Abstract
The risk of stroke and cerebrovascular disease complicating infection with SARS-CoV-2 has been extensively reported since the onset of the pandemic. The striking efforts of many scientists in cooperation with regulators and governments worldwide have rapidly brought the development of a large landscape of vaccines against SARS-CoV-2. The novel DNA and mRNA vaccines have offered great flexibility in terms of antigen production and led to an unprecedented rapidity in effective and safe vaccine production. However, as mass vaccination has progressed, rare but catastrophic cases of thrombosis have occurred in association with thrombocytopenia and antibodies against PF4 (platelet factor 4). This catastrophic syndrome has been named vaccine-induced immune thrombotic thrombocytopenia. Rarely, ischemic stroke can be the symptom onset of vaccine-induced immune thrombotic thrombocytopenia or can complicate the course of the disease. In this review, we provide an overview of stroke and cerebrovascular disease as a complication of the SARS-CoV-2 infection and outline the main clinical and radiological characteristics of cerebrovascular complications of vaccinations, with a focus on vaccine-induced immune thrombotic thrombocytopenia. Based on the available data from the literature and from our experience, we propose a therapeutic protocol to manage this challenging condition. Finally, we highlight the overlapping pathophysiologic mechanisms of SARS-CoV-2 infection and vaccination leading to thrombosis.
Keywords: COVID, 19, pandemics, SARS, CoV, 2, stroke, vaccines
According to the World Health Organization, almost 5 million people have died from COVID-19, with >245 million confirmed cases.1 A number of vascular and thromboembolic complications of COVID-19 were noted early in the pandemic,2 and this was soon followed by observations suggesting a heightened risk of stroke and other cerebrovascular complications.3 Comparative meta-analytic studies have since been undertaken to confirm that infection with SARS-CoV-2 increases the risk of ischemic stroke relative to noninfected contemporary or historical controls,4 as well as relative historical controls infected with influenza.5 In addition to ischemic stroke, hemorrhagic stroke,6 cerebral venous sinus thrombosis (CVST),7 and posterior reversible encephalopathy syndrome8 have all been reported as possible complications.
Vaccines against SARS-CoV-2 are a milestone in the fight against COVID-19. Response to this global crisis, with devastating health, social, and economic impact, was extraordinary, and thanks to cooperation between companies and governments, within a year, several vaccines against SARS-CoV-2 have shown impressive efficacy in randomized clinical trials that have translated into real-world observations. Unfortunately, extremely rare cases of thrombocytopenia and thromboembolic complications have been reported following administration of the ChAdOx1 nCoV-19 vaccine (Oxford-AstraZeneca) and the Ad26.COV2-S vaccine (Janssen), which has contributed to vaccine hesitancy among the public. The situation, however, is highly nuanced, as the risk of thromboembolic complications from infection with SARS-CoV-2 alone is significant. This is of special relevance to stroke and cerebrovascular complications given the significant morbidity associated with intracranial thromboses and hemorrhage.
In what follows, we review the evidence surrounding stroke and cerebrovascular complications of both SARS-CoV-2 infection and SARS-CoV-2 vaccination. In so doing, we review thromboinflammation and the proposed pathophysiology of stroke as a complication of COVID-19, and vaccine-induced immune thrombotic thrombocytopenia (VITT), its typical clinical presentation, and the cases that have presented with stroke and cerebrovascular complications. We conclude by identifying the main pathophysiologic abnormalities common to the two conditions and compare the risk of stroke related to infection and vaccination.
Stroke as a Complication of SARS-CoV-2 Infection
Early reports of neurological complications of SARS-CoV-2 infection emerged in the pre-peer review literature in March 2020. By April 2020, the first retrospective observational reports from Wuhan were published finding neurological symptoms in as many as 36.4% of the admitted patients, specifically citing both ischemic and hemorrhagic stroke as complications of SARS-CoV-2.3 In this section, we discuss the risk of ischemic stroke and other cerebrovascular disorders, as well as putative pathophysiology for stroke in patients with COVID-19.
Ischemic Stroke
Oxley at al9 soon reported a series of relatively young patients (<50 years old) presenting with large vessel occlusion ischemic strokes during the first peak in New York City, all of whom tested positive for SARS-CoV-2. As time would tell, the risk of such presentations was not as great as was initially feared. In fact, initial retrospective incidence rates varied considerably; Li et al10 reported ischemic strokes in as many as 4.6% of their Wuhan inpatient cohort (n=219), whereas Yaghi et al11 found that only 0.9% of their patients admitted in New York had stroke diagnosed during their admission (n=3556). Cohorts in Italy,12 France,13 Germany,14 Philadelphia,15 and other New York hospital systems5,16 fell within this range. To date, the largest multinational studies17–19 and meta-analytic4 estimates of risk among hospitalized patients are shown to be between 0.5% and 1.3%. However, there are important caveats to these estimates, most notably that the majority of strokes did not present with typical clinically evident focal neurological deficits. Rather, the events were detected on neuroimaging during hospital admission,5,17,20 leading many to dispute the true incidence given that not all patients undergo neuroimaging.21 From our personal experience in New York, this was especially true during the peak periods of COVID-19, social distancing, and airborne isolation rules. Risk has been shown to vary with clinical severity of COVID-19.4,10,14,22 Consistent with this hypothesis, studies that include mild disease (managed in the outpatient setting) have yielded lower estimates of risk.23,24 In terms of relative risk, patients requiring hospitalization for COVID-19 have a 3- to 4-fold greater risk of stroke compared with noninfected hospitalized historical or contemporary control cohorts.4,5,11,25 Compared with patients with influenza requiring hospitalization, COVID-19 patients have a 7- to 8-fold greater risk of stroke, although the CIs of these estimates are wide and overlap significantly with the risk estimates when compared with historical controls.5 Patients with COVID-19 at the highest risk of ischemic stroke appear to be those with a history of ischemic stroke,26 possibly a history of diabetes4 and other traditional stroke risk factors,27 and higher serum d-dimer levels.28,29
Outcomes in strokes occurring in patients with COVID-19 also appear worse, in terms of initial stroke severity (compared with historical controls),30 functional outcome at discharge (compared with contemporary and historical controls),30,31 discharge destination (compared with historical controls),32 and inpatient mortality (compared with both contemporary and historical controls).4,30–33
With regard to etiologic classification, patients with COVID-19–associated ischemic strokes have been shown to present with more embolic-appearing findings on neuroimaging. Specifically, multiple case series have been published, highlighting an increased rate of strokes with large vessel occlusions.9,16,17,31,34–37 The majority of these strokes were classified as cryptogenic or embolic stroke of undetermined source.38 Consistent with this, the risk of specifically cryptogenic strokes has been found to be disproportionately increased in patients with COVID compared with control cohorts.4,5,11,16 Many of these patients were also found to have other systemic evidence of thromboembolic disease39,40 and visceral infarction—a phenomenon that is known to be associated with cardioembolic and cryptogenic strokes.41
Putative Mechanisms of Ischemic Stroke in Patients With SARS-CoV-2
The mechanism leading to cerebrovascular complications in the setting of SARS-CoV-2 is likely multifactorial. First, patients with COVID-19, especially those with severe disease, frequently have comorbid factors that increase their baseline risk of thromboembolism. These include dehydration, immobilization, chronic cardiovascular risk factors, or prior atherosclerotic diseases (ie, coronary artery disease, cerebrovascular disease, and chronic kidney disease), as well as inherited thrombophilia.42 Patients with severe COVID-19 requiring intensive care unit (ICU) admission have been shown to have significantly higher rates of arterial or venous thromboembolic events,43 presumably due to a combination of factors discussed herein. Interestingly, studies comparing the rates of ischemic stroke between critically ill COVID-19 patients and other acute respiratory distress syndrome patients have not detected a significant difference, suggesting acute respiratory distress syndrome or critical illness itself likely confers some risk for stroke.44
However, more causal mechanisms have also been proposed given that SARS-CoV-2 both increases the risk of cardiac pathology and impacts all 3 factors comprising Virchow triad (endothelial injury, stasis, and hypercoagulable state), ultimately promoting thrombosis.
Regarding cardiac complications, SARS-CoV-2 has been shown to increase the risk of developing atrial fibrillation,45 which is a well-established risk factor for ischemic stroke.46 In addition, myocardial infarction,47 myocarditis,48 and Takotsubo cardiomyopathy48 have been reported in hospitalized patients with COVID-19, all of which predispose to the formation of left ventricular thrombi and subsequent cardiac embolism.49 Additionally, bacterial superinfection is common in patients with severe COVID-19,50 which increases the risk of bacteremia and infective endocarditis; both of which increase the risk of ischemic stroke.51
In addition, vascular injury is a recognized hallmark of COVID-19. The precise pathophysiology of this remains unclear, but both ACE2 (angiotensin-converting enzyme 2)-dependent and independent processes have been implicated, with some suggesting direct platelet activation via the SP (spike protein) itself.52,53 Within the pulmonary circulation, postmortem analysis has found severe endothelial injury, disrupted cell membranes, with diffuse vascular thrombosis and occlusion of alveolar capillaries.54 Within the cerebral microvasculature specifically, combined imaging and histopathologic assessments have revealed thinning of the basal lamina of the endothelial cells, capillary congestion with fibrinogen leakage, and perivascular inflammation associated with macrophage infiltrates and CD3+ and CD8+ T cells.55 This mild-to-moderate, nonspecific inflammation without clear evidence of vasculitis is a consistent finding in autopsy studies. In contrast to the pulmonary pathology, however, cerebral histopathology has only rarely revealed frank vascular occlusion55,56 or florid cerebrovascular inflammation as seen in the pulmonary microcirculation.57,58 Despite this, these studies have frequently noted mild-to-moderate hypoxic-ischemic injury and microhemorrhages, as well as a stroke phenotype involving multiple small infarcts, including in the corpus callosum, presumed to be a manifestation of microvascular occlusion secondary to thromboinflammation. There are reports of SARS-CoV-2–like particles being found in the brain and endothelium using ultrastructural analysis of tissue from SARS-CoV-2–infected patients with neurological symptoms59; however, diagnosis is challenging due to similar appearing normal cellular structures.60
Consequent to this vascular endothelial inflammation,61 whether systemically or in the cerebral microcirculation, patients with COVID-19 can demonstrate a coagulopathy with an increased risk of in situ thrombosis.62 Specifically, endothelial release of proinflammatory cytokines (the so-called cytokine storm) has been shown to be associated with a hypercoagulable state as evidenced by deranged levels of VWF (von Willebrand factor), D-dimer, fibrinogen, and factor VIII.63 Specifically, small case series of patients with severe COVID-19 have shown exaggerated interleukin-mediated release of VWF, and suppression of the function of ADAMTS13 (a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13), promoting thrombosis via a thrombotic microangiopathy-like process.64,65 This increases the risk of both arterial and venous thrombosis with or without paradoxical embolism to the cerebral circulation.
Other Cerebrovascular Complications
Hemorrhagic Stroke
Studies evaluating the risk of hemorrhagic stroke, which includes intracerebral, subdural, and subarachnoid hemorrhage, have been more limited. The largest studies to date suggest the prevalence among hospitalized patients is rare (as low as 0.2%) and was more likely to occur in older patients and those receiving therapeutic anticoagulation.6 Those hospitalized with ICH and COVID-19 had worse outcomes than those with COVID-19 alone. Studies with more granular hospital course data have suggested that hemorrhages tend to occur during the hospitalization, and the mechanism of these hemorrhages tends to be related to coagulopathy or supratherapeutic anticoagulation, as opposed to primary causes of intracerebral hemorrhages.66
Cerebral Venous Sinus Thrombosis
In a self-controlled case series including 29.1 million people in the United Kingdom comparing rates of thrombotic complications of COVID-19 and vaccinations for COVID-19, testing positive for SARS-CoV-2 was associated with an increased risk of CVST.67 While the point estimate of this risk was high, the CIs were broad, suggesting significant uncertainty of the precise risk. In a meta-analysis of 67 845 patients, the pooled rate of CVST was 0.03%.4 Case series7,68 and reviews have highlighted relatively few (<50) CVST cases reported in the literature to date and suggest it is a relatively rare complication of COVID-19 that is not associated to severe disease as with ischemic stroke. Almost 90% of the reported cases have occurred in women and are mostly found in the transverse sinus. Clinical presentations, however, are subtle, and authors recommend that high suspicion is maintained when encountering patients with COVID-19, headache, and focal neurological deficits.
Posterior Reversible Encephalopathy Syndrome
Posterior reversible encephalopathy syndrome is another relatively rare neurovascular complication that has been linked to hospitalized patients with COVID-19. Almost all the data on this phenomenon are at the level of case series with relatively few cases reported in the literature in total (<50).69 Cases have mostly presented with prolonged or otherwise unexplained encephalopathy and poor level of consciousness, with some complicated by seizures or a focal neurological deficit.8 Most have been in the setting of critically ill patients who have traditional risk factors for posterior reversible encephalopathy syndrome (acute kidney injury and uncontrolled hypertension), and it remains unclear whether the rate of posterior reversible encephalopathy syndrome in patients with COVID-19 is any greater than in patients with other critical illness or multiorgan failure.
Stroke as a Complication of COVID-19 Vaccination
To date, the European Medicines Agency has approved 5 vaccines:70 (1) Comirnaty (BNT 162b2 mRNA vaccine) by Pfizer BionTech; (2) Ad26.COV2.S adenovirus vaccine by Johnson & Johnson/Janssen; (3) Spikevax (mRNA-1273 vaccine) by Moderna; (4) Vaxzevria (ChAdOx1 nCoV-19 vaccine) by Oxford-AstraZeneca, and more recently, (5) Nuvaxovid (NVX-CoV2373) by Novovax. The Food and Drug Administration similarly approved these excluding Vaxzevria and Nuvaxovid.71 Both the European Medicines Agency (EudraVigilance) and the Food and Drug Administration are monitoring the safety of authorized COVID-19 vaccines. This enables the detection of any side effects that may emerge during the mass vaccination. At the time of writing, a total of 6 838 727 352 vaccine doses have been administered worldwide.1 Although most of the reported vaccine-related side effects are mild and transient, concerns progressively emerged about post-Vaxzevria embolic and thrombotic events associated with thrombocytopenia (thrombosis with thrombocytopenia syndrome [TTS], renamed VITT). On April 14, 2021, the European Medicines Agency’s Safety Committee (Pharmacovigilance Risk Assessment Committee) concluded that a causal relationship between Vaxzevria vaccination and rare cases of venous thrombosis in unusual sites (ie, CVST and splanchnic vein thrombosis) and less frequently arterial thrombosis was plausible. As of July 31, 2021, 1503 cases of suspected TTS with Vaxzevria out of about 592 million administered doses were globally reported.70
With a much lower incidence, TTS has also been described after Janssen vaccination. In the United States, by July 8, 2021, 38 TTS confirmed cases occurring within 15 days after vaccination were reported to the Vaccine Adverse Event Reporting System, four of which resulted in death. The overall calculated rate was 3.0 TTS cases per million administered doses, with a higher reporting rate of 8.8 TTS cases per million administered doses among women aged 30 to 49 years.72,73 Nevertheless, a population-level risk-benefit analysis has shown a large population benefit of Janssen vaccination as compared with rare occurrence of TTS.72 In Europe, as of June 27, 2021, 21 cases over about 7 million administered doses were spontaneously reported to EudraVigilance, four of which were fatal.70 Though further case ascertainment is required to confirm TTS in these reported cases, the relationship between the administration of DNA vaccines and TTS has now been established. In fact, according to the Bradford-Hill criteria, which are the accepted criteria for assessing causality of an association, the link between the ChAdOx1 nCoV-19 vaccine and TTS has recently been demonstrated.74 However, the precise estimate of this association is not known, since the incidence rates vary from 0.5 to 25 per 100 000 vaccinated individuals, depending on the different countries.74 Nevertheless, benefits of adenoviral vector vaccines, clearly demonstrated in randomized controlled trials,75,76 still outweigh the risks of these rare thrombotic events, especially in subjects >30 years.77 Interestingly, Hippisley-Cox et al67 found an increased risk of ischemic stroke after 15 to 21 days from BNT162b2 mRNA vaccination (Pfizer BionTech), and after a positive SARS-CoV-2 test, but not after ChAdOx1 nCov-19 vaccination (Oxford-AstraZeneca). The same authors found an increased risk of thrombocytopenia after ChAdOx1 nCov-19 vaccination, and of CVST after ChAdOx1 nCoV-19 vaccination (at 8–14 days), after BNT162b2 mRNA vaccination (at 15–21 days), and after a positive SARS-CoV-2 test.67 More data on these adverse events from BNT162b2 mRNA vaccination are needed.
COVID-19 Vaccines and Target Proteins
All the approved vaccines are based on the full-length homotrimeric SARS-CoV-2 SP. SP plays a key role in viral infection and pathogenesis, since it mediates the entrance of the virus into the host cells via the binding with ACE2. SP, which is located on the viral envelope, comprises 3 S1/S2 heterodimers: S1 harbors the N-terminal domain and the receptor-binding domain.78–80 Interestingly, the SP ectodomain consists of a head where receptor-binding domains are located and a stalk with 3 flexible hinges connecting SP to the viral membrane. This high degree of conformational freedom of SP on the viral surface may interfere with antibody access to the stalk, add strength to the virus, and facilitate the binding of the SP with the host receptor.81
In December 2020, Wajnberg et al82 found that most of the infected individuals with mild-to-moderate COVID-19 had developed a robust IgG antibody response against the viral SP. These authors also showed that titers were long-lasting (several months) and that anti-SP binding titers significantly correlated with neutralization of SARS-CoV-2.82 All these data confirmed the SP as the main target of vaccine development.78
The three vaccines approved by the United States and 4 of the 5 approved by the European Union (EU) are DNA or mRNA vaccines encoding the SARS-CoV-2 SP. In DNA vaccines (Janssen and Oxford-AstraZeneca), the genetic materials need to pass through the nucleus to create mRNA with subsequent transcription of the protein in the cytoplasm.83 In mRNA vaccines (Pfizer and Moderna), the nuclear step is missing, making the process even simpler.84 Pfizer and Moderna vaccines consist of a lipid-enclosed nucleoside-modified mRNA encoding a different mutated SP, whereas the AstraZeneca and Janssen vaccines utilize a chimpanzee nonreplicating adenovirus and a type 26 nonreplicating recombinant adenovirus vector, respectively. Moreover, the AstraZeneca vaccine has the complete coding sequence of SP plus a sequence of a tissue-type plasminogen activator, and the Janssen vaccine has mutations for stabilizing the SP.83 Nuvaxovid is based on the SP produced by recombinant DNA technology using a baculovirus expression system in an insect cell line and is adjuvanted with Matrix-M. Effectiveness and safety of this vaccine have been demonstrated in clinical trials,85 but real-world evidence is still lacking.
Efficacy data of DNA- and mRNA-based vaccines against SARS-CoV-2 from clinical trials seem to be consistent with data on vaccine effectiveness from the real world. However, more data are urgently needed, considering both the rapidly emerging appearance of SARS-CoV-2 novel variants and the temporal waning of immunity after vaccination.86
Vaccine-Induced Immune Thrombotic Thrombocytopenia
By the end of March 2021, several scientific papers from different countries reported cases of devastating thrombosis in unusual sites, especially CVST, 5 to 30 days after the administration of the first dose of the ChAdOx1 nCoV-19 vaccine. These patients, who were otherwise young and healthy, also presented with thrombocytopenia, elevated D-dimer, sometimes low fibrinogen, and high levels of antibodies against PF4 (platelet factor 4)-heparin.87–89 Similar syndromes have also been reported after Janssen vaccination90 and after Moderna’s mRNA-1273 vaccine.91
The syndrome was named VITT92 since it resembles the heparin-induced immune thrombocytopenia (HIT),93 although in the absence of exposure to heparin.
Pathogenetic Hypothesis
PF4 is a cationic chemokine consisting of 4 monomers, released from the α-granules of activated platelets as an immune defense mechanism. It is capable of opsonizing negatively charged surfaces of bacteria, ultimately facilitating binding of anti-PF4 antibodies. In HIT, heparin, acting as a polyanion, causes a conformational change of PF4 tetramers and consequently the anti-PF4/heparin antibody induction.93 Things other than heparin, such as chondroitin sulphate, DNA and RNA, bacterial wall components, and high concentration of PF4 per se, can induce the exposure of HIT antigens leading to spontaneous or autoimmune HIT. Sera from patients with autoimmune HIT typically contain high-avidity IgG antibodies, which strongly activate platelets from healthy donors via FcγRIIa, one of the receptors for the Fc domain of IgG antibodies. As a consequence, platelet-derived procoagulant microvesicles are released, resulting in severe thrombocytopenia, leading to an increased frequency of disseminated intravascular coagulation, and atypical thrombotic events.93,94
Similarly to autoimmune HIT, sera from VITT patients contain high levels of PF4-heparin antibodies that activates platelets in the presence of, but also in the absence of, heparin. This activation is greatly enhanced in the presence of PF4.87–89 Notably, a cross-reaction between the anti–SARS-CoV-2 SP antibodies and PF4 or PF4/heparin complexes has been ruled out,95 and no correlation has been found between the anti-PF4 and the anti–SARS-CoV-2 neutralizing antibody levels after ChAdOx1 nCoV-19 vaccination.96 These data exclude the possibility that the anti-PF4 antibodies are a side product of the vaccine immune response.
Recently published data suggest that vaccine components, including the adenovirus hexon protein and also the adenovirus per se,97 can generate neoantigen complexes with PF4, thus inducing anti-PF4 antibody production.98 Anti-PF4 antibodies stimulate platelet aggregation. Cross talk of platelets and anti-PF4 antibodies activates neutrophils, leading to the formation of neutrophil extracellular traps and ultimately to the activation of monocytes and endothelial cells, further amplifying the activation of the coagulation cascade. The ChAdOx1 nCov-19 vaccine also contains EDTA, which increases the capillary leakage at the inoculation site, allowing the virus to spread via the bloodstream.98 Data based on an intriguing hypothesis about a possible transcription of spliceosome-mediated soluble SP fragments with thrombogenic properties in DNA vector vaccines have not yet been peer reviewed.99 However, preprinted data from our group seem to support this hypothesis. In fact, a soluble SP has been found in sera from 3 VITT patients and on a platelet-rich thrombus retrieved from middle cerebral artery (MCA) of 1 VITT patient, suggesting that SP could be one of the platelet activation triggers in VITT.100 Undoubtedly, additional experiments are required to fully understand the pathogenesis of this rare, devastating syndrome.
Clinical Characteristics, Diagnosis, and Therapy
As of April 2021, descriptions of clinical features of patients affected by VITT in case series and case reports from different countries allowed us to delineate precise diagnostic criteria and inform the therapeutic approach.73,87–89,101–103 Guidelines from different medical societies have been developed,87,104–106 although some of the published cases do not strictly meet classification criteria. VITT is an evolving condition, and a low-probability diagnosis of VITT at presentation can rapidly evolve to a fully blown VITT in the subsequent days. Close monitoring of patients is then mandatory to rule out this diagnosis.107 Recently, a pre-VITT syndrome characterized by severe headache without associated CVST or other thrombosis has been described, highlighting the need for clinicians to be aware of different presenting onsets of VITT, and intravenous immunoglobulin (IVIG) therapy promptly.108
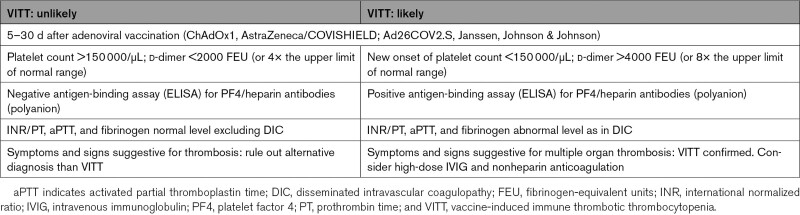
Diagnosis of VITT is clinical and radiological. Typical blood test results are needed to confirm the clinical suspicion. Patients with VITT typically present with a classical clinical triad of thrombosis (mainly CVST, pulmonary and splanchnic), thrombocytopenia (<150 000/µL), and elevated D-dimers (>4000 fibrinogen-equivalent units [FEU] or 4–8× the upper limit of normal range). As addressed by Greinacher et al,87 the different combination of these elements leads to 2 different scenarios: VITT likely and VITT unlikely (Table 1). Diagnosis of VITT is confirmed by demonstration of anti-PF4 antibodies by ELISA plate–based PF4/heparin (polyanion) antibody test (but a negative test still does not definitively rule out the diagnosis)87 and functional platelet activation assay.87–89,92,106
Table 1.
Criteria to Consider When Risk Stratifying Patients With Suspected VITT
Prompt recognition and treatment of this syndrome may reduce mortality. Education of the public and clinicians has reduced mortality of VITT from 50% in the first case series in April 2021 to 22% in June 2021 in the United Kingdom.87 The pillars of VITT therapy are nonheparin anticoagulants (direct oral anticoagulants, danaparoid, argatroban or fondaparinux, continued for at least 3 months) and high-dose IVIG (0.5–1 g/kg of actual body weight for 1 or 2 consecutive days).87,104,105 Steroids may be useful (especially if platelets are <50 000), and plasma exchange can be considered in selected cases.87,104,105 Rituximab can be prescribed in patients who are refractory to repeated doses of IVIG and plasma exchange, although evidence is limited.104
Ischemic Stroke as VITT Atypical Presentation
Ischemic stroke can be a rare and challenging symptom onset of VITT or can complicate its course. The real incidence of this serious and life-threatening condition is unknown.
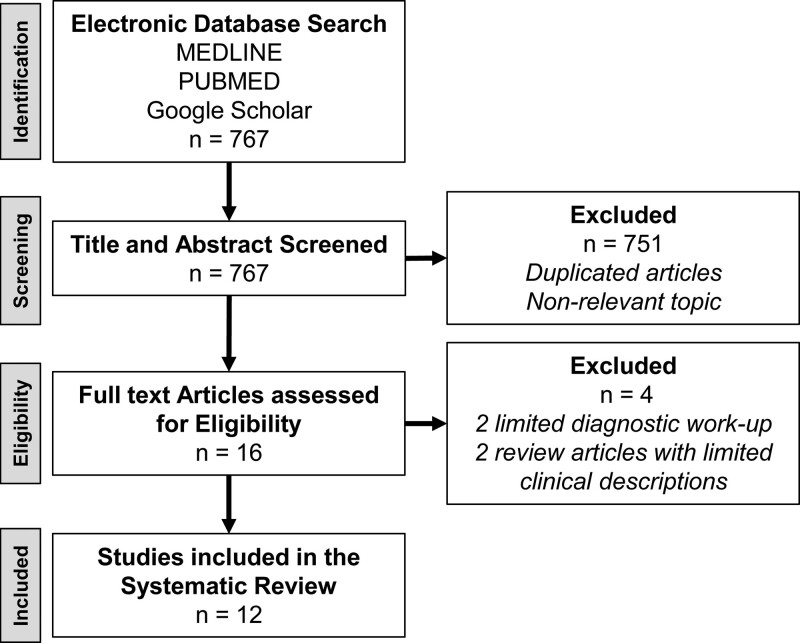
We performed a systematic review using MEDLINE, PUBMED, and Google Scholar databases to collect all the published articles related to the development of ischemic stroke after vaccination against SARS-CoV-2. The search process was done on October 27, 2021 by the authors using the following terms in various combinations: “ChAdOx1 ncov19 vaccination,” “stroke,” “vaccine induced immune thrombotic thrombocytopenia,” “infarct,” “VITT,” “AstraZeneca,” “SARS-CoV-2 vaccination,” “COVID-19,” and “PF4.” Overall, 161 published articles were identified, but only 13 were relevant to this review. One article was removed due to insufficient workup to rule out other causes of stroke and for not testing anti-PF4 polyanion antibodies. Consequently, the search and sorting processes were finalized with 12 articles with data on 16 patients (Figure 1).
Figure 1.
Systematic review flowchart.
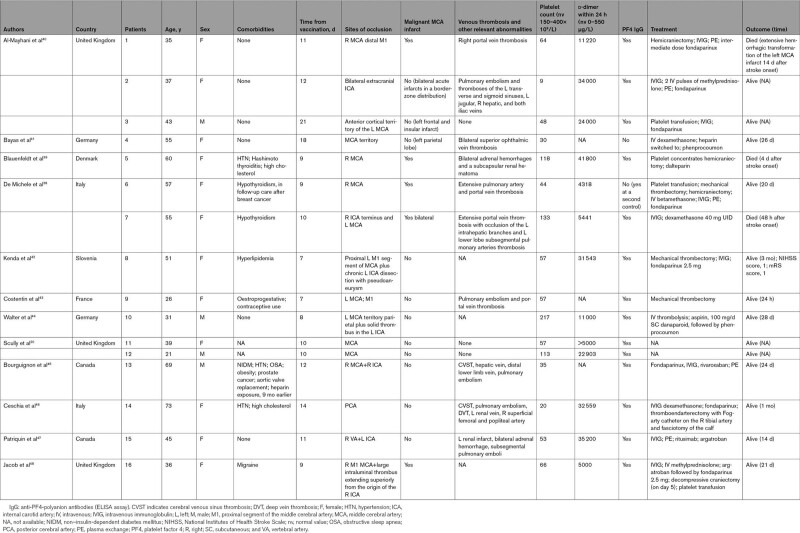
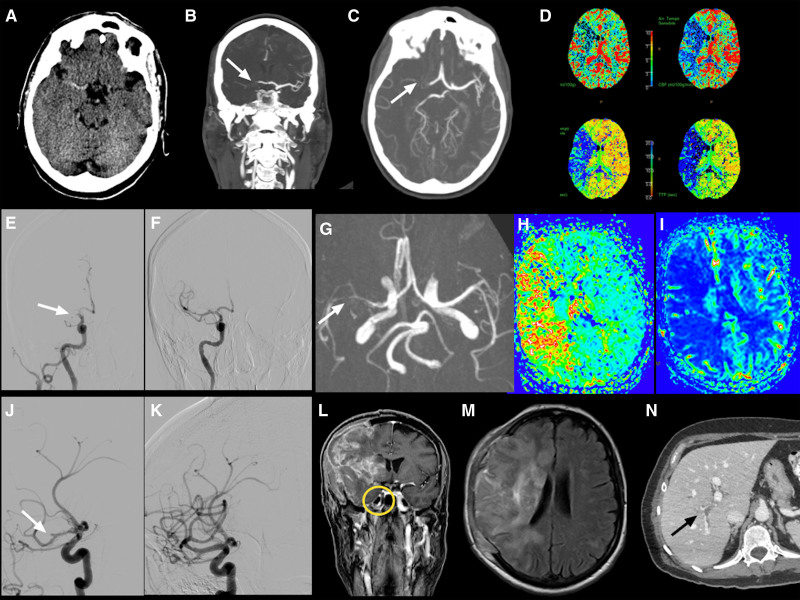
In Table 2, we have summarized the demographic information and clinical features of the 16 patients affected by ischemic stroke with VITT confirmed diagnosis, identified from case reports or case series published in peer-reviewed journals. All the patients had received the first dose of the ChAdOx1 ncov19 vaccine. Three cases were reported from Italy, 6 from the United Kingdom, 2 from Germany, 1 from Slovenia, 1 from France, 1 from Denmark, and 2 from Canada. The mean age of the reported patients was 46.6 (SD, ±15.2; range, 21–73) years. Twelve of 16 patients were women (75%). Median time between vaccination and onset of stroke was 10 days. All the individuals were healthy before vaccination, and half of them had no preexisting comorbidities in their medical history. Three of them experienced hypertension and three had hyperlipidemia. Three subjects had thyropathy (1 case of Hashimoto thyroiditis and 2 cases of hypothyroidism). One patient was in follow-up care after breast cancer, and another one had recently received the diagnosis of prostate cancer (not yet staged). Only 1 patient, a 69-year-old man, had multiple vascular risk factors (hypertension, diabetes, obstructive sleep apnea, obesity, and aortic valve replacement) and previous exposure to heparin (9 months earlier). One patient experienced migraine and the another one was on estroprogestative contraceptives. Most of the patients had occlusion of the MCA or its branches (81%), and 7 of them (43.7%) also had thrombotic occlusion of the intracranial internal carotid artery. Five of 11 patients with proximal MCA occlusion (45.4%) developed a malignant MCA infarct involving the whole territory of the MCA with space-occupying cerebral edema and rapid neurological deterioration, successfully treated with hemicraniectomy in 4 cases. In 1 case (case 7), surgical intervention was not performed since the malignant infarct was bilateral (Figure 2).109 Ten of 13 cases in which the data have been reported presented multiple sites of venous thrombosis, particularly of the splanchnic, portal, and hepatic veins and pulmonary embolism, while only 3 of them had CVST. Fifteen patients had low platelet count at admission (mean±SD, 70.33±54×109/L; range, 9–133×109/L), while 1 patient had 217×109/L platelets at admission, with subsequent decrease to a nadir of 152×109/L 2 days after stroke onset. Very high level of D-dimer (mean value, 21 580 μg/L; normal range, 0–550) was present in all 14 patients in which the data have been reported. ELISA for PF4 autoantibodies was positive in 14 of the 16 tested patients. In 1 negative patient at baseline, high levels of antibodies were found at day 15 from admission. Unfortunately, it is not possible to draw a precise follow-up of these patients since outcome at 3 months has only been reported for case 8. Three of the 16 patients died. Patient 6, who survived and was described from our group,109 died 2 months later from an unexpected cardiac arrest while she was hospitalized in a rehabilitation center. Brain computed tomography scan did not show any new vascular events, and platelet counts were in the normal range. Her relatives denied autopsy. Only 1 patient (case 10) with distal MCA occlusion received intravenous thrombolysis with alteplase since platelet count was in the normal range, while 3 patients underwent successful mechanical thrombectomy. Case 6, reported from our group,109 underwent a second mechanical thrombectomy 2 hours after a first successful endovascular procedure (and 3 hours after the symptom onset), due to worsening of the neurological conditions and evidence on brain magnetic resonance imaging of a reocclusion of the same vessel (M1 segment of right MCA), with salvageable penumbra. Unexpectedly, despite a second complete reperfusion of the MCA territory, the patient developed a malignant infarct because of a third reocclusion of the right MCA and extension of the clot to the ipsilateral internal carotid artery terminus 12 hours apart (Figure 3). We hypothesized that the postthrombectomy injured arterial endothelium, combined with the high prothrombotic state and endothelium dysfunction characteristic of VITT, could have been the cause of the repeated arterial occlusions of the same vessel. Unfortunately, at the time the patient was admitted to our hospital, articles on VITT had not still been published and, due to very low baseline platelet count (44×109/L), platelet transfusion was performed before the first thrombectomy, which may have contributed to exacerbate the thrombotic event. At present, guidelines recommend that prophylactic platelet transfusions should be avoided in the context of VITT but should be provided before major surgical interventions (ie, hemicraniectomy) or if life-threatening bleeding is present.87,105
Table 2.
Demographic, Clinical Characteristics, Baseline Blood Samples, Radiological Features, Treatment, and Outcome of Patients With Vaccine-Induced Immune Thrombotic Thrombocytopenia and Ischemic Stroke Described in the Literature
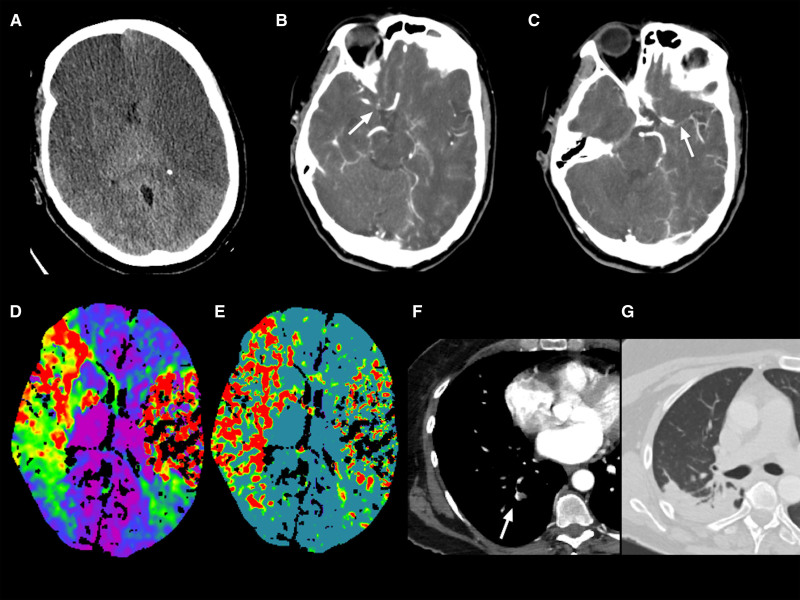
Figure 2.
Radiological findings from patient 7.
Case reported by De Michele et al.109 A, Computed tomography (CT) demonstrated extensive ischemic changes in the bilateral middle cerebral artery (MCA) distribution with general hypodensity and loss of gray-white matter differentiation. CT angiography showed the occlusion of the right internal carotid artery terminus (white arrow in B) and the proximal M1 segment occlusion of the left MCA (white arrow in C); time-to-maximum (D) and mean transit time (E) in CT perfusion showed hypoperfusion without treatable penumbra; pulmonary artery thrombosis (white arrow in F) with pulmonary consolidation in the right lobe (G).
Figure 3.
Radiological findings from patient 6.
Case reported by De Michele et al.109 A, Computed tomography (CT) showed hyperdensity in the right middle cerebral artery. The CT angiography revealed proximal M1 segment occlusion of the right middle cerebral artery (MCA; white arrow in B and C); CT perfusion maps showed a large area of mismatch indicating salvageable penumbra (D); digital subtraction angiography confirming a proximal MCA occlusion (F) of the MCA occlusion (white arrow in E); MCA reocclusion on M2 segment 2 h after the procedure, 3-dimensional time-of-flight magnetic resonance imaging (MRI) sequence (white arrow in G), with extensive ischemic penumbra (time to peak map in H and cerebral blood flow in I). Second endovascular recanalization, oblique views showed occlusion of M2 segment (white arrow in J) with reopening of the vessel after the mechanical thrombectomy (K). Fourteen-day MRI follow-up after craniectomy showed the extension of ischemia to superficial and deep right MCA territory (M) with occlusion of right internal carotid artery at postcontrast sequences (yellow circle in L). N, Right portal vein thrombosis (black arrow).
Additional Information
Through the abovementioned systematic review of the literature, we also identified 2 additional studies relevant for the scope of this review, 1 from United Kingdom and 1 from Germany. A prospective multicenter cohort study from the United Kingdom evaluated VITT patients who presented to the hospital between March 22 and June 6, 2021.102 Among 220 patients with diagnosis of VITT classified as definite (ie, all 5 of the following criteria: [1] onset of symptoms 5–30 days after vaccination against SARS-CoV-2; [2] presence of thrombosis; [3] thrombocytopenia [platelet count <150 000 per mm3]; [4] D-dimer level >4000 FEU; [5] positive anti-PF4 antibodies on ELISA) or probable (ie, D-dimer level >4000 FEU but absence of one of the abovementioned criteria or D-dimer level unknown or 2000–4000 FEU and the presence of all other criteria), 17 subjects (7.7%) experienced cerebrovascular accidents. Unfortunately, no more details about these 17 patients have been reported by the authors, since data reported in the article are cumulative and related to all 220 VITT patients.
The second study comes from the German Society of Neurology SARS-CoV-2 Vaccination Study Group.101 Nine cases of ischemic stroke (mean age, 55.6; range, 31.0–82.0) of 62 cerebrovascular events (14.5%) within 31 days from a first dose of COVID-19 vaccination have been reported in the December 28, 2020, to April 14, 2021, time period. Eight of these patients had received the ChAdOx1 ncov19 vaccination, and only 1 received the BNT162b2 vaccine. Majority of cases were females (66.7%). Among these 9 ischemic patients, 5 with embolic stroke had a VITT score of >2 (which means a highly probable VITT defined as the presence of the following 2 criteria: [1] time from shot administration between 1 and 16 days; [2] thrombocytopenia, <150×109/L or relative thrombocytopenia, drop of thrombocytes of at least 50%; [3] positive ELISA to detect PF4-polyanion antibodies; [4] positive modified platelet activation assay) without signs of CVST. In four of them, thrombotic occlusion of the MCA or internal carotid artery and recurrent thrombotic material in duplex ultrasound were described.
Therapeutic Implications
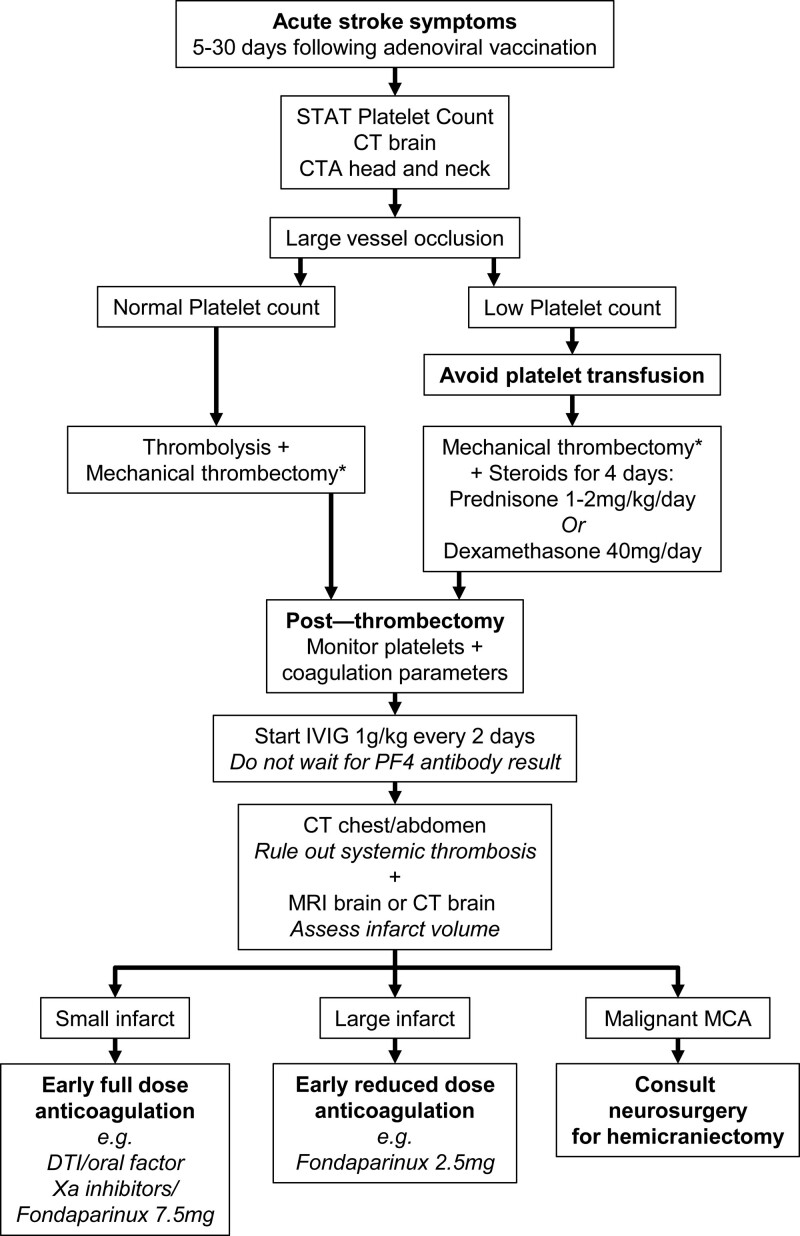
Therapeutic approach of acute ischemic stroke due to large vessel occlusion in VITT is challenging. Based on the available literature89,110–119 and our personal experience,109 we propose the following management protocol for acute stroke patients presenting to the Emergency Department within the time window for reperfusion strategies (Figure 4):
Figure 4.
Management flowchart of patients with acute ischemic stroke and suspected vaccine-induced thrombotic thrombocytopenia presenting to the Emergency Department within the time windows for reperfusion interventions. CT indicates computed tomography; CTA, computed tomography angiography; DTI, direct thrombin inhibitor; IVIG, intravenous immunoglobulin; MCA, middle cerebral artery; MRI, magnetic resonance imaging; and PF4, platelet factor 4. *According to the current international guidelines.
Keep in mind that ischemic stroke can be the first presentation symptom at onset of VITT.
If a patient has received the first dose of DNA vector vaccination against SARS-CoV-2 within the previous 5 to 30 days, wait for platelet count results before starting thrombolysis.
If large vessel occlusion is evident at cerebral computed tomography angiography without signs of malignant MCA infarct, mechanical thrombectomy is indicated according to guidelines from professional medical societies.
If low platelet count is evident, avoid platelet transfusion and consider steroid administration (prednisone 1–2 mg/kg per day or dexamethasone 40 mg/day for 4 days) possibly before the endovascular procedure, monitoring blood pressure and blood glucose.
Monitor the patient closely after mechanical thrombectomy since the risk of reocclusion and neurological deterioration is high.
Schedule a control brain computed tomography scan or magnetic resonance imaging in the next 12 hours to decide the timing for starting anticoagulation.
Start early full-dose anticoagulation with oral or parenteral direct thrombin inhibitors, or oral factor Xa inhibitors, or fondaparinux, only if brain infarct is small. If brain infarct is large, start with a reduced dose of anticoagulant (ie, fondaparinux, 2.5 mg daily) and increase the dosage after 2 weeks from stroke onset (ie, fondaparinux, 7.5 mg daily), due to the high risk of hemorrhagic transformation of the ischemic lesion.
Thrombocytopenia seems not to be a contraindication to therapeutic dose anticoagulation in VITT, since subjects with the lowest platelet count are at the highest risk of thrombosis.87 However, some of the available current guidelines suggest low-dose anticoagulants if platelet counts are <30 to 50×109/L.87
Consider IVIG treatment (1 g/kg for 2 consecutive days) immediately after reperfusion therapies if VITT diagnosis is probable (thrombosis, thrombocytopenia, high D-dimer after vaccination), without awaiting confirmation from PF4 antibodies ELISA immunoassay. Repeated IVIG may be required.
Perform anti-PF4 ELISA immunoassay and functional assay of platelet activation as soon as possible to confirm diagnosis (blood sample for functional assay should be obtained before IVIG administration since IVIG inhibits functional immunoassay).87
Consider plasma exchange (daily for up to ≥5 days) if extensive thrombosis and platelet count is <30×109/L.
Consider rituximab for patients who are refractory to repeat doses of IVIG and plasma exchange, although evidence of its efficacy in VITT is scarce.
Expert consultation from hematologist is necessary.
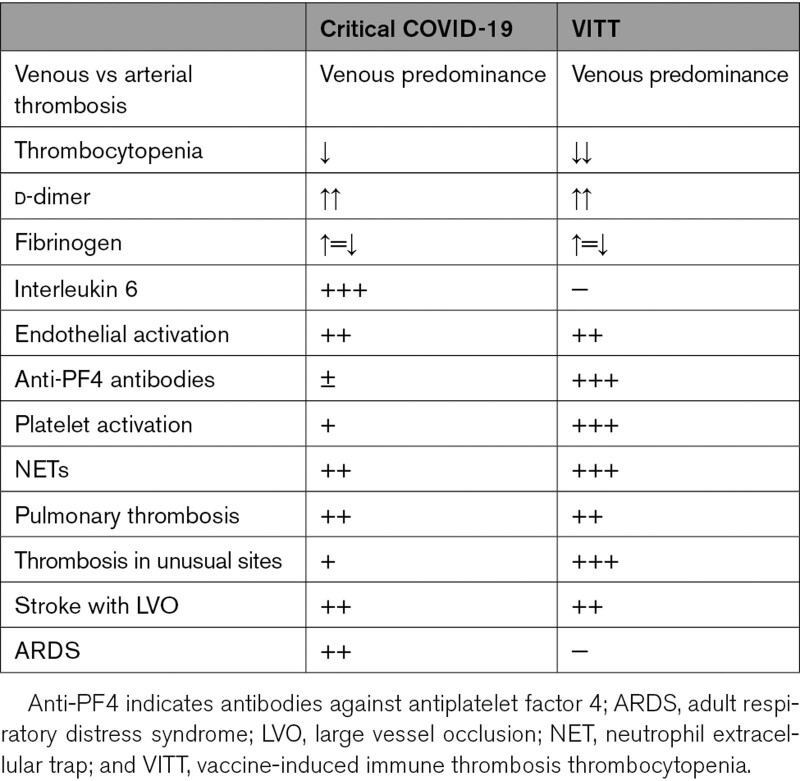
Pathophysiology of Stroke in COVID-19 and VITT: Similarities and Differences
COVID-19 and VITT show some common elements that lead to hypercoagulability and vascular occlusion. The abnormal interaction between platelets, innate immune effectors (neutrophils, macrophages, and complement), and coagulation factors are the key features of both pathological conditions. The ultimate consequence yields clot formation—a phenomenon known as thromboinflammation.120
In contrast to patients with COVID-19 and ischemic stroke, VITT patients do not show the typical elevations in interleukins. Rather, as discussed, the combination between soluble SP, adenovirus, and vaccine excipients probably acts as trigger for platelet activation.99 This distinct mechanism yields very different histopathologic findings postmortem. Specifically, postmortem studies on VITT patients found diffuse vascular thrombosis with endothelial activation, dense recruitment of inflammatory cells, and complement pathway activation in multiple organs.121
While thrombocytopenia is a typical finding of VITT, it is less frequently seen in patients with COVID-19 but is associated with increased risk of serious illness and death.122 Mechanisms of thrombocytopenia in COVID-19 are different, however, and are speculated to be mostly secondary to cytokine release, viral bone marrow infiltration, and increased platelet consumption.123 More rarely, thrombocytopenia in patients with COVID-19 can result from anti-PF4 antibody production as a complication of prolonged exposure to unfractionated heparin. There are also reports of platelet internalization of SARS-CoV-2 inducing platelet apoptosis, release of granular content, reduced platelet functionality, and high prothrombotic and proinflammatory immune response.124
Other common characteristics to both pathologies are the marked endothelial activation with elevated VWF, coagulation abnormalities (which can culminate to disseminated intravascular coagulation), and increased production of neutrophil extracellular traps.123,125 A comparison is summarized in Table 3.
Table 3.
Comparison Between COVID-19 Critically Ill and VITT Patients
Conclusions
Stroke and other cerebrovascular complications of SARS-CoV-2 infection are a highly morbid problem, with multifactorial pathophysiology. Given the association between severe SARS-CoV-2 infection and cardiovascular risk factors common to stroke, there is some degree of confounding; however, multiple studies at this point suggest that SARS-CoV-2 infection is an independent risk factor for ischemic stroke.4
Cerebrovascular complications of vaccination, specifically VITT is a rare but devastating syndrome occurring more frequently in young people after inoculation of DNA adenoviral vector vaccines that should be promptly recognized. The VITT variant causing arterial stroke is an even rarer but catastrophic event, whose management in the acute phase is complex and challenging.
Some European Union countries have restricted the use of adenovirus vector vaccines to older age groups. In Italy, the Government’s Technical and Scientific Committee has limited the use of Oxford-AstraZeneca vaccines to people over 60 years of age, whereas in the United Kingdom, the Joint Committee on Vaccination and Immunization recommended that the Oxford-AstraZeneca vaccine should not be given to people under 40 years of age. Canada and France have restricted the use of this vaccine to people 55 years of age and over, while Germany has set the bar at 60 and Iceland at 70 years of age.
Despite this, to date, studies have demonstrated that the risk of stroke and other prespecified outcomes of interest (thrombocytopenia, venous thromboembolism, arterial thrombosis, CVST, and myocardial infarction) following a SARS-CoV-2 infection were significantly higher than following vaccination with either the Oxford-AstraZeneca or Pfizer vaccines.67 As such, because benefits of mass vaccination against COVID-19 far outweighed the risks of VITT, no age restrictions were announced either by the European Medicines Agency or the Food and Drug Administration.
A global immunization campaign is urgently needed, particularly in low-income countries, and all currently available vaccines are approved by emergency authorizations. Nevertheless, more studies about the pathogenesis of VITT are mandatory to ameliorate the risk of adenovirus-based vaccines and to identify those most at risk of VITT.
Article Information
Acknowledgments
We would like to acknowledge Natalie Pacheco LeMoss and Jed Kaiser for their assistance with formatting and editing of this article.
Disclosures
None.
Nonstandard Abbreviations and Acronyms
- ACE2
- angiotensin-converting enzyme 2
- CVST
- cerebral venous sinus thrombosis
- HIT
- heparin-induced thrombocytopenia
- MCA
- middle cerebral artery
- PF4
- platelet factor 4
- SP
- spike protein
- TTS
- thrombosis with thrombocytopenia syndrome
- VITT
- vaccine-induced immune thrombotic thrombocytopenia
- VWF
- von Willebrand factor
D. Toni and A.E. Merkler contributed equally.
For Disclosures, see page 1200.
References
- 1.WHO, Others. COVID-19 weekly epidemiological update. Accessed November 12, 2021. https://apps.who.int/iris/bitstream/handle/10665/341525/CoV-weekly-sitrep25May21-eng.pdf?sequence=1
- 2.Nopp S, Moik F, Jilma B, Pabinger I, Ay C. Risk of venous thromboembolism in patients with COVID-19: a systematic review and meta-analysis. Res Pract Thromb Haemost. 2020;4:1178–1191. doi: 10.1002/rth2.12439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, Chang J, Hong C, Zhou Y, Wang D, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77:683–690. doi: 10.1001/jamaneurol.2020.1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Katsanos AH, Palaiodimou L, Zand R, Yaghi S, Kamel H, Navi BB, Turc G, Romoli M, Sharma VK, Mavridis D, et al. The impact of SARS-CoV-2 on stroke epidemiology and care: a meta-analysis. Ann Neurol. 2021;89:380–388. doi: 10.1002/ana.25967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Merkler AE, Parikh NS, Mir S, Gupta A, Kamel H, Lin E, Lantos J, Schenck EJ, Goyal P, Bruce SS, et al. Risk of ischemic stroke in patients with coronavirus Disease 2019 (COVID-19) vs patients with influenza. JAMA Neurol. 2020;77:1–7. doi: 10.1001/jamaneurol.2020.2730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leasure AC, Khan YM, Iyer R, Elkind MSV, Sansing LH, Falcone GJ, Sheth KN. Intracerebral hemorrhage in patients with COVID-19: an analysis from the COVID-19 cardiovascular disease registry. Stroke. 2021;52:e321–e323. doi: 10.1161/STROKEAHA.121.034215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abdalkader M, Shaikh SP, Siegler JE, Cervantes-Arslanian AM, Tiu C, Radu RA, Tiu VE, Jillella DV, Mansour OY, Vera V, et al. Cerebral venous sinus thrombosis in COVID-19 patients: a multicenter study and review of literature. J Stroke Cerebrovasc Dis. 2021;30:105733. doi: 10.1016/j.jstrokecerebrovasdis.2021.105733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parauda SC, Gao V, Gewirtz AN, Parikh NS, Merkler AE, Lantos J, White H, Leifer D, Navi BB, Segal AZ. Posterior reversible encephalopathy syndrome in patients with COVID-19. J Neurol Sci. 2020;416:117019. doi: 10.1016/j.jns.2020.117019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oxley TJ, Mocco J, Majidi S, Kellner CP, Shoirah H, Singh IP, De Leacy RA, Shigematsu T, Ladner TR, Yaeger KA, et al. Large-vessel stroke as a presenting feature of Covid-19 in the young. N Engl J Med. 2020;382:e60. doi: 10.1056/NEJMc2009787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Y, Li M, Wang M, Zhou Y, Chang J, Xian Y, Wang D, Mao L, Jin H, Hu B. Acute cerebrovascular disease following COVID-19: a single center, retrospective, observational study. Stroke Vasc Neurol. 2020;5:279–284. doi: 10.1136/svn-2020-000431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yaghi S, Ishida K, Torres J, Mac Grory B, Raz E, Humbert K, Henninger N, Trivedi T, Lillemoe K, Alam S, et al. SARS-CoV-2 and stroke in a New York Healthcare System. Stroke. 2020;51:2002–2011. doi: 10.1161/STROKEAHA.120.030335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lodigiani C, Iapichino G, Carenzo L, Cecconi M, Ferrazzi P, Sebastian T, Kucher N, Studt JD, Sacco C, Bertuzzi A, et al. ; Humanitas COVID-19 Task Force. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Helms J, Kremer S, Merdji H, Clere-Jehl R, Schenck M, Kummerlen C, Collange O, Boulay C, Fafi-Kremer S, Ohana M, et al. Neurologic features in severe SARS-CoV-2 infection. N Engl J Med. 2020;382:2268–2270. doi: 10.1056/NEJMc2008597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Siepmann T, Sedghi A, Simon E, Winzer S, Barlinn J, de With K, Mirow L, Wolz M, Gruenewald T, Schroettner P, et al. Increased risk of acute stroke among patients with severe COVID-19: a multicenter study and meta-analysis. Eur J Neurol. 2021;28:238–247. doi: 10.1111/ene.14535 [DOI] [PubMed] [Google Scholar]
- 15.Rothstein A, Oldridge O, Schwennesen H, Do D, Cucchiara BL. Acute cerebrovascular events in hospitalized COVID-19 patients. Stroke. 2020;51:e219–e222. doi: 10.1161/STROKEAHA.120.030995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dhamoon MS, Thaler A, Gururangan K, Kohli A, Sisniega D, Wheelwright D, Mensching C, Fifi JT, Fara MG, Jette N, et al. ; Mount Sinai Stroke Investigators*. Acute cerebrovascular events with COVID-19 infection. Stroke. 2021;52:48–56. doi: 10.1161/STROKEAHA.120.031668 [DOI] [PubMed] [Google Scholar]
- 17.Shahjouei S, Naderi S, Li J, Khan A, Chaudhary D, Farahmand G, Male S, Griessenauer C, Sabra M, Mondello S, et al. Risk of stroke in hospitalized SARS-CoV-2 infected patients: a multinational study. EBioMedicine. 2020;59:102939. doi: 10.1016/j.ebiom.2020.102939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nogueira RG, Abdalkader M, Qureshi MM, Frankel MR, Mansour OY, Yamagami H, Qiu Z, Farhoudi M, Siegler JE, Yaghi S, et al. Global impact of COVID-19 on stroke care. Int J Stroke. 2021;16:573–584. doi: 10.1177/1747493021991652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qureshi AI, Baskett WI, Huang W, Shyu D, Myers D, Raju M, Lobanova I, Suri MFK, Naqvi SH, French BR, et al. Acute ischemic stroke and COVID-19: an analysis of 27 676 patients. Stroke. 2021;52:905–912. doi: 10.1161/STROKEAHA.120.031786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katz JM, Libman RB, Wang JJ, Sanelli P, Filippi CG, Gribko M, Pacia SV, Kuzniecky RI, Najjar S, Azhar S. Cerebrovascular complications of COVID-19. Stroke. 2020;51:e227–e231. doi: 10.1161/STROKEAHA.120.031265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shtaya A, Trippier S, Ghatala R, Cluckie G, Zhang L. Comment on “Stroke in patients with SARS-CoV-2 infection: case series” from a London hospital experience. J Neurol. 2021;268:424–430. doi: 10.1007/s00415-020-10105-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qiu F, Wu Y, Zhang A, Xie G, Cao H, Du M, Jiang H, Li S, Ding M. Changes of coagulation function and risk of stroke in patients with COVID-19. Brain Behav. 2021;11:e02185. doi: 10.1002/brb3.2185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Modin D, Claggett B, Sindet-Pedersen C, Lassen MCH, Skaarup KG, Jensen JUS, Fralick M, Schou M, Lamberts M, Gerds T, et al. Acute COVID-19 and the incidence of ischemic stroke and acute myocardial infarction. Circulation. 2020;142:2080–2082. doi: 10.1161/CIRCULATIONAHA.120.050809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Katsoularis I, Fonseca-Rodríguez O, Farrington P, Lindmark K, Fors Connolly AM. Risk of acute myocardial infarction and ischaemic stroke following COVID-19 in Sweden: a self-controlled case series and matched cohort study. Lancet. 2021;398:599–607. doi: 10.1016/S0140-6736(21)00896-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benussi A, Pilotto A, Premi E, Libri I, Giunta M, Agosti C, Alberici A, Baldelli E, Benini M, Bonacina S, et al. Clinical characteristics and outcomes of inpatients with neurologic disease and COVID-19 in Brescia, Lombardy, Italy. Neurology. 2020;95:e910–e920. doi: 10.1212/WNL.0000000000009848 [DOI] [PubMed] [Google Scholar]
- 26.Shen J, Hou Y, Zhou Y, Mehra R, Jehi L, Cheng F. The epidemiological and mechanistic understanding of the neurological manifestations of COVID-19: a comprehensive meta-analysis and a network medicine observation. Front Neurosci. 2021;15:606926. doi: 10.3389/fnins.2021.606926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Annie F, Bates MC, Nanjundappa A, Bhatt DL, Alkhouli M. Prevalence and outcomes of acute ischemic stroke among patients ≤50 Years of age with laboratory confirmed COVID-19 infection. Am J Cardiol. 2020;130:169–170. doi: 10.1016/j.amjcard.2020.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Esenwa C, Cheng NT, Luna J, Willey J, Boehme AK, Kirchoff-Torres K, Labovitz D, Liberman AL, Mabie P, Moncrieffe K, et al. Biomarkers of coagulation and inflammation in COVID-19-Associated ischemic stroke. Stroke. 2021;52:e706–e709. doi: 10.1161/STROKEAHA.121.035045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim Y, Khose S, Abdelkhaleq R, Salazar-Marioni S, Zhang GQ, Sheth SA. Predicting in-hospital mortality using D-dimer in COVID-19 patients with acute ischemic stroke. Front Neurol. 2021;12:702927. doi: 10.3389/fneur.2021.702927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ntaios G, Michel P, Georgiopoulos G, Guo Y, Li W, Xiong J, Calleja P, Ostos F, González-Ortega G, Fuentes B, et al. Characteristics and outcomes in patients with COVID-19 and acute ischemic stroke: the global COVID-19 stroke registry. Stroke. 2020;51:e254–e258. doi: 10.1161/STROKEAHA.120.031208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perry RJ, Smith CJ, Roffe C, Simister R, Narayanamoorthi S, Marigold R, Willmot M, Dixit A, Hassan A, Quinn TJ, et al. ; SETICOS Collaborators. Characteristics and outcomes of COVID-19 associated stroke: a UK multicentre case-control study. J Neurol Neurosurg Psychiatry. 2021;92:242–248. doi: 10.1136/jnnp-2020-324927 [DOI] [PubMed] [Google Scholar]
- 32.de Havenon A, Ney JP, Callaghan B, Delic A, Hohmann S, Shippey E, Esper GJ, Stulberg E, Tirschwell D, Frontera J, et al. Impact of COVID-19 on outcomes in ischemic stroke patients in the United States. J Stroke Cerebrovasc Dis. 2021;30:105535. doi: 10.1016/j.jstrokecerebrovasdis.2020.105535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yao X, Liu S, Wang J, Zhao K, Long X, He X, Kang H, Yang Y, Ma X, Yue P, et al. The clinical characteristics and prognosis of COVID-19 patients with cerebral stroke: a retrospective study of 113 cases from one single-centre. Eur J Neurosci. 2021;53:1350–1361. doi: 10.1111/ejn.15007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hernández-Fernández F, Sandoval Valencia H, Barbella-Aponte RA, Collado-Jiménez R, Ayo-Martín Ó, Barrena C, Molina-Nuevo JD, García-García J, Lozano-Setién E, Alcahut-Rodriguez C, et al. Cerebrovascular disease in patients with COVID-19: neuroimaging, histological and clinical description. Brain. 2020;143:3089–3103. doi: 10.1093/brain/awaa239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Majidi S, Fifi JT, Ladner TR, Lara-Reyna J, Yaeger KA, Yim B, Dangayach N, Oxley TJ, Shigematsu T, Kummer BR, et al. Emergent large vessel occlusion stroke during New York City’s COVID-19 outbreak: clinical characteristics and paraclinical findings. Stroke. 2020;51:2656–2663. doi: 10.1161/STROKEAHA.120.030397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Escalard S, Chalumeau V, Escalard C, Redjem H, Delvoye F, Hébert S, Smajda S, Ciccio G, Desilles JP, Mazighi M, et al. Early brain imaging shows increased severity of acute ischemic strokes with large vessel occlusion in COVID-19 patients. Stroke. 2020;51:3366–3370. doi: 10.1161/STROKEAHA.120.031011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kihira S, Schefflein J, Mahmoudi K, Rigney B, N Delman B, Mocco J, Doshi A, Belani P. Association of coronavirus disease (COVID-19) with large vessel occlusion strokes: a case-control study. AJR Am J Roentgenol. 2021;216:150–156. doi: 10.2214/AJR.20.23847 [DOI] [PubMed] [Google Scholar]
- 38.Hart RG, Catanese L, Perera KS, Ntaios G, Connolly SJ. Embolic stroke of undetermined source: a systematic review and clinical update. Stroke. 2017;48:867–872. doi: 10.1161/STROKEAHA.116.016414 [DOI] [PubMed] [Google Scholar]
- 39.John S, Kesav P, Mifsud VA, Piechowski-Jozwiak B, Dibu J, Bayrlee A, Elkambergy H, Roser F, Elhammady MS, Zahra K, et al. Characteristics of large-vessel occlusion associated with COVID-19 and ischemic stroke. AJNR Am J Neuroradiol. 2020;41:2263–2268. doi: 10.3174/ajnr.A6799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McAlpine LS, Zubair AS, Maran I, Chojecka P, Lleva P, Jasne AS, Navaratnam D, Matouk C, Schindler J, Sheth KN, et al. Ischemic stroke, inflammation, and endotheliopathy in COVID-19 patients. Stroke. 2021;52:e233–e238. doi: 10.1161/STROKEAHA.120.031971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Finn C, Hung P, Patel P, Gupta A, Kamel H. Relationship between visceral infarction and ischemic stroke subtype. Stroke. 2018;49:727–729. doi: 10.1161/STROKEAHA.117.020035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Allegra A, Innao V, Allegra AG, Musolino C. Coagulopathy and thromboembolic events in patients with SARS-CoV-2 infection: pathogenesis and management strategies. Ann Hematol. 2020;99:1953–1965. doi: 10.1007/s00277-020-04182-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Piazza G, Campia U, Hurwitz S, Snyder JE, Rizzo SM, Pfeferman MB, Morrison RB, Leiva O, Fanikos J, Nauffal V, et al. Registry of Arterial and Venous Thromboembolic Complications in Patients With COVID-19. J Am Coll Cardiol. 2020;76:2060–2072. doi: 10.1016/j.jacc.2020.08.070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Helms J, Tacquard C, Severac F, Leonard-Lorant I, Ohana M, Delabranche X, Merdji H, Clere-Jehl R, Schenck M, Fagot Gandet F, et al. ; CRICS TRIGGERSEP Group (Clinical Research in Intensive Care and Sepsis Trial Group for Global Evaluation and Research in Sepsis). High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46:1089–1098. doi: 10.1007/s00134-020-06062-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Linschoten M, Peters S, van Smeden M, Jewbali LS, Schaap J, Siebelink HM, Smits PC, Tieleman RG, van der Harst P, van Gilst WH, et al. ; CAPACITY-COVID Collaborative Consortium. Cardiac complications in patients hospitalised with COVID-19. Eur Heart J Acute Cardiovasc Care. 2020;9:817–823. doi: 10.1177/2048872620974605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wolf PA, Kannel WB, McGee DL, Meeks SL, Bharucha NE, McNamara PM. Duration of atrial fibrillation and imminence of stroke: the Framingham study. Stroke. 1983;14:664–667. doi: 10.1161/01.str.14.5.664 [DOI] [PubMed] [Google Scholar]
- 47.Solomon MD, McNulty EJ, Rana JS, Leong TK, Lee C, Sung SH, Ambrosy AP, Sidney S, Go AS. The Covid-19 pandemic and the incidence of acute myocardial infarction. N Engl J Med. 2020;383:691–693. doi: 10.1056/NEJMc2015630 [DOI] [PubMed] [Google Scholar]
- 48.Haussner W, DeRosa AP, Haussner D, Tran J, Torres-Lavoro J, Kamler J, Shah K. COVID-19 associated myocarditis: a systematic review. Am J Emerg Med. 2022;51:150–155. doi: 10.1016/j.ajem.2021.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ramasamy S, Yaghi S, Salehi Omran S, Lerario MP, Devereux R, Okin PM, Gupta A, Navi BB, Kamel H, Merkler AE. Association between left ventricular ejection fraction, wall motion abnormality, and embolic stroke of undetermined source. J Am Heart Assoc. 2019;8:e011593. doi: 10.1161/JAHA.118.011593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hou C, Hu Y, Yang H, Chen W, Zeng Y, Ying Z, Hu Y, Sun Y, Qu Y, Gottfreðsson M, et al. COVID-19 and risk of subsequent life-threatening secondary infections: a matched cohort study in UK Biobank. BMC Med. 2021;19:301. doi: 10.1186/s12916-021-02177-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shao IY, Elkind MSV, Boehme AK. Risk Factors for stroke in patients with sepsis and bloodstream infections. Stroke. 2019;50:1046–1051. doi: 10.1161/STROKEAHA.118.023443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang S, Liu Y, Wang X, Yang L, Li H, Wang Y, Liu M, Zhao X, Xie Y, Yang Y, et al. SARS-CoV-2 binds platelet ACE2 to enhance thrombosis in COVID-19. J Hematol Oncol. 2020;13:120. doi: 10.1186/s13045-020-00954-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Letarov AV, Babenko VV, Kulikov EE. Free SARS-CoV-2 spike protein S1 particles may play a role in the pathogenesis of COVID-19 infection. Biochemistry (Mosc). 2021;86:257–261. doi: 10.1134/S0006297921030032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, Vanstapel A, Werlein C, Stark H, Tzankov A, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee MH, Perl DP, Nair G, Li W, Maric D, Murray H, Dodd SJ, Koretsky AP, Watts JA, Cheung V, et al. Microvascular injury in the brains of patients with Covid-19. N Engl J Med. 2021;384:481–483. doi: 10.1056/NEJMc2033369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mukerji SS, Solomon IH. What can we learn from brain autopsies in COVID-19? Neurosci Lett. 2021;742:135528. doi: 10.1016/j.neulet.2020.135528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bryce C, Grimes Z, Pujadas E, Ahuja S, Beasley MB, Albrecht R, Hernandez T, Stock A, Zhao Z, AlRasheed MR, et al. Pathophysiology of SARS-CoV-2: the Mount Sinai COVID-19 autopsy experience. Mod Pathol. 2021;34:1456–1467. doi: 10.1038/s41379-021-00793-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Solomon IH, Normandin E, Bhattacharyya S, Mukerji SS, Keller K, Ali AS, Adams G, Hornick JL, Padera RF, Jr, Sabeti P. Neuropathological features of Covid-19. N Engl J Med. 2020;383:989–992. doi: 10.1056/NEJMc2019373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Paniz-Mondolfi A, Bryce C, Grimes Z, Gordon RE, Reidy J, Lednicky J, Sordillo EM, Fowkes M. Central nervous system involvement by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). J Med Virol. 2020;92:699–702. doi: 10.1002/jmv.25915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Miller SE, Goldsmith CS. Caution in identifying coronaviruses by electron microscopy. J Am Soc Nephrol. 2020;31:2223–2224. doi: 10.1681/ASN.2020050755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, Mehra MR, Schuepbach RA, Ruschitzka F, Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Evans PC, Rainger GE, Mason JC, Guzik TJ, Osto E, Stamataki Z, Neil D, Hoefer IE, Fragiadaki M, Waltenberger J, et al. Endothelial dysfunction in COVID-19: a position paper of the ESC Working Group for Atherosclerosis and Vascular Biology, and the ESC Council of Basic Cardiovascular Science. Cardiovasc Res. 2020;116:2177–2184. doi: 10.1093/cvr/cvaa230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Klok FA, Kruip MJHA, van der Meer NJM, Arbous MS, Gommers D, Kant KM, Kaptein FHJ, van Paassen J, Stals MAM, Huisman MV, et al. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: an updated analysis. Thromb Res. 2020;191:148–150. doi: 10.1016/j.thromres.2020.04.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Huisman A, Beun R, Sikma M, Westerink J, Kusadasi N. Involvement of ADAMTS13 and von Willebrand factor in thromboembolic events in patients infected with SARS-CoV-2. Int J Lab Hematol. 2020;42:e211–e212. doi: 10.1111/ijlh.13244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.South K, McCulloch L, McColl BW, Elkind MS, Allan SM, Smith CJ. Preceding infection and risk of stroke: an old concept revived by the COVID-19 pandemic. Int J Stroke. 2020;15:722–732. doi: 10.1177/1747493020943815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kvernland A, Kumar A, Yaghi S, Raz E, Frontera J, Lewis A, Czeisler B, Kahn DE, Zhou T, Ishida K, et al. Anticoagulation use and hemorrhagic stroke in SARS-CoV-2 patients treated at a New York Healthcare System. Neurocrit Care. 2021;34:748–759. doi: 10.1007/s12028-020-01077-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hippisley-Cox J, Patone M, Mei XW, Saatci D, Dixon S, Khunti K, Zaccardi F, Watkinson P, Shankar-Hari M, Doidge J, et al. Risk of thrombocytopenia and thromboembolism after covid-19 vaccination and SARS-CoV-2 positive testing: self-controlled case series study. BMJ. 2021;374:n1931. doi: 10.1136/bmj.n1931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mowla A, Shakibajahromi B, Shahjouei S, Borhani-Haghighi A, Rahimian N, Baharvahdat H, Naderi S, Khorvash F, Altafi D, Ebrahimzadeh SA, et al. Cerebral venous sinus thrombosis associated with SARS-CoV-2; a multinational case series. J Neurol Sci. 2020;419:117183. doi: 10.1016/j.jns.2020.117183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hixon AM, Thaker AA, Pelak VS. Persistent visual dysfunction following posterior reversible encephalopathy syndrome due to COVID-19: case series and literature review. Eur J Neurol. 2021;28:3289–3302. doi: 10.1111/ene.14965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.COVID-19 vaccines [Internet]. Accessed November 12, 2021. https://vaccination-info.eu/en/covid-19/covid-19-vaccines.
- 71.Office of the Commissioner. Learn more about COVID-19 vaccines from the FDA [Internet]. Authored on behalf of The United States Food and Drug Administration. Accessed November 12, 2021. https://www.fda.gov/consumers/consumer-updates/learn-more-about-covid-19-vaccines-fda
- 72.Rosenblum HG, Hadler SC, Moulia D, Shimabukuro TT, Su JR, Tepper NK, Ess KC, Woo EJ, Mba-Jonas A, Alimchandani M, et al. Use of COVID-19 vaccines after reports of adverse events among adult recipients of Janssen (Johnson & Johnson) and mRNA COVID-19 vaccines (Pfizer-BioNTech and Moderna): update from the advisory committee on immunization practices - United States, July 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1094–1099. doi: 10.15585/mmwr.mm7032e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.See I, Su JR, Lale A, Woo EJ, Guh AY, Shimabukuro TT, Streiff MB, Rao AK, Wheeler AP, Beavers SF, et al. US case reports of cerebral venous sinus thrombosis with thrombocytopenia after Ad26.COV2.S vaccination, March 2 to April 21, 2021. JAMA. 2021;325:2448–2456. doi: 10.1001/jama.2021.7517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.MacIntyre CR. Using the Bradford-Hill criteria to assess causality in the association between CHADOX1 NCOV-19 vaccine and thrombotic immune thrombocytopenia. Glob Biosecur. 2021;3. doi: 10.31646/gbio.109 [Google Scholar]
- 75.Sadoff J, Gray G, Vandebosch A, Cárdenas V, Shukarev G, Grinsztejn B, Goepfert PA, Truyers C, Fennema H, Spiessens B, et al. ; ENSEMBLE Study Group. Safety and efficacy of single-dose Ad26.COV2.S vaccine against Covid-19. N Engl J Med. 2021;384:2187–2201. doi: 10.1056/NEJMoa2101544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK, Angus B, Baillie VL, Barnabas SL, Bhorat QE, et al. ; Oxford COVID Vaccine Trial Group. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Palladino R, Ceriotti D, De Ambrosi D, De Vito M, Farsoni M, Seminara G, Barone-Adesi F. A quantitative risk-benefit analysis of ChAdOx1 nCoV-19 vaccine among people under 60 in Italy. medRxiv. Preprint posted online May 10, 2021. doi: 10.1101/2021.05.07.21256826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yang Y, Du L. SARS-CoV-2 spike protein: a key target for eliciting persistent neutralizing antibodies. Signal Transduct Target Ther. 2021;6:95. doi: 10.1038/s41392-021-00523-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Song W, Gui M, Wang X, Xiang Y. Cryo-EM structure of the SARS coronavirus spike glycoprotein in complex with its host cell receptor ACE2. PLoS Pathog. 2018;14:e1007236. doi: 10.1371/journal.ppat.1007236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lan J, Ge J, Yu J, Shan S, Zhou H, Fan S, Zhang Q, Shi X, Wang Q, Zhang L, et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020;581:215–220. doi: 10.1038/s41586-020-2180-5 [DOI] [PubMed] [Google Scholar]
- 81.Turoňová B, Sikora M, Schürmann C, Hagen WJH, Welsch S, Blanc FEC, von Bülow S, Gecht M, Bagola K, Hörner C, et al. In situ structural analysis of SARS-CoV-2 spike reveals flexibility mediated by three hinges. Science. 2020;370:203–208. doi: 10.1126/science.abd5223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wajnberg A, Amanat F, Firpo A, Altman DR, Bailey MJ, Mansour M, McMahon M, Meade P, Mendu DR, Muellers K, et al. Robust neutralizing antibodies to SARS-CoV-2 infection persist for months. Science. 2020;370:1227–1230. doi: 10.1126/science.abd7728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Silveira MM, Moreira GMSG, Mendonça M. DNA vaccines against COVID-19: perspectives and challenges. Life Sci. 2021;267:118919. doi: 10.1016/j.lfs.2020.118919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Abbasi J. COVID-19 and mRNA vaccines-first large test for a new approach. JAMA. 2020;324:1125–1127. doi: 10.1001/jama.2020.16866 [DOI] [PubMed] [Google Scholar]
- 85.Heath PT, Galiza EP, Baxter DN, Boffito M, Browne D, Burns F, Chadwick DR, Clark R, Cosgrove C, Galloway J, et al. ; 2019nCoV-302 Study Group. Safety and efficacy of NVX-CoV2373 Covid-19 vaccine. N Engl J Med. 2021;385:1172–1183. doi: 10.1056/NEJMoa2107659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tregoning JS, Flight KE, Higham SL, Wang Z, Pierce BF. Progress of the COVID-19 vaccine effort: viruses, vaccines and variants versus efficacy, effectiveness and escape. Nat Rev Immunol. 2021;21:626–636. doi: 10.1038/s41577-021-00592-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Greinacher A, Langer F, Makris M, Pai M, Pavord S, Tran H, Warkentin TE. Vaccine-induced immune thrombotic thrombocytopenia (VITT): update on diagnosis and management considering different resources. J Thromb Haemost. 2022;20:149–156. doi: 10.1111/jth.15572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schultz NH, Sørvoll IH, Michelsen AE, Munthe LA, Lund-Johansen F, Ahlen MT, Wiedmann M, Aamodt AH, Skattør TH, Tjønnfjord GE, et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021;384:2124–2130. doi: 10.1056/NEJMoa2104882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Scully M, Singh D, Lown R, Poles A, Solomon T, Levi M, Goldblatt D, Kotoucek P, Thomas W, Lester W. Pathologic antibodies to platelet factor 4 after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021;384:2202–2211. doi: 10.1056/NEJMoa2105385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Muir KL, Kallam A, Koepsell SA, Gundabolu K. Thrombotic thrombocytopenia after Ad26.COV2.S vaccination. N Engl J Med. 2021;384:1964–1965. doi: 10.1056/NEJMc2105869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sangli S, Virani A, Cheronis N, Vannatter B, Minich C, Noronha S, Bhagavatula R, Speredelozzi D, Sareen M, Kaplan RB. Thrombosis with thrombocytopenia after the messenger RNA-1273 vaccine. Ann Intern Med. 2021;174:1480–1482. doi: 10.7326/L21-0244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med. 2021;384:2092–2101. doi: 10.1056/NEJMoa2104840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Greinacher A, Selleng K, Warkentin TE. Autoimmune heparin-induced thrombocytopenia. J Thromb Haemost. 2017;15:2099–2114. doi: 10.1111/jth.13813 [DOI] [PubMed] [Google Scholar]
- 94.Mullier F, Minet V, Bailly N, Devalet B, Douxfils J, Chatelain C, Elalamy I, Dogné JM, Chatelain B. Platelet microparticle generation assay: a valuable test for immune heparin-induced thrombocytopenia diagnosis. Thromb Res. 2014;133:1068–1073. doi: 10.1016/j.thromres.2013.12.009 [DOI] [PubMed] [Google Scholar]
- 95.Greinacher A, Selleng K, Mayerle J, Palankar R, Wesche J, Reiche S, Aebischer A, Warkentin TE, Muenchhoff M, Hellmuth JC, et al. ; Immune-Response in COVID-19 Vaccination Study Group. Anti-platelet factor 4 antibodies causing VITT do not cross-react with SARS-CoV-2 spike protein. Blood. 2021;138:1269–1277. doi: 10.1182/blood.2021012938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Uzun G, Althaus K, Bakchoul T. No correlation between anti-PF4 and anti–SARS-CoV-2 antibodies after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021;385:1334–1336. doi: 10.1056/NEJMc2111305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Baker AT, Boyd RJ, Sarkar D, Teijeira-Crespo A, Chan CK, Bates E, Waraich K, Vant J, Wilson E, Truong CD, et al. ChAdOx1 interacts with CAR and PF4 with implications for thrombosis with thrombocytopenia syndrome. Sci Adv. 2021;7:eabl8213. doi: 10.1126/sciadv.abl8213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Greinacher A, Selleng K, Palankar R, Wesche J, Handtke S, Wolff M, Aurich K, Lalk M, Methling K, Völker U, et al. Insights in ChAdOx1 nCoV-19 vaccine-induced immune thrombotic thrombocytopenia. Blood. 2021;138:2256–2268. doi: 10.1182/blood.2021013231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kowarz E, Krutzke L, Külp M, Streb P, Larghero P, Reis J, Bracharz S, Engler T, Kochanek S, Marschalek R. Vaccine-induced COVID-19 mimicry syndrome. e-life. 2022;11:e74974. 10.7554/eLife.74974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.De Michele M, Piscopo P, Crestini A, Rivabene R, d’Amati G, Leopizzi M, Stefanini L, Flego D, Pulcinelli F, Chistolini A, et al. Vaccine-induced immune thrombotic thrombocytopenia and spike protein. Res Sq. 2021. doi: 10.21203/rs.3.rs-887779/v1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schulz JB, Berlit P, Diener HC, Gerloff C, Greinacher A, Klein C, Petzold GC, Piccininni M, Poli S, Röhrig R, et al. ; German Society of Neurology SARS-CoV-2 Vaccination Study Group. COVID-19 vaccine-associated cerebral venous thrombosis in germany. Ann Neurol. 2021;90:627–639. doi: 10.1002/ana.26172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pavord S, Scully M, Hunt BJ, Lester W, Bagot C, Craven B, Rampotas A, Ambler G, Makris M. Clinical features of vaccine-induced immune thrombocytopenia and thrombosis. N Engl J Med. 2021;385:1680–1689. doi: 10.1056/NEJMoa2109908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.van Kammen MS, de Sousa DA, Poli S, Cordonnier C, Heldner MR, van de Munckhof A, Krzywicka K, van Haaps T, Ciccone A, Middeldorp S, et al. Characteristics and outcomes of patients with cerebral venous sinus thrombosis in SARS-CoV-2 vaccine–induced immune thrombotic thrombocytopenia. JAMA Neurol. 2021;78:1314–1323. doi: 10.1001/jamaneurol.2021.3619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Guidance produced from the Expert Haematology Panel (EHP) focussed on syndrome of thrombosis and thrombocytopenia occurring after coronavirus vaccination [Internet]. Authored on behalf of the Expert Haematology Panel. Accessed November 12, 2021. https://b-s-h.org.uk/about-us/news/guidance-produced-by-the-expert-haematology-panel-ehp-focussed-on-vaccine-induced-thrombosis-and-thrombocytopenia-vitt/
- 105.The ISTH releases interim guidance on vaccine-induced immune thrombotic thrombocytopenia (VITT) - International Society on Thrombosis and Haemostasis Inc [Internet]. Authored on behalf of the The International Society on Thrombosis and Haemostasis. Accessed November 12, 2021. https://www.isth.org/news/561406/The-ISTH-Releases-Interim-Guidance-on-Vaccine-Induced-Immune-Thrombotic-Thrombocytopenia-VITT-.htm.
- 106.Oldenburg J, Klamroth R, Langer F, Albisetti M, von Auer C, Ay C, Korte W, Scharf RE, Pötzsch B, Greinacher A. Diagnosis and management of vaccine-related thrombosis following AstraZeneca COVID-19 vaccination: guidance statement from the GTH. Hamostaseologie. 2021;41:184–189. doi: 10.1055/a-1469-7481 [DOI] [PubMed] [Google Scholar]
- 107.Lavin M, Elder PT, O’Keeffe D, Enright H, Ryan E, Kelly A, El Hassadi E, McNicholl FP, Benson G, Le GN, et al. Vaccine-induced immune thrombotic thrombocytopenia (VITT) - a novel clinico-pathological entity with heterogeneous clinical presentations. Br J Haematol. 2021;195:76–84. doi: 10.1111/bjh.17613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Salih F, Schönborn L, Kohler S, Franke C, Möckel M, Dörner T, Bauknecht HC, Pille C, Graw JA, Alonso A, et al. Vaccine-induced thrombocytopenia with severe headache. N Engl J Med. 2021;385:2103–2105. doi: 10.1056/NEJMc2112974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.De Michele M, Iacobucci M, Chistolini A, Nicolini E, Pulcinelli F, Cerbelli B, Merenda E, Schiavo OG, Sbardella E, Berto I, et al. Malignant cerebral infarction after ChAdOx1 nCov-19 vaccination: a catastrophic variant of vaccine-induced immune thrombotic thrombocytopenia. Nat Commun. 2021;12:4663. doi: 10.1038/s41467-021-25010-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Blauenfeldt RA, Kristensen SR, Ernstsen SL, Kristensen CCH, Simonsen CZ, Hvas AM. Thrombocytopenia with acute ischemic stroke and bleeding in a patient newly vaccinated with an adenoviral vector-based COVID-19 vaccine. J Thromb Haemost. 2021;19:1771–1775. doi: 10.1111/jth.15347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Al-Mayhani T, Saber S, Stubbs MJ, Losseff NA, Perry RJ, Simister RJ, Gull D, Jäger HR, Scully MA, Werring DJ. Ischaemic stroke as a presenting feature of ChAdOx1 nCoV-19 vaccine-induced immune thrombotic thrombocytopenia. J Neurol Neurosurg Psychiatry. 2021;92:1247–1248. doi: 10.1136/jnnp-2021-326984 [DOI] [PubMed] [Google Scholar]
- 112.Bayas A, Menacher M, Christ M, Behrens L, Rank A, Naumann M. Bilateral superior ophthalmic vein thrombosis, ischaemic stroke, and immune thrombocytopenia after ChAdOx1 nCoV-19 vaccination. Lancet. 2021;397:e11. doi: 10.1016/S0140-6736(21)00872-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kenda J, Lovrič D, Škerget M, Milivojević N. Treatment of ChAdOx1 nCoV-19 vaccine-induced immune thrombotic thrombocytopenia related acute ischemic stroke. J Stroke Cerebrovasc Dis. 2021;30:106072. doi: 10.1016/j.jstrokecerebrovasdis.2021.106072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Costentin G, Ozkul-Wermester O, Triquenot A, Cam-Duchez VL, Massy N, Benhamou Y, Massardier E. Acute ischemic stroke revealing ChAdOx1 nCov-19 vaccine-induced immune thrombotic thrombocytopenia: impact on recanalization strategy. J Stroke Cerebrovasc Dis. 2021;30:105942. doi: 10.1016/j.jstrokecerebrovasdis.2021.105942 [DOI] [PubMed] [Google Scholar]
- 115.Walter U, Fuchs M, Grossmann A, Walter M, Thiele T, Storch A, Wittstock M. Adenovirus-Vectored COVID-19 vaccine–induced immune thrombosis of carotid artery. Neurology. 2021;97:716–719. [DOI] [PubMed] [Google Scholar]
- 116.Bourguignon A, Arnold DM, Warkentin TE, Smith JW, Pannu T, Shrum JM, Al Maqrashi ZAA, Shroff A, Lessard MC, Blais N, et al. Adjunct immune globulin for vaccine-induced immune thrombotic thrombocytopenia. N Engl J Med. 2021;385:720–728. doi: 10.1056/NEJMoa2107051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ceschia N, Scheggi V, Gori AM, Rogolino AA, Cesari F, Giusti B, Cipollini F, Marchionni N, Alterini B, Marcucci R. Diffuse prothrombotic syndrome after ChAdOx1 nCoV-19 vaccine administration: a case report. J Med Case Rep. 2021;15:496. doi: 10.1186/s13256-021-03083-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Patriquin CJ, Laroche V, Selby R, Pendergrast J, Barth D, Côté B, Gagnon N, Roberge G, Carrier M, Castellucci LA, et al. Therapeutic plasma exchange in vaccine-induced immune thrombotic thrombocytopenia. N Engl J Med. 2021;385:857–859. doi: 10.1056/NEJMc2109465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Jacob C, Rani KA, Holton PJ, Boyce SR, Weir NU, Griffith CR, Eynon CA. Malignant middle cerebral artery syndrome with thrombotic thrombocytopenia following vaccination against SARS-CoV-2. Pediatr Crit Care Med. 2021. doi: 10.1177/17511437211027496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jackson SP, Darbousset R, Schoenwaelder SM. Thromboinflammation: challenges of therapeutically targeting coagulation and other host defense mechanisms. Blood. 2019;133:906–918. doi: 10.1182/blood-2018-11-882993 [DOI] [PubMed] [Google Scholar]
- 121.Pomara C, Sessa F, Ciaccio M, Dieli F, Esposito M, Garozzo SF, Giarratano A, Prati D, Rappa F, Salerno M, et al. Post-mortem findings in vaccine-induced thrombotic thombocytopenia. Haematologica. 2021;106:2291–2293. doi: 10.3324/haematol.2021.279075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Xu P, Zhou Q, Xu J. Mechanism of thrombocytopenia in COVID-19 patients. Ann Hematol. 2020;99:1205–1208. doi: 10.1007/s00277-020-04019-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Warkentin TE, Kaatz S. COVID-19 versus HIT hypercoagulability. Thromb Res. 2020;196:38–51. doi: 10.1016/j.thromres.2020.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Koupenova M, Corkrey HA, Vitseva O, Tanriverdi K, Somasundaran M, Liu P, Soofi S, Bhandari R, Godwin M, Parsi KM, et al. SARS-CoV-2 initiates programmed cell death in platelets. Circ Res. 2021;129:631–646. doi: 10.1161/CIRCRESAHA.121.319117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Iadecola C, Anrather J, Kamel H. Effects of COVID-19 on the nervous system. Cell. 2020;183:16–27.e1. doi: 10.1016/j.cell.2020.08.028 [DOI] [PMC free article] [PubMed] [Google Scholar]