Objective
The purpose of this study was to investigate the therapeutic effects of transcranial direct current stimulation on swallowing function in poststroke patients.
Design
We searched for potentially eligible randomized controlled trials from electronic databases, including the PubMed, Embase, Web of Science, Cochrane Library, China National Knowledge Infrastructure, Wanfang, and Chinese Science and Technology Periodical (VIP) databases, from their inception to January 15, 2021. All statistical analyses were performed using RevMan 5.4, and the standardized mean difference with 95% confidence intervals was estimated for the swallowing function outcomes and to understand the mean effect size.
Results
Ten studies involving 343 participants were included in this meta-analysis. The overall analyses demonstrated a significant effect size for swallowing function. Subgroup analyses suggested that both acute and chronic stroke patients showed significant effects on swallowing function after transcranial direct current stimulation. Furthermore, compared with sham stimulation, transcranial direct current stimulation anodal to the affected, unaffected, and bilateral hemispheres can produce a significant effect size for swallowing function in stroke patients.
Conclusions
This meta-analysis showed that transcranial direct current stimulation is likely to be effective for the recovery of dysphagia in poststroke patients, in the acute or chronic phase, and that the effect of anodal transcranial direct current stimulation to unaffected hemispheres is larger.
Key Words: Dysphagia, Transcranial Direct Current Stimulation, Swallowing Function, Meta-analysis
What Is Known
Transcranial direct current stimulation (tDCS) is effective for the recovery of dysphagia in the acute and chronic stages after stroke.
What Is New
When the unaffected hemisphere was anodized, the therapeutic effect of TDCS on the swallowing function after stroke was greater.
Dysphagia is a potentially fatal complication of stroke, and approximately 50% of poststroke patients experience swallowing disorders.1,2 Prolonged swallowing problems in stroke patients are associated with high institutionalization rates, greater healthcare costs, poor outcomes, and many life-threatening complications, including dehydration, malnutrition, aspiration, respiratory infection, and death.3,4 The development of effective interventions that can improve swallowing problems after stroke can not only help regain swallowing control but also reduce the complications of dysphagia.
Currently, traditional therapies include body position adjustment, diet adjustment, oral exercise training, swallowing training, acupuncture, and electrical stimulation, and their positive effects have been confirmed to some extent.5,6 Over the past decade, noninvasive brain stimulation techniques, which regulate the excitability of the cerebral cortex, have been used to investigate the physiology and pathology of swallowing and as a therapeutic tool for improving swallowing function in the different poststroke stages.2 As a noninvasive neuromodulatory approach, transcranial direct current stimulation (tDCS) is a promising treatment for poststroke swallowing function recovery, with many advantages over other stimulation procedures, including economic efficiency, feasibility, ease of administration, and portability.7,8
Ever since the first pilot study on the positive effect of tDCS combined with swallowing exercises in acute stroke patients with dysphagia was reported in 2011, there have been many large sample-size, well-designed randomized clinical trial (RCT) studies reporting its clinical effects.9 Although most of these studies showed that tDCS had a beneficial effect on swallowing function in stroke patients,9–15 one study showed that tDCS had little effect on swallowing function,16,17 and another showed that tDCS had no effect on the recovery of swallowing function.18
A recent systematic review evaluated the effectiveness of tDCS for poststroke dysphagia; however, some meaningful studies were not included in its meta-analyses, and further studies are needed to verify these results.19 Therefore, we conducted this systematic review and meta-analysis to systematically synthesize evidence on the effectiveness of tDCS on swallowing function after stroke.
METHODS
We conducted this systematic review and meta-analysis following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement for RCTs20 and registered our review protocol at the PROSPERO (https://www.crd.york.ac.uk/PROSPERO/, ID CRD42021232205). This study conforms to all Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines and reports the required information accordingly (see Supplementary Checklist, Supplemental Digital Content 1, http://links.lww.com/PHM/B349).
Search Strategy
We searched for potentially eligible RCTs from electronic databases, including the PubMed, Embase, Web of Science, Cochrane Library, China National Knowledge Infrastructure, Wanfang, and Chinese Science and Technology Periodical (VIP) databases, from their inception to January 15, 2021. The key words were “tDCS” or “transcranial direct current stimulation,” “stroke,” “post-stroke,” “cerebrovascular disorders,” or “cerebrovascular accident.” The detailed search strategies for all the electronic database searches are listed in Supplemental Appendix S1 (Supplemental Digital Content 2, http://links.lww.com/PHM/B350; also available online at https://www.crd.york.ac.uk/PROSPERO/).
Eligibility Criteria
Two reviewers independently reviewed the abstracts of each article for the initial selection. To ensure the quality of the included literature, only articles that met the following inclusion criteria were retained for full-length text examination: (1) all of the participants were adults (≥18 yrs) and were diagnosed with ischemic or hemorrhagic stroke by computed tomography or magnetic resonance imaging; (2) the articles were focused on the effect of tDCS on the recovery of swallowing function; (3) the trials were RCTs with crossover and parallel designs; and (4) the outcome measures were standardized, validated dysphagia scales.
The exclusion criteria were as follows: (1) other study designs, such as reviews, meta-analyses, or case reports; (2) studies that were not published in English or Chinese; and (3) studies in which the required data were unavailable.
Any disagreement was settled by the two reviewers through discussion and negotiation.
Figure 1 is the Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram of the study selection procedure.
FIGURE 1.
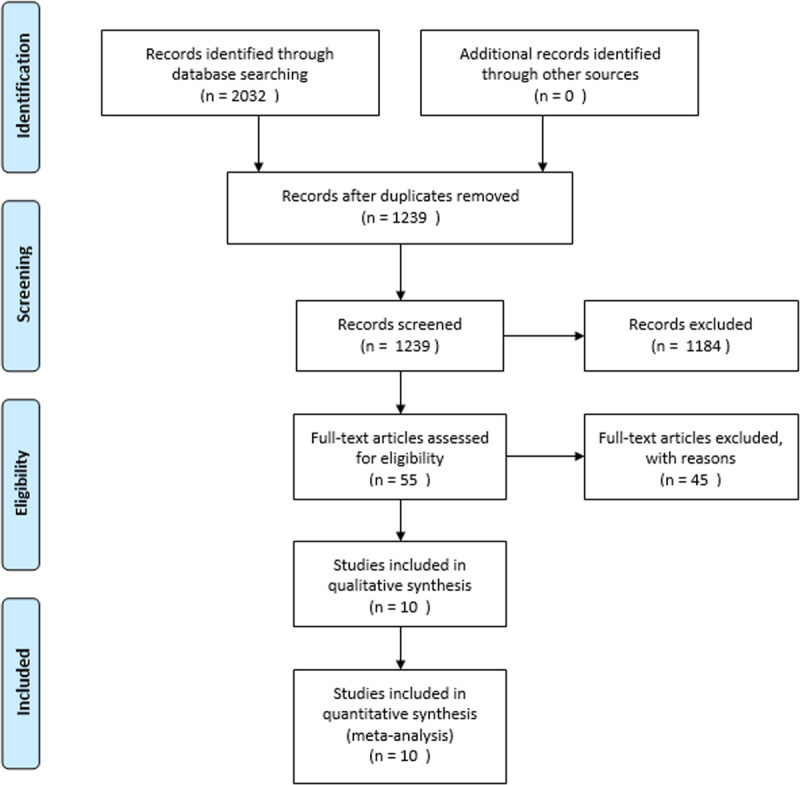
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram of the study selection procedure.
Data Extraction
Two reviewers independently extracted data from the selected full-text studies using predefined data extraction sheets. The following data were extracted: study information (first author, publication year, country, sample size), patient characteristics (age, sex, stroke type), interventions (treatment, stimulation site, dosage, duration, concurrent exercises/therapy), and main outcome measure.
Assessment of Risk of Bias in Individual Studies
Two reviewers independently assessed the quality of the methodology of the extracted studies using the Cochrane Collaboration Risk of Bias Tool. Any disagreements were resolved through discussion and negotiation. The assessment contents included six aspects: selective bias, performance bias, detection bias, attrition bias, reporting bias, and other biases. The overall judgment of each item for each study was categorized as “low,” “high,” and “unclear,” according to the level of bias. Any disagreements were resolved through discussion and negotiation. In addition, we tested for publication bias using a simple funnel plot.
Statistical Analyses
All statistical analyses were performed using Reviewer Manager Software 5.4. A pooled estimate of the mean difference (MD) with the 95% confidence interval (CI) for continuous data from the same measure was calculated. If studies did not use the same measure for an outcome, then the standard MD (SMD) with the 95% CI was calculated instead. Heterogeneity among the included studies was assessed using the I2 statistic, for which P < 0.10 and I2 > 50% represented substantial heterogeneity. A random-effects model was used regardless of the level of heterogeneity.
RESULTS
Study Selection and Characteristics
The search identified a total of 1239 abstracts for screening and yielded 55 relevant studies for full-text review. After careful screening and assessment, 10 studies were finally included in the meta-analysis. The search results are shown in the flowchart in Figure 1, and detailed results are presented in Table 1. Of the 10 included RCTs, three were conducted in China, three in South Korea, and one each in the United States, Japan, Italy, and Germany. Three were published in English, and seven were published in Chinese. The included trials were conducted from 2011 to 2020. The eligible cases in the included studies totaled 343 patients: 187 in the experimental group and 156 in the control group. The groups consisted of 169 men and 114 women with an average age of 62.27 ± 3.17 yrs (1 study had incomplete data). The distribution of the stroke types was as follows: 73 subjects had acute stroke and 270 had chronic stroke. The outcome measures used in the literature in this study were different. Five RCTs included in this meta-analysis used the Dysphagia Outcome and Severity Scale (DOSS)21 as the outcome measure. Two studies used the Functional Dysphagia Scale22 as their outcome measure. The remaining three studies used the Fiberoptic Endoscopic Dysphagia Severity Scale,23 the modified Mann Assessment of Swallowing Ability,24 and the Kubota Water Swallowing Test.25 If possible, we extracted DOSS data as the main outcome indicator because this scale was the most frequently used in this particular group of trials. Otherwise, we evaluated the severity of dysphagia instead of measuring feeding status. Because of the different scales included in the analysis, the directionality of the scales was different. We defined a greater number as indicating positive improvement, but in the inverse situation (greater number meaning decline), we multiplied the effect size by −1 to orient the scales of all the trials in the same direction.
TABLE 1.
Characteristics of the included studies
| Article, Year | Sample Size | Mean Age, yr | Sex | Stroke Type | Country/Region | Intervention | Stimulation Site | Duration of Treatment | Dosage | Electrode Size, cm2 | Main Outcome Measure |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ahn et al.17 (2017) | 26 | 64 | 11 F/15 M | Chronic poststroke | Korea | Anodal to both hemispheres | Pharyngeal motor cortex | 20 mins, 10 d | 1 mA | 25 | DOSS |
| Kumar et al.9 (2011) | 14 | 70 | 7 F/7 M | Acute poststroke | America | Anodal to unaffected | Inferior sensorimotor cortex and premotor brain regions | 30 mins, 5 d | 2 mA | 15 | DOSS |
| Lai14 (2017) | 40 | 67.8 | 19 F/21 M | Chronic poststroke | China | Anodal to affected | Oral region of brain | 20 mins, 20 d | 0–2 mA | NA | Kubota Water Swallowing Test |
| Pingue et al.16 (2018) | 40 | 65.25 | 20 F/20 M | Chronic poststroke | Italy | Anodal to affected, cathodal to unaffected | Pharyngeal motor cortex | 30 mins, 10 d | 2 mA | 25 | DOSS and PAS |
| Shigematsu et al.12 (2013) | 20 | 65.8 | 7 F/13 M | Chronic poststroke | Japan | Anodal to affected | Pharyngeal motor cortex | 20 mins, 10 d | 1 mA | 35 | DOSS |
| Suntrup-Krueger et al.11 (2018) | 59 | 68.05 | 25 F/34 M | Acute poststroke | Germany | Anodal to unaffected | Pharyngeal motor cortex | 20 mins, 4 d | 1 mA | 35 | FEDSS, DSRS, and FOIS |
| Wang et al.15 (2019) | 40 | 62.8 | 12 F/28 M | Chronic poststroke | China | Anodal to unaffected | Pharyngeal motor cortex | 40 mins, 10 d | 1 .5 mA | 35 | MMASA |
| Wang et al.13 (2020) | 28 | 61.8 | 7 F/21 M | Chronic poststroke | China | Anodal to both hemispheres | Bilateral esophageal cortical area | 40 mins, 20 d | 1 mA | 25 | FDS, FOIS, and PESO |
| Yang et al.18 (2012) | 16 | 71 | 6 F/10 M | Chronic poststroke | South Korea | Anodal to affected | Pharyngeal motor cortex | 20 mins, 10 d | 1 mA | 25 | FDS |
| Ko et al.10 (2016) | 60 | NA | NA | Chronic poststroke | Korea | Anodal to both hemispheres | Pharyngeal motor cortex | 20 mins, 10 d | 1 mA | NA | DOSS |
DSRS, Dysphagia Severity Rating Scale; F, female; FDS, Functional Dysphagia Scale; FEDSS, Fiberoptic Endoscopic Dysphagia Severity Scale; FOIS, Functional Oral Intake Scale; M, male; NA, not available; PAS, Penetration-Aspiration Scale.
Results of the Meta-analysis
Risk of Bias Within the Studies
Figure 2 shows a summary of the risk of bias of the included studies. All the included trials reported randomized allocation, but only four of them described the method of the randomization sequence generation.13–16 Two studies clearly reported allocation concealment.13,16 Six studies pointed out that the single-blind method was used,9,11,13,16,18 and five of them pointed out that blinding was used for outcome assessment.11,13,16,18 The risk of attrition bias was low in six studies because the research data were complete or because the amount of missing data and the reasons for their absence were described. There was uncertainty about the reporting bias and other sources of bias because no previously published trial protocols for the included studies were found.
FIGURE 2.
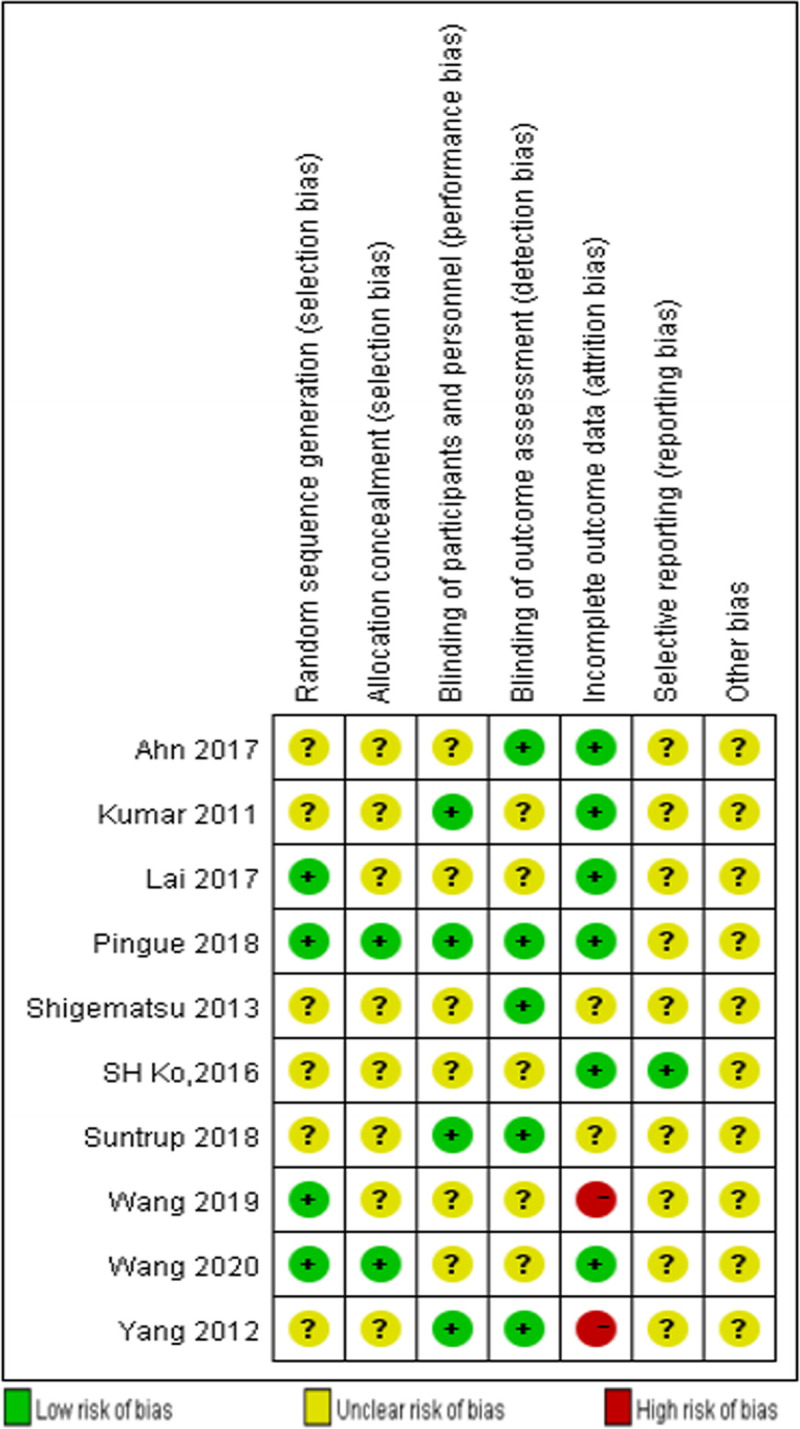
Risk of bias summary: review authors’ judgments about each risk of bias item for each included study.
The funnel plot appeared symmetric as a whole, because an asymmetric plot suggests a publication bias, which is usually positive. In our analysis results, the dots were approximately symmetrically scattered around the pooled effect size (vertical line), indicating that there was no significant publication bias in our review (Fig. 3).
FIGURE 3.
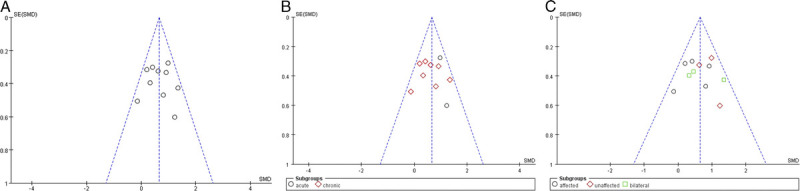
The funnel plot assesses publication bias in the 10 included trials. A, Overall analysis of 10 included trials. B, Subgroup analysis on the stroke stages (acute vs. chronic stroke). C, Subgroup analysis using tDCS on the affected, unaffected, or bilateral hemispheres.
Overall Effect of tDCS
The pooled results on combining all 10 RCTs showed that tDCS was effective for swallowing function (SMD = 0.66, 95% CI = 0.40 to 0.92, P < 0.00001) in stroke patients. The I2, a measure of statistical heterogeneity, was 22%, indicating good homogeneity between all trials. The pooled results showed that of the four trials found to have a small effect size,10,16–18 three were positive10,16,17 and one was negative (SMD = −0.13, 95% CI = −1.12 to 0.86).18 One trial had a moderate positive effect size (SMD = 0.62, 95% CI = −0.01 to 1.26)15 and five trials had large positive effect sizes ranging from 0.81 to 1.35.9,11,12,14,26 Of these, only four trials were statistically significant (Fig. 4).
FIGURE 4.
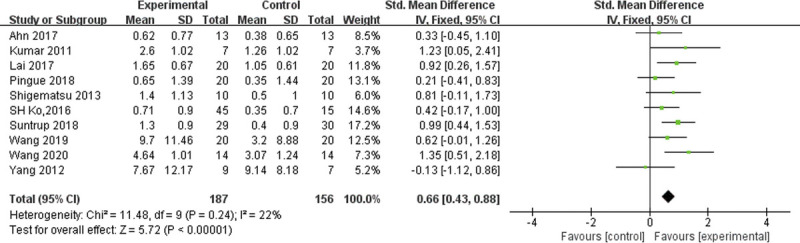
Forest plot of the overall effects of tDCS on poststroke dysphagia.
Acute Versus Chronic Stroke Patients
On further subgroup analysis on the stroke stages (acute vs. chronic stroke), we found a moderate positive effect size (SMD = 0.56, 95% CI = 0.28 to 0.85) in chronic stroke patients and a large positive effect size (SMD = 1.03, 95% CI = 0.54 to 1.52) in acute stroke patients. Both were statistically significant. The I2 values for these two groups were 19% and 0%, respectively (Fig. 5).
FIGURE 5.
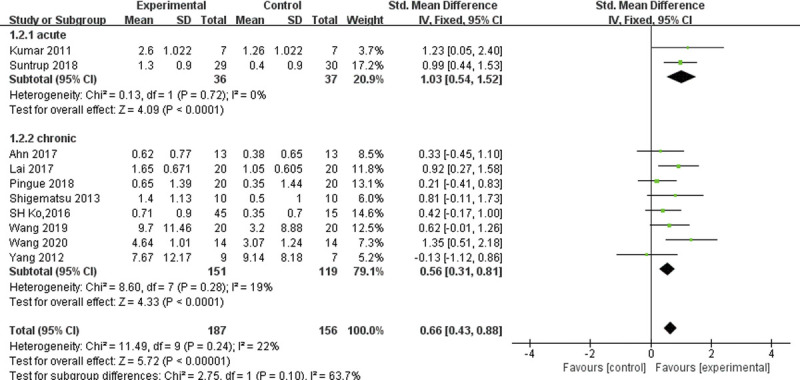
Forest plot of subgroup analysis, which shows the effect sizes for stimulation during the acute versus chronic stroke phase.
Effect of Different tDCS Stimulation Schemes on Swallowing Function
On further subgroup analysis using tDCS on the affected, unaffected, or bilateral hemispheres, we found that compared with anodal tDCS in the affected hemisphere or anodal tDCS in the bilateral hemispheres, patients with anodal tDCS in the unaffected hemisphere were more likely to have greater improvement on the swallowing function test (SMD = 0.88, 95% CI = 0.49 to 1.27, P < 0.0001, I2 = 0%; Fig. 6). We pooled the results, combining the three RCTs in the group with anodal tDCS in the bilateral hemisphere (SMD = 0.68, 95% CI = 0.08 to 1.29, P = 0.03, I2 = 45%). Sensitivity analysis showed that the study by Wang et al.13 (2020) was a major source of heterogeneity. After removing this study, the SMD for swallowing function improvement was 0.39 (95% CI = −0.14 to 0.92, P = 0.151, I2 = 0%).
FIGURE 6.
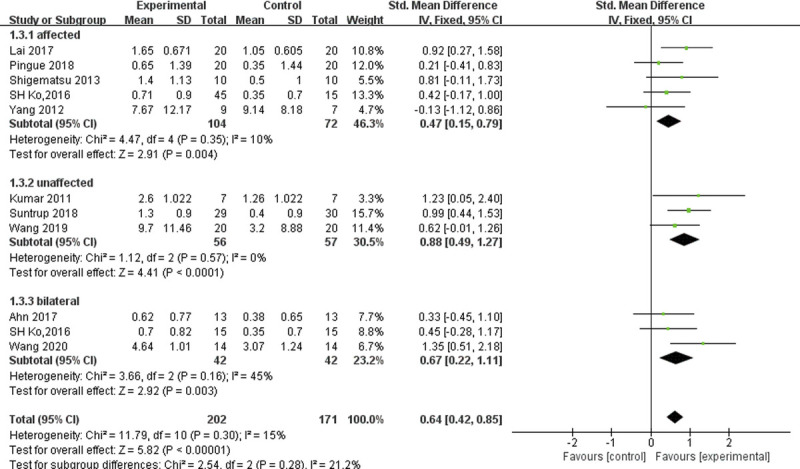
Forest plot of subgroup analysis, which shows the effect sizes for affected, unaffected, and bilateral hemisphere stimulation.
DISCUSSION
Summary of Evidence
The present systematic review and meta-analysis of 10 studies showed that tDCS was associated with improved swallowing function in stroke patients. This was consistent with a previous review by Marchina et al.,19 but some differences existed in the tDCS subgroup analysis. A moderate effect size of 0.66 (95% CI = 0.40 to 0.92, P < 0.00001) of tDCS on poststroke dysphagia was demonstrated in our systematic review. However, another meta-analysis reported a small effect size of 0.31 (95% CI = 0.03 to 0.59, P = 0.03).19 Comparing the two meta-studies, we found that our study basically contains the previous literature, except for those from which we could not obtain full-text information. It is important to point out that most of the newly added literature were mainly published in China. One of these trials had a moderate positive effect size,15 and two trials had large positive effect sizes ranging from 0.92 to 1.35.13,14 Our research is very meaningful, because we have included new literature, and our results further confirm the effectiveness of tDCS on the recovery of dysphagia after stroke. However, the result of the specific effect size of tDCS on swallowing function after stroke still requires further confirmation with clinical trials of large sample sizes and with standardized methodology.
Acute Versus Chronic Stroke Patients
There was a statistically significant effect in both groups with relation to time after stroke: acute patients had a value of 1.03 (95% CI = 0.54 to 1.52, Z = 4.09, P < 0.001), and chronic patients had a value of 0.56 (95% CI = 0.28 to 0.85, Z = 3.89, P = 0.0001). Both were statistically significant. Based on the pooled results of our study, we can conclude that it is safe and effective in improving swallowing function in both acute and chronic stroke patients, and the effect of tDCS is larger in the acute phase of stroke. The current research tends to consider neuromodulatory techniques (such as tDCS) as beneficial for cortical reorganization and increasing the pharyngeal activity of the contralateral motor cortex, which may be the potential mechanism of swallowing function rehabilitation during the acute phase of stroke.19 Consistent with previous suggestions,27–29 our meta-analysis findings support the hypothesis that the rehabilitation effects of tDCS on the contralesional hemisphere are different based on recovery stages. Interestingly, the previous meta-studies revealed a small nonsignificant effect size for both groups. Through further comparison, we found that the main outcome measures of the article that we did not include were Penetration-Aspiration Scale and Functional Oral Intake Scale, which was different from the two other studies. The I2, a measure of statistical heterogeneity, was 50%, indicating medium heterogeneity between all trials. These factors may have affected the interpretation of the results. Furthermore, it is worth mentioning that both meta-analyses combined the chronic and subacute groups together because of their small sample size. Whether the effect of tDCS is greater in the acute versus chronic phase of stroke requires further investigation.
Effect of Different tDCS Stimulation Schemes on Swallowing Function
The subgroup analyses demonstrated that tDCS anodal to the affected hemisphere, unaffected hemisphere, and bilateral hemispheres can produce a significant effect size of swallowing function in stroke patients. Consistent with previous studies on the recovery of swallowing function after stroke, the reorganization of the swallowing motor cortex in the unaffected cerebral hemisphere can promote the improvement of swallowing function.19 However, because swallowing has a bihemispheric representation, the reorganization of the damaged cerebral hemisphere may also play an important role in the recovery of swallowing function after stroke.30–32 The weighted effect size for the unaffected hemisphere was large at 0.88, compared with the medium effect size of 0.47 for the affected hemisphere and 0.68 for bilateral hemispheres. This suggests that tDCS anodal to the unaffected hemisphere is superior to the affected hemisphere and bilateral hemispheres in improving swallowing function after stroke. This is consistent with the previous studies showing that the application of tDCS in the unaffected hemisphere may have some inherent advantages over applying it to the affected hemisphere, as the distribution of current density is unaffected by an underlying stroke with nonhomogeneous tissue, abnormal topography, or impaired intracortical connections.33 Of course, this result requires further confirmation.
This meta-analysis still has the following limitations: (1) we excluded some studies published in languages other than English or Chinese, which led to the inclusion of research that is not very comprehensive; (2) the scale of outcome index used in this meta-analysis is different, and the tDCS stimulation scheme is also different, which leads to greater clinical heterogeneity; and (3) because of the relatively small number of studies that we eventually included and the inconsistent outcome indicators, these results cannot be confidently interpreted.
In conclusion, this systematic review and meta-analysis provided evidence that tDCS is likely to be effective for the recovery of dysphagia in poststroke patients in both the acute and chronic phases and that the effect of tDCS is larger when anodal to the unaffected hemisphere. More high-quality and large-scale studies in this area are required to determine whether this intervention has more significant benefits in certain patient subgroups and with specific stimulation protocols.
Supplementary Material
Footnotes
Qian Lin is the first author.
This study was financed by Fujian Health and Family Planning for Rural and Urban Communities to Promote Appropriate Technology Projects (No. 2020TG019).
Dun-Bing Huang did the conceptualization, funding acquisition, project administration, and resources. Qian Lin and Xiao-Fei Jia did the data curation and formal analysis. Qian Lin did the investigation. Shu-Fang Lin and Xiao-Fei Jia did the methodology. Qian Lin and Shu-Fang Lin did the writing of the original draft. Xiao-Fei Jia and Dun-Bing Huang did the writing review and editing.
Financial disclosure statements have been obtained, and no conflicts of interest have been reported by the authors or by any individuals in control of the content of this article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.ajpmr.com).
Contributor Information
Qian Lin, Email: 29738019@qq.com.
Shu-Fang Lin, Email: 1095704951@qq.com.
Xiao-Hua Ke, Email: xh.ke@outlook.com.
Xiao-Fei Jia, Email: 495067109@qq.com.
REFERENCES
- 1.Arnold M Liesirova K Broeg-Morvay A, et al. : Dysphagia in acute stroke: incidence, burden and impact on clinical outcome. PLoS One 2016;11:e0148424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chiang CF Lin MT Hsiao MY, et al. : Comparative efficacy of noninvasive neurostimulation therapies for acute and subacute poststroke dysphagia: a systematic review and network meta-analysis. Arch Phys Med Rehabil 2019;100:739–50.e4 [DOI] [PubMed] [Google Scholar]
- 3.Clavé P Rofes L Carrión S, et al. : Pathophysiology, relevance and natural history of oropharyngeal dysphagia among older people. Nestle Nutr Inst Workshop Ser 2012;72:57–66 [DOI] [PubMed] [Google Scholar]
- 4.Kushner DS Peters K Eroglu ST, et al. : Neuromuscular electrical stimulation efficacy in acute stroke feeding tube-dependent dysphagia during inpatient rehabilitation. Am J Phys Med Rehabil 2013;92:486–95 [DOI] [PubMed] [Google Scholar]
- 5.Tarameshlu M Ghelichi L Azimi AR, et al. : The effect of traditional dysphagia therapy on the swallowing function in patients with multiple sclerosis: a pilot double-blinded randomized controlled trial. J Bodyw Mov Ther 2019;23:171–6 [DOI] [PubMed] [Google Scholar]
- 6.Banik AA, Hattiangadi GA: Transcutaneous electrical neuromuscular stimulation (TENS) along with traditional dysphagia therapy in patients with posterior stroke: a case study. Indian J Otolaryngol Head Neck Surg 2020;72:279–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aparício L Guarienti F Razza LB, et al. : A systematic review on the acceptability and tolerability of transcranial direct current stimulation treatment in neuropsychiatry trials. Brain Stimul 2016;9:671–81 [DOI] [PubMed] [Google Scholar]
- 8.Erfmann K Macrae PR Jones RD, et al. : Effects of cerebellar transcranial direct current stimulation (tDCS) on motor skill learning in swallowing. Disabil Rehabil 2020:1–9. doi: 10.1080/09638288.2020.1827303 [DOI] [PubMed] [Google Scholar]
- 9.Kumar S Wagner CW Frayne C, et al. : Noninvasive brain stimulation may improve stroke-related dysphagia: a pilot study. Stroke 2011;42:1035–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ko SH Kim SY Park M, et al. : Effect of transcranial direct current stimulation for swallowing function in the stroke patients[J]. Arch Phys Med Rehabil 2016;97:e104 [Google Scholar]
- 11.Suntrup-Krueger S Ringmaier C Muhle P, et al. : Randomized trial of transcranial direct current stimulation for poststroke dysphagia. Ann Neurol 2018;83:328–40 [DOI] [PubMed] [Google Scholar]
- 12.Shigematsu T, Fujishima I, Ohno K: Transcranial direct current stimulation improves swallowing function in stroke patients. Neurorehabil Neural Repair 2013;27:363–9 [DOI] [PubMed] [Google Scholar]
- 13.Wang ZY Chen JM Lin ZK, et al. : Transcranial direct current stimulation improves the swallowing function in patients with cricopharyngeal muscle dysfunction following a brainstem stroke. Neurol Sci 2020;41:569–74 [DOI] [PubMed] [Google Scholar]
- 14.Lai R: Effects of electric acupuncture with transcranial direct current stimulation on dysphagia after stroke [Doctoral dissertation]. Guangzhou, China, Guangzhou University of Traditional Chinese Medicine, 2017 [Google Scholar]
- 15.Wang S Shen X D Mo, et al. : Effect of transcranial direct current stimulation combined with swallowing training on swallowing dysfunction after stroke. Nerve Inj Funct Reconstruction 2019;14:209–11 [Google Scholar]
- 16.Pingue V Priori A Malovini A, et al. : Dual transcranial direct current stimulation for poststroke dysphagia: a randomized controlled trial. Neurorehab Neural Repair 2018;32:635–44 [DOI] [PubMed] [Google Scholar]
- 17.Ahn YH Sohn HJ Park JS, et al. : Effect of bihemispheric anodal transcranial direct current stimulation for dysphagia in chronic stroke patients: a randomized clinical trial. J Rehabil Med 2017;49:30–5 [DOI] [PubMed] [Google Scholar]
- 18.Yang EJ Baek SR Shin J, et al. : Effects of transcranial direct current stimulation (tDCS) on post-stroke dysphagia. Restor Neurol Neurosci 2012;30:303–11 [DOI] [PubMed] [Google Scholar]
- 19.Marchina S Pisegna JM Massaro JM, et al. : Transcranial direct current stimulation for post-stroke dysphagia: a systematic review and meta-analysis of randomized controlled trials. J Neurol 2021;268:293–304 [DOI] [PubMed] [Google Scholar]
- 20.Moher D Liberati A Tetzlaff J, et al. : Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. BMJ 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Neil KH Purdy M Falk J, et al. : The Dysphagia Outcome and Severity Scale. Dysphagia 1999;14:139–45 [DOI] [PubMed] [Google Scholar]
- 22.Han TR, Paik NJ, Park JW: Quantifying swallowing function after stroke: a functional dysphagia scale based on videofluoroscopic studies. Arch Phys Med Rehabil 2001;82:677–82 [DOI] [PubMed] [Google Scholar]
- 23.Dziewas R Warnecke T Olenberg S, et al. : Towards a basic endoscopic assessment of swallowing in acute stroke—development and evaluation of a simple dysphagia score. Cerebrovasc Dis 2008;26:41–7 [DOI] [PubMed] [Google Scholar]
- 24.Antonios N Carnaby-Mann G Crary M, et al. : Analysis of a physician tool for evaluating dysphagia on an inpatient stroke unit: the modified Mann Assessment of Swallowing Ability. J Stroke Cerebrovasc Dis 2010;19:49–57 [DOI] [PubMed] [Google Scholar]
- 25.Dong Y Zhang CJ Shi J, et al. : Clinical application of ICF key codes to evaluate patients with dysphagia following stroke. Medicine (Baltimore) 2016;95:e4479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li N Fang R Chen C, et al. : Observation on the clinical effect of acupuncture Lianquan (RN23) combined with transcranial direct current stimulation in the treatment of dysphagia after stroke. Guangming Tradit Chin Med 2020;35:3607–10 [Google Scholar]
- 27.Hamdy S Aziz Q Rothwell JC, et al. : Recovery of swallowing after dysphagic stroke relates to functional reorganization in the intact motor cortex. Gastroenterology 1998;115:1104–12 [DOI] [PubMed] [Google Scholar]
- 28.Hamdy S Rothwell JC Aziz Q, et al. : Organization and reorganization of human swallowing motor cortex: implications for recovery after stroke. Clin Sci 2000;99:151–7 [PubMed] [Google Scholar]
- 29.Nyeonju K, Amelia W, Cauraugh JH: Transcranial direct current stimulation and suppression of contralesional primary motor cortex post-stroke: a systematic review and meta-analysis. Brain Inj 2018;32:1–8 [DOI] [PubMed] [Google Scholar]
- 30.Takeuchi N, Izumi SI: Noninvasive brain stimulation for motor recovery after stroke: mechanisms and future views. Stroke Res Treat 2012;2012:584727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hamdy S Aziz Q Rothwell JC, et al. : The cortical topography of human swallowing musculature in health and disease. Nat Med 1996;2:1217–24 [DOI] [PubMed] [Google Scholar]
- 32.Bastani A, Jaberzadeh S: Does anodal transcranial direct current stimulation enhance excitability of the motor cortex and motor function in healthy individuals and subjects with stroke: a systematic review and meta-analysis. Clin Neurophysiol 2012;123:644–57 [DOI] [PubMed] [Google Scholar]
- 33.Schlaug G, Renga V, Nair D: Transcranial direct current stimulation in stroke recovery. Arch Neurol 2008;65:1571–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


