging. Cell 2020; 180:1–16. [DOI] [PubMed] [Google Scholar]
- 92. Zhang J-J, Liu X, Chen L, Zhang S, Zhang X, Hao C, Miao Y-L. Advanced maternal age alters expression of maternal effect genes that are essential for human oocyte quality. Aging 2020; 12:3950–3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Llonch S, Barragan M, Nieto P, Mallol A, Elosua-Bayes M, Lorden P, Ruiz S, Zambelli F, Heyn H, Vassena R, Payer B. Single human oocyte transcriptome analysis reveals distinct maturation stage-dependent pathways impacted by age. Aging Cell 2021; 20:e13360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Harris SE, Adriaens I, Leese HJ, Gosden RG, Picton HM. Carbohydrate metabolism by murine follicles and oocytes grown in vitro. Reproduction 2007; 134:415–424. [DOI] [PubMed] [Google Scholar]
- 95. Gook DA, Edgar DH, Lewis K, Sheedy JR, Gardner DK. Impact of oxygen concentration on adult murine pre-antral follicle development in vitro and the corresponding metabolic profile. Mol Hum Reprod 2014; 20:31–41. [DOI] [PubMed] [Google Scholar]
- 96. Dunning KR, Russell DL, Robker RL. Lipid and oocyte developmental competence: the role of fatty acids and β-oxidation. Reproduction 2014; 148:R15–R27. [DOI] [PubMed] [Google Scholar]
- 97. Pantasri T, Wu LL, Hull ML, Sullivan TR, Barry M, Norman RJ, Robker RL. Distinct localisation of lipids in the ovarian follicular environment. Reprod Fertil Dev 2015; 27:593–601. [DOI] [PubMed] [Google Scholar]
- 98. Dunning KR, Akison LK, Russell DL, Norman RJ, Robker RL. Increased beta-oxidation and improved oocyte developmental competence in response to L-carnitine during ovarian in vitro follicle development in mice. Biol Reprod 2011; 85:548–555. [DOI] [PubMed] [Google Scholar]
- 99. Richani D, Dunning KR, Thompson JG, Gilchrist RB. Metabolic co-dependence of the oocyte and cumulus cells: essential role in determining oocyte developmental competence. Hum Reprod Update 2021; 27:27–47. [DOI] [PubMed] [Google Scholar]
- 100. Choi JK, Agarwal P, Huang H, Zhao S, He X. The crucial role of mechanical heterogeneity in regulating follicle development and ovulation with engineered ovarian microtissue. Biomaterials 2014; 35:5122–5128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Aziz AUR, Fu M, Deng J, Geng C, Luo Y, Lin B, Yu X, Liu B. A microfluidic device for culturing an encapsulated ovarian follicle. Micromachines 2017; 8:335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Healy MW, Dolitsky SN, Villancio-Wolter M, Raghavan M, Tillman AR, Morgan NY, DeCherney AH, Park S, Wolff EF. Creating an artificial 3-dimensional ovarian follicle culture system using a microfluidic system. Micromachines 2021; 12:261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Xiao S, Coppeta JR, Rogers HB, Isenberg BC, Zhu J, Olalekan SA, McKinnon KE, Dokic D, Rashedi AS, Haisenleder DJ, Malpani SS, Arnold-Murray CA et al. A microfluidic culture model of the human reproductive tract and 28-day menstrual cycle. Nat Commun 2017; 8:14584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Rasmussen LM, Sen N, Vera JC, Liu X, Craig ZR. Effects of in vitro exposure to dibutyl phthalate, mono-butyl phthalate, and acetyl tributyl citrate on ovarian antral follicle growth and viability. Biol Reprod 2017; 96:1105–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Apollini LB, BrunoJB ABG, Ferreira ACA, Paes VM, Moreno JRC, Aguiar FLN, Brandao FZ, Smitz J, Apgar G, Figueiredo JR. Accelerated follicle growth during the culture of isolated caprine preantral follicles is detrimental to follicular survival and oocyte meiotic resumption. Theriogenology 2016; 86:1530–1540. [DOI] [PubMed] [Google Scholar]
- 106. Passos MJ, Vasconcelos GL, Silva AWB, Brito IR, Saraiva MVA, Magalhaes DM, Costa JJN, Donato MAM, Ribeiro RP, Cunha EV, Peixoto CA, Campello CC et al. Accelerated growth of bovine prantral follicles in vitro after stimulation with both FSH and BMP-15 is accompanied by ultrastructural changes and increased atresia. Theriogenology 2013; 79:1269–1277. [DOI] [PubMed] [Google Scholar]
- 107. Xu J, Bernuci MP, Lawson MS, Yeoman RR, Fisher TE, Zelinski MB, Stouffer RL. Survival, growth, and maturation of secondary follicles from prepubertal, young, and older adult rhesus monkeys during encapsulated three-dimensional culture: effects of gonadotropins and insulin. Reproduction 2010; 140:685–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Xu M, Barrett SL, West-Farrell E, Kondapalli LA, Kiesewetter SE, Shea LD, Woodruff TK. In vitro grown human ovarian follicles from cancer patients support oocyte growth. Hum Reprod 2009; 24:2531–2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Sánchez F, Romero S, De Vos M, Verheyen G, Smitz J. Human cumulus-enclosed germinal vesicle oocytes from early antral follicles reveal heterogeneous cellular and molecular features associated with in vitro maturation capacity. Hum Reprod 2015; 30:1396–1409. [DOI] [PubMed] [Google Scholar]
- 110. Sanfins A, Rodrigues P, Albertini DF. GDF-9 and BMP-15 direct the follicle symphony. J Assisted Reprod Genetics 2018; 35:741–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Peluffo MC, Barrett SL, Stouffer RL, Hennebold JD, Zelinski MB. Cumulus-oocyte complexes from small antral follicles during the early follicular phase of menstrual cycles in rhesus monkeys yield oocytes that reinitiate meiosis and fertilize in vitro. Biol Reprod 2010; 83:525–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Guzman L, Adriaenssens T, Ortega-Hrepich C, Albuz FK, Mateizel I, Devroey P, De Vos M, Smitz J. Human antral follicles <6 mm: a comparison between in vivo maturation and in vitro maturation in non-hCG primed cycles using cumulus cell gene expression. Mol Hum Reprod 2013; 19:7–16. [DOI] [PubMed] [Google Scholar]
- 113. Sanchez F, Le AH, Ho VNA, Romero S, Van Ranst H, De Vos M, Gilchrist RB, Ho TM, Vuong LN, Smitz J. Biphasic in vitro maturation (CAPA-IVM) specifically improves the developmental capacity of oocytes from small antral follicles. J Assist Reprod Genet 2019; 36:2135–2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Richani D, Gilchrist RB. Approaches to oocyte meiotic arrest in vitro and impact on oocyte developmental competence. Biol Reprod 2021, online ahead of print. 10.1093/biolre/ioab176. [DOI] [PubMed] [Google Scholar]
- 115. Gine R, Capellades J, Badia JM, Vughs D, Schwaiger-Haber M, Alexandrov T, Vinaixa M, Brunner AM, Patti GJ, Yanes O. HERMES: a molecular-formula-orientated method to target the metabolome. Nature Meth 2021; 18:1370–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Anastácio A, Rodriguez-Wallberg KA, Chardonnet S, Pionneau C, Fédérici C, Almeida Santos T, Poirot C. Protein profile of mouse ovarian follicles grown in vitro. Mol Hum Reprod 2017; 23:827–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Paulini F, Araujo MS, Silva LP, Lucci CM. Initial steps on mapping differentially expressed proteins in bovine preantral follicles and ovarian tissue: an approach using single-follicle MALDI-MS and mass spectrometry imaging (MSI) analysis. Reprod Domes Anim 2021, online ahead of print. 10.1111/rda.14025. [DOI] [PubMed] [Google Scholar]
- 118. Zhou H, Decker JT, Lemke MM, Tomaszweski CE, Shea LD, Arnold KB, Shikanov A. Synergy of paracrine signaling during early-stage mouse ovarian follicle development in vitro. Cell Mol Bioeng 2018; 11:435–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Strack R. Revealing the secretome. Nature Meth 2021; 18:1273. [DOI] [PubMed] [Google Scholar]
- 120. Wei W, Riley NM, Yang AC, Kim JT, Terrell SM, Li VL, Garcia-Contreras M, Bertozzi CR, Long JZ. Cell-type selective secretome profiling in vivo. Nat Chem Biol 2021; 17:326–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Biase FH, Kimble KM. Functional signaling and gene regulatory networks between the oocyte and the surrounding cumulus cells. BMC Genomics 2018; 19:351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Akin N, Von Mengden L, Herta A-C, Billoove K, van Leersum J, Cava-Cami B, Saucedo-Cuevas L, Klamt F, Smitz J, Anckaert E. Glucose metabolism characterization during mouse in vitro maturation identifies alterations in cumulus cells. Biol Reprod 2021; 104:902–913. [DOI] [PubMed] [Google Scholar]
- 123. Walter J, Huwiler F, Fortes C, Grossmann J, Roschitzki B, Hu J, Naegeli H, Laczko E, Bleul U. Analysis of the equine "cumulome" reveals major metabolic aberrations after maturation in vitro. BMC Genomics 2019; 20:588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Diaz FJ, Wigglesworth K, Eppig JJ. Oocytes determine cumulus cell lineage in mouse ovarian follicles. J Cell Sci 2007; 120:1330–1340. [DOI] [PubMed] [Google Scholar]
- 125. Eppig JJ, Schultz RM, O’Brien M, Chesnel F. Relationship between the developmental programs controlling nuclear and cytoplasmic maturation of mouse oocytes. Dev Biol 1994; 164:1–9. [DOI] [PubMed] [Google Scholar]
- 126. El-Hayek S, Yang Q, Abbassi L, FitzHarris G, Clarke HJ. Mammalian oocytes locally remodel follicular architecture to provide the foundation for germline-soma communication. Curr Biol 2018; 28:1124–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Cinco R, Digman MA, Gratton E, Luderer U. Spatial characterization of bioenergetics and metabolism of primordial to preovulatory follicles in whole ex vivo murine ovary. Biol Reprod 2016; 95:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Suzuki T, Sasano H, Takaya R, Fukaya T, Yajima A, Nagura H. Cyclic changes of vasculature and vascular phenotypes in normal human ovaries. Hum Reprod 1998; 13:953–959. [DOI] [PubMed] [Google Scholar]
- 129. Herrmann G, Spanel-Borowski K. A sparsely vascularised zone in the cortex of the bovine ovary. Anat Histol Embryol 1998; 27:143–146. [DOI] [PubMed] [Google Scholar]
- 130. Lutzmann M, Grey C, Traver S, Ganier O, Maya-Mendoza A, Ranisavljevic N, Bernex F, Nishiyama A, Montel N, Gavois E, Forichon L, de Massy B et al. MCM8- and MCM9-deficient mice reveal gametogenesis defects and genome instability due to impaired homologous recombination. Mol Cell 2012; 47:523–534. [DOI] [PubMed] [Google Scholar]
- 131. Stubbs SA, Stark J, Dilworth SM, Franks S, Hardy K. Abnormal preantral folliculogenesis in polycystic ovaries is associated with increased granulosa cell division. J Clin Endocrinol Metab 2007; 92:4418–4426. [DOI] [PubMed] [Google Scholar]
- 132. Naumoff PA, Stevenson PM. Creatine kinase, steroidogenesis and the developing ovarian follicle. Int J Biochem 1985; 17:1363–1367. [DOI] [PubMed] [Google Scholar]
- 133. Wang RS, Chang HY, Kao SH, Kao CH, Wu YC, Yeh S, Tzeng CR, Chang C. Abnormal mitochondrial function and impaired granulosa cell differentiation in androgen receptor knockout mice. Int J Mol Sci 2015; 16:9831–9849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Van Blerkom J, Antczak M, Schrader R. The developmental potential of the human oocyte is related to the dissolved oxygen content of follicular fluid: association with vascular endothelial growth factor levels and perifollicular blood flow characteristics. Hum Reprod 1997; 12:1047–1055. [DOI] [PubMed] [Google Scholar]
- 135. Eppig JJ, Wigglesworth K. Factors affecting the developmental competence of mouse oocytes grown in vitro: oxygen concentration. Mol Reprod Dev 1995; 42:447–456. [DOI] [PubMed] [Google Scholar]
- 136. Dolmans MM, Amorim CA. Construction and use of artificial ovaries. Reproduction 2019; 158:F15–F25. [DOI] [PubMed] [Google Scholar]
- 137. Jones ASK, Shikanov A. Follicle development as an orchestrated signaling network in a 3D organoid. J Biol Eng 2019; 13:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Laronda MM. Engineering a bioprosthetic ovary for fertility and hormone restoration. Theriogenology 2020; 150:8–14. [DOI] [PubMed] [Google Scholar]
- 139. Carroll J, Gosden RG. Transplantation of frozen-thawed mouse primordial follicles. Hum Reprod 1993; 8:1163–1167. [DOI] [PubMed] [Google Scholar]
- 140. de la Peña EC, Takahashi Y, Katagiri S, Atabay EC, Nagano M. Birth of pups after transfer of mouse embryos derived from vitrified preantral follicles. Reproduction 2002; 123:593–600. [DOI] [PubMed] [Google Scholar]
- 141. Bao RM, Yamasaka E, Moniruzzaman M, Hamawaki A, Yoshikawa M, Takashi MT. Development of vitrified bovine secondary and primordial follicles in xenografts. Theriogenology 2010; 74:817–827. [DOI] [PubMed] [Google Scholar]
- 142. Bulgarelli DM, Ting AY, Zelinski MB. Post-thaw function during 3-dimensional (3D) culture of isolated secondary follicles vitrified in a closed system: a new option for fertility preservation in young patients with cancer. Fertil Steril 2014; 102:E125–E126. [Google Scholar]
- 143. Bus A, Szymanska K, Pintelon I, Leroy JLMR, Leybaert L, Bols PEJ. Preservation of connexin 43 and transzonal projections in isolated bovine pre-antral follicles before and following vitrification. J Assist Reprod Genet 2021; 38:479–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Xu M, Banc A, Woodruff TK, Shea LD. Secondary follicle growth and oocyte maturation by culture in alginate hydrogel following cryopreservation of the ovary or individual follicles. Biotechnol Bioeng 2009; 103:378–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Wang Y, Xu J, Stanley JE, Xu M, Brooks BW, Scott GI, Chatterjee S, Zhang Q, Zelinski MB, Xiao S. A closed vitrification system enables a murine ovarian follicle bank for high-throughput ovotoxicity screening, which identifies endocrine disrupting activity of microcystins. Reprod Toxicol 2020; 93:118–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Wang Y, Russo DD, Pattarawat P, Zhang Q, Zelinski MB, Shalek AK, Goods BA, Xiao S. Vitrification preserves murine ovarian follicular cell transcriptome in a 3D encapsulated in vitro follicle growth system. Biol Reprod 2021; 105:1378–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Lunardi FO, de Aguiar FLN, Apolloni LB, Duarte ABG, de Sá NAR, Leal ÉSS, Sales AD, Lobo CH, Campello CC, Smitz J, Apgar GA, de Figueiredo JR et al. Sheep isolated secondary follicles are able to produce metaphase II oocytes after vitrification and long-term in vitro growth. Biopreserv Biobank 2017; 15:321–331. [DOI] [PubMed] [Google Scholar]
- 148. Lopes EPF, Rodrigues GQ, de Brito DCC, Rocha RMP, Ferreira ACA, de Sá NAR, Silva RFD, de Alcântara GLH, Alves BG, Figueiredo JR, Zelinski M, Rodrigues APR. Vitrification of caprine secondary and early antral follicles as a perspective to preserve fertility function. Reprod Biol 2020; 20:371–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article are available in the article.