Quinupristin-dalfopristin is a streptogramin and has recently been approved for human treatment (7). Streptogramins have been considered valuable antimicrobial drugs for treatment of infections with methicillin-resistant Staphylococcus aureus (MRSA) and multiresistant enterococci (8). Low frequencies of streptogramin resistance have been detected among Enterococcus faecium strains of human origin in Europe and the United States (8, 11), whereas streptogramin resistance has been detected frequently among E. faecium strains of animal origin (2, 12, 14), especially among poultry isolates (2, 14). The streptogramin virginiamycin has been used as a growth promoter in both Europe and the United States. Streptogramins consist of two compounds: streptogramin A and streptogramin B (4). Several genes encoding resistance to streptogramins in E. faecium have been described (10). Two genes encoding resistance to streptogramin A in E. faecium have been identified. These are vat(D) (formerly called satA [9]) and vat(E) (formerly called satG [15]). For streptogramin B resistance, the erm genes are known to encode macrolide, lincosamide, and streptogramin B resistance (MLSB) (13). Another gene encoding streptogramin B resistance in enterococci is vgb (1).
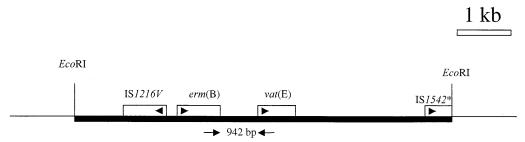
In an attempt to determine the genetic background for streptogramin resistance in E. faecium of poultry origin, a 7.5-kb EcoRI-digested DNA fragment was cloned from isolate F9731349-1 into pUC18Not (6), resulting in the plasmid pVIR1. The streptogramin-sensitive E. coli DB10 (9) (MIC of virginiamycin, 8 μg/ml; MIC of pristinamycin, 4 μg/ml) was used as a recipient. E. coli DB10 containing pVIR1 (MIC of virginiamycin, 64 μg/ml; MIC of pristinamycin, 128 μg/ml) was resistant to streptogramins. The entire DNA fragment was sequenced, and the sequence was deposited in GenBank under the accession number AF242872. The sequence revealed the presence of two insertion sequences (IS1216V and part of IS1542), erm(B), and vat(E) (Fig. 1). No other open reading frames were detected. This is the first evidence of a direct physical linking of the vat(E) and the erm(B) genes. A previous publication did not find a similar link between an erm(B) gene and the vat(D) gene in a streptogramin-resistant E. faecium strain (HM1032) of human origin (3).
FIG. 1.
pVIR1. The sizes and positions of the genes and insertion sequences are indicated. Filled bar, 7.5-kb insert cloned in the EcoRI site of pUC18Not. Restriction enzyme sites and open reading frames for IS1216V, erm(B), vat(E), and IS1542 are indicated. Only the sequence to the internal EcoRI site in IS1542 is present (asterisk). Arrowheads, orientations of the genes; arrows positions and orientations of the primers.
The linkage of erm(B) and vat(E) was tested in 102 vat(E)-positive streptogramin-resistant E. faecium poultry isolates from Denmark isolated in 1997. The linkage was found in 74% of the isolates using the new primers satG1-out (5′-GCATTTGCGTCAGGTATAGT-3′) and ermB2-1 (5′-CGCCATACCACAGATGTTCC-3′) on the basis of the finding that, in all PCR-positive isolates, an amplicon of 942 bp was obtained. Previously described vat(E)-positive strains from different origins and countries in Europe were also tested for the presence of the link between vat(E) and erm(B). The 942-bp PCR amplicon was obtained from UW1965K1 (a sewage isolate from Germany) (15), K14syn (a poultry isolate from The Netherlands) (5), and A33 (a chicken isolate from the United Kingdom) (12) (data not shown). This indicates the presence of a highly conserved genetic element, containing erm(B) and vat(E) and mediating resistance to both streptogramins and macrolides, in Europe.
REFERENCES
- 1.Allignet J, Loncle V, Mazodier P, El Sohl N. Nucleotide sequence of a staphylococcal plasmid gene vgb, encoding a hydrolase inactivating the B components of virginiamycin-like antibiotics. Plasmid. 1988;20:271–275. doi: 10.1016/0147-619x(88)90034-0. [DOI] [PubMed] [Google Scholar]
- 2.Anonymous. DANMAP 1998. Consumption of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from food animals, food and humans in Denmark. Copenhagen, Denmark: Danish Zoonosis Centre; 1999. [Google Scholar]
- 3.Bozdogan B, Leclercq R, Lozniewski A, Weber M. Plasmid-mediated coresistance to streptogramins and vancomycin in Enterococcus faecium HM1032. Antimicrob Agents Chemother. 1999;43:2097–2098. doi: 10.1128/aac.43.8.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cocito C. Antibiotics of the virginiamycin family, inhibitors which contain synergistic compounds. Microbiol Rev. 1979;43:145–198. doi: 10.1128/mr.43.2.145-192.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haroche J, Allignet J, Aubert S, van den Bogaard A E, El Sohl N. satG, conferring resistance to streptogramin A, is widely distributed in Enterococcus faecium strains but not in staphylococci. Antimicrob Agents Chemother. 2000;44:190–191. doi: 10.1128/aac.44.1.190-191.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herrero M, de Lorenzo V, Timmis K N. Transposon vectors containing non-antibiotic resistance selection markers for cloning and stable chromosomal insertion of foreign genes in gram-negative bacteria. J Bacteriol. 1990;172:6557–6567. doi: 10.1128/jb.172.11.6557-6567.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson A P, Livermore D M. Quinupristin/dalfopristin, a new addition to the antimicrobial arsenal. Lancet. 1999;354:2012–2013. doi: 10.1016/s0140-6736(99)00344-x. [DOI] [PubMed] [Google Scholar]
- 8.Jones R N, Ballow C H, Biedenbach D J, Deinhart J A, Schentag J J. Antimicrobial activity of quinupristin-dalfopristin (RP 59500 Synercid®) tested against over 28,000 recent clinical isolates from 200 medical centers in the United States and Canada. Diagn Microbiol Infect Dis. 1998;30:437–451. doi: 10.1016/s0732-8893(98)80002-3. [DOI] [PubMed] [Google Scholar]
- 9.Rende-Fournier R, Leclercq R, Galimand M, Duval J, Courvalin P. Identification of the satA gene encoding a streptogramin A acetyltransferase in Enterococcus faecium BM4145. Antimicrob Agents Chemother. 1993;37:2119–2125. doi: 10.1128/aac.37.10.2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roberts M C, Sutcliffe J, Courvalin P, Jensen L B, Rood J, Seppala H. Nomenclature for macrolide and macrolide-lincosamide-streptogramin B resistance determinants. Antimicrob Agents Chemother. 1999;43:2823–2830. doi: 10.1128/aac.43.12.2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schouten M A, Voss A, Hoogkamp-Korstanje J A A The European VRE Study Group. Antimicrobial susceptibility patterns of enterococci causing infections in Europe. Antimicrob Agents Chemother. 1999;43:2542–2546. doi: 10.1128/aac.43.10.2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soltani M, Beighton D, Philpott-Howard J, Woodford N. Mechanisms of resistance to quinupristin-dalfopristin among isolates of Enterococcus faecium from animals, raw meat, and hospital patients in Western Europe. Antimicrob Agents Chemother. 2000;44:433–436. doi: 10.1128/aac.44.2.433-436.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weisblum B. Erythromycin resistance by ribosome modification. Antimicrob Agents Chemother. 1995;39:577–585. doi: 10.1128/AAC.39.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Welton L A, Thal L A, Perri M B, Donabedian S, McMahon J, Chow J W, Zervos M J. Antimicrobial resistance in enterococci isolated from turkey flocks fed virginiamycin. Antimicrob Agents Chemother. 1998;42:705–708. doi: 10.1128/aac.42.3.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Werner G, Witte W. Characterization of a new enterococcal gene, satG, encoding a putative acetyltransferase conferring resistance to streptogramin A compounds. Antimicrob Agents Chemother. 1999;43:1813–1814. doi: 10.1128/aac.43.7.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]