Abstract
Mitochondria are essential organelles for cellular energy and metabolism. Like with any organ, the liver highly depends on the function of these cellular powerhouses. Hepatotoxic insults often lead to an impairment of mitochondrial activity and an increase in oxidative stress, thereby compromising the metabolic and synthetic functions. Mitochondria play a critical role in ATP synthesis and the production or scavenging of free radicals. Mitochondria orchestrate many cellular signaling pathways involved in the regulation of cell death, metabolism, cell division, and progenitor cell differentiation. Mitochondrial dysfunction and oxidative stress are closely associated with ischemia-reperfusion injury during organ transplantation and with different liver diseases, including cholestasis, steatosis, viral hepatitis, and drug-induced liver injury. To develop novel mitochondria-targeting therapies or interventions, a better understanding of mitochondrial dysfunction and oxidative stress in hepatic pathogenesis is very much needed. Therapies targeting mitochondria impairment and oxidative imbalance in liver diseases have been extensively studied in preclinical and clinical research. In this review, we provide an overview of how oxidative stress and mitochondrial dysfunction affect liver diseases and liver transplantation. Furthermore, we summarize recent developments of antioxidant and mitochondria-targeted interventions.
INTRODUCTION
The liver plays a pivotal role in detoxification and blood purification to protect the organism from endogenous and exogenous toxic compounds. Related to its high metabolic activity, liver parenchymal/epithelial cells including hepatocytes and cholangiocytes contain high numbers of mitochondria. These mitochondria maintain homeostasis of cellular energy but also contribute hepatic pathogenesis once their metabolic function is impaired. Components of electron transport chain (ECT) of mitochondrial can be affected by numerous triggers such as drugs, high lipid intake, viruses, or ischemia.1 Mitochondrial perturbation results in oxidative stress, defined as imbalanced redox homeostasis caused by excessive synthesis of oxidative products or exhaustion of antioxidant substances. Oxidative substances, mostly reactive oxygen species (ROS) and reactive nitrogen species (RNS), are mainly produced in parenchymal cells and Kupffer cells in the liver and can damage biologically relevant macromolecules such as carbohydrates, DNA, proteins, and lipids.2 Mitochondrial dysfunction and oxidative stress are inseparable processes and play a critical role in cell death, inflammation and fibrosis during many liver diseases such as steatohepatitis, alcoholic liver disease (ALD), cholestasis, cirrhosis, and hepatic malignancies.3 Also, ischemia-reperfusion injury (IRI), a major cause of liver transplantation failure, is also strongly associated with oxidative injury.4 However, the crosstalk between redox signaling and mitochondrial defects and its precise role in hepatic pathogenesis remain unclear. In addition, over the last decades, various therapies have been developed to eliminate ROS/RNS, enhance antioxidant defense or restore mitochondrial homeostasis. Several antioxidative agents, such as vitamin E and ursodeoxycholate (UDCA), have been clinically utilized as the first-line drugs in the treatment of liver diseases.5 Nevertheless, numerous antioxidative therapies failed to get into clinical application due to unsatisfactory clinical outcome or even side effects in patients. This phenomena, also known as the “antioxidant paradox,” highlights the gaps in our current knowledge and the incomplete understanding of the pathogenetic role of mitochondrial dysfunction and oxidative stress in liver transplantation and underlying diseases.6 In this review, we first summarize the evolving concepts and underlying mechanisms of mitochondrial impairment and redox imbalance and interpret their involvement in cell death, inflammation, and fibrogenesis. We then emphasize the pathogenetic role of mitochondrial dysfunction and oxidative stress in major liver diseases and liver transplantation. Finally, the recent advancement of antioxidative and mitochondria-targeted therapeutic regimens will also be discussed.
MITOCHONDRIAL DYSFUNCTION AND OXIDATIVE STRESS IN HEPATO-PATHOGENESIS
Mitochondria as the Main Source of ROS/RNS
The ROS/RNS family includes superoxide (O2−), hydrogen peroxide (H2O2), peroxynitrite, and nitric oxide (NO).7 Continuous generation of ROS/RNS is a part of normal aerobic metabolism and serves as signal-transducing molecules in metabolism, gene transcription/translation, cell cycle, and differentiation.5 Excessive ROS/RNS are scavenged by the antioxidant defense system. The imbalance between ROS/RNS production and scavenging, however, is detrimental to cells. The shift from being beneficial to detrimental is orchestrated by the amount and duration of ROS/RNS production.8
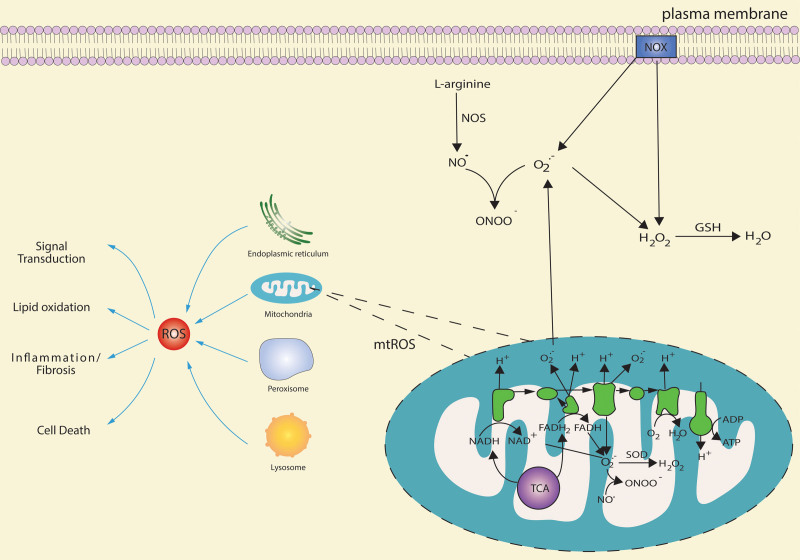
Mitochondria are intracellular organelles serving as cellular powerhouses and are the main source of ATP production in a cell. In addition to this role in energy metabolism, mitochondria are involved in calcium homeostasis, signal transduction, and controlling programmed cell death.9 Most intracellular ROS/RNS are produced by impaired mitochondria10 as byproducts of oxidative metabolism (Figure 1). They are mainly generated at the site of oxidative phosphorylation (OXPHOS) of the ETC, which takes place on the inner mitochondrial membrane.11,12 Approximately 0.2%–2.0% of electrons still escape from complex I and III of ETC.13 Electron leakage generates O2, which is rapidly divided into H2O2 and O2 by superoxide dismutase (SOD).12
Figure 1.
The main intracellular sources of ROS/RNS. Many cell organelles can produce ROS, acting as a signal-transducing molecule. Excessive ROS can induce lipid oxidation, inflammatory responses, fibrosis, or cell death in the liver. The main source of ROS is generated by ETC, NADH, and FADH2 are respectively involved in the TCA cycle, and β-oxidation of fatty acids donates electron and H+ to mitochondrial ETC. With the process of electron transfer from complex I/complex II to complex IV, electrons leak from ETC attack O2, leading to O2− formation. O2− has a short half-life that is rapidly divided into H2O2 and O2 by SOD. NOX is also the main ROS source that transfers an electron to O2 to produce O2− and H2O2. Increasing mtROS activate NOXs, which, in turn, enhances mtROS generation. This feed-forward cycle between NOXs and mitochondria maintains the cellular redox homeostasis. O2− produced by ETC can respond to NO•, which is produced from L-arginine by NOS catalysis to generate ONOO−. Besides, ONOO− can also be produced in mitochondria, which is mainly catalyzed by mtNOS. ETC, electron transport chain; FADH2, flavin adenine dinucleotide; mtNOS, mitochondrial NOS; ONOO−, peroxynitrite; NADH, nicotinamide adenine dinucleotide; NADPH, nicotinamide adenine dinucleotide phosphate; NOXs, NADPH oxidases; RNS, reactive nitrogen species; ROS, reactive oxygen species; SOD, superoxide dismutase; TCA, tricarboxylic acid.
Another primary source of ROS is through nicotinamide adenine dinucleotide phosphate oxidases (NOXs), which catalyzes electron transfer from nicotinamide adenine dinucleotide phosphate to molecular oxygen to produce O2− and H2O2.14 Activated NOXs increase mitochondrial ROS (mtROS) production, and increased mtROS level, in turn, activates NOXs, suggesting a feed-forward cycle between mtROS and NOXs.15,16
The RNS family, including NO, also plays a critical role in liver redox reactions. NO is produced from L-arginine catalyzed by NO synthases. Mitochondrial NO synthases activity is regulated by complex I of ETC,17,18 and mitochondria have been suggested as a primary source of RNS.19
Mitochondrial Dysfunction is Associated With Programmed Cell Death
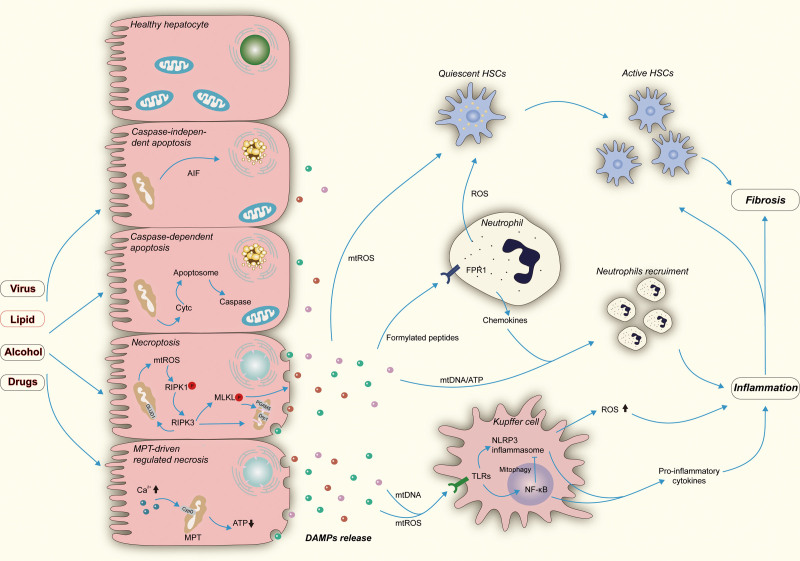
Cell death is a crucial feature of liver injury. Mitochondrial dysfunction and oxidative stress contribute to apoptosis, a well-characterized type of programmed cell death. Increased mtROS/RNS oxidize cardiolipin, a mitochondrial lipid, which augments mitochondrial membrane permeability and the release of cytochrome c (Cytc). Released Cytc induces the formation of apoptosome complexes and subsequent activation of caspase-3 and -9, leading to caspase-dependent apoptosis (Figure 2).20,21 Alternatively, pro-apoptotic proteins, such as apoptosis-inducing factor (AIF), translocated from the mitochondria to the cytosol through permeabilization of the mitochondrial membrane, mediating the fragmentation of DNA and causing caspase-independent apoptosis (Figure 2).22
Figure 2.
The role of oxidative stress and mitochondrial dysfunction in hepatic cell death, inflammation, and fibrogenesis. A, Mitochondrial function could be impaired during chronic liver injury induced by the hepatitis virus, fatty acid, alcohol, and drugs. Defected mitochondrial and subsequent oxidative stress are involved in the execution of multiple programmed cell death. Proapoptotic proteins such as AIF could translocate from mitochondrial to the cytosol via permeabilization of the mitochondrial membrane and ultimately elicit caspase-independent apoptosis. Likewise, released Cytc from mitochondrial can induce caspase-dependent apoptosis by forming apoptosome and activating caspase effectors. mtROS released from impaired mitochondrial facilitates the auto-phosphorylation of RIPK1 and thus promotes necroptosis. RIPK3 could result in a massive generation of ROS by activating mitochondrial GLUD1. MLKL could mediate fragmentation of mitochondrial through activation of PGAM5 and Drp1 and consequently execute necroptosis. Besides, overloaded Ca2+ triggers CYPD-mediated MPT, leading to a dramatic drop of ATP and ultimately causes MPT-driven regulated necrosis. Subsequent released DAMPs from dying cells are critical effectors in hepatic inflammation and fibrogenesis. B, mtDNA and mtROS can interact with TLRs on Kupffer cells, activating NF-κB pathways and NLRP3 inflammasomes, promoting the production of pro-inflammatory cytokines. Activated NF-κB could, in turn, suppress NLRP3 inflammasomes by the elimination of defective mitochondrial, mediated by mitophagy. Activated Kupffer cells serve as an essential ROS source, amplifying inflammation and spreading cell death. C, Mitochondrial formylated peptides conjunct with FPR1 on neutrophils, which promotes neutrophil chemotaxis. Chemokines collaborated with formylated peptides, ATP and mtDNA, lead to neutrophils recruitment. D, ROS produced by Kupffer cells, neutrophils, and injured hepatocytes could transform HSCs from quiescent to functional status and further promote the proliferation of active HSCs, consequently causing hepatic fibrosis. AIF, apoptosis-inducing factor; CYPD, cyclophilin D; Cytc, cytochrome c; DAMPs, damage-associated molecular patterns; Drp1, dynamin-related protein 1; FPR1, formyl peptide receptor 1; GLUD1, glutamate dehydrogenase 1; HSC, hepatic stellate cell; MLKL, pseudokinase mixed lineage kinase domain-like; MPT, mitochondrial permeability transition; mtDNA, mitochondrial DNA; mtROS, mitochondrial ROS; NF-κB, nuclear factor kappa B; NLRP3, NOD-, LRR- and pyrin domain-containing protein 3; PGAM5, phosphoglycerate mutase family member 5; RIPK1, receptor-interacting protein kinase 1; RIPK3, receptor-interacting protein kinase 3; ROS, reactive oxygen species; TLRs, toll-like receptors.
There are several nonapoptotic forms of programmed cell death, also known as “regulated necrosis,” including necroptosis, ferroptosis, pyroptosis, and mitochondrial permeability transition-driven necrosis. These nonapoptotic programmed cell death share similar morphologic features with accidental necrosis but are regulated by specific signaling.23 Emerging evidence suggests a critical role of nonapoptotic programmed cell death in hepatic pathogenesis.23,24 Mitochondrial dysfunction essentially contributes to MPT-driven programmed cell death (Figure 2),25 but not ferroptosis, an iron-dependent form of programmed cell death.26,27
The role of mitochondrial dysfunction and oxidative stress in programmed cell death is multifaceted. For instance, necroptosis is mediated by receptor-interacting protein kinase 1, receptor-interacting protein kinase 3, and pseudokinase mixed lineage kinase domain-like, in the case caspase 8 activity is absent or blocked. MtROS functions in a positive feedback loop to promote necroptosis by facilitating the auto-phosphorylation of receptor-interacting protein kinase 1 and necrosome formation. A burst of ROS could be triggered by receptor-interacting protein kinase 3 via the activation of mitochondrial glutamate dehydrogenase 1 and serves as an effector of necroptosis.28,29 Pseudokinase mixed lineage kinase domain-like activity may result in mitochondria fragmentation by activating phosphoglycerate mutase family member 5 and dynamin-related protein 1 to execute necroptosis (Figure 2).30 Nonetheless, recent studies suggest that necroptosis cannot be retarded by the depletion of either mitochondria or phosphoglycerate mutase family member 5, implicating that mitochondria do not play a role in necroptosis.31,32 MtROS production is supposed to accompany, rather than cause, necroptosis.32 Hence, there may be multiple mechanisms involved in necroptosis, and the precise role of mitochondrial dysfunction and oxidative stress remains to be further studied.
Mitochondrial Dysfunction and Oxidative Stress in Hepatic Inflammatory Response
Mitochondrial dysfunction and oxidative stress participate in hepatic inflammatory responses via damage-associated molecular patterns (DAMPs) released from damaged hepatocytes. DAMPs bind to various cell surface or intracellular receptors to activate innate and adaptive immune responses.33 In parallel, passive release or active secretion of mitochondria DAMPs (mtDAMPs) into cytosol or extracellular space trigger inflammatory responses by directly binding to the cell surface and intracellular receptors.34 Interaction of mitochondrial DNA (mtDNA) and mtROS with toll-like receptors results in activation of pro-inflammatory signaling through nuclear factor kappa B, NOD-, LRR- and pyrin domain-containing protein 3 inflammasomes.35,36 Passively released mitochondrial formylated peptides from necrotic cells can interact with formyl peptide receptor 1 on neutrophils, driving neutrophil chemotaxis and neutrophil-dominant sterile inflammation.34,37 Mitochondrial formyl peptides, ATP, and mtDNA can collaborate with chemokines including chemokine (C-X-C motif) ligand-1 (CXCL1) and -8 (CXCL8) to mediate neutrophil recruitment. This further amplifies hepatocyte necrosis and may elicit a systemic inflammatory response and remote tissue injury.38 mtROS also serves as a secondary messenger to regulate inflammasome-(in)dependent pro-inflammatory cytokine production by modulating the equilibrium between positive and negative regulators.39,40 NF-κB has been shown to restrain NOD-, LRR- and pyrin domain-containing protein 3 activation through mitophagy by deposition of dysfunctional mitochondria, revealing a critical anti-inflammatory mechanism of mitophagy (Figure 2).41,42
Mitochondrial Dysfunction in Liver Regeneration and Fibrosis
The liver has remarkable regenerative capacity to maintain tissue homeostasis when exposed to toxins or partial hepatectomy.43 Currently, little is known about the role of mitochondrial dysfunction and oxidative stress in liver regeneration, which is controlled by the delicate balance of hepatocyte division and apoptosis. Typical compensatory cellular hyperplasia is accompanied by increased mitochondrial oxidation. However, a recent study revealed impaired mitochondrial function upon the arrest of hepatocyte division. To maintain liver size, synthesis of alanine and a-ketoglutarate from pyruvate is boosted to induce compensatory cellular hypertrophy, rather than hyperplasia.44
In contrast to a “transient” regenerative response evoked by acute cellular injury, chronic injury by alcohol, fatty acid, or virus promotes a fibrogenic response characterized by inflammation and remodeling of the liver extracellular matrix. Mitochondrial dysfunction and oxidative stress have been recognized as critical mediators of liver fibrosis.45 ROS produced by hepatocytes and Kupffer cells as a response to damage is involved in cell death and subsequent amplification of paracrine inflammation to promote fibrogenesis.46 Hepatic stellate cells (HSCs) are the main effectors in liver fibrosis through the transformation from quiescent to a functional state in response to damage. HSC activation involves the excessive generation of ROS, mainly NOX-dependent ROS, in neighboring cells such as neutrophils, Kupffer cells, and damaged hepatocytes.47 However, once HSCs are activated, their response to ROS is deflected. ROS provides a critical proapoptotic trigger to activated HSCs, possibly by decreasing intracellular glutathione (GSH) levels.48-50 As current antifibrotic therapies are based on using antioxidants, this paradox should be critically assessed. Of note, a recent study demonstrates that mtDAMPs released from injured hepatocytes are regulated by phagocytic hepatic macrophages and could directly activate HSCs, thus promoting liver fibrosis.51 This suggests that direct targeting mtDAMPs or modulating mtDAMPs by phagocytic hepatic macrophages may be a potential antifibrotic strategy.
MITOCHONDRIAL DYSFUNCTION AND OXIDATIVE STRESS IN LIVER TRANSPLANTATION
Liver transplantation using conventional organ procurement and preservation methods is always associated with IRI. In addition to liver transplantation, hepatic IRI is a major risk in clinical settings such as shock, trauma, and liver resection. The damage to parenchymal cells is elicited by deprivation of oxygen and becomes augmented once the oxygen supply is restored. In particular, hepatic warm and cold ischemia followed by reperfusion in liver transplantation represents the main factor determining graft function and survival after transplantation.52 The mechanism of hepatic IRI encompasses exacerbated oxidative injury, impairment of mitochondrial function, and activation of the immune system. Clinical evidence has shown that circulating mtDAMPs are associated with the onset of early allograft dysfunction in liver transplant recipients.53 In light of organ shortage, grafts from extended criteria donors, such as older, steatotic, and brain death donors, have been increasingly applied in liver transplantation. These are considered to be more susceptible to IRI, especially as a result of oxidative injury.54
Interruption of the mitochondrial respiratory chain in hepatocytes lacking sufficient oxygen is the first event in (warm) ischemia. Consequently, OXPHOS is abrupted quickly, which causes depletion of ATP, acceleration of glycolysis, and production of lactate, leading to hepatocyte death and DAMPs release.55 Sinusoidal endothelial cells play a protective role in liver homeostasis and remain the most injured cell type in cold ischemia, also known as “preservation injury,” which can induce ROS production and further Kupffer cell activation.55 Recent studies show that endothelial dysfunction during cold ischemia is mediated by downregulation of Kruppel-like Factor-2, a cellular growth and differentiation regulator, which impairs the antioxidant system by suppressing transcription of protective genes such as endothelial synthase of NO and Nrf2.56,57 Multiple studies revealed the beneficial effect of pretreatment of donors after circulatory death with Kruppel-like Factor-2 inducers as a supplement in the cold storage solution, attenuating oxidative stress and improving graft liver function.57,58 Subsequently, reperfusion injury is initiated by ischemia and represents a dramatically vulnerable phase with massive oxidative injury and inflammatory response. A major event in this phase is the excessive production of ROS in the recovered and viable cells.59 In the early stage of reperfusion, damaged endothelial cells and Kupffer cells are the key cell type producing ROS, which further actives Kupffer cells and initiates neutrophil recruitment, ultimately leading to a larger portion of damage and inflammatory response in the late stage of reperfusion.60 Emerging evidence indicates that the burst of ROS generation and oxidative stress in grafts from extended criteria donors under IRI stress could be ameliorated by ex vivo machine perfusion.61 Moreover, both antioxidative treatment of donor and recipient and supplementation of antioxidants during machine perfusion exhibit promising therapeutic potential to diminish hepatic IRI.62,63
Defective mitochondria play a critical role in hepatic IRI. Mitochondrial permeability transition pore (MPTP) opening could be induced by alkaline pH, mtROS (rather than cytosolic ROS), and calcium overload during IRI, leading to depolarization, uncoupling and swelling of mitochondria and ultimately cause ATP depletion and necrosis, which is the dominant cell death type in reperfusion phase.4 Primarily, after the onset of MPTP, the subsequent extracellular release of Cytc can trigger ATP- and caspase-dependent apoptosis. However, once ATP is depleted due to sudden ischemia, necrotic cell death is induced in hepatocyte. Thus, in the context of hepatic IRI, the depletion status of ATP serves as a switch between ATP-dependent apoptosis and ATP depletion-dependent necrosis.64 As mentioned previously, mtDAMPs released from dead cells are capable to trigger localized and systematic inflammation. A recent study suggests that the post-transplant level of serum mtDNA fragment is associated with the recovery of patients undergoing liver transplantation and predicts the onset of postoperative multiorgan dysfunction syndrome.65 Additionally, blockage of MPTP to restore mitochondrial function has been studied for a long time as a promising therapeutic approach against hepatic IRI. Cyclophilin D is a critical regulator of MPTP opening in the inner mitochondrial membrane. A recent study demonstrated that the small-molecule inhibitors of cyclophilin D reduce calcium-induced mitochondria swelling, restore hepatic calcium retention and OXPHOS parameters, and consequently attenuate hepatic IRI.66
Innate and adaptive immune responses are strongly associated with immediate graft function and long-term outcome in liver transplantation. The involvement of mitochondrial in this process cannot be ignored. The metabolism and functions of innate and adaptive immune cells are extensively modulated by mitochondria, in which mtROS acts as the critical signaling molecules. For instance, excessive ROS and perturbation of mitochondrial function are detrimental to neutrophil migration and promote neutrophils apoptosis.67,68 Similar results are seen in macrophages, where defective mitochondria prevent repolarization from a pro-inflammatory macrophage phenotype to an anti-inflammatory phenotype.69 In contrast, the production of mtROS is not detrimental, but an essential step for T cells activation.70 Likewise, mitochondrial function instructs the activation and cell fates of B cells.71 A recent study observed an increased expression of mitochondrial-encoded genes in peripheral blood mononuclear cell of recipients with acute rejection after kidney transplantation, potentially indicating a predictive value of mitochondrial gene expression for the onset of rejection.72 In rodent orthotopic liver transplant model, oxidative DNA damage and mtDNA mutation in hepatocytes were found to be caused by tumor necrosis factor-alpha, which further promotes graft rejection.73
MITOCHONDRIAL DYSFUNCTION AND OXIDATIVE STRESS IN LIVER DISEASES
Nonalcoholic Fatty Liver Disease
The burden of nonalcoholic fatty liver disease (NAFLD) is rapidly growing worldwide.74 Alterations of mitochondrial morphology and function have been widely observed in hepatocytes from NAFLD patients and animal models.75 At the early stage of NAFLD, increasing lipid levels are accompanied by higher numbers of mitochondria. This is associated with increased OXPHOS, hepatic cardiolipin, ubiquinone, and mitochondrial DNA.76-79
Mitochondrial dysfunction and oxidative stress feature the progression from steatosis to nonalcoholic steatohepatitis (NASH). In these patients, mitochondrial adaptation is impaired possibly because of increased ROS levels and the triggered inflammatory response.80 In NASH patients, hepatic mitochondria have a swollen, rounded shape, and multilamellar membranes with loss of cristae and accumulation of paracrystalline inclusion bodies.75,81 These morphologic changes might involve membrane permeabilization and mitochondrial impairment. Hepatic cardiolipin, a unique phospholipid located at the inner membrane interacts with many other mitochondrial inner membrane proteins, enzymes, and metabolite carriers, and is particularly susceptible to ROS-induced oxidation.82-84 Oxidative cardiolipin affects ETC complex activity, ETC super-complex stability, and cooperates with Ca2+ to induce MPTP opening.85-87 Moreover, cardiolipin provides a recognition site for Bcl2 proteins to mediate apoptosis.88
Uncoupling protein plays a vital role in ETC uncoupling to regulate ATP synthesis, the NAD+/NADH ratio, and other metabolic pathways.89 Uncoupling protein-2 (UCP2) is a mitochondrial inner membrane protein related to oxidative stress.90 Under physiologic conditions, UCP2 is expressed in Kupffer cells rather than hepatocytes.91 However, it becomes abundant in hepatocytes of fatty liver.92 Suppression of UCP2 limits hepatic IRI in ob/ob mice.93 These findings indicate that UCP2-dependent mitochondria uncoupling seems an essential factor in NASH development.
Alcoholic Liver Disease
Continuous consumption of alcohol drives hepatocyte-injury and liver inflammation. ALD can progress to fibrosis, cirrhosis, and the development of hepatocellular carcinoma (HCC).94 Similar to NAFLD and NASH, ALD involves mitochondrial dysfunction and oxidative stress.
There are 2 major oxidative pathways of alcohol metabolism in the liver. Ethanol is converted to acetaldehyde by alcohol dehydrogenase, or is metabolized by cytochrome P-450 2E1 through the microsomal ethanol-oxidizing system.3,95 Hepatic mitochondria are vulnerable targets by ethanol, which consumes NAD+ leading to increased NADH/NAD+ ratio. This inhibits the TCA cycle and fatty acid oxidation in the liver and stimulates lipogenesis to cause steatosis.3,96 Alternatively, acetaldehyde, the metabolite of ethanol, induces mitochondrial damage and cytotoxicity through increasing ROS and Ca2+ levels.97 Oxidative stress, triggered by acetaldehyde-induced mitochondrial functionality loss, sensitizes hepatocytes to further oxidative damage.98
As a result of chronic alcohol intake, lipopolysaccharide is released from the gut99,100 and increases ROS production. This induces mtDNA oxidative damage and inhibits mitochondrial gene transcription, eventually leading to mitochondrial dysfunction.101 In parallel, lipopolysaccharide also activates Kupffer cells that produce large amounts of ROS, chemokines, and pro-inflammatory cytokines augmenting inflammation, fibrosis, and cell death.99,102
Viral Hepatitis
Hepatitis B virus (HBV) and hepatitis C virus (HCV) infections are frequently accompanied by mitochondrial injury.103,104 These infections promote mitochondrial fission by inducing dynamin-related protein 1 phosphorylation and induce Parkin-dependent mitophagy.103,105 But they have different ways to alter mitochondrial functions. HBV infection induces alterations of mitochondrial functions and transmembrane potential, increases mtROS levels, and regulates calcium homeostasis.106-108 HCV infection stimulates fatty acid synthesis to support viral RNA replication109,110 and overload of fatty acids triggers mitochondrial damage. NS5A, an HCV nonstructural protein, activates NF-κB and STAT-3 by inducing oxidative stress and alteration of calcium levels,111 resulting in inflammatory response and subsequent HCC induction.112
Drug-induced Liver Injury
Acetaminophen overdose is the most frequent cause of drug-induced liver failure in Western medicine.113 Mitochondrial dysfunction and oxidative stress play a critical role in acetaminophen hepatoxicity.114 Generally, acetaminophen is metabolized into the reactive metabolite, N-acetyl-p-benzoquinone imine, primarily by cytochrome P-450 2E1, which can be deposed by GSH under a therapeutic dose of acetaminophen. In the context of acetaminophen overdose, N-acetyl-p-benzoquinone imine is continuously generated due to the overwhelmed GSH, which contributes to the depletion of GSH, the formation of adducts and subsequently binds to mitochondrial proteins, causing mitochondrial dysfunction and increased ROS/RNS generation.3 N-acetylcysteine, the GSH precursor, has been introduced and clinically applied for the treatment of acetaminophen hepatoxicity since the 1970s.115 It replenishes GSH stores, improves hemodynamics, scavenges ROS/RNS, recovers mitochondrial GSH, and supports mitochondrial bioenergetics.116,117 Moreover, excessive ROS/RNS actives mitogen-activated protein kinases, especially c-jun N-terminal kinase (JNK) whereby phosphorylated JNK interplays with mitochondrial and augments oxidative stress and further promotes MPTP opening and release of AIF.118 Oncolytic necrosis, a dominant type of cell death in acetaminophen hepatotoxicity, can be induced by AIF and elicits the release of DAMPs, including mtDAMPs, inducing sterile inflammation.34 Circulating glutamate dehydrogenase, mtDNA and nDNA fragments are useful indicators to evaluate mitochondrial damage in acetaminophen hepatoxicity and are strongly associated with the outcome in patients.34
Cholestatic Liver Diseases
Cholestatic liver diseases (CLD) are featured by an overload of detrimental bile acid in liver and circulation due to alteration of bile formation or intrahepatic/extrahepatic obstruction of bile ducts.119 Several studies uncovered the central role of mitochondrial dysfunction and oxidative stress in CLD. A striking increase of oxidative injury markers has been reported in patients with chronic CLD, while the levels of antioxidants decreased.120 Similar results were found in bile duct ligated rats, an experimental CLD model, in which a systemic elevation of lipid peroxidation products occurred in the liver and extrahepatic tissues such as kidney and heart.121 In the context of moderate CLD, intrahepatic accumulation of low concentrations of hydrophobic bile acid triggers hepatocyte apoptosis via massive production of mtROS.122 With regard to severe CLD, dramatically increased levels of bile acid triggers necrosis mainly by lipid peroxidation.123,124 Additionally, MPT can be induced and thus impairs mitochondrial OXPHOS, initiating disruption of mitochondria.123,125 It has been shown that inhibition of mitochondrial fission effectively diminishes ROS levels, hepatocellular injury, and fibrosis in CLD.126 Nuclear factor-erythroid 2-related factor-2 (Nrf2), a critical regulator of antioxidative genes, has been identified as a potential target for treating CLD. Sustained activation of Nrf2 shows hepatoprotective effects in experimental CLD models.127 One of the critical beneficial mechanisms of UDCA is believed to stimulate Nrf2, which subsequently restores detoxification and antioxidative defense systems in the liver.128
Liver Cancer
Primary liver cancer, mainly including HCC and cholangiocarcinoma (CCA), becomes the second major cause for cancer-related mortality worldwide.129 HCC and CCA are highly heterogeneous and featured by aggressive progression and poor prognosis, but the underlying mechanisms remain elusive. Accumulating evidence suggests that mitochondrial dysfunction and oxidative stress act as initiator or promoter of the tumor initiation, progression, recurrence, and metastasis.130 Toxic mtROS is produced in aberrant mitochondria under continuous exposure to insults, and subsequently elicits irreversible damage for both mtDNA and nDNA, and mutation of proto-oncogenes and tumor-suppressor genes, promoting cell transformation and onset of carcinogenesis.131 Additionally, mtROS could also promote the survival, angiogenesis, and proliferation of tumor cells by activating NF-κB and stabilize HIF-1α.132,133 ROS-induced cell death could further amplify hepatic inflammation by the release of DMAPs and formation of a ROS-related vicious inflammatory loop, resulting in an oxidative and inflammatory microenvironment.34 This microenvironment has been proven to favor the development of HCC or CCA and is essential to direct lineage commitment in liver cancer.134 A recent study showed that the level of Kupffer cell-derived ROS is particularly higher in human CCA and surrounding hepatocytes, which causes JNK-dependent cholangiocellular proliferation and oncogenic transformation.135 However, the involvement of ROS in liver cancer cannot be interpreted by a simple notion of “pro-carcinogenesis.” For instance, due to depletion of mitochondrial GSH, the hypoxic environment within the tumor can be reversed from pro-carcinogenesis to anti-carcinogenesis by overproduction of mtROS, whereby tumor cells are preferentially killed.136
THERAPEUTIC STRATEGIES
Silymarin/Silibinin
Silymarin is a natural extract from milk thistle (Silybum marianum) that has been used for centuries for treating liver diseases. Silymarin is a complex mixture of compounds in which silibinin represents the most prevalent and biologically active structural component.137 Silymarin is a potent ROS/RNS scavenger and exhibits hepatoprotective properties in various experimental models.138,139 Production of O2− and NO in isolated rat Kupffer cells could be effectively inhibited by silibinin.139 Silymarin can also enhance the enzymatic antioxidant defense system by increasing GSH synthesis and attenuate acetaminophen hepatoxicity in mice.138 A recent study identified silymarin as an inducer of Nrf2 and regulator of the redox signaling pathway.140 Additionally, by blocking the activation of NF-κB and inflammatory metabolites, silymarin is capable of restraining the generation of cytokines and thus reducing liver fibrosis induced by carbon tetrachloride toxicity.141 Numerous clinical investigations have been conducted to evaluate the hepatoprotective effect of silymarin in liver diseases such as NAFLD, NASH, ALD, drug-induced liver injury, and viral hepatitis. For instance, in a randomized and double-blind study in patients with chronic ALD, a 6-mo treatment of silymarin significantly restored the diminished expression of SOD, increased the level of serum GSH peroxidase, and reduced the serum malondialdehyde.142 In contrast, another 2-y period and multicenter trial indicated that silymarin has no beneficial effect on either liver function or mortality in ALD patients with cirrhosis.143 This paradox may arise from the various administration periods and dosage of silymarin in different studies. Besides, the distinct silymarin formulations used should also be considered as the oral bioavailability of crude silymarin extract is far lower than Eurosil 85, a commercial formulation of silymarin, which has been applied widely in recent trials. Altogether, silymarin represents a promising therapy for chronic liver diseases which remains to be confirmed in more clinical trials.
Vitamin E
Vitamin E is a lipid-soluble antioxidant, which has been extensively investigated during the last decade, especially in the field of steatohepatitis. The inherited antioxidative mechanism of vitamin E results from the deposition of ROS/RNS by donating a hydrogen ion from its chromanol ring.144 Vitamin E regulates antioxidative enzymes such as manganese SOD and GSH to enhance an antioxidant defense.145 In addition to its antioxidative capacity, vitamin E is also able to suppress hepatic fibrogenesis by retarding inflammation and inhibiting apoptosis.144 Accumulating evidence demonstrates that vitamin E could reduce lipid accumulation, lipid peroxidation, insulin resistance, oxidative stress, necroinflammation, and thus ameliorate experimental hepatic steatosis.146,147 However, the effect of vitamin E in the treatment of steatohepatitis seems controversial. Two large-scale clinical trials, also known as Pioglitazone versus Vitamin E versus Placebo for the Treatment of Non-Diabetic Patients with Nonalcoholic Steatohepatitis and Treatment of Nonalcoholic Fatty Liver Disease in Children, have been conducted. In the Pioglitazone versus Vitamin E versus Placebo for the Treatment of Non-Diabetic Patients with Nonalcoholic Steatohepatitis trial, vitamin E significantly improves the outcome of biopsy-confirmed adult NASH patients without diabetes but did not affect fibrosis.148 Hence, vitamin E has been recommended as first-line pharmacotherapy for non-diabetic patients with biopsy-proven NASH.149 However, in the Treatment of Nonalcoholic Fatty Liver Disease in Children trial, vitamin E improved both liver histology and NAFLD activity scores in pediatric patients with NASH but failed to reduce the level of transaminases.150 Besides, the increased mortality after the treatment of vitamin E in several clinical trials cannot be neglected.151
MitoQ
MitoQ is a ubiquinone-derived mitochondria-specific antioxidant, which attaches to a lipophilic triphenylphosphonium cation and selectively blocks mitochondrial oxidative damage.152 MitoQ inhibits lipid peroxidation and hydrogen peroxide-induced apoptosis and protects mitochondria from oxidative damage.152,153 Intestinal barrier disruption is a known risk for oxidative stress in liver diseases. As shown in previous studies on intestinal IRI, mitoQ protects the intestinal barrier by meliorating mtDNA damage through the Nrf2/ARE signaling pathway.154 It also has a noticeable improvement of mitochondrial function and antiviral CD8 functions in HBV infection.155 In mice experiments, mitoQ showed inhibition of HSCs activation and transforming growth factor-beta 1 expression, both key factors in hepatic fibrosis.156,157
Targeting Farnesoid X Receptor
The farnesoid X receptor (FXR) is a nuclear receptor that plays a critical role in energy metabolism, especially in bile acids, fats, and hydrocarbon metabolism. FXR and FXR agonists can reduce oxidative stress, protect mitochondrial function and antagonize the SOD4-activated JNK signaling pathway.158-160 Obeticholic acid (OCA), an FXR agonist, is studied in patients with PBC that have an inadequate response to UDCA. Time will show the long-term safety and clinical efficacy of OCA.161 OCA was already assessed in NASH patients who had alleviated fibrosis, hepatocellular ballooning, steatosis, and lobular inflammation compared with placebo groups. Still to be analyzed is the effect of OCA on serum cholesterol levels and insulin resistance, as well as the risk of atherogenesis.162
Nonpharmacologic Interventions
Pharmacologic antioxidants have been extensively investigated in experimental models and clinical trials. Beyond that, nonpharmacologic interventions, such as nanocarrier-coated antioxidants, ischemia preconditioning (IPC), gene therapy, machine perfusion, and gaseous supplements, represent unconventional approaches to suppress mitochondrial dysfunction and oxidative stress in liver diseases and transplantation. Notably, the clinical application of current antioxidative therapies is hampered mainly by the instability, poor membrane permeability, short half-life in circulation, and poor solubility and hydrophilicity of the agents.163 To solve this, antioxidants can be encapsulated by a nanocarrier to improve its bioavailability. For example, SOD, an enzymatic antioxidant, could be encapsulated into poly(D, L-lactideco-glycolide) nanoparticles and reduced neuron IRI by detoxifying free radicals, of which efficiency of SOD is significantly enhanced.164
Antioxidative therapies are of importance to alleviate IRI in liver surgery and could be employed specifically for the treatment of donors, graft livers, and recipients during liver transplantation.62 IPC is a surgical procedure that is initiated by a short period of nonlethal ischemia to the target organ before a much longer time of ischemia elicited by surgery such as hepatectomy and graft liver procurement. IPC has been proven to protect the liver from subsequent IRI by regulating redox balance, preventing nuclear damage, and inhibiting cell death.165 IPC could increase the tolerance of the fatty rat liver to IRI by activating antioxidative enzymes. Multiple lines of evidence in clinical trials indicate that the IPC on donor could attenuate liver injury following liver transplantation and decrease the mortality of recipients.166-168 Machine perfusion remains a promising approach to protect the graft liver from IRI. A recent systematic review demonstrates that both hypothermic machine perfusion and normothermic machine perfusion are superior to static cold storage on improving early graft function in liver transplantation.169 Compared with static cold storage, hypothermic machine perfusion could significantly reduce oxidative stress in liver grafts.170 Of note, the antioxidative effect of machine perfusion is primarily determined by the temperature because the mitochondrial activity is known to be temperature-dependent.63 Indeed, increased production of ROS has been observed in normothermic machine perfusion due to the induction of reverse, instead of forward, electron transfer through the mitochondrial respiratory chain. In contrast, during hypothermic or subnormothermic machine perfusion, the mitochondrial respiratory function is significantly suppressed while the ATP pool is enhanced, leading to less ROS generation.171 However, normothermic machine perfusion remains to be superior in terms of its capability to restore metabolism under normothermic condition, thus allowing the monitoring of function of graft liver.63 Hence, combination of hypothermic and normothermic machine perfusion represents an optimized protocol to alleviate oxidative injury. A recent study showed that application of normothermic after hypothermic machine perfusion could mitigate oxidative injury and restore mitochondrial function.172
SUMMARY AND CONCLUSION
Endogenous and exogenous hepatic insults are known to cause mitochondrial dysfunction in the liver involving loss of ATP synthesis capacity and increasing ROS/RNS generation. This, in turn, results in oxidative stress and further forms a vicious cycle, which is believed to be the critical pathogenic factors in liver diseases, as well as liver transplantation. Although ROS/RNS are involved in normal cell homeostasis, they are also related to the regulation of apoptosis, necroptosis, and MPT-driven regulated necrosis. Also, ROS/RNS are also capable to indirectly affect hepatocyte function by promoting an inflammatory response and fibrogenesis. The balance of ROS/RNS being either harmful or beneficial largely depends on the intensity and duration of ROS/RNS production. Although numerous antioxidative interventions have been developed for the treatment of liver diseases by either scavenging ROS/RNS or restoring mitochondrial homeostasis, not many showed beneficial effects in clinical trials.2 Of note, intake of dietary antioxidant supplements such as beta carotene, vitamin A, and vitamin E might even increase the mortality of patients.151 The precise role of mitochondrial dysfunction and oxidative stress in hepatic pathogenesis should be studied in more detail, and the development of therapeutic regimens should be thoroughly examined to not only alleviate detrimental effects of free radicals but also to preserve its biologic function.
Footnotes
S.S. and L.W. drafted the article and designed the figures. All authors contributed to the final article.
The authors declare no conflicts of interest.
This study was supported by the China Scholarship Council for funding PhD fellowship to S.S. (no. 201706230252) and L.W. (no. 201708530248).
S.S. and L.W. contributed equally to this manuscript and shared the first authorship.
REFERENCES
- 1.Ayala-Peña S, Torres-Ramos CA.Preedy VR, ed. Oxidative stress, aging and mitochondrial dysfunction in liver pathology. In: Aging 2014Academic Press; 39–48. [Google Scholar]
- 2. Mello T, Zanieri F, Ceni E, et al. Oxidative stress in the healthy and wounded hepatocyte: a cellular organelles perspective. Oxid Med Cell Longev. 2016;2016:8327410. doi: 10.1155/2016/8327410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mansouri A, Gattolliat CH, Asselah T. Mitochondrial dysfunction and signaling in chronic liver diseases. Gastroenterology. 2018;155:629–647. doi: 10.1053/j.gastro.2018.06.083. [DOI] [PubMed] [Google Scholar]
- 4. Go KL, Lee S, Zendejas I, et al. Mitochondrial dysfunction and autophagy in hepatic ischemia/reperfusion injury. Biomed Res Int. 2015;2015:183469. doi: 10.1155/2015/183469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jadeja RN, Devkar RV, Nammi S. Oxidative stress in liver diseases: pathogenesis, prevention, and therapeutics. Oxid Med Cell Longev. 2017;2017:8341286. doi: 10.1155/2017/8341286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bonner MY, Arbiser JL. The antioxidant paradox: what are antioxidants and how should they be used in a therapeutic context for cancer. Future Med Chem. 2014;6:1413–1422. doi: 10.4155/fmc.14.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lee J, Giordano S, Zhang J. Autophagy, mitochondria and oxidative stress: cross-talk and redox signalling. Biochem J. 2012;441:523–540. doi: 10.1042/BJ20111451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Finkel T. Signal transduction by mitochondrial oxidants. J Biol Chem. 2012;287:4434–4440. doi: 10.1074/jbc.R111.271999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bhatti JS, Bhatti GK, Reddy PH. Mitochondrial dysfunction and oxidative stress in metabolic disorders — a step towards mitochondria based therapeutic strategies. Biochim Biophys Acta - Mol Basis Dis. 2017;1863:1066. 1077. doi: 10.1016/j.bbadis.2016.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mishra P, Chan DC. Metabolic regulation of mitochondrial dynamics. J Cell Biol. 2016;212:379–387. doi: 10.1083/jcb.201511036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dröge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 12. Cadenas E, Davies KJA. Mitochondrial free radical generation, oxidative stress, and aging. Free Radic Biol Med. 2000;29:222–230. doi: 10.1016/s0891-5849(00)00317-8. [DOI] [PubMed] [Google Scholar]
- 13. Madamanchi NR, Runge MS. Mitochondrial dysfunction in atherosclerosis. Circ Res. 2007;100:460–473. doi: 10.1161/01.RES.0000258450.44413.96. [DOI] [PubMed] [Google Scholar]
- 14. Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 15. Dikalova AE, Bikineyeva AT, Budzyn K, et al. Therapeutic targeting of mitochondrial superoxide in hypertension. Circ Res. 2010;107:106–116. doi: 10.1161/CIRCRESAHA.109.214601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Doughan AK, Harrison DG, Dikalov SI. Molecular mechanisms of angiotensin II-mediated mitochondrial dysfunction: linking mitochondrial oxidative damage and vascular endothelial dysfunction. Circ Res. 2008;102:488–496. doi: 10.1161/CIRCRESAHA.107.162800. [DOI] [PubMed] [Google Scholar]
- 17. Valdez LB, Zaobornyj T, Boveris A. Mitochondrial metabolic states and membrane potential modulate mtNOS activity. Biochim Biophys Acta. 2006;1757:166–172. doi: 10.1016/j.bbabio.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 18. Parihar MS, Nazarewicz RR, Kincaid E, et al. Association of mitochondrial nitric oxide synthase activity with respiratory chain complex I. Biochem Biophys Res Commun. 2008;366:23–28. doi: 10.1016/j.bbrc.2007.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Radi R, Rodriguez M, Castro L, et al. Inhibition of mitochondrial electron transport by peroxynitrite. Arch Biochem Biophys. 1994;308:89–95. doi: 10.1006/abbi.1994.1013. [DOI] [PubMed] [Google Scholar]
- 20. Yuan S, Topf M, Reubold TF, et al. Changes in Apaf-1 conformation that drive apoptosome assembly. Biochemistry. 2013;52:2319–2327. doi: 10.1021/bi301721g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li P, Nijhawan D, Budihardjo I, et al. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 22. Sevrioukova IF. Apoptosis-inducing factor: structure, function, and redox regulation. Antioxid Redox Signal. 2011;14:2545–2579. doi: 10.1089/ars.2010.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Karch J, Molkentin JD. Regulated necrotic cell death: the passive aggressive side of Bax and Bak. Circ Res. 2015;116:1800–1809. doi: 10.1161/CIRCRESAHA.116.305421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Guicciardi ME, Malhi H, Mott JL, et al. Apoptosis and necrosis in the liver. Compr Physiol. 2013;3:977–1010. doi: 10.1002/cphy.c120020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Izzo V, Bravo-San Pedro JM, Sica V, et al. Mitochondrial permeability transition: new findings and persisting uncertainties. Trends Cell Biol. 2016;26:655–667. doi: 10.1016/j.tcb.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 26. Skouta R, Dixon SJ, Wang J, et al. Ferrostatins inhibit oxidative lipid damage and cell death in diverse disease models. J Am Chem Soc. 2014;136:4551–4556. doi: 10.1021/ja411006a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dixon SJ, Stockwell BR. The role of iron and reactive oxygen species in cell death. Nat Chem Biol. 2014;10:9–17. doi: 10.1038/nchembio.1416. [DOI] [PubMed] [Google Scholar]
- 28. Zhang DW, Shao J, Lin J, et al. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science. 2009;325:332–336. doi: 10.1126/science.1172308. [DOI] [PubMed] [Google Scholar]
- 29. Shindo R, Kakehashi H, Okumura K, et al. Critical contribution of oxidative stress to TNFα-induced necroptosis downstream of RIPK1 activation. Biochem Biophys Res Commun. 2013;436:212–216. doi: 10.1016/j.bbrc.2013.05.075. [DOI] [PubMed] [Google Scholar]
- 30. Wang Z, Jiang H, Chen S, et al. The mitochondrial phosphatase PGAM5 functions at the convergence point of multiple necrotic death pathways. Cell. 2012;148:228–243. doi: 10.1016/j.cell.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 31. Moriwaki K, Farias Luz N, Balaji S, et al. The mitochondrial phosphatase PGAM5 is dispensable for necroptosis but promotes inflammasome activation in macrophages. J Immunol. 2016;196:407–415. doi: 10.4049/jimmunol.1501662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tait SW, Oberst A, Quarato G, et al. Widespread mitochondrial depletion via mitophagy does not compromise necroptosis. Cell Rep. 2013;5:878–885. doi: 10.1016/j.celrep.2013.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mihm S. Danger-associated molecular patterns (DAMPs): molecular triggers for sterile inflammation in the liver. Int J Mol Sci. 2018;19 doi: 10.3390/ijms19103104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Grazioli S, Pugin J. Mitochondrial damage-associated molecular patterns: from inflammatory signaling to human diseases. Front Immunol. 2018;9:832. doi: 10.3389/fimmu.2018.00832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shimada K, Crother TR, Karlin J, et al. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity. 2012;36:401–414. doi: 10.1016/j.immuni.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Agod Z, Fekete T, Budai MM, et al. Regulation of type I interferon responses by mitochondria-derived reactive oxygen species in plasmacytoid dendritic cells. Redox Biol. 2017;13:633–645. doi: 10.1016/j.redox.2017.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dorward DA, Lucas CD, Chapman GB, et al. The role of formylated peptides and formyl peptide receptor 1 in governing neutrophil function during acute inflammation. Am J Pathol. 2015;185:1172–1184. doi: 10.1016/j.ajpath.2015.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Marques PE, Amaral SS, Pires DA, et al. Chemokines and mitochondrial products activate neutrophils to amplify organ injury during mouse acute liver failure. Hepatology. 2012;56:1971–1982. doi: 10.1002/hep.25801. [DOI] [PubMed] [Google Scholar]
- 39. Naik E, Dixit VM. Mitochondrial reactive oxygen species drive proinflammatory cytokine production. J Exp Med. 2011;208:417–420. doi: 10.1084/jem.20110367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhou R, Yazdi AS, Menu P, et al. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469:221–225. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]
- 41. Minton K. Inflammasome: anti-inflammatory effect of mitophagy. Nat Rev Immunol. 2016;16:206. doi: 10.1038/nri.2016.33. [DOI] [PubMed] [Google Scholar]
- 42. Harris J, Deen N, Zamani S, et al. Mitophagy and the release of inflammatory cytokines. Mitochondrion. 2018;41:2–8. doi: 10.1016/j.mito.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 43. Cordero-Espinoza L, Huch M. The balancing act of the liver: tissue regeneration versus fibrosis. J Clin Invest. 2018;128:85–96. doi: 10.1172/JCI93562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Caldez MJ, Van Hul N, Koh HWL, et al. Metabolic remodeling during liver regeneration. Dev Cell. 2018;47:425–438.e5. doi: 10.1016/j.devcel.2018.09.020. [DOI] [PubMed] [Google Scholar]
- 45. Luangmonkong T, Suriguga S, Mutsaers HAM, et al. Targeting oxidative stress for the treatment of liver fibrosis. Rev Physiol Biochem Pharmacol. 2018;175:71–102. doi: 10.1007/112_2018_10. [DOI] [PubMed] [Google Scholar]
- 46. Mortezaee K. Nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (NOX) and liver fibrosis: a review. Cell Biochem Funct. 2018;36:292–302. doi: 10.1002/cbf.3351. [DOI] [PubMed] [Google Scholar]
- 47. Bataller R, Schwabe RF, Choi YH, et al. NADPH oxidase signal transduces angiotensin II in hepatic stellate cells and is critical in hepatic fibrosis. J Clin Invest. 2003;112:1383–1394. doi: 10.1172/JCI18212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Thirunavukkarasu C, Watkins S, Harvey SA, et al. Superoxide-induced apoptosis of activated rat hepatic stellate cells. J Hepatol. 2004;41:567–575. doi: 10.1016/j.jhep.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 49. Jameel NM, Thirunavukkarasu C, Wu T, et al. p38-MAPK- and caspase-3-mediated superoxide-induced apoptosis of rat hepatic stellate cells: reversal by retinoic acid. J Cell Physiol. 2009;218:157–166. doi: 10.1002/jcp.21581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gandhi CR. Oxidative stress and hepatic stellate cells: a paradoxical relationship. Trends Cell Mol Biol. 2012;7:1–10. [PMC free article] [PubMed] [Google Scholar]
- 51. An P, Wei LL, Zhao S, et al. Hepatocyte mitochondria-derived danger signals directly activate hepatic stellate cells and drive progression of liver fibrosis. Nat Commun. 2020;11:2362. doi: 10.1038/s41467-020-16092-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Dar WA, Sullivan E, Bynon JS, et al. Ischaemia reperfusion injury in liver transplantation: cellular and molecular mechanisms. Liver Int. 2019;39:788–801. doi: 10.1111/liv.14091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Pollara J, Edwards RW, Lin L, et al. Circulating mitochondria in deceased organ donors are associated with immune activation and early allograft dysfunction. JCI Insight. 2018;3:e121622. doi: 10.1172/jci.insight.121622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Badawy A, Kaido T, Uemoto S. Current status of liver transplantation using marginal grafts. J Invest Surg. 2020;33:553–564. doi: 10.1080/08941939.2018.1517197. [DOI] [PubMed] [Google Scholar]
- 55. Peralta C, Jiménez-Castro MB, Gracia-Sancho J. Hepatic ischemia and reperfusion injury: effects on the liver sinusoidal milieu. J Hepatol. 2013;59:1094–1106. doi: 10.1016/j.jhep.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 56. Gracia-Sancho J, Villarreal G, Jr, Zhang Y, et al. Flow cessation triggers endothelial dysfunction during organ cold storage conditions: strategies for pharmacologic intervention. Transplantation. 2010;90:142–149. doi: 10.1097/TP.0b013e3181e228db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Russo L, Gracia-Sancho J, García-Calderó H, et al. Addition of simvastatin to cold storage solution prevents endothelial dysfunction in explanted rat livers. Hepatology. 2012;55:921–930. doi: 10.1002/hep.24755. [DOI] [PubMed] [Google Scholar]
- 58. Liu Z, Zhang X, Xiao Q, et al. Pretreatment donors after circulatory death with simvastatin alleviates liver ischemia reperfusion injury through a KLF2-dependent mechanism in rat. Oxid Med Cell Longev. 2017;2017:3861914. doi: 10.1155/2017/3861914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Klune JR, Tsung A. Molecular biology of liver ischemia/reperfusion injury: established mechanisms and recent advancements. Surg Clin North Am. 2010;90:665–677. doi: 10.1016/j.suc.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 60. Jaeschke H, Smith CV, Mitchell JR. Reactive oxygen species during ischemia-reflow injury in isolated perfused rat liver. J Clin Invest. 1988;81:1240–1246. doi: 10.1172/JCI113441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Boteon YL, Laing RW, Schlegel A, et al. Combined hypothermic and normothermic machine perfusion improves functional recovery of extended criteria donor livers. Liver Transpl. 2018;24:1699–1715. doi: 10.1002/lt.25315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Shi S, Xue F. Current antioxidant treatments in organ transplantation. Oxid Med Cell Longev. 2016;2016:8678510. doi: 10.1155/2016/8678510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hofmann J, Otarashvili G, Meszaros A, et al. Restoring mitochondrial function while avoiding redox stress: the key to preventing ischemia/reperfusion injury in machine perfused liver grafts? Int J Mol Sci. 2020;21:3132. doi: 10.3390/ijms21093132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kim JS, He L, Qian T, et al. Role of the mitochondrial permeability transition in apoptotic and necrotic death after ischemia/reperfusion injury to hepatocytes. Curr Mol Med. 2003;3:527–535. doi: 10.2174/1566524033479564. [DOI] [PubMed] [Google Scholar]
- 65. Nagakawa K, Soyama A, Hidaka M, et al. Elevated plasma levels of mitochondria-derived damage-associated molecular patterns during liver transplantation: predictors for postoperative multi-organ dysfunction syndrome. Tohoku J Exp Med. 2020;250:87–93. doi: 10.1620/tjem.250.87. [DOI] [PubMed] [Google Scholar]
- 66. Panel M, Ruiz I, Brillet R, et al. Small-molecule inhibitors of cyclophilins block opening of the mitochondrial permeability transition pore and protect mice from hepatic ischemia/reperfusion injury. Gastroenterology. 2019;157:1368–1382. doi: 10.1053/j.gastro.2019.07.026. [DOI] [PubMed] [Google Scholar]
- 67. Fossati G, Moulding DA, Spiller DG, et al. The mitochondrial network of human neutrophils: role in chemotaxis, phagocytosis, respiratory burst activation, and commitment to apoptosis. J Immunol. 2003;170:1964–1972. doi: 10.4049/jimmunol.170.4.1964. [DOI] [PubMed] [Google Scholar]
- 68. Maianski NA, Geissler J, Srinivasula SM, et al. Functional characterization of mitochondria in neutrophils: a role restricted to apoptosis. Cell Death Differ. 2004;11:143–153. doi: 10.1038/sj.cdd.4401320. [DOI] [PubMed] [Google Scholar]
- 69. Van den Bossche J, Baardman J, Otto NA, et al. Mitochondrial dysfunction prevents repolarization of inflammatory macrophages. Cell Rep. 2016;17:684–696. doi: 10.1016/j.celrep.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 70. Sena LA, Li S, Jairaman A, et al. Mitochondria are required for antigen-specific T cell activation through reactive oxygen species signaling. Immunity. 2013;38:225–236. doi: 10.1016/j.immuni.2012.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Jang KJ, Mano H, Aoki K, et al. Mitochondrial function provides instructive signals for activation-induced B-cell fates. Nat Commun. 2015;6:6750. doi: 10.1038/ncomms7750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Roedder S, Sigdel T, Hsieh SC, et al. Expression of mitochondrial-encoded genes in blood differentiate acute renal allograft rejection. Front Med (Lausanne) 2017;4:185. doi: 10.3389/fmed.2017.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Nagakawa Y, Williams GM, Zheng Q, et al. Oxidative mitochondrial DNA damage and deletion in hepatocytes of rejecting liver allografts in rats: role of TNF-alpha. Hepatology. 2005;42:208–215. doi: 10.1002/hep.20755. [DOI] [PubMed] [Google Scholar]
- 74. Younossi Z, Anstee QM, Marietti M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15:11–20. doi: 10.1038/nrgastro.2017.109. [DOI] [PubMed] [Google Scholar]
- 75. Caldwell SH, de Freitas LA, Park SH, et al. Intramitochondrial crystalline inclusions in nonalcoholic steatohepatitis. Hepatology. 2009;49:1888–1895. doi: 10.1002/hep.22851. [DOI] [PubMed] [Google Scholar]
- 76. Rolo AP, Teodoro JS, Palmeira CM. Role of oxidative stress in the pathogenesis of nonalcoholic steatohepatitis. Free Radic Biol Med. 2012;52:59–69. doi: 10.1016/j.freeradbiomed.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 77. Peng KY, Watt MJ, Rensen S, et al. Mitochondrial dysfunction-related lipid changes occur in nonalcoholic fatty liver disease progression. J Lipid Res. 2018;59:1977–1986. doi: 10.1194/jlr.M085613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Chiappini F, Barrier A, Saffroy R, et al. Exploration of global gene expression in human liver steatosis by high-density oligonucleotide microarray. Lab Invest. 2006;86:154–165. doi: 10.1038/labinvest.3700374. [DOI] [PubMed] [Google Scholar]
- 79. Wang Y, Oxer D, Hekimi S. Mitochondrial function and lifespan of mice with controlled ubiquinone biosynthesis. Nat Commun. 2015;6:6393. doi: 10.1038/ncomms7393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Koliaki C, Szendroedi J, Kaul K, et al. Adaptation of hepatic mitochondrial function in humans with non-alcoholic fatty liver is lost in steatohepatitis. Cell Metab. 2015;21:739–746. doi: 10.1016/j.cmet.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 81. Sanyal AJ, Campbell-Sargent C, Mirshahi F, et al. Nonalcoholic steatohepatitis: association of insulin resistance and mitochondrial abnormalities. Gastroenterology. 2001;120:1183–1192. doi: 10.1053/gast.2001.23256. [DOI] [PubMed] [Google Scholar]
- 82. Schlame M, Rua D, Greenberg ML. The biosynthesis and functional role of cardiolipin. Prog Lipid Res. 2000;39:257–288. doi: 10.1016/s0163-7827(00)00005-9. [DOI] [PubMed] [Google Scholar]
- 83. Paradies G, Petrosillo G, Paradies V, et al. Role of cardiolipin peroxidation and Ca2+ in mitochondrial dysfunction and disease. Cell Calcium. 2009;45:643–650. doi: 10.1016/j.ceca.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 84. Paradies G, Paradies V, De Benedictis V, et al. Functional role of cardiolipin in mitochondrial bioenergetics. Biochim Biophys Acta. 2014;1837:408–417. doi: 10.1016/j.bbabio.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 85. Paradies G, Petrosillo G, Pistolese M, et al. Reactive oxygen species affect mitochondrial electron transport complex I activity through oxidative cardiolipin damage. Gene. 2002;286:135–141. doi: 10.1016/s0378-1119(01)00814-9. [DOI] [PubMed] [Google Scholar]
- 86. Paradies G, Petrosillo G, Pistolese M, et al. Reactive oxygen species generated by the mitochondrial respiratory chain affect the complex III activity via cardiolipin peroxidation in beef-heart submitochondrial particles. Mitochondrion. 2001;1:151–159. doi: 10.1016/s1567-7249(01)00011-3. [DOI] [PubMed] [Google Scholar]
- 87. McKenzie M, Lazarou M, Thorburn DR, et al. Mitochondrial respiratory chain supercomplexes are destabilized in Barth Syndrome patients. J Mol Biol. 2006;361:462–469. doi: 10.1016/j.jmb.2006.06.057. [DOI] [PubMed] [Google Scholar]
- 88. Schlattner U, Tokarska-Schlattner M, Ramirez S, et al. Mitochondrial kinases and their molecular interaction with cardiolipin. Biochim Biophys Acta. 2009;1788:2032–2047. doi: 10.1016/j.bbamem.2009.04.018. [DOI] [PubMed] [Google Scholar]
- 89. Ricquier D, Bouillaud F. The uncoupling protein homologues: UCP1, UCP2, UCP3, StUCP and AtUCP. Biochem J. 2000;345 Pt 2:161–179. [PMC free article] [PubMed] [Google Scholar]
- 90. Echtay KS, Roussel D, St-Pierre J, et al. Superoxide activates mitochondrial uncoupling proteins. Nature. 2002;415:96–99. doi: 10.1038/415096a. [DOI] [PubMed] [Google Scholar]
- 91. Larrouy D, Laharrague P, Carrera G, et al. Kupffer cells are a dominant site of uncoupling protein 2 expression in rat liver. Biochem Biophys Res Commun. 1997;235:760–764. doi: 10.1006/bbrc.1997.6852. [DOI] [PubMed] [Google Scholar]
- 92. Serviddio G, Bellanti F, Tamborra R, et al. Uncoupling protein-2 (UCP2) induces mitochondrial proton leak and increases susceptibility of non-alcoholic steatohepatitis (NASH) liver to ischaemia-reperfusion injury. Gut. 2008;57:957–965. doi: 10.1136/gut.2007.147496. [DOI] [PubMed] [Google Scholar]
- 93. Wan CD, Wang CY, Liu T, et al. Alleviation of ischemia/reperfusion injury in ob/ob mice by inhibiting UCP-2 expression in fatty liver. World J Gastroenterol. 2008;14:590–594. doi: 10.3748/wjg.14.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Seitz HK, Bataller R, Cortez-Pinto H, et al. Alcoholic liver disease. Nat Rev Dis Primers. 2018;4:16. doi: 10.1038/s41572-018-0014-7. [DOI] [PubMed] [Google Scholar]
- 95. Kawaratani H, Tsujimoto T, Douhara A, et al. The effect of inflammatory cytokines in alcoholic liver disease. Mediators Inflamm. 2013;2013:495156. doi: 10.1155/2013/495156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. You M, Crabb DW. Recent advances in alcoholic liver disease II. Minireview: molecular mechanisms of alcoholic fatty liver. Am J Physiol Gastrointest Liver Physiol. 2004;287:G1–G6. doi: 10.1152/ajpgi.00056.2004. [DOI] [PubMed] [Google Scholar]
- 97. Yan T, Zhao Y. Acetaldehyde induces phosphorylation of dynamin-related protein 1 and mitochondrial dysfunction via elevating intracellular ROS and Ca2+ levels. Redox Biol. 2020;28:101381. doi: 10.1016/j.redox.2019.101381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Farfán Labonne BE, Gutiérrez M, Gómez-Quiroz LE, et al. Acetaldehyde-induced mitochondrial dysfunction sensitizes hepatocytes to oxidative damage. Cell Biol Toxicol. 2009;25:599–609. doi: 10.1007/s10565-008-9115-5. [DOI] [PubMed] [Google Scholar]
- 99. Lucey MR, Mathurin P, Morgan TR. Alcoholic hepatitis. N Engl J Med. 2009;360:2758–2769. doi: 10.1056/NEJMra0805786. [DOI] [PubMed] [Google Scholar]
- 100. Szabo G. Gut-liver axis in alcoholic liver disease. Gastroenterology. 2015;148:30–36. doi: 10.1053/j.gastro.2014.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Choumar A, Tarhuni A, Lettéron P, et al. Lipopolysaccharide-induced mitochondrial DNA depletion. Antioxid Redox Signal. 2011;15:2837–2854. doi: 10.1089/ars.2010.3713. [DOI] [PubMed] [Google Scholar]
- 102. Zeng T, Zhang CL, Xiao M, et al. Critical roles of Kupffer cells in the pathogenesis of alcoholic liver disease: from basic science to clinical trials. Front Immunol. 2016;7:538. doi: 10.3389/fimmu.2016.00538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Kim SJ, Khan M, Quan J, et al. Hepatitis B virus disrupts mitochondrial dynamics: induces fission and mitophagy to attenuate apoptosis. Plos Pathog. 2013;9:e1003722. doi: 10.1371/journal.ppat.1003722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Kim SJ, Syed GH, Khan M, et al. Hepatitis C virus triggers mitochondrial fission and attenuates apoptosis to promote viral persistence. Proc Natl Acad Sci U S A. 2014;111:6413–6418. doi: 10.1073/pnas.1321114111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Kim SJ, Syed GH, Siddiqui A. Hepatitis C virus induces the mitochondrial translocation of Parkin and subsequent mitophagy. Plos Pathog. 2013;9:e1003285. doi: 10.1371/journal.ppat.1003285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Bouchard MJ, Wang LH, Schneider RJ. Calcium signaling by HBx protein in hepatitis B virus DNA replication. Science. 2001;294:2376–2378. doi: 10.1126/science.294.5550.2376. [DOI] [PubMed] [Google Scholar]
- 107. Chen J, Siddiqui A. Hepatitis B virus X protein stimulates the mitochondrial translocation of Raf-1 via oxidative stress. J Virol. 2007;81:6757–6760. doi: 10.1128/JVI.00172-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Waris G, Huh KW, Siddiqui A. Mitochondrially associated hepatitis B virus X protein constitutively activates transcription factors STAT-3 and NF-kappa B via oxidative stress. Mol Cell Biol. 2001;21:7721–7730. doi: 10.1128/MCB.21.22.7721-7730.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Kapadia SB, Chisari FV. Hepatitis C virus RNA replication is regulated by host geranylgeranylation and fatty acids. Proc Natl Acad Sci U S A. 2005;102:2561–2566. doi: 10.1073/pnas.0409834102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Yang W, Hood BL, Chadwick SL, et al. Fatty acid synthase is up-regulated during hepatitis C virus infection and regulates hepatitis C virus entry and production. Hepatology. 2008;48:1396–1403. doi: 10.1002/hep.22508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Gong G, Waris G, Tanveer R, et al. Human hepatitis C virus NS5A protein alters intracellular calcium levels, induces oxidative stress, and activates STAT-3 and NF-kappa B. Proc Natl Acad Sci U S A. 2001;98:9599–9604. doi: 10.1073/pnas.171311298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. He G, Karin M. NF-κB and STAT3 - key players in liver inflammation and cancer. Cell Res. 2011;21:159–168. doi: 10.1038/cr.2010.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Andrade RJ, Aithal GP, Björnsson ES, et al. EASL clinical practice guidelines: drug-induced liver injury. J Hepatol. 2019;70:1222–1261. doi: 10.1016/j.jhep.2019.02.014. [DOI] [PubMed] [Google Scholar]
- 114. Jaeschke H, McGill MR, Ramachandran A. Oxidant stress, mitochondria, and cell death mechanisms in drug-induced liver injury: lessons learned from acetaminophen hepatotoxicity. Drug Metab Rev. 2012;44:88–106. doi: 10.3109/03602532.2011.602688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Prescott LF, Park J, Ballantyne A, et al. Treatment of paracetamol (acetaminophen) poisoning with N-acetylcysteine. Lancet. 1977;2:432–434. doi: 10.1016/s0140-6736(77)90612-2. [DOI] [PubMed] [Google Scholar]
- 116. Saito C, Zwingmann C, Jaeschke H. Novel mechanisms of protection against acetaminophen hepatotoxicity in mice by glutathione and N-acetylcysteine. Hepatology. 2010;51:246–254. doi: 10.1002/hep.23267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Harrison PM, Wendon JA, Gimson AE, et al. Improvement by acetylcysteine of hemodynamics and oxygen transport in fulminant hepatic failure. N Engl J Med. 1991;324:1852–1857. doi: 10.1056/NEJM199106273242604. [DOI] [PubMed] [Google Scholar]
- 118. Jaeschke H. Mitochondrial dysfunction as a mechanism of drug-induced hepatotoxicity: current understanding and future perspectives. J Clin Transl Res. 2018;4:75–100. doi: 10.18053/jctres.04.201801.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. European Association for the Study of the Liver. EASL clinical practice guidelines: management of cholestatic liver diseases. J Hepatol. 2009;51:237–267. doi: 10.1016/j.jhep.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 120. Kaffe ET, Rigopoulou EI, Koukoulis GK, et al. Oxidative stress and antioxidant status in patients with autoimmune liver diseases. Redox Rep. 2015;20:33–41. doi: 10.1179/1351000214Y.0000000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Ljubuncic P, Tanne Z, Bomzon A. Evidence of a systemic phenomenon for oxidative stress in cholestatic liver disease. Gut. 2000;47:710–716. doi: 10.1136/gut.47.5.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Liu HX, Keane R, Sheng L, et al. Implications of microbiota and bile acid in liver injury and regeneration. J Hepatol. 2015;63:1502–1510. doi: 10.1016/j.jhep.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Li Y, Tang R, Leung PSC, et al. Bile acids and intestinal microbiota in autoimmune cholestatic liver diseases. Autoimmun Rev. 2017;16:885–896. doi: 10.1016/j.autrev.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 124. Lemasters JJ, Qian T, He L, et al. Role of mitochondrial inner membrane permeabilization in necrotic cell death, apoptosis, and autophagy. Antioxid Redox Signal. 2002;4:769–781. doi: 10.1089/152308602760598918. [DOI] [PubMed] [Google Scholar]
- 125. Botla R, Spivey JR, Aguilar H, et al. Ursodeoxycholate (UDCA) inhibits the mitochondrial membrane permeability transition induced by glycochenodeoxycholate: a mechanism of UDCA cytoprotection. J Pharmacol Exp Ther. 1995;272:930–938. [PubMed] [Google Scholar]
- 126. Yu T, Wang L, Lee H, et al. Decreasing mitochondrial fission prevents cholestatic liver injury. J Biol Chem. 2014;289:34074–34088. doi: 10.1074/jbc.M114.588616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Okada K, Shoda J, Taguchi K, et al. Nrf2 counteracts cholestatic liver injury via stimulation of hepatic defense systems. Biochem Biophys Res Commun. 2009;389:431–436. doi: 10.1016/j.bbrc.2009.08.156. [DOI] [PubMed] [Google Scholar]
- 128. Okada K, Shoda J, Taguchi K, et al. Ursodeoxycholic acid stimulates Nrf2-mediated hepatocellular transport, detoxification, and antioxidative stress systems in mice. Am J Physiol Gastrointest Liver Physiol. 2008;295:G735–G747. doi: 10.1152/ajpgi.90321.2008. [DOI] [PubMed] [Google Scholar]
- 129. Sia D, Villanueva A, Friedman SL, et al. Liver cancer cell of origin, molecular class, and effects on patient prognosis. Gastroenterology. 2017;152:745–761. doi: 10.1053/j.gastro.2016.11.048. [DOI] [PubMed] [Google Scholar]
- 130. Chang CW, Lo JF, Wang XW. Roles of mitochondria in liver cancer stem cells. Differentiation. 2019;107:35–41. doi: 10.1016/j.diff.2019.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Li S, Hong M, Tan HY, et al. Insights into the role and interdependence of oxidative stress and inflammation in liver diseases. Oxid Med Cell Longev. 2016;2016:4234061. doi: 10.1155/2016/4234061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Marí M, Colell A, Morales A, et al. Redox control of liver function in health and disease. Antioxid Redox Signal. 2010;12:1295–1331. doi: 10.1089/ars.2009.2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Li HS, Zhou YN, Li L, et al. HIF-1α protects against oxidative stress by directly targeting mitochondria. Redox Biol. 2019;25:101109. doi: 10.1016/j.redox.2019.101109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Seehawer M, Heinzmann F, D’Artista L, et al. Necroptosis microenvironment directs lineage commitment in liver cancer. Nature. 2018;562:69–75. doi: 10.1038/s41586-018-0519-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Yuan D, Huang S, Berger E, et al. Kupffer cell-derived Tnf triggers cholangiocellular tumorigenesis through JNK due to chronic mitochondrial dysfunction and ROS. Cancer Cell. 2017;31:771–789.e6. doi: 10.1016/j.ccell.2017.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Trachootham D, Zhou Y, Zhang H, et al. Selective killing of oncogenically transformed cells through a ROS-mediated mechanism by beta-phenylethyl isothiocyanate. Cancer Cell. 2006;10:241–252. doi: 10.1016/j.ccr.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 137. Gillessen A, Schmidt HH. Silymarin as supportive treatment in liver diseases: a narrative review. Adv Ther. 2020;37:1279–1301. doi: 10.1007/s12325-020-01251-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Kwon D, Jun D, Kim Y. A novel mechanism involved in the enhancement of glutathione synthesis in liver by silymarin and its pharmacological significance. Planta Med. 2014;80:SL5. [Google Scholar]
- 139. Dehmlow C, Erhard J, de Groot H. Inhibition of Kupffer cell functions as an explanation for the hepatoprotective properties of silibinin. Hepatology. 1996;23:749–754. doi: 10.1053/jhep.1996.v23.pm0008666328. [DOI] [PubMed] [Google Scholar]
- 140. Vargas-Mendoza N, Morales-González Á, Morales-Martínez M, et al. Flavolignans from silymarin as Nrf2 bioactivators and their therapeutic applications. Biomedicines. 2020;8:122. doi: 10.3390/biomedicines8050122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Li CC, Hsiang CY, Wu SL, et al. Identification of novel mechanisms of silymarin on the carbon tetrachloride-induced liver fibrosis in mice by nuclear factor-κB bioluminescent imaging-guided transcriptomic analysis. Food Chem Toxicol. 2012;50:1568–1575. doi: 10.1016/j.fct.2012.02.025. [DOI] [PubMed] [Google Scholar]
- 142. Müzes G, Deák G, Láng I, et al. [Effect of silimarin (Legalon) therapy on the antioxidant defense mechanism and lipid peroxidation in alcoholic liver disease (double blind protocol)]. Orv Hetil. 1990;131:863–866. [PubMed] [Google Scholar]
- 143. Parés A, Planas R, Torres M, et al. Effects of silymarin in alcoholic patients with cirrhosis of the liver: results of a controlled, double-blind, randomized and multicenter trial. J Hepatol. 1998;28:615–621. doi: 10.1016/s0168-8278(98)80285-7. [DOI] [PubMed] [Google Scholar]
- 144. Perumpail B, Li A, John N, et al. The role of vitamin E in the treatment of NAFLD. Diseases. 2018;6:86. doi: 10.3390/diseases6040086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Singh N, Chander Narula S, Kumar Sharma R, et al. Vitamin E supplementation, superoxide dismutase status, and outcome of scaling and root planing in patients with chronic periodontitis: a randomized clinical trial. J Periodontol. 2014;85:242–249. doi: 10.1902/jop.2013.120727. [DOI] [PubMed] [Google Scholar]
- 146. Chung MY, Yeung SF, Park HJ, et al. Dietary α- and γ-tocopherol supplementation attenuates lipopolysaccharide-induced oxidative stress and inflammatory-related responses in an obese mouse model of nonalcoholic steatohepatitis. J Nutr Biochem. 2010;21:1200–1206. doi: 10.1016/j.jnutbio.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 147. He W, Xu Y, Ren X, et al. Vitamin E ameliorates lipid metabolism in mice with nonalcoholic fatty liver disease via Nrf2/CES1 signaling pathway. Dig Dis Sci. 2019;64:3182–3191. doi: 10.1007/s10620-019-05657-9. [DOI] [PubMed] [Google Scholar]
- 148. Sanyal AJ, Chalasani N, Kowdley KV, et al. NASH CRN. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675–1685. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005–2023. doi: 10.1002/hep.25762. [DOI] [PubMed] [Google Scholar]
- 150. Lavine JE, Schwimmer JB, Van Natta ML, et al. Nonalcoholic Steatohepatitis Clinical Research Network. Effect of vitamin E or metformin for treatment of nonalcoholic fatty liver disease in children and adolescents: the TONIC randomized controlled trial. JAMA. 2011;305:1659–1668. doi: 10.1001/jama.2011.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Bjelakovic G, Nikolova D, Gluud LL, et al. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. JAMA. 2007;297:842–857. doi: 10.1001/jama.297.8.842. [DOI] [PubMed] [Google Scholar]
- 152. Kelso GF, Porteous CM, Coulter CV, et al. Selective targeting of a redox-active ubiquinone to mitochondria within cells: antioxidant and antiapoptotic properties. J Biol Chem. 2001;276:4588–4596. doi: 10.1074/jbc.M009093200. [DOI] [PubMed] [Google Scholar]
- 153. Kelso GF, Porteous CM, Hughes G, et al. Prevention of mitochondrial oxidative damage using targeted antioxidants. Ann N Y Acad Sci. 2002;959:263–274. doi: 10.1111/j.1749-6632.2002.tb02098.x. [DOI] [PubMed] [Google Scholar]
- 154. Hu Q, Ren J, Li G, et al. The mitochondrially targeted antioxidant MitoQ protects the intestinal barrier by ameliorating mitochondrial DNA damage via the Nrf2/ARE signaling pathway. Cell Death Dis. 2018;9:403. doi: 10.1038/s41419-018-0436-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Fisicaro P, Barili V, Montanini B, et al. Targeting mitochondrial dysfunction can restore antiviral activity of exhausted HBV-specific CD8 T cells in chronic hepatitis B. Nat Med. 2017;23:327–336. doi: 10.1038/nm.4275. [DOI] [PubMed] [Google Scholar]
- 156. Xu F, Liu C, Zhou D, et al. TGF-β/SMAD pathway and its regulation in hepatic fibrosis. J Histochem Cytochem. 2016;64:157–167. doi: 10.1369/0022155415627681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Rehman H, Liu Q, Krishnasamy Y, et al. The mitochondria-targeted antioxidant MitoQ attenuates liver fibrosis in mice. Int J Physiol Pathophysiol Pharmacol. 2016;8:14–27. [PMC free article] [PubMed] [Google Scholar]
- 158. Lívero FA, Stolf AM, Dreifuss AA, et al. The FXR agonist 6ECDCA reduces hepatic steatosis and oxidative stress induced by ethanol and low-protein diet in mice. Chem Biol Interact. 2014;217:19–27. doi: 10.1016/j.cbi.2014.03.014. [DOI] [PubMed] [Google Scholar]
- 159. Wang YD, Chen WD, Li C, et al. Farnesoid X receptor antagonizes JNK signaling pathway in liver carcinogenesis by activating SOD3. Mol Endocrinol. 2015;29:322–331. doi: 10.1210/me.2014-1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Comeglio P, Cellai I, Mello T, et al. INT-767 prevents NASH and promotes visceral fat brown adipogenesis and mitochondrial function. J Endocrinol. 2018;238:107–127. doi: 10.1530/JOE-17-0557. [DOI] [PubMed] [Google Scholar]
- 161. Hirschfield GM, Mason A, Luketic V, et al. Efficacy of obeticholic acid in patients with primary biliary cirrhosis and inadequate response to ursodeoxycholic acid. Gastroenterology. 2015;148:751–61.e8. doi: 10.1053/j.gastro.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 162. Neuschwander-Tetri BA, Loomba R, Sanyal AJ, et al. NASH Clinical Research Network. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet. 2015;385:956–965. doi: 10.1016/S0140-6736(14)61933-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Li S, Li H, Xu X, et al. Nanocarrier-mediated antioxidant delivery for liver diseases. Theranostics. 2020;10:1262–1280. doi: 10.7150/thno.38834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Reddy MK, Labhasetwar V. Nanoparticle-mediated delivery of superoxide dismutase to the brain: an effective strategy to reduce ischemia-reperfusion injury. Faseb J. 2009;23:1384–1395. doi: 10.1096/fj.08-116947. [DOI] [PubMed] [Google Scholar]
- 165. Xue TM, Tao LD, Zhang J, et al. Intestinal ischemic preconditioning reduces liver ischemia reperfusion injury in rats. Mol Med Rep. 2016;13:2511–2517. doi: 10.3892/mmr.2016.4817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Robertson FP, Magill LJ, Wright GP, et al. A systematic review and meta-analysis of donor ischaemic preconditioning in liver transplantation. Transpl Int. 2016;29:1147–1154. doi: 10.1111/tri.12849. [DOI] [PubMed] [Google Scholar]
- 167. Cescon M, Carini R, Grazi G, et al. Variable activation of phosphoinositide 3-kinase influences the response of liver grafts to ischemic preconditioning. J Hepatol. 2009;50:937–947. doi: 10.1016/j.jhep.2008.11.016. [DOI] [PubMed] [Google Scholar]
- 168. Amador A, Grande L, Martí J, et al. Ischemic pre-conditioning in deceased donor liver transplantation: a prospective randomized clinical trial. Am J Transplant. 2007;7:2180–2189. doi: 10.1111/j.1600-6143.2007.01914.x. [DOI] [PubMed] [Google Scholar]
- 169. Desai KK, Dikdan GS, Shareef A, et al. Ischemic preconditioning of the liver: a few perspectives from the bench to bedside translation. Liver Transpl. 2008;14:1569–1577. doi: 10.1002/lt.21630. [DOI] [PubMed] [Google Scholar]
- 170. Zhao DF, Dong Q, Zhang T. Effects of static cold storage and hypothermic machine perfusion on oxidative stress factors, adhesion molecules, and zinc finger transcription factor proteins before and after liver transplantation. Ann Transplant. 2017;22:96–100. doi: 10.12659/aot.901897. [DOI] [PubMed] [Google Scholar]
- 171. Dutkowski P, Guarrera JV, de Jonge J, et al. Evolving trends in machine perfusion for liver transplantation. Gastroenterology. 2019;156:1542–1547. doi: 10.1053/j.gastro.2018.12.037. [DOI] [PubMed] [Google Scholar]
- 172. Boteon YL, Laing RW, Schlegel A, et al. The impact on the bioenergetic status and oxidative-mediated tissue injury of a combined protocol of hypothermic and normothermic machine perfusion using an acellular haemoglobin-based oxygen carrier: the cold-to-warm machine perfusion of the liver. PLoS One. 2019;14:e0224066. doi: 10.1371/journal.pone.0224066. [DOI] [PMC free article] [PubMed] [Google Scholar]