Summary
Background
Many patients report persistent symptoms after COVID-19. Our aim was to determine whether some of these symptoms were more associated with past SARS-CoV-2 infection compared to other conditions.
Methods
This prospective survey was nested in CONSTANCES, a randomly selected French population-based cohort, started in 2012. All participants being followed-up by internet completed 2 questionnaires during the first wave of the pandemic focusing on the acute symptoms of their COVID-19-like illness. Serological tests for SARS-CoV-2 were then performed (May-Nov 2020). Between December 2020 and January 2021, participants completed a third questionnaire about symptoms that had lasted more than 2 months. Participants were classified into four groups according to both European Center for Diseases Control (ECDC) criteria for COVID-19 (ECDC+ or ECDC-) and serological SARS-CoV-2 test results (Sero+ or Sero-). To compare the risk of each persistent symptom among the groups, logistic regression models were adjusted for age, sex, educational level, comorbidities, and the number of acute symptoms declared during the first wave of the epidemic. A mediation analysis was performed to estimate the direct effect of the infection on persistent symptoms and its indirect effect via the initial clinical presentation.
Findings
The analysis was performed in 25,910 participants. There was a higher risk of persistent dysgeusia/anosmia, dyspnea and asthenia in the ECDC+/Sero+ group than in the ECDC+/Sero- group (OR: 6.83 [4.47–10.42], 1.69 [1.07–2.6] and 1.48 [1.05–2.07], respectively). Abdominal pain, sensory symptoms or sleep disorders were at lower risk in the ECDC+/Sero+ group than in the ECDC+/Sero- group (0.51 [0.24–0.96], 0.40 [0.16–0.85], and 0.69 [0.49–0.95], respectively). The mediation analysis revealed that the association of the serological test results with each symptom was mainly mediated by ECDC symptoms (proportion mediated range 50–107%).
Conclusion
A greater risk of persistent dysgeusia/anosmia, dyspnea and asthenia was observed in SARS-CoV-2 infected people. The initial clinical presentation substantially drives the association of positive serological test results with persistent symptoms.
Funding
French National Research Agency
Keywords: COVID-19, Long-covid, Post-infectious symptoms, Persistent symptoms, Populationbased study, Constances, Post-covid condition
Research in context.
Evidence before this study
To identify existing studies on post-acute symptoms due to SARS-CoV-2 infection, we searched PubMed from January, 2020 to December 20, 2021. We used the following search string: (COVID-19[title/abstract] OR SARS-CoV-2[title/abstract]) AND ((long-term[title/abstract] AND complications[title/abstract]) OR ((persistent[title/abstract] OR persistence[title/abstract]) AND symptoms[title/abstract])) OR (long-covid [title/abstract]) OR (long-hauler [title/abstract]) OR (post-covid condition [title/abstract])). We took into account observational studies that described cohorts of outpatients or studies that compared persistent symptoms between individuals with COVID-19 and with other conditions.
Most of the literature described cohorts of patients with no comparison groups. Other studies comparing patients with COVID-19 and uninfected individuals were performed in populations that had access to testing during the first wave of the pandemic, thus creating a selection bias. These studies suggested associations between persistent symptoms and a history of COVID-19, but may have been confounded by severity of the acute illness, age, sex, or comorbidities. In addition, there were no studies comparing individuals from the general population who received serological tests or PCR in a research context.
Added value of this study
This population-based cohort study included 25910 individuals divided in 4 groups defined by the symptoms they presented during the first wave of the pandemic and by their serological status for SARS-CoV-2 infection. All individuals received serological tests and prospectively completed questionnaires. We compared the risk of presenting with several persistent symptoms 7 to 8 months after the first wave of the pandemic in France. We used adjusted logistic regression models to compare the risk of having each of the symptoms between the groups. This showed that individuals in all groups had a similar risk of having at least one symptom. However, dysgeusia/anosmia, dyspnea, and asthenia were more frequent in individuals with a history of COVID-19. Some symptoms were more frequent in other groups (sleep disorders, abdominal pain, sensory symptoms).
Implications of the available evidence
This study shows that persistent symptoms are common in the general population, irrespective of COVID-19 history. Individuals with a history of COVID-19 have however an increased risk of persistent dysgeusia/anosmia, dyspnea and asthenia. Acute clinical presentation is a strong determinant of these long-term symptoms. This should prompt policy makers to develop holistic management strategies for patients with post-COVID-19 conditions as well as for other patients with similar complaints. Promoting therapeutic and preventive strategies that reduce the symptoms of the acute phase of the disease could have an effect on the post-COVID symptoms.
Alt-text: Unlabelled box
Introduction
Post-covid-19 condition is potentially an emerging issue in the Covid-19 pandemic. Months after the acute illness caused by SARS-CoV-2 infection, clinical symptoms may persist whether or not the acute phase required hospitalization.1, 2, 3, 4 The main persistent symptoms are asthenia, pain, dyspnea, cognitive complaints, and dysgeusia/anosmia.5 Other symptoms such as neurological or digestive complaints have also been described.6 The pathophysiology of these persistent symptoms is poorly understood. Except for dysgeusia/anosmia, these persistent symptoms are not specific to SARS-CoV-2 infection but may be related to other conditions, or the consequence of changes in behavior, wellbeing, or access to care due to the pandemic. In this context, characterizing the phenotypes represented by the association of these persistent symptoms in persons with (versus without) SARS-CoV-2 past infection has rarely been studied. Therefore, to better understand the persistent symptoms after COVID-19, it is important to determine whether some of these symptoms are more specifically associated with SARS-CoV-2 infection than with other conditions.7
The aim of this study using extensive data from a large population-based cohort study was to compare the risk of each persistent symptom depending on serological status for SARS-CoV-2 and depending on the history of acute symptoms during the first pandemic wave.
Methods
Design and participants
This survey is nested in the French CONSTANCES population-based cohort8 which includes approximately 200,000 adults aged 18 to 69 at inception. Since 2012, participants have been selected from the French adult population affiliated with the National Fund for Health Insurance according to a random sampling scheme stratified for age, gender, socioeconomic status and region of France to be representative of the source population, and have been followed by repeated yearly questionnaires and linked by administrative databases. CONSTANCES is also one of the three adult cohorts that is involved in the SAPRIS-SERO ("Santé, Pratiques, Relations et Inégalités Sociales en population générale pendant la crise COVID-19" - Serology) survey, whose aim is to quantify the cumulative incidence of SARS-CoV-2 infection in the French population using the dried blood spot (DBS) test for anti-SARS-CoV-2 antibodies.9 In this specific COVID-19 survey, 66,848 CONSTANCES participants being followed-up by internet were asked to complete two self-administered e-questionnaires between April 6, 2020, and June 15, 2020. Variables included socio-demographics, comorbidities, COVID-19 diagnosis, and a detailed collection of 12 acute symptoms present in the participants during the 15 days before each questionnaire. These two questionnaires were used to classify participants according to whether or not they experienced a COVID-19-like illness during the first wave of the epidemic, but also to evaluate the number of acute symptoms experienced during the initial episode. Between May 4, 2020, and November 30, 2020, DBS was also collected. Then, between December 2020 and February 2021, a third follow-up internet questionnaire including questions on clinical symptoms was completed by the participants. Overall, 35,852 participants completed the first 2 questionnaires, had a serology performed and were eligible for this survey on persistent symptoms.
Ethical approval and written or electronic informed consent were obtained from each participant before enrolment in the original cohort. The SAPRIS-SERO study was approved by the Sud-Mediterranée III ethics committee (approval #20.04.22.74247) and electronic informed consent was obtained from all participants for DBS testing.
Classification of participants
Participants were considered “Sero+” or “Sero-” if the serological test was positive for SARS-CoV-2 infection. COVID-19-like illness during the first pandemic wave was defined according to the European Centre for Disease Prevention and Control (ECDC) case definition. Participants were considered “ECDC+” if they reported at least one of the following symptoms that lasted at least 3 days: dysgeusia/anosmia, dyspnea, fever, and cough in at least one of the two first questionnaires.10 Other acute symptoms included headache, unusual asthenia, myalgia, joint pain, runny nose, nausea/vomiting, diarrhea and skin problems.
The seropositive and seronegative status was defined by IgG results against the spike protein of the virus (i.e., optical density ratio ≥1.1 and <0.7, respectively) using the (Euroimmun®, Lübeck, Germany) test.9 In case of indeterminate results (i.e., optical density ratio ≥0.7 and <1.1), an in-house microneutralization assay to detect neutralizing anti-SARS-CoV-2 antibodies was performed. Participants with neutralizing antibody titers ≥40 were also considered to be seropositive. Most seropositive participants were assumed to have been infected during the first wave of the pandemic, between February 2020 and May 2020.
We selected all seropositive and seronegative participants who completed the follow-up questionnaire excluding participants who reported a diagnosis of COVID-19 after the serological test. There were 4 groups of participants: the ECDC+/Sero+ group defined by ECDC symptoms and seropositivity to anti-SARS-CoV-2 antibodies; the ECDC+/Sero- group defined by ECDC symptoms and seronegativity; the ECDC-/Sero+ group defined by the absence of ECDC symptoms, seropositivity and no history of COVID-19 reported on the third questionnaire; and the ECDC-/Sero- goup defined by the absence of ECDC symptoms, seronegativity and no history of COVID-19 reported on the third questionnaire (see supplementary appendix p 1).
Persistent symptoms
To identify persistent symptoms, we used symptoms reported in the third questionnaire. The list of symptoms was based on symptoms described in the literature on “post-covid-19 syndrome” and “long-covid”.6 It explored dysgeusia/anosmia, cardiothoracic symptoms (cough, thoracic pain, palpitations), pains (backpain, arthralgia, myalgia, headache), digestive disorders (nausea, diarrhea, constipation, abdominal pain), and others symptoms that were frequently associated with long-covid (asthenia, fever, cognitive complaints, cranial nerves abnormalities, sensory disorders, talk abnormalities, auditory disorders, dizziness, sleep disorders, skin disorders).
Persistent symptoms were defined as those that began after March 2020, lasted at least two months, and were still present at the time of the third questionnaire.
Data analysis
Because our goal was to compare persistent symptoms according to infection assessed by SARS-CoV-2 serological status and to highlight the specific association with past infection in patients with a history of acute symptoms during the first pandemic wave, the risk of presenting each persistent symptom was compared between Sero+ and Sero- groups, and between ECDC+/Sero+ and ECDC+/Sero- groups. Comparisons involving ECDC-/Sero+ and ECDC-/Sero- are presented in the supplementary appendix (ECDC-/Sero+ VS ECDC-/Sero-; ECDC+/Sero+ VS ECDC-/Sero-). Groups were compared using t-test for continuous variables and Chi-square test for qualitative variables. Univariable and multivariable logistic regression models were performed to estimate the strength of association (odds-ratio (OR)) between each persistent symptoms (the dependent variable) and the group (independent variable). Multivariable OR estimates were adjusted for sex, age, educational level, comorbidities lasting more than 6 months and the number of symptoms reported during the first wave of the pandemic (aOR). Indeed, persistent symptoms seem to be epidemiologically associated with the number of acute symptoms in hospitalized patients and in outpatients with COVID-19.11,12 All tests were two-sided and P < 0.05 was considered statistically significant.
We also used longitudinal data for the 10 symptoms that were collected in all questionnaires to compare the persistence of each of these symptoms between Sero- and Sero+ participants.
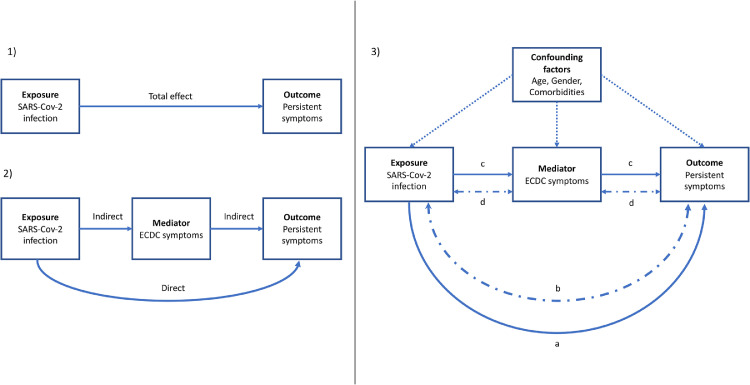
We finally conducted a mediation analysis for all persistent symptoms associated with the serological status in univariable analysis. In this analysis, we assumed that a part of the association of the serological status on persistent symptoms was mediated by the acute symptoms caused by the infection; in other words, the acute symptoms are supposed to be in the causal pathway between the infection and the persistent symptoms and cannot be considered as a confounding factor (Figure 1).
Figure 1.
Concept and design of the mediation analysis for the association of SARS-COV-2 infection with persistent symptoms. (1) Total effect model (2) Basic mediation model (3) Full mediation model used in the study.
The mediation analysis allows us to evaluate the part of the direct and indirect effect (2) in the total effect (1).
The full model (3) took also into account the interaction between the exposure and the mediator (b and d) and was adjusted on confounding factors. We calculated the total effect (TE= a+b+c+d), the direct effect of the exposure (a+b: natural direct effect, NDE) and the indirect effect mediated by the ECDC symptoms (c+d: natural indirect effect, NIE). The proportion mediated is represented by NIE/TE. The proportion due to interaction is NDE/TE.
Mediation analysis has been widely used in social and medical sciences.13, 14, 15, 16, 17 It relies on a set of regression equations to model the relationships between the dependent variable, the mediator and the independent variable, while allowing adjustments for confounding factors.
We analyzed the association of past infection (defined by the SARS-CoV-2 serology result) on persistent symptoms, mediated by the initial clinical presentation, defined by ECDC status. The “effect” of past infection status can thus be direct, not passing through the initial symptoms, or indirect, passing through the initial clinical presentation.
We estimated the direct and indirect effect by calculating the following parameters: 16,17
-
-
The natural direct effect (NDE), defined as the effect of a positive serology versus a negative serology on the probability of persistent symptoms when the ECDC status is negative. The NDE includes an interaction effect of serology with ECDC status which is not mediated;
-
-
The natural indirect effect (NIE), defined as the effect of ECDC positive versus ECDC negative on the probability of persistent symptoms when the serological status is positive. The NIE includes an interaction effect of serology with ECDC status which is mediated;
-
-
The total effect (TE) defined as the overall effect of serology on persistent symptoms, which is the product of NDE and NIE, when they are calculated as adjusted OR (aORTE=aORNDEx aORNIE).
-
-
The proportion of the total effect that was mediated using the formula on the risk difference scale, (100xaORNDE x(aORNIE -1)/(aORTE -1))
-
-
The proportion of the effect due to interaction (see Figure 1).
For each persistent symptom, the estimation of direct and indirect effects was based on two logistic models. A first logistic model predicted the probability of persistent symptom according to serological status, ECDC status, an interaction term between these two covariates, and the confounding factors. The second model predicted the probability of ECDC status according to serological status and the confounding factors. Direct and indirect aOR estimates were deduced from these two logistic models (formulas can be found in Ref 16.). All the mediation models were adjusted on age, sex, and comorbidities. Confidence intervals for estimates were calculated using bootstrap with 10 000 replicates.
Statistical analyses were performed using R software 4.0.5. Mediation analysis was performed using the CAUSALMED procedure in SAS 9.4.
Role of the funding source
The sponsor of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all data in the study and had final responsibility for the decision to submit for publication.
Results
Overall, 29,438 of 35,852 (82%) eligible participants completed the follow-up questionnaire. After excluding those whose serology was indeterminate or who reported a diagnosis of COVID-19 after serology, 25,910 (72%) participants were classified into one of the four groups of interest (see supplementary appendix p 1).
Participant characteristics according to the presence of at least one persistent symptom reported through the follow-up questionnaire are summarized in Table 1. All variables were statistically different between the groups declaring no persistent symptom and the group declaring at least one persistent symptom. However, the most important difference was in the number of acute symptoms presented during the acute phase of the epidemic (70.1 and 50.2% declared no acute symptoms in the group having no persistent symptoms and in the group having at least one persistent symptom, respectively). The median time between completion of the first questionnaire and the third follow-up questionnaire was 242 days [IQR:238–251]. Overall, 4028 (15.5%) participants experienced COVID-19-like illness during the first wave and 1022 (3.9%) participants had a positive SARS-CoV-2 serology. Overall, 6643 (25.6%) reported having at least one persistent symptom in the follow-up questionnaire (in those participants, the median number (IQR) of persistent symptoms was 1 (1-2)).
Table 1.
Main characteristics of participants, serological status (Sero) and experience of COVID-19-like illness during the first wave (ECDC) according to the presentation of at least one persistent symptom in the follow-up questionnaire.
| All participants | At least one persistent symptom lasting more than 2 months - 12/2020-02/2021 |
|||
|---|---|---|---|---|
| No | Yes | p | ||
| N = 25910 | N = 19267 | N = 6643 | ||
| Mean age (SD) | 49.5 (12.9) | 48.9 (13.0) | 51.1 (12.2) | <0.0001 |
| Sex (female) | 13299 (51.3%) | 9509 (49.4%) | 3790 (57.1%) | <0.0001 |
| Educational level | <0.0001 | |||
| Other | 294 (1.13%) | 222 (1.2%) | 72 (1.1%) | |
| Under high school diploma | 7027 (27.1%) | 5109 (26.5%) | 1918 (28.9%) | |
| Under graduate degree | 9948 (38.4%) | 7326 (38.0%) | 2622 (39.5%) | |
| Post graduate degree | 8641 (33.4%) | 6610 (34.3%) | 2031 (30.6%) | |
| At least one Comorbidity⁎⁎ | 6989 (27.2%) | 4541 (23.7%) | 2448 (37.3%) | <0.0001 |
| Groups | <0.0001 | |||
| ECDC-/Sero- | 21354 (82.4%) | 16393 (85.1%) | 4961 (74.7%) | |
| ECDC-/Sero+ | 528 (2.0%) | 423 (2.2%) | 105 (1.6%) | |
| ECDC+/Sero- | 3534 (13.6%) | 2170 (11.3%) | 1364 (20.5%) | |
| ECDC+/Sero+ | 494 (1.9%) | 281 (1.5%) | 213 (3.2%) | |
| Number of acute symptoms⁎⁎⁎ | <0.0001 | |||
| 0 | 16054 (65.0%) | 12919 (70.1%) | 3135 (50.2%) | |
| 1 | 5085 (20.6%) | 3484 (18.9%) | 1601 (25.7%) | |
| 2 | 1926 (7.8%) | 1206 (6.5%) | 720 (11.5%) | |
| 3 | 753 (3.1%) | 427 (2.3%) | 326 (5.2%) | |
| 4 or more | 863 (3.50%) | 405 (2.2%) | 458 (7.3%) | |
Groups were compared using t-test for continuous variables and Chi-square test for qualitative variables.
Missing value: n=227 (0.9%).
missing value n=1229 (4.7%).
The association between SARS-CoV-2 serological status and each persistent symptom is presented in Table 2. At least one persistent symptom was present in 318 (31.1%) and 5926 (24.7%) participants in the Sero+ and the Sero- groups, respectively (P<0.0001). Dysgeusia/anosmia, dyspnea, palpitations, asthenia, and cognitive complaints were positively associated with a positive serology in multivariable adjusted models, while abdominal pain or skin disorders were negatively associated. The association was strong for persistent dysgeusia/anosmia (with an aOR of 8.98 (95%CI 6.03–13.28)), while it was weak for other symptoms with aOR ranging from 1.82 (95%CI 1.20–2.68) for dyspnea to 0.42 (95%CI 0.21–0.74) for abdominal pain.
Table 2.
Persistent symptoms lasting more than 2 months according to serological results.
| Sero- | Sero+ | OR | p | aOR* | p | |
|---|---|---|---|---|---|---|
| N = 24888 | N = 1022 | [95% CI] | [95% CI] | |||
| Number of persistent symptoms (med [IQR] | 0 [0-2] | 0 [0-1] | - | - | - | - |
| At least one persistent symptom | 5926 (24.7%) | 318 (31.1%) | 1.33 [1.16-1.52] | <0.0001 | 1.04 [0.90-1.21] | 0.57 |
| Dysgeusia/anosmia | 101 (0.4%) | 65 (6.4%) | 16.67 [12.08-22.85] | <0.0001 | 8.98 [6.03-13.28] | <0.0001 |
| Cardiothoracic complaints | ||||||
| Cough | 178 (0.7%) | 14 (1.4%) | 1.93 [1.06-3.21] | 0.019 | 0.90 [0.46-1.64] | 0.76 |
| Dyspnea | 235 (0.9%) | 39 (3.8%) | 4.16 [2.91-5.8] | <0.0001 | 1.82 [1.20-2.68] | 0.004 |
| Thoracic pain | 172 (0.7%) | 21 (2.1%) | 3.01 [1.85-4.65] | <0.0001 | 1.27 [0.73-2.10] | 0.38 |
| Palpitations | 195 (0.8%) | 22 (2.2%) | 2.79 [1.74-4.25] | <0.0001 | 1.40 [0.82-2.30] | 0.20 |
| Pains | ||||||
| Backpain | 1531 (6.2%) | 63 (6.2%) | 1 [0.77-1.29] | 0.99 | 0.79 [0.59-1.04] | 0.11 |
| Arthralgia | 1790 (7.2%) | 65 (6.4%) | 0.88 [0.67-1.12] | 0.31 | 0.90 [0.67-1.18] | 0.45 |
| Myalgia | 834 (3.4%) | 40 (3.9%) | 1.17 [0.84-1.6] | 0.33 | 0.91 [0.62-1.30] | 0.61 |
| Headache | 356 (1.4%) | 28 (2.7%) | 1.94 [1.29-2.81] | 0.0008 | 1.01 [0.63-1.54] | 0.97 |
| Digestive complaints | ||||||
| Nausea | 56 (0.2%) | 3 (0.3%) | 1.31 [0.32-3.54] | 0.65 | 0.68 [0.16-1.95] | 0.53 |
| Diarrhoea | 155 (0.6%) | 8 (0.8%) | 1.26 [0.57-2.4] | 0.53 | 0.61 [0.26-1.27] | 0.22 |
| Constipation | 374 (1.5%) | 16 (1.6%) | 1.04 [0.6-1.67] | 0.87 | 0.78 [0.42-1.33] | 0.39 |
| Abdominal pain | 390 (1.6%) | 12 (1.2%) | 0.75 [0.4-1.27] | 0.32 | 0.42 [0.21-0.74] | 0.006 |
| Other complaints | ||||||
| Asthenia | 694 (2.8%) | 86 (8.4%) | 3.2 [2.52-4.02] | <0.0001 | 1.43 [1.08-1.86] | 0.01 |
| Cognitive complaints | 590 (2.4%) | 57 (5.6%) | 2.43 [1.82-3.19] | <0.0001 | 1.27 [0.91-1.74] | 0.15 |
| Fever | 23 (0.1%) | 2 (0.2%) | 2.12 [0.34-7.17] | 0.31 | 0.64 [0.09-2.72] | 0.60 |
| Cranial nerves abnormalities | 16 (0.1%) | 0 (0.0%) | - | - | - | - |
| Auditive disorders | 455 (1.8%) | 12 (1.2%) | 0.64 [0.34-1.08] | 0.13 | 0.66 [0.33-1.18] | 0.20 |
| Sensory disorders | 491 (2.0%) | 14 (1.4%) | 0.69 [0.39-1.13] | 0.17 | 0.62 [0.33-1.05] | 0.10 |
| Talk abnormalities | 54 (0.2%) | 4 (0.4%) | 1.81 [0.55-4.42] | 0.25 | 0.80 [0.18-2.48] | 0.73 |
| Dizziness | 160 (0.6%) | 13 (1.3%) | 1.99 [1.07-3.38] | 0.017 | 1.40 [0.69-2.60] | 0.31 |
| Vertigo | 13 (0.1%) | 0 (0.0%) | - | - | - | - |
| Sleep disorders | 2589 (10.4%) | 103 (10.1%) | 0.97 [0.78-1.18] | 0.74 | 0.83 [0.66-1.04] | 0.11 |
| Skin disorders | 607 (2.4%) | 17 (1.7%) | 0.68 [0.4-1.06] | 0.12 | 0.50 [0.28-0.82] | 0.01 |
aOR were adjusted for sex, age, educational level, comorbidities lasting more than 6 months and the number of symptoms declared during the first wave of the pandemic. OR odds-ratio; aOR: Adjusted odds-ratio; CI: confidence interval.
At least one persistent symptom was reported in 213/494 (43.1%) ECDC+/Sero+ participants, 1334/3534 (37.7%) ECDC+/Sero- participants, 105/528 (19.9%) ECDC-/Sero+ participants and 4961/21,354 (23.2%), ECDC-/Sero- participants (p < 0.0001).
More than 80% of the symptoms reported during the first wave of the pandemic disappeared before the follow-up questionnaire, regardless of the serological result, with the exception of dysgeusia/anosmia and asthenia in the seropositive group (59/246 (24%) and 75/354 (20.3%), respectively -Supplementary Appendix p 5). Dysgeusia/anosmia, asthenia and persistent thoracic pain were still more frequent at the follow-up in seropositive participants than in seronegative participants who experienced these symptoms during the first wave.
Table 3 presents the association between serological status and each persistent symptom in participants who reported a COVID-19-like illness during the first wave of the pandemic (ECDC+). Dysgeusia/anosmia, dyspnea, and asthenia were positively associated with a positive serology while abdominal pain, sensory complaints, and sleep disorders were negatively associated. The association appeared strong only with dysgeusia/anosmia (aOR= 6.83 (95%CI 4.47–10.42)).
Table 3.
Persistent symptoms lasting more than 2 months according to the presence of ECDC symptoms during the first wave of the pandemic and to the serological results.
| ECDC+/Sero- | ECDC+/Sero+ | OR | aOR* | |||
|---|---|---|---|---|---|---|
| N=3534 | N=494 | [95% CI] | p | [95% CI] | p | |
| At least one persistent symptom | 1364 (38.6%) | 213 (43.1%) | 1.21 [1-1.46] | 0.05 | 1.14 [0.91-1.42] | 0.25 |
| Dysgeusia/anosmia | 65 (1.8%) | 64 (13.0%) | 7.94 [5.54-11.39] | <0.0001 | 6.83 [4.47-10.42] | <0.0001 |
| Cardiothoracic complaints | ||||||
| Cough | 118 (3.3%) | 13 (2.63%) | 0.78 [0.42-1.35] | 0.41 | 0.70 [0.33-1.31] | 0.29 |
| Dyspnea | 134 (3.8%) | 35 (7.1%) | 1.93 [1.32-2.81] | 0.0008 | 1.69 [1.07-2.60] | 0.02 |
| Thoracic pain | 85 (2.4%) | 19 (3.9%) | 1.62 [0.95-2.63] | 0.06 | 1.15 [0.61-2.06] | 0.65 |
| Palpitations | 74 (2.1%) | 16 (3.2%) | 1.57 [0.87-2.64] | 0.11 | 1.23 [0.63-2.26] | 0.53 |
| Pains | ||||||
| Backpain | 356 (10.1%) | 38 (7.7%) | 0.74 [0.52-1.04] | 0.10 | 0.78 [0.52-1.15] | 0.22 |
| Arthralgia | 364 (10.3%) | 48 (9.7%) | 0.94 [0.68-1.27] | 0.69 | 1.02 [0.69-1.46] | 0.92 |
| Myalgia | 206 (5.8%) | 28 (5.7%) | 0.97 [0.63-1.43] | 0.89 | 0.82 [0.49-1.31] | 0.42 |
| Headache | 119 (3.37%) | 23 (4.66%) | 1.40 [0.87-2.17] | 0.15 | 1.11 [0.64-1.85] | 0.69 |
| Digestive complaints | ||||||
| Nausea | 17 (0.5%) | 0 (0.0%) | - | - | - | - |
| Diarrhoea | 51 (1.4%) | 6 (1.2%) | 0.84 [0.32-1.82] | 0.68 | 0.61 [0.21-1.48] | 0.31 |
| Constipation | 75 (2.1%) | 7 (1.4%) | 0.66 [0.28-1.35] | 0.30 | 0.38 [0.11-0.99] | 0.08 |
| Abdominal pain | 126 (3.6%) | 11 (2.2%) | 0.62 [0.31-1.10] | 0.13 | 0.51 [0.24-0.96] | 0.05 |
| Other complaints | ||||||
| Asthenia | 260 (7.4%) | 70 (14.2%) | 2.08 [1.56-2.74] | <0.0001 | 1.48 [1.05-2.07] | 0.02 |
| Cognitive complaints | 190 (5.4%) | 45 (9.1%) | 1.76 [1.24-2.45] | 0.0011 | 1.47 [0.98-2.16] | 0.06 |
| Fever | 17 (0.5%) | 2 (0.4%) | 0.84 [0.13-2.94] | 0.82 | 0.68 [0.10-2.84] | 0.64 |
| Cranial nerves abnormalities | 9 (0.3%) | 0 (0.0%) | - | - | - | - |
| Sensory disorders | 126 (3.6%) | 7 (1.4%) | 0.39 [0.16-0.78] | 0.02 | 0.40 [0.16-0.85] | 0.03 |
| Talk abnormalities | 22 (0.6%) | 4 (0.8%) | 1.30 [0.38-3.42] | 0.63 | 1.00 [0.21-3.41] | >0.99 |
| Auditive disorders | 107 (3.0%) | 9 (1.8%) | 0.59 [0.28-1.12] | 0.14 | 0.62 [0.25-1.32] | 0.26 |
| Dizziness | 45 (1.3%) | 10 (2.0%) | 1.60 [0.76-3.07] | 0.18 | 1.54 [0.65-3.35] | 0.30 |
| Vertigo | 7 (0.2%) | 0 (0.0%) | - | - | - | - |
| Sleep disorders | 556 (15.7%) | 56 (11.3%) | 0.68 [0.51-0.91] | 0.01 | 0.69 [0.49-0.95] | 0.02 |
| Skin disorders | 136 (3.9%) | 13 (2.6%) | 0.68 [0.36-1.16] | 0.18 | 0.61 [0.29-1.15] | 0.15 |
aOR were adjusted for sex, age, educational level, comorbidities, and the number of symptoms at the acute phase. OR odds-ratio; aOR: Adjusted odds-ratio; CI: confidence interval.
Other comparisons between groups defined according to a COVID-19-like illness during the first wave and serological status are presented in supplementary appendix (p 6). Of note, there was no difference in any of the persistent symptoms between ECDC-/Sero+ and ECDC-/Sero- participants except for palpitations which appear to be more frequent in seropositive than in seronegative participants (aOR: 2.67 (95%CI 1.15, 6.22), p = 0.02).
The mediation analysis is presented on Table 4 and was performed for dysgeusia/anosmia, cough, dyspnea, thoracic pain, palpitations, headache, asthenia, and cognitive complaints.
Table 4.
Mediation analysis. Estimates of direct and indirect effect for persistent symptoms associated with the serological status.
| Symptoms | Total effectaOR* [95% CI] | NDEaOR* [95% CI] | NIEaOR* [95% CI] | Proportion mediated% [95% CI] | Proportion due to the interaction% [95% CI] |
|---|---|---|---|---|---|
| At least one persistent symptom | 1.57 [1.34-1.83] | 1.05 [0.88-1.23] | 1.50 [1.34-1.69] | 92 [68-131] | 55 [20-85] |
| Dysgeusia/anosmia | 21.24 [14.81-29.28] | 6.87 [4.72-9.70] | 3.09 [2.60-3.47] | 71 [65-75] | 92 [83-97] |
| Cough | 2.22 [1.14-3.69] | 0.91 [0.40-1.82] | 2.45 [1.46-3.46] | 107 [45-310] | -12 [-440-61] |
| Dyspnea | 4.16 [2.83-5.83] | 1.83 [2.65-2.80] | 2.28 [1.70-3.02] | 74 [54-93] | 54 [16-77] |
| Palpitations | 3.15 [1.82-4.85] | 1.37 [0.69-2.41] | 2.30 [1.50-3.38] | 83 [51-127] | 58 [-10-90] |
| Thoracic pain | 2.89 [1.72-4.41] | 1.95 [0.98-3.35] | 1.48 [1.05-2.30] | 50 [6-101] | 25 [-59-80] |
| Headache | 1.90 [1.20-2.72] | 1.03 [0.57-1.67] | 1.84 [1.31-2.74] | 96 [48-239] | 62 [-36-134] |
| Asthenia | 3.05 [2.36-3.91] | 1.62 [1.19-2.19] | 1.88 [1.53-2.32] | 70 [51-88] | 58 [29-80] |
| Cognitive complaints | 2.42 [1.76-3.22] | 1.40 [0.94-1.99] | 1.73 [1.36-2.25] | 72 [45-105] | 57 [14-92] |
ECDC status is used as the mediator variable.
Estimates are adjusted for age, gender and comorbidities and interaction are taken into account (reference interaction and mediated interaction). NDE: natural direct effect; NIE: natural indirect effect; aOR: Adjusted odds-ratio; CI: confidence interval.
The aOR for NDE were in the range of those found in previous analyses with an aOR of 6.87 (95%CI 4.72–9.70) for dysgeusia/anosmia (aOR) and lower aORs > 1 for dyspnea and asthenia. All aOR for NIE were > 1, meaning that the experience of ECDC symptoms was associated with persistent symptoms in those with positive serology. The effect of positive serology on each symptom was mainly mediated by ECDC symptoms (proportion mediated range 50–107%) with a high proportion of the effect due to a positive interaction between serology and ECDC symptoms in all symptoms except cough.
Discussion
This population-based study quantifies the prevalence of persistent symptoms and examine symptoms according to ECDC COVID-19-like illness criteria and SARS-CoV-2 serological status. The results confirm the high prevalence of persistent symptoms in this population, especially in participants who experienced ECDC symptoms during the first pandemic wave. The mediation analysis showed that persistent symptoms associated with a seropositive status are mainly driven by the acute symptoms during the first pandemic wave.
Some of these persistent symptoms were more frequent in participants with ECDC+/Sero+ than in the other groups, independently of the number of acute symptoms. It confirmed the importance of dysgeusia/anosmia, dyspnea, and asthenia in persistent symptoms.12,18,19 The risk of having the other symptoms that have been suggested as related to “long covid “ were similar or higher in ECDC+/Sero- than in ECDC+/Sero+. This suggest that these persistent symptoms are common and are often not specific to the causative agent. During the first wave of the epidemic in France in 2020, viruses other than SARS-CoV-2 were circulating.20 Furthermore, the lack of specificity of most symptoms or their combinations to the post-covid condition emphasizes the importance of studying post-infectious states. Chronic post-viral fatigue (CMV, EBV, HIV) is a known but poorly explained phenomenon related to a persistent inflammatory state, a latent infection, a particular psychological context, or functional disorders. It has been suggested that persistent symptoms are driven by the initial intensity of the disease as well as other multidimensional and indirect factors.21 This is in line with another study based on the CONSTANCES cohort which shows that, except for dysgeusia/anosmia, a self-reported infection of SARS-CoV-2 was associated with persistent physical symptoms regardless of the result of serological tests.22 Our results are complementary to these, by classifying individuals according to their symptoms in the acute phase rather than a classification according to the diagnosis, which may be affected by the beliefs of the patient and/or the doctor. Here we demonstrated that some symptoms are more associated with SARS-COV-2 infection, especially in case of symptomatic acute symptoms. However, it is interesting to note that the individuals with asymptomatic SARS-CoV-2 infection were like ECDC-/Sero-, except for having a higher risk of palpitations. These results should be considered with caution, although cardiac consequences in asymptomatic or mild infections have been suggested.23
Unlike in other results in the literature evaluating outpatients with covid-19, the median number of persistent symptoms was low. This could be due to the population-based design of the present study. Most of the studies published in the literature were performed in specific populations (e.g. population with access to a PCR test during the first wave, healthcare workers) or in individuals who used the healthcare system for symptoms.12,19,24, 25, 26 This suggests that the intensity and the number of acute symptoms, which are associated with persistent symptoms, might have been higher in these previous studies than in the general population.11,12 The follow-up in the present survey was also longer. Thus, certain symptoms might have disappeared over time.
Persistent symptoms are known to be associated with the acute disease.12 Here, we take the analysis a step further by quantifying the direct impact of SARS-CoV-2 infection on symptom persistence and its indirect effect, mediated by the symptoms present at the initial phase of infection. Interestingly, it appears that the effect is mainly indirect for all symptoms, even for those that are not included in the ECDC's definition. This strong mediation effect underlines the importance of symptoms developed during the initial episode on the risk of presenting persistent symptoms. In addition, the association of persistent symptoms with serology was higher in case of initial ECDC symptoms except for cough. Thus, it can be speculated that all interventions reducing the intensity and/or the number of symptoms of the acute phase of the disease might have an impact on the incidence of post-covid persistent complaints. If this is the case, the individual benefit of vaccination could be enhanced even in people at low risk of severe COVID-19, as already suggested.27
The strength of this study relies in its design, which was based on a large sample from the general population followed prospectively, thus allowing comparison of different groups of individuals. SARS-CoV-2 infection was evaluated by serological tests in all individuals. Data were not collected through the healthcare system, making the reported symptoms more representative of those perceived by the general population than in previous studies using healthcare databases.18,26
This work has certain limitations. First, symptoms were self-declared and were not evaluated with specific scales. Also the declarations may have differed depending on the recent medical history, especially during the pandemic where participants were more apt to believe that they had had COVID-19.22 In addition, we could not separate persistent symptoms related to an infection (COVID-19 or other) from those related to otherwise poor health or a change in access to health care. Moreover, the possibility of serology misclassifications cannot be excluded.9,22
However, the fact that difference in the risk of anosmia is high between ECDC+/Sero+ and ECDC+/Sero- suggest that the prevalence for SARS-COV-2 infection is likely to be highest in participants with both positive serology results and ECDC symptoms declared during the first phase of the pandemic. Although this is one of the largest surveys on persistent symptoms after Covid-19 first pandemic wave, the number of individuals with this condition remained low in relation to the marked heterogeneity of the number and types of symptoms. Thus, we might have been unable to detect associations between the less frequent symptoms and COVID-19. We compared COVID-19 (ECDC+/Sero+) consequences to those of COVID-Like illness (ECDC+/Sero-). We assumed that this latter group was heterogenous. The consequences of COVID-19 need to be compared with other upper respiratory infection. A recent survey compared the consequences of COVID-19 and influenza in hospitalized patients.26 Although it showed that the burden of COVID-19 was greater, the comparison was performed between two different periods, and did not take into account the possible influence of the pandemic on other factors that could affect health and the health system. Finally, some symptoms following acute infection may not have been reported by participants - particularly if they appeared late after the initial two questionnaires were collected. In any case, this will only reinforce the fact that most of the effect of infection on persistent symptoms is mediated by the symptoms experienced in the acute phase.
In conclusion, there is an increased risk of persistent anosmia/dysgeusia, dyspnea, and asthenia in individuals with a history of COVID-19. Persistent symptoms are mainly driven by the acute phase of the disease. Further studies are needed to determine the origin of these persistent symptoms which could be associated with various pathophysiological causes that could also interact, from an inappropriate inflammatory response to a persistent functional disorder.
Authors’ contributions
OR and FC take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation. OR and EW accessed and verified all the data . OR designed the study; EW, MG, FC, MT, GS, and MZ acquired the data; OR performed statistical analysis. All authors contributed to the interpretation of data and to the design of the questionnaires. OR, EW, CL and FC drafted the article. All authors have read and approved the final manuscript.
Funding
The Constances cohort benefits from a grant from the French National Research Agency [Grant Number ANR-11-INBS-0002]: It is supported by the Caisse Nationale d'Assurance Maladie (CNAM), the French Ministry of Health, the Ministry of Research, the Institut national de la santé et de la recherche médicale. CONSTANCES and is also partly funded by MSD, AstraZeneca, Lundbeck and L'Oreal.
The SAPRIS-SERO study was supported by Agence Nationale de la Recherche, #ANR-10-COHO-06, Santé Publique France: N°20DMIA014-0, Fondation pour la Recherche Médicale (#20RR052-00), Inserm (Institut National de la Santé et de la Recherche Médicale), #C20-26.
Data availability statement
Data from this study are protected by health data regulations from the French National Commission on Informatics and Liberty (Commission Nationale de l'Informatique et des Libertés, CNIL). Data can be made available upon reasonable request to the steering committee of the CONSTANCES cohort study. While French law forbids providing free access to raw data; access could be provided by the steering committee after legal verification of the use of the data. The informed consent form are available in French on the CONSTANCES website.
Declaration of interests
OR reports personal fees and non-financial support from ViiV healthcare, Gilead, MSD. CL reports personal fees and non-financial support from Boehringer Ingelheim, Janssen-Cilag, Lundbeck and Otsuka Pharmaceutical, outside the submitted work. The other authors declare that they have no competing interest.
Acknowledgements
We thank the SAPRIS-SERO study group for its involvement in this work (see the supplementary appendix p 8 for details)
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.lanepe.2022.100363.
Appendix. Supplementary materials
References
- 1.Nehme M, Braillard O, Alcoba G, et al. COVID-19 Symptoms: Longitudinal Evolution and Persistence in Outpatient Settings. Ann Intern Med. 2021;174:723–725. doi: 10.7326/M20-5926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Petersen MS, Kristiansen MF, Hanusson KD, et al. Long COVID in the Faroe Islands - a longitudinal study among non-hospitalized patients. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1792. published online Nov 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang L, Yao Q, Gu X, et al. 1-year outcomes in hospital survivors with COVID-19: a longitudinal cohort study. Lancet. 2021;398:747–758. doi: 10.1016/S0140-6736(21)01755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nehme M, Braillard O, Chappuis F, Courvoisier DS, Guessous I. Prevalence of Symptoms More Than Seven Months After Diagnosis of Symptomatic COVID-19 in an Outpatient Setting. Ann Intern Med. 2021;174:1252–1260. doi: 10.7326/M21-0878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wanga V, Chevinsky JR, Dimitrov LV, et al. Long-term symptoms among adults tested for SARS-CoV-2 - United States. MMWR Morb Mortal Wkly Rep. 2021;70:1235–1241. doi: 10.15585/mmwr.mm7036a1. January 2020-April 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tran V-T, Riveros C, Clepier B, et al. Development and validation of the long covid symptom and impact tools, a set of patient-reported instruments constructed from patients’ lived experience. Clin Infect Dis. 2021:ciab352. doi: 10.1093/cid/ciab352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mahase E. Covid-19: What do we know about ‘long covid’? BMJ. 2020;370:m2815. doi: 10.1136/bmj.m2815. [DOI] [PubMed] [Google Scholar]
- 8.Zins M, Goldberg M, team CONSTANCES. The French CONSTANCES population-based cohort: design, inclusion and follow-up. Eur J Epidemiol. 2015;30:1317–1328. doi: 10.1007/s10654-015-0096-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carrat F, de Lamballerie X, Rahib D, et al. Antibody status and cumulative incidence of SARS-CoV-2 infection among adults in three regions of France following the first lockdown and associated risk factors: a multicohort study. Int J Epidemiol. 2021;50:1458–1472. doi: 10.1093/ije/dyab110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Case definition for coronavirus disease 2019 (COVID-19), as of 3 December 2020. European Centre for Disease Prevention and Control. https://www.ecdc.europa.eu/en/covid-19/surveillance/case-definition. Accessed 4 April 2021.
- 11.Ghosn J, Piroth L, Epaulard O, et al. Persistent COVID-19 symptoms are highly prevalent 6 months after hospitalization: results from a large prospective cohort. Clin Microbiol Infect. 2021;27 doi: 10.1016/j.cmi.2021.03.012. 1041.e1-e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sudre CH, Murray B, Varsavsky T, et al. Attributes and predictors of long COVID. Nat Med. 2021 doi: 10.1038/s41591-021-01292-y. Published online March 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robins JM, Greenland S. Identifiability and exchangeability for direct and indirect effects. Epidemiology. 1992;3:143–155. doi: 10.1097/00001648-199203000-00013. [DOI] [PubMed] [Google Scholar]
- 14.Robins JM, Mark SD, Newey WK. Estimating exposure effects by modelling the expectation of exposure conditional on confounders. Biometrics. 1992;48:479–495. [PubMed] [Google Scholar]
- 15.Pearl J. Direct and indirect effects. Proceedings of the Seventeenth conference on Uncertainty in artificial intelligence; San Francisco, CA, USA; Morgan Kaufmann Publishers Inc.; 2001. pp. 411–420. [Google Scholar]
- 16.Valeri L, VanderWeele TJ. Mediation analysis allowing for exposure-mediator interactions and causal interpretation: theoretical assumptions and implementation with SAS and SPSS macros. Psychol Methods. 2013;18:137–150. doi: 10.1037/a0031034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vanderweele TJ, Vansteelandt S. Odds ratios for mediation analysis for a dichotomous outcome. Am J Epidemiol. 2010;172:1339–1348. doi: 10.1093/aje/kwq332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lund LC, Hallas J, Nielsen H, et al. Post-acute effects of SARS-CoV-2 infection in individuals not requiring hospital admission: a Danish population-based cohort study. Lancet Infect Dis. 2021 doi: 10.1016/S1473-3099(21)00211-5. Published online May 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blomberg B, Mohn KG-I, Brokstad KA, et al. Long COVID in a prospective cohort of home-isolated patients. Nat Med. 2021 doi: 10.1038/s41591-021-01433-3. Published online June 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Le Hingrat Q, Bouzid D, Choquet C, et al. Viral epidemiology and SARS-CoV-2 co-infections with other respiratory viruses during the first COVID-19 wave in Paris, France. Influenza Other Respir Viruses. 2021;15:425–428. doi: 10.1111/irv.12853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Figueroa JD, Brennan PM, Theodoratou E, et al. Distinguishing between direct and indirect consequences of covid-19. BMJ. 2020;369:m2377. doi: 10.1136/bmj.m2377. [DOI] [PubMed] [Google Scholar]
- 22.Matta J, Wiernik E, Robineau O, et al. Association of self-reported COVID-19 infection and SARS-CoV-2 serology test results with persistent physical symptoms among french adults during the COVID-19 pandemic. JAMA Intern Med. 2021 doi: 10.1001/jamainternmed.2021.6454. Published online Nov 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rajpal S, Tong MS, Borchers J, et al. Cardiovascular magnetic resonance findings in competitive athletes recovering from COVID-19 infection. JAMA Cardiology. 2021;6:116–118. doi: 10.1001/jamacardio.2020.4916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nehme M, Braillard O, Alcoba G, et al. COVID-19 Symptoms: longitudinal evolution and persistence in outpatient settings. Ann Intern Med. 2020 doi: 10.7326/M20-5926. Published online Dec 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Havervall S, Rosell A, Phillipson M, et al. Symptoms and functional impairment assessed 8 months after mild COVID-19 among health care workers. JAMA. 2021 doi: 10.1001/jama.2021.5612. Published online April 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Al-Aly Z, Xie Y, Bowe B. High-dimensional characterization of post-acute sequalae of COVID-19. Nature. 2021:1–8. doi: 10.1038/s41586-021-03553-9. [DOI] [PubMed] [Google Scholar]
- 27.Antonelli M, Penfold RS, Merino J, et al. Risk factors and disease profile of post-vaccination SARS-CoV-2 infection in UK users of the COVID Symptom Study app: a prospective, community-based, nested, case-control study. The Lancet Infectious Diseases. 2022;22:43–55. doi: 10.1016/S1473-3099(21)00460-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data from this study are protected by health data regulations from the French National Commission on Informatics and Liberty (Commission Nationale de l'Informatique et des Libertés, CNIL). Data can be made available upon reasonable request to the steering committee of the CONSTANCES cohort study. While French law forbids providing free access to raw data; access could be provided by the steering committee after legal verification of the use of the data. The informed consent form are available in French on the CONSTANCES website.