Abstract
A transferable β-lactamase produced by a multidrug-resistant clinical isolate of Enterobacter cloacae was studied. The bla gene was carried by a large (>80-kb) transmissible plasmid. Nucleotide sequence analysis of cloned fragments revealed that it was part of a gene cassette carried by a class 1 integron along with other resistance genes, including aac(6′)-Ib. The encoded β-lactamase, designated IBC-1, was a novel class A enzyme that hydrolyzed ceftazidime and cefotaxime and was inhibited by tazobactam and, to a lesser extent, by clavulanate. Also, imipenem exhibited potent inhibitory activity against IBC-1. The enzyme consisted of 287 amino acid residues, including Ser-237, cysteines at positions 69 and 237a, and Arg-244, which may be implicated in its interaction with β-lactams. In amino acid sequence comparisons, IBC-1 displayed the highest similarity with the chromosomal penicillinase of Yersinia enterocolitica, a carbenicillinase from Proteus mirabilis GN79, the species-specific β-lactamases of Klebsiella oxytoca, and the carbapenemase Sme-1. However, a phylogenetic association with established β-lactamase clusters could not be conclusively shown.
Production of extended-spectrum (ES) β-lactamases (ESBLs) is one of the major causes of resistance to the newer oxyimino-β-lactams in enterobacteria. β-Lactamases hydrolyzing broad-spectrum β-lactams and inhibited by clavulanic acid are considered to have ES properties (3). Except for the class D enzymes OXA-11 (9) and OXA-18 (22), which display ES properties, the ESBLs described to date belong to molecular class A. The most clinically important enzymes in this group are the ES descendants of the common plasmid-mediated TEM-1 and SHV-1 β-lactamases. Various non-TEM, non-SHV class A β-lactamases exhibiting ES activities have also been described. CTX-M-type (1, 8) and SFO-1 (13) β-lactamases can be placed in a cluster of clavulanate-inhibited enzymes that preferentially hydrolyze cefotaxime and are related to the species-specific β-lactamases of Klebsiella oxytoca (7) and other enterobacterial species. PER (2, 19) and VEB-1 (23) β-lactamases and their distant relatives CME-1 from Chryseobacterium meningosepticum (28) and the cephalosporinases of Bacteroides spp. (21) also exhibit ES properties. VEB-1 is the first class A ESBL that was found to be encoded by an integron-associated gene (23). Resistance genes found in the variable region of class 1 integrons are parts of cassettes (27) that also include an integrase-specific recombination segment called the 59-base element (59-be) (35). The bla genes that are most frequently associated with these structures belong to the pse, carb, and oxa types (27).
In this work we describe IBC-1, a novel class A ESBL that was encoded by a class 1 integron-associated gene. The integron was located in a multidrug-resistant transferable plasmid found in an Enterobacter cloacae clinical strain.
MATERIALS AND METHODS
Bacterial strains and plasmids.
E. cloacae HT9 was isolated in January 1999 in Hippokration General Hospital, Thessaloniki, Greece from the blood of a hospitalized patient suffering from leukemia. Escherichia coli strains 14R519 (Nalr lac) (40) and DH5α (GIBCO-BRL) were used as recipients in conjugation and transformation experiments, respectively. E. coli strains producing TEM-1 (5), SHV-5 (40), and CTX-M-4 (8) β-lactamases were also used as sources of these enzymes. The chloramphenicol-resistant plasmid pBCSK(+) (Stratagene) was used as a cloning vector.
Antibiotic susceptibility testing.
Minimum inhibitory concentrations of β-lactam antibiotics were determined by an agar dilution method, according to the recommendations of the National Committee for Clinical Laboratory Standards (15). Double-disk synergy tests were applied by placing disks of oxyimino-β-lactams at 30 and 20 mm from a disk containing amoxicillin (20 μg) plus clavulanate (10 μg). Susceptibility to non-β-lactam antibiotics was determined by a disk diffusion method (16).
Conjugal transfer of resistance and plasmid DNA analysis.
Mating experiments were carried out in broth cultures as described previously (40). Transconjugant clones were selected in Mueller-Hinton agar containing nalidixic acid (100 μg/ml) plus ampicillin (50 μg/ml). Plasmid content of the donor E. cloacae strain and E. coli transconjugants was analyzed by an alkaline lysis procedure (25).
β-Lactamase studies.
β-Lactamase preparations were obtained by mild ultrasonic treatment of cells from mid-log-phase cultures in tryptone soy broth. The extracts were clarified by centrifugation. The protein content was determined by a protein assay kit (Bio-Rad). β-Lactamase activity was quantified using nitrocefin (Oxoid) and expressed as units of activity. One unit was the amount of enzyme hydrolyzing 1 μmol of nitrocefin per min per mg of protein at 30°C and pH 7.0. Isoelectric focusing (IEF) of β-lactamases was performed in polyacrylamide gels containing ampholytes (Pharmacia-LKB) covering a pH range from 3.5 to 9.5. β-Lactamase bands were visualized with nitrocefin. Hydrolytic activity against penicillin G, ampicillin, cephalothin, cefotaxime, and ceftazidime was examined by UV spectrophotometry at pH 7.0 and 30°C. At least six concentrations of each substrate were used. The respective wavelengths and extinction coefficients were as described elsewhere (4). Kinetic parameters were determined by Lineweaver-Burk plots. Inhibition by clavulanic acid, tazobactam, and imipenem was assessed as described previously (20). Enzyme preparations (100 U each) were incubated with the inhibitor for 5 min. Nitrocefin was used as the reporter substrate at a concentration of 50 μM. The 50% inhibitory concentrations (IC50s) were calculated from plots of inhibitor concentration versus percent inhibition.
DNA techniques.
Recombinant DNA techniques were performed according to standard protocols. Nucleotide sequencing was performed by the dideoxy chain termination method with a Sequenase 2.0 kit (U.S. Biochemical Corp.) and a set of custom synthesized oligonucleotide primers. Integron mapping was carried out by PCR using primers specific for genes and DNA segments found in class 1 integrons (31).
Computer-assisted nucleotide and amino acid sequence analysis.
Sequence similarity searches were carried out with the BLAST program found at the website of the National Center for Biotechnology Information. Synonymous codon usage bias was assessed by the codon adaptation index (33). Prediction of the signal peptides of deduced protein sequences was done as described previously (17). Multiple alignment of amino acid sequences was performed with the Clustal W program (38). The alignment was used for the construction of a dendrogram by the neighbor-joining method (29) with the bootstrap tree option allowing for 1,000 trials (this program was found at the website of the Institut Pasteur).
Nucleotide sequence accession number.
The sequence of the blaIBC-1 gene was submitted to the GenBank and assigned number AF208529.
RESULTS
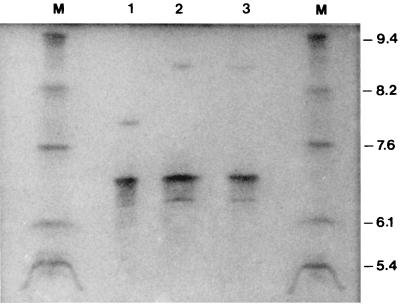
E. cloacae HT9 was resistant to penicillins, penicillin-clavulanate combinations, cefoxitin, and ceftazidime. The MICs of cefotaxime, ceftriaxone, aztreonam, and cefpirome, although elevated, were below the resistance breakpoints. The strain was susceptible to piperacillin-tazobactam, cefepime, and imipenem. E. cloacae HT9 was resistant to various non-β-lactam antibiotics, including aminoglycosides, and susceptible to nalidixic acid and fluorinated quinolones (Table 1). β-Lactam resistance was transferred by conjugation to E. coli at a frequency of 5 × 10−5 per donor cell. Transconjugants displayed a resistance phenotype similar to that of the donor, except that they were susceptible to amoxicillin-clavulanate and cefoxitin. Also, potentiation of ceftazidime activity by clavulanate and tazobactam was more pronounced in transconjugants than in the donor strain (Table 1). Double-disk synergy tests in the clinical isolate and the transconjugants appeared positive only by application of the cephalosporin disks at 20 mm from amoxicillin-clavulanate. IEF showed production of a β-lactamase with an apparent isoelectric point (pI) of 6.9 in both E. cloacae HT9 and the transconjugant clones. A second enzyme with a pI of 7.8 was also produced by HT9, most probably representing an Enterobacter AmpC chromosomal β-lactamase (Fig. 1). Analysis of plasmid DNA indicated transfer of a >80-kb plasmid (pHT9).
TABLE 1.
MICs of β-lactams and other antibiotics
| Strain | MIC (μg/ml) ofa:
|
Additional resistance markersb | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AMP | AMC | TIC | TIM | PIP | TZP | FOX | CAZ | CAZ-CLA | CAZ-TZB | CTX | CRO | ATM | FEP | CPO | IPM | ||
| E. cloacae HT9 | >256 | 128 | >256 | >256 | 128 | 8 | >256 | >256 | 128 | 64 | 16 | 16 | 8 | 2 | 16 | 0.5 | KAN AMK NET TOB TET TMP S |
| E. coli 14R519(pHT9) | >256 | 8 | >256 | 128 | 128 | 2 | 4 | >256 | 16 | 2 | 4 | 4 | 4 | 0.5 | 4 | 0.25 | KAN AMK NET TOB TET TMP S |
| E. coli DH5α(pHT9-1) | >256 | 16 | >256 | 128 | 128 | 2 | 4 | >256 | 32 | 4 | 8 | 8 | 16 | 1 | 8 | 0.25 | KAN AMK NET TOB TMP S |
| E. coli DH5α(pHT9-2) | >256 | 16 | >256 | 128 | 128 | 2 | 4 | >256 | 32 | 4 | 8 | 8 | 16 | 1 | 8 | 0.25 | KAN AMK NET TOB |
| E. coli 14R519 | 2 | 2 | 1 | 1 | 1 | 1 | 4 | 0.25 | NDc | ND | 0.06 | 0.06 | 0.12 | 0.03 | 0.06 | 0.25 | |
| E. coli DH5α | 1 | 1 | 1 | 1 | 0.5 | 0.5 | 2 | 0.12 | ND | ND | 0.03 | 0.06 | 0.12 | 0.03 | 0.03 | 0.25 | |
Abbreviations: AMP, ampicillin; AMC, amoxicillin-clavulanic acid (2:1); TIC, ticarcillin; TCC, ticarcillin-clavulanic acid (inhibitor fixed at 2 μg/ml); PIP, piperacillin; TZP, piperacillin-tazobactam (inhibitor fixed at 4 μg/ml); FOX, cefoxitin; CAZ, ceftazidime; CAZ-CLA, ceftazidime-clavulanic acid (inhibitor fixed at 4 μg/ml); CAZ-TZB, ceftazidime-tazobactam (inhibitor fixed at 4 μg/ml); CTX, cefotaxime; CRO, ceftriaxone; ATM, aztreonam; FEP, cefepime; CPO, cefpirome; IPM, imipenem.
KAN, kanamycin; AMK, amikacin; NET, netilmicin; TOB, tobramycin; TET, tetracycline; TMP, trimethoprim; S, sulfonamides.
ND, not determined.
FIG. 1.
IEF of β-lactamases produced by E. cloacae HT9, E. coli 14R519(pHT9), and E. coli DH5α(pHT9-2) (lanes 1 to 3, respectively). β-Lactamases with known pIs (TEM-1, PSE-2, SHV-1, SHV-5, and LAT-1) are shown in lanes M. Isoelectric points are indicated on the right.
Cloning of an 8-kb PstI-PstI fragment of pHT9 yielded a pBCSK(+) derivative (pHT9-1), which mediated the resistance phenotype of the pHT9 except for resistance to sulfonamides. Subcloning of a 3.3-kb SalI-HindIII fragment yielded pHT9-2, which conferred resistance to β-lactams and aminoglycosides (Table 1) and encoded a β-lactamase with a pI of 6.9 (Fig. 1).
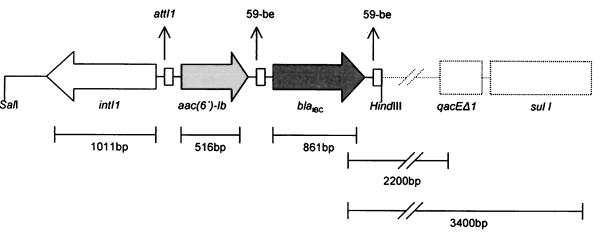
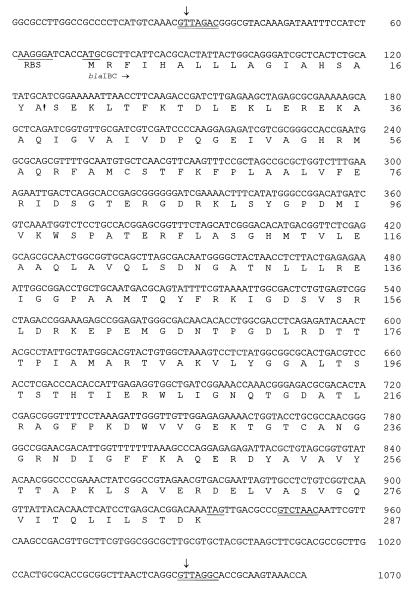
Nucleotide sequencing showed that the latter fragment included an intI1 allele, which encoded a type 1 integrase. At the 5′ end of intI1 there were at least two gene cassettes (Fig. 2). The first included an allele of the aac(6′)-Ib gene. The deduced polypeptide was identical with the previously described acetyltransferase AAC(6′)-Ib from Klebsiella pneumoniae (18). The nucleotide sequence of the following cassette is shown in Fig. 3. It was 1,021 bp long and included an open reading frame of 861 bp. At the cassette's boundaries two core sites for recombination crossover were observed. Immediately downstream of the open reading frame, an inverse core site and a core site were detected, bounding a 59-be that was 110 nucleotides long and contained putative IntI1-binding domains. The deduced polypeptide (Fig. 3) comprised 287 amino acids and possessed the characteristic motifs of class A β-lactamases, i.e., 70SXXK73, 130SDN132, E-166, and 234KTG236. The enzyme was designated IBC-1 for integron-borne cephalosporinase.
FIG. 2.
Schematic presentation of the IBC-1-encoding integron. Part of the variable region and the 3′ conserved sequence of the integron (dashed lines) were postulated from the results of the PCR assays.
FIG. 3.
Nucleotide sequence of the IBC-1 gene cassette. The boundaries of the cassette are indicated with vertical arrows. The putative ribosome binding site (RBS) and the start and stop codons of the blaIBC-1 are underlined. The core sites for recombination are double underlined. The deduced amino acid sequence of IBC-1 is also shown. The exclamation point indicates the cleavage site of the putative signal peptide.
The relative efficiency of hydrolysis of ceftazidime by IBC-1 was half that observed for penicillin G. Cefotaxime was hydrolyzed more efficiently than ceftazidime due to a lower apparent Km. The highest relative hydrolytic efficiency was observed with cephalothin. The relative Vmax and Km values for cephalosporins were higher than those for penicillin G and ampicillin (Table 2).
TABLE 2.
Kinetic parameters of hydrolysis of β-lactams by IBC-1a
| Antibiotic | Relative Vmax | Km (μM) | Relative Vmax/Km |
|---|---|---|---|
| Penicillin G | 100 | 118 | 100 |
| Ampicillin | 160 | 180 | 105 |
| Cephalothin | 1,170 | 586 | 235 |
| Cefotaxime | 582 | 782 | 88 |
| Ceftazidime | 594 | 1,300 | 54 |
Vmax values and physiologic efficiencies are relative to that of penicillin G, which were set at 100. Values are the means of three independent measurements differing by ≤10%.
IC50s (Table 3) showed that tazobactam was more effective than clavulanic acid against IBC-1. This was also indicated in the low piperacillin-tazobactam and ceftazidime-tazobactam MICs for the IBC-1-producing strains (Table 1). Compared to SHV-5, CTX-M-4, and TEM-1, IBC-1 was less susceptible to inhibition by clavulanic acid and tazobactam. Also, imipenem appeared to be a potent inhibitor of IBC-1.
TABLE 3.
Inhibition profiles of IBC-1 compared with other β-lactamases
| β-Lactamase | IC50 (μM) of:
|
||
|---|---|---|---|
| Clavulanic acid | Tazobactam | Imipenem | |
| IBC-1 | 1.05 | 0.12 | 0.06 |
| SHV-5 | 0.01 | 0.06 | 10.5 |
| CTX-M-4 | 0.18 | 0.01 | 3.7 |
| TEM-1 | 0.08 | 0.05 | 11.8 |
The G+C content of blaIBC-1 was 53%, and the codon adaptation index was 2.32. The likely secretory signal sequence of IBC-1 comprised 18 amino acid residues (Fig. 3). The mature β-lactamase would have a molecular weight of 29,258. The calculated pI (6.35) differed from the apparent pI of the native form of IBC-1. Such discrepancies may arise from charged amino acid residues that are not exposed.
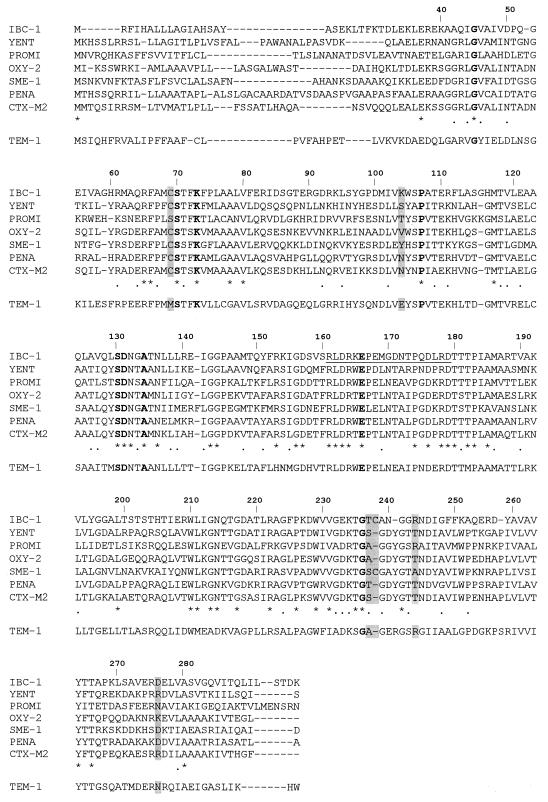
IBC-1 shared similarity (BLASTP scores, >450) with the chromosomal penicillinase of Yersinia enterocolitica, (32), the carbenicillinase of Proteus mirabilis GN79 (30), the chromosomal OXY-type enzymes of K. oxytoca (7), and the carbapenem-hydrolyzing β-lactamase Sme-1 (14). Slightly lower similarity scores were observed with the class A β-lactamase of Burkholderia cepacia (39), CTX-M-type plasmid-mediated β-lactamases (1), and the L2 penicillinase of Xanthomonas maltophilia (41) (Table 4).
TABLE 4.
Similarity of the deduced amino acid sequence of IBC-1 with various β-lactamasesa
| β-Lactamase | Organism | Accession no.b | BLAST score | No. of identical residues |
|---|---|---|---|---|
| IBC-1 | E. cloacae | AF208529 (GB) | ||
| YENT | Y. enterocolitica | Q01165 (SP) | 475 | 115 |
| PROMI | P. mirabilis | D13209 (GB) | 469 | 99 |
| OXY-2c | K. oxytoca | P23954 (SP) | 458 | 107 |
| Sme-1 | Serratia marcescens | Z28968 (GB) | 453 | 108 |
| PenA | B. cepacia | U85041 (EM) | 450 | 102 |
| CTX-M-2c | Salmonella enterica serovar Typhimurium | X92507 (EM) | 444 | 97 |
| L2 | X. maltophilia | P96465 (SP) | 441 | 107 |
| CITDI | Citrobacter diversus | P22390 (SP) | 438 | 94 |
| SHV-2 | Salmonella enterica serovar Typhimurium | P14558 (SP) | 429 | 97 |
β-Lactamases used were those that exhibited the highest similarity scores (≥450) as determined with BLASTP. Other β-lactamases with lower similarity are also included.
Accession numbers are from GenBank (GB), EMBL (EM), and SwissProt (SP) databases.
Similar scores were also obtained with other β-lactamases of this type.
A comparison of the deduced amino acid sequence of IBC-1 with other class A β-lactamases is shown in Fig. 4. IBC-1 possessed a Cys residue at position 69. Another cysteine was found at 237a. Position 237 was occupied by a threonine. Other amino acid residues that could be involved in the interaction of IBC-1 with β-lactams were a lysine at position 104, an arginine at position 244, and an aspartic acid at position 276. The segment from position 161 to 179, which included Arg-164 and Asp-179, showed extensive similarity with the putative Ω loops of other class A β-lactamases.
FIG. 4.
Amino acid sequence alignment of IBC-1 and six of the β-lactamases exhibiting the highest similarity scores with it. The sequence of TEM-1 is included for comparison. The dashes indicate gaps introduced to optimize alignment. Identical amino acids are indicated by asterisks. The dots indicate conservative amino acid substitutions corresponding to the exchange groups described in reference 6. Residues that are strictly conserved in class A β-lactamases are shown in boldface type. The putative Ω loop of IBC-1 is underlined. Other amino acid residues of potential importance are shaded. Ambler's numbering scheme was followed.
To examine potential phylogenetic relationships of IBC-1 with other β-lactamases, a dendrogram was constructed by the neighbor-joining method. Various β-lactamases representing the major class A clusters were used in this analysis. IBC-1 could not be clearly associated with any of the established groups of β-lactamases (data not shown).
The nucleotide sequence downstream of the IBC-1 cassette was not determined. PCR amplification products using pHT9-1 as a template and a blaIBC-1 internal primer paired with a sul1- and a qacEΔ1-specific primer were 3.4 and 2.2 kb, respectively (Fig. 2). This indicated colinearity of the studied sequence with a part of the 3′ conserved region of this integron.
DISCUSSION
Overproduction of the species-specific cephalosporinase of E. cloacae affects susceptibility to most broad-spectrum cephalosporins. Hence, the β-lactam-resistance phenotype of E. cloacae HT9 suggested production of a secondary enzyme with ceftazidime-hydrolyzing activity. Also, the results of the ESBL-detecting tests were equivocal. These observations prompted us to study the responsible β-lactamase.
Cloning experiments indicated a compact array of resistance genes. Nucleotide sequence analysis showed that the ESBL IBC-1 was encoded by a gene located in the variable region of a class 1 integron. The blaIBC-1 gene was part of a segment that exhibited the typical characteristics of an integron-associated gene cassette including also a 59-be. The outer ends of the 59-be of the IBC-1 cassette conform to the ends of other 59-bes. As has also been described for many 59-bes found in various class 1 integrons, the internal part does not share significant homology with analogous regions (35). A cassette that included an aac(6′)-Ib allele, which was, most likely, the last one added to this integron, preceded the IBC-1 cassette. Expression of the respective 6-N-acetyltransferase was, possibly, the sole cause of resistance to aminoglycosides. AAC(6′)-I-type enzymes are of special clinical importance because they inactivate tobramycin, netilmicin, and amikacin.
The resistance phenotype conferred by IBC-1 was consistent with its substrate profile. Although the enzyme hydrolyzed cefotaxime and ceftazidime at comparable efficiencies, it conferred higher levels of resistance to the latter drug. This apparent discrepancy has also been observed with VEB-1 (23) and some of the initially designated CAZ enzymes, such as SHV-4, SHV-5, and TEM-8 (3). This is presumably due to the faster diffusion of cefotaxime through the enterobacterial outer membrane (42). There are two notable points concerning the interaction of IBC-1 with β-lactams. (i) For cephalosporins, the relative Vmax values were high while the affinity was low compared with the respective parameters for penicillins. This characteristic can also be seen in various non-TEM non-SHV ESBLs, including VEB-1 and the related enzymes (23, 28). (ii) IBC-1 was inhibited by low concentrations of imipenem, suggesting a high affinity for this antibiotic. Imipenem hydrolysis was neither detected by spectrophotometry nor indicated from the susceptibility data. Imipenem may act as an inhibitor of class A β-lactamases (10, 36). However, compared with other class A enzymes, IBC-1 was significantly more sensitive to the inhibitory activity of imipenem.
Presuming that the IBC-1 structure shares extensive similarity with studied structures of various class A β-lactamases, some associations of specific amino acid residues with the properties of IBC-1 can be inferred. A hydrogen bond donor such as Ser or Thr at position 237 has been connected with an increase in catalytic efficiency on cephems over penems in class A β-lactamases and with an enhancement of cefotaxime-hydrolyzing activity in complex ES TEM mutants (11). Also, a Ser-237 in the P. vulgaris chromosomal cefuroxime-hydrolyzing (37), CTX-M-type enzymes (8), and class A carbapenemases (34) seems to influence the substrate preferences of these β-lactamases. Based on the significance of residue 237 in different class A enzymes, it could be speculated that Thr-237 in IBC-1 is associated with the ES activity of the enzyme. In IBC-1, position 104 is occupied by a lysine. A Lys-for-Glu-104 substitution complements ES activity in TEM variants by enhancing binding of ceftazidime and aztreonam (11). An analogous role of Lys-104 in IBC-1 would be compatible with the enzyme's activity against ceftazidime. An Arg-244 is found in IBC-1 as in most class A enzymes. This residue, or an arginine at an equivalent position, is considered critical in both hydrolysis and inhibition in most class A β-lactamases (12). In TEM and the related enzymes, Arg-244 interacts with residue 276. In IBC-1 the latter position is occupied by an aspartic acid as in the inhibitor-resistant TEM mutants (11). Also, the cysteines at positions 69 and 237a could form a disulfide bridge that may enable the active site of IBC-1 to bind imipenem, as has been suggested for the class A carbapenemase Sme-1 (26). It should be stressed that these analogies are purely speculative. IBC-1 shares a moderate similarity with various β-lactamases that, in addition, belong to distinct class A subgroups. Therefore, its active site may be different from those of the β-lactamases with which it was compared.
The available data do not provide clear indications as to the origin of IBC-1 or its potential phylogenetic association with other β-lactamases. The value of the codon adaptation index, although low, and the G+C content of blaIBC-1 do not preclude an enterobacterial origin. Also, in the neighbor-joining dendrogram, IBC-1 appears as a divergent species not clearly associated with any of the established clusters of class A enzymes. There is a recent report describing GES-1, an integron-associated ESBL from K. pneumoniae that exhibited similarity with the inherent penicillinase of Y. enterocolitica and the carbenicillinase of P. mirabilis GN79 and, additionally, was inhibited by imipenem (P. L. Nordmann, Abstr. 39th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 2050, 1999). Characterization of more enzymes similar to IBC-1 will further the understanding of their interaction with β-lactams and the tracing of their phylogenetic relations.
Identification of new β-lactamases with intrinsic ES activity underlines the diversity of bla genes in the bacterial gene pool. The β-lactam selective pressure facilitates the spread of these genes and their establishment in pathogenic microorganisms. Association of the bla genes with multidrug-resistant integrons and mobile elements is bound to be critical in this process.
ADDENDUM
The sequence of GES-1 has been recently published (24) (GenBank accession no. AF156486). GES-1 differs from IBC-1 in two amino acid residues (Glu instead of Lys-104 and Ala instead of Leu-125).
REFERENCES
- 1.Bauernfeind A, Stemplinger I, Jungwirth R, Ernst S, Casellas J M. Sequence of β-lactamase genes encoding CTX-M-1 (MEN-1) and CTX-M-2 and relationship of their amino acid sequence with those of other β-lactamases. Antimicrob Agents Chemother. 1996;40:509–513. doi: 10.1128/aac.40.2.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bauernfeind A, Stemplinger I, Jungwirth R, Mangold P, Amann S, Akalin E, Ang O, Bal C, Casellas J M. Characterization of β-lactamase gene blaPER-2, which encodes an extended-spectrum class A β-lactamase. Antimicrob Agents Chemother. 1996;40:616–620. doi: 10.1128/aac.40.3.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bush K, Jacoby G A, Medeiros A A. A functional classification scheme for β-lactamases and its correlation with molecular structure. Antimicrob Agents Chemother. 1995;39:1211–1233. doi: 10.1128/aac.39.6.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cantu C, III, Palzkill T. The role of residue 238 of TEM-1 β-lactamase in the hydrolysis of extended-spectrum antibiotics. J Biol Chem. 1998;273:26603–26609. doi: 10.1074/jbc.273.41.26603. [DOI] [PubMed] [Google Scholar]
- 5.Datta N, Kontomichalou P. Penicillinase synthesis controlled by infectious R factors in Enterobacteriaceae. Nature. 1965;208:239–242. doi: 10.1038/208239a0. [DOI] [PubMed] [Google Scholar]
- 6.Dayhoff M O, Schwartz R M, Orcott B L. A model of evolutionary change in proteins. In: Dayhoff M O, editor. Atlas of protein sequence and structure. 5, suppl. 3. Washington, D.C.: National Biochemical Research Foundation; 1978. pp. 345–352. [Google Scholar]
- 7.Fournier B, Roy P H. Variability of chromosomally encoded β-lactamases from Klebsiella oxytoca. Antimicrob Agents Chemother. 1997;41:1641–1648. doi: 10.1128/aac.41.8.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gazouli M, Tzelepi E, Sidorenko S V, Tzouvelekis L S. Sequence of the gene encoding a plasmid-mediated cefotaxime-hydrolyzing class A β-lactamase (CTX-M-4): involvement of serine 237 in cephalosporin hydrolysis. Antimicrob Agents Chemother. 1998;42:1259–1262. doi: 10.1128/aac.42.5.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hall L M C, Livermore D M, Gur D, Akova M, Akalin H E. OXA-11, an extended-spectrum variant of OXA-10 (PSE-2) β-lactamase from Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1993;37:1637–1644. doi: 10.1128/aac.37.8.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hashizume T, Yamaguchi A, Hirata T, Sawai T. Kinetic studies on the inhibition of Proteus vulgaris β-lactamase by imipenem. Antimicrob Agents Chemother. 1984;42:1259–1262. doi: 10.1128/aac.25.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Knox J R. Extended-spectrum and inhibitor-resistant TEM-type β-lactamases: mutations, specificity, and three-dimensional structure. Antimicrob Agents Chemother. 1995;39:2593–2601. doi: 10.1128/aac.39.12.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matagne A, Lamotte-Brasseur J, Frere J-M. Catalytic properties of class A β-lactamases: efficiency and diversity. Biochem J. 1998;330:581–598. doi: 10.1042/bj3300581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matsumoto Y, Inoue M. Characterization of SFO-1, a plasmid-mediated inducible class A β-lactamase from Enterobacter cloacae. Antimicrob Agents Chemother. 1999;43:307–313. doi: 10.1128/aac.43.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Naas T, Vandel L, Sougakoff W, Livermore D M, Nordmann P. Cloning and sequence analysis of the gene for a carbapenem-hydrolyzing class A β-lactamase, Sme-1, from Serratia marcescens S6. Antimicrob Agents Chemother. 1994;38:1262–1270. doi: 10.1128/aac.38.6.1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 4th ed. Approved standard M7-A4 (M100-S7). Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 16.National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial disk susceptibility tests, 6th ed. Approved standard M2-A6 (M100-S7). Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 17.Nielsen H, Engelbrecht J, Brunak S, von Heijne G. Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng. 1997;10:1–6. doi: 10.1093/protein/10.1.1. [DOI] [PubMed] [Google Scholar]
- 18.Nobuta K, Tolmasky M E, Crosa L M, Crosa J H. Sequencing and expression of the 6′-N-acetyltransferase gene of transposon Tn1331 from Klebsiella pneumoniae. J Bacteriol. 1988;170:3769–3773. doi: 10.1128/jb.170.8.3769-3773.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nordmann P, Naas T. Sequence analysis of PER-1 extended-spectrum β-lactamase from Pseudomonas aeruginosa and comparison with class A β-lactamases. Antimicrob Agents Chemother. 1994;38:104–114. doi: 10.1128/aac.38.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Papanicolaou G A, Medeiros A A, Jacoby G A. Novel plasmid-mediated β-lactamase (MIR-1) conferring resistance to oxyimino- and α-methoxy β-lactams in clinical isolates of Klebsiella pneumoniae. Antimicrob Agents Chemother. 1990;30:119–127. doi: 10.1128/aac.34.11.2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parker A C, Smith C J. Genetic and biochemical analysis of a novel Ambler class A β-lactamase responsible for cefoxitin resistance in Bacteroides species. Antimicrob Agents Chemother. 1993;37:1028–1036. doi: 10.1128/aac.37.5.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Philippon L N, Naas T, Buthors A T, Barakett V, Nordmann P. OXA-18, a class D clavulanic acid-inhibited extended-spectrum β-lactamase from Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1997;41:2188–2195. doi: 10.1128/aac.41.10.2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Poirel L, Naas T, Guibert M, Chaibi E B, Labia R, Nordmann P. Molecular and biochemical characterization of VEB-1, a novel class A extended-spectrum β-lactamase encoded by an Escherichia coli integron gene. Antimicrob Agents Chemother. 1999;43:573–581. doi: 10.1128/aac.43.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poirel L, Le Thomas I, Naas T, Karim A, Nordmann P. Biochemical sequence analyses of GES-1, a novel class A extended-spectrum β-lactamase, and the class 1 integron In52 from Klebsiella pneumoniae. Antimicrob Agents Chemother. 2000;44:622–632. doi: 10.1128/aac.44.3.622-632.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Portnoy D A, Moseley S L, Falkow S. Characterization of plasmids and plasmid-associated determinants of Yersinia enterocolitica pathogenesis. Infect Immun. 1981;31:775–782. doi: 10.1128/iai.31.2.775-782.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raquet X, Lamotte-Brasseur J, Bouillenne F, Frere J-M. A disulfide bridge near the active site of carbapenem-hydrolyzing class A β-lactamases might explain their unusual substrate profile. Proteins. 1997;27:47–58. doi: 10.1002/(sici)1097-0134(199701)27:1<47::aid-prot6>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 27.Recchia G D, Hall R M. Gene cassettes: a new class of mobile element. Microbiology. 1995;141:3015–3027. doi: 10.1099/13500872-141-12-3015. [DOI] [PubMed] [Google Scholar]
- 28.Rossolini G M, Franceschini N, Lauretti L, Caravelli B, Piccio M L, Galleni M, Frere J-M, Amicosante G. Cloning of a Chryseobacterium (Flavobacterium) meningosepticum chromosomal gene (blaACME) encoding an extended-spectrum class A β-lactamase related to the Bacteroides cephalosporinases and the VEB-1 and PER β-lactamases. Antimicrob Agents Chemother. 1999;43:2193–2199. doi: 10.1128/aac.43.9.2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 30.Sakuray Y, Tsukamoto K, Sawai T. Nucleotide sequence and characterization of a carbenicillin-hydrolyzing penicillinase gene from Proteus mirabilis. J Bacteriol. 1991;173:7038–7041. doi: 10.1128/jb.173.21.7038-7041.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sandvang D, Aarestrup F M, Larsen L B. Characterisation of integrons and antibiotic resistance genes in Danish multiresistant Salmonella enterica Typhimurium DT104. FEMS Microbiol Lett. 1998;160:37–41. doi: 10.1111/j.1574-6968.1998.tb12887.x. [DOI] [PubMed] [Google Scholar]
- 32.Seoane A, Garcia Lobo J M. Nucleotide sequence of new class A β-lactamase from the chromosome of Yersinia enterocolitica: implications for the evolution of class A β-lactamases. Mol Gen Genet. 1991;228:215–220. doi: 10.1007/BF00282468. [DOI] [PubMed] [Google Scholar]
- 33.Sharp P M, Li W-H. The codon adaptation index—a measure of directional synonymous codon usage bias, and its potential applications. Nucleic Acids Res. 1987;15:1281–1295. doi: 10.1093/nar/15.3.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sougakoff W, Naas T, Nordmann P, Collatz E, Jarlier V. Role of Ser-237 in the substrate specificity of the carbapenem-hydrolyzing class A β-lactamase Sme-1. Biochim Biophys Acta. 1999;1433:153–158. doi: 10.1016/s0167-4838(99)00138-7. [DOI] [PubMed] [Google Scholar]
- 35.Stokes H W, O'Gorman D B, Recchia G D, Parsekhian M, Hall R M. Structure and function of 59-base element recombination sites associated with mobile gene cassettes. Mol Microbiol. 1997;26:731–745. doi: 10.1046/j.1365-2958.1997.6091980.x. [DOI] [PubMed] [Google Scholar]
- 36.Taibi P, Mobashery S. Mechanism of turnover of imipenem by the TEM β-lactamase revisited. J Am Chem Soc. 1995;117:7600–7605. [Google Scholar]
- 37.Tamaki M, Nukaga M, Sawai T. Replacement of serine 237 in class A β-lactamase of Proteus vulgaris modifies its unique substrate specificity. Biochemistry. 1994;33:10200–10206. doi: 10.1021/bi00199a049. [DOI] [PubMed] [Google Scholar]
- 38.Thompson J D, Higgins D G, Gibson T J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Trepanier S, Prince A, Huletsky A. Characterization of the penA and penR genes of Burkholderia cepacia 249, which encode the chromosomal class A penicillinase and its Lys-R type transcriptional regulator. Antimicrob Agents Chemother. 1997;41:2399–2405. doi: 10.1128/aac.41.11.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vatopoulos A C, Philippon A, Tzouvelekis L S, Komninou Z, Legakis N J. Prevalence of a transferable SHV-5 type β-lactamase in clinical isolates of Klebsiella pneumoniae and Escherichia coli in Greece. J Antimicrob Chemother. 1990;26:635–648. doi: 10.1093/jac/26.5.635. [DOI] [PubMed] [Google Scholar]
- 41.Walsh T R, MacGowan A P, Bennett P M. Sequence analysis and enzyme kinetics of the L2 serine beta-lactamase from Stenotrophomonas maltophilia. Antimicrob Agents Chemother. 1997;41:1460–1464. doi: 10.1128/aac.41.7.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yoshimura F, Nikaido H. Diffusion of β-lactam antibiotics through the porin channels of Escherichia coli K-12. Antimicrob Agents Chemother. 1985;27:84–92. doi: 10.1128/aac.27.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]