Abstract
Importance
The ongoing pandemic of COVID-19 is still affecting our life, but the effects of lockdown measures on gestational diabetes mellitus (GDM) in pregnant women remain unclear.
Aim
To investigate the association between COVID-19 lockdown and GDM.
Subjects and Methods
Medical records of 140844 pregnant women during 2015-2020 were extracted from 5 hospitals in Guangdong Province, China. Pregnant women who underwent the COVID-19 Level I lockdown (1/23 - 2/24/2020) during pregnancy were defined as the exposed group (N=20472) and pregnant women who underwent the same calendar months during 2015-2019 (1/23 - 2/24) were defined as the unexposed group (N=120372). Subgroup analyses were used to explore the potential susceptible exposure window of COVID-19 lockdown on GDM. Cumulative exposure is quantitatively estimated by assigning different weights to response periods with different exposure intensities. A logistic regression model was used to estimate the association between COVID-19 lockdown exposure and GDM.
Results
The rates of GDM in the exposed and unexposed groups were 15.2% and 12.4%, respectively. The overall analyses showed positive associations (odds ratio, OR=1.22, 95%CI: 1.17, 1.27) between lockdown exposure and GDM risk in all pregnant women. More pronounced associations were found in women who underwent the COVID-19 lockdown in their first four months of pregnancy, and the adjusted OR values ranged from 1.24 (95%CI: 1.10, 1.39) in women with 5-8 gestational weeks (GWs) to 1.35 (95%CI: 1.20, 1.52) with < 5 GWs. In addition, we found a positive exposure-response association of cumulative lockdown exposure with the risk of GDM.
Conclusions
The COVID-19 lockdown was associated with an increased risk of GDM, and the first four months of pregnancy may be the window for sensitive exposure.
Keywords: COVID-19, lockdown, pregnant woman, gestational diabetes mellitus, China
Introduction
Gestational diabetes mellitus (GDM) is temporary hyperglycemia induced by glucose intolerance with onset or first monitor during pregnancy (1). As one of the most common complications in pregnant women, GDM is widely prevalent around the world. The median estimated prevalence of GDM in the Middle East and North Africa region is 12.9% versus 5.8% in Europe, while in the Western Pacific region, prevalence estimates vary from 4.5% in Japan to 25.1% in Singapore (2). In China, the prevalence of GDM is also not optimistic. A meta-analyses conducted by Gao et al. in 2019 found a prevalence of GDM of 14.8% across China (3). According to the data released by the International Diabetes Federation (IDF), more than 1 million Chinese women were affected by GDM in 2013, ranking second in the world after India (4).
Although the degree of blood glucose elevation is usually not as high as that of diabetes mellitus combined with pregnancy, it can still cause serious harm to both women and fetuses (4). The short-term effects of GDM on mothers and infants include increased maternal pregnancy complications, such as gestational hypertensive disease and polyhydramnios, as well as increased risk of fetal macrosomia, and neonatal respiratory distress syndrome (4). The long-term threat to maternal and child health is mainly the increased risk of long-term type 2 diabetes mellitus (T2DM) and metabolic syndrome in mothers after postpartum and offspring (2, 4, 5). Thus, reducing the prevalence of GDM is an important public health issue.
Since the early 2020, the COVID-19 pandemic and the corresponding catastrophic effect have challenged the view of public health of the world’s people. At the time of the COVID-19 pandemic, a series of special measures have been adopted by governments and health-care leaders around the world to decrease the pandemic of the virus. For example, many cities and regions have fully or partially implemented lockdown measures, with large venues closed and traffic restricted. Apart from the control of pandemic, these measures during the lockdown have not only led to economic recessions, but also the strain on medical resources (6–8), which has substantially affected health in the public such as glycemic control in diabetic patients (9, 10).
Pregnant women go through huge physiological and psychological changes during pregnancy, and are more potentially affected by extreme events (11). A few epidemiological studies have reported significant associations of COVID-19 lockdown with maternal health and pregnancy outcomes, such as stillbirth, and preterm delivery (12, 13). While as a common complication of pregnancy, few studies have assessed the association of COVID-19 lockdown measure with GDM. A study had found the association between COVID-19 and blood sugar control in pregnant women (14). Moreover, there are several research issues or limitations that need to be fully addressed or investigated in future studies. First, previous studies focused on changes in glycemic control in patients with GDM and changes in GDM prevalence during COVID-19 need to be further evaluated. Second, the impact of environmental changes on maternal health is related to the stage of pregnancy (15, 16). The sensitive window exposure period when COVID-19 affects pregnant women’s GDM remains unknown. Third, the intensity and duration of the lockdown varied constantly, and its impact on GDM should be considered.
Accordingly, to fill in these research gaps, we comprehensively evaluated the association between the COVID-19 lockdown and the risk of GDM by quantifying the duration and intensity of exposure among pregnant women in Guangdong Province, South China. Moreover, we considered seasonal effects and adequate follow-up time in this study.
Materials and Methods
Subjects
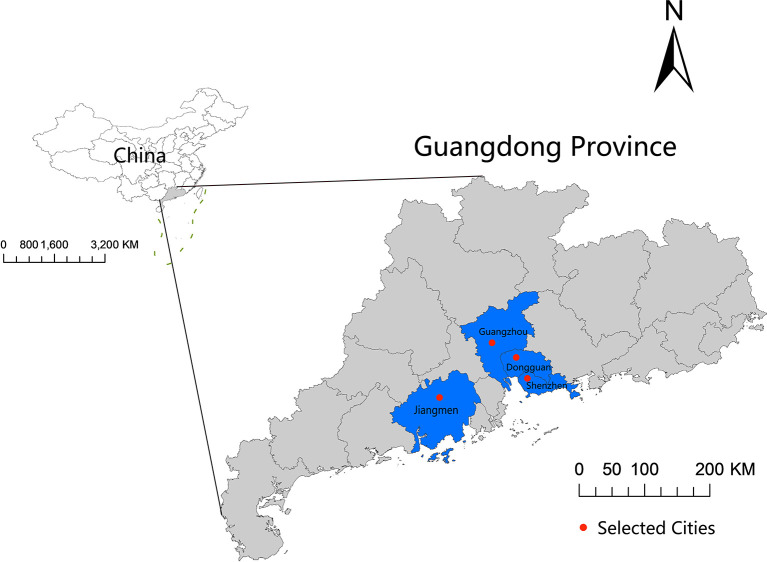
Medical records of 222126 pregnant women between 2015 and 2020 were obtained from 5 hospitals [Guangzhou (n=1), Shenzhen (n=1), Dongguan (n=2), and Jiangmen (n=1)] located in Guangdong Province, China ( Figure 1 ). We excluded 1318 women with missing information on important variables, and 37487 women whose gestational period did not overlap with the COVID-19 lockdown period in 2020 or the same period in 2015-2019. Because GDM is usually diagnosed during the 24th to 28th gestational week (GW) (17), we further excluded those (n=42353) pregnant women whose gestational age was larger than 28GWs at the time of COVID-19 lockdown and the corresponding period in 2015-2019, and those (n=124) preterm pregnant women with less than 28 GWs (not screened for GDM) when had their childbirth. Finally, 140844 pregnant women were included in the data analyses. None of these pregnant women was infected with SARS-CoV-2 ( Supplementary Figure 1 ).
Figure 1.
Geographic locations of the four included study cities in Guangdong Province, China.
Data Collection
We extracted the following individual information from maternal medical records: maternal age, gestational weeks (GWs), marital status, parity, and gestational diabetes mellitus (GDM). For this study, the data is imported into R3.6.1 software to clean up the data information mentioned above. Unreasonable or abnormal values were either amended or defined as missing.
Exposure Assessment
According to the National Emergency Plan for Public Emergencies, the emergency response for public health emergencies is divided into four levels: Level I (especially serious), Level II (serious), Level III (relatively serious), and Level IV (general) (18). In response to the COVID-19 outbreak, Guangdong Province launched a Level I response on 1/23/2020. Then the public health emergency response level was adjusted to Level II on 2/24/2020 and Level III on 5/9/2020. During the Level I response, the government implemented control measures to minimize public gatherings and stopped public gatherings rigorously (19). Shopping malls, bars, schools, and other establishments were closed, and traffic was restricted. After the Level I response, fewer restrictions were imposed. During the Level II response, public places at risk of cross-infection were temporarily closed or disinfected. During the Level III response, except for masks and temperature checks in certain places such as hospitals and shopping malls, life is gradually returning to pre-pandemic conditions ( Supplementary Table 1 ).
The days with Level I response (1/23-2/24/2020) were defined as Level I lockdown. We identified the exposure group (N=20472) based on the time of pregnancy crossed with the Level I lockdown. The unexposed group (N=120372) experienced the same calendar months as the exposed group between 2015 and 2019. This helps to control for seasonal effects, as our data suggest that the GDM rate is related to the season of pregnancy ( Supplementary Figure 2 ).
To explore the potential susceptible exposure window, we divided the exposed group into 8 subgroups according to GWs and the crossover of 1/23/2020. We calculated the date of conception based on GWs and birth date. For instance, the first subgroup consisted of women who conceived during the Level I lockdown, and the second group was pregnant women in the first four GWs of gestational age at 2020/1/23 ( Supplementary Figure 3 ). The grouping ended at 28 GWs. Similarly, the unexposed group was also divided into 8 subgroups (considering the same calendar month) and matched with the exposed group. For each pair of subsets (exposed vs unexposed), we calculated the associations between lockdown exposure and GDM risk.
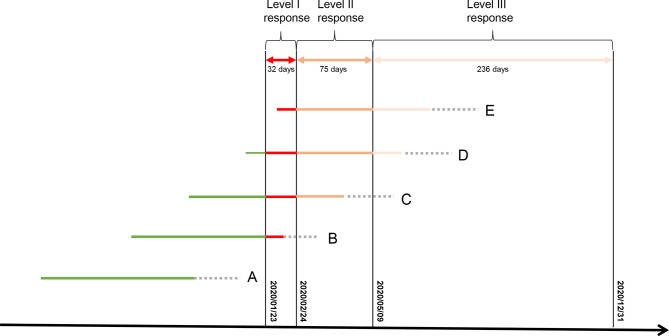
The lockdown measures during the Level II (2/25-5/9/2020) and Level III (5/10-12/31/2020) responses might also adversely affect GDM risk. Therefore, we assigned different weights to different response times, and multiply response times with weights to quantitatively estimate the cumulative exposure: (no response, weighting=0), (Level I, weighting=3), (Level II, weighting=2), and (Level III, weighting=1). Because GDM is usually diagnosed between 24 and 28 GWs (17), we only estimated the amount of cumulative exposure before 28 GWs ( Figure 2 ). Supplementary Figure 4 shows the distribution of cumulative exposures to COVID-19 lockdown.
Figure 2.
Approach to calculating individual cumulative exposure dose to lockdown in the first 28 GWs. Weeks after 28 GWs. (A–E) represent subgroups of pregnant women with different GWs during the Level I lockdown; We assigned a weighting value of 3 to the days with Level I response, 2 to the days with Level II response, 1 to the days with Level III response, and 0 to days before lockdown (no exposure).
Outcome Measures
Individual information on GDM was extracted from each woman’s medical record. Gestational diabetes was diagnosed when the blood glucose was higher than the standard at any of the three time points: fasting blood glucose 5.1 mmol/L; 1-h plasma glucose 10.0 mmol/L following a 75 g oral glucose tolerance test; or 2-h plasma glucose 8.5 mmol/L following a 75 g oral glucose tolerance test (OGTT) (20).
Potential Confounders
We probe into the following confounders potentially associated with GDM based on biological plausibility, literature review, and data availability: maternal age, marital status, parity, and residential city.
Statistical Analyses
Chi-square test (for categorical variables) or t-test (for continuous variables) were applied to detect the difference in the distribution of maternal characteristics between exposed (n = 20472) and unexposed (n = 120372) groups. An unconditional logistic regression model was implemented to estimate the associations of lockdown exposure with GDM, after adjusting for potential confounders. The logistic regression model was also implemented to analyze the association between the cumulative exposure dose and GDM risk. The cumulative exposure was treated as a continuous variable and categorical variables in the logistical regression model. For the categorical variable, the cumulative exposure was divided into four groups [Q1 (<25% centile), Q2 (≥25% centile and <50% centile), Q3(≥50% centile and <75% centile), and Q4 (≥75% centile)] according to the quartiles. The trend test is performed by inputting the four groups as continuous variables. We employed a generalized additive model (GAM) with a binomial link function to estimate the potential nonlinear exposure-response association between cumulative lockdown exposure and the risk of GDM. A penalized smoothing spline with 3 degrees of freedom (df) was used to estimate the potential nonlinear effect of cumulative lockdown exposure.
Sensitivity Analysis
It was reported that the worldwide prevalence of GDM is constantly increasing (3). Thus, it is expected that prevalence of 2020 may be significantly higher of those of 2015, independently from lockdown. To test the potential impact of long-term trend of GDM prevalence on the association between COVID-19 lockdown and GDM risk, we selected those pregnant women only in 2019 as the control group.
'We performed all the analyses in R3.6.1 (R Development Core Team 2019). And all p values were 2-sided, and a P-value <0.05 was considered statistically significant.
Ethics Statement
This study was approved by the Ethics Committee of Guangdong Provincial Center for Disease Control and Prevention (No. W96-027E-2020004). Written informed consent was obtained from all participants.
Results
General Characteristics of Study Subjects
Out of the total included 140844 pregnant women, 20472 were identified as exposed group and 120372 were defined as unexposed group ( Table 1 ). Compared with the unexposed group, the exposed group had a significantly higher proportion of women aged 30 years or older (59.5% vs 54.1%) and a lower proportion of married (94.5% vs 96.6%).
Table 1.
General characteristics of study participants.
| Unexposed group (n = 120372) No. of participants (%) |
Exposed group (n = 20472) No. of participants0 (%) |
χ 2 | P | |
|---|---|---|---|---|
| Maternal age (years) | ||||
| <24 | 8670 (7.2) | 1150 (5.6) | 228.61 | <0.001 |
| 24–26 | 17309 (14.4) | 2596 (12.7) | ||
| 27–29 | 29234 (24.3) | 4546 (22.2) | ||
| 30–32 | 26770 (22.2) | 5089 (24.9) | ||
| 33–35 | 20511 (17.0) | 3696 (18.0) | ||
| >35 | 17878 (14.9) | 3395 (16.6) | ||
| Residential city | ||||
| Guangzhou | 15381 (12.8) | 2346 (11.5) | 37.267 | <0.001 |
| Dongguan | 27843 (23.1) | 4624 (22.6) | ||
| Jiangmen | 15230 (12.7) | 2725 (13.3) | ||
| Shenzhen | 61918 (51.4) | 10777 (52.6) | ||
| Gestational diabetes mellitus (GDM) | 123.449 | <0.001 | ||
| No | 105413 (87.6) | 17352 (84.8) | ||
| Yes | 14959 (12.4) | 3120 (15.2) | ||
| Marital status | 750.81 | <0.001 | ||
| Married | 116223 (96.6) | 19346 (94.5) | ||
| Unmarried | 3403 (2.8) | 598 (2.9) | ||
| Other | 746 (0.6) | 528 (2.6) | ||
| Parity | 2.6951 | 0.260 | ||
| 0 (Primiparas) | 57293 (47.6) | 9858 (48.2) | ||
| 1 (Multiparas) | 50676 (42.1) | 8559 (41.8) | ||
| 2-4 (Multiparas) | 12403 (10.3) | 2055 (10.0) | ||
| Mean ± SD | Mean ± SD | t | P | |
| Maternal age (years) | 30.31 ± 4.85 | 30.80 ± 4.84 | 13.462 | <0.001 |
Associations of COVID-19 Lockdown Exposure With GDM
We observed a greater prevalent GDM in the exposed group (15.2%) than the unexposed group (12.4%). Multivariable analyses showed a positive association [adjusted odds ratio (OR)= 1.22, 95%CI: 1.17, 1.27] of lockdown exposure with GDM in the total pregnant women, after adjustment for maternal age, marital status, parity, and residential city. Subgroup analyses showed that the significant associations were only found in pregnant women who experienced the Level I lockdown in the first four months of pregnancy. The adjusted ORs varied from 1.35 (95%CI: 1.20, 1.52) in women with less than 5 GWs to 1.24 (95%CI: 1.10, 1.39) in women with 5-8 GWs on 1/23/2020, the beginning of Level I response ( Table 2 ).
Table 2.
Associations of exposure to the COVID-19 lockdown with gestational diabetes mellitus.
| Unexposed group (n, %) | Exposed group (n, %) a | OR for GDM (95%CI) | ||||
|---|---|---|---|---|---|---|
| GDM (-) | GDM (+) | GDM (-) | GDM (+) | Crude OR(95% CI) | Adjusted OR* (95% CI) | |
| Gestational week at the beginning of the Level I lockdown | ||||||
| All | 105413 (87.6) | 14959 (12.4) | 17352 (84.8) | 3120 (15.2) | 1.27 (1.22, 1.32) | 1.22 (1.17, 1.27) |
| Conception during the lockdown | 16228 (87.6) | 2298 (12.4) | 2271 (84.0) | 432 (16.0) | 1.34 (1.20, 1.50) | 1.30 (1.16, 1.46) |
| Prior to 5th | 13431 (87.6) | 1905 (12.4) | 2229 (83.5) | 439 (16.5) | 1.38 (1.24, 1.55) | 1.35 (1.20, 1.52) |
| 5th -8th | 13188 (86.9) | 1988 (13.1) | 2293 (83.9) | 441 (16.1) | 1.27 (1.14, 1.43) | 1.24 (1.10, 1.39) |
| 9th -12nd | 12881 (87.8) | 1783 (12.2) | 2159 (84.8) | 387 (15.2) | 1.29 (1.15, 1.46) | 1.25 (1.11, 1.41) |
| 13rd -16th | 12743 (88.6) | 1643 (11.4) | 2332 (86.0) | 379 (14.0) | 1.26 (1.12, 1.42) | 1.26 (1.11, 1.42) |
| 17th -20th | 12946 (87.9) | 1787 (12.1) | 2187 (86.6) | 337 (13.4) | 1.11 (0.98, 1.26) | 1.04 (0.92,1.19) |
| 21st -24th | 12058 (87.3) | 1760 (12.7) | 2036 (85.3) | 352 (14.7) | 1.18 (1.05, 1.34) | 1.11 (0.97, 1.26) |
| 25th -28th | 11938 (86.9) | 1795 (13.1) | 1845 (83.9) | 353 (16.1) | 1.27 (1.12, 1.44) | 1.20 (1.06, 1.36) |
*Adjusted for maternal age, marital status, parity, residential city.
GDM, gestational diabetes mellitus.
Pregnant women who have experienced the COVID-19 lockdown (from 1/23/2020 to 2/24/2020) during any period of their pregnancy were defined as the exposed group. We further divided the exposed group into subgroups according to their gestational weeks (GW) on 1/23/2020, the beginning of lockdown.
Association of Cumulative Exposures to COVID-19 Lockdown With GDM
We also found significant positive associations between cumulative exposure dose and GDM risk ( Table 3 ). The risk of GDM increased by 1.09 (95%CI:1.07, 1.11) times for each additional 100 units of cumulative exposure during the first 28 GWs. Compared with the unexposed group, the adjusted ORs of GDM in the Q1, Q2, Q3, and Q4 groups were 1.17 (95%CI: 1.08, 1.27), 1.10 (95%CI: 1.02, 1.20), 1.22 (95%CI: 1.13, 1.32), and 1.39 (95%CI: 1.29, 1.50), respectively. In addition, the nonlinear exposure-response relationship showed that higher cumulative lockdown exposure was associated with a higher risk of GDM ( Figure 3 ).
Table 3.
Associations of cumulative exposure to the COVID-19 lockdown with gestational diabetes mellitus.
| Exposure dose in | Exposure dose in | OR for GDM (95%CI) | |||||
|---|---|---|---|---|---|---|---|
| Unexposed group (Mean ± SD) | Exposed Group (Mean ± SD) | No. of participants (%) | Crude OR (95% CI) | Adjusted OR* (95% CI) | |||
| GDM (-) +GDM (+) | GDM (-) | GDM (+) | GDM (-) | GDM (+) | |||
| Cumulative exposure dose in the first 28 weeks during the Level I to the Level III lockdown a | |||||||
| Per 100 unit increase in all participants | 0 ± 0 | 223.94 ± 90.67 | 227.11 ± 92.55 | 1.10 (1.08, 1.12) | 1.09 (1.07, 1.11) | ||
| Categories of cumulative exposure dose | |||||||
| Unexposed group | 0 ± 0 | – | – | 105413 (87.6) | 14959 (12.4) | Reference | Reference |
| Q1 (<158) | – | 89.35 ± 44.45 | 86.75 ± 43.89 | 4310 (84.9) | 769 (15.1) | 1.26 (1.16, 1.36) | 1.17 (1.08, 1.27) |
| Q2 (158-256) | – | 211.19 ± 29.87 | 213.54 ± 30.62 | 4448 (86.1) | 720 (13.9) | 1.14 (1.05, 1.24) | 1.10 (1.02, 1.20) |
| Q3 (257-298) | – | 279.07 ± 11.64 | 279.16 ± 11.48 | 4306 (84.9) | 763 (15.1) | 1.25 (1.15, 1.35) | 1.22 (1.13, 1.32) |
| Q4 (≥299) | – | 317.10 ± 10.74 | 316.98 ± 10.97 | 4288 (83.2) | 868 (16.8) | 1.43 (1.32, 1.54) | 1.39 (1.29, 1.50) |
| P for trend test | < 0.001 | ||||||
*Adjusted for maternal age, marital status, parity, residential city.
GDM, gestational diabetes mellitus.
The exposed group refers to the pregnant women who have experienced the COVID-19 lockdown in their first 28 GWs. The other participants were defined as the unexposed group. The individual cumulative exposure dose was calculated by combining the weightings with the overlap between their pregnancy period ≤28 GWs and the three levels of responses. Q1-Q4 were defined as the cumulative exposure dose of the exposed group classified by quartiles, and the unexposed group was used as reference.
-Not applicable.
Figure 3.
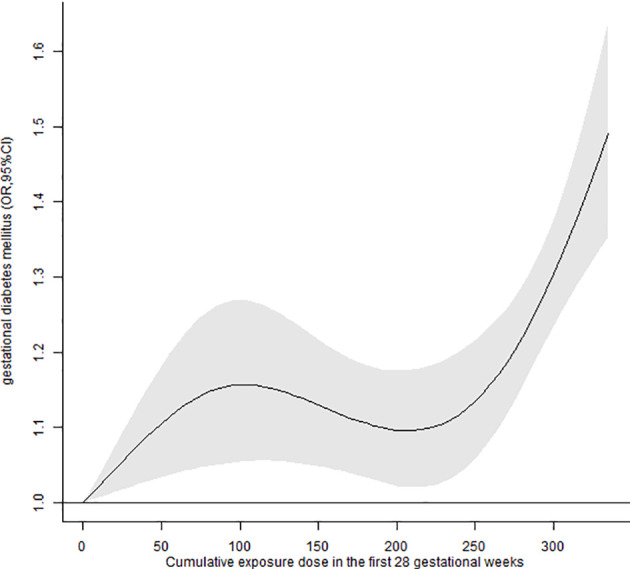
Associations between cumulative exposure dose to lockdown in the first 28 GWs and the risk of GDM.
Sensitivity Analyses
The results of sensitivity analysis suggest that the associations between COVID-19 lockdown and GDM were attenuated, and subgroup analyses suggested that the significant association was found only during the first five GWs ( Supplementary Table 2 ). However, the adjusted OR of GDM in all pregnant women was also statistically significant (1.07, 95%CI: 1.02, 1.14), which indicated the solid effect of COVID-19 lockdown on the risk of GDM.
Discussion
This study comprehensively investigates the effect of COVID-19 lockdown measures on GDM risks in pregnant women using a large database from South China. The results suggested that the COVID-19 lockdown measures were associated with an increased risk of GDM in pregnant women. The association was stronger in pregnant women within the first four months of pregnancy during the Level I lockdown period. In addition, we observed a significant exposure-response association between cumulative exposures to lockdown and GDM risk. These findings extend our understanding of the effects of COVID-19 lockdown measures on maternal and fetal health, and suggest taking actions to prevent the risk of GDM in pregnant women during COVID-19 lockdown periods.
A population study in Italy is consistent with our results. Zanardo et al. found a significant increase in the prevalence of GDM among pregnant women during the COVID-19 pandemic. Experiencing lockdown during the first trimester of pregnancy plays an important role in increasing the GDM risk in pregnant women (21). Moreover, several previous studies had estimated the associations of disasters or the COVID-19 pandemic with adverse human health including pregnancy complications. For example, a study in New York State reported an increased risk of GDM after massive power outages during Hurricane Sandy (22). Another study found a 42.3% (95% CI: 15.0%, 76.0%) increase in emergency department visits for diabetes or abnormal blood sugar in New York State during Hurricane Sandy (23). A study of the Great East Japan Earthquake of 2011 showed a 5% increase in the prevalence of GDM among the most affected residents compared to those who were not affected (24). On top of that, during the COVID-19 lockdown, an Indian cohort study found an increased risk of type 2 diabetes (25), and some other studies found that lockdown measures designed to avoid SARS-CoV-2 transmission may contribute to the deterioration of control in patients with diabetes (9, 10).
These previous studies suggest the plausible causal association between COVID-19 lockdown and GDM, which may relate to several reasons. First, during the COVID-19 lockdown period, most medical services were allocated to tackle the pandemic, and it is difficult for pregnant women to receive timely and adequate prenatal care (26). Pregnant women may also cut back on prenatal care for reasons such as fear of contracting COVID-19 patients in the hospital, following government recommendations to stay home, and restricting transportation (27, 28). Second, social distancing and family economic stress during the lockdown may induce psychological problems in pregnant women who could not attend entertainment venues, play team sports, or meet friends to relax (7, 29). Mental disorders have been regarded as a common risk factor of GDM (30). Third, there is a lot published data, including from China (31), to show that people gain weight during the lockdown. Maternal BMI was an independent risk factor for GDM (32). During the lockdown, snacks and carbohydrates are consumed more (33, 34), and the movement range and mode were greatly restricted (14, 35), which can lead to an elevated maternal BMI.
We further observed that women in the first four months during the Level I lockdown were at a greater risk of developing GDM, which is consistent with previous studies. For instance, Abdo et al. also reported a positive association between exposure to wildfire smoke during early pregnancy and GDM (36). These findings suggest that early pregnancy might be a susceptible exposure window for environmental factors affecting GDM in pregnant women. Changes in environments, behaviors, and the psychological status during the lockdown, such as physical inactivity, low sleep levels, poor diet, and mental health problems, may disturb the normal glycometabolism, and lead to GDM (37). In addition, these women in the early pregnancy during the Level I lockdown would continue to experience lockdown measures even though the Level I lockdown was over, and therefore get more cumulative exposures to lockdown measures in the first 28 GWs. We also observed a positive exposure-response association between cumulative exposure to COVID-19 lockdown and the risk of GDM, which also suggests a higher risk of GDM in women who have experienced the most cumulative exposures to lockdown. Therefore, the government and others should consider how to provide economic, medical treatment, and psychological assistance to pregnant women to reduce the risk of GDM.
Strengths and Limitations
There are several strengths in this study. First, this is the first study to quantitatively assess the exposure to COVID-19 lockdown, and investigate the association with GDM risk in a Chinese population of pregnant women. We not only estimated the association of exposure to COVID-19 lockdown as an event with the risk of GDM but also provided the exposure-response association between cumulative exposure to lockdown and GDM risk. Second, we applied a large dataset with detailed individual information to investigate the association between lockdown and GDM risk. The dataset covered a wide enough timespan, in which GDM information of all women who have experienced the lockdown was recorded. The large sample size also provided us an adequate statistical power to implement subgroup analyses and identify the potential susceptible exposure window. Third, we used strict contemporaneous controls to reduce the impact of seasonal effects on the occurrence of GDM. To test the seasonal impacts, we estimated the difference in GDM rates between the exposed group and all pregnant women in 2015-2019 (rather than matching calendar months). After adjustment for maternal age etc., we found no statistical association between lockdown and GDM risk ( Supplementary Table 3 ). These strengths could provide a stronger causal argument for our findings.
Several limitations should be considered. First, our study is a retrospective study, due to the unexpected emergence of the COVID-19 and the related lockdown measures, which limited our ability to infer the causal relationship between lockdown and GDM. Second, information of all participants was extracted from their medical recodes. Hence, several individual covariates such as maternal BMI, heredity for T2DM, smoking, alcohol consumption were not obtained in this study, and the influence of these confounding factors on the association was unknown. Third, the COVID-19 lockdown measures were implemented across countries with substantial variation in timing, content, and comprehensiveness. But this study was conducted in only four south cities, which limits the generalization of our findings. Fourth, some countries used alternative criteria for diabetes screening to avoid pregnant women staying in the hospital for long time during the COVID-19 pandemic (38, 39). However, it was not clear whether the diagnostic criteria for GDM were modified during the lockdown in this study, which may be a potential bias in this study. Some studies reported that using alternative criteria can increase the missed diagnosis rate of GDM by as much as 30-50% (40, 41). Meanwhile, a prospective study by Molina-Vega et al. found that the rate of missed diagnosis of GDM did not substantially change when comparing conventional criteria used before the pandemic with alternative diagnostic criteria used during the COVID-19 pandemic (42). Therefore, more studies are needed to examine the effects of diagnosis criteria on the association between COVID-19 lockdown and GDM. Fifth, the COVID-19 lockdown included many measures which were usually implemented simultaneously. As a result, we cannot determine their individual impact on the risk of GDM. The COVID-19 is still ongoing throughout the world, and the lockdown measures have been implemented in many countries. Therefore, more research works are needed to demonstrate the effect of COVID-19 lockdown measures on GDM.
In conclusion, we found that the COVID-19 lockdown was associated with a moderately higher risk of GDM, and the first four months might be a susceptible exposure window. Now that the global pandemic of COVID-19 is not over, and we are also confronted with the challenge of the Delta variant B.1.617.2. A study had shown that non-pharmaceutical interventions have made a huge difference in controlling the epidemic (43), so that the lockdown measures will continue to affect our lives. Our findings suggest the critical importance of planning for strong maternal services in the future lockdown. Governments and women’s health care providers must take action to reduce the risk of pregnant women developing GDM. Given the nature of this study, more investigation is needed to clarify the association between the lockdown measures and GDM, which is critical to maternal health.
Data Availability Statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding authors.
Author Contributions
TL and XS conceived study hypotheses. ZH, YL, SZ, YP, and QL conceptualized and designed the study. TL, XS, ZH, and YL edited the first draft of the manuscript. ZH, HZ, MD, JW, JF, YY, and HC did formal analyses, interpreted the results. RQ, JJ, YC, GC, GH, and SC contributed to data curation and did statistical analyses. JH, JX, and WM helped interpret and discuss the results. All authors critically revised and approved the final manuscript.
Funding
The study was funded by the National Natural Science Foundation of China (81874276, 42175181); Natural Science Foundation of Guangdong Province (2019A1515011264); Key-Area Research and Development Program of Guangdong Province (2019B111103001); Science and Technology Program of Guangzhou (202102080565); Chinese Postdoctoral Science Foundation (2020T130020ZX); and Foshan Key Technology Project for COVID-19 (2020001000376). These funders had no role in the design of the study, data collection, and analyses, or preparation of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We express our appreciation to all study participants.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.824245/full#supplementary-material
References
- 1. Metzger BE, Gabbe SG, Persson B, Buchanan TA, Catalano PA, Damm P, et al. International Association of Diabetes and Pregnancy Study Groups Recommendations on the Diagnosis and Classification of Hyperglycemia in Pregnancy. Diabetes Care (2010) 33(3):676–82. doi: 10.2337/dc09-1848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhu Y, Zhang C. Prevalence of Gestational Diabetes and Risk of Progression to Type 2 Diabetes: A Global Perspective. Curr Diabetes Rep (2016) 16(1):7. doi: 10.1007/s11892-015-0699-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gao C, Sun X, Lu L, Liu F, Yuan J. Prevalence of Gestational Diabetes Mellitus in Mainland China: A Systematic Review and Meta-Analyses. J Diabetes Investig (2019) 10(1):154–62. doi: 10.1111/jdi.12854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Guariguata L, Linnenkamp U, Beagley J, Whiting DR, Cho NH. Global Estimates of the Prevalence of Hyperglycaemia in Pregnancy. Diabetes Res Clin Pract (2014) 103(2):176–85. doi: 10.1016/j.diabres.2013.11.003 [DOI] [PubMed] [Google Scholar]
- 5. Metzger BE, Coustan DR, Trimble ER. Hyperglycemia and Adverse Pregnancy Outcomes. Clin Chem (2019) 65(7):937–8. doi: 10.1373/clinchem.2019.303990 [DOI] [PubMed] [Google Scholar]
- 6. The World Bank . The Global Economic Outlook During the COVID-19 Pandemic: A Changed World (2020). Available at: https://www.worldbank.org/en/news/feature/2020/06/08/the-global-economic-outlook-during-the-covid-19-pandemic-a-changed-world (Accessed July 12, 2021).
- 7. Pfefferbaum B, North CS. Mental Health and the Covid-19 Pandemic. N Engl J Med (2020) 383(6):510–2. doi: 10.1056/NEJMp2008017 [DOI] [PubMed] [Google Scholar]
- 8. Sun Y, Hu X, Xie J. Spatial Inequalities of COVID-19 Mortality Rate in Relation to Socioeconomic and Environmental Factors Across England. Sci Total Environ (2021) 758:143595. doi: 10.1016/j.scitotenv.2020.143595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bellido V, Pérez A. Consequences of COVID-19 on People With Diabetes. Endocrinol Diabetes Nutr (Engl Ed) (2020) 67(6):355–6. doi: 10.1016/j.endinu.2020.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ghosal S, Sinha B, Majumder M, Misra A. Estimation of Effects of Nationwide Lockdown for Containing Coronavirus Infection on Worsening of Glycosylated Haemoglobin and Increase in Diabetes-Related Complications: A Simulation Model Using Multivariate Regression Analyses. Diabetes Metab Syndr (2020) 14(4):319–23. doi: 10.1016/j.dsx.2020.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Oni O, Harville E, Xiong X, Buekens P. Relationships Among Stress Coping Styles and Pregnancy Complications Among Women Exposed to Hurricane Katrina. J Obstet Gynecol Neonatal Nurs (2015) 44(2):256–67. doi: 10.1111/1552-6909.12560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Khalil A, von Dadelszen P, Draycott T, Ugwumadu A, O’Brien P, Magee L. Change in the Incidence of Stillbirth and Preterm Delivery During the COVID-19 Pandemic. JAMA (2020) 324(7):705–6. doi: 10.1001/jama.2020.12746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Been JV, Burgos Ochoa L, Bertens LCM, Schoenmakers S, Steegers EAP, Reiss IKM. Impact of COVID-19 Mitigation Measures on the Incidence of Preterm Birth: A National Quasi-Experimental Study. Lancet Public Health (2020) 5(11):e604–11. doi: 10.1016/s2468-2667(20)30223-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ghesquière L, Garabedian C, Drumez E, Lemaître M, Cazaubiel M, Bengler C, et al. Effects of COVID-19 Pandemic Lockdown on Gestational Diabetes Mellitus: A Retrospective Study. Diabetes Metab (2021) 47(2):101201. doi: 10.1016/j.diabet.2020.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pereira G, Belanger K, Ebisu K, Bell ML. Fine Particulate Matter and Risk of Preterm Birth in Connecticut in 2000-2006: A Longitudinal Study. Am J Epidemiol (2014) 179(1):67–74. doi: 10.1093/aje/kwt216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang Y, Ma ZF. Psychological Responses and Lifestyle Changes Among Pregnant Women With Respect to the Early Stages of COVID-19 Pandemic. Int J Soc Psychiatry (2021) 67(4):344–50. doi: 10.1177/0020764020952116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chinese Medical Association . Guidelines for Diagnosis and Treatment of Gestational Diabetes Mellitus (2014). Chin J Obstet Gynecol (2014) 49(08):561–9. In Chinese. [Google Scholar]
- 18. Health Emergency Response Office . National Emergency Response Plan for Public Emergencies (2006). Available at: http://www.nhc.gov.cn/yjb/s3577/201501/a32bbe5e9b7e4478aded668f0338c027.shtml (Accessed July 1, 2021).
- 19. Office of Guangdong Province Leading Group on Prevention and Control of COVID-19 . Guangdong Province has Issued 16 Level I Measures for the Prevention and Control of COVID-19 (2020). Available at: http://wsjkw.gd.gov.cn/zwyw_gzdt/content/post_2878979.html (Accessed June 5, 2021).
- 20. World Health Organization . Diagnostic Criteria and Classification of Hyperglycaemia First Detected in Pregnancy. Geneva: WHO Press; (2013). 63p. [PubMed] [Google Scholar]
- 21. Zanardo V, Tortora D, Sandri A, Severino L, Mesirca P, Straface G. COVID-19 Pandemic: Impact on Gestational Diabetes Mellitus Prevalence. Diabetes Res Clin Pract (2021) 183:109149. doi: 10.1016/j.diabres.2021.109149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Xiao J, Zhang W, Huang M, Lu Y, Lawrence WR, Lin Z, et al. Increased Risk of Multiple Pregnancy Complications Following Large-Scale Power Outages During Hurricane Sandy in New York State. Sci Total Environ (2021) 770:145359. doi: 10.1016/j.scitotenv.2021.145359 [DOI] [PubMed] [Google Scholar]
- 23. Xiao J, Huang M, Zhang W, Rosenblum A, Ma W, Meng X, et al. The Immediate and Lasting Impact of Hurricane Sandy on Pregnancy Complications in Eight Affected Counties of New York State. Sci Total Environ (2019) 678:755–60. doi: 10.1016/j.scitotenv.2019.04.436 [DOI] [PubMed] [Google Scholar]
- 24. Ishikuro M, Obara T, Murakami K, Ueno F, Noda A, Kikuya M, et al. Relation Between Disaster Exposure, Maternal Characteristics, and Obstetric Outcomes: The Tohoku Medical Megabank Project Birth and Three-Generation Cohort Study. J Epidemiol (2021). doi: 10.2188/jea.JE20210052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ghosal S, Arora B, Dutta K, Ghosh A, Sinha B, Misra A. Increase in the Risk of Type 2 Diabetes During Lockdown for the COVID19 Pandemic in India: A Cohort Analyses. Diabetes Metab Syndr (2020) 14(5):949–52. doi: 10.1016/j.dsx.2020.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Khalil A, von Dadelszen P, Kalafat E, Sebghati M, Ladhani S, Ugwumadu A, et al. Change in Obstetric Attendance and Activities During the COVID-19 Pandemic. Lancet Infect Dis (2021) 21(5):e115. doi: 10.1016/s1473-3099(20)30779-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Corbett GA, Milne SJ, Hehir MP, Lindow SW, O’Connell MP. Health Anxiety and Behavioural Changes of Pregnant Women During the COVID-19 Pandemic. Eur J Obstet Gynecol Reprod Biol (2020) 249:96–7. doi: 10.1016/j.ejogrb.2020.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li M, Yin H, Jin Z, Zhang H, Leng B, Luo Y, et al. Impact of Wuhan Lockdown on the Indications of Cesarean Delivery and Newborn Weights During the Epidemic Period of COVID-19. PloS One (2020) 15(8):e0237420. doi: 10.1371/journal.pone.0237420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Thapa SB, Mainali A, Schwank SE, Acharya G. Maternal Mental Health in the Time of the COVID-19 Pandemic. Acta Obstet Gynecol Scand (2020) 99(7):817–8. doi: 10.1111/aogs.13894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schmitt A, Reimer A, Hermanns N, Kulzer B, Ehrmann D, Krichbaum M, et al. Depression Is Linked to Hyperglycaemia via Suboptimal Diabetes Self-Management: A Cross-Sectional Mediation Analyses. J Psychosom Res (2017) 94:17–23. doi: 10.1016/j.jpsychores.2016.12.015 [DOI] [PubMed] [Google Scholar]
- 31. Dun Y, Ripley-Gonzalez JW, Zhou N, You B, Li Q, Li H, et al. Weight Gain in Chinese Youth During a 4-Month COVID-19 Lockdown: A Retrospective Observational Study. BMJ Open (2021) 11(7):e052451. doi: 10.1136/bmjopen-2021-052451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Aydın H, Çelik Ö, Yazıcı D, Altunok Ç, Tarçın Ö, Deyneli O, et al. Prevalence and Predictors of Gestational Diabetes Mellitus: A Nationwide Multicentre Prospective Study. Diabetes Med (2019) 36(2):221–7. doi: 10.1111/dme.13857 [DOI] [PubMed] [Google Scholar]
- 33. Pietrobelli A, Pecoraro L, Ferruzzi A, Heo M, Faith M, Zoller T, et al. Effects of COVID-19 Lockdown on Lifestyle Behaviors in Children With Obesity Living in Verona, Italy: A Longitudinal Study. Obes (Silver Spring) (2020) 28(8):1382–5. doi: 10.1002/oby.22861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ghosh A, Arora B, Gupta R, Anoop S, Misra A. Effects of Nationwide Lockdown During COVID-19 Epidemic on Lifestyle and Other Medical Issues of Patients With Type 2 Diabetes in North India. Diabetes Metab Syndr (2020) 14(5):917–20. doi: 10.1016/j.dsx.2020.05.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Leppänen M, Aittasalo M, Raitanen J, Kinnunen TI, Kujala UM, Luoto R. Physical Activity During Pregnancy: Predictors of Change, Perceived Support and Barriers Among Women at Increased Risk of Gestational Diabetes. Matern Child Health J (2014) 18(9):2158–66. doi: 10.1007/s10995-014-1464-5 [DOI] [PubMed] [Google Scholar]
- 36. Abdo M, Ward I, O’Dell K, Ford B, Pierce JR, Fischer EV, et al. Impact of Wildfire Smoke on Adverse Pregnancy Outcomes in Colorado, 2007-2015. Int J Environ Res Public Health (2019) 16(19):3720. doi: 10.3390/ijerph16193720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yawen W, Yahui F, Sansan W, Shuya C, Liangkun M, Yu J, et al. A Prospective Cohort Study of the Relationship Between Unhealthy Lifestyle and Gestational Diabetes Mellitus. Chin J Dis Control Prev (2020) 24(01):14–9. doi: 10.16462/j.cnki.zhjbkz.2020.01.004 [DOI] [Google Scholar]
- 38. Thangaratinam S, Cooray SD, Sukumar N, Huda M, Devlieger R, Benhalima K, et al. ENDOCRINOLOGY in the TIME of COVID-19: Diagnosis and Management of Gestational Diabetes Mellitus. Eur J Endocrinol (2020) 183(2):G49–56. doi: 10.1530/eje-20-0401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kasuga Y, Saisho Y, Ikenoue S, Ochiai D, Tanaka M. A New Diagnostic Strategy for Gestational Diabetes During the COVID-19 Pandemic for the Japanese Population. Diabetes Metab Res Rev (2020) 36(8):e3351. doi: 10.1002/dmrr.3351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. van Gemert TE, Moses RG, Pape AV, Morris GJ. Gestational Diabetes Mellitus Testing in the COVID-19 Pandemic: The Problems With Simplifying the Diagnostic Process. Aust N Z J Obstet Gynaecol (2020) 60(5):671–4. doi: 10.1111/ajo.13203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. van-de-l’Isle Y, Steer PJ, Watt Coote I, Cauldwell M. Impact of Changes to National UK Guidance on Testing for Gestational Diabetes Screening During a Pandemic: A Single-Centre Observational Study. BJOG (2021) 128(5):917–20. doi: 10.1111/1471-0528.16482 [DOI] [PubMed] [Google Scholar]
- 42. Molina-Vega M, Gutiérrez-Repiso C, Lima-Rubio F, Suárez-Arana M, Linares-Pineda TM, Cobos Díaz A, et al. Impact of the Gestational Diabetes Diagnostic Criteria During the Pandemic: An Observational Study. J Clin Med (2021) 10(21):4904. doi: 10.3390/jcm10214904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Meng Z, Jianpeng X, Aiping D, Yingtao Z, Yali Z, Ting H, et al. Transmission Dynamics of an Outbreak of the COVID-19 Delta Variant B.1.617.2 — Guangdong Province, China, may–June 2021. China CDC Weekly (2021) 3(27):584–6. doi: 10.46234/ccdcw2021.148 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding authors.