Abstract
Purpose
Dexmedetomidine combined with opioids has been extensively used to blunt cardiovascular responses to endotracheal intubation. To determine their interaction, we aimed to develop a response surface model between dexmedetomidine and sufentanil.
Methods
One hundred and twenty patients undergoing scheduled gynaecological surgery were recruited. According to a simulation of slice design, patients received different dose pairs of dexmedetomidine (0 to 1.1 μg/kg) and sufentanil (.1 to .5 μg/kg). The mean arterial blood pressure and heart rate of patients were recorded just before endotracheal intubation, immediately after intubation, and during the first 3 min after intubation. The primary outcomes were haemodynamic changes. The full dose–response relationship between dexmedetomidine and sufentanil was analysed using a logit model.
Results
This response surface model revealed that the interaction between dexmedetomidine and sufentanil was additive. The dose pairs that could effectively attenuate the haemodynamic response to endotracheal intubation primarily ranged from .3 to .4 μg/kg and .5 to 1.1 μg/kg for sufentanil and dexmedetomidine, respectively.
Conclusion
When used propofol as the main hypnotic drug during anaesthesia induction, dexmedetomidine could effectively reduce the requirement of sufentanil in an additive manner. However, it is not an effective drug for ablating the cardiovascular response to endotracheal intubation when used alone. The clinical trial registry. The trial registry name: Chinese Clinical Trial Registry. Registration number: ChiCTR1800015273. URL:http://www.chictr.org.cn
Keywords: dexmedetomidine, sufentanil, response surface, endotracheal intubation
Introduction
Laryngoscopy and endotracheal intubation remain the gold standard for ensuring a secure airway in general anaesthesia. However, the sympathetic response of increased blood pressure and heart rate caused by endotracheal intubation may lead to dysrhythmia in some cases. 1 Moreover, it may be dangerous, sometimes even fatal, in patients susceptible to coronary artery disease, hypertension or cerebrovascular diseases.2,3 During anaesthesia induction, opioids are the most commonly used drugs to inhibit the haemodynamic response. 4 However, opioid-based anaesthesia is associated with postoperative nausea and vomiting, drowsiness, respiratory depression and increased analgesic requirements. Currently, there has been a trend to reduce perioperative usage of opioids through various methods, such as regional blockage, ketamine and dexmedetomidine.
Dexmedetomidine is a highly selective α2-adrenergic agonist with analgesic, sedative and sympatholytic properties, as well as minimal respiratory depression. Previous studies have reported that dexmedetomidine combined with opioids could effectively attenuate haemodynamic responses to endotracheal intubation.5,6 When co-administered with two or more drugs, it is necessary to consider their interaction to accurately predict the dose–response relationship. However, the pharmacodynamic interaction between opioids and dexmedetomidine has not been well-investigated.
Response surface models are powerful representations for evaluating drug–drug interactions. They allow the complete characterisation of pharmacodynamic interactions over the entire spectrum of possible dose pairs. In addition, this three-dimensional model provides a comprehensive description of the dose–response relationship in the presence of multiple drugs,7,8 which is helpful for guiding anaesthesiologists in more effective drug-dosing practice. In this prospective, double-blind, randomised study, we developed a response surface model to investigate the interaction between dexmedetomidine and sufentanil for attenuating the haemodynamic response to endotracheal intubation.
Material and Methods
Study Design and Participants
This study was approved by the Institutional Review Board of Tongji Medical College, Huazhong University of Science and Technology. Written informed consent was obtained from all participants. The trial was registered prior to patient enrolment at the Chinese Clinical Trail Registry (ChiCTR1800015273). The study was conducted at Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China.
Patients aged 18 to 60 years, American Society of Anesthesiologists status I or II, deviating from ideal body weight by no more than 25%, and undergoing scheduled gynaecological surgery were eligible to be included in this study. Patients were excluded if they met one of the following criteria: anticipated difficult airway, alcohol or drug addiction, allergy to opioids or dexmedetomidine, severe cardiovascular disease (preoperative left ventricular ejection fraction less than 30%), hypertension, sick sinus syndrome, sinus bradycardia (<60 beats per min), second degree or greater atrioventricular block, serious hepatic dysfunction (Child–Pugh class C), already receiving medicines with cardiovascular effects or serious renal dysfunction (undergoing dialysis before surgery). Demographic information and clinical characteristics of the patients were recorded.
Study Protocol
We adopted a design that was a simulation of the ‘slices’ type, as previously described. 7 As shown in Table 1, we designed 24 drug pairs, with 5 patients for each pair. 9 Each patient randomly received a prespecified combination of sufentanil (.1 to .5 μg/kg) and dexmedetomidine (0 to 1.1 μg/kg).
Table 1.
Dose Grid of Study Design.
| Patients (n) | Dex (μg/kg) | Suf (μg/kg [n]) |
|---|---|---|
| 20 | 0 | .2 (5), .3 (5), .4 (5), .5 (5) |
| 20 | 0.3 | .1 (5), .2 (5), .3 (5), .4 (5) |
| 20 | 0.5 | .1 (5), .2 (5), .3 (5), .4 (5) |
| 20 | 0.7 | .1 (5), .2 (5), .3 (5), .4 (5) |
| 20 | 0.9 | .1 (5), .2 (5), .3 (5), .4 (5) |
| 20 | 1.1 | .1 (5), .15 (5), .2 (5), .3 (5) |
Dex: dexmedetomidine. Suf: sufentanil.
Randomisation and Blinding
Randomisation was performed through a computer-generated random number table, which was generated by SPSS 14.0 software (SPSS Inc., Chicago, IL). The group assignment numbers were sealed in opaque envelopes and kept by the study supervisor. After written consent was obtained, the envelope was unsealed to determine which drug pair would be used. Dexmedetomidine (Hengrui Medicine Co., Ltd. Jiangsu, China), labelled Drug A, was diluted in 40 mL of normal saline, and sufentanil (Humanwell Health care (Group) Co., Ltd. Yichang, China), labelled Drug B, was diluted in 5 mL of normal saline. The random sequence generation and preparation of the drugs were completed by an investigator who did not participate in general anaesthesia management. The drugs were administered by another investigator who was blinded to the group assignment of patients. Endotracheal intubation was performed by a faculty anaesthesiologist for all patients. The haemodynamic parameters of patients during anaesthesia induction were recorded by the third investigator who was unaware of the study design. All the data were analysed by a research member who was not involved in the clinical care of patients. Study personnel and patients were blinded to group assignment throughout the study period.
General Anaesthesia
None of the patients was premedicated. After patients arrived at the operating theatre, 18-gauge IV cannulas were placed in forearm veins, and 10 mL kg−1 Ringer’s lactate solution was infused. Patients were monitored with non-invasive blood pressure, electrocardiography and pulse oxygen saturation. The mean arterial blood pressure (MAP) and heart rate (HR) were recorded every 5 min 3 times, and the averages were considered the baseline values.
Patients received 100% oxygen at a flow rate of 5 L min−1 via a mask during continuous administration of dexmedetomidine, which lasted 15 min and was completed 10 min before endotracheal intubation. 10 Target-controlled infusion (TCI) of propofol with a Graseby 3500 Anaesthesia Pump (Smiths Medical MD, Inc. USA) was used for anaesthesia induction. The targeted plasma concentration of propofol was set to 4 μg ml−1 and was maintained for at least 12 min before intubation. 11 Sufentanil was administered 5 min before intubation. 12 Rocuronium (.6 mg kg−1) was administered to provide muscle relaxation. The peak effects of drugs for anaesthesia induction were achieved when an endotracheal tube was inserted.
General anaesthesia was maintained with sevoflurane in a mixture fresh gas at a flow rate of 1 L min−1 oxygen and 1 L min−1 medical air. Remifentanil was continuously infused to maintain perioperative changes in MAP and HR within 20% of baseline values. Muscle relaxants were administered when needed. Tramadol (1.5 mg kg−1) and tropisetron (2 mg) were administered 30 min prior to the end of surgery. The vapourizer was switched off, and the concentration of oxygen was increased to 100% with oxygen flow at 6 L min−1 at the end of surgery. The endotracheal tube was removed when the patient demonstrated purposeful movement and spontaneous and regular breathing. The patients were transferred to the post-anaesthesia care unit and discharged after a modified Aldrete score of 9 was attained.
Outcomes
The primary outcome was haemodynamic changes. The MAP and HR of patients were recorded before endotracheal intubation, immediately after intubation, and at 1-min intervals during the first 3 min after intubation. If the changes in MAP and HR at all time points after intubation were within 15% of pre-stimulation values, the patient was considered to have no haemodynamic response, and the measurement of drug effect was labelled 1; otherwise, the patient was labelled . 13 If hypotension (MAP ≤50 mm Hg) or bradycardia (HR ≤ 45 beats per min) occurred during anaesthesia induction, 5 mg of ephedrine or .5 mg of atropine was intravenously administered, respectively. Patients who experienced hypotension or bradycardia were excluded from this study. Patients with unanticipated difficult airways who required multiple attempts or prolonged endotracheal intubation (>30 s) were also excluded.
Response Surface Model
A logit response surface model, which allowed data analysis with different baseline responses to endotracheal intubation, was used to minimise the influence of different baseline effects on our results. 14 The model and calculation of the 50% effective doses (ED50) for dexmedetomidine and sufentanil are shown as follows. In the logit model, the β3 coefficient controls the interaction between the 2 drugs, where β3 = 0, β3 > 0, and β3 < 0 indicate that the interaction is additive, synergistic and antagonistic, respectively
where P represents the probability that there would be no haemodynamic response to endotracheal intubation. Dsuf and Ddex are the doses of dexmedetomidine and sufentanil, respectively. βi is the parameter that describes the response surface (i = 0, 1, 2, and 3). ED50suf and ED50dex are the doses for sufentanil and dexmedetomidine that inhibit the haemodynamic responses to tracheal intubation in 50% of patients, respectively.
Statistical Analysis
The model parameters and graphics were estimated or generated using MATLAB (R2013a; The MathWorks, Inc., Natick, MA, USA). For each pharmacodynamic response, data were combined to build a three-dimensional response surface using a naïve-pooled technique. Model parameters were determined with an iterative approach minimising the −2 times the logarithm of maximal likelihood (−2LL), 15 as follows
where N is the number for all observations. Ri is the observed response, which is 0 (response to intubation) or 1 (no response to intubation). P represents the estimated probability of loss response.
The mean (μ) and standard deviation (σ) of the 1000 estimates using the bootstrap method were used to compute the coefficient of variance (CV) for the model, 16 as follows
Model building was performed starting with the simplest form (β3 = 0) and expanding the model with β3. Assuming a χ2 distribution with 1 degree of freedom and P = .05, if the difference between the objective function values (OFV) > 3.84, it would be considered significantly different. 15 The correlation coefficient of the regression parameter estimates was used to evaluate how well the final model described the observed data. A value of the correlation coefficient ≥.7 indicates that the observed response could be well predicted by the model. 14 In addition, an assessment of how well the model predictions fitted the observations was arbitrarily defined by calculating the percentage of predictions that agreed with the observations. The predictions and observations were considered to agree with each other if the difference between the prediction and observation values was <.5. 15
Results
From April 2, 2018, to March 10, 2019, 153 patients were screened for this study. A total of 120 patients were enrolled and randomly assigned to receive different dose pairs of dexmedetomidine and sufentanil. Of the 120 patients, 112 completed the study after 3 experiencing hypotension and 5 experiencing bradycardia during anaesthesia induction were excluded. The average age of the patients was 32 ± 7 years. The average body weight and height of the patients were 54 ± 6 kg and 159 ± 5 cm, respectively. All patients were classified as American Society of Anesthesiologists status I.
The haemodynamic responses to endotracheal intubation in all patients were analysed using the logit model. When β3 was not fixed at 0 but estimated simultaneously with other parameters, the OFV was not significantly decreased. These results indicated that patient data could be well characterised by an additive model (β3 = 0). The model parameters and goodness-of-fit analysis are shown in Table 2. The correlation coefficient (.74) indicated that the prediction of the model in this study was reliable.
Table 2.
Model Parameters, Measures of Fitness for Logit Response Surface.
| Parameters | Value |
|---|---|
| β0 | 8.57 (21%) |
| β1 | 22.86 (22%) |
| β2 | 5.21 (22%) |
| OFV (β3 = 0) | 82.66 |
| OFV (β3 ≠ 0) | 80.62 |
| ED50suf (μg/kg) | .37 |
| ED50dex (μg/kg) | 1.64 |
| Correlation coefficient | .72* |
| SP | 84% |
βi (i = 0, 1, 2, or 3) parameters describing response surface, presented as typical values with variance coefficient. OFV: objective function values. ED50: 50% effective dose. Suf: sufentanil. Dex: dexmedetomidine. SP: percentage of successful prediction. *P < .01.
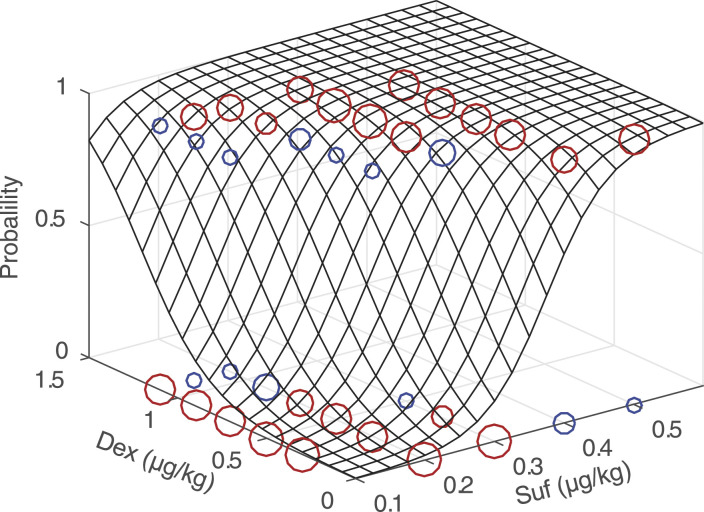
The response surface for the probability of inhibiting the haemodynamic response to endotracheal intubation is shown in Figure 1. The raw data were labelled based on the agreement between the prediction and observation values. This format was used to illustrate the difference between model predictions (ranging from 0 to 1, using Equation (1)) and observed responses (0 or 1). For much of the data, the difference was less than .5. The percentage of model predictions that was consistent with observed responses was 84%.
Figure 1.
Response surface for inhibiting the haemodynamic response to endotracheal intubation. The raw data, model predictions and an assessment of model error were demonstrated. The circles represent the observed responses of patients. The circle at the bottom of the response surface represents the response to endotracheal intubation, and the circle at the top indicates no response. Prediction accuracy was defined as < .5 deviations from the true patient response. The red circle indicates the accurate prediction and the blue one indicates the inaccurate prediction. The size of the circle reflects the number of patients. There are 5 different sizes of circles, which represent the number of patients from 1 to 5. Abbreviations: Dex, dexmedetomidine. Suf, sufentanil.
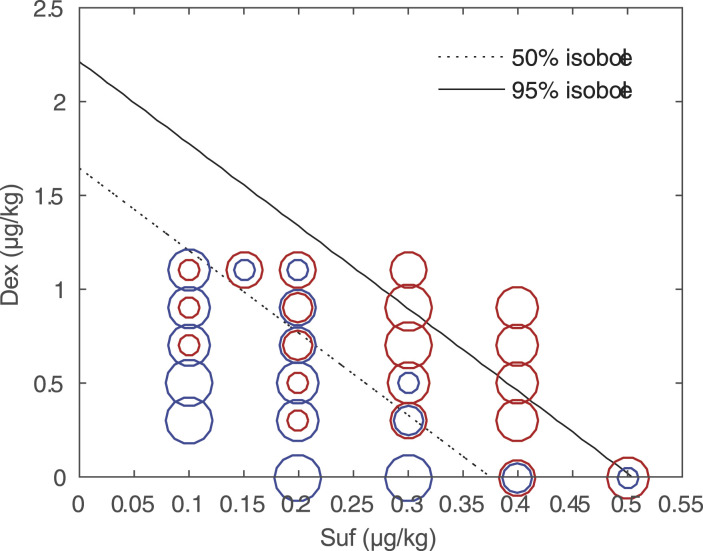
As shown in Figure 2, a topographic view of the response surface was generated by plotting 50% and 95% isoboles, as well as observed data. The raw data were labelled based on the haemodynamic responses to endotracheal intubation. The dose pairs that could effectively attenuate the haemodynamic response primarily clustered in the .5 to 1.1 μg/kg range for dexmedetomidine and the .3 to .4 μg/kg range for sufentanil. In addition, the combinations of dexmedetomidine ranging from .9 to 1.1 μg/kg and sufentanil ranging from .3 to .4 μg/kg, as well as dexmedetomidine ranging from .5 to 1.1 μg/kg and sufentanil .4 μg/kg, were close to the 95% isoboles, indicating that these dose pairs approximated 95% effective doses (ED95).
Figure 2.
Topographical views of the raw data, the 50% and 95% isoboles. The blue circle represents a response to endotracheal intubation, and the red one represents no response. The size of the circles reflects the number of patients. There are 5 different sizes of circles, which represent the number of patients from 1 to 5. Abbreviations: Dex, dexmedetomidine. Suf, sufentanil.
The doses of sufentanil and dexmedetomidine that blocked the sympathetic responses to endotracheal intubation in 50% of cases (ED50) were .37 μg/kg and 1.64 μg/kg, respectively. The simulation results showed that when the dose of dexmedetomidine was increased from 0 to 1 μg/kg, the ED50 and ED95 of sufentanil for inhibiting the haemodynamic response to endotracheal intubation were decreased from .37 to .15 μg/kg and .50 to .28 μg/kg, respectively (Table 3).
Table 3.
Effects of Dexmedetomidine Dosage on ED50suf and ED95suf.
| Dex (μg/kg) | ED50suf (μg/kg) | ED95suf (μg/kg) |
|---|---|---|
| 0 | .37 | .50 |
| 0.1 | .35 | .48 |
| 0.2 | .33 | .46 |
| 0.3 | .30 | .44 |
| 0.4 | .28 | .41 |
| 0.5 | .26 | .39 |
| 0.6 | .24 | .37 |
| 0.7 | .22 | .34 |
| 0.8 | .19 | .32 |
| 0.9 | .17 | .30 |
| 1.0 | .15 | .28 |
ED50: 50% effective dose. ED95: 95% effective dose. Suf: sufentanil. Dex: dexmedetomidine.
Discussion
In this study, we developed a response surface model to characterise the interaction between dexmedetomidine and sufentanil. The results suggested that the interaction was additive when the 2 drugs were co-administered during anaesthesia induction with propofol used as the main hypnotic drug.
In the response surface analysis to investigate the interaction between drugs, the maximum dosage of each drug was approximately the ED95. 7 However, little is known about the ED95 of dexmedetomidine or sufentanil for inhibiting the haemodynamic stress response during intubation. High doses of dexmedetomidine may lead to cardiovascular side effects, including bradycardia, hypotension or even cardiac arrest.17,18 The common dose of dexmedetomidine used in anaesthesia induction should be no more than 1.0 μg/kg.19,20 Based on clinical practice, .5 μg/kg sufentanil is generally sufficient to completely inhibit the stress response during anaesthesia induction. Therefore, the maximum doses of dexmedetomidine and sufentanil in our study were set to be 1.1 μg/kg and .5 μg/kg, respectively. Our results showed that only 8 patients experienced bradycardia or hypotension during anaesthesia induction, indicating that the doses of dexmedetomidine and sufentanil in this study were relatively safe.
Soulard et al reported that the ED50 of sufentanil for intubation after 3% sevoflurane induction was .32 μg/kg. 21 The estimated ED50 of sufentanil (.37 μg/kg) in this study was higher than that in a previous report. This discrepancy could be explained by the different efficiencies between sevoflurane and propofol in attenuating the noxious stress response. 22 Kwak et al reported that the ED50 of dexmedetomidine for successful laryngeal mask (LMA) insertion with propofol 2.0 mg kg−1 was .55 μg/kg. 23 In this study, a standard protocol of propofol TCI combined with sufentanil, dexmedetomidine and rocuronium was used for anaesthesia induction. The intensity of the stress response caused by laryngoscopy and intubation is much higher than that caused by LMA insertion. Therefore, it is reasonable that the ED50 of dexmedetomidine for LMA insertion is lower than that in this study (1.64 μg/kg). In this logit model, the patient data could be well characterised by a simple model (β3 = 0), indicating an additive interaction between dexmedetomidine and sufentanil. This result is consistent with previous studies that found the interactions between dexmedetomidine and other opioids, including fentanyl and morphine, to be additive.24,25
Our estimate of the ED50 of dexmedetomidine was 1.64 μg/kg, which was well beyond the normal recommended dosage. This finding indicates that dexmedetomidine alone may not be able to effectively inhibit the haemodynamic response to endotracheal intubation. Given that the mechanisms of cardiovascular reflex caused by laryngoscopy and endotracheal intubation remain unclear, there might be 2 possible explanations for the effect of dexmedetomidine. First, although dexmedetomidine exerts analgesic effects by binding α2 receptors in the spinal cord, 26 the afferent pathway of the cardiovascular reflex caused by mechanical touch on the laryngeal lumen contains glossopharyngeal and vagal nerves instead of spinal nerves.27,28 Therefore, it is difficult for dexmedetomidine to prevent nociceptive transmission in the afferent pathway. Second, although there is a remarkable correlation between the locus coeruleus (the main action site for dexmedetomidine) and the peripheral sympathetic system for the regulation of cardiovascular function, the parallelism is not absolute. 29 Elam et al reported that the response of the peripheral sympathetic system remained increased in rats that experienced prolonged noxious stimulation, but there was complete attenuation of the locus coeruleus within a few minutes. 30 Therefore, we speculated that the locus coeruleus might rarely be involved in the cardiovascular reflex pathway for endotracheal intubation. As a result, sympathetic stress caused by endotracheal intubation may overshadow the suppressive effect of dexmedetomidine and lead to a robust stress response in patients.
As a preliminary exploration of the response surface model for dexmedetomidine and sufentanil, this study has several limitations. First, due to the lack of TCI devices for dexmedetomidine and sufentanil, the plasma concentration of these drugs may be different among patients, which can increase the variability of measurement. Second, since we are unsure about the efficacy of dexmedetomidine in blunting the haemodynamic response, dexmedetomidine was not administered alone during anaesthesia induction. This makes the identification of the boundary of the response surface for dexmedetomidine difficult. Caution should be taken in predicting the effect of dexmedetomidine when used alone. Third, because of ethics issues, the doses of dexmedetomidine and sufentanil were set in clinically relevant ranges, which limits the extrapolation of our conclusion to areas beyond our dose ranges. Fourth, all participants in our study were American Society of Anesthesiologists status I patients. Thus, the suitability of our conclusion for high-risk patients is unknown. Fifth, all participants in our study were female. Although sex has little effect on the pharmacokinetics or pharmacodynamics of dexmedetomidine, most studies indicate that the analgesic efficacy of opioids is more potent in females than males.31,32 Therefore, the model needs to be investigated in male patients in the future. Finally, to guide drug-dosing practice more accurately, it is more advantageous to develop a triple-drug interaction model to estimate the combined potency of dexmedetomidine, sufentanil and other anaesthetics, such as porofol and volatile anaesthetics. In addition, the study design for a triple-drug response surface model needs further exploration.
Conclusion
Our response surface model provides a comprehensive description of the dose–response relationship between dexmedetomidine and sufentanil. When used propofol as the main hypnotic drug during anaesthesia induction, dexmedetomidine could effectively reduce the requirement of sufentanil in an additive manner. However, dexmedetomidine is not an effective drug to completely inhibit the haemodynamic response to endotracheal intubation when used alone in clinically relevant doses. Our results can optimise the combined usage of dexmedetomidine and sufentanil in general anaesthesia.
Acknowledgements
The authors would like to thank all staff members in the Department of anaesthesia and operating room of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology. The authors sincerely thank Ping Yi, PhD for his contributions to the trial design and his assistance with the operation of statistical software. The authors acknowledge Hao Yang, PhD who helped build the response model and helped review the study design and date analysis.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by National Natural Science Foundation of China (No. 81717191).
ORCID iD
References
- 1.Oczenski W, Krenn H, Dahaba AA, et al. Hemodynamic and catecholamine stress responses to insertion of the Combitube, laryngeal mask airway or tracheal intubation. Anesth Analg. 1999;88(6):1389-1394. [DOI] [PubMed] [Google Scholar]
- 2.Edwards ND, Alford AM, Dobson PMS, Peacock JE, Reilly CS. Myocardial ischaemia during tracheal intubation and extubation. British journal of anaesthesia. 1994;73(4):537-539. [DOI] [PubMed] [Google Scholar]
- 3.Karwacki Z, Witkowska M, Niewiadomski S, et al. Anaesthetic management for endovascular treatment of unruptured intracranial aneurysms. Anaesthesiol Intensive Ther. 2013;45(3):145-148. doi: 10.5603/AIT.2013.0030 [DOI] [PubMed] [Google Scholar]
- 4.Khan FA, Ullah H. Pharmacological agents for preventing morbidity associated with the haemodynamic response to tracheal intubation. Cochrane Database Syst Rev. 2013;7:CD004087. doi: 10.1002/14651858.CD004087.pub2 [DOI] [PubMed] [Google Scholar]
- 5.Kamali A, Taghizadeh M, Esfandiar M, Akhtari AS. A comparison of the effects of dexmedetomidine and propofol in controlling the hemodynamic responses after intubation: a double-blind, randomized, clinical trial study. Open Access Maced J Med Sci. 2018;6(11):2045-2050. doi: 10.3889/oamjms.2018.385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keshri RK, Prasad MK, Choudhary AK, Jheetay GS, Singh Y, Kapoor K. Comparative evaluation of different doses of intravenous dexmedetomidine on hemodynamic response during laryngoscopy and endotracheal intubation in geriatric patients undergoing spine surgeries: a prospective, double-blind study. Anesth Essays Res. 2018;12(4):897-902. doi: 10.4103/aer.AER_156_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Short TG, Ho TY, Minto CF, Schnider TW, Shafer SL. Efficient trial design for eliciting a pharmacokinetic-pharmacodynamic model-based response surface describing the interaction between two intravenous anesthetic drugs. Anesthesiology. 2002;96(2):400-408. [DOI] [PubMed] [Google Scholar]
- 8.Minto CF, Schnider TW, Short TG, Gregg KM, Gentilini A, Shafer SL. Response surface model for anesthetic drug interactions. Anesthesiology. 2000;92(6):1603-1616. [DOI] [PubMed] [Google Scholar]
- 9.Bi SS, Deng CH, Zhou TY, et al. Remifentanil-sevoflurane interaction models of circulatory response to laryngoscopy and circulatory depression. Br J Anaesth. 2013;110(5):729-740. doi: 10.1093/bja/aes504 [DOI] [PubMed] [Google Scholar]
- 10.Xu B, Zhou D, Ren L, Shulman S, Zhang X, Xiong M. Pharmacokinetic and pharmacodynamics of intravenous dexmedetomidine in morbidly obese patients undergoing laparoscopic surgery. J Anesth. 2017;31(6):813-820. doi: 10.1007/s00540-017-2399-y [DOI] [PubMed] [Google Scholar]
- 11.Schumacher PM, Dossche J, Mortier EP, Luginbuehl M, Bouillon TW, Struys MMRF. Response surface modeling of the interaction between propofol and sevoflurane. Anesthesiology. 2009;111(4):790-804. doi: 10.1097/ALN.0b013e3181b799ef [DOI] [PubMed] [Google Scholar]
- 12.Xue FS, Xu YC, Liu Y, et al. Different small-dose sufentanil blunting cardiovascular responses to laryngoscopy and intubation in children: a randomized, double-blind comparison. Br J anaesth. 2008;100(5):717-723. doi: 10.1093/bja/aen032 [DOI] [PubMed] [Google Scholar]
- 13.Albertin A, Casati A, Federica L, et al. The effect-site concentration of remifentanil blunting cardiovascular responses to tracheal intubation and skin incision during bispectral index-guided propofol anesthesia. Anesth Analg. 2005;101(1):125-130. doi: 10.1213/01.ANE.0000153012.35120.FE [DOI] [PubMed] [Google Scholar]
- 14.Manyam SC, Gupta DK, Johnson KB, et al. Opioid-volatile anesthetic synergy: a response surface model with remifentanil and sevoflurane as prototypes. Anesthesiology. 2006;105(2):267-278. [DOI] [PubMed] [Google Scholar]
- 15.Liou J-Y, Ting C-K, Mandell MS, et al. Predicting the best fit: a comparison of response surface models for midazolam and alfentanil sedation in procedures with varying stimulation. Anesth Analg. 2016;123(2):299-308. doi: 10.1213/ANE.0000000000001299 [DOI] [PubMed] [Google Scholar]
- 16.LaPierre CD, Johnson KB, Randall BR, White JL, Egan TD. An exploration of remifentanil-propofol combinations that lead to a loss of response to esophageal instrumentation, a loss of responsiveness, and/or onset of intolerable ventilatory depression. Anesth Analg. 2011;113(3):490-499. doi: 10.1213/ANE.0b013e318210fc45 [DOI] [PubMed] [Google Scholar]
- 17.Davy A, Fessler J, Fischler M, Le Guen M. Dexmedetomidine and general anesthesia: a narrative literature review of its major indications for use in adults undergoing non-cardiac surgery. Minerva Anestesiol. 2017;83(12):1294-1308. doi: 10.23736/S0375-9393.17.12040-7 [DOI] [PubMed] [Google Scholar]
- 18.Jin S, Zhou X. Influence of dexmedetomidine on cardiac complications in non-cardiac surgery: a meta-analysis of randomized trials. Int J Clin Pharm. 2017;39(4):629-640. doi: 10.1007/s11096-017-0493-8 [DOI] [PubMed] [Google Scholar]
- 19.Liu H, Zhou C, Ji J. Effects of using different dose of dexmedetomidine during tracheal extubation for patients with parotidectomy after general anesthesia. Shang Hai Kou Qiang Yi Xue. 2016;25(3):368-372. [PubMed] [Google Scholar]
- 20.Sharma N, Mehta N. Therapeutic efficacy of two different doses of dexmedetomidine on the hemodynamic response to intubation, the intubating conditions, and the effect on the induction dose of propofol: a randomized, double-blind, placebo-controlled study. Anesth Essays Res. 2018;12(2):566-571. doi: 10.4103/aer.AER_45_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soulard A, Babre F, Bordes M, Meymat Y, Sztark F, Cros AM. Optimal dose of sufentanil in children for intubation after sevoflurane induction without neuromuscular block. Br J Anaesth. 2009;102(5):680-685. doi: 10.1093/bja/aep044 [DOI] [PubMed] [Google Scholar]
- 22.Lin C-K, Feng Y-T, Hwang S-L, Lin C-L, Lee K-T, Cheng K-I. A comparison of propofol target controlled infusion-based and sevoflurane-based anesthesia in adults undergoing elective anterior cervical discectomy and fusion. Kaohsiung J Med Sci. 2015;31(3):150-155. doi: 10.1016/j.kjms.2014.12.002 [DOI] [PubMed] [Google Scholar]
- 23.Kwak HJ, Min SK, Yoo JY, Park KH, Kim JY. The median effective dose of dexmedetomidine for laryngeal mask airway insertion with propofol 2.0 mg/kg. Acta Anaesthesiol Scand. 2014;58(7):815-819. doi: 10.1111/aas.12338 [DOI] [PubMed] [Google Scholar]
- 24.Ossipov MH, Harris S, Lloyd P, Messineo E, Lin B-S, Bagley J. Antinociceptive interaction between opioids and medetomidine: systemic additivity and spinal synergy. Anesthesiology. 1990;73:1227-1235. [DOI] [PubMed] [Google Scholar]
- 25.Tham SM, Angus JA, Tudor EM, Wright CE. Synergistic and additive interactions of the cannabinoid agonist CP55,940 with mu opioid receptor and alpha2-adrenoceptor agonists in acute pain models in mice. Br J Pharmacol. 2005;144:875-884. doi: 10.1038/sj.bjp.0706045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weerink MAS, Struys MMRF, Hannivoort LN, Barends CRM, Absalom AR, Colin P. Clinical Pharmacokinetics and Pharmacodynamics of Dexmedetomidine. Clin Pharmacokinet. 2017;56(8):893-913. doi: 10.1007/s40262-017-0507-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mutoh T, Tsubone H, Nishimura R, Sasaki N. Cardiovascular reflex mechanisms by topical instillation of capsaicin and distilled water into the larynx in anesthetized dogs. J Vet Med Sci. 1997;59(9):801-806. [DOI] [PubMed] [Google Scholar]
- 28.Nishino T. Physiological and pathophysiological implications of upper airway reflexes in humans. Jpn J Physiol. 2000;50(1):3-14. [DOI] [PubMed] [Google Scholar]
- 29.Wood CS, Valentino RJ, Wood SK. Individual differences in the locus coeruleus-norepinephrine system: relevance to stress-induced cardiovascular vulnerability. Physiol Behav. 2017;172:40-48. doi: 10.1016/j.physbeh.2016.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elam M, Svensson TH, Thorén P. Locus coeruleus neurons and sympathetic nerves: activation by cutaneous sensory afferents. Brain Research. 1986;366(1-2):254-261. [DOI] [PubMed] [Google Scholar]
- 31.Colin PJ, Hannivoort LN, Eleveld DJ, et al. Dexmedetomidine pharmacodynamics in healthy volunteers: 2. Haemodynamic profile. Br J Anaesth. 2017;119(2):211-220. doi: 10.1093/bja/aex086 [DOI] [PubMed] [Google Scholar]
- 32.Dahan A, Kest B, Waxman AR, Sarton E. Sex-specific responses to opiates: animal and human studies. Anesth Analg. 2008;107(1):83-95. [DOI] [PubMed] [Google Scholar]