Abstract
Purpose
To investigate the safety and efficacy of micropulse (MP) macular laser in combination with intravitreal aflibercept for the treatment of center-involved diabetic macular edema (CI-DME).
Methods
A single-blind prospective randomized controlled pilot trial was performed. In total, 30 eyes of 30 patients with CI-DME and best corrected visual acuity (BCVA) between, and including, 20/30 and 20/400 were enrolled. Enrolled eyes were randomized to 2 groups. Group 1 received intravitreal aflibercept injections (IVT-AFL) with sham laser. Group 2 received IVT-AFL with MP laser. Both groups were followed every 4 weeks for 48 weeks and retreatment was performed on pro re nata basis according to preset criteria. The main outcome measure was the average number of intravitreal injections for each group at 48 weeks. Secondary outcome measures included changes in BCVA and central macular thickness (CMT) at 24 and 48 weeks.
Results
The average number of intravitreal injections at 48 weeks was similar between the groups (8.5±3.3 in Group 1 vs 7.9±3.6 in Group 2, p=0.61). After 48 weeks, both groups demonstrated an improvement in BCVA and CMT. However, the difference in improvement between the groups was not statistically significant (p=0.18 for BCVA and p=0.57 for CMT).
Conclusion
Intravitreal injections of aflibercept led to improvements in BCVA and CMT at 24 and 48 weeks. Addition of MP laser to eyes in group 2 did not offer additional benefit in reducing treatment burden or improving CMT. Eyes that received MP laser showed a numerically greater improvement in BCVA, although this was not statistically significant.
Clinicaltrials.gov Identifier
NCT03143192 March 8, 2017.
Keywords: anti-VEGF, diabetic macular edema, micropulse laser, visual acuity
Introduction
Diabetic macular edema (DME) is the leading cause of visual decline in patients with diabetes mellitus.1 Although focal/grid macular laser had long been considered the standard treatment for DME in the past, the focus of current treatments for DME revolves around mitigating the effect of vascular endothelial growth factor (VEGF). More recently, intravitreal injections of anti-VEGF agents, either as monotherapy or in combination with focal/grid macular laser, have proven to be superior for the treatment of DME compared to focal/grid laser alone.2–5 The 5-year results of the Diabetic Retinopathy Clinical Research (DRCR) Network protocol I suggested that in eyes receiving ranibizumab for center-involved DME and vision impairment, deferring focal/grid laser treatment may be more favorable than prompt laser treatment at baseline.6
One possible alternative to the conventional focal/grid laser is the micropulse (MP) diode laser, first reported by Friberg and Karatza in 1997 for the treatment of macular disease.7 Micropulse, or sub-threshold, macular laser involves applying the laser only in a fraction of time (typically 10%-15% duty cycle) within very small (typically 10–20 ms) pockets of energy.8 Unlike traditional focal/grid macular laser, the micropulse method of laser delivery does not leave any visible burns on the retina. Although the mechanism of action of micropulse laser is not quite understood, it is thought to work via stimulation of retinal pigment epithelium (RPE) to absorb the extra retinal fluid and thereby reduce the macular edema. Since it is a non-destructive method of delivery, micropulse laser is thought to have no negative thermal effect on neural retina, RPE or choroid.9
Anti-VEGF medications have changed the treatment paradigm of diabetic macular edema from a laser-based approach to an injection-based one. Even though MP laser has been shown to have favorable results as monotherapy compared to traditional focal laser, with anti-VEGF medications becoming the standard of care, the role of MP laser for the treatment of DME remains unclear.9 MP laser does not have the undesired side effect of leaving laser scars on the macula, warranting further exploration of its use in combination therapy. Our study aimed to determine if prompt MP laser in addition to anti-VEGF injections for the treatment of DME may lead to decreased treatment burden and/or improved visual outcomes without the undesired side effect of macular scarring.
Materials and Methods
Ethics Approval
Institutional Review Board/Ethics Committee approval was obtained from the University of Toronto, and all patients provided informed, written consent before their participation in the clinical trial. The conducted research adhered to the tenets of the Declaration of Helsinki. Finally, all adverse events and safety information were collected and reported to Health Canada and Bayer Inc. in accordance with the Food and Drug Regulations [DiVision 1 (C.01.016 and C.01.017) and Division 8 (C.08.007(h) and C.08.008(c)].
Patient Enrollment
The study (registered at ClinicalTrials.gov with identifier: NCT03143192) was a single-blind, prospective, randomized controlled pilot trial at the Toronto Retina Institute and Mississauga Retina Institute. To be eligible for the study, patients needed to be 18 years of age or older, be diagnosed with Type I or Type II diabetes mellitus, have centre-involved DME with a central macular thickness (CMT) of ≥310 µm on the spectral-domain Optical Coherence Tomography (OCT) measurement, and have a best corrected visual acuity (BCVA) score between, and including, 20/30 (0.18 logMAR) and 20/400 (1.30 logMAR) in the study eye. The main exclusion criteria included patients suffering from other causes of macular edema, a history of ocular surgery in the preceding 6 months, a history of DME treatment in the preceding 4 months, or any history of panretinal photocoagulation. A total of 30 patients were recruited and randomly divided into two groups of 15 by study coordinators in a 1:1 allocation ratio; the randomization was performed using random.org’s coin flip (see Table 1 for baseline characteristics). In addition to patients, those testing visual acuity and OCT were masked to the treatment group by their lack of access to patients’ study files, whereas the coordinators and investigators remained unmasked. Participants were only allowed to enroll one eye in the study. If both eyes were eligible and one of the eyes had never received treatment for DME, that eye was enrolled. If the non-study eye developed DME and required intravitreal anti-VEGF for treatment, 2.0 mg aflibercept was used for that eye. The investigator could have also chosen MP or traditional/focal grid laser for the non-study eye.
Table 1.
Baseline Demographic Characteristics of Both Treatment Groups
| Parameter | Group 1 (n=15) | Group 2 (n=15) | Overall (n=30) |
|---|---|---|---|
| Mean Age (years) | 58.8 | 59.8 | 59.3 |
| Range of Ages (years) | 37–72 | 37–77 | 37–77 |
| SD of Ages (years) | 9.28 | 9.47 | 9.23 |
| Number of Males | 7 | 10 | 17 |
| Number of Females | 8 | 5 | 13 |
| Number of OD Eyes Enrolled | 9 | 9 | 18 |
| Number of OS Eyes Enrolled | 6 | 6 | 12 |
Abbreviations: SD, standard deviation; OD, oculus dexter; OS, oculus sinister.
Treatment and Follow-Up Schedule
At baseline and at each follow-up session, all eyes in Group 1 were scheduled to receive one injection of 2.0 mg of intravitreal aflibercept (IVT-AFL) with one session of sham laser, while all eyes in group 2 received one injection of 2.0 mg of IVT-AFL with one session of MP laser on the same day (see Table 2 for MP specifications). Patients were followed every 4 weeks (± 1 week) up to and including week 48. IVT-AFL was allowed to be administered every 4 weeks (± 1 week), but the time between successive MP treatments was set at a minimum of 12 weeks (see Table 3).
Table 2.
Specifications of the Micropulse Laser Used for Treating Group 2
| Laser Aspect | Specification |
|---|---|
| Size of laser spot | 200 μm |
| Duration of laser pockets | 20 ms |
| Micropulse duty cycle | 10% |
| Laser energy | Micropulsed laser will be used to create light (barely visible) test burns outside the macular area (starting at 100 mw with upward titration). The laser will then be decreased to 90% of that of the test burns. The final laser spots should not be visible. |
| Delivery pattern | 3 x 3 pattern mode (confluent spots) over the entire macular area including the foveal centre. Non-pattern repeat mode with overlapping burns is also permitted. |
| Wavelength | 532 nm |
| Manufacturer | Quantel (SupraScan 532) |
Table 3.
Follow-Up and Treatment Schedule for Both Groups of Patients
| Week # | 0 | 4 | 8 | 12 | 16 | 20 | 24 | 28 | 32 | 36 | 40 | 44 | 48 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BCVA, exam, FP, FAF, OCT; study eye | + | + | + | + | + | + | + | + | + | + | + | + | + |
| BCVA, exam, FP, FAF, OCT; non-study eye | + | + | + | ||||||||||
| Aflibercept Injection | + | ± | ± | ± | ± | ± | ± | ± | ± | ± | ± | ± | ± |
| MP/Sham laser | + | ± | ± | ± | ± | ± | ± | ± | ± | ± | ± |
Abbreviations: BCVA, best corrected visual acuity; FP, fundus photography; FAF, fundus autofluorescence; OCT, optical coherence tomography; MP, micropulse.
During each study visit, IVT-AFL was given if the following re-treatment criteria were met: all eyes continued to receive treatment as long as there was a change (improving or worsening) of visual acuity (1 Snellen line) or a 10% change in OCT central macular thickness (improving or worsening) or a change in the appearance of cystic changes in OCT from the previous visit. The first time that no change was detected in visual acuity or OCT, another treatment was still administered. The second time that no change was detected, treatment was deferred if the macular edema had resolved. However, if the macular edema had still not resolved, treatment continued to be given every 4 weeks. At intervals no shorter than 12 weeks, study eyes also received sham laser (group 1) or MP laser (group 2) based on the same aforementioned re-treatment criteria. All intravitreal injections were performed according to investigators’ routine sterile technique. Investigators were allowed to use pre- or post-injection antibiotics and sterile eyelid speculum at their discretion. They were also given the option to perform anterior chamber paracentesis for patients with a higher risk of intraocular pressure elevation.
The primary outcome measure was the number of intravitreal injections for each group at 48 weeks. Secondary outcome measures included the change in visual acuity from baseline to 24 and 48 weeks (adjusted for baseline visual acuity) and the change in OCT central macular thickness and volume at 24 and 48 weeks, as well as the proportion of eyes with 2 or 3 lines of visual gain or loss at 24 and 48 weeks. BCVA was determined using the participants’ own corrective lenses, if they were current, with verification by pinhole correction. When refraction was not current, the study eye was refracted at the baseline visit and the visual acuity was obtained using the updated refraction at subsequent visits. Fundus photographs and autofluorescent images were taken alongside regular ocular exams during routine examination. In addition to these main efficacy outcomes, investigators recorded any adverse outcomes that were systemic, injection-related, or laser-related.
Statistical Methods and Analysis
Since this is a pilot study, an arbitrary sample size of 15 eyes per group (30 eyes in total) was chosen as the sample size. Paired Student’s t-test was used to compare the results between the two groups.
Results
Between March and October 2017, 30 eyes of 30 patients were enrolled in the trial. A total of 27/30 patients completed follow-up until 48 weeks; two patients passed away before the completion of the trial and one was lost to follow-up. There were no adverse safety events related to any of the interventions.
The primary outcome measure (number of intravitreal injections at 48 weeks) was similar between the groups (8.5 ± 3.3 in group 1 vs 7.9 ± 3.6 in group 2, p= 0.61). The number of sham treatments for group 1 was also similar to the number of MP treatments in group 2 (3.2 ± 1.3 in group 1 vs 3.4 ± 1.4 in group 2, p = 0.69).
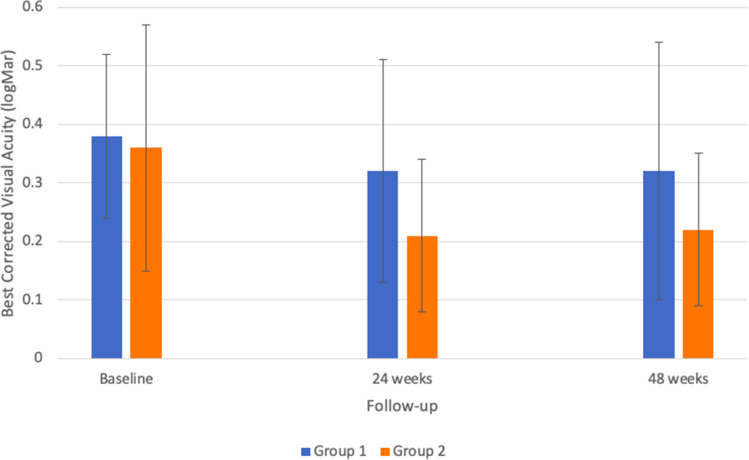
At 24 weeks, group 1 showed a trend in improvement of BCVA (from 0.38 ± 0.14 logMAR (20/48 Snellen) to 0.32 ± 0.19 logMAR (20/42 Snellen), p=0.08) and group 2 demonstrated a larger and significant change in BCVA (from 0.36 ± 0.21 logMAR (20/46 Snellen) to 0.21 ± 0.13 logMAR (20/32 Snellen), p= 0.003). However, this difference between the groups was not statistically significant, p=0.10). When comparing baseline measurements to final measurements at 48 weeks, the average BCVA of group 1 changed from 0.38 ± 0.14 logMAR (20/48 Snellen) to 0.32 ± 0.22 logMAR (20/42 Snellen), p=0.07. For group 2, the average BCVA changed from 0.36 ± 0.21 logMAR (20/46 Snellen) to 0.22 ± 0.13 logMAR (20/33 Snellen), p=0.01. Once again, this difference between the groups was not statistically significant (p=0.18). For both groups, the largest improvements in BCVA were seen in the first 24 weeks (−0.06 for group 1 and −0.15 for group 2). Between 24 and 48 weeks, the difference in average BCVA for group 1 was only 0.00 logMAR, whereas group 2 actually demonstrated an increase of +0.01 logMAR (see Figure 1 for a graph comparing the two groups’ BCVA over time).
Figure 1.
Average best corrected visual acuity of treated eyes in both groups.
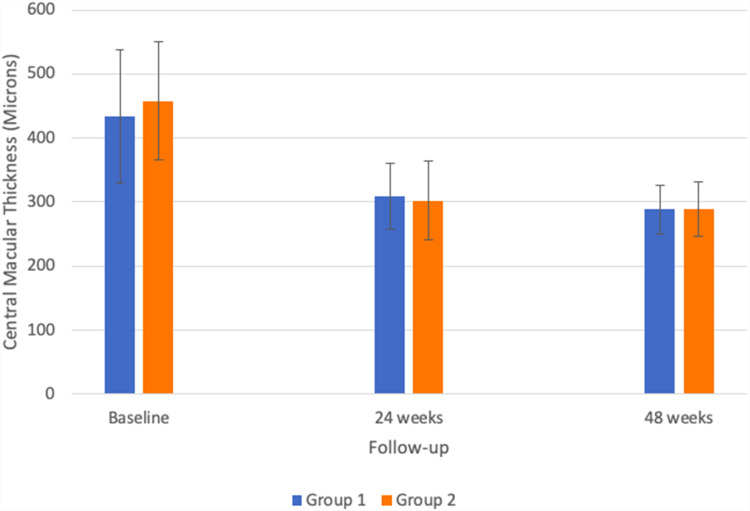
Both groups showed a significant improvement in central macular thickness: group 1 improved from 433.4 ± 103.5 microns at baseline to 309.3 ± 52.0 microns at 24 weeks (P< 0.001) and 288.3 ± 38.2 microns at 48 weeks (P< 0.001). Group 2 also improved from 457.8 ± 92.8 microns at baseline to 302.0 ± 61.5 microns at 24 weeks (P< 0.001) and 289.5 ± 42.7 microns at 48 weeks (p<0.001) (see Figure 2 for a graph comparing the two groups’ CMT over time). The difference in CMT improvement between the groups was not statistically significant at either 24 weeks (p=0.39) or 48 weeks (p= 0.57).
Figure 2.
Average central macular thickness of treated eyes in both groups.
Discussion
In this prospective randomized pilot trial, we investigated the specific combination of micropulse laser with an anti-VEGF agent (aflibercept) for the treatment of DME. Even though MP laser is thought to be effective in reducing macular edema, its efficacy especially in combination with anti-VEGF agents, has not been well established by large clinical studies. Currently, there are a few small studies that highlight the potential of MP laser as a treatment option. A small prospective study involving 23 eyes compared micropulse diode laser (810 nm) and conventional argon laser (514 nm) for the treatment of clinically significant macular edema (CSME) and showed that visual acuity remained stable in both groups.10 The authors suggested that micropulse laser may play a stabilizing role in CSME and may be a good option in combination with the conventional argon laser. Another prospective study on 53 eyes with DME showed that either 577-nm MP or 810-nm MP, combined with direct photocoagulation for microaneurysm closure, was effective in reducing DME, maintaining visual acuity and reducing the additional treatment rate within 12 months.11 Mansouri et al’s prospective study on 63 eyes with DME that received MP laser in combination with rescue bevacizumab concluded that the severity of edema can influence the effects of MP laser.12 The authors suggested that MP monotherapy is safe and effective in treating edema of mild to moderate severity (CMT < 400µm), while they found no significant improvement in CMT or BCVA in eyes with severe baseline edema (CMT > 400 µm). Finally, other smaller retrospective studies looking at MP laser monotherapy for the treatment of DME have also suggested favorable safety as well as efficacy in improving vision and OCT macular thickness.13,14
Some studies have also shown that MP monotherapy without anti-VEGF supplementation may not be enough to adequately treat DME. In the study by Valera-Cornejo et al, although a small decrease in CMT was demonstrated in diabetic eyes receiving MP monotherapy, no significant improvements in BCVA were observed over the course of 3 months.15 Similar findings were reported by Passos et al, who observed only small improvements in BCVA and CMT in eyes receiving MP monotherapy, suggesting the need for combined treatment in most patients.16
In our study, we found that intravitreal injections of aflibercept led to improvements in BCVA and CMT at 24- and 48-week follow-ups, which was expected given the fact that intravitreal anti-VEGF injections are currently the gold standard treatment for DME. The addition of MP laser to eyes in group 2 did not offer additional benefit in reducing the treatment burden or improvement of CMT measurements at 24 or 48 weeks. However, eyes that received MP laser showed a strong trend towards greater BCVA improvement. Interestingly enough, the greatest improvements in BCVA and CMT were seen in the first 24 weeks, as opposed to weeks 24–48. This observation may suggest that the peak therapeutic effect for patients receiving IVT-AFL could be attained early in the course of treatment.
A limitation of this pilot trial is its small sample size. Until larger studies are completed, it remains unclear whether the lack of statistical significance between the two treatment groups can be attributed to low treatment efficacy or the small sample size. Another potential limitation is the lack of data after the 48-week follow-up period. The primary outcome measure, the number of intravitreal injections given to each group at 48 weeks, was based on the expectation that one group might no longer need further treatment by the final follow-up due to resolution of their macular edema. However, at 48 weeks, the macular edema of many patients in both groups did not fully resolve. This suggests that a longer follow-up period might be needed before differences in the disease resolution of both groups are fully revealed. In addition, there is a large variability of settings of micropulse laser among different studies. In this current study, a laser was used that had a wavelength of 532 nm, a laser pocket duration of 20 ms, and an MP duty cycle of 10%. Other appropriate lasers that could have been used include ones with wavelengths of 810 nm and 577 nm with duty cycles varying between 5–15%.8 With such high setting variability, it is currently unclear which specifications can provide patients with the greatest therapeutic effect.
In conclusion, our study found that the addition of MP laser to eyes receiving anti-VEGF therapy for DME did not reduce treatment burden or improve CMT. Eyes that received MP laser showed a numerically greater improvement in BCVA, although this was not statistically significant. As such, clinicians should exercise caution before choosing to provide MP laser as supplemental therapy, as it may not provide the desired added benefit for patients. Further research with larger sample sizes, longer follow-up times, and laser setting variability is recommended to explore the potential added benefits of combining MP laser with standard anti-VEGF therapy.
Funding Statement
This work was supported by Bayer Inc. The funding organization had no role in the design or conduct of this research.
Abbreviations
MP, micropulse; DME, diabetic macular edema; CI-DME, centre-involved diabetic macular edema; IVT-AFL, intravitreal aflibercept injections; BCVA, best corrected visual acuity; CMT, central macular thickness; RPE, retinal pigment epithelium; VEGF, vascular endothelial growth factor; OCT, optical coherence tomography; CSME, clinically significant macular edema.
Data Sharing Statement
Individual de-identified participant data will be available upon request by the corresponding author, in addition to the study protocol immediately following publication. No end date. Anyone can access the data for research purposes.
Ethics Approval
Institutional Review Board/Ethics Committee approval was obtained from the University of Toronto, and all patients provided informed, written consent before their participation in the clinical trial. The conducted research adhered to the tenets of the Declaration of Helsinki. Finally, all adverse events and safety information were collected and reported to Health Canada and Bayer Inc. in accordance with the Food and Drug Regulations [Division 1 (C.01.016 and C.01.017) and Division 8 (C.08.007(h) and C.08.008(c)].
Consent for Publication
Any figures accompanying this manuscript have consent for publication.
Author Contributions
Keyvan Koushan was involved in study conception and data collection. All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors have no conflicts of interest to declare in this work.
References
- 1.Romero-Aroca P. Managing diabetic macular edema: the leading cause of diabetes blindness. World J Diabetes. 2011;2:98. doi: 10.4239/wjd.v2.i6.98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Korobelnik JF, Do DV, Schmidt-Erfurth U, et al. Intravitreal aflibercept for diabetic macular edema. Ophthalmology. 2014;121:2247–2254. doi: 10.1016/j.ophtha.2014.05.006 [DOI] [PubMed] [Google Scholar]
- 3.Brown DM, Nguyen QD, Marcus DM, et al. Long-term outcomes of ranibizumab therapy for diabetic macular edema: the 36-month results from two Phase III trials: RISE and RIDE. Ophthalmology. 2013;120:2013–2022. doi: 10.1016/j.ophtha.2013.02.034 [DOI] [PubMed] [Google Scholar]
- 4.Schmidt-Erfurth U, Lang GE, Holz FG, et al. Three-year outcomes of individualized ranibizumab treatment in patients with diabetic macular edema: the RESTORE extension study. Ophthalmology. 2014;121:1045–1053. doi: 10.1016/j.ophtha.2013.11.041 [DOI] [PubMed] [Google Scholar]
- 5.Massin P, Bandello F, Garweg JG, et al. Safety and efficacy of ranibizumab in diabetic macular edema (RESOLVE study): a 12-month, randomized, controlled, double-masked, multicenter Phase II study. Diabetes Care. 2010;33:2399–2405. doi: 10.2337/dc10-0493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elman MJ, Ayala A, Bressler NM, et al. Intravitreal ranibizumab for diabetic macular edema with prompt versus deferred laser treatment: 5-year randomized trial results. Ophthalmology. 2015;122:375–381. doi: 10.1016/j.ophtha.2014.08.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Friberg TR, Karatza EC. Treatment of macular disease using a micropulsed and continuous wave 810-nm diode laser. Ophthalmology. 1997;104:2030–2038. doi: 10.1016/S0161-6420(97)30061-X [DOI] [PubMed] [Google Scholar]
- 8.Scholz P, Altay L, Fauser S. A Review of Subthreshold Micropulse Laser for Treatment of Macular Disorders. Adv Ther. 2017;34:1528–1555. doi: 10.1007/s12325-017-0559-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sivaprasad S, Elagouz M, McHugh D, Shona O, Dorin G. Micropulsed Diode Laser Therapy: evolution and Clinical Applications. Surv Ophthalmol. 2010;55:516–530. doi: 10.1016/j.survophthal.2010.02.005 [DOI] [PubMed] [Google Scholar]
- 10.Laursen ML, Moeller F, Sander B, Sjoelie AK. Subthreshold micropulse diode laser treatment in diabetic macular oedema. Br J Ophthalmol. 2004;88:1173–1179. doi: 10.1136/bjo.2003.040949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Inagaki K, Ohkoshi K, Ohde S, Deshpande GA, Ebihara N, Murakami A. Comparative efficacy of pure yellow (577-nm) and 810-nm subthreshold micropulse laser photocoagulation combined with yellow (561–577-nm) direct photocoagulation for diabetic macular edema. Jpn J Ophthalmol. 2015;59:21–28. doi: 10.1007/s10384-014-0361-1 [DOI] [PubMed] [Google Scholar]
- 12.Mansouri A, Sampat KM, Malik KJ, Steiner JN, Glaser BM. Efficacy of subthreshold micropulse laser in the treatment of diabetic macular edema is influenced by pre-treatment central foveal thickness. Eye. 2014;28:1418–1424. doi: 10.1038/eye.2014.264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kwon YH, Lee DK, Kwon OW. The short-term efficacy of subthreshold Micropulse yellow (577-nm) laser photocoagulation for diabetic macular edema. Korean J Ophthalmol. 2014;28:379. doi: 10.3341/kjo.2014.28.5.379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luttrull JK, Sinclair SH. Safety of transfoveal subthreshold diode micropulse laser for fovea-involving diabetic macular edema in eyes with good visual acuity. Retina. 2014;34:2010–2020. doi: 10.1097/IAE.0000000000000177 [DOI] [PubMed] [Google Scholar]
- 15.Valera-Cornejo DA, García-Roa M, Quiroz-Mendoza J, et al. Micropulse laser in patients with refractory and treatment-naïve center–involved diabetic macular edema: short terms visual and anatomic outcomes. Ther Adv Ophthalmol. 2021;13:251584142097911. doi: 10.1177/2515841420979112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Passos RM, Malerbi FK, Rocha M, Maia M, Farah ME. Real-life outcomes of subthreshold laser therapy for diabetic macular edema. Int J Retin Vitr. 2021;7. doi: 10.1186/s40942-020-00268-3. [DOI] [PMC free article] [PubMed] [Google Scholar]