Abstract
Introduction:
Acute kidney injury (AKI) affects 10% of patients following major surgery and is independently associated with extra-renal organ injury, development of chronic kidney disease, and death. Perioperative renal ischemia and reperfusion (IR) contributes to AKI by, in part, increasing production of reactive oxygen species (ROS) and leading to oxidative damage. Variations in inhaled oxygen may mediate some aspects of IR injury by affecting tissue oxygenation, ROS production, and oxidative damage. We tested the hypothesis that provision of air (normoxia) compared to 100% oxygen (hyperoxia) during murine renal IR affects renal ROS production and oxidative damage.
Methods:
We administered 100% oxygen or 21% oxygen (air) to 8–9 week-old FVB/N mice and performed dorsal unilateral nephrectomy with contralateral renal ischemia/reperfusion surgery while mice spontaneously ventilated. We subjected mice to 30 minutes of ischemia and 30 minutes of reperfusion prior to sacrifice. We obtained an arterial blood gas (ABG) by performing sternotomy and left cardiac puncture. We stained the kidney with pimonidazole, a marker of tissue hypoxia; 4-HNE, a marker of ROS-production; and we measured F2-isoprostanes in homogenized tissue to quantify oxidative damage.
Results:
Hyperoxia during IR increased arterial oxygen content compared to normoxia, but both groups of mice were hypoventilating at the time of ABG sampling. Renal tissue hypoxia following reperfusion was similar in both treatment groups. ROS production was similar in the cortex of mice (3.8% area in hyperoxia vs. 3.1% in normoxia, P=0.19) but increased in the medulla of hyperoxia-treated animals (6.3% area in hyperoxia vs. 4.5% in nomoxia, P=0.02). Renal F2-isoprostanes were similar in treatment groups (2.2 pg/mg kidney in hyperoxia vs. 2.1 pg/mg in normoxia, P=0.40).
Conclusions:
Hyperoxia during spontaneous ventilation in murine renal IR did not appear to affect renal hypoxia following reperfusion, but hyperoxia increased medullary ROS production compared to normoxia.
Keywords: kidney, AKI, oxygen, ischemia, reperfusion, oxidative damage, organ injury, normoxia, hypoxia, FIO2, mice
1. Introduction
Acute kidney injury (AKI) affects 10% of patients following major surgery and increases healthcare costs, length of hospital stay, organ injury, chronic kidney disease, and death.1–3 Strategies to limit perioperative renal damage are limited.4, 5 This is in part due to the complex pathophysiology that contributes to organ injury in surgical patients. Multiple mechanisms affect the inflammation, tissue edema, mechanical disruption of tissues, and alterations in organ perfusion that culminate in AKI.6, 7
Oxygen may mediate some of these mechanisms of renal injury through changes in tissue oxygenation, reactive oxygen species (ROS) production, and oxidative damage, especially in scenarios of renal ischemia and reperfusion (IR). IR is a phenomenon characterized by transient loss of perfusion and oxygenation followed by restoration of blood flow and oxygenation. IR damages tissues and causes inflammation, and the impact of oxygen tension during reperfusion is incompletely understood. In cardiac, transplant, and vascular surgery, tissues may completely lose blood supply for a portion of the procedure when major vessels are clamped or ligated. Even in routine surgeries, tissues are subject to ischemia during fluctuations in blood pressure and the administration of vasoactive drugs. Reperfusion is necessary for survival of ischemic tissue, but paradoxically, the influx of oxygen-rich blood may create additional injury via vasoconstriction,8, 9 generation of ROS in mitochondria,10, 11 and oxidative damage.12 Consequently, improved strategies for oxygen delivery in the operating room have potential to ameliorate damage caused by unavoidable IR.
During surgery, oxygen is delivered at the discretion of the anesthesiologist. To avoid organ hypoxia, anesthesiologists typically administer supraphysiologic levels of oxygen, leading to hyperoxia.13, 14 Hyperoxia causes vasoconstriction, reduces oxygen consumption, leads to shunting of blood, and may decrease perfusion.9 Hyperoxia may also induce renal damage during IR through modulation of ROS production and oxidative stress. When tissue oxygenation is restored during reperfusion, mitochondria produce ROS.15, 16 ROS directly damage lipids, DNA, and other proteins and induce inflammation and cellular dysfunction. The impact of oxygen content in blood during IR on the quantity of ROS produced or subsequent oxidative tissue damage is unclear.
We designed this study to test the hypothesis that oxygen delivery affects renal tissue oxygenation, ROS production, and oxidative damage in a murine model of renal IR.
2. Methods
We subjected mice to 100% oxygen or air during unilateral renal ischemia and reperfusion and measured and compared arterial oxygenation, renal hypoxia, renal ROS production, and renal oxidative damage between treatment groups.
2.1. Animals
All animal studies and procedures described herein were approved by the Vanderbilt Institutional Animal Care and Use Committee and in compliance with the NIH Guide for the Care and Use of Laboratory Animals. Male 8–9-week-old FVB/N mice were ordered from The Jackson Laboratory (Bar Harbor, ME). The mice were housed in institutional Department of Animal Care facilities without restrictions and with free access to standard chow and water.
2.2. Surgical Model and Oxygen Treatment
In this surgical model, we administered 100% oxygen or air to twenty anesthetized mice and performed a dorsal unilateral nephrectomy prior to contralateral renal ischemia/reperfusion injury as follows.
On the day of surgery, animals were weighed and then anesthetized with an intraperitoneal injection of 100 mg/kg ketamine and 10 mg/kg xylazine. Mice were then randomized by cage to receive either room air or 100% oxygen during the experiment. We prepped the mice for surgery by shaving the dorsal flanks and applying ophthalmic lubricating solution. We then began the assigned oxygen treatment by placing the head of each animal into the back end of a 20 cm3 syringe, such that their snout and head was fully covered. The assigned exposure, 100% oxygen or room air, was delivered through the syringe via attachment to a compressed gas tank containing the respective gas mixtures. Fresh gas flow through the system was maintained at 2 L/min throughout the experiment. Mice maintained spontaneous ventilation (Figure 1).
Figure 1:
Oxygen delivery during renal IR surgery.
Anesthetized mice were placed on a recirculating water heater and prepped for surgery with betadine and chlorhexidine. A dorsal midline incision was created with surgical scissors. The right kidney was palpated and then exposed through a small incision through the right flank muscles. Perirenal fat was dissected from the hilum, which was then ligated and tied with a 4–0 silk suture. The right kidney was removed and discarded. The unilateral nephrectomy model provides a more consistent and reproducible AKI following contralateral IR.17
Through the same midline incision, the left kidney was palpated and exposed through the left flank. The perirenal fat was dissected, exposing the renal vascular pedicle. The renal pedicle was then clamped with a microvascular clamp for 30 minutes. Fifteen minutes after the start of ischemia, we injected a subset of 10 mice with intraperitoneal 60 mg/kg pimonidazole (Hydroxyprobe, Burlington, MA). After 30 minutes of ischemia, the clamp was removed, and animals underwent 30 minutes of reperfusion while continuing the assigned oxygen treatment.
2.3. ABG and Tissue Harvest
After 25 minutes of reperfusion, we prepared the animals for cardiac puncture and sacrifice. A ventral midline laparotomy incision was created to expose the kidney. Then, the chest was opened via sternotomy and heart exposed. At 30 minutes of reperfusion, arterial blood was aspirated from the left ventricle in a heparinized syringe, and the animal expired. The kidney was immediately harvested. We bisected the kidney and placed half in formalin phosphate acetate salt (FPAS) for fixation and immunohistochemical analysis and half in liquid nitrogen for F2-isoprostane quantification by gas chromatography-mass spectrometry (GC-MS). We measured an arterial blood gas (ABG) with the left ventricle blood sample.
2.4. Tissue Oxygenation
We stained kidneys with pimonidazole to determine the pattern and extent of renal hypoxia. Pimonidazole is a 2-nitroimidazole molecule stable in the presence of oxygen. In the absence of oxygen, the nitro groups reduce to form stable thiol adducts that are visible under light microscopy. Thus, pimonidazole serves as a robust hypoxia marker.18 A subset of 10 mice (5 assigned normoxia, 5 assigned hyperoxia) were injected with pimonidazole. Because pimonidazole was administered during renal artery clamping, the staining pattern represents hypoxia during the 30-minute reperfusion period. Following 18–24 hours of FPAS fixation, the tissues were processed, embedded in paraffin, sectioned, and stained.
2.5. ROS Production
We performed antigen retrieval via 3×5 minutes of microwave boiling in 100 mM citric acid buffer at pH 6.0 and stained kidneys for 4-hydroxy-nonenol (4-HNE) to quantify ROS production in renal tissues. ROS generate 4-HNE through decomposition of cellular polyunsaturated fatty acids such as arachidonic and linoleic acid.19 Slides were counterstained with toluene blue for 6 minutes.
We analyzed 4-HNE staining and calculated the percent area in 10 randomly selected frames in the cortex and medulla of each kidney at 400× magnification using automated CellSens software.
2.6. F2-isoprostanes
We measured renal tissue F2-isoprotanes to quantify oxidative damage following IR. F2-isoprostanes are end-products of non-enzymatic arachidonic acid peroxidation.20 We homogenized snap-frozen renal tissue, isolated and purified arachidonic acid metabolites via sequential washings and thin layer chromatography, and quantified F2-isoprostanes using GC-MS, as previously described.21
2.7. Statistical Analysis
We summarized continuous data as mean ± standard deviation of the mean. Statistical significance for between group comparisons was determined with a 2-tailed Student’s t-test, with P <0.05 considered significant.
3. Results
Of the 20 mice in the study, one mouse assigned hyperoxia died after induction of anesthesia. The remaining 19 mice survived to the completion of IR surgery and constitute the study sample.
3.1. Arterial Blood Gas
Arterial blood gas samples were obtained in a subset of 6 mice via left cardiac puncture. (N=2 normoxia, N=4 hyperoxia; Table 1). Our findings indicated that spontaneously ventilating mice under ketamine/xylazine general anesthesia were significantly acidemic and hypercarbic in both groups at the time of blood sampling. The acidemia was more pronounced in mice assigned 21% oxygen, with an average pH 6.94 compared to an average pH of 7.06 in the mice assigned 100% oxygen. The degree of hypercarbia was similar in both groups, with an average pCO2 of 93 mmHg and 97 mmHg in the mice given 21% oxygen and 100% oxygen, respectively. It should be noted that following laparotomy and sternotomy prior to blood sampling, mice become agonal with poor expansion of lungs following disruption of the diaphragm and chest wall and visual hypoventilation. Arterial oxygen tension was lower in mice breathing air compared to mice breathing 100% oxygen. In the mice given air, the average pO2 and oxygen saturation were 20 mmHg and 15%, versus the mice given 100% oxygen with a pO2 and oxygen saturation of 119 mmHg and 96%.
Table 1:
Arterial Blood Gas results from left cardiac puncture 30 minutes post-reperfusion in mice breathing air and mice breathing 100% oxygen.
| Oxygen Treatment | pH | pCO2 | pO2 | Sat (%) |
|---|---|---|---|---|
| 21% | 6.9 | 87 | 27 | 23 |
| 21% | 6.97 | 98 | 13 | 7 |
| 100% | 7.06 | 93 | 144 | 98 |
| 100% | 7.08 | 93 | 113 | 95 |
| 100% | 7.06 | 90 | 101 | 94 |
| 100% | 7.05 | 113 | 117 | 95 |
3.2. Tissue Oxygenation
Pimonidazole staining, a marker of tissue hypoxia, was primarily localized to the renal cortex and the outer medulla. The inner medulla did not have appreciable uptake of pimonidazole stain. This staining pattern was similar between mice assigned to air and those assigned 100% oxygen. A representative image of pimonidazole staining is shown in Figure 2.
Figure 2:
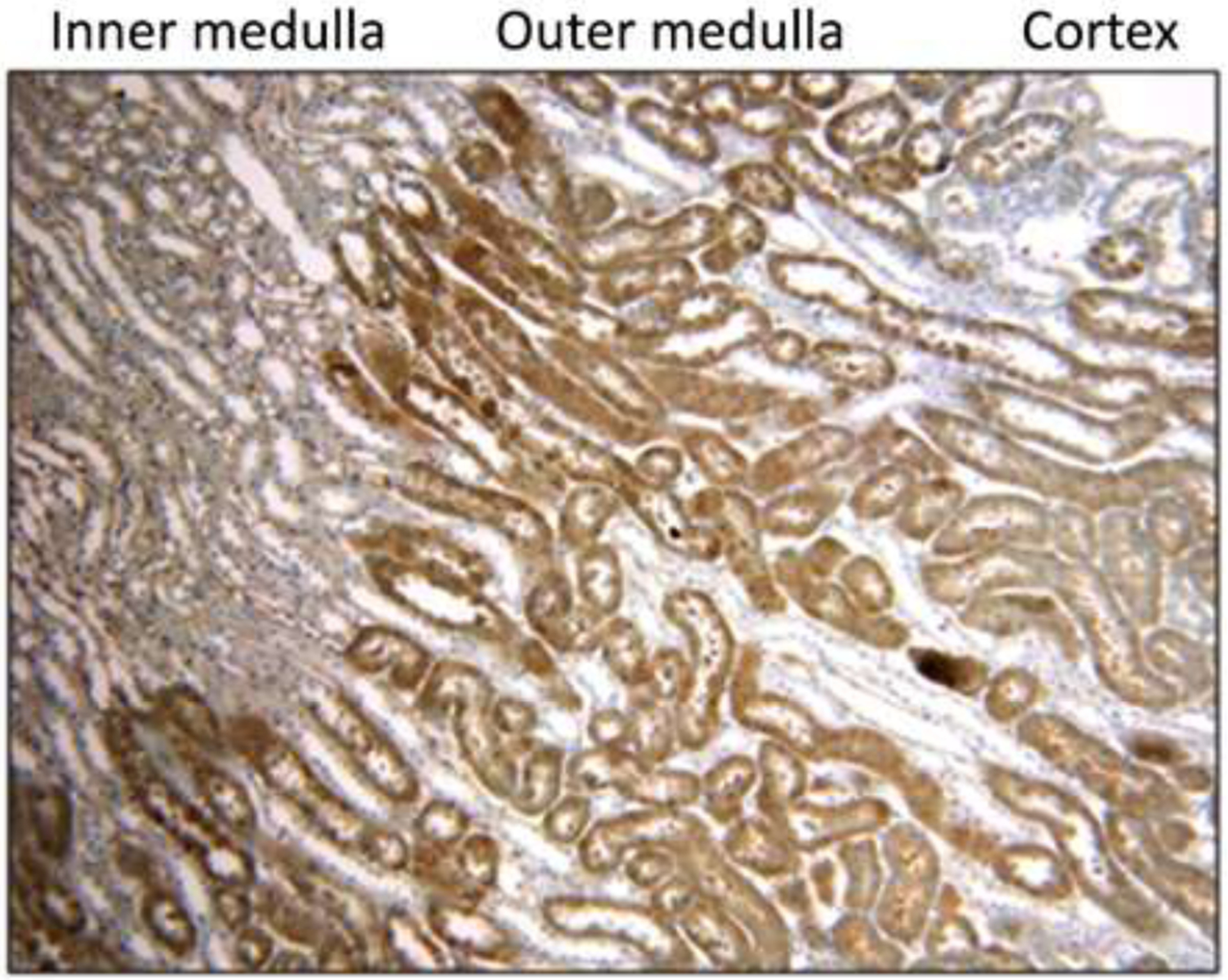
Representative image of pimonidazole staining in the murine corticomedullary junction (100× magnification). Pimonidazole staining was primarily localized to the outer medulla. The staining pattern was similar between mice administered hyperoxia and normoxia.
3.3. ROS Production
4-HNE, the marker of ROS production, was similar in the renal cortex of mice assigned to air (4-HNE % area 3.1 ± 2.0%) or to 100% oxygen (3.8 ± 2.8%, P=0.19). However, 4-HNE staining was increased in the outer medulla compared to the cortex and increased the outer medulla in mice assigned to 100% oxygen (6.3 ± 4.1% area) compared to the outer medulla of mice assigned air (4.5 ± 3.2% area, P=0.02). Administration of 100% oxygen also increased tubular vacuolization in the medulla, an early sign of cellular damage. Representative images of 4-HNE staining and 4-HNE quantification are displayed in Figure 3.
Figure 3:
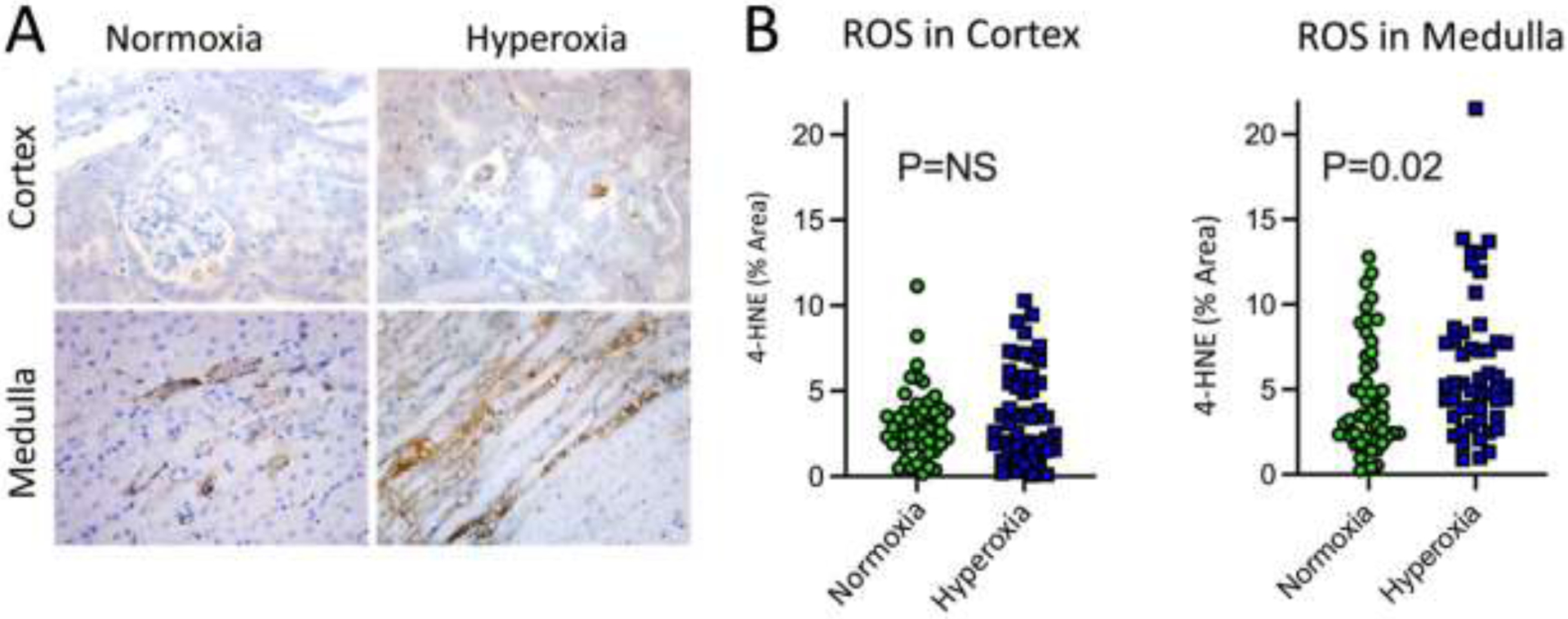
Representative images and quantification of 4-HNE staining in murine kidney following renal ischemia and reperfusion. A) Cortex and medulla in mice exposed to normoxia or hyperoxia during renal IR. 4-HNE stained cells were primarily located in the medulla in animals administered hyperoxia. B) Percent area of 4-HNE staining in cortex and medulla of mice exposed to normoxia or hyperoxia. Each dot represents a random field in the cortex or medulla (10 random fields in the cortex or medulla for each mouse).
3.4. Oxidative Damage
Renal F2-isoprostanes, markers of oxidative damage in vivo, were 2.2 ± 0.4 pg/mg kidney in mice treated with 100% oxygen and 2.1 ± 0.5 pg/mg kidney in mice treated with normoxia (P=0.40; Figure 4). Quantification was performed on homogenized, snap-frozen kidney and thus not localized to any specific location within the renal cortex or medulla.
Figure 4:
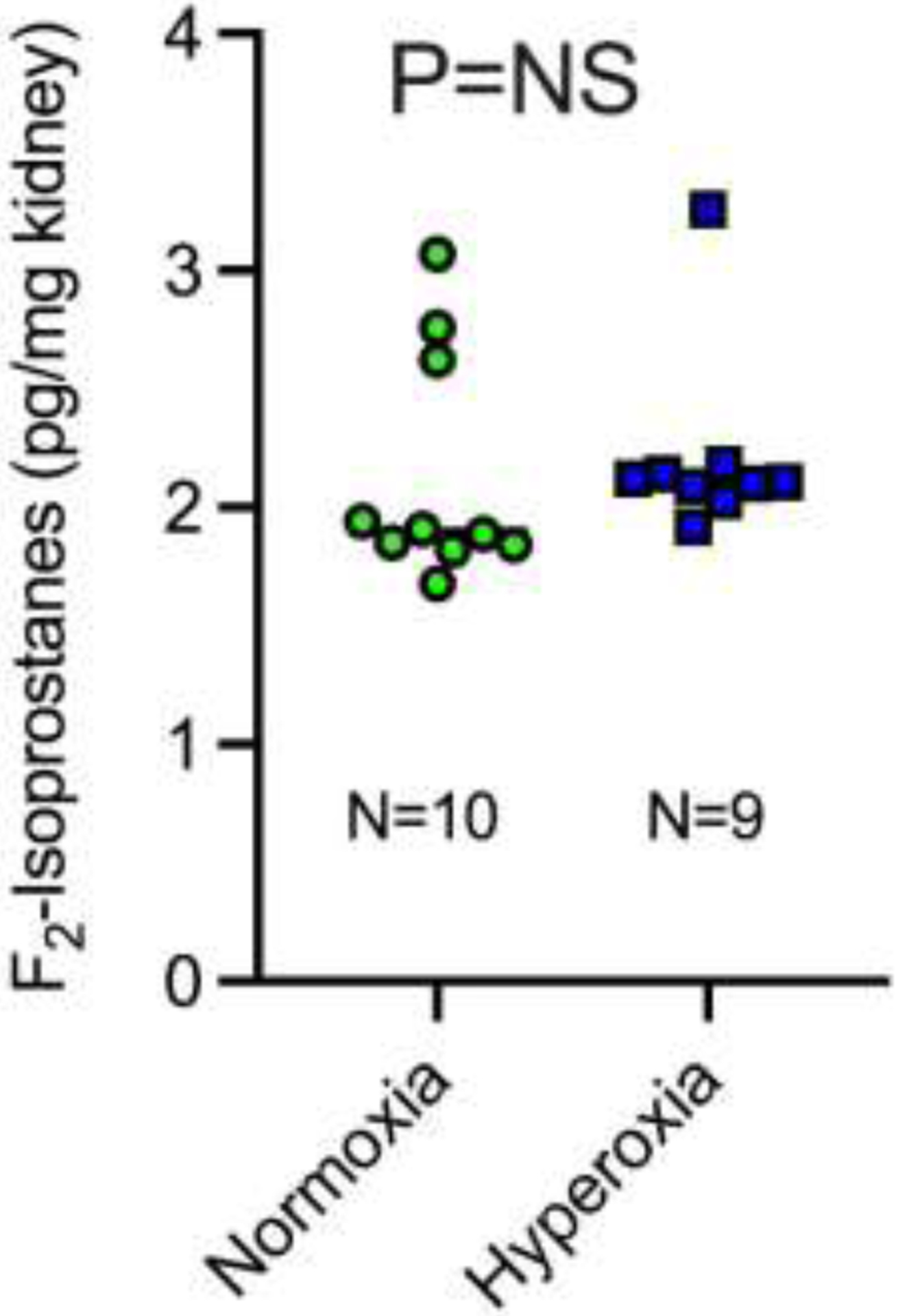
F2-isoprostanes in whole kidney in mice treated with normoxia or hyperoxia during renal ischemia and reperfusion
4. Discussion
In these initial findings from a murine model of renal IR, administration of hyperoxia as compared to normoxia increased arterial partial pressure of oxygen but did not appear to impact renal hypoxia post reperfusion. Hyperoxia also increased ROS production in the renal medulla but not the cortex, and oxidative damage in the whole kidney appeared similar between treatment groups.
We collected ABG data to confirm that animals received the appropriate oxygen treatment to which they were assigned. While breathing air, murine blood pH should be around 7.4, with corresponding partial pressure of carbon dioxide (pCO2) 30–40 mmHg and bicarbonate levels (HCO3) 15–20 mEq/L.22 There are limited data to suggest appropriate values for mice breathing 100% oxygen, but in corresponding human surgery, the partial pressure of oxygen in arterial blood (pO2) is typically 300–500 mmHg. Our ABG results in the current study demonstrate that mice in both groups were significantly acidemic and hypercarbic, indicators of inadequate ventilation. Additionally, the oxygen tension was much lower than expected in both groups, although it was greater in the mice breathing 100% oxygen. Therefore, although treatment with 100% oxygen increased the oxygen saturation and pO2, these findings further suggest that mice were hypoventilating at the time of sacrifice. This may be due to hypoventilation during spontaneous ventilation secondary to ketamine/xylazine anesthesia, which has been shown previously,23 or loss of chest wall integrity and diaphragm function at the time of laparotomy and sternotomy prior to left ventricle puncture. Thus, the quality of ventilation immediately preceding arterial sampling, and therefore the ABG results, were altered. We do not know the pH, pCO2, and pO2 during the majority of the IR experiments.
Pimonidazole, the marker of hypoxia, primarily stained the cortex and outer medulla, indicative of corticomedullary ischemia. This finding is consistent with prior studies,24, 25 as the corticomedullary junction is the region of the kidney most sensitive to hypoxia. Pimonidazole was administered during renal ischemia so its first opportunity to access the kidney was during reperfusion immediately after unclamping the renal pedicle. The pattern and intensity of pimonidazole staining did not differ between the hyperoxia and normoxia groups. This implies that following total cessation of renal perfusion, hyperoxia as compared to normoxia during reperfusion may not reduce renal hypoxia. We harvested the kidney for analysis after 30 minutes of reperfusion, so it is possible that over a longer period of reperfusion, for example after hours or days of reperfusion, oxygen treatment may differentially affect renal hypoxia.
4-HNE, the marker used to visualize ROS production, generated minimal stain in the cortex of mice assigned to both hyperoxia and normoxia. This indicates that regardless of oxygen treatment, there is low ROS production in the cortex during renal IR. In the medulla, however, ROS production was significantly higher in hyperoxia-treated animals compared to normoxia. These results are consistent with prior studies where pretreatment with the antioxidant allopurinol significantly abrogated plasma creatinine elevation in hyperoxia-treated rabbits 24 hours after IR injury.26 Taken together, these findings suggest that hyperoxia may increase ROS-production, which could be partly responsible for the damage caused in ischemia and reperfusion injury. However, there is no clear consensus on optimal oxygenation therapy in IR injury, as results of preclinical studies are not consistnet. Another study found that intrarenal tissue oxygenation is directly related to GFR in the acute period after reperfusion, and that hyperoxia can increase tissue pO2 when measured 2 hours after reperfusion.14 That finding suggests that hyperoxia may ameliorate some aspect of acute functional damage immediately following IR.
We quantified oxidative damage by measuring renal F2-isoprostanes and found no significant difference between mice assigned hyperoxia versus normoxia. We would expect oxidative damage to be increased in the hyperoxia group since ROS production was increased in those animals, but it is possible that any difference in the medulla was masked by homogenizing the entire kidney. Additionally, oxidative damage may require hours to days of post-reperfusion to manifest as increased F2-isoprostanes after initial IR. Lastly, we also may have been underpowered to detect the small difference observed.
This pilot study has several limitations, including the efficacy of oxygen treatment on systemic and renal oxygen tensions in spontaneously breathing animals. In future studies, we will intubate and mechanically ventilate mice to ensure precision of oxygen treatment. Our study is additionally limited by the short duration of reperfusion. Animals were only subjected to 30 minutes of reperfusion before sacrifice and tissue harvest, but this does provide opportunity to assess the proximal contributors to mechanisms of IR injury. The short duration of reperfusion prior to animal sacrifice and tissue sampling may not be sufficient to appreciate the full difference in tissue oxygenation, ROS production, and oxidative damage.
In conclusion, spontaneous ventilation with oxygen concentrations common in clinical practice impacted murine systemic oxygenation and medullary production of ROS. Future studies will further investigate the impact of inspired oxygen on mechanisms of renal IR injury.
Acknowledgements:
We acknowledge Aolei Niu for assistance performing the ischemia reperfusion model and grant support from a 2018 Burroughs Wellcome Fund Physician-Scientist Institutional Award to Vanderbilt University (ID: 1018894) (MJK), the National Institutes of Health (T32GM108554 [EHM and MBB], R01DK96765 and R01DK114809 [RCH and MZZ], K08GM123345 [AH], and R01GM112871 [FTB]), and the Veterans Administration (RCH).
References
- 1.Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute Kidney Injury, Mortality, Length of Stay, and Costs in Hospitalized Patients. 2005–11–01 2005;doi: 10.1681/ASN.2004090740 [DOI] [PubMed] [Google Scholar]
- 2.Fortrie G, de Geus HRH, Betjes MGH. The aftermath of acute kidney injury: a narrative review of long-term mortality and renal function. ReviewPaper. Critical Care. 2019–01–24 2019;23(1):1–11. doi:doi: 10.1186/s13054-019-2314-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meersch M, Schmidt C, Zarbock A. Perioperative Acute Kidney Injury: An Under-Recognized Problem. Anesthesia and analgesia. 2017 Oct 2017;125(4)doi: 10.1213/ANE.0000000000002369 [DOI] [PubMed] [Google Scholar]
- 4.Molinari L, Sakhuja A, Kellum JA. Perioperative Renoprotection: General Mechanisms and Treatment Approaches. Anesthesia & Analgesia. 2020;131(6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saadat-Gilani K, Zarbock A, Meersch M. Perioperative Renoprotection: Clinical Implications. Anesthesia & Analgesia. 2020;131(6) [DOI] [PubMed] [Google Scholar]
- 6.O’Neal J, Shaw A, Billings F. Acute kidney injury following cardiac surgery: current understanding and future directions. Critical care (London, England). 07/04/2016 2016;20(1)doi: 10.1186/s13054-016-1352-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conrad C, Eltzschig HK. Disease Mechanisms of Perioperative Organ Injury. Anesthesia & Analgesia. 2020;131(6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mouren S, Souktani R, Beaussier M, et al. Mechanisms of coronary vasoconstriction induced by high arterial oxygen tension. Am J Physiol. Jan 1997;272(1 Pt 2):H67–75. doi: 10.1152/ajpheart.1997.272.1.H67 [DOI] [PubMed] [Google Scholar]
- 9.Smit B, Smulders YM, van der Wouden JC, Oudemans-van Straaten HM, Spoelstra-de Man AME. Hemodynamic effects of acute hyperoxia: systematic review and meta-analysis. ReviewPaper. Critical Care. 2018–02–25 2018;22(1):1–10. doi:doi: 10.1186/s13054-018-1968-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cadenas S ROS and redox signaling in myocardial ischemia-reperfusion injury and cardioprotection. Free radical biology & medicine. 2018 Mar 2018;117 doi: 10.1016/j.freeradbiomed.2018.01.024 [DOI] [PubMed] [Google Scholar]
- 11.Granger D, Kvietys P. Reperfusion injury and reactive oxygen species: The evolution of a concept. Redox biology. 2015 Dec 2015;6 doi: 10.1016/j.redox.2015.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meng-Yu W, Giou-Teng Y, Wan-Ting L, et al. Current Mechanistic Concepts in Ischemia and Reperfusion Injury. Cellular Physiology and Biochemistry. 2021;46(4):1650–1667. doi: 10.1159/000489241 [DOI] [PubMed] [Google Scholar]
- 13.Grocott HP, Faraoni D. Avoidance of Hyperoxemia during Cardiopulmonary Bypass: Why Does Pathophysiology Not Always Translate into Clinical Outcome? Anesthesiology. 2021;128(2):419–419. doi: 10.1097/ALN.0000000000001990 [DOI] [PubMed] [Google Scholar]
- 14.Nensén O, Hansell P, Palm F. Intrarenal oxygenation determines kidney function during the recovery from an ischemic insult. American journal of physiology Renal physiology. 12/01/2020 2020;319(6)doi: 10.1152/ajprenal.00162.2020 [DOI] [PubMed] [Google Scholar]
- 15.Chouchani ET, Pell VR, Gaude E, et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature. 2014/11/01 2014;515(7527):431–435. doi: 10.1038/nature13909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eltzschig HK, Eckle T. Ischemia and reperfusion—from mechanism to translation. Nature Medicine. 2011/11/01 2011;17(11):1391–1401. doi: 10.1038/nm.2507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Skrypnyk NI, Harris RC, de Caestecker MP. Ischemia-reperfusion model of acute kidney injury and post injury fibrosis in mice. J Vis Exp. Aug 9 2013;(78)doi: 10.3791/50495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Varia M, Calkins-Adams D, Rinker L, et al. Pimonidazole: a novel hypoxia marker for complementary study of tumor hypoxia and cell proliferation in cervical carcinoma. Gynecologic oncology. 1998 Nov 1998;71(2)doi: 10.1006/gyno.1998.5163 [DOI] [PubMed] [Google Scholar]
- 19.Dalleau S, Baradat M, Guéraud F, Huc L. Cell death and diseases related to oxidative stress:4-hydroxynonenal (HNE) in the balance. Cell Death & Differentiation. 2013/12/01 2013;20(12):1615–1630. doi: 10.1038/cdd.2013.138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Milne GL, Musiek ES, Morrow JD. F2-isoprostanes as markers of oxidative stress in vivo: an overview. Biomarkers. Nov 2005;10 Suppl 1:S10–23. doi: 10.1080/13547500500216546 [DOI] [PubMed] [Google Scholar]
- 21.Milne G, Sanchez S, Musiek E, Morrow J. Quantification of F2-isoprostanes as a biomarker of oxidative stress. Nature protocols. 2007 2007;2(1)doi: 10.1038/nprot.2006.375 [DOI] [PubMed] [Google Scholar]
- 22.Iversen N, Malte H, Baatrup E, Wang T. The normal acid-base status of mice. Respiratory physiology & neurobiology. 03/15/2012 2012;180(2–3)doi: 10.1016/j.resp.2011.11.015 [DOI] [PubMed] [Google Scholar]
- 23.Massey CA, Richerson GB. Isoflurane, ketamine-xylazine, and urethane markedly alter breathing even at subtherapeutic doses. J Neurophysiol. 2017;118(4):2389–2401. doi: 10.1152/jn.00350.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Munshi R, Hsu C, Himmelfarb J. Advances in understanding ischemic acute kidney injury. ReviewPaper. BMC Medicine. 2011–02–02 2011;9(1):1–6. doi:doi: 10.1186/1741-7015-9-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Molitoris B, Sandoval R, Sutton T. Endothelial injury and dysfunction in ischemic acute renal failure. Critical care medicine. 2002 May 2002;30(5 Suppl)doi: 10.1097/00003246-200205001-00011 [DOI] [PubMed] [Google Scholar]
- 26.Zwemer CF, Shoemaker JL Jr., Hazard SW 3rd, Davis RE, Bartoletti AG, Davis RE, Phillips CL. Hyperoxic reperfusion exacerbates postischemic renal dysfunction. Surgery. Nov 2000;128(5):815–21. doi: 10.1067/msy.2000.109117 [DOI] [PubMed] [Google Scholar]


