Abstract
We have used the hamster model of antibiotic-induced Clostridium difficile intestinal disease to evaluate nitazoxanide (NTZ), a nitrothiazole benzamide antimicrobial agent. The following in vitro and in vivo activities of NTZ in the adult hamster were examined and compared to those of metronidazole and vancomycin: (i) MICs and minimum bactericidal concentrations (MBCs) against C. difficile, (ii) toxicity, (iii) ability to prevent C. difficile-associated ileocecitis, and (iv) propensity to induce C. difficile-associated ileocecitis. The MICs and MBCs of NTZ against 15 toxigenic strains of C. difficile were comparable to those of vancomycin or metronidazole. C. difficile-associated ileocecitis was induced with oral clindamycin and toxigenic C. difficile in a group of 60 hamsters. Subgroups of 10 hamsters were given six daily intragastric treatments of NTZ (15, 7.5, and 3.0 mg/100 g of body weight [gbw]), metronidazole (15 mg/100 gbw), vancomycin (5 mg/100 gbw), or saline (1 ml/100 gbw). Animals receiving saline died 3 days post-C. difficile challenge. During the treatment period, NTZ (≥7.5 mg/100 gbw), like metronidazole and vancomycin, prevented outward manifestations of clindamycin-induced C. difficile intestinal disease. Six of ten hamsters on a scheduled dose of 3.0 mg of NTZ/100 gbw survived for the complete treatment period. Of these surviving animals, all but three died of C. difficile disease by between 3 and 12 days following discontinuation of antibiotic therapy. Another group of hamsters received six similar daily doses of the three antibiotics, followed by an inoculation with toxigenic C. difficile. All of the NTZ-treated animals survived the 15-day postinfection period. Upon necropsy, all hamsters appeared normal: there were no gross signs of toxicity or C. difficile intestinal disease, nor was C. difficile detected in the cultures of the ceca of these animals. By contrast, vancomycin and metronidazole treatment induced fatal C. difficile intestinal disease in 20 and 70% of recipients, respectively.
Clostridium difficile is an important cause of hospital-acquired infectious diarrhea (17, 26). Treatment with antimicrobials is the primary risk factor contributing to the development of C. difficile diarrheal disease, which ranges from a mild self-limiting disease to the severe, life-threatening condition called pseudomembranous colitis. The antimicrobials most often implicated are clindamycin, ampicillin, and cephalosporins; however, C. difficile intestinal disease can occur following exposure to a wide variety of antimicrobials (11, 15). Currently, therapy for patients with antibiotic-induced C. difficile intestinal disease includes treatment with vancomycin or metronidazole, agents which inhibit the growth of C. difficile (8, 29). Successful resolution of C. difficile intestinal disease using these antimicrobials, however, is compromised by several factors: (i) ca. 20% of those patients who initially respond to metronidazole or vancomycin suffer a relapse with C. difficile intestinal disease following the cessation of antimicrobial therapy (2); (ii) metronidazole and vancomycin are, themselves, capable of inducing C. difficile intestinal disease (8); and (iii) some patients do not respond to therapy with these antimicrobials, and these patients risk development of more severe disease (24). Additionally, vancomycin is the only antibiotic active against some serious life-threatening pathogenic bacteria (3, 5). Therefore, in an effort to minimize the emergence of resistant enterococci or Staphylococcus aureus, the medical community discourages the use of vancomycin except when absolutely necessary (4). The problems associated with the current therapy for antibiotic-induced C. difficile intestinal disease have led to searches for alternative treatments.
Nitazoxanide (NTZ), a compound first synthesized by Rossignol and Cavier, is a nitrothiazole benzamide (J. F. Rossignol and R. Cavier, Chem. Abstr. 83:28216n, 1975). The in vitro and in vivo antimicrobial activities of NTZ have been shown to encompass a wide range of helminthic and protozoan intestinal parasites, as well as aerobic and anaerobic bacterial enteric pathogens, including C. difficile (6, 7, 18, 23).
The aim of this study was to evaluate the in vivo efficacy of NTZ in the hamster model of antibiotic-induced C. difficile intestinal disease relative to the efficacies of metronidazole and vancomycin in this model. We also examined the relative in vitro susceptibilities of 15 toxigenic strains of C. difficile to NTZ, metronidazole, and vancomycin.
MATERIALS AND METHODS
Strains.
C. difficile strains used in in vitro susceptibility studies were from the Texas Tech University Health Sciences Center culture collection. All strains were isolated from patients with C. difficile-associated diarrheal disease and were collected locally and from hospitals in other cities. C. difficile strain TTU 614 was used to challenge hamsters orogastrically (12). Strains were maintained in Wilkins-Chalgren broth (Oxoid-Unipath, Dardilly, France) under anaerobic conditions (80% nitrogen, 10% carbon dioxide, and 10% hydrogen). C. difficile organisms present in the ceca of hamsters were isolated on cycloserine-cefoxitin-fructose agar (10).
Antimicrobial agents.
Metronidazole and vancomycin were obtained from Sigma (St. Louis, Mo.). NTZ was provided by Romark Laboratories, L.C., Tampa, Fla. NTZ was dissolved in dimethyl sulfoxide (DMSO) at a concentration of ≤45 mg/ml and then further diluted to an appropriate concentration in Wilkins-Chalgren broth or normal saline (30). Metronidazole and vancomycin were dissolved in Wilkins-Chalgren broth or normal saline.
Determination of MICs and minimum bactericidal concentrations (MBCs).
MICs were determined by the microbroth dilution method (21). Briefly, 50 μl of antimicrobial agents, diluted in Wilkins-Chalgren broth, was added to wells of a microtiter plate such that concentrations in the wells ranged from 0.24 to 250 μg/ml. Microtiter plates containing antimicrobials were allowed to equilibrate in anaerobic chamber for 24 h before C. difficile cells were added. Twenty-four-hour cultures of C. difficile were adjusted to an A600 of 0.65 (ca. 108 CFU/ml) in Wilkins-Chalgren broth (12). Then, 50 μl of the cell suspensions, further diluted to 106 CFU/ml, was added to wells in 96-well microtiter plates containing equilibrated antimicrobials. The final concentration of antimicrobials in the wells thus ranged from 0.12 to 125 μg/ml. Inoculated microtiter plates were incubated at 37°C in anaerobic conditions for 48 h. The MIC was defined as the lowest concentration of antimicrobial agent inhibiting the total growth of the strain. After 48 h of incubation, aliquots from microtiter wells were plated on Wilkins-Chalgren agar. Visible colonies were counted at the end of 48 h of incubation at 37°C. The MBC was defined as the lowest concentration of antimicrobial agent at which 99.9% of the organisms in the original inoculum were killed. MICs and MBCs were also determined for antibiotics dissolved in a 5% suspension filtered (0.45-μm pore size) cecal contents in Wilkins-Chalgren broth.
Hamster model.
The hamster model of antibiotic-induced C. difficile intestinal disease is well characterized (20). Clindamycin-treated hamsters challenged with toxigenic C. difficile will consistently develop a fatal ileocecitis. Several other antimicrobial agents, including ampicillin, the cephalosporins, gentamicin, and erythromycin, are also capable of inducing C. difficile-associated ileocecitis in hamsters (1). Studies of this model have been invaluable in gaining an understanding of C. difficile intestinal disease in humans. Age-matched male Syrian hamsters (80 to 100 g each) used in the present study were purchased from Harlan Sprague-Dawley (Indianapolis, Ind.). All hamsters were housed individually in plastic cages and were provided a commercial laboratory ration and water ad libitum. NTZ was first dissolved in DMSO at a concentration of 45 μg/ml. This was further diluted to 15, 7.5, or 3.0 mg/ml in normal saline. The resulting preparations, which were 33 to 6.7% DMSO, were suspensions rather than solutions. Metronidazole and vancomycin were diluted to solutions of 15 and 5 mg/ml in normal saline, respectively. These suspensions and solutions were maintained on ice and each dose mixed well just prior to administration. Flexible tubing was used to administer antimicrobials and C. difficile orogastrically (1 ml/100 g of body weight [gbw]) (16). All inoculations were performed under ether anesthesia.
Assays for C. difficile toxins in hamster cecal contents.
Fifty-percent (vol/vol) suspensions of cecal contents were prepared in sterile phosphate-buffered saline (PBS). Suspensions were then clarified by centrifugation, and the resulting supernatants were filtered (0.45 μm [pore size]) and then stored at −20°C. The presence C. difficile toxin A in hamster cecal content extracts was detected using a commercially available enzyme-linked immunoassay (ImmunoCard; Meridian Diagnostics, Inc., Cincinnati, Ohio). The presence of C. difficile toxin B was confirmed in a cytotoxicity assay, using human fibroblast (HF) cells (Toxi-Titer; Bartels, Inc., Issaquah, Wash.). For cytotoxicity assays, extracts of cecal contents were further diluted to 1:200 in PBS containing gentamicin and amphotericin B. Portions (50 μl) of extracts were added to microtiter wells containing confluent HF cells in 50 μl of culture medium, giving a final extract dilution of 1:400. Cells with extracts were incubated at 37°C for 24 h. The toxicity of extracts, indicated microscopically by cytopathic effect (CPE), was noted after the incubation period. In similar assays, 25 μl of extracts from cecal contents (diluted 1:100) was preincubated with 25 μl of C. difficile toxin-specific antibody before addition to HF cells. Abrogation of CPE by the addition of toxin-specific antibody confirmed that CPE with extract alone was due to the toxicity of C. difficile toxin.
Induction of C. difficile ileocecitis in hamsters.
NTZ, metronidazole, and vancomycin were examined for their propensity to induce C. difficile-associated intestinal disease in hamsters. Groups of 10 animals each received six daily inoculations of antimicrobials according to the following regimen: three different concentrations of NTZ (15, 7.5, or 3.0 mg/100 gbw), metronidazole (15 mg/100 gbw), and vancomycin (5.0 mg/100 gbw). Another group of 10 hamsters received six daily doses of saline. At 24 h after the sixth antimicrobial or saline treatment, each hamster received 105 C. difficile strain 614. Suspensions of strain 614 were prepared from a 24-h culture as described above. Hamsters were monitored daily for signs of C. difficile disease (diarrhea, ruffled fur, lethargy). Moribund animals were sacrificed immediately by cervical dislocation under ether anesthesia. All surviving hamsters were similarly sacrificed 15 days after C. difficile challenge. Necropsies were performed on all animals, which were examined for gross evidence of intestinal disease. In addition, cecal contents from each animal were examined for the presence of C. difficile, toxin A (ImmunoCard Toxin A; Meridian Diagnostics), and cytotoxicity to cultured HF cells (Bartels, Inc.).
Prevention of ileocecitis in hamsters.
We also examined the ability of NTZ, metronidazole, and vancomycin to prevent clindamycin-induced C. difficile-associated ileocecitis in hamsters. Sixty hamsters were given a single oral inoculation of 3.0 mg of clindamycin/100 gbw, followed 24 h later by a single dose of 105 C. difficile strain 614. After 24 h treatments with antimicrobials or saline (administered once daily for 6 days, as described above) were initiated. Animals were monitored daily for signs of C. difficile disease. All moribund animals were sacrificed immediately. At 35 days after challenge with C. difficile, all surviving animals were sacrificed. Necropsies were performed on each animal, and the gross signs of disease and the presence of C. difficile were determined as described above.
Statistical analysis.
The mean of MBCs for the toxigenic C. difficile strains were determined for each antimicrobial agent. The means of MBCs were compared by analysis of variance, and significant differences between antimicrobial groups were identified by Scheffé's test.
RESULTS
Susceptibility studies.
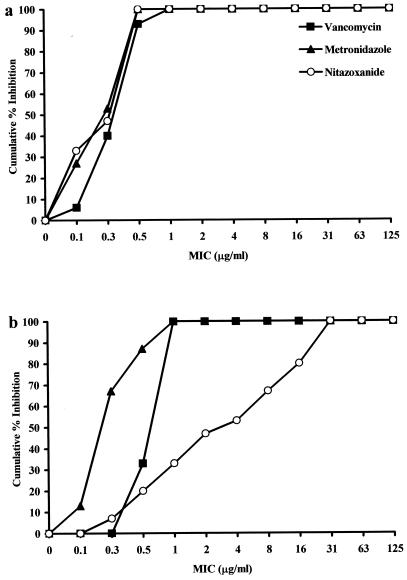
The MICs at which 50% (MIC50) or 90% (MIC90) of the toxigenic C. difficile strains were inhibited are shown in Table 1. Strains were as susceptible to NTZ as they were to metronidazole or vancomycin; the MIC90s of all three antimicrobials were 0.50 μg/ml. There was no statistical difference between the means of the MBCs of the three antimicrobials for the C. difficile strains. The means MBCs ± the standard deviations (SD) for NTZ, vancomycin, and metronidazole were 0.48 ± 0.47, 0.82 ± 0.25, 0.37 ± 0.21, respectively. The cumulative MICs, shown in Fig. 1a, further demonstrate that the potencies of three antimicrobials in inhibiting the growth of the C. difficile strains were similar.
TABLE 1.
MIC50 and MIC90 values and ranges of antimicrobial agents for 15 toxigenic C. difficile strains
| Antimicrobial agent | MIC (μg/ml)
|
||
|---|---|---|---|
| MIC50 | MIC90 | Range | |
| NTZ | 0.25 | 0.50 | 0.13–1 |
| Metronidazole | 0.25 | 0.50 | 0.13–0.50 |
| Vancomycin | 0.50 | 0.50 | 0.13–1.0 |
| NTZ in 5% cecal contents | 4 | 32 | 0.50–32 |
| Metronidazole in 5% cecal contents | 0.25 | 0.50 | 0.13–1 |
| Vancomycin in 5% cecal contents | 1 | 1 | 0.50–1 |
FIG. 1.
MICs of vancomycin, metronidazole, and NTZ for 15 strains of C. difficile in Wilkins-Chalgren broth (a) and 5% filtered hamster cecal contents in Wilkins-Chalgren broth (b).
The use of NTZ in the hamster model of C. difficile intestinal disease is undocumented, and the stability of NTZ in the hamster is also unknown. As a preliminary examination of this stability, we determined the MICs of NTZ and the other two antimicrobials in a 5% suspension of filtered hamster cecal contents. The MIC50 and the MIC90 for vancomycin and metronidazole in 5% cecal contents were increased about twofold compared to these values in medium alone (Table 1). The MIC50 and MIC90 of NTZ in 5% cecal contents were considerably higher than those in medium alone. The increased MICs and the decreased potency of NTZ is also reflected in the cumulative MICs shown in Fig. 1b. The mean MBC ± the SD of NTZ in 5% cecal contents was 13.6 ± 13, a value which differed significantly from the means ± the SD of MBCs for vancomycin (1.0 ± 36) and metronidazole (0.49 ± 0.34) in cecal contents (P < 0.03).
Induction of C. difficile-associated ileocecitis.
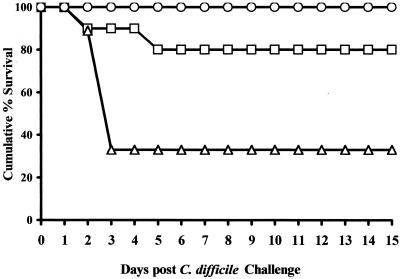
In a preliminary study, groups of 10 hamsters each were given a single dose of the antimicrobials or saline as described in Materials and Methods. Twenty-four hours later, each hamster was challenged orogastrically with 105 C. difficile 614. All of the animals survived 35 days post-C. difficile challenge with no apparent signs of intestinal disease (data not shown). We then examined the propensity of the antimicrobials to induce C. difficile disease when these agents were administered in multiple doses. Hamsters treated with six doses of 15, 7.5, or 3.0 mg of NTZ/100 gbw, as well as hamsters given saline, survived the C. difficile challenge (Fig. 2). By contrast, 2 of 10 hamsters receiving multiple doses of 5 mg of vancomycin/100 gbw and 7 of 9 hamsters receiving multiple doses of 15 mg of metronidazole/100 gbw died within 5 days of C. difficile challenge (Fig. 2). Necropsy of these animals revealed greatly enlarged and hemorrhagic ceca, with necrotic foci. The cecal contents of these animals were positive for C. difficile and C. difficile toxin A. The MICs of the antimicrobials for these recovered C. difficile isolates were within 1 dilution of these values of these agents for C. difficile 614 (0.13 to 0.50 μg/ml versus 0.25 to 0.50 μg/ml). Additionally, a 1:400 dilution of the cecal contents was toxic for cultured human fibroblasts. This in vitro toxicity was neutralized by the addition of anti-toxin A antibodies, confirming that the toxicity was due to the presence of toxin A in the cecal contents.
FIG. 2.
Cumulative survival of hamsters after induction with six daily treatments of antimicrobials prior to challenge with 105 C. difficile. Symbols: ○, 15 mg/100 gbw (NTZ); □, 5 mg/100 gbw (vancomycin); ▵, 15 mg/100 gbw (metronidazole). Animals were challenged with 105 C. difficile on day 0.
The balance of the animals, sacrificed 15 days following C. difficile challenge, appeared normal upon necropsy. Further, the cecal contents of these surviving animals were negative for C. difficile and C. difficile toxin A.
Prevention of clindamycin-induced ileocecitis.
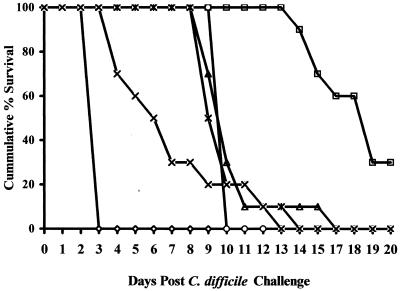
C. difficile intestinal disease was induced in hamsters by treatment with clindamycin and challenge with C. difficile (see Materials and Methods). The control animals, those treated postchallenge with saline, all died by between 60 and 72 h following C. difficile challenge (Fig. 3). The necropsy findings of these animals included grossly enlarged and hemorrhagic ceca, suggesting C. difficile intestinal disease. The presence of C. difficile and C. difficile toxin A was confirmed in all six animals.
FIG. 3.
Cumulative survival of hamsters with clindamycin-induced C. difficile ileocecitis. Relapses of animals following saline treatments (◊), treatment with vancomycin (□) or metronidazole (▵), or treatment with NZT at 15 mg/100 gbw (○), 7.5 mg/100 gbw (✠), or 3.0 mg/100 gbw (×) are indicated. Animals were challenged with 105 C. difficile on day 0.
The outward manifestations of C. difficile disease were prevented during daily treatments of 15.0 or 7.5 mg of NTZ, vancomycin, and metronidazole per 100 gbw. Only 50% of the animals on the 3.0-mg/100 gbw NTZ regimen survived the 6-day treatment period. The surviving NTZ-treated hamsters all died within a week of cessation of antimicrobial therapy (Fig. 3). Similarly, metronidazole-treated hamsters died within 5 to 11 days after the end of therapy. Of the vancomycin-treated hamsters, 70% died within 8 to 12 days posttherapy (Fig. 3). The three surviving hamsters were sacrificed at the end of the experimental period (35 days post-C. difficile challenge). Necropsy of animals which died or were sacrificed in moribund condition during the 2-week period following cessation of antimicrobial therapy showed gross signs of intestinal disease. As described for the induction phase of these experiments, the presence of C. difficile and C. difficile toxin A was confirmed by in vitro assay. By contrast, the three vancomycin-treated hamsters surviving the experiment appeared robust and showed no gross signs of intestinal disease upon necropsy. Additionally, no C. difficile or toxin A was detected in the cecal contents of these animals.
The MICs of the three antimicrobials for the C. difficile isolates detected in the cecal contents of animals succumbing to intestinal disease were within 1 log of the MICs for the challenge strain (C. difficile 614).
DISCUSSION
The goal of this study was to evaluate the antimicrobial NTZ in the hamster model of C. difficile-associated intestinal disease. An ideal agent for the treatment of this disease would be effective in controlling the in vivo growth of C. difficile and the ensuing pathogenic sequelae. This agent should also protect against relapse and not predispose the recipient to C. difficile disease. As a frame of reference, we evaluated NTZ in meeting these criteria and compared it to the antimicrobials metronidazole and vancomycin.
NTZ, a thiazolide derivative, was initially developed as an antiparasitic drug (13, 22, 23). It is active against a variety of enteric parasitic pathogens of humans and animals, including protozoa, nematodes, cestodes, and trematodes. In the United States, NTZ has investigational status for the treatment of diarrheal diseases, particularly cryptosporidiosis, in AIDS patients (27). NTZ has been shown to be nontoxic in preclinical trials (19). In human clinical trials, NTZ appears to be well tolerated, with only minimal side effects, and no abnormalities in blood chemistry or formed elements of the blood have been reported (26).
More recently, the activity of NTZ against bacteria has been investigated. NTZ was shown to inhibit 241 obligate and facultative anaerobic bacteria in vitro (7). These NTZ-susceptible bacteria, isolated from human clinical samples, included 21 strains of C. difficile. In other studies, NTZ inhibited Helicobacter pylori in vitro (18). When given in combination with omeprazole, NTZ was effective in the treatment of human H. pylori infections, some of which were resistant to metronidazole (18).
In the preliminary in vitro phase of our study, the susceptibilities of 15 clinical isolates of toxigenic C. difficile to NTZ were determined and compared with those to metronidazole and vancomycin. The MICs of NTZ for the isolates are within the range of those of metronidazole and vancomycin. The MICs of metronidazole and vancomycin determined in our study agree with those previously reported for C. difficile (7, 9). The increase in the MICs of NTZ in 5% cecal contents is difficult to explain. It is possible that the pH or salt concentration of the cecal contents may not have been optimal for NTZ activity. In addition, in the process of collecting the cecal contents, we may have released a substance capable of degrading NTZ or interfering with its activity. In any case, if this apparent reduction in potency of NTZ did occur in vivo, it did not abrogate the activity of NTZ in preventing C. difficile-associated intestinal disease in the hamster.
Previous reports of susceptibilities of C. difficile to NTZ are scant. In an evaluation of the activity of NTZ against several anaerobic bacterial isolates, Dubreuil et al. reported both the MIC50 and the MIC90 of NTZ for 21 isolates of C. difficile to be 0.06 μg/ml, with a range of 0.06 to 0.125 μg/ml (7). While the values from our assays were comparable to those of this previous study, they were slightly higher, suggesting that our C. difficile isolates were less susceptible to NTZ.
In the in vivo phases of this examination of NTZ, we utilized the hamster model of C. difficile-associated intestinal disease. In our experiments, animals pretreated with clindamycin, and posttreated with saline died within 60 to 70 h post-challenge with C. difficile. By contrast, postchallenge administration of NTZ, as well as metronidazole or vancomycin, was effective in preventing this rapid onset of disease manifestations during the treatment period. However, most animals died within 2 weeks of cessation of antimicrobial therapy. It is not clear from these experiments if the relapse of hamsters was due to reinfection with environmental C. difficile or to failure to eradicate the existing infection. Opportunities for the spread of C. difficile were minimized by housing only one animal per cage, but neither food nor water was autoclaved, nor were the animals handled in sterile fields. While all the animals receiving NTZ or metronidazole died from C. difficile-associated ileocecitis, 3 of 10 animals receiving vancomycin therapy survived the experimental period, possibly cured of C. difficile infection. Furthermore, relapse of vancomycin-treated animals was delayed compared to NTZ- or metronidazole-treated animals. These findings suggest that of the three antimicrobials tested in this study, vancomycin may be the most effective in the treatment of clindamycin-induced cecitis in hamsters. In a previous study examining several antibiotics for the treatment of C. difficile disease in hamsters, Fekety et al. reported similar contrasting efficacies for vancomycin and metronidazole (9). These authors discussed the possibility that vancomycin, which is poorly absorbed when administered orally, reaches higher concentrations in the gut than metronidazole, which is efficiently absorbed (14, 28). The resulting concentrations of vancomycin in the hamster gut may be sufficient to eliminate the C. difficile organism.
Certainly one of the paradoxes of treating C. difficile-associated intestinal disease is that most agents used to control the disease are also capable of precipitating the disease. This proved to be the case with metronidazole and vancomycin in our induction study. Twenty percent of the animals receiving vancomycin and seventy-seven percent of those receiving metronidazole died within 5 days of C. difficile challenge. Fekety et al. reported similar results in the hamster following multiple doses of these two antimicrobials (9). It was an unexpected finding then that NTZ, even at doses which prevented disease, did not induce C. difficile in the hamsters. The reasons for this are not clear from our experiments. That therapy with broad-spectrum antimicrobials disrupts normal gut flora and predisposes for colonization of C. difficile is a widely held and supportable theory. While NTZ has proven to be therapeutic for an unusually wide range of parasitic and bacterial intestinal infections, it is possible that NTZ does not affect a key component of normal gut flora required for the colonization by C. difficile. The route of excretion of orally administered NTZ in the hamster is unknown. In the rat, however, ca. 66% of an orally administered dose of NTZ is excreted in the feces (Marc Ayers, Romark Laboratories, personal communication). If this holds true for hamsters, it is reasonable to speculate that residual NTZ in the gut may be toxic to C. difficile in the challenge inoculation.
In summary, NTZ compares well with metronidazole and vancomycin for the treatment of C. difficile-associated ileocecitis in hamsters. NTZ was well tolerated and was effective in preventing clindamycin-induced intestinal disease. Patterns of relapse of C. difficile disease in NTZ- and metronidazole-treated animals were similar; however, neither of these agents was as effective as vancomycin in preventing relapse. It is perhaps more promising that, in contrast to treatment with vancomycin and metronidazole, treatment induction of C. difficile intestinal disease was not observed with NTZ.
ACKNOWLEDGMENTS
This research was supported by a grant from Romark Laboratories, L.C., Tampa, Fla.
We thank Brendan Headd for technical assistance.
REFERENCES
- 1.Bartlett J G, Chang T, Moon N, Onderdonk A B. Antibiotic-induced lethal enterocolitis in hamsters: studies with eleven agents and evidence to support the pathogenic role of toxin-producing clostridia. Am J Vet Res. 1978;39:1525–1530. [PubMed] [Google Scholar]
- 2.Buggy B D. Clostridium difficile colitis: Causes, cures. JAMA. 1993;269:2088. [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Nosocomial enterococci resistant to vancomycin—United States, 1989. Morb Mortal Wkly Rep. 1993;42:597–599. [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Recommendations for preventing the spread of vancomycin resistance: recommendations of the Hospital Infection Control Practices Advisory Committee (HICPAC) Morb Mortal Wkly Rep. 1995;44:1–13. [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. Staphylococcus aureus with reduced susceptibility to vancomycin—United States, 1997. Morb Mortal Wkly Rep. 1997;46:471–476. [Google Scholar]
- 6.Doumbo O, Rossignol J F, Pichard E, Traore H A, Dembele M, Diakite M, Traore F, Diallo D A. Nitrazoxanide in the treatment of cryptosporidial diarrhea and other intestinal parasitic infections associated with acquired immunodeficiency syndrome in tropical Africa. Am J Trop Med Hyg. 1997;56:637–639. doi: 10.4269/ajtmh.1997.56.637. [DOI] [PubMed] [Google Scholar]
- 7.Dubreuil L, Houcke X, Mouton Y, Rossignol J F. In vitro evaluation of nitazoxanide and tizoxanide against anaerobes and aerobic organism. Antimicrob Agents Chemother. 1996;40:2266–2270. doi: 10.1128/aac.40.10.2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fekety R. Guidelines for the diagnosis and management of Clostridium difficile-associated diarrhea and colitis. Am J Gastroenterol. 1997;92:739–750. [PubMed] [Google Scholar]
- 9.Fekety R, Silva J, Toshniwal R, Allo M, Armstrong J, Browne R, Ebright J, Rifkin G. Antibiotic-associated colitis: effects of antibiotics on Clostridium difficile and the disease in hamsters. Rev Infect Dis. 1979;1:386–397. doi: 10.1093/clinids/1.2.386. [DOI] [PubMed] [Google Scholar]
- 10.George W L, Sutter V L, Citron D, Finegold S M. Selective and differential medium for isolation of Clostridium difficile. J Clin Microbiol. 1979;9:214–219. doi: 10.1128/jcm.9.2.214-219.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hecht J R. Clostridium difficile colitis secondary to intravenous vancomycin. Dig Dis Sci. 1989;34:148–149. doi: 10.1007/BF01536172. [DOI] [PubMed] [Google Scholar]
- 12.Iaconis J P, Rolfe R D. Clostridium difficile-associated ileocecitis in clindamycin-treated infant hamsters. Curr Microbiol. 1986;13:327–332. [Google Scholar]
- 13.Kabil S M, Abd El-Salam F, Nassar A, Abd El-Bast M. Effects of nitazoxanide on the treatment of common human helminthic and protozoal infections. J Trop Med. 1994;3:7–10. [Google Scholar]
- 14.Kapusnik-Uner J E, Sande M A, Chambers H F. Antimicrobial agents. In: Hardman J G, Limbird L E, Molinoff P B, Ruddon R W, editors. Goodman Gilman's the pharmacological basis of therapeutics. 9th ed. New York, N.Y: McGraw-Hill, Health Professions Division; 1996. pp. 1123–1153. [Google Scholar]
- 15.Kelly C P, Pothoulakis C, LaMont J T. Clostridium difficile colitis. N Engl J Med. 1994;330:257–262. doi: 10.1056/NEJM199401273300406. [DOI] [PubMed] [Google Scholar]
- 16.Kim P-H, Iaconis J P, Rolfe R D. Immunization of adult hamsters against Clostridium difficile-associated ileocecitis and transfer of protection to infant hamsters. Infect Immun. 1987;55:2984–2992. doi: 10.1128/iai.55.12.2984-2992.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McFarland L V, Mulligan M E, Kwok R Y, Stamm W E. Nosocomial acquisition of Clostridium difficile infection. N Engl J Med. 1989;320:204–210. doi: 10.1056/NEJM198901263200402. [DOI] [PubMed] [Google Scholar]
- 18.Mégraud, Occhialini F A, Rossignol J F. Nitazoxanide, a potential drug for eradication of Helicobacter pylori with no cross-resistance to metronidazole. Antimicrob Agents Chemother. 1998;42:2836–2840. doi: 10.1128/aac.42.11.2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murphy J R, Friedman J C. Pre-clinical toxicology of nitazoxanide—a new antiparasitic compound. J Appl Toxicol. 1985;5:49–52. doi: 10.1002/jat.2550050202. [DOI] [PubMed] [Google Scholar]
- 20.Onderdonk A B. Role of the hamster model of antibiotic-associated colitis in defining the etiology of the disease. In: Rolfe R D, Finegold S M, editors. Clostridium difficile: its role in disease. New York, N.Y: Academic Press, Inc.; 1988. pp. 115–125. [Google Scholar]
- 21.Rosenblatt J E. Antimicrobial susceptibility testing of anaerobes. In: Lorian V, editor. Antibiotics in laboratory medicine. 2nd ed. Baltimore, Md: Williams & Wilkins; 1986. pp. 159–180. [Google Scholar]
- 22.Rossignol J F. Nitazoxanide, a new broad spectrum antibacterial-antiparasiticidal drug: pre-clinical pharmacology. J Trop Med. 1994;3:1–6. [Google Scholar]
- 23.Rossignol J F, Maisonneuve H. Nitazoxanide in the treatment of Taenia saginata and Hymenolepsis nana. Am J Trop Med Hyg. 1984;33:511–512. doi: 10.4269/ajtmh.1984.33.511. [DOI] [PubMed] [Google Scholar]
- 24.Salcedo J, Keates S, Potlhoulakis C, Warny M, Castagliuolo I, LaMont J T, Kelly C P. Intravenous immunoglobulin therapy for severe Clostridium difficile colitis. Gut. 1997;41:366–370. doi: 10.1136/gut.41.3.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Samore M H. Epidemiology of nosocomial Clostridium difficile infection. Compr Ther. 1993;19:151–156. [PubMed] [Google Scholar]
- 26.Stockis A, Lins R, Deroubaix X, Jeanbaptiste B, Calderon P, Rossignol J F. Pharmacokinetics of nitazoxanide after a single oral dose administration in healthy volunteers. Int J Clin Pharmacol Ther. 1996;34:349–351. [PubMed] [Google Scholar]
- 27.Theodos C M, Griffiths J K, D'Onfro J, Fairfield A, Tzipori S. Efficacy of nitazoxanide against Cryptosporidium parvum in cell culture and in animal models. Antimicrob Agents Chemother. 1998;42:1959–1965. doi: 10.1128/aac.42.8.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tracy J W, Webster L T., Jr . Drugs used in chemotherapy of protozoal infections. In: Hardman J G, Limbird L E, Molinoff P B, Ruddon R W, editors. Goodman Gilman's the pharmacological basis of therapeutics. 9th ed. New York, N.Y: McGraw-Hill, Health Professions Division; 1996. p. 996. [Google Scholar]
- 29.Wenisch C, Parschalk B, Hasenhundl H, Hirschl A M, Graninger W. Comparison of vancomycin, teicoplanin, metronidazole, and fusidic acid for treatment of Clostridium difficile-associated diarrhea. Clin Infect Dis. 1996;22:813–818. doi: 10.1093/clinids/22.5.813. [DOI] [PubMed] [Google Scholar]
- 30.Wilkins T D, Chalgren S. Medium for use in antibiotic susceptibility testing of anaerobic bacteria. Antimicrob Agents Chemother. 1976;10:926–928. doi: 10.1128/aac.10.6.926. [DOI] [PMC free article] [PubMed] [Google Scholar]