Abstract
Background & objectives
The persistence of the severe acute respiratory syndrome coronavirus (SARS-CoV)-2 pandemic, partly due to the appearance of highly infectious variants, has made booster vaccinations necessary for vulnerable groups. Here, we present data regarding the decline of the SARS-CoV-2 BNT162b2 mRNA vaccine-induced humoral immune response in a monocentric cohort of MS patients.
Methods
96 MS patients undergoing eight different DMTs, all without previous SARS-CoV-2 infection, were evaluated for anti-Spike IgG levels, 21 days (T1) and 5–6 months (T2) after the second SARS-CoV-2 BNT162b2 mRNA vaccine dose. The anti-Spike IgG titre from MS subjects was compared with 21 age- and sex-matched healthy controls (HC).
Results
When compared with SARS-CoV-2 IgG levels at T2 in HC, we observed comparable levels in interferon-β 1a-, dimethyl fumarate-, teriflunomide- and natalizumab-treated MS subjects, but an impaired humoral response in MS subjects undergoing glatiramer acetate-, cladribine-, fingolimod- and ocrelizumab-treatments. Moreover, comparison between SARS-CoV-2 IgG Spike titre at T1 and T2 revealed a faster decline of the humoral response in patients undergoing dimethyl fumarate-, interferon-β 1a- and glatiramer acetate-therapies, while those receiving teriflunomide and natalizumab showed higher persistence compared to healthy controls.
Conclusion
The prominent decline in humoral response in MS subjects undergoing dimethyl fumarate-, interferon-β 1a- and glatiramer acetate-therapies should be considered when formulating booster regimens as these subjects would benefit of early booster vaccinations.
Keywords: BNT162b2-mRNA vaccine, Coronavirus-19, Disease modifying therapies, Multiple sclerosis, Humoral persistence, SARS-CoV-2 spike protein
Abbreviations: CLAD, Cladribine; COVID-19, Coronavirus disease 2019; DMF, Dimethyl fumarate; DMTs, Disease modifying therapies; EDSS, Expanded disability status scale; FTY, Fingolimod; GA, Glatiramer acetate; HC, Healthy controls; IgG, Immunoglobulin G; INF, Interferon-β 1a; IQR, Interquartile range; MS, Multiple sclerosis; NAT, Natalizumab; OCRE, Ocrelizumab; PPMS, Primary progressive multiple sclerosis; RRMS, Relapsing remitting multiple sclerosis; SARS-CoV-2, severe acute respiratory syndrome coronavirus-2; SPMS, Secondary progressive multiple sclerosis; TERI, Teriflunomide
1. Introduction
Vaccination against the severe acute respiratory syndrome coronavirus (SARS-CoV)−2 has become a global priority as coronavirus disease 2019 (COVID-19) presents a severe or life-threatening disease course up to 5–10% of cases (Gavriatopoulou et al., 2020). Among all the available vaccines, BNT162b2 mRNA has been shown to induce high levels of the anti-SARS-CoV-2 Spike-receptor binding domain (RBD) IgG neutralizing antibodies (NAbs) that strongly correlate with clinical immune protection from COVID-19 in healthy subjects (Borobia et al., 2021; Walsh et al., 2020; Khoury et al., 2021). Furthermore, NAbs also induce a good level of seroprotection in Multiple Sclerosis (MS) patients, although with different effectiveness depending on the disease-modifying therapy (DMT). Indeed, while MS patients treated with immunosuppressive therapies such as cladribine (CLAD), fingolimod (FTY) and ocrelizumab (OCRE) exhibited an impaired secretion of SARS-CoV-2 anti-Spike IgG (Tortorella et al., 2021; Sormani et al., 2021; Maniscalco et al., 2021), an increased humoral vaccine response has been reported in interferon-β 1a (IFN)-treated MS subjects (Maniscalco et al., 2021). In light of the current discussion regarding SARS-CoV-2 booster vaccinations, several studies have evaluated the duration of anti-SARS-CoV-2 immune protection in healthy individuals (A Achiron et al., 2021; Andrews et al., 2022); however clinical data suggest an attenuated short-term humoral response to SARS-CoV-2 vaccines in patients with MS receiving DMTs (Tortorella et al., 2021; Capuano et al., 2022).
Here we present a prospective study aimed at assessing the six-months persistence of the humoral response in a monocentric cohort of MS patients treated with eight different DMTs compared with healthy subjects, all receiving BNT162b2 mRNA vaccine and without previous SARS-CoV-2 infection.
2. Materials and methods
2.1. Subjects and study design
This is a prospective monocentric study to evaluate the kinetics of SARS-CoV-2 IgG Spike titre 5–6 months after the second dose of BNT162b2 vaccine in MS subjects undergoing vaccination at the Multiple Sclerosis centre of the Cardarelli Hospital (Naples, Italy) from March to November 2021. All human subjects were enrolled after obtaining informed consent. The study was approved by the Institutional Review Board of the Cardarelli Hospital. We enrolled 96 MS and 21 healthy controls receiving the two doses of BNT162b2 vaccine according to the recommendations of Italian Authority of Health, all without previous SARS-CoV-2 infection. MS subjects were vaccinated according to specific timing; more in detail, MS subjects treated with IFN, GA, TERI, DMF, FTY and NAT were vaccinated without any interruption of immunomodulatory treatment, while CLAD- and OCRE-treated MS subjects were vaccinated at least 1 or 3 months respectively after the last therapeutic administration, according to the recommendations of Italian Authority of Health. Blood samples were collected at 9:00AM into heparinized Vacutainers (BD Biosciences) and processed within the following 4 h. Demographic and clinical characteristics of the study cohort are shown in Table 1 . Inclusion criteria were patients aged between 18 and 65 years, diagnosed with multiple sclerosis treated with DMTs for at least 6 months. Exclusion criteria were previous SARS-CoV-2 infection (antibody screening), any relapse and/or steroid use in the last 30 days before enrolment. Healthy subjects were matched for age and sex and had no history of inflammation, endocrine or autoimmune disease. The ethnic distribution among the groups was comparable, with all participants being Caucasian.
Table 1.
Clinical and demographic characteristics of the study cohort.
| MS patients (N = 96) | Healthy Controls (N = 21) | |
|---|---|---|
| Gender, n (%) | ||
| ▒Female | 57 (59.4) | 11 (52.4) |
| ▒Male | 39 (40.6) | 10 (47.6) |
| Age, years | ||
| ▒Mean age (±SD) | 40.7 (±10.5) | 48.3 (±10.8) |
| ▒IQR (25–75) | 14.5 | 20.5 |
| EDSS, mean (range) | 1.9 (0–6.5) | |
| DMTs duration, mean (months ± SD) | 50.2 (±41.5) | |
| MS type, n (%) | ||
| ▒RRMS | 91 (94.8) | |
| ▒PPMS | 1 (1.04) | |
| ▒SPMS | 4 (4.2) | |
| DMTs, n (%) | ||
| ▒Interferon -β 1a | 19 (19.8) | |
| ▒Natalizumab | 14 (14.6) | |
| ▒Dimethyl fumarate | 14 (14.6) | |
| ▒Ocrelizumab | 13 (13.5) | |
| ▒Fingolimod | 13 (13.5) | |
| ▒Teriflunomide | 8 (8.3) | |
| ▒Cladribine | 8 (8.3) | |
| ▒Glatiramer acetate | 7 (7.4) |
DMTs: Disease modifying therapy; EDSS: Expanded disability status scale; IQR: Interquartile range; MS: Multiple sclerosis; PPMS: Primary progressive multiple sclerosis; RRMS: Relapsing remitting multiple sclerosis; SPMS: Secondary progressive multiple sclerosis.
2.2. SARS-CoV-2 IgG spike detection
Quantitative determination of antibodies to the SARS-CoV-2 Spike protein was carried out by Roche Elecsys® Anti‑SARS‑CoV‑2 S assay (Roche Diagnostics International Ltd, Rotkreuz, Switzerland). The assay was performed using a recombinant protein representing the RBD of the S antigen leading to a double-antigen sandwich assay complex which favors detection of high affinity antibodies against SARS-CoV-2 (range between 0.4 to 250 U/mL), resulting in a sensitivity of 98.8% (95% CI: 98.1 – 99.3%).
2.3. Ethics (standard protocol approvals, registrations and patient consents)
The study was conducted according the Good Clinical Practice guidelines and the ethical principles of the Declaration of Helsinki. Investigators obtained ethic committee approval for the study protocol and amendments by the local Ethic Committee of A.O.R.N. A. Cardarelli/Santobono-Pausilipon (protocol number 2821). All subjects give written informed consent to participate to the study.
2.4. Statistical analysis
Descriptive analyses were presented as mean (± standard error of the mean), median and interquartile range (IQR). Categorial variables were described as frequency and percentage. A Shapiro-wilk test was performed to assess the normal distribution of data. In case of not-normal distribution appropriate non-parametric tests were performed (Wilcoxon test). The fold persistence of SARS-CoV-2 Spike IgG titre was calculated as the percentage ratio between IgG titre at T2 and T1, relative to HC (100%). P-value less of 0.05 indicated significance. A multilinear regression model was used to compare the antibody levels across subjects treated with differerent DMTs after adjusting for age, sex, EDSS levels, disease and DMT duration and antibody levels in the pre-booster samples. Data analyses were performed using Graphpad Prism (version 8).
3. Results
3.1. Study cohort
Data were collected from March to November 2021. In this prospective monocentric study, we excluded subjects previously infected with SARS-CoV-2, through the measurement of nucleocapsid-specific antibodies. After assessment, 96 MS patients and 21 HC were evaluated for anti-Spike IgG levels, 21 days (T1) and 5–6 months (T2) after the second SARS-CoV-2 BNT162b2 mRNA vaccine dose. The demographic and clinical characteristics are reported in Table 1. In the MS group, 57 were female (59.4%) and 39 male (40.6%) and the mean age was 40.7 ± 10.5 (mean ± SD) years. In the control group (HC), 11 subjects were females (52.4%) and 10 males (47.6%), with a mean age of 48.3 ± 10.8 (mean ± SD) years. In the MS cohort, 91 had relapsing-remitting (RR) (94.8%), 1 primary-progressive (PP) (1.04%) and 4 secondary-progressive (SP) (4.2%) MS. Different types of disease-modifying therapies (DMTs) were: interferon-β 1a (IFN) (n = 19; 19.8%), natalizumab (NAT) (n = 14; 14.6%), dimethyl fumarate (DMF) (n = 14; 14.6%), ocrelizumab (OCRE) (n = 13; 13.5%), fingolimod (FTY) (n = 13; 13.5%), teriflunomide (TERI) (n = 8; 8.3%), cladribine (CLAD) (n = 8; 8.3%), and glatiramer acetate (GA) (n = 7; 7.4%).
3.2. SARS-CoV-2 - specific humoral response declines differently in MS subjects undergoing distinct disease modifying therapies (DMTs)
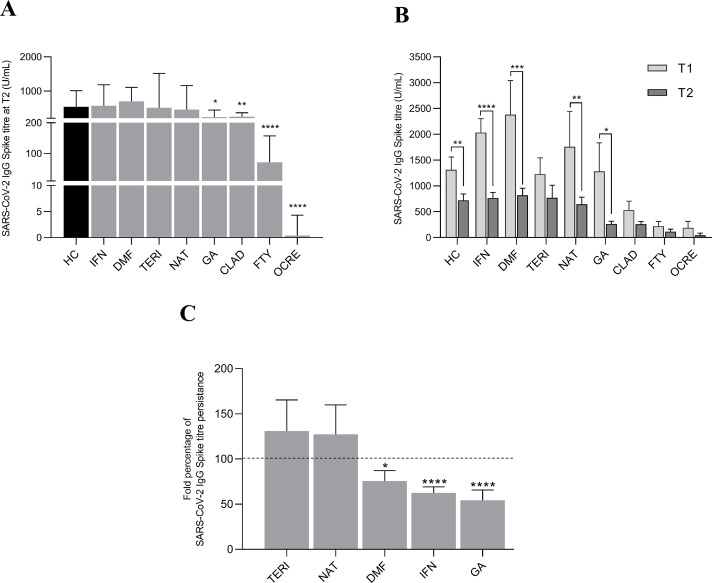
We previously reported that IFN-treated MS subjects showed a significant increase of anti-Spike IgG levels compared to HC, while humoral response was profoundly affected in MS subjects undergoing CLAD, FTY and OCRE, 21 days after the second BNT162b2 mRNA vaccine dose (Sormani et al., 2021). We aimed at evaluating the persistence of the SARS-CoV-2 IgG titre 5–6 months after the second vaccine dose (T2), in our monocentric cohort of MS subjects undergoing different DMTs, respect to age- and sex-matched HC. We found that SARS-CoV-2 IgG levels in MS subjects under treatment with IFN (median 566.6 (IQR: 427.5–1182) U/mL), DMF (median 694.6 (IQR: 438.3.5–1103) U/mL) TERI (median 507.8 (IQR: 278.7–1514) U/mL) and NAT (median 456.4 (IQR: 198.6–1156) U/mL) were comparable to HC (median 533.8 (IQR: 366.4–1008) U/mL) (Fig. 1 A and Table 2 ). On the contrary, humoral response at T2 was reduced in GA- (median 232.9 (IQR: 167.5–440.4) U/mL), CLAD- (median 246.5 (IQR: 145.3–363.3) U/mL) and FTY- (median 70.71 (IQR: 13.22–157.3) U/mL) and almost undetectable in OCRE- (median 0.4 (IQR: 0.4–4.3) U/mL) treated MS subjects (Fig. 1 A and Table 2). Follow-up of our monocentric cohort at 6 months revealed a significant drop in anti-Spike IgG titre compared to the relative T1 IgG values in HC (median 533.8 (IQR: 366.4 – 1008) vs 1087 (IQR:640.3 – 1542) U/mL), IFN- (median 566.6 (IQR: 427.5 – 1182) vs 1909 (IQR: 1073 – 2758) U/mL), DMF- (median 694.6 (IQR: 438.3 – 1103) vs 1587 (IQR:767.7 – 3472) U/mL), NAT- (median 456.4 (IQR: 198.6 – 1156) vs 735.1 (IQR: 351.3 – 2151) U/mL) and GA- (median 232.9 (IQR: 167.5 – 440.4) vs 446.5 (IQR: 417.7 – 3034) U/mL) treated MS patients (Table 2). The reduction was not significant in TERI-, CLAD- and OCRE-treated MS groups, while subjects under FTY showed a slight - albeit irrelevant - increase of anti-Spike IgG at T2 compared to their T1 levels (Fig. 1 B e Table 2). Finally, in those groups of DMT-treated MS subjects having shown a significant antibody production after the second SARS-CoV-2 BNT162b2 mRNA vaccine dose, we evaluated the persistence of the humoral response at 6 months, expressed as the percentage ratio between IgG titre at T2 and T1, relative to HC (Fig. 1 C). When compared to healthy controls, we observed a faster decline of anti-Spike IgG levels in patients undergoing DMF- (75.7 ± 11.5%), IFN- (62.4 ± 6.7%) and GA- (54.3 ± 11.3%) therapies, while those receiving TERI and NAT showed an increased persistence (130.9 ± 34.4% and 127.2 ± 32.6%, respectively), still not reaching the statistical significance (Fig. 1 C). We performed linear regression analyses to rule out that individual factors or clinical variables (age, sex, EDSS, DMT duration, time to last therapeutic administration) could affect the SARS-CoV-2 vaccine humoral response at T2, but we did not find any significant correlation between these parameters and IgG-Spike titre in the different DMT groups (data not shown). Our data highlight how DMTs could differentially promote the persistence of protective SARS-CoV-2 humoral response in MS subjects.
Fig. 1.
(A) SARS-CoV-2 IgG Spike titre (median with IQR) in MS subjects treated with different DMTs, 5–6 months after the second vaccine dose (T2) compared to healthy controls (HC). Wilcoxon two-tailed test vs HC subjects was performed and a p-value less than 0.05 was considered statistically significant. * p<0.05; **p<0.01; ****p<0.0001. (B) SARS-CoV-2 IgG Spike titre kinetics (mean ± SEM) of MS subjects treated with different DMTs at 21 days (T1) and 5–6 months (T2) after the second vaccine dose, compared to HC. Wilcoxon two-tailed test was performed to compare T1 and T2 levels and a p-value less than 0.05 was considered statistically significant. * p<0.05; **p<0.01; *** p<0.005; ****p<0.0001. (C) Fold percentage of SARS-CoV-2 IgG Spike titre persistance (mean ± SEM) of MS subjects treated with different DMTs at T2, relative to HC (100%). Wilcoxon two-tailed test vs HC subjects was performed and a p-value less than 0.05 was considered statistically significant. * p<0.05; ****p<0.0001.
Table 2.
Kinetics of SARS-CoV-2 IgG Spike titre at T1 ant T2, respectively 21 days and 5–6 months after the second vaccine dose in HC and MS subjects treated with different DMTs.
| DMTs | SARS-CoV-2 IgG Spike titre (U/mL) | p-value | |
|---|---|---|---|
| Median (IQR) T1 | Median (IQR) T2 | ||
| Healthy Controls | 1087 (640.3 – 1542) | 533.8 (366.4 – 1008) | 0.003 |
| Interferon-β 1a | 1909 (1073 – 2758) | 566.6 (427.5 – 1182) | <0.0001 |
| Dimethyl fumarate | 1587 (767.7 – 3472) | 694.6 (438.3 – 1103) | 0.009 |
| Teriflunomide | 1312 (288.4 – 1920) | 507.8 (278.7 – 1514) | 0.38 |
| Natalizumab | 735.1 (351.3 – 2151) | 456.4 (198.6 – 1156) | 0.002 |
| Glatiramer Acetate | 446.5 (417.7 – 3034) | 232.9 (167.5 – 440.4) | 0.015 |
| Cladribine | 468.2 (65.5 – 1021) | 246.5 (145.3 – 363.3) | 0.31 |
| Fingolimod | 20.6 (5.9 – 456.3) | 70.7 (13.2 – 157.3) | 0.94 |
| Ocrelizumab | 1.2 (0.4 – 119.4) | 0.4 (0.4 – 4.3) | 0.10 |
Wilcoxon two-tailed test was performed to compare SARS-CoV-2 IgG Spike titre at T2 and T1 in HC and MS subjects treated with different DMTs. Data are reported as median and IQR at T1 and T2. A p-value less than 0.05 was considered statistically significant.
DMTs: Disease modifying therapies; IQR: Interquartile range.
4. Discussion
Our study shows that antibody responses to SARS-CoV-2 BNT162b2 mRNA vaccine are broadly distributed and declined substantially in most individuals over time. Antibodies are key immune effectors that confer protection against pathogens (Chen et al., 2020). The longevity of the antibody response to SARS-CoV-2 vaccine in multiple sclerosis (MS) subjects are still not well defined. In this context, decisions regarding initiation or continuation of specific disease modifying therapy (DMT) have to consider the potential relevance to the pandemic. Understanding the possible distinctive effects of each therapeutic agent on the immune response to the vaccine is essential during this special time.
In this monocentric study, we charted longitudinal anti-Spike specific IgG antibody response to SARS-CoV-2 BNT162b2 mRNA vaccine in MS subjects undergoing eight different DMTs, compared to healthy controls (HC). 96 MS subjects followed longitudinally from day 21 (T1) to 5–6 months (T2) after the second SARS-CoV-2 BNT162b2 vaccine dose showed marked heterogeneity in antibody duration dynamics. Anti-Spike IgG decayed substantially in most individuals, whereas distinct groups had stable or increasing antibody levels in the same time frame (T2), despite the initial antibody magnitudes at T1. More in detail, anti-Spike IgG levels at T2 were comparable among IFN-, DMF-, TERI- and NAT-treated MS subjects and reflect those of HC. Conversely, antibody levels were significantly reduced in MS subjects under GA-, CLAD-, FTY-treatments and almost absent in OCRE-treated MS groups. Vaccination of cladribine-treated subjects occurred at least one month after the last therapy, in line with the Italian Authority of Health recommendations (Centonze et al., 2021); as these subjects have reduced anti-Spike IgG levels, this aspect should be considered in the selection of the booster time administration.
However, based on their anti-Spike IgG levels at T1, we evaluated the antibody persistence in those DMT-groups exhibiting an adequate humoral response after vaccination. We observed that, when compared to HC, TERI- and NAT-treated MS subjects showed an increased persistence of anti-Spike specific IgG antibody response to SARS-CoV-2 BNT162b2 mRNA vaccine after 5–6 months, while DMF-, IFN- and GA-therapies affected the retention of the humoral response overtime. An important implication of our data is the possible existence of an efficient SARS-CoV-2 vaccine ‘‘holder’’ phenotype, defined as individuals who experience relatively sustained anti-SARS-CoV-2 IgG production. This seems to be the case of MS under teriflunomide, which selectively inhibits dihydro-orotate dehydrogenase (DHODH), an important mitochondrial enzyme in the de novo pyrimidine synthesis pathway. The downstream effect is the reduced proliferation of rapidly dividing cells, including activated T and B lymphocytes. It has been reported that TERI-treated MS patients developed effective immunity to seasonal influenza after vaccination. In addition, there is emerging evidence suggesting a direct antiviral effect for teriflunomide and other DHODH inhibitors against a range of viruses such as Theiler's, respiratory syncytial, Ebola, cytomegalovirus, Epstein–Barr, and picornavirus (Maghzi et al., 2020). Moreover, our data are in line with the successful development of anti-SARS-CoV-2 antibodies described in patients under teriflunomide after COVID-19 infection (Bollo et al., 2020). Regarding natalizumab, it acts as an antagonist to alpha-4 integrin on leukocyte surface, blocking their interaction with vascular cell adhesion molecules and preventing leukocyte migration to the CNS. Data from influenza vaccine studies strongly support its treatment under the COVID-19 pandemic; however, some concerns have been raised regarding the potential increase of viral shedding due to the reduction of lymphocyte trafficking in the lungs (Aguirre et al., 2020). Since our data unveiled a slight increase in both T1 and T2 anti-Spike IgG levels compared to HC and a good humoral persistence, this could suggest a delayed booster timing for NAT-treated MS subjects. The same holds true also for TERI-treated MS subjects which, despite a lower antibody production at T1, retained an excellent humoral reservoir at T2. According to the literature, our study shows that different humoral persistence could be observed among immuno-modulating DMTs (Tortorella et al., 2021; Capuano et al., 2022; A Achiron et al., 2021); this offers new evidence that, if confirmed in a larger cohort of patients, should be considered when formulating booster regimens in MS subjects.
5. Data access, responsibility, and analysis
The corresponding author had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Author contributions
G.T. Maniscalco, A.L. Ferrara, V. De Rosa: Conceptualization; A.L. Ferrara, A. Liotti, D. Di Giulio Cesare, O. Moreggia: Data curation; A.L. Ferrara, A. Liotti, D. Di Giulio Cesare: Formal analysis; G.T. Maniscalco, V. De Rosa: Investigation; G.T. Maniscalco, V. De Rosa: Methodology; V. Manzo, M. E. Di Battista, S. Salvatore, D. Graziano, A. Viola, G. Amato, G. Alfieri, W. Di Iorio, G. Della Rocca, V. Andreone: Project administration; D. Graziano, A. Viola, G. Amato: Resources; A.L. Ferrara, A. Liotti, D. Di Giulio Cesare, O. Moreggia: Software; G.T. Maniscalco, V. De Rosa, V. Andreone: Supervision; V. Manzo, M. E. Di Battista, S. Salvatore, D. Graziano, A. Viola, G. Amato, O. Moreggia, G. Alfieri, W. Di Iorio, G. Della Rocca, V. Andreone: Validation; V. Manzo, M. E. Di Battista, S. Salvatore, D. Graziano, A. Viola, G. Amato, G. Alfieri, W. Di Iorio, G. Della Rocca, V. Andreone: Visualization; G.T. Maniscalco, A.L. Ferrara, V. De Rosa: Roles/Writing - original draft; A. Liotti, V. Manzo, M. E. Di Battista, S. Salvatore, D. Graziano, A. Viola, G. Amato, G. Alfieri, W. Di Iorio, G. Della Rocca, V. Andreone: Writing - review & editing.
Funding
This work was supported by FISM 2018/R/4 from Fondazione Italiana Sclerosi Multipla, STAR Program Linea 1-2018 by UniNA and by Compagnia di San Paolo from the Università degli Studi di Napoli “Federico II”, Bando PRIN 2017 Prot. 2017K7FSYB from Ministry of Education, University and Research (MIUR).
Ethical standards
All procedures were performed in accordance with the institutional ethics committee and the Declaration of Helsinki.
CRediT authorship contribution statement
Giorgia Teresa Maniscalco: Conceptualization, Investigation, Methodology, Supervision, Writing – original draft. Anne Lise Ferrara: Conceptualization, Data curation, Formal analysis, Software, Writing – original draft. Antonietta Liotti: Data curation, Formal analysis, Software, Writing – review & editing. Valentino Manzo: Project administration, Validation, Visualization, Writing – review & editing. Maria Elena Di Battista: Project administration, Validation, Visualization, Writing – review & editing. Simona Salvatore: Project administration, Validation, Visualization, Writing – review & editing. Daniela Graziano: Project administration, Resources, Validation, Visualization, Writing – review & editing. Assunta Viola: Project administration, Resources, Validation, Visualization, Writing – review & editing. Gerardino Amato: Project administration, Resources, Validation, Visualization, Writing – review & editing. Ornella Moreggia: Data curation, Software, Validation. Daniele Di Giulio Cesare: Data curation, Formal analysis, Software. Gennaro Alfieri: Project administration, Validation, Visualization, Writing – review & editing. Walter Di Iorio: Project administration, Validation, Visualization, Writing – review & editing. Gennaro Della Rocca: Project administration, Validation, Visualization, Writing – review & editing. Vincenzo Andreone: Project administration, Supervision, Validation, Visualization, Writing – review & editing. Veronica De Rosa: Conceptualization, Investigation, Methodology, Supervision, Writing – original draft.
Declaration of Competing Interest
G.T. Maniscalco received personal compensations from Serono, Biogen, Novartis, Roche and TEVA for public speaking and advisory boards. The other authors have nothing to disclose.
References
- Gavriatopoulou M., Korompoki E., Fotiou D., Ntanasis-Stathopoulos I., Psaltopoulou T., Kastritis E., Terpos E., Dimopoulos M.A. Organ-specific manifestations of COVID-19 infection. Clin. Exp. Med. 2020;20(4):493–506. doi: 10.1007/s10238-020-00648-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borobia A.M., Carcas A.J., Perez-Olmeda M., Castano L., Bertran M.J., Garcia-Perez J., Campins M., Portoles A., Gonzalez-Perez M., Garcia Morales M.T., Arana-Arri E., Aldea M., Diez-Fuertes F., Fuentes I., Ascaso A., Lora D., Imaz-Ayo N., Baron-Mira L.E., Agusti A., Perez-Ingidua C., Gomez de la Camara A., Arribas J.R., Ochando J., Alcami J., Belda-Iniesta C., Frias J., CombiVac S.S.G. Immunogenicity and reactogenicity of BNT162b2 booster in ChAdOx1-S-primed participants (CombiVacS): a multicentre, open-label, randomised, controlled, phase 2 trial. Lancet. 2021;398(10295):121–130. doi: 10.1016/S01406736(21)01420-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh E.E., Frenck R.W., Jr., Falsey A.R., Kitchin N., Absalon J., Gurtman A., Lockhart S., Neuzil K., Mulligan M.J., Bailey R., Swanson K.A., Li P., Koury K., Kalina W., Cooper D., Fontes-Garfias C., Shi P.Y., Tureci O., Tompkins K.R., Lyke K.E., Raabe V., Dormitzer P.R., Jansen K.U., Sahin U., Gruber W.C. Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. N. Engl. J. Med. 2020;383(25):2439–2450. doi: 10.1056/NEJMoa2027906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury D.S., Cromer D., Reynaldi A., Schlub T.E., Wheatley A.K., Juno J.A., Subbarao K., Kent S.J., Triccas J.A., Davenport M.P. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021;27(7):1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- Tortorella C., Aiello A., Gasperini C., Agrati C., Castilletti C., Ruggieri S., Meschi S., Matusali G., Colavita F., Farroni C., Cuzzi G., Cimini E., Tartaglia E., Vanini V., Prosperini L., Haggiag S., Galgani S., Quartuccio M.E., Salmi A., Repele F., Altera A.M.G., Cristofanelli F., D'Abramo A., Bevilacqua N., Corpolongo A., Puro V., Vaia F., Capobianchi M.R., Ippolito G., Nicastri E., Goletti D., INMI COVID-19 Vaccine Study Group Humoral- and T-cell-specific immune responses to SARS-CoV-2 mRNA vaccination in patients with MS using different disease-modifying therapies. Neurol. 2021;98(5):e541–e554. doi: 10.1212/WNL.0000000000013108. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sormani M.P., Inglese M., Schiavetti I., Carmisciano L., Laroni A., Lapucci C., Da Rin G., Serrati C., Gandoglia I., Tassinari T., Perego G., Brichetto G., Gazzola P., Mannironi A., Stromillo M.L., Cordioli C., Landi D., Clerico M., Signoriello E., Frau J., Ferro M.T., Di Sapio A., Pasquali L., Ulivelli M., Marinelli F., Callari G., Iodice R., Liberatore G., Caleri F., Repice A.M., Cordera S., Battaglia M.A., Salvetti M., Franciotta D., Uccelli A., M. S. study group on behalf of the Italian Covid-Alliance in M. S. CovaXi Effect of SARS-CoV-2 Mrna vaccination in MS patients treated with disease modifying therapies. EBioMedicine. 2021;72 doi: 10.1016/j.ebiom.2021.103581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniscalco G.T., Manzo V., Ferrara A.L., Perrella A., Di Battista M., Salvatore S., Graziano D., Viola A., Amato G., Moreggia O., Di Giulio Cesare D., Barbato S., Servillo G., Longo K., Di Giovanni M., Scarpati B., Muggianu S.M., Longo G., Russo G., Andreone V., De Rosa V. Interferon Beta-1a treatment promotes SARS-CoV-2 mRNA vaccine response in multiple sclerosis subjects. Mult. Scler. Relat. Disord. 2021;58 doi: 10.1016/j.msard.2021.103455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achiron A., Mandel M., Dreyer-Alster S., Harari G., Gurevich M. Humoral SARS-COV-2 IgG decay within 6 months in COVID-19 healthy vaccinees: the need for a booster vaccine dose? Eur. J. Intern. Med. 2021;94:105–107. doi: 10.1016/j.ejim.2021.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews N., Stowe J., Kirsebom F., Toffa S., Rickeard T., Gallagher E., Gower C., Kall M., Groves N., O'Connell A.M., Simons D., Blomquist P.B., Zaidi A., Nash S., Iwani Binti Abdul Aziz N., Thelwall S., Dabrera G., Myers R., Amirthalingam G., Gharbia S., Barrett J.C., Elson R., Ladhani S.N., Ferguson N., Zambon M., Campbell C.N.J., Brown K., Hopkins S., Chand M., Ramsay M., Lopez Bernal J. Covid-19 vaccine effectiveness against the Omicron (B.1.1.529) variant. N. Engl. J. Med. 2022 doi: 10.1056/NEJMoa2119451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capuano R., Bisecco A., Conte M., Donnarumma G., Altieri M., Grimaldi E., Franci G., Chianese A., Galdiero M., Coppola N., Tedeschi G., Gallo A. Six-month humoral response to mRNA SARS-CoV-2 vaccination in patients with multiple sclerosis treated with ocrelizumab and fingolimod. Mult. Scler. Relat. Disord. 2022;4(60) doi: 10.1016/j.msard.2022.103724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Zuiani A., Fischinger S., Mullur J., Atyeo C., Travers M., Lelis F.J.N., Pullen K.M., Martin H., Tong P., Gautam A., Habibi S., Bensko J., Gakpo D., Feldman J., Hauser B.M., Caradonna T.M., Cai Y., Burke J.S., Lin J., Lederer J.A., Lam E.C., Lavine C.L., Seaman M.S., Chen B., Schmidt A.G., Balazs A.B., Lauffenburger D.A., Alter G., Wesemann D.R. Quick COVID-19 healers sustain anti-SARS-CoV-2 antibody production. Cell. Dec. 2020;10(6):1496–1507. doi: 10.1016/j.cell.2020.10.051. 183e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centonze D., Rocca M.A., Gasperini C., Kappos L., Hartung H.P., Magyari M., Oreja-Guevara C., Trojano M., Wiendl H., Filippi M. Disease-modifying therapies and SARS-CoV-2 vaccination in multiple sclerosis: an expert consensus. J. Neurol. 2021;268(11):3961–3968. doi: 10.1007/s00415-021-10545-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maghzi A.H., Houtchens M.K., Preziosa P., Ionete C., Beretich B.D., Stankiewicz J.M., Tauhid S., Cabot A., Berriosmorales I., Schwartz T.H.W., Sloane J.A., Freedman M.S., Filippi M., Weiner H.L., Bakshi R. COVID-19 in teriflunomide-treated patients with multiple sclerosis. J. Neurol. 2020;267(10):2790–2796. doi: 10.1007/s00415-020-09944-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollo L., Guerra T., Bavaro D.F., Monno L., Saracino A., Angarano G., Paolicelli D., Trojano M., Iaffaldano P. Seroconversion and indolent course of COVID-19 in patients with multiple sclerosis treated with fingolimod and teriflunomide. J. Neurol. Sci. 2020;416 doi: 10.1016/j.jns.2020.117011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguirre C., Meca-Lallana V., Barrios-Blandino A., Del Rio B., Vivancos J. Covid-19 in a patient with multiple sclerosis treated with natalizumab: may the blockade of integrins have a protective role? Mult. Scler. Relat. Disord. 2020;44 doi: 10.1016/j.msard.2020.102250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achiron A., Mandel M., Dreyer-Alster S., Harari G., Dolev M., Menascu S., Magalashvili D., Flechter S., Givon U., Guber D., Sonis P., Zilkha-Falb R., Gurevich M. Humoral immune response in multiple sclerosis patients following PfizerBNT162b2 COVID19 vaccination: up to 6 months cross-sectional study. J. Neuroimmunol. 2021;361 doi: 10.1016/j.jneuroim.2021.577746. [DOI] [PMC free article] [PubMed] [Google Scholar]