Abstract
Background
Booster doses for COVID-19 vaccinations are currently recommended and approved in many countries. However, we need more evidence on the immune response of individuals to booster doses of inactivated vaccines and the neutralizing effect against the variants of concerns of SARS-CoV-2.
Objective
To compare the fold reduction in antibody titers against the variants of concerns of SARS-CoV-2 between the primary doses and booster dose vaccine cohorts of inactivated BBIBP-CorV vaccine.
Study design
In this observational study Plaque Reduction Neutralization Test (PRNT) assay was done on pooled serum samples of the recipients of primary two doses of inactivated BBIBP-CorV and on the pooled serum samples of recipients of a booster dose of inactive BBIBP-CorV. The neutralizing antibody titers against the wild (Wuhan) strain and the variants of concern (alpha, beta and delta) were compared.
Results
The serum sample pool from the booster cohort had high neutralizing antibody titers against the SARS-CoV-2 variants compared to the pooled serum samples of the recipients of primary two doses of inactivated BBIBP-CorV and the difference was statistically significant. The observed fold reduction in antibody titers from the serum pool of recipients of two doses of BBIBP-CorV vaccine were 3.7-fold, 14.6-fold and 10.4-fold compared to 1.8 -fold, 6.5-fold and 3.8-fold reduction against the alpha, beta and delta lineages respectively in the serum pool of recipient of a booster dose (three doses of BBIBP-CorV).
Conclusion
Booster doses of inactive BBIBP-CORV offered better protection against the variants of concern of SARS-CoV-2.
Keywords: Neutralizing antibodies, booster dose, SARS-CoV-2, variants of concern, COVID-19
1. Background
The BBIBP-CORV is an inactivated vaccine prepared by multiplication of SARS-CoV-2 WIV04/HB02 strain in African green monkey kidney cells, the virus is further inactivated by beta-propiolactone and is mixed with an aluminum-based adjuvant. [1] Phase III trials reports of the inactivated vaccine (BBIBP-CORV) demonstrated a vaccine efficacy of 79% against symptomatic COVID-19 infection. [2] However we are yet to completely understand the humoral immunity offered by COVID-19 vaccination, studies have shown that there is a difference in humoral immunogenicity with regards to vaccine type and the vaccine-induced antibody response is less effective against the novel variants of SARS-CoV-2 than the wild strain. [3, 4]
The newer, novel strains of SARS-CoV-2, particularly those harboring various mutations in the spike protein receptor binding domain (RBD) that increase viral affinity for ACE2 on target cells, have shown to mediate escape from vaccine-induced humoral immunity. Additionally, the population becomes more vulnerable to the novel strains due to a gradual decrease in the antibody titers over months post-vaccination. Both of these factors lead to the decreased effectiveness of the vaccines with less pronounced protection against the newer SARS-CoV-2 variants of concern. [5]
In the current global situation where booster doses of the COVID-19 vaccines are widely recommended, we are challenged to answer the question of whether a booster dose is needed for inactivated vaccines and whether a booster dose is more effective in inducing a greater immune response and offer better protection against these emerging variants of SARS-CoV-2. [6,7] Neutralizing antibody titers against the SARS-CoV-2 virus have shown to be highly predictive of the host immune protection against infection. [8] Therefore in this study, neutralizing antibody titers in pooled serum samples from individuals immunized with primary 2 doses of inactivated BBIBP-CORV and from individuals who have received a booster dose of inactivated BBIBP-CORV were compared against the Wild (Wuhan) and the Alpha (B.1.1.7, UK Variant), Beta (B.1.351, South Africa Variant), Delta (B.1.617.2, Indian Variant) variants of concerns (VOCs) of SARS-CoV-2.
2. Method
The study was approved by the medical research review board, Department of Health (DOH), Abu Dhabi, United Arab Emirates. Approval number: DOH/CVDC/2021/1424
2.1. Study design and study participants
An observational study was conducted as a pilot study on a minimal number of samples to understand the reduction in antibody titers against the VOCs compared to the wild strain of SARS-Cov-2. The SARS-CoV-2 viral strains used in this study were obtained from nasopharyngeal samples collected for the purpose of PCR testing for COVID-19 infection. SARS-CoV-2-positive samples were sequenced to identify the viral strains, of which samples containing the Wild, Alpha, Beta, or Delta variants were used in our study. These three variants were chosen based on the World health organization (WHO) classification of the VOCs that was of greater concern during our study period from September to October, 2021 and the Delta variant was the most predominant lineage obtained from the COVID-19 nasopharyngeal test samples. [9] Therefore, in this study, 45 different isolates of the circulating Delta lineages, 15 different isolates of the Beta lineages, and 13 different isolates of Alpha lineages were used to study the effect of neutralising antibodies against each of the strains. Antibody titers were estimated from the pooled serum samples of post-vaccination individuals using a Plaque Reduction Neutralization Test (PRNT) assay. The serum samples pooled were chosen by convenience sampling based on the availability of the stored samples.
PRNT was performed using the sera of vaccinated individuals who were categorized into two vaccine cohorts: 1) participants vaccinated with 2 doses of BBIBP-CORV vaccine (n= 35), this cohort included 15 (42.9%) females and 20 (57.1% male) participants and the mean age was 41.84 ± 10.45. The average interval post second dose of of BBIBP-CorV vaccine of the pooled serum sample was 73.35 ± 26.91 days. 2) participants vaccinated with 3 doses (2 doses plus one booster dose) of BBIBP-CORV vaccine (n= 20), this cohort included 8 (33.3%) females and 16 (66.1% male) participants and the mean age was 41.71 ± 9.86. The average interval post booster dose of of BBIBP-CorV vaccine of the pooled serum sample was 82 days.
2.2. Viral culture
SARS-CoV-2 was isolated from the clinical sample received in laboratory. The virus was propagated at the biosafety level 3 by inoculating Vero E6 cells (ATCC CRL 1586), acquired from the ECACC. The infected cells were incubated for 2 – 3 days in Dulbecco's Modified Eagle Medium with F12 and Glutamax containing 5% heat-inactivated fetal bovine serum and 1% Antibiotic at 37°C with 5% carbon dioxide (CO2). The supernatant was clarified by centrifugation and 250uL aliquoted into cryovials. The resulting stock was quantified by plaque assay by serially diluting the viral stock and infecting Vero E6 cells in 12 well tissue culture plate with a 0.6% Agarose overlay and stained after 2 – 3 days with 0.5% crystal violet stain. Virus stocks were sequenced to identify the lineages.
2.3. The PRNT assay
The PRNT was performed in duplicate using 12-well tissue culture plates. Serial dilutions of serum samples were incubated with 60–100 plaque-forming units of virus for 1h at 37°C. The virus–serum mixtures were added onto Vero E6 cell monolayers and incubated 2 h at 37°C in 5% CO2 incubator. Then the plates were overlaid with 0.6% agarose in cell culture medium and incubated for 2 - 3 days when the plates were fixed and stained with 0.5% crystal violet. Antibody titers were defined as the highest serum dilution that resulted in > 50% (PRNT50) reduction in the number of plaques compared to negative control.
The neutralizing antibody titer against the Wild strain and against the variant strains were observed using the same serum sample pool and the average titers were recorded. The reduction in antibody titres against the variants of concern of SARS-CoV-2 in comparison to the Wild strain was estimated and compared among the vaccine cohorts. Independent sample t-test was performed to compare the antibody titers between the two vaccine groups. Analysis was carried out using R software version 4.0.4 and p values <0.05 were considered significant.
3. Results
The antibody titers against the Wild strain were 1:320 in the two primary doses of BIBP-CORV vaccine cohort and 1:640 in the booster cohort of BBIBP-CORV. The mean titers of BBIBP-CorV two doses cohort against alpha, beta and delta lineages were 138.46 ± 94.68, 34.12 ± 21.52 and 45.78 ± 29.96 repectively, similarly the mean titers of BBIBP-CorV three doses cohort against Alpha, Beta and Delta lineages were 289.23 ±186.30, 103.53 ± 62.94 and 156.89 ± 104.44 respectively.
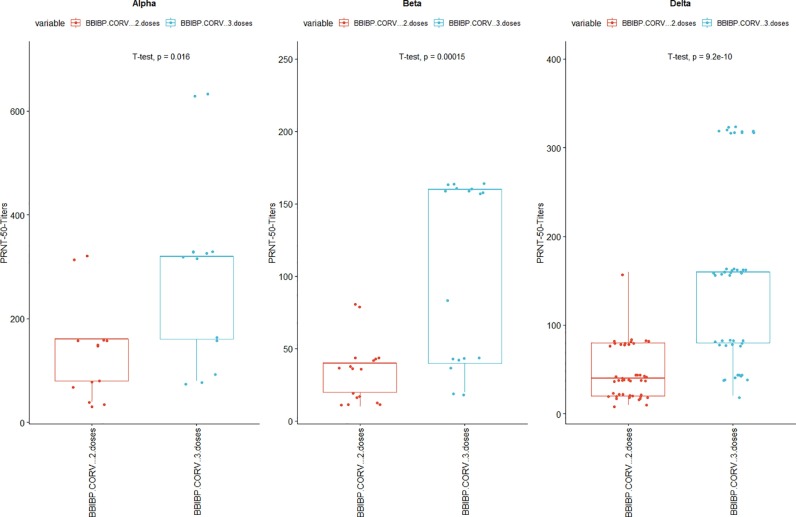
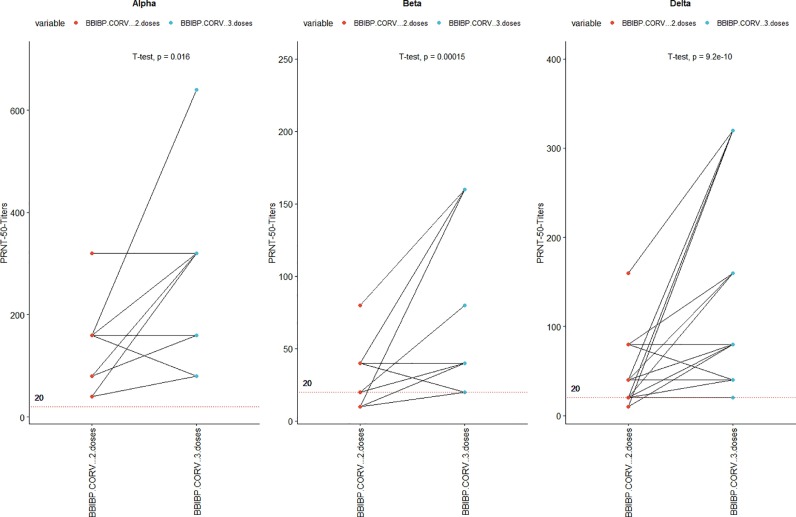
The antibody titers were higher in the booster vaccine cohort compared to the primary two doses of the inactivated vaccine cohort. The average fold reduction in antibody titers from the serum pool of 2 doses of BBIBP-CORV vaccine were 3.7-fold, 14.6-fold and 10.4-fold against the Alpha, Beta and Delta lineages respectively when compared to the titers against the wild strain. Similarly average fold reduction in antibody titers from the serum pool of the booster dose (2 doses of BBIBP-CORV plus one booster dose after 6 months) were 1.8 -fold, 6.5-fold and 3.8-fold against the Alpha, Beta and Delta lineages respectively. In both the vaccination cohorts the fold reduction in antibody titers against the variants of concern were least pronounced against the Alpha variant and most pronounced against the Beta variants. Against all the variants of concern of SARS-COV-2 booster vaccine cohort showed statistically significant higher antibody titers compared to the primary vaccine cohorts. This shows that against the variants booster doses offered better protection than primary 2 doses of vaccination. [Fig.1 ]. The serum pool of recipients of booster dose of BBIBP-CORV vaccine neutralized all isolates of Alpha, Beta and Delta variants at titers of at least 1:20. [Fig. 2 ].
Fig. 1.
Neutralizing antibody titers against the variants of concern, Reciprocal titers of neutralizing antibodies against the Alpha, Beta and Delta lineages in the serum pool of Primary 2 doses of BIBP-CORV vaccine and in the serum pool after booster dose of the same vaccine. t-test was used test the difference in titers between the two vaccine cohorts. The mean titers of BBIBP-CorV two doses cohort against alpha, beta and delta lineages were 138.46 ± 94.68, 34.12 ± 21.52 and 45.78 ± 29.96 repectively, similarly the mean titers of BBIBP-CorV three doses cohort against alpha, beta and delta lineages were 289.23 ±186.30, 103.53 ± 62.94 and 156.89 ± 104.44 respectively
Fig. 2.
Paired comparison of the antibody titers against the same variant isolates from both the Primary and booster dose vaccine cohorts, Reciprocal titers of neutralizing antibodies against the Alpha, Beta and Delta lineages. Data are from 13 different isolates of Alpha; 15 different isolates of the Beta and 45 different isolates of the circulating Delta lineages and identical titer values may overlap. The reciprocal titer of 1:20 is marked to show titers below the cut-off value for PRNT assay.
4. Discussion
Our study results show that booster doses had better neutralizing effect against variants of concern thus emphasizing the need of booster doses for inactivated vaccines. The Delta variant is potentially a greater concern as it is less sensitive to antibodies in sera from individuals who had received only primary 2 doses of BBIBP-CORV, our findings were similar to the findings of another study on inactivated vaccines that showed that neutralizing antibodies titers against the Delta variant were several folds lower compared to the Wild and Alpha variants. [10] This could be because the Delta variant strains of SARS-CoV-2 with changing antigenicity of the spike protein can affect neutralization or escape post-vaccination antibodies. [11] However, newer evidence shows that reduction in vaccine effectiveness against Delta infections would probably be due to reducing antibody levels and immunity with time post-vaccination rather than the Delta variant more effectively escaping the antibodies. [12]
This study showed that the fold reduction in antibody titers were more pronounced against the Beta variant in comparison to the Alpha variant. This might be due to the fact that the Beta variant was more severe and more resistant to immune response generated by vaccines. [13] Studies showed that compared to Alpha variant the odds of progressing to severe disease, requiring critical care admissions and death due to COVID-19 were higher with the Beta variant. [13,14]
Supporting our study findings that booster doses for BBIBP-CORV vaccine increases antibody titers and showed lower fold reduction against the SARS-CoV-2 variants, studies on inactivated vaccine have also shown that antibody concentrations vigorously elevated along with spike-specific circulating follicular helper T cells after a booster dose of inactivated vaccine [15]
The strength of our study is that our comparisons were standardized by comparing the neutralizing activity and the fold reductions between the two vaccine cohorts against the same viral isolate of all three variants of concern. This study has also some limitations as it did not take into consideration the influence of factors like age, gender, comorbidities or prior SARS-CoV-2 infections that could have influenced the antibody titers. However, the study used the same serum sample pool to understand the reduction in antibody titers against the variant strains compared to the wild strain. Therefore, these factors are unlikely to have made significant difference in the interpretation of the results.
We conclude that while post-vaccination sera have reduced efficacy against variants of concern when compared to the wild strain, there is still highly neutralizing effect against the variants of concern. The Delta variant is potentially a greater concern as it is less sensitive to antibodies in sera from individuals who had received only primary 2 doses. However, sera of patients who have received booster dose have largely preserved protection against the Delta variant. This study suggest that booster doses can be recommended for vaccine recipients of inactivated vaccines for augmenting protection against the newer variants of concern. Further studies are needed to evaluate the impact of various other factors that can affect the antibody titers and the optimal timing of the booster doses.
Authors contribution
SM,SG - contributed to the study concept and design; SN, PG, AM, LB – contribute to data collection; SG, SE - contributed to the data analysis and data visualization; SM, SG- contributed to the original draft; NA, SN, KW, WZ, FA - revised the manuscript, SM, SG, NA, SN, SE, PG, AM, LB, KW, WZ, FA- critically reviewed and edited the final draft of the manuscript. All authors approved the final version of the manuscript.
Data sharing
Individual data reported in this study are not publicly shared. Request for accessing data should be submitted to the corresponding author (sally.mahmoud@g42.ai) and upon approval of the request de-identified data will be shared.
Competing interest statement
No funding was received for this study. The authors declare no competing financial or non-financial interests.
Acknowledgment
We thank Ms. Flavia Catarutti and all lab personnel at Biogenix lab for their support.
References
- 1.Wang H, Zhang Y, Huang B, Deng W, Quan Y, Wang W, et al. Development of an inactivated vaccine candidate, BBIBP-CorV, with potent protection against SARS-CoV-2. Cell. 2020 Aug 6;182(3):713–721. doi: 10.1016/j.cell.2020.06.008. e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al Kaabi N, Zhang Y, Xia S, et al. Effect of 2 Inactivated SARS-CoV-2 vaccines on symptomatic COVID-19 infection in adults: a randomized clinical trial. JAMA. 2021;326(1):35–45. doi: 10.1001/jama.2021.8565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jalkanen P, Kolehmainen P, Häkkinen H, Huttunen M, Tähtinen P, Lundberg R, et al. COVID-19 mRNA vaccine induced antibody responses against three SARS-CoV-2 variants. Nat. Commun. 2021 June 28;12(3991) doi: 10.1038/s41467-021-24285-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Planas D, Veyer D, Baidaliuk A, Staropoli I, Guivel-Benhassine F, Rajah MM, Planchais C, Porrot F, Robillard N, Puech J, Prot M. Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization. Nature. 2021 Aug;596(7871):276–280. doi: 10.1038/s41586-021-03777-9. [DOI] [PubMed] [Google Scholar]
- 5.Barros-Martins J, Hammerschmidt SI, Cossmann A, Odak I, Stankov MV, Morillas Ramos G, Dopfer-Jablonka A, Heidemann A, Ritter C, Friedrichsen M, Schultze-Florey C. Immune responses against SARS-CoV-2 variants after heterologous and homologous ChAdOx1 nCoV-19/BNT162b2 vaccination. Nat. Med. 2021 Jul 14:1–5. doi: 10.1038/s41591-021-01449-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Callaway E. COVID vaccine boosters: the most important questions. Nature. 2021;596(7871):178–180. doi: 10.1038/d41586-021-02158-6. [DOI] [PubMed] [Google Scholar]
- 7.Schmidt T, Klemis V, Schub D, Mihm J, Hielscher F, Marx S, Abu-Omar A, Ziegler L, Guckelmus C, Urschel R, Schneitler S. Immunogenicity and reactogenicity of heterologous ChAdOx1 nCoV-19/mRNA vaccination. Nat. Med. 2021 Sep;27(9):1530–1535. doi: 10.1038/s41591-021-01464-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khoury D.S., Cromer D., Reynaldi A., et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021;27:1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization (WHO) 20 August 2022. Tracking SARS-CoV-2 variants.https://www.who.int/en/activities/tracking-SARS-CoV-2-variants/ Available at: Accessed on. [Google Scholar]
- 10.Chen Y, Shen H, Huang R, Tong X, Wu C. Serum neutralizing activity against SARS-CoV-2 variants elicited by CoronaVac. Lancet Infect. Dis. 2021 May 27;21(8):1071–1072. doi: 10.1016/S1473-3099(21)00287-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harvey WT, Carabelli AM, Jackson B, Gupta RK, Thomson EC, Harrison EM, Ludden C, Reeve R, Rambaut A, Peacock SJ, Robertson DL. SARS-CoV-2 variants, spike mutations and immune escape. Nat. Rev. Microbiol. 2021 Jul;19(7):409–424. doi: 10.1038/s41579-021-00573-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tartof SY, Slezak JM, Fischer H, Hong V, Ackerson BK, Ranasinghe ON, Frankland TB, Ogun OA, Zamparo JM, Gray S, Valluri SR. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: a retrospective cohort study. Lancet North Am. Ed. 2021 Oct 4 doi: 10.1016/S0140-6736(21)02183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Callaway E. Remember Beta? New data reveal variant's deadly powers. Nature. 2021 Aug 9 doi: 10.1038/d41586-021-02177-3. https://www.nature.com/articles/d41586-021-02177-3#ref-CR1 Available at. Accessed 18 March 2022. [DOI] [PubMed] [Google Scholar]
- 14.Abu-Raddad LJ, Chemaitelly H, Ayoub HH, Yassine HM, Benslimane FM, HA Al Khatib, Tang P, Hasan MR, Coyle P, AlMukdad S, Severity Al Kanaani Z. Criticality, and fatality of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) beta variant. Clin. Infect. Dis. 2021 May 31 doi: 10.1093/cid/ciab909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Y, Zeng Q, Deng C, Li M, Li L, Liu D, Mei J, Mo R, Zhou Q, Liu M, Peng S. Robust induction of B cell and T cell responses by a third dose of inactivated SARS-CoV-2 vaccine. medRxiv. 2021 Jan 1 doi: 10.1038/s41421-022-00373-7. [DOI] [PMC free article] [PubMed] [Google Scholar]