Abstract
Background
Botulinum toxin A (BoNT-A) injections are a popular non-surgical procedure for facial rejuvenation. Its increase in popularity and utilization is met with limited regulations, potentially posing a significant risk to patient safety and public health.
Objectives
The authors sought to assess the safety profile of cosmetic glabellar and forehead BoNT-A injections and evaluate BoNT-A type on complication rate.
Methods
A systematic search of MEDLINE and EMBASE was performed for studies reporting complications after cosmetic BoNT-A in the glabellar or in the forehead region in the glabellar or in the forehead region. A random effects meta-analysis was carried out to assess complication rate. Where there were sufficient randomized-controlled trials, a network meta-analysis was performed.
Results
Of 556 identified articles, 24 were included in the final quantitative analysis, with 4268 BoNT-A injection sessions and 1234 placebos. Frequently observed treatment-related complications in the BoNT-A intervention group included headache, local skin reactions, and facial neuromuscular symptoms. The overall BoNT-A complication rate was 16%. The odds ratio of developing complications from abobotulinum toxin injections compared with placebo was 1.62 (1.15, 2.27; P > 0.05) and that from onabotulinum toxin injections compared with placebo was 1.34 (0.52, 3.48; P > 0.05). In 30% of the studies, the injectors were doctors, whereas the training status of the practitioner was not reported in the remaining 70%.
Conclusions
Cosmetic BoNT-A injections in the glabellar and forehead region appear to be safe, and most complications are mild and transient. Nevertheless, the literature demonstrates heterogeneous reporting of complications and a lack of consistency of the definition of treatment-related complications.
Level of Evidence: 2

The global market for anti-aging products is steadily increasing.1 Botulinum toxin A (BoNT-A) injections are a popular non-surgical but still invasive treatment to optimize and change facial appearance and achieve rejuvenation. BoNT-A injections are preferred to other surgical and non-surgical procedures due to their satisfying results, relatively safe complication profile, and minimal downtime.2,3 Also, BoNT-A injections are an affordable alternative to more expensive surgical procedures and still profitable for the practitioner.3 BoNT-A, with its numerous formulations, is estimated to increase its market value for rejuvenation up to 6.9 billion dollars by 2026.4-10
Despite the globally increasing number of cosmetic BoNT-A injections, regulations for BoNT-A administration are variable or even absent in different countries.11 For example, the 2015 Keogh report raised concerns about patient safety and insufficient protection of patients due to the lack of BoNT-A regulations in the United Kingdom.12 Regulation guidelines were subsequently published.13 To establish regulations, it is imperative to study the safety profile of a product or intervention and to put in place an efficient standardized national reporting system for complications. National reporting systems and retrospective studies tend to underestimate products’ complication rates compared with prospective studies. A comparison of data provided by the Medicines and Healthcare products Regulatory Agency with BoNT-A complication rates in the literature corroborated an underestimation of complication rates.
In this paper, we seek to amalgamate and identify the overall complication rate of cosmetic BoNT-A across randomized controlled trials, thereby helping establish the safety profile of BoNT-A. Given the increase in utilization of BoNT-A and the paucity of robust regulations, we believe that establishing its safety profile is critical to ensuring an up-to-date understanding of the overall complication rate. This empowers practitioners to be more informed as part of their consent process.
To ensure high-quality care, it is crucial to train skilled and responsible practitioners and to work with validated and safe products. It is important to formulate accurate recommendations and set regulations based on safety profiles and to inform patients about risks and benefits.12 Although BoNT-A appears safe, it is arguable whether there are enough data on long-term complications.14 We aim to analyze the safety profile of cosmetic BoNT-A injections. Additionally, we assess factors potentially influencing the complication rate.
METHODS
Overview
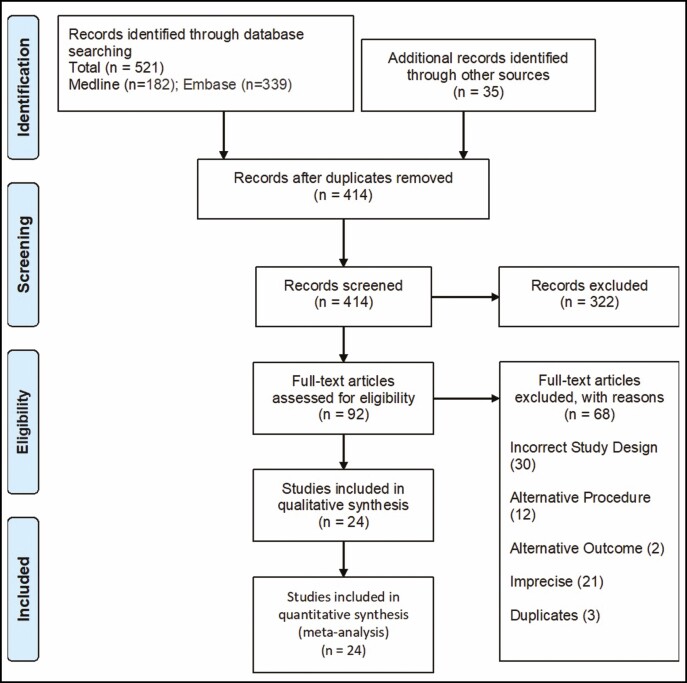
A systematic review was carried out to identify any studies reporting short-term or long-term complications of facial-cosmetic BoNT-A injections. Title and abstract screening, full-text review, and data extraction were handled independently by 2 reviewers (D.Z. and F.Z.). The kappa score for interobserver reliability was 1.0. We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses protocol.15 This review was registered on PROSPERO (www.crd.york.ac.uk/prospero, Record ID: CRD42021219425).
Search Strategy
The PubMed/MEDLINE (United States National Library of Medicine, Bethesda, MD) and EMBASE (Elsevier, Amsterdam, the Netherlands) databases were searched to identify eligible articles. The search strategy included combinations of the following terms: Botox (Allergan, Irvine, CA); botulinum toxin A; onabotulinum; aesthetic; cosmetic; plastic surgery; complications; adverse (see Figure 1). Word variations and exploded medical subject headings were searched for whenever feasible. Additionally, reference lists were hand-searched to identify further studies of interest. Furthermore, appropriate studies that were not identified in our search but known to us were included. The last comprehensive search was conducted on October 2, 2020.
Figure 1.
Flow chart of the reviewing process.
Study Selection
Only in vivo studies in English enrolling adult humans over 18 years were considered. Studies before 1989 were not included. Only randomized placebo-controlled trials and randomized dose-ranging trials were included. To be considered, patients had to undergo cosmetic facial BoNT-A injection in the glabellar or forehead region. Studies had to assess at least 1 complication. In this way, we were able to rate the risk of complications after the injections. Studies reporting therapeutic botulinum toxin injections were excluded. Exact cohort duplicates were excluded.
Data Extraction and Quality Assessment
The following information was extracted, where available, from all included publications: study design and year of publication, number of patients, patient age, gender distribution, number of BoNT-A injections, BoNT-A formulation, BoNT-A dose, practitioner, specific complications as well as the total complication rate. Practitioners were categorized as doctor, nurse, or non-medical professional. Observed BoNT-A formulations were onabotulinum (ONA), abobotulinum (ABO), and incobotulinum (INCO), and doses were categorized as 0 to 10 U, 11 to 20 U, 21 to 30 U, 31 to 40 U, 41 to 50 U, and 50+ U. Complications were categorized in subgroups: 1) localized skin reaction (erythema, eczema, hematoma, bruising, or contusion); 2) remote skin reactions (rash or edema); 3) wound infection; 4) asymmetric or unsatisfying result; 5) facial neuromuscular symptoms (stiffness, weakness, pain, spasm, paresis, ptosis, dysesthesia); 6) headache; 7) ocular symptoms and infections; 8) pulmonary symptoms and infections; 9) gastrointestinal symptoms; 10) cardiovascular symptoms (hypertension, hypotension, tachycardia, myocardial infarction); 11) general symptoms (influenza-like symptoms, asthenia, chills, pyrexia, fatigue); 12) anaphylactic reaction; and 13) others (nausea, vertigo, etc). Complications were additionally rated as severe or non-severe. Severe complications were defined as those resulting in hospitalization, death, life-threatening conditions, disability, permanent damage, congenital anomaly, or requiring an intervention to prevent permanent impairment.16 When explicitly defined in the study whether a complication was treatment related or not, only probable or possible treatment-related complications were analyzed in the complication rate. When the total complication rate was not reported by the study, the rate was calculated based on the number of single complications identified. The complication rate was defined as the number of injection sessions with at least 1 complication divided by the total number of injection sessions. We did not analyze second cycles or open-label top-up cycles because of the risk of introducing bias, assuming that additional cycles are more likely to be taken by patients who had positive experiences without any complications after the first injection. Methodological quality of included studies was graded employing the quality assessment tool of the Effective Public Healthcare Panacea Project.17 We selected this tool because it is applicable to quantitative literature studies and has implications for public health. Given the increasing role of BoNT-A, we believe that its safety profile is critical to update and establish, and this has profound public health implications.
Meta-Analysis
We first calculated the complication rates per study for each reported complication category alone as well as for total complications. These effect sizes were then meta-analyzed if enough appropriate data from at least 3 studies was available. Because major heterogeneity among the studies was expected, a random-effects meta-analysis was decided on. Complication rates were meta-analyzed utilizing the generic inverse variance method, with a Freeman-Tukey double arcsine transformation to estimate overall proportions.18
We also assessed the influence of different BoNT-A formulations on the complication rate. If enough controlled studies were available, we conducted a network meta-analysis. Otherwise, we conducted subgroup meta-analysis employing a mixed-effects model (random-effects model within subgroups, fixed-effects model between subgroups). Statistical analyses were carried out in R with the “meta” package.19 Forest plots were generated to illustrate the main results of the meta-analysis.
RESULTS
Our search resulted in 182 studies found in MEDLINE and 379 studies in EMBASE. After exclusion of the papers that did not meet the inclusion and exclusion criteria, the final analysis included 24 studies (Supplemental Table 1, available online at www.aestheticsurgeryjournal.com).20-43 The review process is shown in Figure 1 and Appendix (available online at www.aestheticsurgeryjournal.com). Included studies were all randomized and double-blinded, except 1 study that was only investigator blinded. Quality of every included study was rated as strong according to the tool of the Effective Public Healthcare Panacea Project.17 In 7 studies, a doctor (unclear if a dermatologist, general practitioner, or plastic surgeon) administered BoNT-A. In 16 studies, the practitioner was unknown. No data of injections administered by nurses or non-medical professionals were identified.
BoNT-A Injections
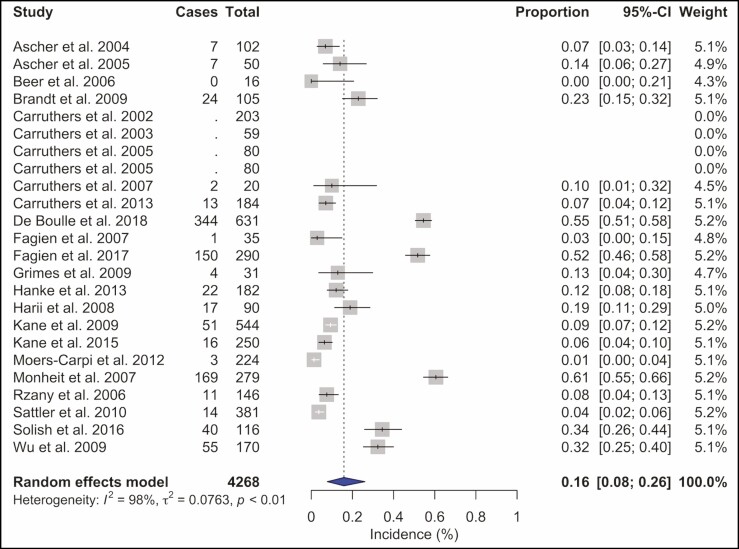
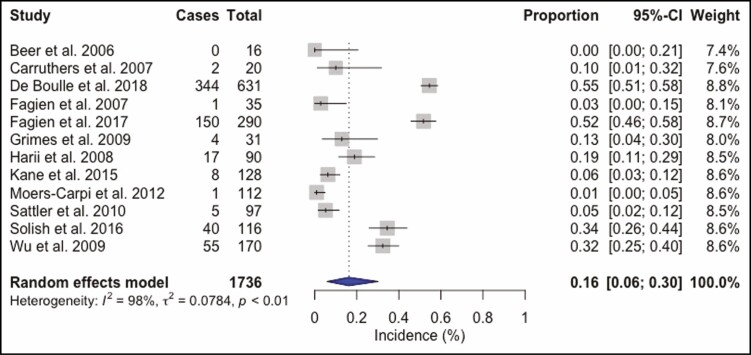
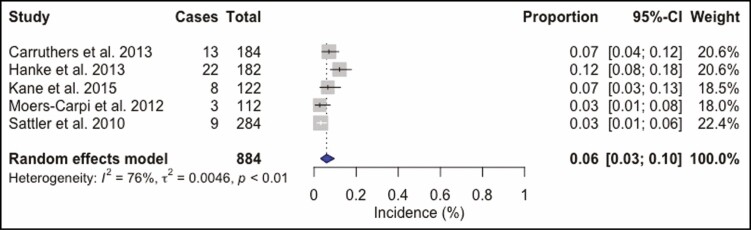
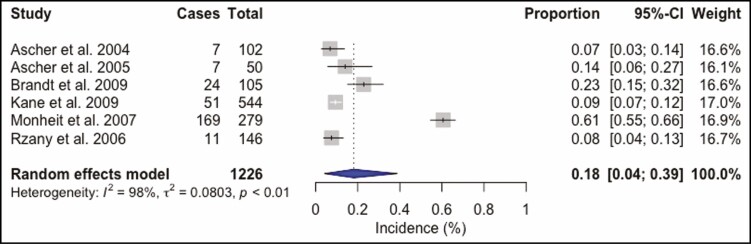
A total of 4268 BoNT-A injection sessions were analyzed. The most frequently used BoNT-A formulation was ONA in 50.6% (n = 2158) of all injections. ABO was utilized in 28.7% (n = 1226) and INCO in 20.7% (n = 884) injection sessions. Doses ranged from 10 to 80 U for ONA injections into the glabellar and forehead region. The total complication rate was 16% (95% confidence interval [CI] = 8% to 26%, I2 = 98%), Figure 2. Headache and migraine were the most frequently reported adverse event and were recorded in 269 (6.3%) injection sessions, followed by local skin reactions such as bruising or hematoma at the injection site, reported in 163 (3.8%) patients, and facial neuromuscular symptoms in 141 (3.3%) injections. Other observed adverse events were pulmonary symptoms after 91 (2.1%) injection sessions and ocular symptoms in 39 (0.9%) cases. Cardiovascular symptoms were recorded in 22 (0.5%) patients, gastrointestinal symptoms in 18 (0.4%) cases, remote skin reactions after 11 (0.3%) injections, face asymmetry in 6 (0.1%) injection sessions, and general symptoms such as fatigue occurred as well after 6 (0.1%) injections (Supplemental Table 2, available online at www.aestheticsurgeryjournal.com). ONA injections (Figure 3) showed a complication rate of 16% (95% CI = 6% to 30%, I2 = 98%), INCO (Figure 4) of 6% (95% CI = 3% to 10%, I2 = 76%), and for ABO (Figure 5) an overall complication rate of 18% (95% CI = 4% to 39%, I2 = 98%) was reported.
Figure 2.
Forest plot. Complication rate of botulinum toxin A.
Figure 3.
Forest plot. Complication rate of onabotulinum toxin.
Figure 4.
Forest plot. Complication rate of incobotulinum toxin.
Figure 5.
Forest plot. Complication rate of abobotulinum toxin.
Placebo
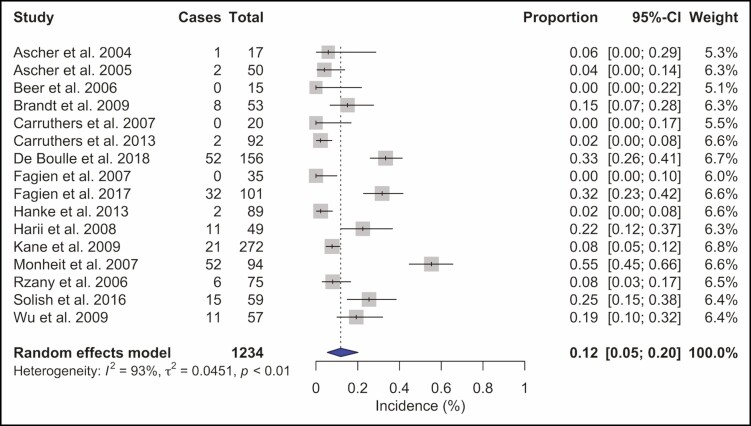
A total 1234 placebo injection sessions were analyzed. The total complication rate was 12% (95% CI = 5% to 20%, I2 = 93%) (Figure 6). Headache and migraine were the most frequently reported adverse events and were recorded in 49 (4%) injection sessions, followed by local skin reactions, such as bruising or hematoma at the injection site, reported in 30 (2.4%) patients. Other observed adverse events were pulmonary symptoms after 19 (1.5%) injection sessions and facial paresis or paralysis in 10 (0.8%) injections. Cardiovascular symptoms were recorded in 7 (0.6%) patients, general symptoms such as fatigue occurred after 5 (0.4%) injections, gastrointestinal symptoms and remote skin reactions were each observed in 2 patients (0.2%), and 1 patient (0.1%) had ocular symptoms (Supplemental Table 2).
Figure 6.
Forest plot. Complication rate placebo.
Comparison of BoNT-A and Placebo
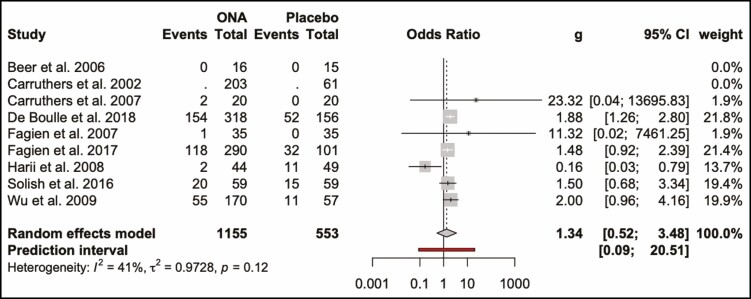
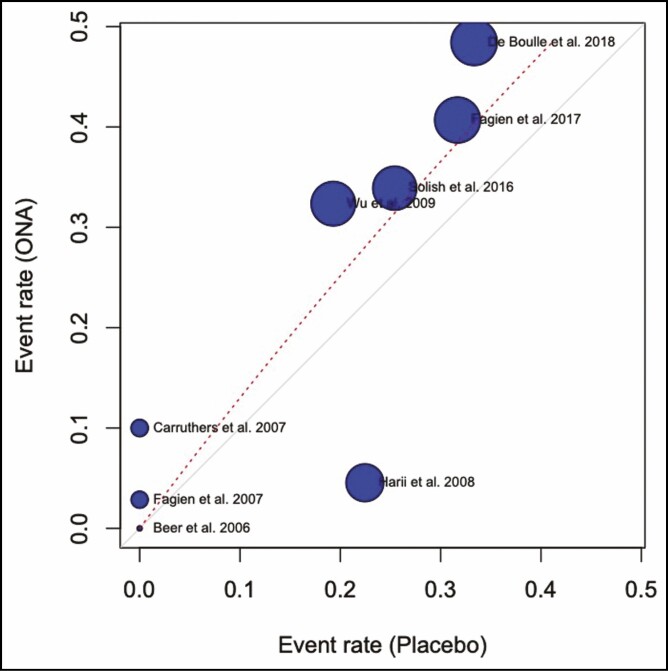
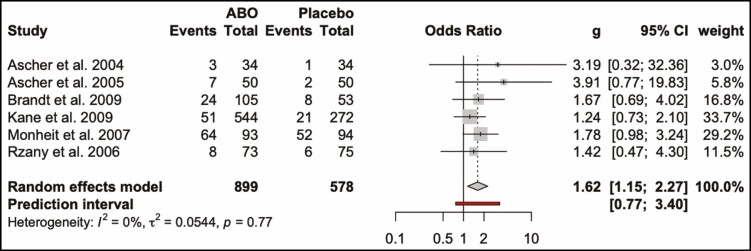
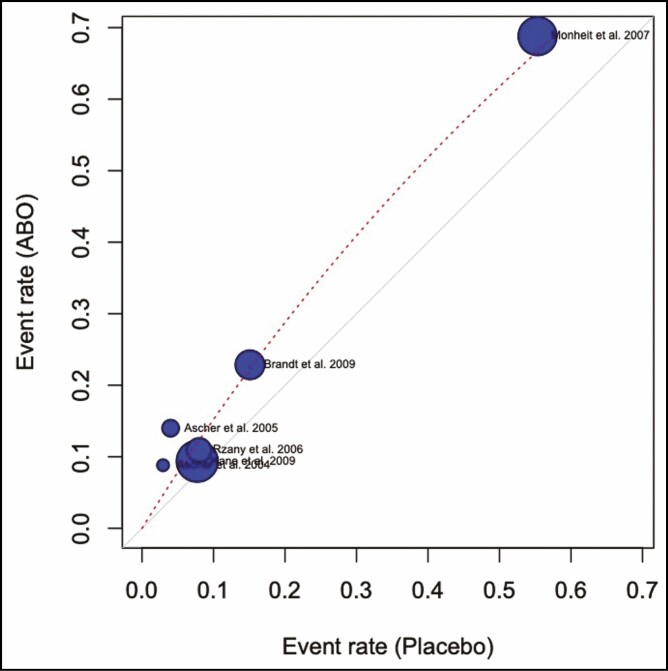
The odds ratio for developing complications from ONA injections compared with placebo was 1.34 (0.52, 3.48; P > 0.05). This is visualized in a forest plot (Figure 7) and a L’Abbé (Figure 8) plot. The dashed red line signifies the pooled effect estimate of the meta-analysis and is running through the top-left sector of the L’Abbé plot, meaning that the intervention with ONA has a higher complication rate. The odds ratio of developing complications from ABO injections compared with placebo was 1.62 (1.15, 2.27; P > 0.05) (Figures 9, 10).
Figure 7.
Forest plot. Complication rate of onabotulinum toxin and placebo.
Figure 8.
L’Abbé plot. Complication rate of onabotulinum toxin and placebo.
Figure 9.
Forest plot. Complication rate of abobotulinum toxin and placebo.
Figure 10.
L’Abbé plot. Complication rate abobotulinum toxin and placebo.
DISCUSSION
In a systematic analysis of the available literature, we found that the incidence of adverse events related to glabellar and forehead BoNT-A injection is approximately 16%, and only a very few severe complications possibly or probably related to the BoNT-A treatment occurred. Headache and local skin reactions were the most frequently reported complications in BoNT-A patients as well as in patients receiving placebo. In BoNT-A injections, facial neuromuscular symptoms and facial asymmetry occurred more often compared with placebo treatment. This implies that local skin reactions and headache might be unrelated to the botulinum toxin itself but more likely caused by the syringe injection, whereas asymmetry and neuromuscular effects are attributable to the toxin. The placebo had a lower complication rate compared with BoNT-A injections, although results were not significant. There were very few severe treatment-related complications in both placebo injections and BoNT-A injections in this review.
Nevertheless, complication rates must be interpreted with caution. The included studies in our systematic review utilized different definitions for complications, which may result in difficulties in the interpretation and analysis of the safety profile. Furthermore, in many of the included studies, treatment-related and non-related complications are not consistently defined or even not distinguished between. For example, Carruthers et al and De Boulle showed high complication rates of 27% to 43% because final complication rates also included non-related treatment complications.29,30 By contrast, Sattler et al distinguished between treatment-related and non-related complications and reported them separately.41 This leads to difficulties in the comparison of complication rates. In addition, an analysis of complications reported to the Medicines and Healthcare Products Regulatory Agency in the United Kingdom showed a lack of a standardized reporting system for adverse events from a procedure or medication. Incorrect and non-standardized reporting of adverse events can lead to an overestimation of a medical product’s safety profile.44 For this reason, it is crucial to create a uniform complication reporting system with guidelines to ensure the capture of all complications in the context of a specific treatment and enable comparison of complication rates of future studies.
The injection method was described accurately and detailed in many studies, but most studies in this systematic review did not provide information about the BoNT-A administrator.23 All studies were conducted in a practice or hospital setting; therefore, it may be assumed that only people with a medical background, such as nurses, doctors, or dentists, administered BoNT-A. We could not identify studies reporting the safety profile or complications of BoNT-A injections administered by non-medical professionals or nurses, although in some countries, for example in the United Kingdom, not only medical doctors but also beauticians and other practitioners without a medical background are allowed to inject BoNT-A if they have a Level 6 (degree level) qualification.13 Unfortunately, despite our initial aims, few of the primary papers included the additional factors we sought as outlined in our methodology. We hope that through drawing attention to this matter, further research could evaluate this. Research is crucial to improve and maintain treatment quality, and it is reasonable to expect that professionals administering a medical treatment should have suitable training to recognize and manage its complications. Furthermore, bias may be introduced in complication rates and safety rating of BoNT-A injections if there are no data available for non-medical professional administrators, yet they are administering a significant proportion of injections to the public. Adverse event rates are shown to be higher when BoNT-A is injected by inexperienced practitioners without sufficient knowledge of anatomy.45 This practitioner factor effect on the prevalence of adverse events has not been well studied, and it is an important area for further study.
Apart from the practitioner factor, we additionally analyzed complication rates for the 3 most common BoNT-A formulations—ONA (Botox/Vistabel, Allergan Inc., Irvine, CA, USA), ABO (Dysport/Ipsen Limited, Slough Berkshire, UK), and INCO (Xeomin/Bocouture, Merz Pharmaceuticals GmbH, Frankfurt, Germany)—and conducted a network meta-analysis. Efficacies of these different BoNT-A types are similar, but nevertheless, the comparability of various preparations is dubious. INCO and ONA show the same efficacy and safety profile when a clinical conversion ratio of 1:1 is utilized; in contrast, a conversion ratio of 3:1 is suggested for ABO to ONA.46 Studies included in our analysis mainly report the utilization of ONA, although ABO has a better cost-efficacy profile.46 There is a steadily increasing BoNT-A market and big global competition of a wide range of products.47
Further research is warranted to establish a standardized complication reporting system to make complication rates across future studies comparable. Moreover, the influence of practitioners’ experience on the complication rate and the impact of BoNT-A dose and top-up treatments on BoNT-A’s safety profile should be analyzed in detail. Complication rates of injections into other locations such as crowfeet and masseter also represent an important area for further study. We only included studies with glabellar or forehead area as the BoNT-A administration site, whereas other regions such as masseter, crowfeet, or nasolabial area are likely to show a different complication profile.
Limitations
Our search strategy included terms such as complication, adverse event, or adverse drug reaction. With this search strategy, we found studies specifically focusing on the safety profile of BoNT-A. Nevertheless, there might be multiple other studies reporting complications of BoNT-A not as their primary outcome and therefore not in the title or abstract, but as a secondary outcome. We sought to mitigate this by including applicable studies in reference lists and other studies known to the authors.
Another limitation is that some studies, for example Kane et al, included patients with past BoNT-A treatments.36 This can introduce population bias, because patients who did not experience complications in their previous treatments may be more likely to participate in a second study, whereas patients who tend to develop complications and who experienced complications in their previous treatments may be less likely to pursue further treatment. Furthermore, the impact of top-up or frequently repeated BoNT-A injections on the complication rate remains unclear; therefore, we did not include second treatment cycles and top-up injections in this study.
Studies did not adopt a consistent and uniform definition of treatment-related complications and reporting of these. Some studies did not report possibly or probably treatment-related and treatment-emerged complications separately.30,32,33,39,43 Therefore, the ability to perform a robust comparison of complication rates of different studies is limited.
CONCLUSIONS
Cosmetic BoNT-A injections in the glabellar and forehead region appear to be safe, and most complications are mild and transient. The overall complication rate was 16%, with no significant difference between BoNT-A formulations in our network meta-analysis. However, the literature demonstrates a heterogenous reporting of complications and a lack of consistency of the definition of treatment-related complications as well as a lack of detail on practitioner profile. There are important areas for further study to uphold the highest standards of patient safety in this rapidly expanding field.
Supplementary Material
Disclosures
The authors declared no potential conflicts of interest with respect to the research, authorship, and publication of this article. Dr Rahman is an Evidence-Based Medicine section co-editor for Aesthetic Surgery Journal. Dr Mosahebi is the Research section co-editor for Aesthetic Surgery Journal.
Funding
The authors received no financial support for the research, authorship, and publication of this article.
REFERENCES
- 1. Statista. Size of the anti-aging market worldwide 2018-2023. Accessed November 30, 2020. https://www.statista.com/statistics/509679/value-of-the-global-anti-aging-market/.
- 2. Statista. U.S. patient satisfaction: top nonsurgical cosmetic procedures 2019. Accessed November 30, 2020. https://www.statista.com/statistics/281434/satisfaction-among-patients-of-top-us-nonsurgical-cosmetic-procedures/.
- 3. Mendez-Eastman SK. BOTOX: a review. Plast Surg Nurs. 2003;23(2):64-69. doi: 10.1097/00006527-200323020-00006 [DOI] [PubMed] [Google Scholar]
- 4. BOTOX 100 units powder for solution for injection—summary of product characteristics (SmPC)—(emc). Accessed November 10, 2020. https://www.medicines.org.uk/emc/product/859/smpc#gref.
- 5. Xeomin 200 units powder for solution for injection—summary of product characteristics (SmPC)—(emc). Accessed November 10, 2020. https://www.medicines.org.uk/emc/product/2162/smpc#gref.
- 6. Bocouture 100 units—summary of product characteristics (SmPC)—(emc). Accessed November 10, 2020. https://www.medicines.org.uk/emc/product/7418/smpc#gref.
- 7. Vistabel | SPC | Allergan Ltd | Medicines.ie. Accessed November 10, 2020. https://www.medicines.ie/medicines/vistabel-34207/smpc.
- 8. Azzalure—summary of product characteristics (SmPC)—(emc). Accessed November 10, 2020. https://www.medicines.org.uk/emc/medicine/21985#gref.
- 9. Dysport 300 units powder for solution for injection—summary of product characteristics (SmPC) —(emc). Accessed November 10, 2020. https://www.medicines.org.uk/emc/product/964/smpc#gref.
- 10. Statista. Botulinum toxin global market size 2016-2026. Accessed November 30, 2020. https://www.statista.com/statistics/988433/facial-rejuvenation-botulinum-toxin-global-market/.
- 11. Boon Harold Tan K. Aesthetic medicine: a health regulator’s perspective. Clin Gov Int J. 2007;12(1):13-25. doi: 10.1108/14777270710725364 [DOI] [Google Scholar]
- 12. Bruce Keogh. Review_of_the_Regulation_of_Cosmetic_Interventions.pdf. 2013. Accessed November 17, 2020. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/192028/Review_of_the_Regulation_of_Cosmetic_Interventions.pdf.
- 13. Qualification requirements for delivery of cosmetic procedures: non-surgical cosmetic interventions and hair restoration surgery. 2015. Accessed November 17, 2020. https://www.hee.nhs.uk/sites/default/files/documents/HEE%20Cosmetic%20publication%20part%20one.pdf.
- 14. Yiannakopoulou E. Serious and long-term adverse events associated with the therapeutic and cosmetic use of botulinum toxin. Pharmacology. 2015;95(1-2):65-69. doi: 10.1159/000370245 [DOI] [PubMed] [Google Scholar]
- 15. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. What is a serious adverse event? FDA. 2020. Accessed December 2, 2020. https://www.fda.gov/safety/reporting-serious-problems-fda/what-serious-adverse-event.
- 17. Quality Assessment Tool for Quantitative Studies. Effective public healthcare panacea project. Accessed December 3, 2020. https://www.ephpp.ca/quality-assessment-tool-for-quantitative-studies/.
- 18. Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods. 2010;1(2):97-111. doi: 10.1002/jrsm.12 [DOI] [PubMed] [Google Scholar]
- 19. Schwarzer G. Meta: general package for meta-analysis.2020. Accessed March 16, 2020. https://CRAN.R-project.org/package=meta.
- 20. Ascher B, Zakine B, Kestemont P, Baspeyras M, Bougara A, Santini JA. Multicenter, randomized, double-blind, placebo-controlled study of efficacy and safety of 3 doses of botulinum toxin A in the treatment of glabellar lines. J Am Acad Dermatol. 2004;51(2):223-233. doi: 10.1016/j.jaad.2003.11.084 [DOI] [PubMed] [Google Scholar]
- 21. Ascher B, Zakine B, Kestemont P, et al. . Botulinum toxin A in the treatment of glabellar lines: scheduling the next injection. Aesthet Surg J. 2005;25(4):365-375. doi: 10.1016/j.asj.2005.05.004 [DOI] [PubMed] [Google Scholar]
- 22. Beer KR. Comparative evaluation of the safety and efficacy of botulinum toxin type A and topical creams for treating moderate-to-severe glabellar rhytids. Dermatol Surg. 2006;32(2):184-197. [DOI] [PubMed] [Google Scholar]
- 23. Brandt F, Swanson N, Baumann L, Huber B. Randomized, placebo-controlled study of a new botulinum toxin type A for treatment of glabellar lines: efficacy and safety. Dermatol Surg. 2009;35(12):1893-1901. doi: 10.1111/j.1524-4725.2009.01235.x [DOI] [PubMed] [Google Scholar]
- 24. Carruthers JA, Lowe NJ, Menter MA, et al. . A multicenter, double-blind, randomized, placebo-controlled study of the efficacy and safety of botulinum toxin type A in the treatment of glabellar lines. J Am Acad Dermatol. 2002;46(6):840-849. doi: 10.1067/mjd.2002.121356 [DOI] [PubMed] [Google Scholar]
- 25. Carruthers A, Carruthers J, Cohen J. A prospective, double-blind, randomized, parallel- group, dose-ranging study of botulinum toxin type a in female subjects with horizontal forehead rhytides. Dermatol Surg. 2003;29(5):461-467. doi: 10.1046/j.1524-4725.2003.29114.x [DOI] [PubMed] [Google Scholar]
- 26. Carruthers A, Carruthers J, Said S. Dose-ranging study of botulinum toxin type a in the treatment of glabellar rhytids in females. Dermatol Surg. 2005;31(4):414-422, discussion 422. [DOI] [PubMed] [Google Scholar]
- 27. Carruthers A, Carruthers J. Prospective, double-blind, randomized, parallel-group, dose-ranging study of botulinum toxin type A in men with glabellar rhytids. Dermatol Surg. 2005;31(10):1297-1303. doi: 10.1097/00042728-200510000-00006 [DOI] [PubMed] [Google Scholar]
- 28. Carruthers J. Botulinum toxin type A treatment of multiple upper facial sites: patient-reported outcomes. Dermatol Surg. 2007;33(1 Spec No.): S10-7. Accessed November 12, 2020. https://insights.ovid.com. [DOI] [PubMed] [Google Scholar]
- 29. Carruthers A, Carruthers J, Coleman WP, et al. . Multicenter, randomized, phase III study of a single dose of IncobotulinumtoxinA, free from complexing proteins, in the treatment of glabellar frown lines. Dermatol Surg. 2013;39(4):551-558. doi: 10.1111/dsu.12100 [DOI] [PubMed] [Google Scholar]
- 30. De Boulle K, Werschler WP, Gold MH, et al. . Phase 3 study of OnabotulinumtoxinA distributed between frontalis, glabellar complex, and lateral canthal areas for treatment of upper facial lines. Dermatol Surg. 2018;44(11):1437-1448. doi: 10.1097/DSS.0000000000001612 [DOI] [PubMed] [Google Scholar]
- 31. Fagien S, Cox SE, Finn JC, Werschler WP, Kowalski JW. Patient-reported outcomes with botulinum toxin type A treatment of glabellar rhytids: a double-blind, randomized, placebo-controlled study. Dermatol Surg. 2007;33(s1):S2-S9. doi: 10.1111/j.1524-4725.2006.32325.x. [DOI] [PubMed] [Google Scholar]
- 32. Fagien S, Cohen JL, Coleman W, et al. . Forehead line treatment with onabotulinumtoxina in subjects with forehead and glabellar facial rhytids: a phase 3 study. Dermatol Surg. 2017;43:S274-S284. doi: 10.1097/DSS.0000000000001414 [DOI] [PubMed] [Google Scholar]
- 33. Grimes PE, Shabazz DA. Four-month randomized, double-blind evaluation of the efficacy of botulinum toxin type A for the treatment of glabellar lines in women with skin types V and VI. Dermatol Surg. 2009;35(3):429-436. doi: 10.1111/j.1524-4725.2009.01063.x. [DOI] [PubMed] [Google Scholar]
- 34. Hanke CW, Narins RS, Brandt F, et al. . A randomized, placebo-controlled, double-blind phase III trial investigating the efficacy and safety of incobotulinumtoxinA in the treatment of glabellar frown lines using a stringent composite endpoint. Dermatol Surg. 2013;39(6):891-899. doi: 10.1111/dsu.12160 [DOI] [PubMed] [Google Scholar]
- 35. Harii K, Kawashima MA. Double-blind, randomized, placebo-controlled, two-dose comparative study of botulinum toxin type A for treating glabellar lines in Japanese subjects. Aesthetic Plast Surg. 2008;32(5):724. doi: 10.1007/s00266-008-9199-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kane MAC, Brandt F, Rohrich RJ, et al. . Evaluation of variable-dose treatment with a new U.S. botulinum toxin type A (Dysport) for correction of moderate to severe glabellar lines: results from a phase III, randomized, double-blind, placebo-controlled study. Plast Reconstr Surg. 2009;124(5):1619-1629. doi: 10.1097/PRS.0b013e3181b5641b [DOI] [PubMed] [Google Scholar]
- 37. Kane MAC, Gold MH, Coleman WPI, et al. . A randomized, double-blind trial to investigate the equivalence of incobotulinumtoxinA and onabotulinumtoxinA for glabellar frown lines. Dermatol Surg. 2015;41(11):1310-1319. doi: 10.1097/DSS.0000000000000531 [DOI] [PubMed] [Google Scholar]
- 38. Moers-Carpi M, Dirschka T, Feller-Heppt G, et al. . A randomised, double-blind comparison of 20 units of onabotulinumtoxinA with 30 units of incobotulinumtoxinA for glabellar lines. J Cosmet Laser Ther. 2012;14(6): 296-303. doi: 10.3109/14764172.2012.738913 [DOI] [PubMed] [Google Scholar]
- 39. Monheit G, Carruthers A, Brandt F, Rand R. A randomized, double-blind, placebo-controlled study of botulinum toxin type A for the treatment of glabellar lines: determination of optimal dose. Dermatol Surg. 2007;33(s1):S51-S59. doi: 10.1111/j.1524-4725.2006.32332.x [DOI] [PubMed] [Google Scholar]
- 40. Rzany B, Ascher B, Fratila A, et al. . Efficacy and safety of 3- and 5-injection patterns (30 and 50 U) of botulinum toxin A (Dysport) for the treatment of wrinkles in the glabella and the central forehead region. Arch Dermatol. 2006;142(3):320-326. doi: 10.1001/archderm.142.3.320 [DOI] [PubMed] [Google Scholar]
- 41. Sattler G, Callander MJ, Grablowitz D, et al. . Noninferiority of incobotulinumtoxinA, free from complexing proteins, compared with another botulinum toxin type A in the treatment of glabellar frown lines. Dermatol Surg. 2010;36(SUPPL. 4):2146-2154. doi: 10.1111/j.1524-4725.2010.01706.x [DOI] [PubMed] [Google Scholar]
- 42. Solish N, Rivers JK, Humphrey S, et al. . Efficacy and safety of onabotulinumtoxinA treatment of forehead lines: a multicenter, randomized, dose-ranging controlled trial. Dermatol Surg. 2016;42(3):410-419. doi: 10.1097/DSS.0000000000000626 [DOI] [PubMed] [Google Scholar]
- 43. Wu Y, Zhao G, Li H, et al. . Botulinum toxin type A for the treatment of glabellar lines in Chinese: a double-blind, randomized, placebo-controlled study. Dermatol Surg. 2010;36(1):102-108. [DOI] [PubMed] [Google Scholar]
- 44. Gliklich RE, Dreyer NA, Leavy MB. Registry design. Agency for Healthcare Research and Quality (US).2014. Accessed November 13, 2020. https://www.ncbi.nlm.nih.gov/books/NBK208632/.
- 45. Sethi N, Singh S, DeBoulle K, Rahman EA. Review of complications due to the use of botulinum toxin A for cosmetic indications. Aesthetic Plast Surg. 2021;45(3):1210-1220. doi: 10.1007/s00266-020-01983-w [DOI] [PubMed] [Google Scholar]
- 46. Scaglione F. Conversion ratio between Botox®, Dysport®, and Xeomin® in clinical practice. Toxins. 2016;8(3):65. doi: 10.3390/toxins8030065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dressler D. Botulinum toxin drugs: brief history and outlook. J Neural Transm. 2016;123(3):277-279. doi: 10.1007/s00702-015-1478-1 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.