Abstract
The presence of multinucleated cells has never been demonstrated in renal tissue, although, polyploid cells were recently observed in the tubules of normal and pathological human kidney. Therefore, the aim of the present study is to identify and quantify, by electron microscopy, multinucleated cells in the cortical tissue of normal human kidney i.e., in the three compartments of renal tubule: the proximal tubule (PT), the distal tubule (DT), and the collecting duct (CD), as well as, in the glomerulus (podocytes). The percentage of the multinucleated cells observed was 5% (95%CI: 3.6%–6.7%) in renal cortical tubules with distribution in each tubular compartment of 6% in PT, 4% in DT and 3% in CD with no statistically significant difference in the distribution of multinucleated cells according to tubular compartments. Four percent of analysed podocytes (in total 149 podocytes) were multinucleated (95%CI: 1.5%−8.6%). In conclusion, multinucleated cells were identified and quantified in functionally normal kidneys, as previously demonstrated in other organs such as the liver.
Keywords: electron microscopy, kidney, multinucleate cells, podocytes, quantitative analysis
Multinucleate cells were identified and quantified in the cortical renal tubules and podocytes of normal human kidney. Five and four percent of multinucleate cells were detected in renal cortical tubules and podocytes, respectively. We suggest that the renal multinucleate cells can be involved in tissue repairing of the kidney as previously described in the liver.
1. INTRODUCTION
Tubular multinucleated cells (Seaman, 1986) as well as bi‐ and multinucleated podocytes (Bonsib & Horvath, 1999; Chandra et al., 2010; Muhldorfer et al., 2018; Nagata et al., 1995) were identified in pathological human kidneys. Our unpublished electron microscopy observations, obtained analysing diagnostic kidney biopsies from 1985 to date at our electron microscopy unit (Electron Microscopy Unit, Università Politecnica delle Marche) suggested the presence of multinucleated cells in the nephron, i.e. in glomeruli, in proximal (PT) and distal (DT) tubules, including collecting ducts (CD), of patients affected by glomerulonephritis. Since only mononucleated polyploid tubular cells were detected in healthy kidneys (Lazzeri et al., 2018), we used standard morphological techniques, including light and electron microscopy, to identify and quantify multinucleated cells in the renal cortex of normal human kidney. We considered glomeruli, PTs, DTs and CDs separately. Multinucleated cells are polyploid cells that arise from cell cycle‐dependent mechanisms (such as endocycle and endomitosis) or from cell cycle‐independent mechanisms (such as cell fusion) (Dörnen et al., 2020; Edgar et al., 2014). At present, it is suggested that polyploid cells could play a pivotal role in maintaining tissue function performance as shown by differentiated parenchymal cells that survive to organ failure (Gjelsvik et al., 2019; Lee et al., 2009; Ovrebo & Edgar, 2018; Shu et al., 2018). Although present, the real function of polyploid cells in tissue regeneration is not sufficiently demonstrated (Ovrebo & Edgar, 2018). We hypothesized that multinucleated cells could be considered resident cells in the kidney with physiological normal function, perhaps to assure renal tissue homeostasis as observed in the liver (Diril et al., 2012; Miyaoka et al., 2012).
2. MATERIALS AND METHODS
2.1. Tissue collection
Four normal‐looking human tissue samples, with no macroscopic and microscopic cancer foci or ischaemic lesions, as confirmed by the analysis of frozen sections by a pathologist (Section of Pathological Anatomy, Università Politecnica delle Marche, School of Medicine, United Hospitals, Ancona, Italy), were immediately collected after surgical resection of the neoplastic lesion (Division of Urology, Università Politecnica delle Marche). All samples were fixed and accurately prepared for electron microscopy analyses, following standard methods. Table 1 summarizes the characteristics of the patients. Normal renal function with creatinine values between 0.8 and 1.0 mg/dl was recorded at the time of sample harvesting in all the patients. Normal‐looking renal tissue was obtained at the bench right after partial nephrectomy, distancing at least 10 mm from the renal tumour. This approach was performed in accordance with the ethical standards as reported in the manuscript.
TABLE 1.
Characteristics of tissue samples studied (Lorenzi et al., 2020)
| Cases | Gender | Age | Tumour histopathology |
|---|---|---|---|
| Case 1 | Female | 46 | Clear cell carcinoma |
| Case 2 | Male | 60 | Clear cell carcinoma |
| Case 3 | Male | 60 | Oncocytoma |
| Case 4 | Male | 75 | Clear cell carcinoma |
2.2. Transmission electron microscopy
Renal specimens were collected and processed as previously described (Cangiotti et al., 2018). Briefly, for transmission electron microscopy, four kidney and one liver (positive control) specimens were fixed in 2% glutaraldehyde/2% paraformaldehyde in 0.1 M phosphate buffer for 3 h at 4°C, postfixed in 1% osmium tetroxide in the same buffer solution, dehydrated in graded alcohols, and embedded in an Epon‐Araldite mixture. Semithin sections (2 μm) were obtained from each specimen with a MICROM HM 355 microtome (Zeiss) and stained with toluidine blue. Thin sections were cut by an MTX ultramicrotome (RMC), stained with lead citrate, and examined with a CM10 transmission electron microscope (Philips, Eindhoven, The Netherlands).
2.3. Cell counting
For the quantitative analysis of multinucleated cells, we examined two ultrathin sections for each sample (from a total of 8 sections). The total tubular mono‐ and multinucleated cells and their percentage were derived (Table 2). In addition, the percentage of mono‐ and multinucleated cells for each tubular compartment of the cortex (PT, DT and CD) was calculated (Table 3). Moreover, 19 glomeruli were examined for the quantitative analysis of the mono‐ and multinucleated podocytes. Then, the total number of podocytes, the mononucleated and multinucleated podocytes number, and therefore the percentage of multinucleated podocytes were calculated (Table 2).
TABLE 2.
Percentage of multinucleate cells in podocytes and tubules of renal cortex
| Multinucleate cells in tubules of cortex | |
|---|---|
| Number of cells analysed | 835 |
| Number of multinucleate cells | 42 |
| % of multinucleate cells | 5% |
| Multinucleate cells in Podocytes | |
| Number of glomeruli analysed | 19 |
| Number of podocytes studied | 149 |
| Number of mononucleate podocytes | 143 |
| Number of multinucleate podocytes | 6 |
| % of multinucleate podocytes | 4% |
TABLE 3.
Distribution of the multinucleate cells in each tubular compartment of the renal cortex
| Cell type, n (%) | PT | DT | CD | p a |
|---|---|---|---|---|
| MoC | 447 (93.9) | 216 (96.0) | 130 (97.0) | 0.247 |
| MuC | 29 (6.1) | 9 (4.0) | 4 (3.0) | |
| Total | 476 | 225 | 134 |
Abbreviations: CD, collecting ducts; DT, distal tubule; MoC, mononucleate cells; MuC, multinucleate cells; PT, proximal tubule.
Chi‐squared test.
2.4. Statistical analysis
The percentage of multinucleated cells in cortical tubules and podocytes was estimated by means of 95% confidence interval (95%CI). The comparison of multinucleated cells among tubular compartments (PT, DT and CD) of the renal cortex was performed using chi‐square test. R statistical package 4.0.2 (R Core Team, 2020. A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R‐project.org/) was used for the analysis and the statistical significance was assessed by considering a level of probability of 5% (p = 0.05).
3. RESULTS AND DISCUSSION
In this study, we demonstrated for the first time that multinucleated cells are present in the cortical tubules and in the podocytes of normal human renal tissues, as previously demonstrated in pathological tissues (Bonsib & Horvath, 1999; Chandra et al., 2010; Muhldorfer et al., 2018; Nagata et al., 1995; Seaman, 1986). Kidney semithin sections showed evidence of multinucleated tubular cells (Figure 1a) while no mitoses were observed. These multinucleated cells, frequently hypertrophic, showed euchromatin in the nucleus, a hypertrophic nucleolus, and sometimes up to five nuclei (see Figure S1). These nuclei were positioned either far from each other or were wedged with one other (the concave part of a nucleus adapted to the convexity of the other nucleus). In addition, ultrastructural investigation allowed a clear identification of the cortical tubular multinucleated cells in the different comparts of nephron (PT, DT and CD) (Figure 2a–c). Ninety‐nine PTs, 31 DTs and 15 CDs and a total of 835 cells were analysed. Four hundred and seventy‐six, 225 and 134 cells were analysed in PT, DT and CD, respectively. The total number of the multinucleated cells was 42 (5%; 95%CI: 3.6%–6.7%) (Table 2). The average percentage of the multinucleate cells was 5.70%, 4.50%, 4.90% and 5.30% for each of the four distinct patient samples analysed. The distribution of multinucleated cells in each tubular compartment of the renal cortex was: 6% in PT, 4% in DT and 3% in CD. No significant differences were found in the distribution of multinucleated cells according to tubular compartments (Table 3). Consistently, we observed rare binucleated cells also in the macula densa (Figure 3a) by ultrathin serial sections of normal human kidney tissue (unpublished data).
FIGURE 1.
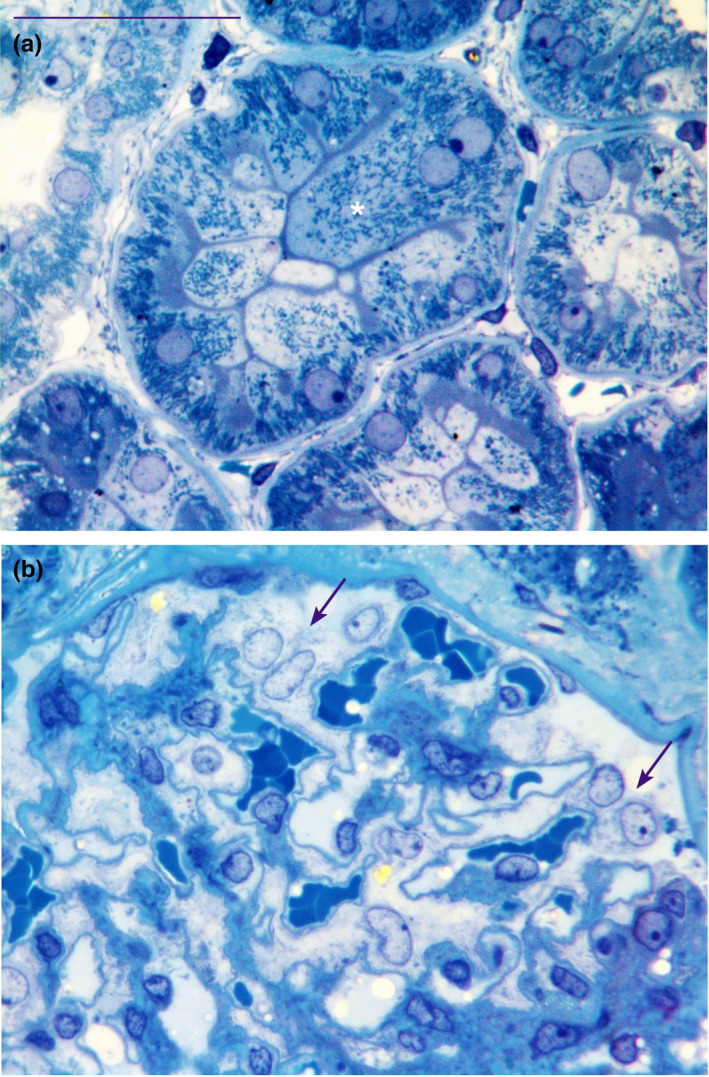
Semithin sections stained with toluidine blue of normal human renal cortical tissue that show in (a) a binucleate hypertrophic cell (white asterisk) in the proxmal tubule and in (b) two binucleate podocytes (arrows) in the glomerulus. Scale bar: a and b = 40 µm
FIGURE 2.
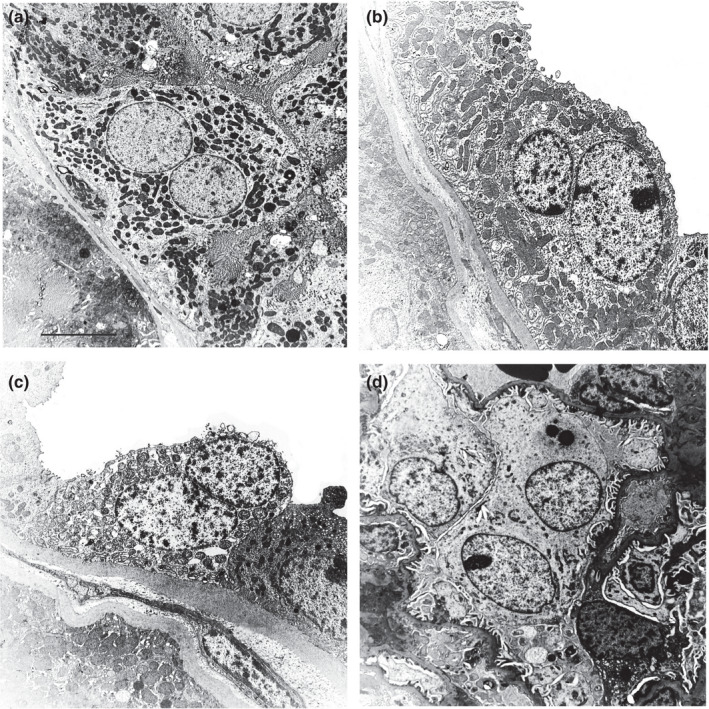
Normal human renal tissue. Ultrastructure of binucleate cells identified in proximal tubule (a), distal tubule (b), collecting duct (c) and in a podocyte of the glomerulus (d). Scale bar: a = 6.5 µm; b = 5 µm; c = 4.5 µm; d = 6.5 µm
FIGURE 3.
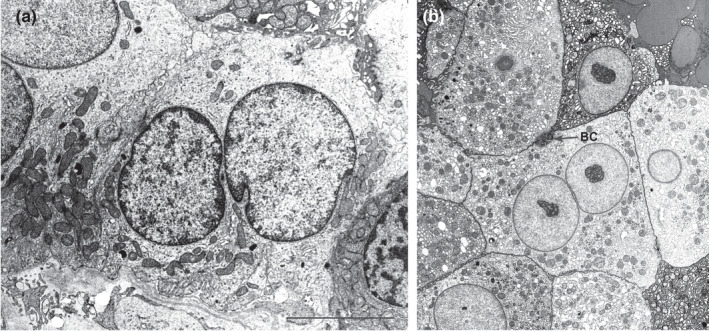
Ultrastructure of a binucleate cell contained in the macula densa (a) of normal human renal tissue. A binucleate hepatocyte of normal human liver (b) is shown as control. BC, bile canaliculus. Scale bar: a and b = 3 µm
We confirmed the presence of multinucleated podocytes (Figures 1b and 2d) even if some nuclei showed irregular profile (Muhldorfer et al., 2018). One‐hundred and forty‐nine podocytes were analysed and six were multinucleated (4%; 95%CI: 1.5%–8.6%) (Table 2). We suggest that these multinucleated cells can be considered as resident parenchymal cells that together with scattered tubular cells (Angelotti et al., 2012; Hansson et al., 2014; Kang et al., 2016; Lazzeri et al., 2018, 2019; Lindgren et al., 2011; Lorenzi et al., 2020; Rinkevich et al., 2014; Smeets et al., 2013) have the capacity to maintain renal tissue homeostasis. The presence of multinucleated cells has been previously demonstrated in the liver (Figure 3b) (Kudryavtsev et al., 1993; Wang et al., 2017) and polyploidy was demonstrated in accordance to the different grades of hepatocyte maturity, i.e. progenitors were diploid, while differentiated hepatocytes become polyploid via endoreplication (Wang et al., 2015). Polyploid differentiated hepatocytes drive the recovery of liver function, while the diploid hepatocyte progenitors drive the restoration of the liver mass by proliferation and differentiation of new hepatocytes (Lazzeri et al., 2019). In addition, recent studies have demonstrated an increased percentage of polyploid cells in the damaged kidney compared to the healthy one (Lazzeri et al., 2018, 2019).
The limit of this study is due to the use of normal‐looking human renal samples taken from kidneys affected by benign and malignant lesions. However, all patients had a normal renal function at the time of sampling; the samples were obtained from tissue distancing at least 10 mm from the lesion; these tissues had a normal histological and ultrastructural morphology.
In conclusion, multinucleated cells were identified and quantified in the cortical tissue of functionally normal kidneys. Further studies will be needed to identify the function of these cells in the physiology of the kidney; nevertheless, we hypothesize that these cells could be involved in renal tissue homeostasis as previously demonstrated in other organs such as the liver (Orr‐Weaver, 2015).
CONFLICT OF INTEREST
The authors report no relationships with companies that may have a financial interest in the information contained in the manuscript.
AUTHOR CONTRIBUTIONS
S.F., G.T., E.S., L.G.: performed the experiments, analysed and interpreted the data. A.B.G.: provided the tissue samples and critically revisioned the manuscript. D.M.: critically revisioned the final version of the manuscript. M.M.: conceptualized and supervisioned the study, analysed and interpreted the data, and wrote the manuscript. All authors approved the final version of the manuscript.
ETHICAL STANDARDS
All procedures performed in this study involving human subjects were in accordance with the ethical standards of the Università Politecnica delle Marche and with the 1964 Helsinki declaration and its later amendments on comparable ethical standards. Written informed consent was obtained from all the subjects included in this study.
Supporting information
Fig S1
ACKNOWLEDGEMENTS
We are grateful to Dr Nicola Alesi (Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA) for his excellent final revision of the manuscript.
Fantone, S. , Tossetta, G. , Graciotti, L. , Galosi, A.B. , Skrami, E. , Marzioni, D. et al. (2022) Identification of multinucleated cells in human kidney cortex: a way for tissue repairing? Journal of Anatomy, 240, 985–990. Available from: 10.1111/joa.13595
Giovanni Tossetta and Sonia Fantone equally contributed as first authors.
Funding information
This work was supported by grant from Università Politecnica delle Marche (Grant number: RSA 2021 to M.M and D.M.)
Contributor Information
Daniela Marzioni, Email: d.marzioni@staff.univpm.it.
Manrico Morroni, Email: m.morroni@univpm.it.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding authors upon reasonable request.
REFERENCES
- Angelotti, M.L. , Ronconi, E. , Ballerini, L. , Peired, A. , Mazzinghi, B. , Sagrinati, C. et al. (2012) Characterization of renal progenitors committed toward tubular lineage and their regenerative potential in renal tubular injury. Stem Cells, 30, 1714–1725. [DOI] [PubMed] [Google Scholar]
- Bonsib, S.M. & Horvath, F. Jr (1999) Multinucleated podocytes in a child with nephrotic syndrome and Fanconi's syndrome: a unique clue to the diagnosis. American Journal of Kidney Diseases, 34, 966–971. [DOI] [PubMed] [Google Scholar]
- Cangiotti, A.M. , Lorenzi, T. , Zingaretti, M.C. , Fabri, M. & Morroni, M. (2018) Polarized ends of human macula densa cells: ultrastructural investigation and morphofunctional correlations. Anatomical Record (Hoboken), 301, 922–931. [DOI] [PubMed] [Google Scholar]
- Chandra, M. , Stokes, M.B. & Kaskel, F. (2010) Multinucleated podocytes: a diagnostic clue to cystinosis. Kidney International, 78, 1052. [DOI] [PubMed] [Google Scholar]
- Diril, M.K. , Ratnacaram, C.K. , Padmakumar, V.C. , Du, T. , Wasser, M. , Coppola, V. et al. (2012) Cyclin‐dependent kinase 1 (Cdk1) is essential for cell division and suppression of DNA re‐replication but not for liver regeneration. Proceedings of the National Academy of Sciences, 109, 3826–3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dörnen, J. , Sieler, M. , Weiler, J. , Keil, S. & Dittmar, T. (2020) Cell fusion‐mediated tissue regeneration as an inducer of polyploidy and aneuploidy. International Journal of Molecular Sciences, 21, 1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar, B.A. , Zielke, N. & Gutierrez, C. (2014) Endocycles: a recurrent evolutionary innovation for post‐mitotic cell growth. Nature Reviews Molecular Cell Biology, 15, 197–210. [DOI] [PubMed] [Google Scholar]
- Gjelsvik, K.J. , Besen‐McNally, R. & Losick, V.P. (2019) Solving the polyploid mystery in health and disease. Trends in Genetics, 35, 6–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson, J. , Hultenby, K. , Cramnert, C. , Pontén, F. , Jansson, H. , Lindgren, D. et al. (2014) Evidence for a morphologically distinct and functionally robust cell type in the proximal tubules of human kidney. Human Pathology, 45, 382–393. [DOI] [PubMed] [Google Scholar]
- Kang, H.M. , Huang, S. , Reidy, K. , Han, S.H. , Chinga, F. & Susztak, K. (2016) Sox9‐positive progenitor cells play a key role in renal tubule epithelial regeneration in mice. Cell Reports, 14, 861–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudryavtsev, B.N. , Kudryavtseva, M.V. , Sakuta, G.A. & Stein, G.I. (1993) Human hepatocyte polyploidization kinetics in the course of life cycle. Virchows Archiv B Cell Pathology including Molecular Pathology, 64, 387–393. [DOI] [PubMed] [Google Scholar]
- Lazzeri, E. , Angelotti, M.L. , Conte, C. , Anders, H.J. & Romagnani, P. (2019) Surviving acute organ failure: cell polyploidization and progenitor proliferation. Trends in Molecular Medicine, 25, 366–381. [DOI] [PubMed] [Google Scholar]
- Lazzeri, E. , Angelotti, M.L. , Peired, A. , Conte, C. , Marschner, J.A. , Maggi, L. et al. (2018) Endocycle‐related tubular cell hypertrophy and progenitor proliferation recover renal function after acute kidney injury. Nature Communications, 9, 1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, H.O. , Davidson, J.M. & Duronio, R.J. (2009) Endoreplication: polyploidy with purpose. Genes & Development, 23, 2461–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindgren, D. , Boström, A.K. , Nilsson, K. , Hansson, J. , Sjölund, J. , Möller, C. et al. (2011) Isolation and characterization of progenitor‐like cells from human renal proximal tubules. American Journal of Pathology, 178, 828–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzi, T. , Graciotti, L. , Sagrati, A. , Reguzzoni, M. , Protasoni, M. , Minardi, D. et al. (2020) Normal human macula densa morphology and cell turnover: a histological, ultrastructural, and immunohistochemical investigation. Anatomical Record (Hoboken), 303, 2904–2916. [DOI] [PubMed] [Google Scholar]
- Miyaoka, Y. , Ebato, K. , Kato, H. , Arakawa, S. , Shimizu, S. & Miyajima, A. (2012) Hypertrophy and unconventional cell division of hepatocytes underlie liver regeneration. Current Biology, 22, 1166–1175. [DOI] [PubMed] [Google Scholar]
- Muhldorfer, J. , Pfister, E. , Buttner‐Herold, M. , Klewer, M. , Amann, K. & Daniel, C. (2018) Bi‐nucleation of podocytes is uniformly accompanied by foot processes widening in renal disease. Nephrology, Dialysis, Transplantation, 33, 796–803. [DOI] [PubMed] [Google Scholar]
- Nagata, M. , Yamaguchi, Y. , Komatsu, Y. & Ito, K. (1995) Mitosis and the presence of binucleate cells among glomerular podocytes in diseased human kidneys. Nephron, 70, 68–71. [DOI] [PubMed] [Google Scholar]
- Orr‐Weaver, T.L. (2015) When bigger is better: the role of polyploidy in organogenesis. Trends in Genetics, 31, 307–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovrebo, J.I. & Edgar, B.A. (2018) Polyploidy in tissue homeostasis and regeneration. Development, 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinkevich, Y. , Montoro, D.T. , Contreras‐Trujillo, H. , Harari‐Steinberg, O. , Newman, A.M. , Tsai, J.M. et al. (2014) In vivo clonal analysis reveals lineage‐restricted progenitor characteristics in mammalian kidney development, maintenance, and regeneration. Cell Reports, 7, 1270–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaman, W.J. (1986) Multinucleated epithelial cells of renal collecting tubules in macaques. Veterinary Pathology, 23, 87–88. [DOI] [PubMed] [Google Scholar]
- Shu, Z. , Row, S. & Deng, W.M. (2018) Endoreplication: the good, the bad, and the ugly. Trends in Cell Biology, 28, 465–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeets, B. , Boor, P. , Dijkman, H. , Sharma, S.V. , Jirak, P. , Mooren, F. et al. (2013) Proximal tubular cells contain a phenotypically distinct, scattered cell population involved in tubular regeneration. The Journal of Pathology, 229, 645–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, B. , Zhao, L. , Fish, M. , Logan, C.Y. & Nusse, R. (2015) Self‐renewing diploid Axin2(+) cells fuel homeostatic renewal of the liver. Nature, 524, 180–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, M.J. , Chen, F. , Lau, J.T.Y. & Hu, Y.P. (2017) Hepatocyte polyploidization and its association with pathophysiological processes. Cell Death & Disease, 8, e2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Data Availability Statement
The data that support the findings of this study are available from the corresponding authors upon reasonable request.


