Abstract
Background
Recently, researchers have conducted many studies on the potential contribution of the retina and other eye structures on schizophrenia. This study aimed to evaluate differences in iris characteristics between patients with schizophrenia and healthy individuals so as to find more easily accessible and easily measurable biomarkers with a view to improving clinical assessments and furthering our understanding of the disease.
Methods
Overall, 80 patients with schizophrenia and 52 healthy individuals were included in the case group and the control group, respectively. Iris images were collected from all subjects to compare differences in the structure and color of the iris. The Positive and Negative Symptom Scale (PANSS) and the Modified Overt Aggression Scale (MOAS) were used to evaluate the clinical symptoms and characteristics of 45 first-episode untreated schizophrenics, and analyzed correlations between iris characteristics and schizophrenia symptoms.
Results
There were significant differences in iris crypts (P<0.05) and pigment spots (P<0.01) between the case and control group, but no significant difference was found in iris wrinkles (P<0.05). The logistic regression analysis demonstrated that the total iris crypts [odds ratio (OR) 1.166, 95% confidence interval (CI) 1.022–1.330] and total iris pigment spots (OR 1.815, 95% CI 1.186–2.775) increased the risk of suffering from schizophrenia. Furthermore, it was demonstrated that the number of iris crypts was positively associated with the MOAS score (r=0.474, P<0.01). Moreover, the number of the iris pigment spots (r=0.395, P<0.01) and wrinkles (r=0.309, P<0.05) were positively correlated with the subjects’ negative symptom scores, respectively.
Conclusion
Iris crypts and pigment spots were identified as potential biomarkers for detecting schizophrenia. In patients with first-episode untreated schizophrenia, iris characteristics may help psychiatrists to identify the illness and its severity, and to detect characteristic clinical symptoms.
Keywords: schizophrenia, biomarker, iris, crypt, pigment spot
Introduction
Schizophrenia has long been recognized as a severe psychiatric disease with high heritability, and it is one of the leading causes of global disease-related disability. According to the results of one epidemiological investigation, there are over 20 million cases of schizophrenia and the prevalence rate of worldwide around 1%.1 Additionally, the findings of another national epidemiological survey that was carried out in 31 provinces in China demonstrated that the life-time and 12-month prevalence rates of schizophrenia were the same, at 0.6% (95% CI: 0.1–1.0).2 As schizophrenia is associated with a series of severe clinical symptoms, such as fixed false beliefs, perceptual abnormalities, negative symptoms and cognitive deficits, patients with schizophrenia are at a higher risk of disability, recurrence, and a reduced quality of life, which ultimately shortens the entire life span.3 However, its underlying etiology and pathogenesis have not been fully explored. Biological, psychological, and social factors, such as genetic factors, intrauterine infection during pregnancy,4 early neurodevelopmental disorders, and psychological stress5,6 have been proven to be closely related to the development of schizophrenia. Moreover, there is considerable overlap of schizophrenia with other mental disorders, which means that early diagnosis proves difficult. Therefore, it is imperative to explore easily measurable biological indicators that could improve the early detection and early diagnosis of schizophrenia.
It is broadly accepted that the eyes and brain develop from the same layer (neural ectoderm), and many genes are implicated in both the eyes and normal neuronal development,7,8 such as Semaphorin 3A (SEMA3A),9 Paired box 6 (Pax6),10,11 SIX homeobox 3 (SIX3),12,13 HECT and RLD domain containing E3 ubiquitin protein ligase 2 (HERC2),14,15 and Oculocutaneous Albinism II (OCA2).16,17 Importantly, these genes are also related to schizophrenia and other mental disorders.9,18–22 The link between structural eye characteristics and psychiatric disorders has been increasingly confirmed in recent years.23 Systematic evaluation and meta-analyses have detected specific changes in the retinal structure of schizophrenic patients, who had thinner retinas and retinal blood vessels that were smaller in diameter compared with healthy people.24–27 Meanwhile, significant differences have also been observed between schizophrenic patients and healthy people in respect to pupil distance, corneal volume, anterior chamber volume, anterior chamber depth, and central corneal thickness.28
The human iris is the most specific tissue in the eye and its structure is characterized by8,14,29,30 crypts (defect of iris stroma), pigment spots, and wrinkles, as shown in Figure 1. Since 1956, studies have been carried out to examine the relationship between schizophrenia and iris color. For example, Kent et al31 found that among hospitalized schizophrenic patients, the mixed iris pigmentation patients accounted for the highest proportion. Compared with healthy people, Trixler et al32 found a higher rate of pigment spots, concentric furrows, and Wolfflin nodules in the iris structure of schizophrenic patients. He further speculated that a denser iris is expected more in healthy individuals, meaning low number of Fuchs crypts. However, as far as we know, very few studies have investigated the relationship between iris structure and schizophrenia, and the relationship between iris structure and psychotic symptoms in schizophrenia remains unclear. Thus, further research in this area is necessary.
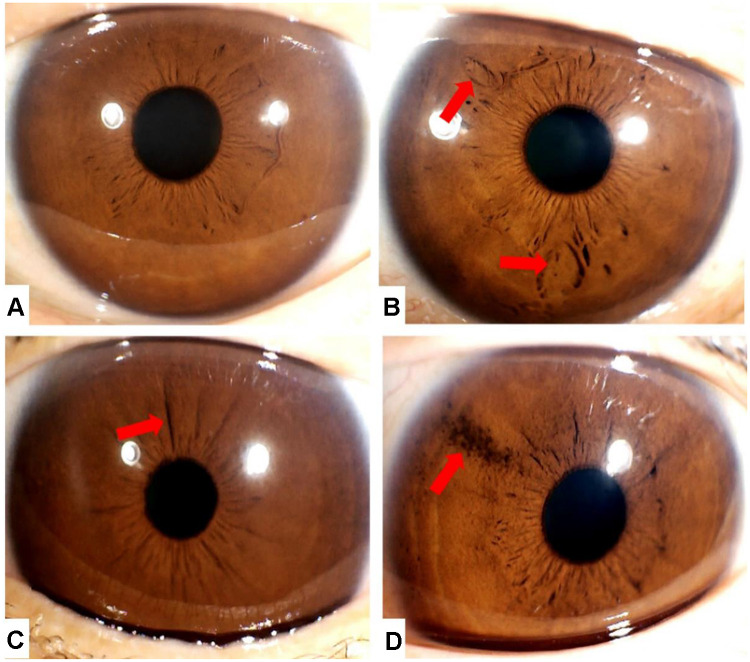
Figure 1.
Iris characteristics.
Notes: (A) The iris does not have any special structure; iris under normal conditions. (B) The two arrows point to iris crypts, which are formed by defects in epithelial and stroma tissues. (C) The arrow points to an iris wrinkle, which is formed by the folding of iris epithelium. (D) The arrow points to iris pigment spots, which are formed by iris melanin aggregation.
Subjects and Methods
Participants and Recruitment Procedure
The participants in this study included outpatients and inpatients from Shenyang Mental Health Center of Liaoning Province of China who were recruited between August 2019 and August 2020. The inclusion criteria were as follows: 1) aged between 18–60 years at the time of enrollment; 2) after independent assessments by two senior psychiatrists, participants were required to satisfy the diagnostic criteria for schizophrenia according to the Diagnostic and Statistical Manual for Mental Disorders-5 (DSM-5); 3) there was no kin relationship between subjects in the case group; 4) patients with other neuropsychiatric disorders and eye diseases (eg, iritis, glaucoma, and diabetes) were excluded; 5) the length of education is more than 9 years; 6) the patients or their guardians were able to liaise with the psychiatrists to complete the consultation, eye photography, and questionnaire survey; and 6) the patients or their guardians volunteered to participate in the study and provided their written informed consent.
In addition, 52 healthy subjects with no mental disorders, or neurological or physical diseases were age- and gender-matched with the schizophrenic patients. All participants completed a face-to-face interview to exclude psychiatric disorders. Moreover, all participants had a negative personal and family history of mental illness.
Principles of Ethical Review
This research was carried out in accordance with the Declaration of Helsinki. All participants were informed of the purpose, experimental methods, benefits and risks of the experiment, and their rights as participants in the experiment. The researchers obtained the written informed consent of all participants after a detailed explanation of the study was provided. This study was approved by the Ethics Committee of China Medical University (approval No. [2020] 2020-352-2).
Measurements
Iris Characteristics
For all participants, iris photographs were taken in the same ophthalmic examination room. Two ophthalmologists used Iris Image Collector EH–9100 (Yiheng Electronics Co., Ltd. Dongguan, China) to capture images of the iris.
The surface characteristics of the iris were counted by two senior ophthalmologists. Based on the findings of previous studies on genes related to iris structure and color, our study mainly classified iris characteristics as crypts, wrinkles, and pigment spots, as shown in Figure 1.
Iris Characteristics Statistical Method
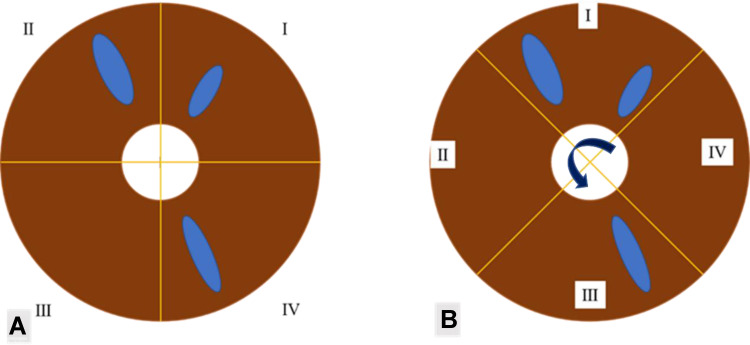
The rotating coordinate statistics method was used to assess the iris characteristics consistently. As shown in Figure 2, this method takes the pupil center as the origin and makes two vertical intersection lines, so that the rectangular coordinate axis is formed by the iris boundary. Considering when iris is divided with a fixed rectangular coordinate axis, it is inevitable that a feature will be exactly divided in the quadrants on both sides by the vertical line, resulting in more statistical quadrants than the actual ones, as shown in Figure 2. To avoid the above situation, the vertical coordinate axis shall be rotated and adjusted with the origin as the center, and the iris should be divided into four quadrants with equal area but not fixed. Only when the vertical coordinate rotates no matter how, the quadrants involved in the iris characteristics are greater than 1, or greater than 2, or greater than 3, can it be counted as 2, or 3, or 4. That is, actually count the minimum number of quadrants involved in each characteristic (not the number of iris characteristics themselves), and then add the number of left and right iris statistics to mark the total frequency of iris characteristics.
Figure 2.
Iris characteristic statistical method.
Notes: (A) This picture shows fixed rectangular coordinate axis, this method resulting in more statistical quadrants than the actual ones. It can be seen that the crypts exist in three quadrants. (B) This picture shows rotating coordinate statistics method, it can be seen that the crypts exist in two quadrants.
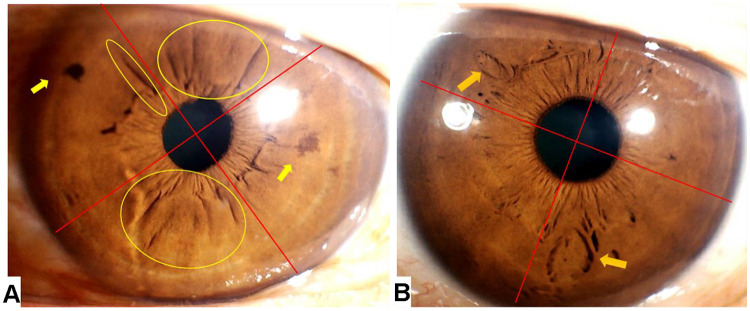
An example of statistical method is shown in Table 1, Figure 3.
Table 1.
Number of Total Iris Characteristics
| Iris Characteristic | Number of Quadrants Occupied | Total Number of Quadrants For Each Characteristic | |
|---|---|---|---|
| Left Iris | Right Iris | ||
| Crypt | 0 | 2 | 2 |
| Spot | 2 | 0 | 2 |
| Wrinkle | 3 | 0 | 3 |
Notes: The number of total iris crypts, total iris pigment spots, and total iris wrinkles represents the sum of the number of quadrants occupied by the left iris and right iris, respectively.
Figure 3.
Example for Iris characteristic statistical method.
Notes: (A) The left iris has pigment spots in two quadrants (indicated by the two yellow arrows), iris wrinkles in three quadrants (as indicated by the three yellow circles). (B) The right iris has crypts in two quadrants (as indicated by the two yellow arrows). The total number of quadrants for each characteristic: Total iris crypt frequency = 2; Total iris pigment spot frequency = 2; Total iris wrinkle frequency = 3.
Clinical Measurements
The Positive and Negative Symptom Scale (PANSS) is mainly used to assess the development of psychiatric symptoms in adults, and the severity of each symptom. The PANSS also distinguishes between positive symptoms and negative symptoms.33 It consists of 30 items, of which seven items are related to positive symptoms, seven items to negative symptoms, and 16 items to general psychopathology. Each item is rated according to a Likert-type scale ranging from 1 (none) to 7 (extremely severe). The entire assessment takes approximately 30 to 50 minutes to complete. By calculating the sum of the ratings of each item, we obtained the score for each subscale, which ranged from 7–49 for the positive and negative subscales, and 16–112 for the general psychopathology subscale.
The Modified Overt Aggression Scale (MOAS) was used to evaluate aggressive and violent behavior. It consists of four subscales which include verbal aggression, aggression against objects, physical aggression against oneself, and physical aggression against others. Each subscale measures the level of impulsive behavior according to a five-point Likert scale ranging from 0 (non-impulsive behavior) to 4 (the most severe impulsive performance). This scale has been proven to have better reliability and validity in the Chinese population.34
Statistical Analysis
The statistical analyses were performed using SPSS version 25.0 (SPSS Inc., Chicago). Independent samples t-tests and chi-square tests were conducted to compare demographic variables between patients and controls. For iris characteristics, the Mann–Whitney U-test was carried out to compare the significance of differences found between the two groups. Moreover, bivariate logistic regression analyses were performed to assess the association between the outcome variable (whether or not subjects developed schizophrenia), and potential predictors (eg, demographic characteristics, number of iris crypts and pigment spots). Taking the occurrence of schizophrenia as the dependent variable, demographic characteristics were used to predict the correlation between iris characteristics and schizophrenia. In the first-episode untreated schizophrenia group, the relationship between the PANSS, MOAS, and the total frequencies of iris characteristics were respectively analyzed using Spearman correlation analysis. All of these tests were two-tailed, and the level of statistical significance was set as P < 0.05.
Results
The sample size of the case group included 80 patients with schizophrenia who were recruited from the Shenyang Mental Health Center. As shown in Table 2, the subjects comprised 28 males (35.0%) and 52 females (65.0%) with a mean age of 38.85 (SD = 12.00) years, ranging from 18- to 60-years-old. The case group included 45 first-episode untreated patients, namely, 18 males and 27 females with a mean age of 29.87 (SD = 7.32, range: 18–43 years). A further in-depth analysis demonstrated that there was no kin relationship between the subjects in the case group. In addition, 52 healthy subjects of Chinese Han ethnicity (aged 18–58 years) were enrolled in the control group, and these subjects included 18 males and 34 females with a mean age of 40.37 (SD = 11.33) years. Moreover, as shown in Table 2, there was no significant difference in the mean age between the case group and healthy controls (P>0.05), and the chi-square test revealed no significant difference in gender between schizophrenic patients and controls (P>0.05). The male-to-female ratio in the schizophrenia group and control group was 28:52 and 18:34, respectively.
Table 2.
Demographics of Case Group and Healthy Control Group
| Factors | Healthy Control | Case Group | P |
|---|---|---|---|
| Age (Mean ± SD) | 40.37±11.33 | 38.85±12.00 | 0.470a |
| Gender | |||
| Male | 18 | 28 | 0.964b |
| Female | 34 | 52 |
Note: “a” presents Student’s t-test analysis; “b” presents chi-square analysis.
Table 3 shows that iris crypts were found in 39 patients, 33 patients had iris pigment spots, and 27 patients had iris wrinkles. In the control group, iris crypts were observed in 14 patients, seven patients had iris pigment spots, and 11 patients had iris wrinkles. The distribution of iris crypts was significantly different between the two groups (P<0.05). A significant difference was also found in iris pigment spots between the two groups (P<0.01). There was no significant difference in iris wrinkles between the two groups (P>0.05).
Table 3.
Iris Characteristics of Healthy Control Group (N=52) and Case Group (N=80)
| Whether Have Iris Character | Number of Subjects | P | |
|---|---|---|---|
| Healthy Control Group | Case Group | ||
| Have crypts/Not | 14/38 | 39/41 | 0.013a |
| Have pigment spots/Not | 7/45 | 33/47 | 0.001a |
| Have wrinkles/Not | 11/41 | 27/53 | 0.120a |
Note: “a” presents the Mann–Whitney U-test.
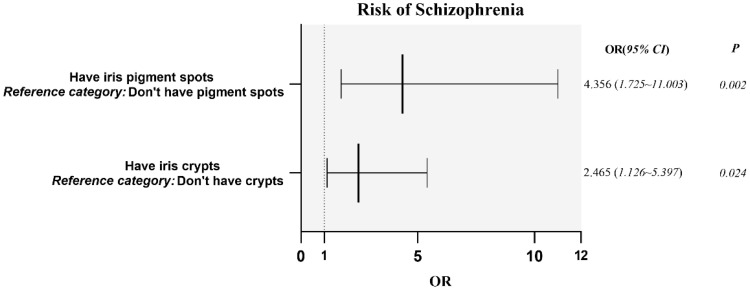
As shown in Table 4 the number of iris crypts was positively correlated with MOAS scores, and the correlation coefficient was 0.474 (P<0.01); the number of iris pigment spots was positively correlated with negative symptom scores, and the correlation coefficient was 0.395 (P<0.01); the number of iris wrinkles was positively correlated with negative symptom scores, the correlation coefficient was 0.309 and P was<0.05. As illustrated in Figure 4, iris crypts (OR 2.465, 95% CI 1.126–5.397) and iris pigment spots (OR 4.356, 95% CI 1.725–11.003) were closely associated with the risk of contracting schizophrenia. Moreover, as shown in Table 5, the regression analysis revealed that increasing iris crypt and pigment spot grade increased the odds of contracting schizophrenia by 1.166 and 1.815, respectively.
Table 4.
Correlations Between PANSS, MOAS, and the Total Frequencies of Each Iris Characteristics in Case Group (N=45)
| Variable | Total Iris Crypts | Total Iris Spots | Total Iris Wrinkles | |||
|---|---|---|---|---|---|---|
| r | P | r | P | r | P | |
| PANSS score | −0.056 | 0.713 | 0.128 | 0.402 | −0.068 | 0.656 |
| Positive symptom score | −0.183 | 0.229 | −0.136 | 0.371 | −0.256 | 0.089 |
| Negative symptom score | −0.087 | 0.57 | 0.395 | 0.007** | 0.309 | 0.039* |
| General symptom score | 0.169 | 0.268 | −0.091 | 0.551 | −0.164 | 0.280 |
| MOAS score | 0.474 | 0.001** | 0.102 | 0.504 | 0.002 | 0.989 |
Note: “r” represents the correlation coefficient. *P<0.05; **P<0.01.
Figure 4.
Correlation between iris characteristics and schizophrenia.
Notes: Binary regression analysis to predict iris characteristics and risk of schizophrenia. Illness presence was taken as the dependent variable. Measured covariates included the presence of iris crypts and pigment spots.
Table 5.
Total Number of Iris Characteristics and the Risk of Schizophrenia
| Variables | B | S.E. | Wals | OR (95% CI) | P |
|---|---|---|---|---|---|
| Total crypts | 0.154 | 0.067 | 5.232 | 1.166 (1.022–1.330) | 0.022 |
| Total pigment spots | 0.596 | 0.217 | 7.556 | 1.815 (1.186–2.775) | 0.006 |
Notes: Binary regression analysis was performed to predict the total number of iris characteristics and the risk of schizophrenia. Illness presence was taken as the dependent variable. Measured covariates included the number of quadrants occupied by iris crypts and pigment spots, respectively.
Discussion
This study carried out a comparison of iris surface features between schizophrenics and healthy individuals, and analyzed the correlation between iris characteristics and the severity of symptoms, particularly with regard to impulsive behavior in patients with first-episode untreated schizophrenia. The results revealed significant differences in the frequency of iris crypts and pigment spots between the case group and healthy controls, which was consistent with the findings of Daniel Trixler et al.32 In other words, the frequency of pigment spots was higher in schizophrenic patients than in healthy controls. However, no significant correlation was found between iris wrinkles and subjects in the case group or the control group. This finding may be attributed to ethnic differences. For instance, Edwards et al30 found that the distribution of iris wrinkles was similar among individuals of South Asian and European ancestry, whereas those of East Asian ancestry had significantly fewer iris wrinkles than those of South Asian and European ancestry. In addition, compared with healthy controls, a higher quantity of iris crypts was detected in the case group. The results of the logistic regression analysis showed that the total number of iris crypts and pigment spots was associated with an increased risk of schizophrenia. Although the exact mechanism of these phenomena is unclear, we speculated that these phenomena may be related to polymorphisms in genes that regulate both eye and brain developments, such as Pax6, Six3, and Sema3A, and the mutation of these genes can lead to brain abnormalities.7 Furthermore, the pathological changes in the iris and brain are parallel:32 who had crypts on their iris, the brain MRI detected abnormal gray matter structure in the anterior cingulate gyrus, cerebellum, and middle temporal lobe.35–38 Similarly, Heyman et al21 found a higher incidence of mental illness and more obvious behavioral abnormalities in individuals with ocular structural abnormalities. The findings of Dou-dou Chen et al39 further confirmed the association between patients with intellectual disabilities and iris defects. The aforementioned theory and research results provide solid theoretical support for the link between schizophrenia and specific iris characteristics which are different to those observed in healthy individuals. Therefore, part of our findings could be explained in terms of a neurodevelopmental perspective.
Moreover, in first-episode untreated schizophrenic patients, iris crypt quantity was positively correlated with MOAS scores, and the frequency of iris pigment spots and wrinkles was positively correlated with negative symptom scores, as shown in Table 5. Previous studies which were not consistent with the results of our study have shown that iris crypts in healthy people were significantly correlated with emotional characteristics, while concentric furrow frequency was positively correlated with impulsivity.40 Thus far, few studies have been carried out to examine the relationship between iris characteristics and mental and behavioral symptoms in schizophrenic patients. However, in these patients, psychotic symptoms, emotional responses, and behavioral traits are associated with neurotransmitters such as dopamine and 5-hydroxytryptamine.41 Meanwhile, considering the correlation between the iris and brain structure, we therefore speculated that iris crypts, pigment spots, and iris wrinkles may be constitute an external manifestation of abnormalities in neurotransmitters and/or in the neural pathways of the brain. In other words, in schizophrenic patients, a higher number of iris crypts indicates a greater tendency towards aggressive behavior.
Lan Kent et al31 found that schizophrenic patients with mixed iris color accounted for the highest proportion of hospitalized schizophrenic patients. Iris color is determined by the concentration of melanin and melanocytes in its tissues,42 and mixed iris color is largely due to the uneven distribution of melanocytes, which also results in the formation of pigment spots on the iris. Additionally, many studies have found that some genes, such as HERC215 and OCA2, are related to both iris color and neural development. HERC2 is a giant E3 ubiquitin protein ligase that is associated with DNA repair regulation,43 pigmentation, and nervous system diseases15,21,44 such as schizophrenia, autism,45 and Down Syndrome.46 OCA2 is not only one of the major pigmentation genes affecting the quantity and quality of melanin in melanocytes, but it is also an independent factor that affects eye color.47 In addition, HERC2 can also regulate the expression of OCA2,48,49 which further affects eye pigmentation. The results of this study also found that first-episode untreated schizophrenic patients who had a higher frequency of iris pigment spots exhibited more prominent negative symptoms. This phenomenon is partly consistent with Lan Kent50 who found that, for assessment purposes, iris pigment spots can be reliable indicators of an individual’s physical and psychological characteristics: schizophrenics with light iris color exhibited paranoid traits, and the study categorized schizophrenics according to the concentration of iris pigmentation. The findings revealed that, as the pigmentation of the iris changes from light to dark, psychotic symptoms also change, manifesting in the form of prominent negative symptoms rather than prominent positive symptoms. In summary, we speculated that HERC2/OCA2 polymorphism may be one of the reasons that a higher incidence of iris pigment spots was detected in schizophrenic patients than in healthy controls. More importantly, it is possible that an excessive incidence of iris pigment spots can be used as an in vitro marker of abnormal brain function.
Limitations
There are several limitations to consider in the current study. First, we developed a novel statistical method to classify the structure of the iris. However, this technique has not been applied to larger study samples to clarify the advantages and disadvantages of this method, and to identify any shortcomings. Second, although there is a large body of evidence to support our reasoning, further genetic research is needed to verify our findings. Third, it was not possible to determine the effect that antipsychotics could have on the structure and color of the iris. Therefore, this paper presented an exploratory study regarding the relationship between schizophrenia and iris characteristics.
Conclusion
In summary, our study further supported the theory of neurodevelopmental disorders in the etiology of schizophrenia by demonstrating a link between specific iris structural features and schizophrenia. Moreover, we recommend that iris crypts and pigmentation spots be used as potential biomarkers to detect schizophrenia, which can help to identify new research directions for risk assessment, and improve early diagnosis and treatment in clinical practice.
Funding Statement
This work was supported by grants awarded by the Major Project of the Department of Science & Technology of Liaoning Province, China (2019JH8/10300019).
Disclosure
The authors declare that they have no conflicts of interest.
References
- 1.James SL, Abate D, Abate KH, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet. 2018;392(10159):1789–1858. doi: 10.1016/S0140-6736(18)32279-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang Y, Wang Y, Wang H, et al. Prevalence of mental disorders in China: a cross-sectional epidemiological study. Lancet Psychiatry. 2019;6(3):211–224. doi: 10.1016/S2215-0366(18)30511-X [DOI] [PubMed] [Google Scholar]
- 3.Duan L, Zhu G. Mapping theme trends and knowledge structure of magnetic resonance imaging studies of schizophrenia: a bibliometric analysis from 2004 to 2018. Front Psychiatry. 2020;11:27. doi: 10.3389/fpsyt.2020.00027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Torrey EF, Yolken RH. Schizophrenia and infections: the eyes have it. Schizophr Bull. 2017;43(2):247–252. doi: 10.1093/schbul/sbw113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stilo SA, Murray RM. Non-genetic factors in schizophrenia. Curr Psychiatry Rep. 2019;21(10):100. doi: 10.1007/s11920-019-1091-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Winkel R, Stefanis NC, Myin-Germeys I. Psychosocial stress and psychosis. A review of the neurobiological mechanisms and the evidence for gene-stress interaction. Schizophr Bull. 2008;34(6):1095–1105. doi: 10.1093/schbul/sbn101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Larsson M, Duffy DL, Zhu G, et al. Findings for human iris patterns: associations with variants in genes that influence normal neuronal pattern development. Am J Hum Genet. 2011;89(2):334–343. doi: 10.1016/j.ajhg.2011.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Larsson M, Pedersen NL. Genetic correlations among texture characteristics in the human iris. Mol Vis. 2004;10:821–831. [PubMed] [Google Scholar]
- 9.Eastwood SL, Law AJ, Everall IP, et al. The axonal chemorepellant semaphorin 3A is increased in the cerebellum in schizophrenia and may contribute to its synaptic pathology. Mol Psychiatry. 2003;8(2):148–155. doi: 10.1038/sj.mp.4001233 [DOI] [PubMed] [Google Scholar]
- 10.Cvekl A, Callaverts P. PAX6: 25th anniversary and more to learn. Exp Eye Res. 2017;156:10–21. doi: 10.1016/j.exer.2016.04.017 [DOI] [PubMed] [Google Scholar]
- 11.Dansault A, David G, Schwartz C, et al. Three new PAX6 mutations including one causing an unusual ophthalmic phenotype associated with neurodevelopmental abnormalities. Mol Vis. 2007;13:511–523. [PMC free article] [PubMed] [Google Scholar]
- 12.Aiajaz S, Allen J, Tregidgo R, et al. Expression analysis of SIX3 and SIX6 in human tissues reveals differences in expression and a novel correlation between the expression of SIX3 and the genes encoding isocitrate dehyhrogenase and cadherin 18. Genomics. 2005;86(1):86–99. doi: 10.1016/j.ygeno.2005.03.002 [DOI] [PubMed] [Google Scholar]
- 13.Li Y, Wang R, Qiao N, et al. Transcriptome analysis reveals determinant stages controlling human embryonic stem cell commitment to neuronal cells. J Biol Chem. 2017;292(48):19590–19604. doi: 10.1074/jbc.M117.796383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sturm RA, Larsson M. Genetics of human iris colour and patterns. Pigment Cell Melanoma Res. 2009;22(5):544–562. doi: 10.1111/j.1755-148X.2009.00606.x [DOI] [PubMed] [Google Scholar]
- 15.Morice-Picard F, Benard G, Rezvani HR, et al. Complete loss of function of the ubiquitin ligase HERC2 causes a severe neurodevelopmental phenotype. Eur J Hum Genet. 2016;25(1):52–58. doi: 10.1038/ejhg.2016.139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsai AL, Agustines D. The coexistence of oculocutaneous albinism with schizophrenia. Cureus. 2020;12(1):e6617. doi: 10.7759/cureus.6617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Donnelly MP, Paschou P, Grigorenko E, et al. A global view of the OCA2-HERC2 region and pigmentation. Hum Genet. 2012;131(5):683–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hung C, Tallerico T, Seeman P. Schizophrenia hippocampus has elevated expression of chondrex glycoprotein gene. Synapse. 2003;50(1):29–34. doi: 10.1002/syn.10228 [DOI] [PubMed] [Google Scholar]
- 19.Davis LK, Meyer KJ, Rudd DS, et al. Pax6 3’ deletion results in aniridia, autism and mental retardation. Hum Genet. 2008;123(4):371–378. doi: 10.1007/s00439-008-0484-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Graw J. From eyeless to neurological diseases. Exp Eye Res. 2017;156:5–9. doi: 10.1016/j.exer.2015.11.006 [DOI] [PubMed] [Google Scholar]
- 21.Heyman I, Frampton I, Van Heyningen V, et al. Psychiatric disorder and cognitive function in a family with an inherited novel mutation of the developmental control gene PAX6. Psychiatr Genet. 1999;9(2):85–90. doi: 10.1097/00041444-199906000-00006 [DOI] [PubMed] [Google Scholar]
- 22.Goudreau G, Petrou P, Reneker LW, et al. Mutually regulated expression of Pax6 and Six3 and its implications for the Pax6 haploinsufficient lens phenotype. Proc Natl Acad Sci USA. 2002;99(13):8719–8724. doi: 10.1073/pnas.132195699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Silverstein SM, Keane BP, CorlettT PR. Oculomics in schizophrenia research. Schizophr Bull. 2021;47(3):577–579. doi: 10.1093/schbul/sbab011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schönfeldt-LecuonaA C, Schmidt A, Pinkhardt EH, et al. Optical coherence tomography (OCT)–A new diagnostic tool in psychiatry? Fortschr Neurol Psychiatr. 2014;82(10):566–571. doi: 10.1055/s-0034-1385024 [DOI] [PubMed] [Google Scholar]
- 25.Lizano P, Bannai D, Lutz O, et al. A meta-analysis of retinal cytoarchitectural abnormalities in schizophrenia and bipolar disorder. Schizophr Bull. 2020;46(1):43–53. doi: 10.1093/schbul/sbz029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pan J, Zhou Y, Xiang Y, et al. Retinal nerve fiber layer thickness changes in Schizophrenia: a meta-analysis of case-control studies. Psychiatry Res. 2018;270:786–791. doi: 10.1016/j.psychres.2018.10.075 [DOI] [PubMed] [Google Scholar]
- 27.Jurisic D, Ćavar I, Sesar A, et al. New insights into schizophrenia: a look at the eye and related structures. Psychiatr Danub. 2020;32(1):60–69. doi: 10.24869/psyd.2020.60 [DOI] [PubMed] [Google Scholar]
- 28.Cumurcu T, Keser S, Cumurcu BE, et al. Refraction and eye anterior segment parameters in schizophrenic patients. Arq Bras Oftalmol. 2015;78(3):180–184. doi: 10.5935/0004-2749.20150046 [DOI] [PubMed] [Google Scholar]
- 29.Sidhartha E, Gupta P, Liao J, et al. Assessment of iris surface features and their relationship with iris thickness in Asian eyes. Ophthalmology. 2014;121(5):1007–1012. doi: 10.1016/j.ophtha.2013.11.028 [DOI] [PubMed] [Google Scholar]
- 30.Edwards M, Cha D, Krithika S, et al. Analysis of iris surface features in populations of diverse ancestry. R Soc Open Sci. 2016;3(1):150424. doi: 10.1098/rsos.150424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kent I, Christie RG, Tunis MM, et al. Human iris pigment. II. Factors in schizophrenia. Can Psychiatr Assoc J. 1956;1(3):105–106. doi: 10.1177/070674375600100302 [DOI] [PubMed] [Google Scholar]
- 32.Trixler D, Tenyi T. Iris structure and minor physical anomalies in schizophrenia. Psychiatry Res. 2017;256:412–416. doi: 10.1016/j.psychres.2017.07.004 [DOI] [PubMed] [Google Scholar]
- 33.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–276. doi: 10.1093/schbul/13.2.261 [DOI] [PubMed] [Google Scholar]
- 34.Huang HC, Wang YT, Chen KC, et al. The reliability and validity of the Chinese version of the modified overt aggression scale. Int J Psychiatry Clin Pract. 2009;13(4):303–306. doi: 10.3109/13651500903056533 [DOI] [PubMed] [Google Scholar]
- 35.Grant MK, Bobilev AM, Pierce JE, et al. Structural brain abnormalities in 12 persons with aniridia. F1000 Res. 2017;6:255. doi: 10.12688/f1000research.11063.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stokes B, Berger SI, Hall BA, et al. SIX3 deletions and incomplete penetrance in families affected by holoprosencephaly. Congenit Anom (Kyoto). 2018;58(1):29–32. doi: 10.1111/cga.12234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Domene S, Roessler E, El-Jaick KB, et al. Mutations in the human SIX3 gene in holoprosencephaly are loss of function. Hum Mol Genet. 2008;17(24):3919–3928. doi: 10.1093/hmg/ddn294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Solomon BD, Lacbawan F, Jain M, et al. A novel SIX3 mutation segregates with holoprosencephaly in a large family. Am J Med Genet A. 2009;149A(5):919–925. doi: 10.1002/ajmg.a.32813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wallis DE, Roessler E, Hehr U, et al. Mutations in the homeodomain of the human SIX3 gene cause holoprosencephaly. Nat Genet. 1999;22(2):196–198. doi: 10.1038/9718 [DOI] [PubMed] [Google Scholar]
- 40.Chen DD, Yang T, Zhu SQ. Recurrent PAX 6 mutation in a Chinese family with congenital aniridia, progressive cataracts and mental retardation. Eur J Ophthalmol. 2020;30(1):181–188. doi: 10.1177/1120672118810998 [DOI] [PubMed] [Google Scholar]
- 41.Larsson M, Pedersen NL, Stattin H. Associations between iris characteristics and personality in adulthood. Biol Psychol. 2007;75(2):165–175. doi: 10.1016/j.biopsycho.2007.01.007 [DOI] [PubMed] [Google Scholar]
- 42.Hehr U, Pineda-Alvarez DE, Uyanik G, et al. Heterozygous mutations in SIX3 and SHH are associated with schizencephaly and further expand the clinical spectrum of holoprosencephaly. Hum Genet. 2010;127(5):555–561. doi: 10.1007/s00439-010-0797-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dorgaleleh S, Naghipoor K, Barahouie A, et al. Molecular and biochemical mechanisms of human iris color: a comprehensive review. J Cell Physiol. 2020;235(12):8972–8982. doi: 10.1002/jcp.29824 [DOI] [PubMed] [Google Scholar]
- 44.Abraham JR, Barnard J, Wang H, et al. Proteomic investigations of human HERC2 mutants: insights into the pathobiology of a neurodevelopmental disorder. Biochem Biophys Res Commun. 2019;512(2):421–427. doi: 10.1016/j.bbrc.2019.02.149 [DOI] [PubMed] [Google Scholar]
- 45.Puffenberger EG, Jinks RN, Wang H, et al. A homozygous missense mutation in HERC2 associated with global developmental delay and autism spectrum disorder. Hum Mutat. 2012;33(12):1639–1646. doi: 10.1002/humu.22237 [DOI] [PubMed] [Google Scholar]
- 46.Markkanen E, Meyer U, Dianov GL. DNA damage and repair in schizophrenia and autism: implications for cancer comorbidity and beyond. Int J Mol Sci. 2016;17(6):856. doi: 10.3390/ijms17060856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang Z, Yang H, Wang H. The histone H2A deubiquitinase USP16 interacts with HERC2 and fine-tunes cellular response to DNA damage. J Biol Chem. 2014;289(47):32883–32894. doi: 10.1074/jbc.M114.599605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Branicki W, Brudnik U, Wojas-Pelc A. Interactions between HERC2, OCA2 and MC1R may influence human pigmentation phenotype. Ann Hum Genet. 2009;73(2):160–170. doi: 10.1111/j.1469-1809.2009.00504.x [DOI] [PubMed] [Google Scholar]
- 49.Visser M, Kayser M, Palstra RJ. HERC2 rs12913832 modulates human pigmentation by attenuating chromatin-loop formation between a long-range enhancer and the OCA2 promoter. Genome Res. 2012;22(3):446–455. doi: 10.1101/gr.128652.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kent I. Human iris pigment I. A concept of individual reactivity with implications in health and disease. Can Psychiatry Assoc J. 1956;1(3):99–104. [DOI] [PubMed] [Google Scholar]