Objective
As the coronavirus disease 2019 pandemic continues to grow, its clinical manifestations are still emerging and are being widely investigated. However, the pain symptoms, including neurological and musculoskeletal pain symptoms, are still poorly understood.
Design
In this cross-sectional study, we investigated the prevalence of musculoskeletal and neurological pain symptoms among hospitalized coronavirus disease 2019 patients. Furthermore, the association of clinical and demographic factors with the prevalence of pain symptoms was also investigated.
Result
We included 182 hospitalized coronavirus disease 2019 patients with a mean age of 48.86 ± 13.98 yrs. Pain symptoms were reported by 61.54% patients (n = 112). Most common symptoms reported were generalized myalgia (n = 60, 32.96%), headache (n = 50, 27.47%), and low back pain (n = 41, 22.53%). Interestingly, neuropathic pain was present in 14 participants (7.69%). Logistic regression analysis revealed an association of pain symptoms with coronavirus disease 2019 severity, male sex, higher body mass index, and a history of addiction.
Conclusions
Pain symptoms are common manifestation of coronavirus disease 2019. Generalized myalgia, headache, and low back pain are the three most common new-onset pain symptoms in hospitalized coronavirus disease 2019 patients. Further investigation of pain symptoms and their predictive factors are recommended, which may guide healthcare workers and policymakers to plan in this direction.
To Claim CME Credits
Complete the self-assessment activity and evaluation online at http://www.physiatry.org/JournalCME
CME Objectives
Upon completion of this article, the reader should be able to: (1) Understand common musculoskeletal and neurological pain symptoms among hospitalized COVID-19 patients; (2) Understand the basic etiopathogenesis of COVID-19 associated pain; and (3) Identify factors associated with presence of COVID-19 pain symptoms.
Level
Advanced
Accreditation
The Association of Academic Physiatrists is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
The Association of Academic Physiatrists designates this Journal-based CME activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should only claim credit commensurate with the extent of their participation in the activity.
Key Words: Coronavirus Disease 2019, COVID-19, Musculoskeletal Pain, Neuropathic Pain, Pain Symptoms
What Is Known
The literature so far has displayed that musculoskeletal pain is among the most common symptoms of COVID-19 with myalgia, headache, and limb pain being the most frequent pain symptoms.
What Is New
This study demonstrated that generalized myalgia, headache, and low back pain are the most frequently reported pain symptoms of COVID-19. In addition, neuropathic pain, though rare, was reported in 7.69% of participants. We also revealed a direct association of new-onset pain symptoms with COVID-19 severity, male sex, body mass index, and a history of addiction.
The coronavirus disease 2019 (COVID-19) outbreak originated in Wuhan, China, and was declared as a global pandemic on March 11, 2020, by the World Health Organization.1 As of August 10, 2021, the disease has spread across more than 220 countries and territories, infecting more than 202 million people across the world with more than 4.2 million deaths.2 The COVID-19 has dramatically changed the way of living and posed a significant threat to public health worldwide. This has drawn unprecedented interest from public health researchers around the world, promoting extensive research on disease characteristics and strategies for management.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the novel coronavirus that causes COVID-19, can affect nearly every organ system, causing respiratory, cardiovascular, gastrointestinal, musculoskeletal (MSK), and neurologic symptoms. Besides the major clinical manifestations indicating a respiratory infection, such as fever, cough, sore throat, and nasal stuffiness, pain is a commonly reported symptom accompanying COVID-19.3,4 Pathophysiology of COVID-19–associated pain can be multifactorial and may include an autoimmune response to the virus, increased cytokine production, and widespread tissue damage (such as muscles and joints).5 Furthermore, a neurotropic property of the virus has been described, which may explain the neurologic pain symptoms of COVID-19 including headache and neuropathic pain.6 If untreated, pain may become chronic and substantially affect activities of daily living, which may further contribute to disease-related disability and reduced quality of life.
Acute pain symptoms associated with COVID-19 can be grouped as localized (e.g., pharyngalgia or sore throat), remote pain (e.g., headache), or generalized (e.g., myalgia, arthralgia, or limb pain). Headache and myalgia are among the most common acute pain symptoms reported so far and can occur in up to 71% of patients.6–8 A recent retrospective study has reported that the head and limbs were the most frequently complained painful sites.9 Neuropathic pain has also been reported in COVID-19 patients,10 although there are very scant data regarding its prevalence and clinical characteristics. Therefore, we aimed to explicitly evaluate the prevalence of MSK and neurologic pain symptoms in the acute phase of COVID-19 and their relationship with demographic and clinical characteristics.
METHODS
This cross-sectional study was conducted at a tertiary care center on hospitalized patients diagnosed with COVID-19 between May 10, 2021, and July 10, 2021. Inclusion criteria were confirmed cases of COVID-19 and age of 18 yrs or greater. Exclusion criteria were known history of chronic pain disorders, known psychiatric illness, serious concurrent illness preventing participation, and unwillingness for participation. A confirmed case was defined as a person with laboratory confirmation of COVID-19 infection, irrespective of clinical signs and symptoms. Real-time reverse transcription–polymerase chain reaction was used to confirm the SARS-CoV-2 infection. Sample size was calculated using a standard formula based on the prevalence from a recent similar study.9 Clearance for the study was taken from institutional ethical committee (reference number: T/IM-NF/PMR/21/19). Patients who satisfied the inclusion and exclusion criteria were approached with the study proposal, and its future implications were explained. The participants signed an informed written consent that was approved by the institutional ethical committee. This study complies with Strengthening the Reporting of Observational Studies in Epidemiology guidelines (see Supplemental Checklist, Supplemental Digital Content 1, http://links.lww.com/PHM/B530).
A physical medicine and rehabilitation resident posted in the COVID-19 dedicated ward collected the data, which included age, sex, height, weight, COVID-19 severity, history of addiction, and known comorbidities. Severity classification was done according to the guidelines issued by the Ministry of Health and Family Welfare, Government of India.11 Mild disease was defined as uncomplicated upper respiratory tract infection without the evidence of breathlessness or hypoxia (with normal saturation). Moderate disease was defined as presence of clinical features of dyspnea and/or hypoxia, fever, cough, including Spo2 90% to 94% on room air and a respiratory rate of 24 breaths/min or greater. Severe disease was identified as presence of clinical signs of pneumonia plus one of the following; respiratory rate of greater than 30 breaths/min, severe respiratory distress, and Spo2 of less than 90% on room air. Patients were asked to report whether they were experiencing any new-onset pain symptoms after the diagnosis of COVID-19. We included only MSK and neurological pain symptoms. If pain was present, its location, character, and severity were noted. For MSK pain and headache, a pain severity of two or more (of 10) on Numeric Rating Scale was considered for inclusion. Furthermore, the Douleur Neuropathique 4 questionnaire score of four or more was used for the confirmation of the diagnosis of neuropathic pain.
Pain localized to a specific part of the body was named according to the anatomical location (e.g., low back pain, knee pain, foot pain, etc). Neck and shoulder pain were considered together as most patients were unable to differentiate between the two. Generalized myalgia was defined as diffuse muscle pain and polyarthralgia was identified as pain involving four or more joints. Headache and neuropathic pain were included under neurologic pain. Pain described as electric shock–like, burning, shooting, prickling, or crawling sensations were identified as neuropathic pain.
Statistical analysis was performed using IBM SPSS version 20.0. Continuous variables are reported as mean ± SD and categorical demographic and clinical data are reported using numbers of participants and percentage. The significance of the two means was compared using an unpaired t test, and a comparison of categorical data is reported using Yate corrected χ2 test. A binary logistic regression analysis was performed to identify the predictors of incidence of the new-onset pain symptoms. The significance level was presented as “P value,” with P < 0.05 considered as significant. Randomization, logistic regression, and odds ratio were used to control for the confounders.
RESULTS
A total of 372 hospitalized patients with laboratory confirmed COVID-19 were screened for the study. Thirty-four patients were of age less than 18 and were excluded from the study. Forty-four patients were having severe illness with either, ventilator support, reduced consciousness, or communication issues. Seventy-nine patients gave history of chronic pain and 12 patients were having concurrent psychiatric illness. Last, 21 patients did not consent to participate. Thus, after exclusion of the previously mentioned patients, a total of 182 patients were included in the final analysis after they satisfied the inclusion and exclusion criteria and consented for participation (Fig. 1). The mean age of the participants was 48.86 ± 13.98 yrs with a predominantly male population (n = 132, 72.53%). Seventy participants (38.46%) had their body mass index (BMI) in either the overweight or obese category with the rest (61.54%) falling into the normal category. The severity of COVID-19 was distributed as 102 mild cases (56.04%), 62 moderate cases (34.07%), and 18 severe cases (9.89%). Type 2 diabetes mellitus was the most commonly reported comorbidity, followed by hypertension. Other comorbidities in decreasing order of prevalence were coronary artery disease, chronic respiratory disease, hypothyroidism, neoplasm, chronic kidney disease, chronic liver disease, and others. Multiple comorbidities (≥2) were present in 36 participants (19.78%). History of addiction with either alcohol or smoking was similar (n = 20, 10.99%) and addiction to both of these was found in 15 participants (8.24%). No addiction history was found in 127 patients (69.78%). The baseline characteristics of all participants have been summarized in Table 1.
FIGURE 1.
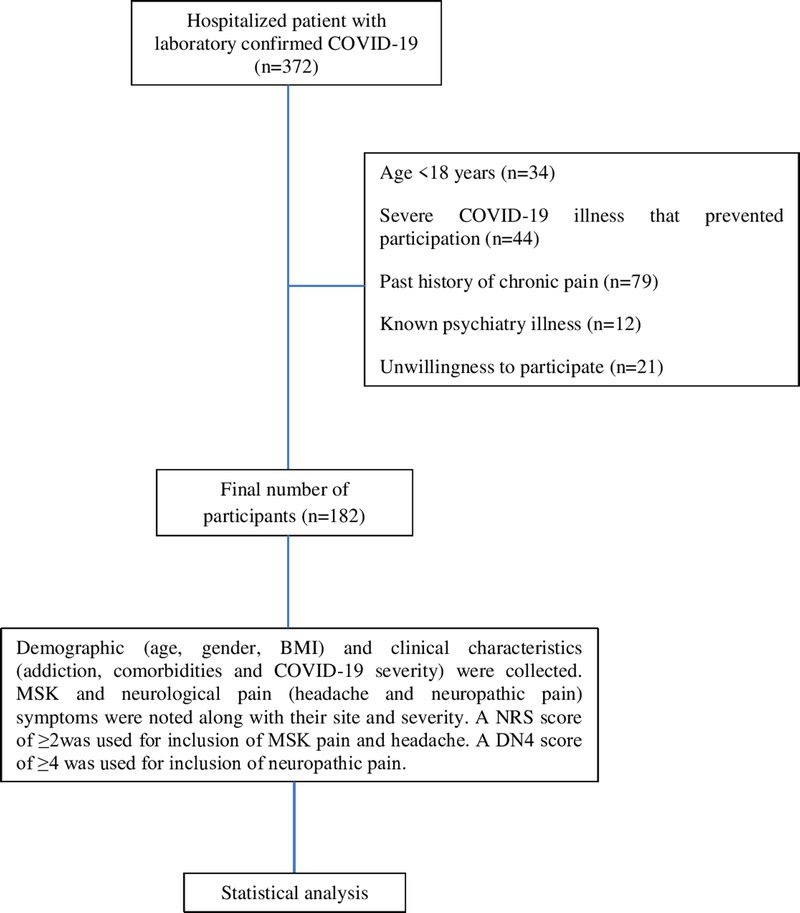
Participant flow diagram.
TABLE 1.
Demographic and clinical characteristics of participants (N = 182)
| Mean age, yr | 48.86 ± 13.98 |
| Sex | |
| Male | 132 (72.53) |
| Female | 50 (27.47) |
| BMI, kg/m2 | |
| Normal | 112 (61.54) |
| Overweight | 61 (33.52) |
| Obese | 9 (4.94) |
| Severity of COVID-19 | |
| Mild | 102 (56.04) |
| Moderate | 62 (34.07) |
| Severe | 18 (9.89) |
| Comorbidities | 109 (59.89) |
| Type 2 diabetes mellitus | 66 (36.26) |
| Hypertension | 42 (23.08) |
| Coronary artery disease | 14 (7.69) |
| Chronic respiratory disease | 11 (6.04) |
| Hypothyroidism | 10 (5.49) |
| Neoplasm | 8 (4.39) |
| Chronic kidney disease | 7 (3.85) |
| Chronic liver disease | 6 (3.30) |
| Others | 11 (6.04) |
| Multiple (≥2 comorbidities) | 36 (19.78) |
| None | 73 (40.11) |
| Addiction | 55 (30.22) |
| Alcohol | 20 (10.99) |
| Smoking | 20 (10.99) |
| Both | 15 (8.24) |
| None | 127 (69.78) |
Data are presented as mean ± SD or n (%).
One or more pain symptoms were reported by 61.54% of patients (n = 112). Three most common symptoms were generalized myalgia (n = 60, 32.96%), headache (n = 50, 27.47%), and low back pain (n = 41, 22.53%). Other pain symptoms in decreasing order of incidence were as follows: leg/calf pain, polyarthralgia, knee pain, hand pain, neck/shoulder pain, neuropathic pain, and foot pain. Neuropathic pain was present in 14 participants (7.69%). The distribution of neuropathic pain was as follows: only upper limb in five patients, only lower limbs in six patients, and diffuse (all limbs and trunk) in three patients. Two patients presented pain (described as tingling sensation) in the median nerve distribution similar to that of carpal tunnel syndrome. Diffuse neuropathic pain (in three patients) was described as burning and/or pin and needles in character involving the whole body below the neck. Three patients presented pain (electric shock–like or tingling sensation) in the distribution of lateral femoral cutaneous nerve. In rest seven patients, no specific nerve distribution could be identified. Objective assessment of neuropathic pain including electrodiagnostic studies and radiological investigations could not be performed because of the COVID-19 regulations. Seventy participants (38.46%) reported no MSK and/or neurologic pain symptoms. Simultaneous presence of four or more pain symptoms was reported by 26 patients (14.29%). In mild cases, generalized myalgia (n = 34, 33.33%) was the most common pain symptom, whereas in moderate and severe cases, headache (n = 25, 40.32%, and n = 12, 66.67%, respectively) was the most commonly reported pain symptom. The distribution of pain symptoms among participants is depicted in Table 2 and Figure 2.
TABLE 2.
Distribution of pain and associated symptoms among the participants (N = 182)
| Musculoskeletal pain symptoms | |
| Generalized myalgia | 70 (38.46) |
| Low back pain | 41 (22.53) |
| Leg pain | 19 (10.44) |
| Polyarthralgia | 17 (9.34) |
| Knee pain | 16 (8.79) |
| Hand pain | 16 (8.79) |
| Neck/shoulder pain | 15 (8.24) |
| Foot pain | 8 (4.39) |
| Neurological pain symptoms | |
| A. Headache | 50 (27.47) |
| B. Neuropathic pain | 14 (7.69) |
| 1. Upper limb | 5 (2.75) |
| 2. Lower limb | 6 (3.30) |
| 3. Diffuse | 3 (1.65) |
| Multiple pain symptoms | |
| 1. Two symptoms | 25 (13.74) |
| 2. Three symptoms | 15 (8.24) |
| 3. Four or more symptoms | 26 (14.29) |
| No pain symptoms | 70 (38.46) |
Data are presented as n (%).
FIGURE 2.
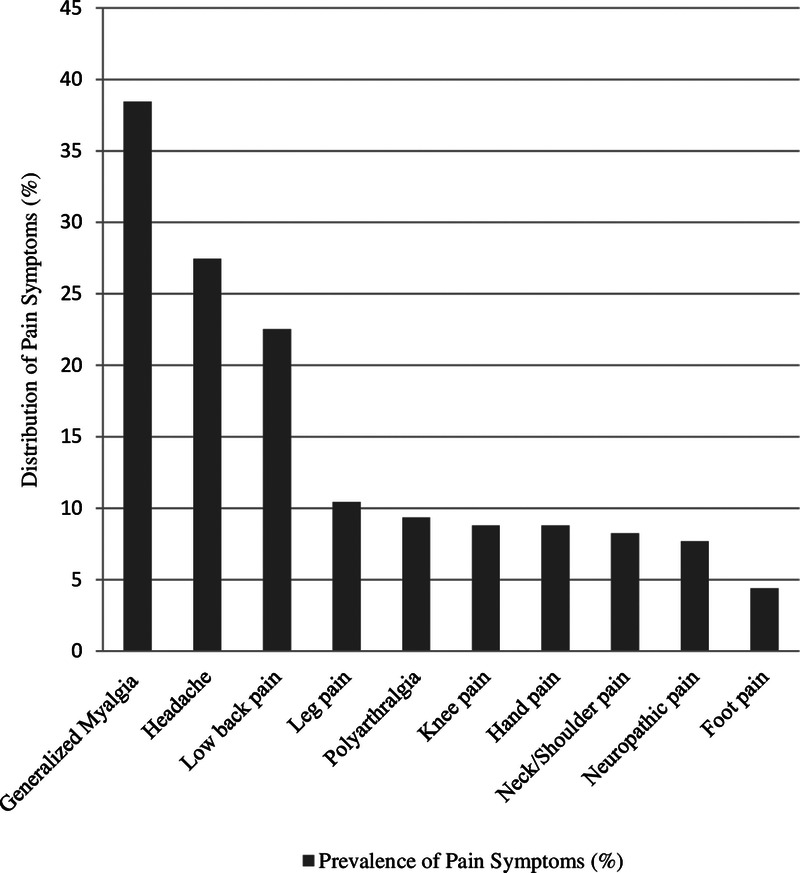
Distribution of pain symptoms among participants (N = 182).
Association of demographic characteristics with COVID-19 severity was analyzed. COVID-19 severity was associated with increased age, presence of comorbidities, and history of addiction (P < 0.05), but no significant association was found with sex and BMI of participants. A logistic regression analysis revealed an association of pain symptoms with male sex, BMI, COVID severity, and history of addiction. No clear association could be demonstrated between the presence of pain symptoms with age and presence of comorbidities (Table 3).
TABLE 3.
Logistic regression analysis demonstrating association of participant variables with pain symptoms (N = 182)
| Variable | Predictor | Coefficient | Standard Error | P | Odds Ratio | 95% Confidence Interval |
|---|---|---|---|---|---|---|
| Age, yr | ≥60 (vs. <60) | 0.4386 | 0.4859 | 0.3667 | 1.5505 | 0.5982–4.0187 |
| Sex | Male (vs. female) | 1.4688 | 0.4532 | 0.0012a | 4.3442 | 1.7871–10.5603 |
| BMI, kg/m2 | ≥25 (vs. <25) | 0.9798 | 0.4168 | 0.0187a | 2.6641 | 1.1770–6.0299 |
| COVID-19 severity | Moderate to severe (vs. mild) | 1.2594 | 0.3836 | 0.0010a | 3.5233 | 1.6612–7.4728 |
| Comorbidity | Present (vs. absent) | 0.2618 | 0.3771 | 0.4875 | 1.2993 | 0.6205–2.7208 |
| Addiction | Present (vs. absent) | 1.2408 | 0.4676 | 0.0080a | 3.4585 | 1.3831–8.6481 |
a P < 0.05 (statistical significance).
DISCUSSION
This study demonstrated a substantial proportion (61.54%) of hospitalized COVID-19 patients presenting with acute pain symptoms. There has been wide variation in reports of COVID-19–associated pain with very few studies evaluating pain symptoms in particular. This discrepancy in the incidence of pain symptoms may be ascribed to a difference in study objectives and surveillance methods.9,12,13 In a recent retrospective study conducted by Şahin et al.,9 the reported prevalence of pain symptoms was 40.7% before the infection that increased to 82.5% during the infection. Knox et al.12 conducted a prospective observational study in which 38.5% of COVID-19 patients presented with an active pain symptom.
The higher prevalence of pain in our study can be attributed to several factors. First, memory bias could be avoided by cross-sectional evaluation of symptoms. Second, we evaluated pain locations in a more precise manner (e.g., hand pain or foot pain) in comparison with previous studies where a more generalized approach was undertaken (e.g., upper limb or lower limb pain).9 Third, the inclusion of neuropathic pain might be another determinant for this high prevalence. Last, a difference in baseline demographic characteristics and virus properties are few other important factors that might have resulted in the difference in the pain incidence.
Our finding is consistent with the fact that myalgia (either localized or generalized) is one of the most frequently encountered pain symptoms among COVID-19 patients. In a recent review of symptoms during COVID-19, myalgia was encountered as the fifth most common symptom.14 Arthralgia is a relatively uncommon symptom of COVID-19 and a recent study reported an incidence of 15.1%. The incidence rate of myalgia or arthralgia has been inconsistent across studies and reported in up to 61.0% of cases.15 These MSK pain symptoms can be a direct result of tissue damage by the virus or secondary to several other factors. Angiotensin-converting enzyme 2 (ACE-2) receptors are known to be widely distributed in the musculoskeletal system.16 These receptors are considered to play a central role in the entry of SARS-CoV-2 into the cells of MSK tissue. This direct invasion through ACE-2 receptors may result in MSK injury, pain, weakness, and fatigue, all of which are known to occur in COVID-19.17–19 In addition, SARS-CoV-2 is known to cause cytokine storm that involves interleukin 6, interleukin 10, and tumor necrosis factor α. This cytokine storm may further induce or aggravate damage in various tissues, such as muscle and joints triggering pain and related symptoms.5 Mao et al.20 reported that COVID-19 patients displaying muscle symptoms demonstrated higher levels of creatine kinase and lactate dehydrogenase than those without muscle symptoms.20 Other potential mechanisms known to cause MSK pain include virus-associated myositis or myopathy, rhabdomyolysis, and steroid-induced myopathy.21–23 In addition, central nervous system involvement by the virus can also manifest as MSK tissue damage and pain.20
Headache is another commonly encountered pain symptom of COVID-19, and the prevalence rate in past studies ranged from 6% to 21%.7 Similar to myalgia/arthralgia, headache can also occur as a result of a direct viral invasion of the central nervous system or because of indirect effects of the virus. The virus has been known to directly invade the trigeminal nerve ending inside the nasal or oral cavity, potentially causing the headache.24 Penetration and damage to central nervous system have been confirmed by isolating SARS-CoV-2 from cerebrospinal fluid by genome sequencing.25 Furthermore, the neurotropism of the virus has been described as a potential mechanism of direct involvement of the nervous system. Besides the virus-specific factors, several triggers may contribute to the occurrence of the headache. These triggers may include psychological stress due to social isolation and/or disease-related anxiety, an adverse effect due to multiple drug prescriptions, or prolonged use of a mask during hospitalization. In a web-based study by Uygun et al.,24 COVID-19–associated headache was frequently long-lasting, bilateral, with a male predominance, and had resistance to analgesics. In our study, a higher prevalence of headache in moderate to severe disease supports the fact that neurological complications are more frequent in severe COVID-19.20
Neuropathic pain is a rare pain symptom of COVID-19 and has been explored in lesser detail so far. Similar to headaches, neuropathic pain may result from direct invasion of the nervous system (central nervous system or peripheral nervous system) or due to virus-mediated immune reactions. SARS-CoV-2 can cause an imbalance between ACE-2 and angiotensin II in the spinal cord that might result in neuropathic pain. Moreover, cytokines and chemokines can lead to activation of nociceptive sensory neurons resulting in pain.25 In addition, prolonged prone positioning deployed to improve oxygenation is known to cause direct injury of peripheral nerves.26 Other well-known neurological complications of COVID-19 that might result in neuropathic pain include transverse myelitis, Guillain-Barre syndrome, etc. Moreover, there are also reports of mixed sensory-motor neuropathy in COVID-19 patients leading to neuropathic pain.10 Nonetheless, the incidence and clinical characteristics of neuropathic pain need further investigation.
On careful analysis, we revealed a direct association of the pain symptoms with male sex, history of addiction, and COVID-19 severity. The predominance of pain symptoms among the male population may be attributable to an increased incidence of COVID-19 among the male population and a more efficient immune activity among females during other viral illnesses. In addition, a sex difference regarding the level of expression of ACE-2 and a protective role of female hormones or the location of ACE-2 on the X chromosome might explain this discrepancy.24 Association of pain symptoms with disease severity is by the fact that cytokine storm is more frequently displayed by severe COVID-19 patients. In the past, addiction history (especially smoking), higher BMI, and medical comorbidities (e.g., depression) have all been linked to the prevalence of pain symptoms.27 Nonetheless, an association of these factors with COVID-19–associated pain is yet to be established. To our knowledge, there is no published literature at this point providing a clear analysis of pain symptoms and their association with patient and disease characteristics.
This study is not without limitations. First, data from a single tertiary care center prevent the generalization of the results. Second, pain severity and their persistence beyond the acute phase were not assessed. Third, a lower proportion of severe COVID-19 patients were included in this study. Nonetheless, the percentage of severe patients is relatively higher than that reported in a similar recent study.9 Fourth, we could not evaluate few important factors including the duration since the diagnosis of COVID-19 to onset of symptoms, presence of systemic commemoratives, and current use of pain medications. Last, lack of a control population can draw less meaningful conclusions from the study. The strength of our study is its cross-sectional nature with a face-to-face interview of participants. Most of the past studies did a retrospective assessment that might have resulted in memory bias and hence underreporting of the symptoms. In addition, a more explicit evaluation of symptoms including evaluation of neuropathic pain adds to the novelty of this study.
Considering the extensive spread of the COVID-19 across the globe and a significantly high prevalence of pain symptoms, healthcare workers must screen and detect such symptoms at the earliest. Chronic pain may lead to psychological distress, sleep impairment, and negatively impact social life. With prompt and efficient treatment, chronic pain and its complications can be prevented, thereby improving health-related quality of life. Furthermore, policy makers should consider reallocation of resources in this direction, which may help prevent a potential pain pandemic in the recent future.
CONCLUSIONS
Generalized myalgia, headache, and low back pain are the most common pain symptoms associated with COVID-19. The cause of the pain is multifactorial. Without treatment, pain can become chronic and add substantially to disease burden and healthcare costs. Importantly, chronic pain can potentially interfere with the rehabilitation process and delay recovery. Therefore, the potential burden of pain associated with COVID-19 cannot be ignored, especially considering the extent of the affected population. Understanding and early detection of these diverse pain symptoms may guide prompt management. The authors encourage future prospective and systematic studies evaluating COVID-19–associated pain further elaborating their risk factors and persistence beyond the acute phase of the disease.
Footnotes
Vikas Patel is in training.
Financial disclosure statements have been obtained, and no conflicts of interest have been reported by the authors or by any individuals in control of the content of this article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.ajpmr.com).
Contributor Information
Debasish Jena, Email: debasish.mbbs@gmail.com.
Jagannatha Sahoo, Email: pmr_jagannath@aiimsbhubaneswar.edu.in.
Apurba Barman, Email: pmr_apurba@aiimsbhubaneswar.edu.in.
Anil Gupta, Email: dranilaiims@yahoo.co.in.
Vikas Patel, Email: vikaiims2012@gmail.com.
REFERENCES
- 1.WHO : WHO Director-General’s opening remarks at the media briefing on COVID-19–11 March 2020; 2020. Available at: https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020. Accessed August 10, 2021
- 2.Johns Hopkins University : Coronavirus resource center. Available at: https://coronavirus.jhu.edu. Accessed August 10, 2021
- 3.Drozdzal S Rosik J Lechowicz K, et al. : COVID-19: pain management in patients with SARSCoV-2 infection-molecular mechanisms, challenges, and perspectives. Brain Sci 2020;10:465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO : Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19). Geneva, Switzerland, World Health Organization, 2020 [Google Scholar]
- 5.Chen G Wu D Guo W, et al. : Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest 2020;130:2620–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu M He P Liu HG, et al. : Clinical characteristics of 30 medical workers infected with new coronavirus pneumonia [in Chinese]. Zhonghua Jie He He Hu Xi Za Zhi 2020;43:209–14 [DOI] [PubMed] [Google Scholar]
- 7.Tolebeyan AS Zhang N Cooper V, et al. : Headache in patients with severe acute respiratory syndrome coronavirus 2 infection: a narrative review. Headache 2020;60:2131–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tostmann A Bradley J Bousema T, et al. : Strong associations and moderate predictive value of early symptoms for SARS-CoV-2 test positivity among healthcare workers, the Netherlands, March 2020. Euro Surveil 2020;25:2000508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Şahin T Ayyıldız A Gencer-Atalay K, et al. : Pain symptoms in COVID-19. Am J Phys Med Rehabil 2021;100:307–12 [DOI] [PubMed] [Google Scholar]
- 10.Bureau BL Obeidat A Dhariwal MS, et al. : Peripheral neuropathy as a complication of SARS-CoV-2. Cureus 2020;12:e11452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ministry of Health and Family Welfare : Available at: https://www.mohfw.gov.in/pdf/ClinicalManagementProtocolforCOVID19dated27062020.pdf. Accessed August 10, 2021
- 12.Knox N Lee CS Moon JY, et al. : Pain manifestations of COVID-19 and their association with mortality: a multicenter prospective observational study. Mayo Clin Proc 2021;96:943–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trigo J García-Azorín D Planchuelo-Gómez Á, et al. : Factors associated with the presence of headache in hospitalized COVID-19 patients and impact on prognosis: a retrospective cohort study. J Headache Pain 2020;21:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu J Ji P Pang J, et al. : Clinical characteristics of 3062 COVID-19 patients: a meta-analysis. J Med Virol 2020;92:1902–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mo P Xing Y Xiao Y, et al. : Clinical characteristics of refractory coronavirus disease 2019 in Wuhan, China. Clin Infect Dis 2021;73:e4208–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li MY Li L Zhang Y, et al. : Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect Dis Poverty 2020;9:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferrandi PJ, Alway SE, Mohamed JS: The interaction between SARSCoV-2 and ACE2 may have consequences for skeletal muscle viral susceptibility and myopathies. J Appl Physiol 2020;129:864–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang C Wang Y Li X, et al. : Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Motta-Santos D Dos Santos RA Oliveira M, et al. : Effects of ACE2 deficiency on physical performance and physiological adaptations of cardiac and skeletal muscle to exercise. Hypertens Res 2016;39:506–12 [DOI] [PubMed] [Google Scholar]
- 20.Mao L Jin H Wang M, et al. : Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol 2020;77:683–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fan CK Yieh KM Peng MY, et al. : Clinical and laboratory features in the early stage of severe acute respiratory syndrome. J Microbiol Immunol Infect 2006;39:45–53 [PubMed] [Google Scholar]
- 22.Wang JT Sheng WH Fang CT, et al. : Clinical manifestations, laboratory findings, and treatment outcomes of SARS patients. Emerg Infect Dis 2004;10:818–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jin M, Tong Q: Rhabdomyolysis as potential late complication associated with COVID-19. Emerg Infect Dis 2020;26:1618–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Uygun Ö Ertaş M Ekizoğlu E, et al. : Headache characteristics in COVID-19 pandemic—a survey study. J Headache Pain 2020;21:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baig AM Khaleeq A Ali U, et al. : Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host-virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci 2020;11:995–8 [DOI] [PubMed] [Google Scholar]
- 26.Malik GR Wolfe AR Soriano R, et al. : Injury-prone: peripheral nerve injuries associated with prone positioning for COVID-19–related acute respiratory distress syndrome. Br J Anaesth 2020;125:e478–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shi Y Hooten MW Roberts RO, et al. : Modifiable risk factors for incidence of pain in older adults. Pain 2010;151:366–71 [DOI] [PubMed] [Google Scholar]


