Abstract
Exposure to heightened inflammation in pregnancy caused by infections or other inflammatory insults has been associated with the onset of neurodevelopmental and psychiatric disorders in children. Rodent models have provided unique insights into how this maternal immune activation (MIA) disrupts brain development. Here, we discuss the key immune factors involved, highlight recent advances in determining the molecular and cellular pathways of MIA, and review how the maternal immune system affects fetal development. We also examine the roles of microbiomes in shaping maternal immune function and the development of autism-like phenotypes. A comprehensive understanding of the gut bacteria-immune-neuro interaction in MIA is essential for developing diagnostic and therapeutic measures for high-risk pregnant women and identifying targets for treating inflammation-induced neurodevelopmental disorders.
Maternal immune activation
A developing fetus absorbs nutrients and other key resources from maternal circulation while avoiding potentially harmful effectors, including those produced from the maternal immune system that might view the fetus as foreign [1]. Mechanisms ensuring mammalian immunological tolerance (e.g., regulatory T cells [Tregs]; See Glossary) contribute to protecting fetuses from pregnancy-associated complications, such as preterm labor, preeclampsia, fetal growth restriction, and miscarriages [2]. Exposure to heightened inflammation during pregnancy, such as respiratory infection and autoimmunity, can lead to various neurological manifestations, including alterations in brain connectivity, variations in cognitive development, and abnormal neural activity [3,4]. Moreover, epidemiological and preclinical studies indicate a strong association between maternal immune activation (MIA) and several psychiatric and neurological disorders, including schizophrenia, bipolar disorder, attention deficit hyperactivity disorder (ADHD), cerebral palsy, developmental delay, cognitive dysfunction, anxiety/depression, and autism spectrum disorders (ASD) [5]. Therefore, determining the underlying mechanisms of MIA is essential for developing preventive and therapeutic measures for various immune-dependent psychiatric disorders. This review summarizes recent findings of how MIA affects brain development and can promote neurodevelopmental illness in the offspring (Figure 1). Postnatal exposure to inflammation also induces long-term consequences in animal health and has been recently reviewed [6]. Here, we focus on the ramifications of prenatal exposure to MIA.
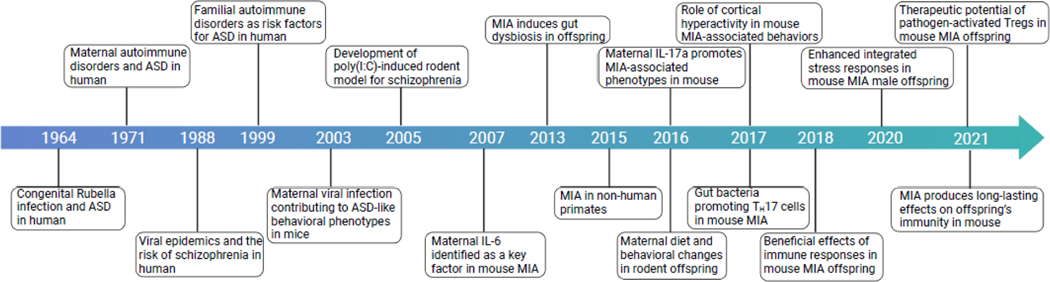
Figure 1. A brief history of MIA research leading to the identification of key players.
Timeline illustrating key events in MIA research. Abbreviations: ASD, Autism spectrum disorder; IL-6, interleukin-6; IL-17a, interleukin-17a; ISR, integrated stress response (ISR); MIA, maternal immune activation; Poly(I:C), polyinosinic:polycytidylic acid.
Maternal infections and neuropsychiatric disorders
In addition to genetic components, environmental factors likely contribute to the onset of neuropsychiatric and neurodevelopmental disorders, such as bipolar disorder, schizophrenia, and ASD. Epidemiological data indicate a strong association between increased infection rates and neurodevelopmental disorders. For example, the 1918–1919 influenza virus pandemic was suggested to increase the risk of schizophrenia [7]. After the 1964 rubella epidemic, the incidence of neurodevelopmental disorders (including ASD and schizophrenia) in children with congenital rubella increased significantly [8]. Consistently, ecological and maternal studies have shown strong associations between prenatal influenza virus infection during the second trimester and a 2- to 8-fold increased risk of schizophrenia [9,10]. Of note, a recent study highlighted seasonal effects in the risk of autism in more than 8,000,000 children born from 1990 to 2002, noting a slight but significant higher risk of ASD in children conceived in December to March, compared to those conceived in July, possibly owing to higher rates of respiratory infections during the colder months [11], although this remains to be fully explored.
The association between infection in pregnancy and an increased risk for neurodevelopmental disorders is not restricted to a specific type of virus [12]. Studies have indicated that both viral- (food-borne viruses, cytomegalovirus, measles, mumps, chickenpox, and polio) and bacterial-induced (Escherichia coli, pneumonia, and Toxoplasma gondii) infections at prenatal stages can trigger psychiatric disorders in mouse offspring [13,14]. A large-scale human study performed on 1,612,342 children born in Denmark from 1980 to 2005 [15] also supports the same notion—infection or inflammation requiring hospitalization of pregnant women was associated with the risk of developing psychiatric disorders in their children. Therefore, pathogen-induced MIA and not specific pathogens, has been deemed a pivotal factor in driving persistent changes in brain development and behaviors in offspring. In line with this idea, various inflammatory conditions during pregnancy are shown to enhance the risk of neurodevelopmental disorders. For instance, maternal autoimmune disorders, allergies, and asthma have been reported as risk factors for developing ASD and schizophrenia in humans [16,17]. Additionally, other factors, such as acute stress [18], exposure to environmental pollutants [19], and nutrient conditions [20] that directly or indirectly elevate immune responses, have also been linked to an increased risk of ASD [2,5]. Mechanistically, a series of recent human studies have shown that pregnant mothers exposed to MIA can have dysregulated cytokine production, such as for interleukins 6 (IL-6) and 17a (IL-17a), which are associated with cognitive impairment and the risk of developing ASD in their children [3,21–23]. Although further studies are needed to clarify which key inflammatory markers or pathways are triggered by different pathogenic infections and which genetic or environmental factors elicit or contribute to the development of certain brain disorders, the available evidence suggests that MIA can act as a prenatal “primer” that can instigate a range of neurodevelopmental and neuropsychiatric disorders in offspring.
Presently, rapid globalization, exploding population growth, emerging novel infectious agents, and a resurgence of classical contagious diseases (including measles, mumps, and polio) may be laying the groundwork for increased rates of opportunistic infections in pregnant women. Combined with the rising rates of inflammatory conditions, which may include autoimmune disorders, allergies, and asthma, these factors might account for or contribute to recent increases in neurodevelopmental disorders in the global pediatric population [24]. The current coronavirus disease 2019 (COVID-19) pandemic has already affected more than 2.5 billion individuals (at the time this article was written), including pregnant women. These alarming cases make it imperative to understand how MIA affects inflammatory responses in pregnant women and can contribute to promoting neurodevelopmental disorders in affected children. Indeed, data already indicate that SARS-CoV-2 infection, like some other respiratory infections, can elicit pregnancy complications, including fetal growth restriction, fetal distress, and preterm birth [25]. We describe various ways of inducing MIA, maternal immune pathways eliciting neurodevelopmental pathologies, and the role of gut bacteria in contributing to MIA-induced pathologies. We also highlight key unanswered questions and future directions.
MIA animal models
Animal models have facilitated studies of complex human brain diseases. Earlier rodent models of MIA were established with an initial focus on schizophrenia [26,27]. A seminal paper reported that infecting mice with influenza virus or treating the animals with the long double-stranded RNA (dsRNA), polyinosinic:polycytidylic acid (poly[I:C]) as a viral mimetic, triggered ASD- and schizophrenia-like behavioral abnormalities in the offspring, as evidenced by cognitive tests, such as the three-chamber and marble-burying assays [28,29]. These and other studies extensively characterized behavioral abnormalities in MIA-affected offspring, particularly those reminiscent of human ASD and schizophrenia patients, including alteration and deficits in pre-pulse inhibition, cognition, sociability, repetitive behavior, and increased sensitivity to antipsychotic drugs compared with controls [13]. Importantly, these MIA-induced behavioral abnormalities have been similarly reproduced in nonhuman primate (NHP) models [30,31]. Subsequently, MIA animal models have been extensively used, allowing researchers to employ various environmental insults to trigger MIA during pregnancy. Those triggers include infectious agents (pathogens, pathogen mimicry), autoimmunity, dietary factors (high-fat, high-salt diet), environmental risk factors (exposure to diesel, fine particles), and even psychiatric stress (social and restraint stressors) [20,32].
Non-infectious stimuli inducing MIA
Metabolic factors
Maternal diabetes conditions, including gestational diabetes mellitus, display a strong co-morbidity with neurodevelopmental deficits, including language delay, poor motor development, impaired recognition memory, and ASD in humans [33]. Indeed, increased incidence of ASD and other developmental delays have significantly correlated with maternal diabetes and hypertension among more than 1000 pregnant women [34]. Consistently, rodent offspring from dams exposed to a high-fat diet (HFD) during gestation display significant deficits in cognitive function, heightened anxiety, and abnormal social behaviors [35]. NHP studies have also resulted in similar outcomes [36,37]. Moreover, maternal obesity in pregnancy has also been attributed to enhanced autism-like phenotypes in offspring. In rodents, a HFD has triggered both systemic and local immune activation in pregnant mice. Specifically, a HFD during gestation increased the production of inflammatory cytokines, including IL-1β, IL-6, and TNF-α, in the plasma, liver, and brain in both the mother and the fetus [38,39]. A HFD also induced changes in immune responses in the placenta, such as the dysregulated NK cell activity [40], and altered gene expression in the mouse fetal brain [41]. Additionally, offspring born to HFD-fed mothers displayed heightened anxiety [35], impaired sociability [42,43], and cognitive decline [43]. In humans, excessive weight gain in pregnancy has been associated with increased concentrations of inflammatory and metabolic factors in maternal serum (e.g., monocyte chemoattractant protein-1, high-sensitivity C-reactive protein, and leptin) [44]. Furthermore, maternal obesity may also affect an offspring’s neurodevelopment; recent epidemiological studies suggest that obesity (defined by body mass index) during pregnancy influences an offspring’s brain development, the dysregulation of which may affect cognitive and motor performance [45,46] and contribute to an increased risk of developing psychiatric disorders, including ASD [47,48], ADHD [49], schizophrenia [50], and anxiety/depression [51].
Altogether, these findings suggest that dysregulated metabolism during pregnancy likely results in heightened inflammation that can negatively impact the proper brain development of fetuses, potentially leading to an enhanced susceptibility to developing certain neurodevelopmental disorders.
Environmental risk factors
Exposure to various environmental factors during pregnancy has been proposed to affect fetal brain development and induce psychiatric disorders in offspring. Risk factors include (but are not limited to) pesticides, air pollutants (fine particles, diesel, NO2, and heavy metals), and phthalates [32,52]. A recent study with more than 1,700 children born from 1990 to 2002 in the U.S. revealed a strong association specifically in the third trimester between maternal exposure to fine particles, or particulate matter 2.5 (PM2.5), and increased ASD prevalence [19]. Moreover, prenatal exposure to PM2.5 during pregnancy has led to behavioral abnormalities in rat offspring [53]. Additionally, exposure to traffic-related air pollutants, such as diesel fumes, during prenatal and early life strongly correlates with the incidence of ASD in humans [54,55] and mice [32,56]. Of note, particulates from air pollution also trigger particle-induced inflammation in humans (e.g., increased cytokine and chemokine responses) [57]. However, it is still unclear, by and large, how environmental risk factors cause excessive inflammation, perturb fetal neurodevelopment, or contribute to neurodevelopmental disorders in children. Therefore, further rigorous investigations are needed to conclusively assess a causal relationship between various environmental risk factors, MIA, and its long-term pediatric consequences.
Stress
In humans, studies have shown a strong association between excessive maternal stress, such as traumatic experiences, anxiety, and depression, with prenatal inflammation, impaired brain development, and psychiatric disorders in offspring [58,59]. These epidemiological findings have been reproduced in the rodent prenatal stress model, enabling researchers to study how maternal stresses might prompt prenatal inflammation and influence fetal development. For instance, exposing pregnant mice to repetitive restraint stress (RSS) during gestation has promoted autism-like behaviors, including anxiety-like behavior, decreased sociability, and locomotor inhibition in offspring [60]. Along with behavioral abnormalities, prenatal RSS has affected immune responses, leading to increased IL-6 and ILβ in maternal serum and altered kynurenine metabolite concentrations in the placenta relative to control mice [61,62]. Blocking the IL-6 pathway with an anti-IL-6 antibody has ameliorated maternal stress-induced microglial dysfunction in offspring, as evidenced by increased sociability and reduced anxiety-like behaviors [63]. Similarly, other prenatal or early life stresses, such as bright light exposure, tail shocks, and noise exposure, increase feto-maternal inflammation as well as neurodevelopmental phenotypes, including irregular brain development and reduced sociability, in laboratory rodents [64]. While fetal microglia are a key mediator producing stress-induced neuroinflammation in fetal brains [65], the identities and functions of the responsible immune cells in pregnant mothers underlying maternal stress-induced neurodevelopmental changes remain to be investigated. These data collectively suggest that the immune system may play a key function in priming maternal stress-induced neurodevelopmental disorders in offspring.
MIA-inducing stimulants
Respiratory viral infections
Influenza viral infection in animal models has been associated with psychiatric and neurodevelopmental diseases in offspring. Rodent and NHP offspring born to influenza virus-infected mothers display core behavioral abnormalities of ASD and schizophrenia, including decreased sensitivity to auditory stimulation, increased anxiety, social deficits, and repetitive behaviors [28,66,67]. Influenza (H1N1) viral infection has led to vascular inflammation, the production of excessive inflammatory mediators such as IL-1β, TNF-α, and IFN-γ, and the infiltration of immune cells, including Ly6Clow and Ly6Chigh monocytes, neutrophils, and T cells, into the vascular tissues of pregnant dams [68]. Studying respiratory viral infection-induced MIA models can enable researchers to further elucidate the molecular and cellular mechanisms of MIA pathogenesis. Of note, it will be essential to test if SARS-CoV-2 infection leads to behavioral abnormalities in pregnant dams expressing SARS-CoV-2 specific receptors, such as human angiotensin-converting enzyme 2 (ACE2)[69].
Non-infectious agents inducing MIA
Unlike pathogenic insults, mimetic agents do not fully recapitulate all the immunological consequences of MIA, such as antigen-specific T and B cell responses and antibody production. However, treating pregnant mice with bacterial and viral mimetics produces a set of core behavioral and neurological abnormalities reminiscent of those observed in human ASD and schizophrenia patients [13,70,71]. Furthermore, non-infectious mimetics have enabled researchers to induce MIA with uniform intensity and in a controlled fashion. Over the years, both viral and bacterial mimetics, including poly(I:C) and LPS (lipopolysaccharides), have been widely used to investigate MIA (Box 1).
Box 1: Widely used agents to stimulate MIA responses in rodent models.
Poly (I:C) is structurally similar to viral double-stranded RNA that stimulates immune responses through TLR3 and MDA5/RIG-1 RNA sensors. Research using poly(I:C) has significantly contributed to understanding the immunological and neurobiological mechanisms of MIA [2,14]. Studies identified a crucial gestation window, between embryonic days 9 to 15, in which poly(I:C) injection promotes behavioral abnormalities in offspring in mice [135]. Extensive efforts have been made to determine the levels of inflammation during which neurodevelopmental abnormalities in offspring ensue following injection with various amounts of poly(I:C) [28]. One study used the poly(I:C) model to demonstrate that IL-6, and not IFN-γ, was a key effector mediating MIA-induced behavioral abnormalities—the first major discovery into the mechanisms of MIA. We and others uncovered pivotal roles for Th17 cells and segmented filamentous bacteria (SFB) in promoting MIA pathogenesis. The poly(I:C) model has also been used to elucidate how MIA influences fetal brain development by affecting cortical architecture [4], ISR in fetal brains [103], and immune cell function in the choroid plexus [136]. Furthermore, this model identified dysregulated tactile hypersensitivity [137] and the emergence of heightened neural activity in the sub-region of the primary somatosensory cortex, the dysgranular zone of the S1 (S1DZ), which is connected to different brain areas (including the temporal association area (TeA) and the striatum) in adult MIA offspring [83] (Figure 2). The effects of poly(I:C)-MIA on offspring include a strong male bias [103] and dysregulated immune responses, such as increased production of inflammatory cytokines upon stimulation [138,139], both of which are observed in human ASD patients [140]. This is an intriguing finding, and the significance of the male bias remains to be elucidated.
Bacterial infections, especially during the second trimester of pregnancy, are strongly associated with an increased risk of developing neurodevelopmental disorders in children [15]. During pregnancy, infections with various bacterial pathogens, including Toxoplasma gondii, Chlamydophila pneumonia, and Mycoplasma, increase the risk of ASD and schizophrenia in humans [141,142]. T. gondii or Escherichia coli infection in pregnant rodent dams promotes feto-maternal inflammation and behavioral abnormalities in offspring [143], and T. gondii antigen induces MIA-associated behavioral and neurodevelopmental changes [144,145]. Likewise, injection of lipopolysaccharide, a cell wall component of gram-negative bacteria, or Staphylococcal Enterotoxin A and B into dams promotes autism- or schizophrenia-like behavioral abnormalities, including deficits in social interaction, pre-pulse inhibition of acoustic startle response, decreased learning and memory, and increased repetitive behavior, in offspring [14,146–148]. While less is known about the downstream effector mechanisms, prenatal administration of LPS elicits the production of inflammatory cytokines (TNF-α, IL-1β, and IL-6) in maternal circulation and fetal brain [100,149]. Indeed, A recent study in mice demonstrated a role for IL-17a in triggering LPS-dependent, autism-like behaviors [100] (see main text).
Other TLR ligands
Injecting pregnant mice with different Toll-like receptor (TLR) ligands, such as synthetic oligodeoxynucleotides containing CpG motifs, has led to fetal demise [72]. However, more extensive analyses are required as different amounts of the CpG motifs might induce MIA and alter immune responses in pregnant mice and developmental phenotypes in offspring. Similarly, it will be informative to assess if fungal or parasitic infections and other inflammatory stimuli induce MIA responses and autism-like phenotypes, and assess whether their actions might converge on IL-6 and IL-17a pathways.
Two-hit MIA models
In the two-hit MIA model, TLR agents (e.g., poly(I:C) and LPS) are used sequentially to induce MIA and postnatal immune activation in mice, leading to the manifestation of core autism-like, behavioral phenotypes in a sex-biased manner [73]. Whether the same immune and neurological pathways that function in the poly(I:C)-MIA model (Box 1) play a role in the two-hit model, however, remains to be investigated. Regardless, given that human fetuses are exposed to distinct pathogens before and after birth, rodent studies employing the two-hit model can provide unique mechanistic insights into the pathogenesis of multi-immune stimuli-induced neurodevelopmental disorders.
Mechanisms underlying MIA-induced neurodevelopmental disorders
Most of the work on MIA has been done using poly(I:C) in rodent models; thus, this section primarily focuses on findings acquired with this model.
Poly(I:C)-driven MIA model
The maternal immune system recognizes and responds to dynamic environmental stimuli to protect the mother and fetus during pregnancy. However, we lack a comprehensive understanding of the cellular, molecular, and immunological mechanisms of how pregnancy shapes the maternal immune system and how, in turn, the immune system affects pregnancy. This gap in knowledge poses a significant challenge to maternal-fetal health. For example, pregnant women are more susceptible to respiratory viral infections and other inflammatory insults compared to age-matched non-pregnant women, warranting revived and refocused efforts to understand the long-term effects of inflammation in pregnancy.
The poly(I:C)-driven MIA rodent model has provided a wealth of information on immune responses in pregnancy following immunological challenges. It is well established that poly(I:C) injection increases inflammatory cytokine concentrations in maternal serum, as well as in amniotic fluid. These observations raise the question of whether maternal cytokines can mediate the effects of MIA on fetal development and cause ensuing behavioral changes in offspring. Administration of IL-6 at embryonic day 12.5 (E12.5) has produced the characteristic behavioral changes of MIA-affected offspring reminiscent of schizophrenia and autism [74]. However, MIA-associated behavioral abnormalities require IL-6 to be present in pregnant mothers given that poly(I:C) injection to IL-6-deficient mice no longer produces MIA-induced behavioral abnormalities in offspring [29].
IL-6
Initially identified as a pleiotropic cytokine that promotes the activation and effector function of various immune cell populations, IL-6 has subsequently been found to play a central role in protecting the host against various infections [75]. In humans, a deficiency in IL-6 function, caused by autoantibodies against IL-6 or autosomal mutations in the Stat3 pathway (the primary IL-6 downstream signaling module), impairs innate and adaptive immunity against viral, parasitic, and bacterial infections, and causes premature death from pneumonia [75]. Such phenotypes have been successfully recapitulated in rodent systems. However, uncontrolled IL-6 activity can also promote chronic inflammation in various inflammatory disorders, such as hyper IgE syndrome, rheumatoid arthritis, psoriasis, systemic lupus erythematosus, colitis, and even cancer.
As stated earlier, IL-6 is a key cytokine in promoting MIA-induced autism-like abnormalities in mice [29]. IL-6 amounts have been reported to be increased in both MIA-affected rodent fetal brains [76] and human brain tissues derived from autistic patients [77]. Furthermore, a functional magnetic resonance imaging study identified elevated IL-6 during pregnancy in maternal sera, suggesting that this cytokine might affect brain connectivity and working memory in human newborns [3]. How does maternal IL-6 bridge maternal inflammation and fetal brain development? IL-6 has been proposed to cross the placenta barrier, leading to the loss of Purkinje cells in fetal hindbrain regions; indeed, the removal of IL-6 receptors in placental trophoblasts has prevented this phenotype [78]. Moreover, Purkinje cell dysfunction has been associated with some cases of autism in humans [79]. A recent finding following Yersinia pseudotuberculosis bacterial infection in mice during pregnancy also showed that maternal IL-6 crosses the placental barrier and acts on its receptor expressed in the fetal gut epithelial cells of offspring, influencing their long-term immunological responses against pathogenic infection occurring in adulthood [80]. Furthermore, IL-6 injection to fetal brains has led to synaptogenesis in glutamatergic neurons [81]. These data suggest that on the one hand, IL-6 might promote MIA phenotypes by directly acting on its receptor in fetal tissues (Figure 2). On the other hand, IL-6 might also indirectly enhance MIA phenotypes. For example, IL-6 functions as an upstream cytokine that stimulates the differentiation of naïve CD4+ T cells into Th17 cells [82], which play a crucial role in MIA (see below section)[83]. Supporting this idea, blocking maternal IL-6 function with an anti-IL-6 antibody injection in pregnant mice has impaired poly(I:C)-induced upregulation of IL-17a protein in maternal circulation; however, intra-ventricle injection of IL-17a, but not IL-6, into fetal brains at E12.5 has recapitulated MIA effects in offspring [83]. These results suggest that there are direct as well as indirect roles of maternal IL-6, as an inflammatory cytokine, in inducing MIA.
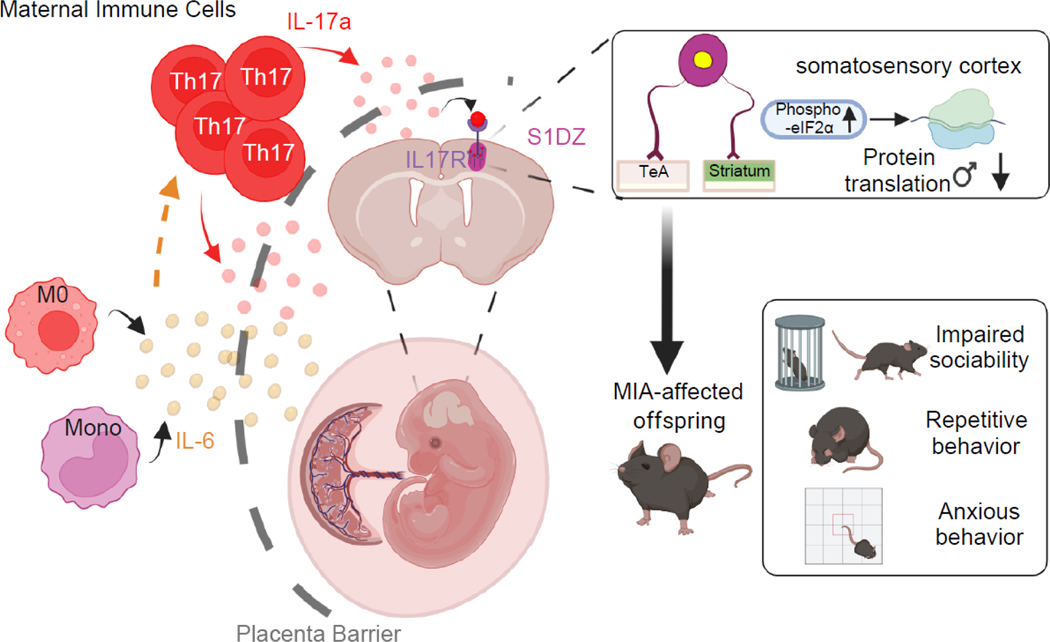
Figure 2. IL-6 and IL-17a promote MIA-associated phenotypes in mice.
This schematic reflects the current understanding of how MIA leads to the abnormal development of the fetal brain and autism-like behavioral abnormalities in offspring. i) MIA leads to enhanced production of interleukin-6 (IL-6) in pregnant dams. ii) IL-6 promotes the differentiation of naïve T cells into TH17 cells, which produce interleukin-17a (IL-17a) in mice exposed to poly(I:C)-induced inflammation. iii) Both IL-6 and IL-17a may act on or across the placental barrier. iv) Exposure to maternally derived IL-17a stimulates the integrated stress response (enhanced phosphorylation of elongation initiation factor 2B [eIF2B]), leading to delayed translation in male, but not female, fetal brains. v) The IL-17-receptor (IL17R) is expressed in the cortical areas, including the primary somatosensory cortex dysgranular zone (S1DZ), which is connected to different brain areas, such as the temporal association area (TeA) and the striatum. Dysregulated neural activity in these regions contributes to autism-like behavioral phenotypes, including impaired sociability and increased repetitive and anxiety behaviors, in MIA-affected offspring. M0, macrophages; Mono, monocytes.
IL-17a
IL-17a received much attention after its discovery as the signature cytokine of a specific subset of T helper cells, later dubbed Th17 cells [84]. IL-17a maintains barrier function, aids in the tissue repair process, and induces innate immune-like defenses by promoting the production of antimicrobial peptides, such as β-defensin, and enhancing the recruitment of neutrophils into infected or injured sites [85]. IL-17a has also been associated with inflammation that drives various autoimmune diseases, such as rheumatoid arthritis (RA), psoriasis, and multiple sclerosis (MS) [85].
Recent mouse studies have also uncovered non-immunological functions of IL-17a, especially in the brain. For example, IL-17a produced from gamma delta T (γδT) cells in the meninges was reported to control synaptic plasticity and short-term memory by increasing the glutamatergic synaptic plasticity of hippocampal neurons [86]. Enhanced production of IL-17a by a high-salt diet has elicited brain endothelial damage and cognitive dysfunction, as measured by behavioral changes [87]. IL-17a-producing cells have also been implicated in promoting anxiety- and depression-like behaviors in mice [88,89]. Along with the extensively studied function of IL-17a in promoting neuroinflammation in the brain [90], these data suggest that IL-17a can directly or indirectly affect brain cells and induce neurological phenotypes.
Both IL-17a and Th17 cells might also play a role in ASD. Certain autistic patients display elevated IL-17a serum concentrations [91,92]. In rodent models, we and others previously showed that poly(I:C)-induced MIA in mice increased IL-17a production in maternal circulation [83,93–96]. Consistent with these findings, increased IL-17a concentrations or enhanced expression of RAR-related orphan receptor gamma t (RORγt)—the key transcription factor driving Th17 cell programming [97]—in pregnant mice, promoted autism-like behavioral abnormalities (e.g., increased repetitive behaviors and reduced sociability) in offspring [98–100] (Figure 2). Conversely, reducing IL-17a production or blocking its activity with anti-IL17a antibody prevented the development of autism-like behavioral abnormalities in MIA offspring [83,101]. Genetic removal of RORγt selectively in maternal CD4+ T cells (CD4-Cre/rorcfl/fl) or intraperitoneal administration of IL-17a blocking antibody in pregnant dams prevented MIA-induced cortical malformation and autism-like behaviors in offspring [83]. Direct introduction of IL-17a into the fetal brain at E14.5 induced MIA-associated phenotypes and microglial activation [83,99]. Conversely, neuron-specific removal of IL-17 receptor subunit A (IL-17RA; Nestin-Cre/il17rafl/fl) in developing embryos prevented poly(I:C)-induced MIA effects, including dysregulated neural activity and behavioral abnormalities [4], indicating that maternally derived IL-17a exerted its function by acting on its receptor expressed in fetal brains. Maternal IL-17a also augmented the integrated stress response (ISR) in mouse fetal brains; ISR was required for the manifestation of MIA-associated behavioral and neurological phenotypes, as MIA offspring carrying eIF2αS51A/+ mutations no longer displayed such phenotypes [102,103]. As mentioned earlier, IL-17a is essential for promoting autism-like symptoms in offspring exposed to LPS-induced MIA [100]. Altogether, these data suggest that IL-17a may represent a relevant diagnostic and therapeutic candidate target to test the prevention of specific neurodevelopmental disorders in offspring exposed to prenatal inflammation. However, whether these findings translate to humans, requires robust research.
Other cytokines
Besides IL-6 and IL-17a, poly(I:C)-MIA triggers the production of other cytokines, including IL-1β, IFN-γ, TNF-α, and type I interferons (IFN) in both maternal and fetal mouse tissues [83,104]. A recent study examining induced MIA in Ifna receptor-deficient mice highlighted a contributing role of IFN signaling in promoting MIA-associated phenotypes [104]. However, more studies are needed to assess epistatic relationships between type I IFNs and other key inflammatory cytokines, such as IL-6 and IL-17a, in MIA. Furthermore, it will be informative to systematically test the modulatory role of each cytokine in MIA by administering individual cytokines to pregnant mice and/or fetal brains.
The gut microbiome and MIA
Mounting evidence supports pivotal roles for the gut microbiome in the developmental and cellular processes in health and disease. Earlier studies primarily focused on possible links between the gut microbiome and various diseases, including type 1 diabetes, asthma, inflammatory bowel disease, and cancer [105]. Recent research suggests that the gut microbiome affects the central nervous system (CNS) and CNS-related disorders, often dubbed as the “gut-brain axis” [106]. Observations made with germ-free mice indicated that commensal bacteria deficiency led to neuro/behavioral abnormalities, including deficits in memory formation [107], increased motor activity [108], reduced anxiety-like behaviors [109], increased stress-induced hypothalamic-pituitary-adrenal responses [110], reduced social behaviors [111], impaired blood-brain barrier function [112], and changes in the expression of various neurotrophic factors (e.g., brain-derived neurotrophic factor and N-methyl-D-aspartate receptor) relative to control mice[108,110]. Moreover, emerging evidence from animal models and clinical samples suggests causative roles for the microbiome in modulating many different neurological conditions, such as depression, anxiety, ASD, schizophrenia, and neurodegenerative disorders [93,113–115] (see below).
Maternal gut bacteria and MIA
The maternal gut microbiome likely functions as one of the key players promoting MIA-associated phenotypes (Figure 3). In pregnant dams, vancomycin antibiotic treatment prevented offspring from developing MIA-induced neural deficits and autism-like behaviors [93]. In 2009, one research group serendipitously observed significant differences in Th17 cell numbers in the intestines of mice obtained from two commercial vendors: mice from the Jackson Laboratory had low Th17 cell numbers while mice from Taconic Biosciences had high Th17 cell numbers [116]. This observation led to the identification of the Th17 cell-promoting role of a single commensal bacterial species, segmented filamentous bacteria (SFB) [116,117]. By inducing MIA in Jackson and Taconic-derived dams and using other means, we and others showed that SFB or other Th17 cell-inducing bacteria in the maternal gut promoted MIA-induced pathogenesis in offspring [93,101]. Consistent with these results, infecting pregnant mice with the food-borne pathogen Yersinia pseudotuberculosis did not induce behavioral changes in offspring if their mothers lacked SFB, while the same inflammation led to long-lasting protective immunity against bacterial pathogens such as Salmonella Typhimurium serovar in affected offspring [80]. These results indicated that maternal gut bacteria were a crucial environmental trigger in an offspring’s increased susceptibility to inflammation-associated neurodevelopmental disorders.
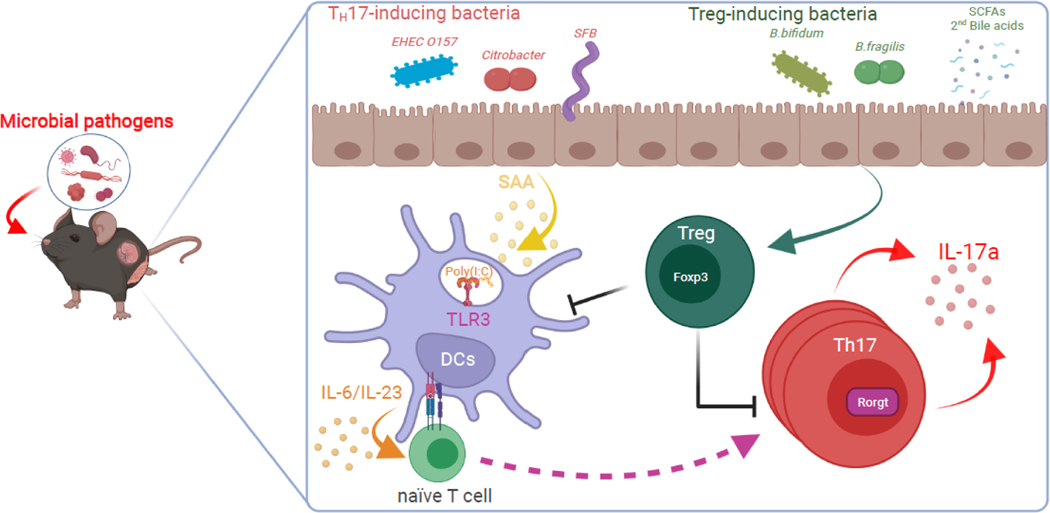
Figure 3. Gut commensal bacteria in pregnant mice contribute to MIA responses by regulating Th17 cell biogenesis and function.
The schematic represents the pathological TH17 responses in the maternal intestine upon exposure to prenatal viral or bacterial infection. i) Gut commensal bacteria promote the differentiation of inflammatory TH17 and anti-inflammatory Treg cells. ii) Commensal bacteria, such as SFB, stimulate intestinal epithelial cells to produce serum amyloid A (SAA). iii) SAA then acts on dendritic cells (DCs) and T cells and differentiates naïve T cells into IL-17a-producing TH17 cells in the gut lamina propria. iv) DCs are further stimulated by poly(I:C) or other pathogenic insults. v) However, commensal bacteria, such as Bifidobacterium bifidum or bacterial metabolites (e.g., short-chain fatty acids [SCFAs] or secondary bile acids), enhance the production of Tregs, which may counteract MIA-stimulated Th17 cell responses.
A recent report summarized various efforts to improve the rigor and reproducibility of MIA models among research groups and institutions [118]. The authors rightly emphasize the importance of using the appropriate immunogen by considering the vendor-to-vendor and lot-to-lot variation of poly(I:C) agents. However, less consideration has been given to harmonizing the efforts to assess the gut bacterial community in mice exposed to MIA, although gut microbiota in pregnant mice can have a significant impact on an offspring’s phenotypes [93,94,101]. Furthermore, given that gut bacteria not only enhance Th17 cell responses but also likely mitigate their activity by augmenting anti-inflammatory Treg pathways [119,120], in an ideal scenario, it might be beneficial to consider the relative ratio of Th17 cell-promoting and -suppressing bacteria before inducing MIA (Box 2). Unfortunately, however, we lack a comprehensive list of gut bacteria that modulate Th17 cell responses. Even Taconic-derived mice, depending on their respective barrier facility, do not always carry SFB in their guts [121]. Therefore, at a minimum, we advocate qPCR testing of SFB amounts in stools from MIA-exposed mice [122] to minimize experiment-to-experiment variation. If SFB is absent in the vivarium, colonizing mice with SFB might likely produce more robust MIA phenotypes than those under different housing conditions—a key parameter for consideration.
Box 2: Anti-inflammatory factors suppressing MIA.
While many studies have shown that inflammatory factors can promote MIA-associated phenotypes, less attention has been given to identifying protective factors that can suppress the development of MIA and prevent offspring from developing MIA-induced neural deficits and psychiatric disorders. In humans, adverse conditions in pregnancy, such as preterm delivery, are strongly associated with loss-of-function mutations in IL-10, a widely studied anti-inflammatory factor [150]. Pregnant IL-10 mutant mice (Il10 knockout) develop more severe inflammation to a low-dose LPS injection at late gestation, leading to significantly enhanced fetal loss, compared to wild-type mice [151]. Conversely, exogenous IL-10 given intraperitoneally to pregnant mice prevents fetal loss by restraining the production of LPS-induced inflammatory cytokines [151]. Likewise, ectopic expression of IL-10 in macrophages is sufficient to attenuate poly(I:C)-induced MIA during pregnancy and protect mouse offspring from long-term behavioral abnormalities [152], highlighting IL-10 as a maternal bulwark against MIA. Together with IL-10, Foxp3+ regulatory T cells (Tregs) (which play key roles in immunological homeostasis) are thought to presumably help maintain a healthy pregnancy and feto-maternal tolerance against MIA [153]. Indeed, the functional impairment of Tregs has been reported to contribute to various human pregnancy complications, including implantation failure, miscarriage, and preeclampsia [153]. Moreover, Earlier studies have described the functional significance of peripherally induced Tregs in maintaining feto-maternal tolerance [153]. Transient depletion of Tregs (using the Foxp3DTR knock-in mouse line) in pregnant mice resulted in fetal resorption phenotypes accompanied by an expansion of IFN-γ-producing, fetal-specific T helper type 1 (Th1) cells in the spleen. Treg depletion in other contexts often triggers devastating inflammation, leading to various types of auto-inflammation. By contrast, Treg supplementation in offspring via an intravenous route has been reported to yield beneficial effects by dampening T cell inflammatory responses in MIA offspring [145]. One consideration is that perhaps Treg dysfunction during pregnancy might also give rise to MIA, potentially leading to neural deficits and psychiatric disorders in offspring, although this remains conjectural.
The gut microbiome in other autism mouse models and in humans
Accumulating data suggest the importance of the host gut microbiome in affecting autism-related phenotypes. Several recent studies have found an altered microbiome in ASD patients, compared with healthy controls, suggesting a putative link between the gut microbiome and autism [123]. Moreover, transplanting gut microbiota from ASD patients into germ-free mice has been shown to promote autism-like behavioral changes in animal recipients [115]. However, a recent large-scale metagenomic study assessing the gut microbiota community in human ASD patients suggests that microbiome differences in ASD may be linked to dietary preference, cautioning against oversimplifying the importance of the gut microbiome in modulating either immunological or neurodevelopmental outcomes [124].
In mice, MIA offspring and several monogenic mouse models for autism such as BTBR or Shank3 mutant mice display gut microbiome dysbiosis [125,126]. Oral gavage with anti-inflammatory bacteria, such as Bacteroides fragilis or Lactobacillus reuteri, has mitigated autism-like behavioral abnormalities, such as social deficits [115,125,127]. Furthermore, oral administration of specific microbial metabolites, including 5AV and taurine, has reduced autism-related repetitive and social behaviors in ASD mouse models [115], while other metabolites, such as 4-ethylphenylsulfate, have exacerbated autism-related anxiety [128]; this has suggested a versatile role of microbial metabolites in affecting brain function and animal behaviors. While a mechanistic understanding is still missing, these studies indicate that non-genetic factors (e.g., microbiome) may interact with genetic factors in modulating ASD pathogenesis.
Concluding remarks
Growing evidence indicates that MIA affects the health of offspring in immunological, metabolic, and neurological disorders [2,129]. In this review, while we primarily focused on autism, many preclinical and human studies have linked MIA with other neurodevelopmental disorders, including anxiety, major depressive disorders, and bipolar disorders [130,131], which often harbor both genetic and environmental contributions to the inherent pathology. Hence, MIA may be considered a surrogate model for studying certain human psychiatric diseases under conditions in which inflammation and immunology are relevant. Accordingly, it would be interesting to investigate whether MIA offspring become susceptible to developing other inflammatory and metabolic disorders, such as diabetes, allergy, multiple sclerosis, and inflammatory bowel disease, since MIA may substantially impact the physiology of the developing fetus. Indeed, we recently demonstrated that pregnant mothers exposed to MIA-induced IL-17a presented altered gut microbiota, and their offspring exhibited primed immunological phenotypes, as evidenced by an enhanced susceptibility to gut inflammation caused by Citrobacter infection in mice [132]. In this study, we reported that this immune priming was due to alterations in the chromatin accessibility of genes linked to the regulation of CD4+ T cell activity. As a result, in experiments such as these, MIA offspring developed heightened susceptibility to autoimmune inflammation in adulthood. By contrast, bacterially-induced MIA in the absence of SFB rendered offspring more resistant to pathogenic Salmonella infection through an IL-6-dependent mechanism by promoting epigenetic memory in intestinal epithelial cells. [80]. Collectively, these data demonstrate the complexities arising from different types of MIA that can modulate maternal gut bacteria (composition and frequencies), and which can lead to different immunological outcomes in the offspring.
From another angle, genetic factors are key drivers for ASD, supported by many twin concordance studies [133]. Hence, ASD research has centered on identifying genetic factors that drive ASD phenotypes with high penetrance. However, major genetic factors that govern ASD pathogenesis remain elusive, despite numerous efforts. The notion that non-CNS-derived factors, such as the gut microbiome, peripheral immune system, and their interactions (known as the gut-immune-brain axis [134]) play a role in ASD pathogenesis is gaining traction. Thus, understanding how these factors combine with or modulate genetic susceptibility and affect brain development represents an imminent research area that might potentially transform how ASD pathologies are diagnosed and prevented (see outstanding questions).
Outstanding questions.
Is there a specific time during fetal development when MIA induces long-lasting behavioral and neurodevelopmental changes? Human and mouse studies suggest that the timing at which developing fetuses are exposed to inflammation matters. However, a mechanistic understanding is lacking. Studies are needed to determine whether this time-dependent “immune sensitivity” reflects differences in the magnitude of maternal immune responses (e.g., amuonts of MIA-inducing cytokines), particular stages of fetal neurogenesis (e.g., cortical formation and neuronal migration), or the transfer of maternal immune molecules into the fetal compartment (e.g., the establishment of the blood-brain barrier in the fetus).
What are the downstream events of MIA? It is largely unknown how IL-17 receptor signaling leads to enhanced ISR and how, in turn, it results in dysregulated neural activity in the S1DZ area. Additionally, the mechanisms by which MIA primes an offspring’s immune responses require further investigation.
What are the upstream events of MIA? Studies need to be done to determine how poly(I:C) injection stimulates IL-6 and IL-17a responses in pregnant mice.
What determines MIA-induced behavioral changes in male but not in female offspring? Male, not female, MIA offspring display enhanced ISR and behavioral abnormalities. The mechanisms underlying the enhanced male susceptibility to MIA-associated changes requires robust investigation.
What other inflammatory stimuli induce MIA? The contributing roles in pregnancy of autoimmune and inflammatory conditions, such as inflammatory bowel disease (IBD), psoriasis, and asthma, need to be extensively tested. Given that the anti-fungal response in humans constitutes a major Th17 cell program, understanding how fungal infections in pregnancy affect fetal brain development remain to be investigated.
What are the main limitations of the current MIA model? Most MIA work has been done using poly(I:C) injection of pregnant mice. Findings made from this model need to be confirmed or expanded to pathogenic viral or bacterial infections. In addition, more studies are needed to dissect the MIA-induced immunological and neurological phenotypes in non-human primate models, which will provide insights towards clinical relevance.
We posit that MIA rodent models can facilitate the development of potential preventive and therapeutic approaches to treating human ASD and other specific psychiatric disorders. Studies have identified maternal factors as potential targets for MIA-induced neural deficits and abnormal behaviors. For example, blocking maternal cytokines (e.g., IL-6 and IL-17a) during pregnancy suppresses MIA-induced autism-like phenotypes in rodent offspring [74,83]. In addition, performing bacteria-driven intervention to affect the maternal microbiome using a particular bacterial species or a defined consortium of the latter might be developed and tested as a putative therapeutic approach to ASD-like maladies. Altogether, we argue that MIA mouse models, in addition to other monogenic mouse models for autism, can provide invaluable avenues and complementary approaches for identifying ways to prevent and treat autism and/or other immune-mediated psychiatric illnesses – certainly representing a fruitful area of future investigation.
Whether or not the findings generated with MIA mouse models can be translated to human patients with neurodevelopmental disorders evidently awaits future validation. Large-scale, longitudinal studies following children born to pregnant mothers infected with pathogens or exposed to severe inflammation are thus warranted. Lastly, a special focus needs to be placed on acquiring samples for immunological and microbiome analyses during the course of pregnancy with or without exposure to inflammation.
Highlights.
An increased risk of neurodevelopmental and psychiatric disorders in children has been associated with their exposure to heightened inflammation in the womb caused by respiratory infections or other inflammatory stimuli.
Rodent models have provided a wealth of information on how maternal immune activation (MIA) influences brain development and animal behaviors.
Maternal gut bacteria shape maternal immune function and influence the development of autism-like phenotypes in offspring.
A comprehensive understanding of the gut bacteria-immune-neuro interaction in MIA is crucial to developing diagnostic and preventive measures for high-risk pregnant women and identifying targets for treating inflammation-induced neurodevelopmental disorders.
Acknowledgments
We thank M. Trombly for scientific editing of this manuscript. H.K.K. was supported by the National Research Foundation of Korea grants 2019M3C9A6091949, 2019R1A6A1A03032869, and 2021R1A2C200450. G.B.C. and J.R.H. were supported by the Jeongho Kim Neurodevelopmental Research Fund, the Simons Foundation Autism Research Initiative, and National Institute of Mental Health grants (R01MH115037 and R01MH119459, respectively). All figures were created with Biorender.com.
Glossary
- Regulatory T cells
specialized subset of T cells that maintain homeostasis and self-tolerance.
- Maternal immune activation
Immune stimulation during pregnancy, due to pathogenic infection or exposure to other inflammatory stimuli.
- Pre-pulse inhibition
neurological phenomenon in which a weaker pre-stimulus inhibits the organism’s reaction to a subsequent, strong, reflex-eliciting stimulus.
- Gestational diabetes mellitus
common metabolic disorder during pregnancy that causes considerable morbidity and pregnancy complications.
- Phthalates
group of chemicals used to make plastics more durable, which induce various pathological effects, including neurotoxicity and anti-androgenic effects.
- Toll-like receptor
class of receptor proteins; play crucial roles in recognizing pathogen-associated molecular patterns derived from various microbes to initiate an innate immune response.
- Integrated stress response
conserved cellular stress response in eukaryotic cells; downregulates protein synthesis and upregulates specific gene expression in response to internal or environmental stressors.
- The primary somatosensory cortex, the dysgranular zone of the S1
area of the cerebral cortex that receives sensory information, including from the somatic, proprioceptive, and from certain visceral senses.
- T helper type 1 (Th1) cells
subset of CD4+ effector T cells; promote cell-mediated immune responses and are required for host defense against intracellular viral and bacterial pathogens.
Footnotes
Declaration of interests
H.K.K. is a scientific advisor member in Enhanced Neo Cell. J.R.H. is a consultant for CJ Research Center, LLC, Interon Laboratories, Inc. and on the scientific advisory board for ChunLab, Inc. G.B.C. is a consultant for Interon Laboratories, Inc.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Erlebacher A. (2013) Mechanisms of T cell tolerance towards the allogeneic fetus. Nat Rev Immunol 13, 23–33. 10.1038/nri3361 [DOI] [PubMed] [Google Scholar]
- 2.Arck PC and Hecher K. (2013) Fetomaternal immune cross-talk and its consequences for maternal and offspring’s health. Nat Med 19, 548–556. 10.1038/nm.3160 [DOI] [PubMed] [Google Scholar]
- 3.Rudolph MD et al. (2018) Maternal IL-6 during pregnancy can be estimated from newborn brain connectivity and predicts future working memory in offspring. Nat Neurosci 21, 765–772. 10.1038/s41593-018-0128-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shin Yim Y. et al. (2017) Reversing behavioural abnormalities in mice exposed to maternal inflammation. Nature 549, 482–487. 10.1038/nature23909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Knuesel I. et al. (2014) Maternal immune activation and abnormal brain development across CNS disorders. Nat Rev Neurol 10, 643–660. 10.1038/nrneurol.2014.187 [DOI] [PubMed] [Google Scholar]
- 6.Bangma JT et al. (2021) Placental programming, perinatal inflammation, and neurodevelopment impairment among those born extremely preterm. Pediatric Research 89, 326–335. 10.1038/s41390-020-01236-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kendell RE and Kemp IW (1989) Maternal Influenza in the Etiology of Schizophrenia. Archives of General Psychiatry 46, 878–882. 10.1001/archpsyc.1989.01810100020004 [DOI] [PubMed] [Google Scholar]
- 8.Chess S. (1971) Autism in children with congenital rubella. J Autism Child Schizophr 1, 33–47 [DOI] [PubMed] [Google Scholar]
- 9.Brown AS and Derkits EJ (2010) Prenatal infection and schizophrenia: a review of epidemiologic and translational studies. Am J Psychiatry 167, 261–280. 10.1176/appi.ajp.2009.09030361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Byrne M. et al. (2007) Obstetric conditions and risk of first admission with schizophrenia: a Danish national register based study. Schizophr Res 97, 51–59. 10.1016/j.schres.2007.07.018 [DOI] [PubMed] [Google Scholar]
- 11.Zerbo O. et al. (2011) Month of conception and risk of autism. Epidemiology 22, 469–475. 10.1097/EDE.0b013e31821d0b53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee BK et al. (2015) Maternal hospitalization with infection during pregnancy and risk of autism spectrum disorders. Brain Behav Immun 44, 100–105. 10.1016/j.bbi.2014.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patterson PH (2009) Immune involvement in schizophrenia and autism: etiology, pathology and animal models. Behav Brain Res 204, 313–321. 10.1016/j.bbr.2008.12.016 [DOI] [PubMed] [Google Scholar]
- 14.Solek CM et al. (2018) Maternal immune activation in neurodevelopmental disorders. Dev Dyn 247, 588–619. 10.1002/dvdy.24612 [DOI] [PubMed] [Google Scholar]
- 15.Atladottir HO et al. (2010) Maternal infection requiring hospitalization during pregnancy and autism spectrum disorders. J Autism Dev Disord 40, 1423–1430. 10.1007/s10803-010-1006-y [DOI] [PubMed] [Google Scholar]
- 16.Croen LA et al. (2005) Maternal autoimmune diseases, asthma and allergies, and childhood autism spectrum disorders: a case-control study. Arch Pediatr Adolesc Med 159, 151–157. 10.1001/archpedi.159.2.151 [DOI] [PubMed] [Google Scholar]
- 17.Patel S. et al. (2018) Social impairments in autism spectrum disorder are related to maternal immune history profile. Mol Psychiatry 23, 1794–1797. 10.1038/mp.2017.201 [DOI] [PubMed] [Google Scholar]
- 18.Varcin KJ et al. (2017) Prenatal maternal stress events and phenotypic outcomes in Autism Spectrum Disorder. Autism Res 10, 1866–1877. 10.1002/aur.1830 [DOI] [PubMed] [Google Scholar]
- 19.Raz R. et al. (2015) Autism spectrum disorder and particulate matter air pollution before, during, and after pregnancy: a nested case-control analysis within the Nurses’ Health Study II Cohort. Environ Health Perspect 123, 264–270. 10.1289/ehp.1408133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lyall K. et al. (2013) Maternal dietary fat intake in association with autism spectrum disorders. Am J Epidemiol 178, 209–220. 10.1093/aje/kws433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spann MN et al. (2018) Maternal Immune Activation During the Third Trimester Is Associated with Neonatal Functional Connectivity of the Salience Network and Fetal to Toddler Behavior. J Neurosci 38, 2877–2886. 10.1523/JNEUROSCI.2272-17.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Graham AM et al. (2018) Maternal Systemic Interleukin-6 During Pregnancy Is Associated With Newborn Amygdala Phenotypes and Subsequent Behavior at 2 Years of Age. Biol Psychiatry 83, 109–119. 10.1016/j.biopsych.2017.05.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Casey S. et al. (2021) Maternal Mid-Gestation Cytokine Dysregulation in Mothers of Children with Autism Spectrum Disorder. J Autism Dev Disord. 10.1007/s10803-021-05271-7 [DOI] [PMC free article] [PubMed]
- 24.Boyle CA et al. (2011) Trends in the prevalence of developmental disabilities in US children, 1997–2008. Pediatrics 127, 1034–1042. 10.1542/peds.2010-2989 [DOI] [PubMed] [Google Scholar]
- 25.Dashraath P. et al. (2020) Coronavirus disease 2019 (COVID-19) pandemic and pregnancy. American journal of obstetrics and gynecology 222, 521–531. 10.1016/j.ajog.2020.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zuckerman L. and Weiner I. (2005) Maternal immune activation leads to behavioral and pharmacological changes in the adult offspring. J Psychiatr Res 39, 311–323 [DOI] [PubMed] [Google Scholar]
- 27.Meyer U. et al. (2006) Prenatal and postnatal maternal contributions in the infection model of schizophrenia. Experimental Brain Research 173, 243–257. 10.1007/s00221-006-0419-5 [DOI] [PubMed] [Google Scholar]
- 28.Shi L. et al. (2003) Maternal influenza infection causes marked behavioral and pharmacological changes in the offspring. J Neurosci 23, 297–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith SE et al. (2007) Maternal immune activation alters fetal brain development through interleukin-6. J Neurosci 27, 10695–10702. 10.1523/JNEUROSCI.2178-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Careaga M. et al. (2017) Maternal Immune Activation and Autism Spectrum Disorder: From Rodents to Nonhuman and Human Primates. Biol Psychiatry 81, 391–401. 10.1016/j.biopsych.2016.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Santana-Coelho D. et al. (2021) Advancing Autism Research From Mice to Marmosets: Behavioral Development of Offspring Following Prenatal Maternal Immune Activation. Frontiers in Psychiatry 12. 10.3389/fpsyt.2021.705554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chang Y-C et al. (2018) Prenatal and early-life diesel exhaust exposure causes autism-like behavioral changes in mice. Particle and fibre toxicology 15, 18–18. 10.1186/s12989-018-0254-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rivera HM et al. (2015) The role of maternal obesity in the risk of neuropsychiatric disorders. Front Neurosci 9, 194. 10.3389/fnins.2015.00194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krakowiak P. et al. (2012) Maternal metabolic conditions and risk for autism and other neurodevelopmental disorders. Pediatrics 129, e1121–1128. 10.1542/peds.2011-2583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bilbo SD and Tsang V. (2010) Enduring consequences of maternal obesity for brain inflammation and behavior of offspring. FASEB J 24, 2104–2115. 10.1096/fj.09-144014 [DOI] [PubMed] [Google Scholar]
- 36.Sullivan EL et al. (2010) Chronic consumption of a high-fat diet during pregnancy causes perturbations in the serotonergic system and increased anxiety-like behavior in nonhuman primate offspring. J Neurosci 30, 3826–3830. 10.1523/JNEUROSCI.5560-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weir RK et al. (2015) Preliminary evidence of neuropathology in nonhuman primates prenatally exposed to maternal immune activation. Brain, behavior, and immunity 48, 139–146. 10.1016/j.bbi.2015.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bordeleau M. et al. (2020) Microglial and peripheral immune priming is partially sexually dimorphic in adolescent mouse offspring exposed to maternal high-fat diet. Journal of Neuroinflammation 17, 264. 10.1186/s12974-020-01914-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xavier S. et al. (2021) Maternal diet before and during pregnancy modulates microglial activation and neurogenesis in the postpartum rat brain. Brain, Behavior, and Immunity 98, 185–197. 10.1016/j.bbi.2021.08.223 [DOI] [PubMed] [Google Scholar]
- 40.Castellana B. et al. (2018) Maternal obesity alters uterine NK activity through a functional KIR2DL1/S1 imbalance. Immunology & Cell Biology 96,805–819. 10.1111/imcb.12041 [DOI] [PubMed] [Google Scholar]
- 41.Fernandes DJ et al. (2021) Exposure to maternal high-fat diet induces extensive changes in the brain of adult offspring. Translational Psychiatry 11, 149. 10.1038/s41398-021-01274-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Buffington SA et al. (2016) Microbial Reconstitution Reverses Maternal Diet-Induced Social and Synaptic Deficits in Offspring. Cell 165, 1762–1775. 10.1016/j.cell.2016.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu X. et al. (2021) High-fiber diet mitigates maternal obesity-induced cognitive and social dysfunction in the offspring via gut-brain axis. Cell Metabolism 33, 923–938.e926. 10.1016/j.cmet.2021.02.002 [DOI] [PubMed] [Google Scholar]
- 44.Madan JC et al. (2009) Maternal obesity and markers of inflammation in pregnancy. Cytokine 47, 61–64. 10.1016/j.cyto.2009.05.004 [DOI] [PubMed] [Google Scholar]
- 45.Bliddal M. et al. (2014) Maternal pre-pregnancy BMI and intelligence quotient (IQ) in 5-year-old children: a cohort based study. PloS one 9, e94498–e94498. 10.1371/journal.pone.0094498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Heikura U. et al. (2007) Variations in Prenatal Sociodemographic Factors associated with Intellectual Disability: A Study of the 20-Year Interval between Two Birth Cohorts in Northern Finland. American Journal of Epidemiology 167, 169–177. 10.1093/aje/kwm291 [DOI] [PubMed] [Google Scholar]
- 47.Connolly N. et al. (2016) Maternal metabolic risk factors for autism spectrum disorder—An analysis of electronic medical records and linked birth data. Autism Research 9, 829–837. 10.1002/aur.1586 [DOI] [PubMed] [Google Scholar]
- 48.Getz KD et al. (2016) Maternal Pre-pregnancy Body Mass Index and Autism Spectrum Disorder among Offspring: A Population-Based Case–Control Study. Paediatric and Perinatal Epidemiology 30, 479–487. 10.1111/ppe.12306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen Q. et al. (2013) Maternal pre-pregnancy body mass index and offspring attention deficit hyperactivity disorder: a population-based cohort study using a sibling-comparison design. International Journal of Epidemiology 43, 83–90. 10.1093/ije/dyt152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kawai M. et al. (2004) Poor maternal care and high maternal body mass index in pregnancy as a risk factor for schizophrenia in offspring. Acta Psychiatrica Scandinavica 110, 257–263. 10.1111/j.1600-0447.2004.00380.x [DOI] [PubMed] [Google Scholar]
- 51.Rodriguez A. (2010) Maternal pre-pregnancy obesity and risk for inattention and negative emotionality in children. Journal of Child Psychology and Psychiatry 51, 134–143. 10.1111/j.1469-7610.2009.02133.x [DOI] [PubMed] [Google Scholar]
- 52.Messerlian C. et al. (2017) Paternal and maternal preconception urinary phthalate metabolite concentrations and child behavior. Environ Res 158, 720–728. 10.1016/j.envres.2017.07.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Emam B. et al. (2020) Effects of PM(2.5) and gases exposure during prenatal and early-life on autism-like phenotypes in male rat offspring. Particle and fibre toxicology 17, 8–8. 10.1186/s12989-020-0336-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Volk HE et al. (2013) Traffic-related air pollution, particulate matter, and autism. JAMA Psychiatry 70, 71–77. 10.1001/jamapsychiatry.2013.266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Roberts AL et al. (2013) Perinatal air pollutant exposures and autism spectrum disorder in the children of Nurses’ Health Study II participants. Environ Health Perspect 121, 978–984. 10.1289/ehp.1206187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bolton JL et al. (2013) Maternal stress and effects of prenatal air pollution on offspring mental health outcomes in mice. Environ Health Perspect 121, 1075–1082. 10.1289/ehp.1306560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Grunig G. et al. (2014) Perspective: ambient air pollution: inflammatory response and effects on the lung’s vasculature. Pulmonary circulation 4, 25–35. 10.1086/674902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Walder DJ et al. (2014) Prenatal maternal stress predicts autism traits in 6½ year-old children: Project Ice Storm. Psychiatry Res 219, 353–360. 10.1016/j.psychres.2014.04.034 [DOI] [PubMed] [Google Scholar]
- 59.Kinney DK et al. (2008) Autism prevalence following prenatal exposure to hurricanes and tropical storms in Louisiana. J Autism Dev Disord 38, 481–488. 10.1007/s10803-007-0414-0 [DOI] [PubMed] [Google Scholar]
- 60.Zuena AR et al. (2008) Prenatal restraint stress generates two distinct behavioral and neurochemical profiles in male and female rats. PLoS One 3, e2170. 10.1371/journal.pone.0002170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bronson SL and Bale TL (2014) Prenatal Stress-Induced Increases in Placental Inflammation and Offspring Hyperactivity Are Male-Specific and Ameliorated by Maternal Antiinflammatory Treatment. Endocrinology 155, 2635–2646. 10.1210/en.2014-1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Notarangelo FM and Schwarcz R. (2016) Restraint Stress during Pregnancy Rapidly Raises Kynurenic Acid Levels in Mouse Placenta and Fetal Brain. Developmental Neuroscience 38, 458–468. 10.1159/000455228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gumusoglu SB et al. (2017) The role of IL-6 in neurodevelopment after prenatal stress. Brain, behavior, and immunity 65, 274–283. 10.1016/j.bbi.2017.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Weinstock M. (2008) The long-term behavioural consequences of prenatal stress. Neurosci Biobehav Rev 32, 1073–1086 [DOI] [PubMed] [Google Scholar]
- 65.Bittle J. and Stevens HE (2018) The role of glucocorticoid, interleukin-1β, and antioxidants in prenatal stress effects on embryonic microglia. Journal of Neuroinflammation 15, 44. 10.1186/s12974-018-1079-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shi L. et al. (2009) Activation of the maternal immune system alters cerebellar development in the offspring. Brain Behav Immun 23, 116–123. 10.1016/j.bbi.2008.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Short SJ et al. (2010) Maternal influenza infection during pregnancy impacts postnatal brain development in the rhesus monkey. Biol Psychiatry 67, 965–973. 10.1016/j.biopsych.2009.11.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liong S. et al. (2020) Influenza A virus causes maternal and fetal pathology via innate and adaptive vascular inflammation in mice. Proceedings of the National Academy of Sciences 117, 24964–24973. 10.1073/pnas.2006905117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Steinman G. (2020) COVID-19 and autism. Med Hypotheses 142, 109797. 10.1016/j.mehy.2020.109797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Patterson PH (2011) Maternal infection and immune involvement in autism. Trends Mol Med 17, 389–394. 10.1016/j.molmed.2011.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Meehan C. et al. (2017) Effects of immune activation during early or late gestation on schizophrenia-related behaviour in adult rat offspring. Brain Behav Immun 63, 8–20. 10.1016/j.bbi.2016.07.144 [DOI] [PubMed] [Google Scholar]
- 72.Prater MR et al. (2006) Maternal treatment with a high dose of CpG ODN during gestation alters fetal craniofacial and distal limb development in C57BL/6 mice. Vaccine 24, 263–271. 10.1016/j.vaccine.2005.07.105 [DOI] [PubMed] [Google Scholar]
- 73.Carlezon WA et al. (2019) Maternal and early postnatal immune activation produce sex-specific effects on autism-like behaviors and neuroimmune function in mice. Scientific Reports 9, 16928. 10.1038/s41598-019-53294-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hsiao EY and Patterson PH (2011) Activation of the maternal immune system induces endocrine changes in the placenta via IL-6. Brain Behav Immun 25, 604–615. 10.1016/j.bbi.2010.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tanaka T. et al. (2014) IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol 6, a016295. 10.1101/cshperspect.a016295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Connor CM et al. (2012) Maternal immune activation alters behavior in adult offspring, with subtle changes in the cortical transcriptome and epigenome. Schizophr Res 140, 175–184. 10.1016/j.schres.2012.06.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wei H. et al. (2011) IL-6 is increased in the cerebellum of autistic brain and alters neural cell adhesion, migration and synaptic formation. J Neuroinflammation 8, 52. 10.1186/1742-2094-8-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wu WL et al. (2017) The placental interleukin-6 signaling controls fetal brain development and behavior. Brain Behav Immun 62, 11–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Courchesne E. (1997) Brainstem, cerebellar and limbic neuroanatomical abnormalities in autism. Current Opinion in Neurobiology 7, 269–278 [DOI] [PubMed] [Google Scholar]
- 80.Lim AI et al. (2021) Prenatal maternal infection promotes tissue-specific immunity and inflammation in offspring. Science 373, eabf3002. doi: 10.1126/science.abf3002 [DOI] [PubMed] [Google Scholar]
- 81.Mirabella F. et al. (2021) Prenatal interleukin 6 elevation increases glutamatergic synapse density and disrupts hippocampal connectivity in offspring. Immunity 54, 2611–2631.e2618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hunter CA and Jones SA (2015) IL-6 as a keystone cytokine in health and disease. Nat Immunol 16, 448–457. 10.1038/ni.3153 [DOI] [PubMed] [Google Scholar]
- 83.Choi GB et al. (2016) The maternal interleukin-17a pathway in mice promotes autism-like phenotypes in offspring. Science 351, 933–939. 10.1126/science.aad0314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Harrington LE et al. (2005) Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol 6, 1123–1132. 10.1038/ni1254 [DOI] [PubMed] [Google Scholar]
- 85.McGeachy MJ et al. (2019) The IL-17 Family of Cytokines in Health and Disease. Immunity 50, 892–906. 10.1016/j.immuni.2019.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ribeiro M. et al. (2019) Meningeal γδ T cell-derived IL-17 controls synaptic plasticity and short-term memory. Sci Immunol 4. 10.1126/sciimmunol.aay5199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Faraco G. et al. (2018) Dietary salt promotes neurovascular and cognitive dysfunction through a gut-initiated TH17 response. Nature Neuroscience 21, 240–249. 10.1038/s41593-017-0059-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Beurel E. et al. (2013) Inflammatory T helper 17 cells promote depression-like behavior in mice. Biol Psychiatry 73, 622–630. 10.1016/j.biopsych.2012.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Alves de Lima K. et al. (2020) Meningeal γδ T cells regulate anxiety-like behavior via IL-17a signaling in neurons. Nature Immunology 21, 1421–1429. 10.1038/s41590-020-0776-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.McGinley AM et al. (2020) Interleukin-17A Serves a Priming Role in Autoimmunity by Recruiting IL-1β-Producing Myeloid Cells that Promote Pathogenic T Cells. Immunity 52, 342–356.e346 [DOI] [PubMed] [Google Scholar]
- 91.Suzuki K. et al. (2011) Plasma cytokine profiles in subjects with high-functioning autism spectrum disorders. PLoS One 6, e20470. 10.1371/journal.pone.0020470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Akintunde ME et al. (2015) Increased production of IL-17 in children with autism spectrum disorders and co-morbid asthma. Journal of neuroimmunology 286, 33–41. 10.1016/j.jneuroim.2015.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kim S. et al. (2017) Maternal gut bacteria promote neurodevelopmental abnormalities in mouse offspring. Nature 549, 528–532. 10.1038/nature23910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang X. et al. (2019) Oral probiotic administration during pregnancy prevents autism-related behaviors in offspring induced by maternal immune activation via anti-inflammation in mice. Autism Research 12, 576–588 [DOI] [PubMed] [Google Scholar]
- 95.Estes ML et al. (2020) Baseline immunoreactivity before pregnancy and poly(I:C) dose combine to dictate susceptibility and resilience of offspring to maternal immune activation. Brain, Behavior, and Immunity 88, 619–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tome S. et al. (2019) Elevated maternal retinoic acid-related orphan receptor-γt enhances the effect of polyinosinic-polycytidylic acid in inducing fetal loss. Experimental Animals 68, 491–497. 10.1538/expanim.19-0038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ivanov II et al. (2006) The Orphan Nuclear Receptor RORgt Directs the Differentiation Program of Proinflammatory IL-17+ T Helper Cells. Cell 126, 1121–1133. 10.1016/j.cell.2006.07.035 [DOI] [PubMed] [Google Scholar]
- 98.Sasaki T. et al. (2021) Effects of RORγt overexpression on the murine central nervous system. Neuropsychopharmacology Reports 41, 102–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sasaki T. et al. (2020) Intraventricular IL-17A administration activates microglia and alters their localization in the mouse embryo cerebral cortex. Molecular Brain 13, 93. 10.1186/s13041-020-00635-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yasumatsu K. et al. (2020) Bacterial-induced maternal interleukin-17A pathway promotes autistic-like behaviors in mouse offspring. Experimental animals 69, 250–260. 10.1538/expanim.19-0156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lammert CR et al. (2018) Cutting Edge: Critical Roles for Microbiota-Mediated Regulation of the Immune System in a Prenatal Immune Activation Model of Autism. J Immunol 201, 845–850. 10.4049/jimmunol.1701755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tsukada T. et al. (2021) Mid-pregnancy maternal immune activation increases Pax6-positive and Tbr2-positive neural progenitor cells and causes integrated stress response in the fetal brain in a mouse model of maternal viral infection. IBRO Neuroscience Reports 11, 73–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kalish BT et al. (2020) Maternal immune activation in mice disrupts proteostasis in the fetal brain. Nature Neuroscience. 10.1038/s41593-020-00762-9 [DOI] [PMC free article] [PubMed]
- 104.Ben-Yehuda H. et al. (2020) Maternal Type-I interferon signaling adversely affects the microglia and the behavior of the offspring accompanied by increased sensitivity to stress. Molecular Psychiatry 25, 1050–1067. 10.1038/s41380-019-0604-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rooks MG and Garrett WS (2016) Gut microbiota, metabolites and host immunity. Nature Reviews Immunology 16, 341–352. 10.1038/nri.2016.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sharon G. et al. (2016) The Central Nervous System and the Gut Microbiome. Cell 167, 915–932. 10.1016/j.cell.2016.10.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gareau MG et al. (2011) Bacterial infection causes stress-induced memory dysfunction in mice. Gut 60, 307–317. 10.1136/gut.2009.202515 [DOI] [PubMed] [Google Scholar]
- 108.Diaz Heijtz R. et al. (2011) Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci U S A 108, 3047–3052. 10.1073/pnas.1010529108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Neufeld KM et al. (2011) Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterol Motil 23, 255–264, e119. 10.1111/j.1365-2982.2010.01620.x [DOI] [PubMed] [Google Scholar]
- 110.Sudo N. et al. (2004) Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J Physiol 558, 263–275. 10.1113/jphysiol.2004.063388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Desbonnet L. et al. (2014) Microbiota is essential for social development in the mouse. Mol Psychiatry 19, 146–148. 10.1038/mp.2013.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Braniste V. et al. (2014) The gut microbiota influences blood-brain barrier permeability in mice. Sci Transl Med 6, 263ra158. 10.1126/scitranslmed.3009759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Blacher E. et al. (2019) Potential roles of gut microbiome and metabolites in modulating ALS in mice. Nature 572, 474–480. 10.1038/s41586-019-1443-5 [DOI] [PubMed] [Google Scholar]
- 114.Hertel J. et al. (2019) Integrated Analyses of Microbiome and Longitudinal Metabolome Data Reveal Microbial-Host Interactions on Sulfur Metabolism in Parkinson’s Disease. Cell Rep 29, 1767–1777.e1768. 10.1016/j.celrep.2019.10.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sharon G. et al. (2019) Human Gut Microbiota from Autism Spectrum Disorder Promote Behavioral Symptoms in Mice. Cell 177, 1600–1618.e1617. 10.1016/j.cell.2019.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ivanov II et al. (2009) Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 139, 485–498. 10.1016/j.cell.2009.09.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gaboriau-Routhiau V. et al. (2009) The Key Role of Segmented Filamentous Bacteria in the Coordinated Maturation of Gut Helper T Cell Responses. Immunity 31, 677–689. 10.1016/j.immuni.2009.08.020 [DOI] [PubMed] [Google Scholar]
- 118.Kentner AC et al. (2019) Maternal immune activation: reporting guidelines to improve the rigor, reproducibility, and transparency of the model. Neuropsychopharmacology 44, 245–258. 10.1038/s41386-018-0185-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Atarashi K. et al. (2013) Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature 500, 232–236. 10.1038/nature12331 [DOI] [PubMed] [Google Scholar]
- 120.Kwon H-K et al. (2010) Generation of regulatory dendritic cells and CD4+Foxp3+ T cells by probiotics administration suppresses immune disorders. Proceedings of the National Academy of Sciences 107, 2159–2164. 10.1073/pnas.0904055107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kriegel MA et al. (2011) Naturally transmitted segmented filamentous bacteria segregate with diabetes protection in nonobese diabetic mice. Proceedings of the National Academy of Sciences 108, 11548–11553. 10.1073/pnas.1108924108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Farkas AM et al. (2015) Induction of Th17 cells by segmented filamentous bacteria in the murine intestine. Journal of Immunological Methods 421, 104–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Xu M. et al. (2019) Association Between Gut Microbiota and Autism Spectrum Disorder: A Systematic Review and Meta-Analysis. Frontiers in psychiatry 10, 473–473. 10.3389/fpsyt.2019.00473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yap CX et al. (2021) Autism-related dietary preferences mediate autism-gut microbiome associations. Cell 184, 5916–5931.e5917 [DOI] [PubMed] [Google Scholar]
- 125.Tabouy L. et al. (2018) Dysbiosis of microbiome and probiotic treatment in a genetic model of autism spectrum disorders. Brain, Behavior, and Immunity 73, 310–319 [DOI] [PubMed] [Google Scholar]
- 126.Coretti L. et al. (2017) Sex-related alterations of gut microbiota composition in the BTBR mouse model of autism spectrum disorder. Scientific Reports 7, 45356. 10.1038/srep45356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sgritta M. et al. (2019) Mechanisms Underlying Microbial-Mediated Changes in Social Behavior in Mouse Models of Autism Spectrum Disorder. Neuron 101, 246–259.e246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hsiao EY et al. (2013) Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell 155, 1451–1463. 10.1016/j.cell.2013.11.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Estes ML and McAllister AK (2016) Maternal immune activation: Implications for neuropsychiatric disorders. Science 353, 772–777. 10.1126/science.aag3194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Parboosing R. et al. (2013) Gestational influenza and bipolar disorder in adult offspring. JAMA Psychiatry 70, 677–685. 10.1001/jamapsychiatry.2013.896 [DOI] [PubMed] [Google Scholar]
- 131.Simanek AM and Meier HC (2015) Association Between Prenatal Exposure to Maternal Infection and Offspring Mood Disorders: A Review of the Literature. Curr Probl Pediatr Adolesc Health Care 45, 325–364. 10.1016/j.cppeds.2015.06.008 [DOI] [PubMed] [Google Scholar]
- 132.Kim E. et al. (2021) Maternal gut bacteria drive intestinal inflammation in offspring with neurodevelopmental disorders by altering the chromatin landscape of CD4+ T cells. Immunity, [DOI] [PMC free article] [PubMed]
- 133.Tick B. et al. (2016) Heritability of autism spectrum disorders: a meta-analysis of twin studies. Journal of Child Psychology and Psychiatry 57, 585–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Huh JR and Veiga-Fernandes H. (2020) Neuroimmune circuits in inter-organ communication. Nature Reviews Immunology 20, 217–228. 10.1038/s41577-019-0247-z [DOI] [PubMed] [Google Scholar]
- 135.Meyer U. et al. (2008) Adult brain and behavioral pathological markers of prenatal immune challenge during early/middle and late fetal development in mice. Brain Behav Immun 22, 469–486. 10.1016/j.bbi.2007.09.012 [DOI] [PubMed] [Google Scholar]
- 136.Cui J. et al. (2020) Inflammation of the Embryonic Choroid Plexus Barrier following Maternal Immune Activation. Developmental Cell 55, 617–628.e616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Orefice LL et al. (2019) Targeting Peripheral Somatosensory Neurons to Improve Tactile-Related Phenotypes in ASD Models. Cell 178, 867–886.e824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hsiao EY et al. (2012) Modeling an autism risk factor in mice leads to permanent immune dysregulation. Proc Natl Acad Sci U S A 109, 12776–12781. 10.1073/pnas.1202556109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Reed MD et al. (2020) IL-17a promotes sociability in mouse models of neurodevelopmental disorders. Nature 577, 249–253. 10.1038/s41586-019-1843-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Becker KG (2012) Male gender bias in autism and pediatric autoimmunity. Autism Res 5, 77–83. 10.1002/aur.1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Nicolson GL et al. (2007) Evidence for Mycoplasma ssp., Chlamydia pneunomiae, and human herpes virus-6 coinfections in the blood of patients with autistic spectrum disorders. J Neurosci Res 85, 1143–1148. 10.1002/jnr.21203 [DOI] [PubMed] [Google Scholar]
- 142.Sabourin KR et al. (2019) Infections in children with autism spectrum disorder: Study to Explore Early Development (SEED). Autism Res 12, 136–146. 10.1002/aur.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wallace K. et al. (2010) Prenatal infection decreases calbindin, decreases Purkinje cell volume and density and produces long-term motor deficits in Sprague-Dawley rats. Dev Neurosci 32, 302–312. 10.1159/000319506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Spini VBMG et al. (2021) Maternal Immune Activation with H1N1 or Toxoplasma gondii Antigens Induces Behavioral Impairments Associated with Mood Disorders in Rodents. Neuropsychobiology 80, 234–241. 10.1159/000510791 [DOI] [PubMed] [Google Scholar]
- 145.Xu Z. et al. (2021) Rescue of maternal immune activation-induced behavioral abnormalities in adult mouse offspring by pathogen-activated maternal Treg cells. Nat Neurosci 24, 818–830. 10.1038/s41593-021-00837-1 [DOI] [PubMed] [Google Scholar]
- 146.Golan HM et al. (2005) Specific neurodevelopmental damage in mice offspring following maternal inflammation during pregnancy. Neuropharmacology 48, 903–917. 10.1016/j.neuropharm.2004.12.023 [DOI] [PubMed] [Google Scholar]
- 147.Glass R. et al. (2019) Maternal immune activation with staphylococcal enterotoxin A produces unique behavioral changes in C57BL/6 mouse offspring. Brain Behav Immun 75, 12–25. 10.1016/j.bbi.2018.05.005 [DOI] [PubMed] [Google Scholar]
- 148.Depino AM (2015) Early prenatal exposure to LPS results in anxiety- and depression-related behaviors in adulthood. Neuroscience 299, 56–65. 10.1016/j.neuroscience.2015.04.065 [DOI] [PubMed] [Google Scholar]
- 149.Rose DR et al. (2017) Long-term altered immune responses following fetal priming in a non-human primate model of maternal immune activation. Brain Behav Immun 63, 60–70. 10.1016/j.bbi.2016.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Cheng SB and Sharma S. (2015) Interleukin-10: a pleiotropic regulator in pregnancy. Am J Reprod Immunol 73, 487–500. 10.1111/aji.12329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Robertson SA et al. (2006) Essential Role for IL-10 in Resistance to Lipopolysaccharide-Induced Preterm Labor in Mice. The Journal of Immunology 177, 4888–4896. 10.4049/jimmunol.177.7.4888 [DOI] [PubMed] [Google Scholar]
- 152.Meyer U. et al. (2008) Adult behavioral and pharmacological dysfunctions following disruption of the fetal brain balance between pro-inflammatory and IL-10-mediated anti-inflammatory signaling. Molecular Psychiatry 13, 208–221. 10.1038/sj.mp.4002042 [DOI] [PubMed] [Google Scholar]
- 153.Josefowicz SZ et al. (2012) Regulatory T Cells: Mechanisms of Differentiation and Function. Annual Review of Immunology 30, 531–564. 10.1146/annurev.immunol.25.022106.141623 [DOI] [PMC free article] [PubMed] [Google Scholar]