Abstract
The prophylactic effect of FK463, a new water-soluble echinocandin-like lipopeptide with inhibitory activity against 1,3-β-d-glucan synthase, against Pneumocystis carinii infection was investigated with the severe combined immunodeficient (SCID) mouse model. Treatment with FK463, pentamidine, and saline only was performed for 6 weeks from the day after the SCID mice were inoculated intranasally with infected lung homogenates. FK463 at 0.2 or 1.0 mg/kg of body weight, pentamidine at 4 mg/kg, or saline was subcutaneously administered daily into the backs of the SCID mice. The effects of the drugs were evaluated by detection of P. carinii cysts in mouse lung homogenates by toluidine blue O staining, lung histology, and PCR amplification of a P. carinii-specific DNA fragment from the lungs. P. carinii cysts were detected in the lungs of all mice administered saline. In contrast, no cysts were detected in mice administered both doses of FK463 and pentamidine. A specific DNA fragment was amplified from all mice administered saline and at least half or more of the mice administered FK463 and pentamidine. These results indicate that FK463 acts on cyst wall formation but not on trophozoite proliferation and is extremely effective in preventing P. carinii-associated pneumonia. These results suggest that FK463 is potentially useful as a prophylactic agent against P. carinii infection.
Pneumocystis carinii is an opportunistic pathogen, and P. carinii-associated pneumonia (PCP) is a frequent cause of morbidity and mortality in immunocompromised patients with and without AIDS. Since the first report of pentamidine by Ivady and Paldy in 1958 (9), several effective chemotherapeutic regimens have become available for the treatment and prophylaxis of PCP. However, conventional therapy such as that with trimethoprim-sulfamethoxazole or parenteral pentamidine is often complicated by adverse reactions in AIDS patients that may require termination of the therapy, and the mortality rate for first episodes of PCP is still 5 to 20% (8). Therefore, special attention is focused on the treatment and prophylaxis of PCP for the current management of human immunodeficiency virus infection (2, 5, 15).
Since the development of alternative drugs that do not cause adverse reactions is necessary, a new strategy to develop an anti-P. carinii drug that interacts with a target not found in other eukaryotic cells has been attempted (4). Such a drug might overcome the adverse reactions caused by conventional chemotherapeutic regimens which act on fungi as well as mammalian cells. This strategy involves selective inhibition of the biosynthesis of important structural elements in the fungal cell. On the basis of this strategy, echinocandins and pneumocandins, inhibitors of the synthesis of 1,3-β-glucan, a major surface component of fungi including P. carinii, have been developed as potential anti-P. carinii drugs (1, 23). Iwamoto et al. (10, 11) isolated water-soluble echinocandin-like lipopeptides produced from Coleophoma empetri and reported that they are effective against fungi. Furuta et al. (7) also reported the therapeutic effectiveness of the natural product FR901379 and of FR131535, a semisynthetic derivative of FR901379, against pneumocystis pneumonia in mice. The earlier study demonstrated the potential efficacy of this novel series of lipopeptides against P. carinii; however, subsequent optimization of the antifungal efficacy by careful tuning of the acyl side chain led to a number of improved analogs. Full pharmacological profiling then led to the discovery of FK463 (21).
In the present study, we evaluated the effectiveness of FK463 as a potential prophylactic agent using the severe combined immunodeficient (SCID) mouse model. This model is used to examine the effectiveness of drugs against P. carinii infection and PCP (6) and is an alternative to the immunosuppressed rat model commonly used (23).
MATERIALS AND METHODS
Mice.
Forty-eight 5-week-old female C.B-17-scid mice were purchased from CLEA Japan Inc., Tokyo, Japan. The mice were maintained at four mice per cage in vinyl isolators under specific-pathogen-free conditions throughout the experiments. The top of each cage was covered with a paper filter (CLEA Japan Inc.) to prevent the transmission of infection between cages. The food, water, and bedding were sterilized with an autoclave. The mice were divided into five groups, consisting of two groups of 8 mice each treated with FK463 (two groups given different dosages), a group of eight mice treated with pentamidine, a group of 12 mice given saline as a control, and a group that was not treated to monitor the severity of P. carinii infection or PCP. For the identification of individuals, the mice were marked with picric acid or by cutting the ears. Mice were weighed each week with a small-scale electronic measurer (Pocket scale, 372-01; Tokyo Glass, Tokyo, Japan).
Compounds.
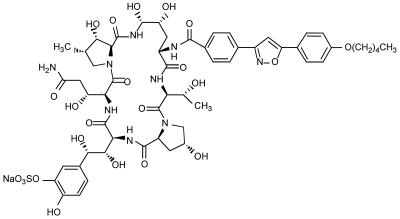
FK463 (Fig. 1) dissolved at 20 or 100 mg/ml in saline was supplied by Fujisawa Pharmaceutical Co. Pentamidine (Benambax 300; Chugai Pharmaceutical Co., Osaka, Japan) was dissolved at 400 mg/ml in saline. These drugs were stored at −20°C until use.
FIG. 1.
Chemical structure of FK463.
Experimental infection of SCID mice.
The experimental infection of SCID mice was undertaken as described previously (6). The lungs of severely P. carinii (P. carinii f. sp. muris [16])-infected SCID mice that had been maintained at −80°C were thawed in a 37°C water bath and then homogenized in 2 ml of saline with a glass homogenizer. The homogenate in a 1.5-ml sample tube was placed in a vinyl isolator after sterilization with 0.5% microquat solution (Ecolab Inc., Tokyo, Japan) outside the tube. Intranasal inoculation of the lung homogenate was performed by dropping 20 μl of the homogenate into the nares of the mice with a micropipette while the mice were under light anesthesia with ether. The number of P. carinii cysts per inoculum was 1.2 × 104 (the cyst-counting method is described below).
Treatment.
The FK463 and pentamidine solutions were thawed at room temperature for 30 min just before use. These drugs were placed in the isolators after sterilization with 0.5% microquat solution outside the tubes. A total of 0.2 ml of FK463, pentamidine, and saline as a control (0.2 ml of each solution per mouse) was subcutaneously injected into the backs of the mice daily. The mice were treated with these drugs for 6 weeks from 1 day after infection.
Monitoring of P. carinii infection.
To observe the severity of infection and to optimize the time of killing, three mice were killed each week from 4 weeks after infection, and the numbers of P. carinii cysts in the lungs and lung histologies were examined.
Evaluation of prophylactic effect. (i) Autopsy.
Thirty-six mice in the treated groups including mice in the group treated with saline were autopsied at 6 weeks after infection and the start of drug treatment. Briefly, the body weights were determined, the mice were bled while they were under anesthesia with ether, and the lungs were aseptically removed. After macroscopic observation, the weights of each lobe were determined. The left lobe was fixed with 10% buffered formalin with cannulation, embedded in paraffin, and sectioned to a thickness of 3 mm. Sections were stained with hematoxylin-eosin or periodic acid-Shiff (PAS) for histology. The remaining right lobes were homogenized in 2 ml of saline with a glass homogenizer, and 1 ml of each homogenate was subjected to determination of the number of P. carinii cysts in the mouse lungs and PCR.
(ii) Determination of number of P. carinii cysts in mouse lungs.
P. carinii cysts in the lung were stained with toluidine blue O (TBO) by the method of Chalvarrjian et al. (3). Ten microliters of the 1-ml homogenate was placed in the wells of a slide (six wells 10 mm in diameter; MS618; Bokusui-Brown Inc., New York, N.Y.), dried, and stained with TBO. After staining, the slide was washed three times with isopropanol and two times with xylene and mounted in MX (Matsunami Glass Ind., Ltd., Tokyo, Japan). To calculate the number of P. carinii cysts per mouse lung, the number of cysts present in the square of a graticule (OC-M-20.4 m/m square; Olympus, Tokyo, Japan) was counted with a microscope (BH2; Olympus) with a magnification of ×400. After counting of the number of cysts in 35 or more fields, the following formula was used for calculation of the number of cysts per lung.
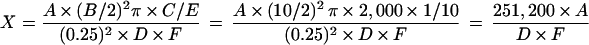 |
where X is the number of P. carinii cysts per mouse lung, A is the total number of counted cysts, B is the diameter (in millimeters) of the well smeared with homogenate, C is the amount of homogenate (in milliliters), D is the number of fields counted, E is the amount of homogenate (in milliliters) smeared on the well, and F is the weight of the right lobe/weight of the lung.
(iii) Lung histology.
To compare the severity of P. carinii infection in mice, the number of PAS staining-positive alveoli in the lung sections was counted. Briefly, at least three of the lung sections from the upper, middle, and lower portions of a left lobe were stained with PAS stain. The number of PAS staining-positive alveoli in 10 fields of each section was counted with a microscope (BH2; Olympus) at a magnification of ×400. The total number of alveoli observed in one field under these conditions was estimated to be 1,000 or more.
(iv) PCR.
Stepwise PCR for amplification of 346 bp of the P. carinii-specific DNA sequence in the region of a large subunit of the mitochondrial rRNA was performed by a modification of the method of Wakefield et al. (22) as described previously (12). DNA was extracted from 1-ml lung homogenates. The homogenates were centrifuged at 13,000 rpm for 20 min, the supernatants were removed, and 1 ml of lysis buffer (50 mM Tris-HCl [pH 8.0], 0.1 M NaCl, 20 mM EDTA, 100 μg of proteinase K per ml, 1% sodium dodecyl sulfate) was added to the pellets. After incubation by shaking overnight in a 50°C water bath, 100 μl of the supernatant was subjected to extraction with an automatic DNA extractor (MagExtractor MFX-2000; Toyobo Inc., Kyoto, Japan). The DNA was recovered as 100 μl of a solution in distilled water, and 10- or 1,000 fold-diluted DNA was used as a template for PCR. The PCR mixture contained 10 mM Tris-HCl (pH 8.3), 50 mM KCl, 2.0 mM MgCl2, 1 mg of gelatin, deoxynucleoside triphosphates at a concentration of 200 mM, 25 pmol of oligonucleotide primers, 2 U of Taq DNA polymerase (Gibco BRL, Tokyo, Japan), and 2 μl of DNA solution in 50 μl of the mixture. The primer sequences were 5′-GATGGCTGTTTCCAAGCCCA-3′ and 5′-GTGTACGTTGCAAAGTACTC-3′. The reaction was performed in steps with a thermal cycler (Perkin-Elmer Cetus, Norwalk, Conn.). The first step was denaturation at 94°C for 1.5 min with annealing at 58°C for 1.5 min and extension at 72°C for 2 min for 10 cycles. The second step was denaturation at 94°C for 1.5 min with annealing at 55°C for 1.5 min and extension at 72°C for 2 min for 10 cycles. The final step was denaturation at 94°C for 1.5 min with annealing at 50°C for 1.5 min and extension at 72°C for 2 min for 20 cycles. The amplified products were subjected to electrophoresis in a 2.5% agarose gel, and the gel was stained with ethidium bromide.
Statistics.
The Kruskal-Wallis analysis of variance and the Dunnett multiple comparison test were used to compare numbers of cysts and PAS staining-positive alveoli in the lung sections for the treatment groups and the control group.
RESULTS
Comparison of number of P. carinii cysts among experimental groups.
The numbers of P. carinii cysts per lung in the mice in each experimental group are shown in Table 1. The cysts could be detected in all 12 mice administered saline as a control. In contrast, P. carinii cysts were detected in only two of eight mice in the pentamidine-treated group and none of the eight mice in both FK463-treated groups. Even in the two positive mice in the pentamidine-treated group, only one or two cysts stained with TBO were detected, indicating that the number of cysts in this group was much lower than that in the control group.
TABLE 1.
Efficacy of FK463 against P. carinii infection in micea
| Compound (dose [mg/kg]) | No. of cysts (log no./lung)b | No. of PAS staining-positive alveoli/lungc |
|---|---|---|
| Saline (control) | 6.04 ± 0.31 | 147 ± 38.7 |
| FK463 (0.2) | <4.00d | 7.25 ± 2.55 |
| FK463 (1.0) | <4.00d | 5.13 ± 1.66 |
| Pentamidine (4.0) | <4.03 ± 0.03d | 0.75 ± 0.37d |
Each group except the control group contained eight 5-week-old female C.B-17-scid mice. The control group contained 12 mice. The lungs of severely P. carinii-infected SCID mice were homogenized in saline, and the lung homogenate (1.2 × 104 cysts) was inoculated intranasally. Treatment was given subcutaneously once daily for about 6 weeks starting at 1 day after infection. The numbers of cysts and PAS staining-positive alveoli were assayed at 6 weeks after infection.
Values are mean ± standard error log numbers of cysts per lung. The limit of detection is 4.00.
Values are mean ± standard error numbers of PAS staining-positive alveoli per lung.
Significantly different from the control (P < 0.01).
Lung histology.
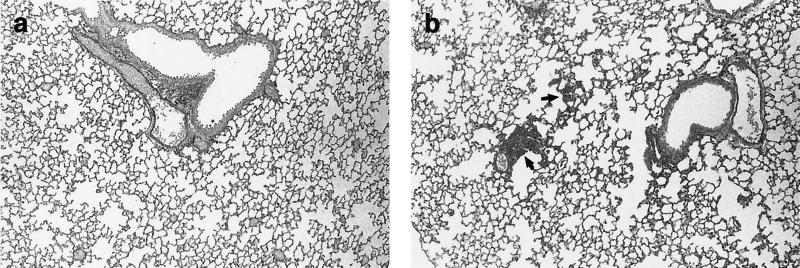
Table 1 also shows the number of PAS staining-positive alveoli per lung section in the mice in each experimental group. In the control group, a large number of PAS staining-positive alveoli per lung were observed compared to the numbers observed in the other groups. Typical pathological findings of the initial stage of PCP, such as enlargement of the interstitial region in the lungs, were observed in the control group but not in the treated groups (Fig. 2).
FIG. 2.
Lung sections from P. carinii-infected SCID mice 8 weeks after treatment with FK463 (1.0 mg/kg) (a) or saline (b). The sections were stained with PAS stain. PAS staining-positive alveoli were observed in control mice but not FK463-treated mice. Magnifications, ×180. Arrows show the PAS staining-positive hyperplastic alveoli.
PCR.
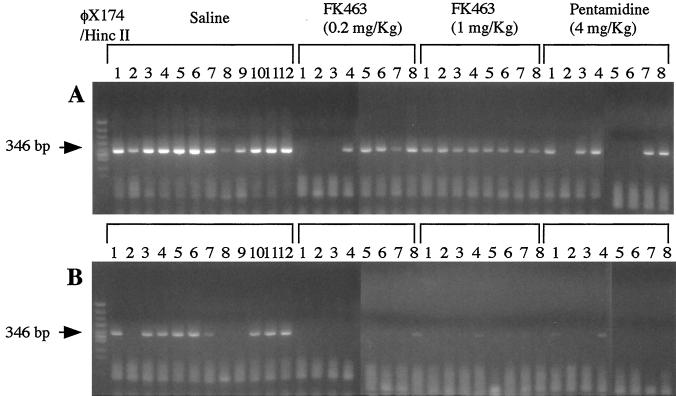
Figure 3 shows the P. carinii-specific DNA fragments amplified from the lungs of the mice in the experimental groups by PCR by using two concentrations of DNA template. By PCR with 10-fold-diluted DNA as a template, a P. carinii-specific DNA fragment was amplified from all 10 mice in the control group whose lungs were tested by PCR. The amplified band was also detected in five, eight, and five of eight mice in the groups treated with FK463 at 0.2 mg/kg, FK463 at 1.0 mg/kg, and pentamidine, respectively (Table 2). However, even in the PCR with 1,000-fold-diluted DNA as the template, the intense bands for the control group were retained, but the faint bands for the treated groups were not.
FIG. 3.
Amplification of P. carinii-specific DNA from the lungs of mice in the experimental groups. PCR was performed with 10-fold-diluted (A) and 1,000-fold-diluted (B) DNA as a template.
TABLE 2.
Results of PCR for detection of P. carinii in lungs of mice treated with FK463 and pentamidine
| Group (dose [mg/kg]) | No. of mice tested | No. of mice positive by PCR |
|---|---|---|
| Control (saline) | 10a | 10 |
| FK463 (0.2) | 8 | 5 |
| FK463 (1.0) | 8 | 8 |
| Pentamidine (4.0) | 8 | 5 |
DNA samples from 10 of 12 mice in the group treated with saline were subjected to PCR.
DISCUSSION
P. carinii has two forms, a trophozoite and a cyst, in its life cycle (13). The cyst form has a thick cell wall composed mainly of polysaccharides, mannans, and glucans, which is the same composition as the cell walls of other fungi (14). Therefore, drugs which have inhibitory activity against cell wall component synthesis are ideal candidates for prophylaxis and treatment of P. carinii infection. Indeed, echinocandin B analogs and pneumocandins, which have inhibitory activity against 1,3-β-d-glucan synthase, have prophylactic effects against P. carinii infection (1, 21). FK463 is a semisynthetic derivative of FR901379, a new water-soluble echinocandin-like lipopeptide that is isolated from the culture broth of C. empedri and that has potent in vitro and in vivo activities against Candida and Aspergillus species (10, 11). FK463 was also shown to have inhibitory activity against 1,3-β-d-glucan synthase, like echinocandin B analogs and pneumocandins. Thus, FK463 is a potential prophylactic agent for P. carinii infection. In this study we attempted to evaluate the efficacy of FK463 as a prophylactic agent for the initial stage of P. carinii infection using a SCID mouse model.
To evaluate the efficacies of drugs against P. carinii infection, animal models have been used in general because the development of drugs that are active against P. carinii has been hampered by the inability to grow this organism in vitro. Among animal models, a rat model of infection with P. carinii provoked by treatment with immunosuppressants has commonly been used (21). The SCID mouse model used in this study is an alternative for evaluation of anti-P. carinii drugs (6). This model has the advantage that the effects of immunosuppressants and other bacteria are not involved and the scale of the experiment can be minimal.
In the SCID mouse model, PCP with a honeycomb structure becomes visible as a typical pathological finding of PCP about 2 or 3 months after infection (18). At this stage, it is often difficult to evaluate the effects of anti-P. carinii drugs. In the present study, the severity of P. carinii infection or PCP was monitored by using lungs from mice killed each week from 4 weeks after infection to determine the time for termination of the experiment.
To evaluate the efficacies of drugs with this model, three techniques were used: determination of the number of P. carinii cysts per lung, lung histology, and amplification of a P. carinii-specific DNA fragment by PCR. The results of these techniques were consistent with the efficacies of the drugs. The sensitivity of PCR was much higher than those of the other two techniques. Although only cysts could be detected by TBO staining, less than 10 organisms including trophozoites could be detected by PCR (unpublished data). The results showed that treatment with FK463 and pentamidine was significantly more effective in preventing the progress of PCP than treatment with saline as a control. In particular, no cysts were detected in any of the mice treated with FK463, but a few alveoli in their lungs were positive by PAS staining and the P. carinii-specific DNA fragment was amplified from the lungs of at least half of the mice. These results indicate that the effect of FK463 is due to inhibition of cyst wall formation.
Although treatment with FK463 effectively prevented or suppressed the progress of P. carinii infection in mice, a few organisms remained in the lungs. This may be attributed to the inhibitory mechanism of FK463 on cyst wall formation. In other words, complete killing of the organism by FK463 was not observed, possibly due to the presence of binary multiplication in the trophozoite form of the life cycle (13).
In summary, pentamidine used as a drug control in this study is now widely used in aerosolized form for primary prophylaxis of PCP, and although its effectiveness is excellent, adverse effects, i.e., bronchospasm, dyspnea, cough, and nausea, are observed in treated patients (17, 19). In contrast, ongoing preclinical and clinical evaluations have demonstrated the favorable pharmacokinetics and safety features of FK463 (S. Suzuki, M. Terakawa, F. Yokobayashi, F. Fujiwara, and T. Hata, Abstr. 38th Intersci Conf. Antimicrob. Agents Chemother., abstr. F144, p. 269, 1998; J. Azuma, I. Yamamoto, M. Ogura, T. Mukai, H. Suematsu, H. Kageyama, H. Nakahara, K. Yoshida, and T. Takaya, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F146, p. 269, 1998), indicating that this agent is a potentially useful prophylactic agent for P. carinii infection.
REFERENCES
- 1.Balkovec J M, Black R M, Hammond M L, Heck J V, Zambias R A, Abruzzo G, Bartizal K, Kropp H, Trainer C, Schwartz R E, et al. Synthesis, stability, and biological evaluation of water-soluble prodrugs of a new echinocandin lipopeptide. Discovery of a potential clinical agent for the treatment of systemic candidiasis and Pneumocystis carinii pneumonia. J Med Chem. 1992;35:194–198. doi: 10.1021/jm00079a027. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Guidelines for prophylaxis against Pneumocystis carinii pneumonia for persons infected with human immunodeficiency virus. JAMA. 1989;262:335–339. [PubMed] [Google Scholar]
- 3.Chalvarrjian A M, et al. A new procedure for the identification of Pc cysts in tissue sections and smear. J Clin Pathol. 1963;16:383–384. doi: 10.1136/jcp.16.4.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Debono M, Gordee R S. Antibiotics that inhibit fungal cell wall development. Annu Rev Microbiol. 1994;48:471–497. doi: 10.1146/annurev.mi.48.100194.002351. [DOI] [PubMed] [Google Scholar]
- 5.Fischi M A. Treatment and prophylaxis of Pneumocystis carinii pneumonia. AIDS. 1988;2(Suppl. 1):S143–S150. doi: 10.1097/00002030-198800001-00022. [DOI] [PubMed] [Google Scholar]
- 6.Furuta T, Ito M, Kuramochi T, Hioki K, Nomura T. Effect of sulfamethoxazole-trimethoprim and sulfadoxine-pyrimethamine against fatal pneumocystosis in SCID mice. J Protozool. 1991;38:221S–222S. [PubMed] [Google Scholar]
- 7.Furuta T, Muramatsu H, Fujie A, Fujihira S, Abudullah N R, Kojima S. Therapeutic effects of water-soluble echinocandin compounds on Pneumocystis pneumonia in mice. Antimicrob Agents Chemother. 1998;42:37–39. doi: 10.1128/aac.42.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gordin F M, Simon G L, Wosfy C B, Mills J. Adverse reactions to trimethoprim-sulfamethoxazole in patients with the acquired immunodeficiency syndrome. Ann Intern Med. 1984;100:495–499. doi: 10.7326/0003-4819-100-4-495. [DOI] [PubMed] [Google Scholar]
- 9.Hughes W T. Pneumocystis carinii pneumonia. Boca Raton, Fla: CRC Press; 1987. pp. 4–5. [Google Scholar]
- 10.Iwamoto T, Fujie A, Sakamoto K, Tsurumi Y, Shigematsu N, Yamashita M, Hashimoto S, Okuhara M, Kohsaka M. WF11899A, B and C, novel antifungal lipopeptides. I. Taxonomy, fermentation, isolation and physico-chemical properties. J Antibiot (Tokyo) 1994;47:1084–1091. doi: 10.7164/antibiotics.47.1084. [DOI] [PubMed] [Google Scholar]
- 11.Iwamoto T, Fujie A, Nitta K, Hashimoto S, Okuhara M, Kohsaka M. WF11899A, B and C, novel antifungal lipopeptides. II. Biological properties. J Antibiot (Tokyo) 1994;47:1092–1097. doi: 10.7164/antibiotics.47.1092. [DOI] [PubMed] [Google Scholar]
- 12.Kuramochi K, Hioki K, Ito M. Pneumocystis carinii cysts are susceptible to inactivation by chemical disinfectants. Exp Anim. 1997;46:241–245. doi: 10.1538/expanim.46.241. [DOI] [PubMed] [Google Scholar]
- 13.Matsumoto Y, Yoshida Y. Advances in Pneumocystis biology. Parasitol Today. 1986;2:137–142. doi: 10.1016/0169-4758(86)90178-x. [DOI] [PubMed] [Google Scholar]
- 14.Matsumoto Y, Matsuda S, Tegoshi T. Yeast glucan in the cyst wall of Pneumocystis carinii. J Protozool. 1989;36:21S–22S. doi: 10.1111/j.1550-7408.1989.tb02674.x. [DOI] [PubMed] [Google Scholar]
- 15.Miller R F, Noury J L, Corbett E L, Felton J M, De Cock K M. Pneumocystis carinii infection: current treatment and prevention. J Antimicrob Chemother. 1996;37(Suppl. B):33–53. doi: 10.1093/jac/37.suppl_b.33. [DOI] [PubMed] [Google Scholar]
- 16.Pneumocystis Workshop (J. D. Stringer, et al.) Revised nomenclature for Pneumocystis carinii. J Eukaryot Microbiol. 1994;41:121S–122S. [PubMed] [Google Scholar]
- 17.Principi N, Marchisio P, Onorato J, Gabiano C, Galli L, Caselli D, Morandi B, Campelli A, Clerici M, Gattinara G C. Long-term administration of aerosolized pentamidine as primary prophylaxis against Pneumocystis carinii pneumonia in infants and children with symptomatic human immunodeficiency virus infection. The Italian Pediatric Collaborative Study Group on Pentamidine. J Acquir Immune Defic Syndr Hum Retrovirol. 1996;12:158–163. doi: 10.1097/00042560-199606010-00009. [DOI] [PubMed] [Google Scholar]
- 18.Roths J B, Marshall J D, Allen R D, Carlson G A, Sidman C L. Spontaneous Pneumocystis carinii pneumonia in immunodeficient mutant scid mice. Natural history and pathobiology. Am J Pathol. 1990;136:1173–1186. [PMC free article] [PubMed] [Google Scholar]
- 19.Saukkonen K, Garland R, Koziel H. Aerosolized pentamidine as alternative primary prophylaxis against Pneumocystis carinii pneumonia in adult hepatic and renal transplant recipients. Chest. 1996;109:1250–1255. doi: 10.1378/chest.109.5.1250. [DOI] [PubMed] [Google Scholar]
- 20.Schmatz D M, Powles M A, McFadden D, Nollstadt K, Bouffard F A, Dropinski J F, Liberator P, Andersen J. New semisynthetic pneumocandins with improved efficacies against Pneumocystis carinii in the rat. Antimicrob Agents Chemother. 1995;39:1320–1323. doi: 10.1128/aac.39.6.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tomishima M, Ohki H, Yamada A, Takasugi H, Maki K, Tawara S, Tanaka H. FK463, a novel water-soluble echinocandin lipopeptide: synthesis and antifungal activity. J Antibiot. 1999;52:674–676. doi: 10.7164/antibiotics.52.674. [DOI] [PubMed] [Google Scholar]
- 22.Wakefield A E, Pixley F J, Banerji S, Shinclair K, Miller R F, Moxon E R, Hopkin J M. Amplification of mitochondrial ribosomal RNA sequences from Pneumocystis carinii DNA of rat and human origin. Mol Biochem Parasitol. 1990;43:69–76. doi: 10.1016/0166-6851(90)90131-5. [DOI] [PubMed] [Google Scholar]
- 23.Walzer P D, Powell R D, Yoneda K, Rutledge M E, Milder J E. Growth characteristics and pathogenesis of experimental Pneumocystis carinii pneumonia. Infect Immun. 1980;27:928–937. doi: 10.1128/iai.27.3.928-937.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]