Abstract
Introduction
The role of definitive radiotherapy in advanced esophageal squamous cell carcinoma (ESCC), especially in the metastatic setting, remains unclear. Therefore, we aimed to investigate the efficacy of chemoradiotherapy (CRT) versus chemotherapy (CT) alone in these selected patients.
Methods
We retrospectively evaluated 194 newly diagnosed advanced ESCC who underwent definitive CRT or CT alone, including 97 patients with locally advanced and 97 patients with distant metastatic disease. Cumulative overall survival (OS) and progression-free survival (PFS) were evaluated with a log-rank test. Propensity score matching was used to simulate random allocation. In addition, we performed subgroup analysis in the locally advanced and metastatic disease.
Results
After matching, 63 well-paired patients were selected. The adjusted median OS (12.5 vs. 7.6 months, p = 0.002) and PFS (9.0 vs. 4.8 months, p = 0.0025) in the CRT group were superior to that in the CT-alone group. Further subgroup analysis revealed that CRT conferred survival benefits to both locally advanced and metastatic cohorts. For patients with distant metastasis, median OS (12.9 vs. 9.3 months, p = 0.029) and PFS (9.9 vs. 4.0 months, p =0.0032) in the CRT group were superior to that in the CT-alone group. In a multivariate Cox regression analysis of the entire cohort, additional definitive radiotherapy was independently associated with better OS (p = 0.041) and PFS (p = 0.007).
Conclusions
In both locally advanced and metastatic ESCC, additional definitive-dose radiotherapy was associated with improved clinical outcomes. Therefore, more consideration should be given to its application in the metastatic setting.
Keywords: esophageal squamous cell carcinoma, advanced, metastatic, chemoradiotherapy, definitive radiotherapy, survival
Introduction
Esophageal cancer (EC) is the seventh most common cancer worldwide and the sixth leading cause of cancer-related death, with approximately 572,000 patients diagnosed in 2018 (1). The prognosis of metastatic EC is inferior, with a 5-year survival rate lower than 5% (2). Definitive radiotherapy (RT) with a dose greater than or equal to 50.4 Gy to the primary tumor is the mainstay of treatment and provides effective symptomatic relief for locally advanced EC (3–5). Since the RTOG 85-01 study, radical chemoradiotherapy (CRT) with a radiation dose of 50 Gy has been established as a curative treatment paradigm for locally advanced patients without evidence of distant metastasis (6). Subsequent clinical trials have further confirmed the clinical efficacy of this combination regimen (7, 8), which is now the standard first-line regimen for patients with locally advanced EC (9, 10). However, the current guidelines generally do not recommend aggressive radiotherapy for the primary tumor in patients with metastatic EC. The latest Chinese Society of Clinical Oncology guideline recommended only system therapy for metastatic EC (11). In the Pan-Asian adapted ESMO Clinical Practice Guidelines, RT was recommended only for palliative care to relieve patients’ dysphagia with metastatic EC (12). Systemic chemotherapy remains the cornerstone treatment for metastatic EC patients, with a median survival time of only 8–12 months (13–15). However, whether combined chemotherapy and aggressive radiotherapy can improve the survival of metastatic esophageal squamous cell carcinoma (ESCC) remains unclear. Therefore, in the present study, we aimed to investigate the efficacy and safety of chemotherapy-based definitive radiotherapy (≥50.4 Gy) in prolonging the survival of patients with advanced ESCC, particularly those with organ metastases.
Materials and Methods
Study Design and Patients
We retrospectively reviewed patients with newly diagnosed advanced ESCC who received CRT or CT alone at the Guangxi Medical University Cancer Hospital between June 2010 and May 2020. The institutional ethics committee approved this study, and informed consent was waived by the board. The eligibility criteria were as follows: (1) ESCC confirmed by histology; (2) clinically confirmed advanced disease (stage IVa or IVb) according to the 8th edition AJCC staging system (16); (3) Eastern Cooperative Oncology Group (ECOG) score 0–2; (4) no history of previous thoracic radiotherapy; (5) received definitive-dose (≥50.4Gy) radiotherapy for primary tumor for the CRT cohort; and (6) received no concurrent targeted therapy or immunotherapy.
Chemotherapy and Radiotherapy Treatment
For all patients, two- or three-drug cisplatin-based chemotherapy was administrated at 3-week intervals for up to 6 cycles as first-line therapy. For patients undergoing CRT, definitive-dose radiotherapy was administrated synchronously with 2 to 3 cycles of cisplatin-based chemotherapy. Radiotherapy was performed with intensity-modulated radiotherapy using a 6-MV linear accelerator (Elekta Synergy, Stockholm, Sweden) at five fractions per week. The gross tumor volume (GTV) and metastatic lymph nodes (GTVnd) were delineated with visible lesions based on contrast-enhanced simulation CT, PET, and endoscopic evaluation results. The clinical target volume (CTV) was defined as a 0.5-cm horizontal expansion from GTV/GTVnd, a 3–5-cm craniocaudal margin from GTV, and a 0.5-cm craniocaudal margin from GTVnd. The planning target volume (PTV) was determined by adding a 0.5-cm margin to the CTV. A median total dose of 60 Gy (range, 56–66 Gy) with a median per dose of 2.0 Gy (range, 1.8–2.2 Gy) in a median fraction of 30 (range, 25–33) was prescribed to the PGTV for five consecutive days in a given week. The dose constraints for organs at risk (OARs) were as follows: (1) lung: the whole lung V20 <28%, V30 <20%, and Dmean <15–17 Gy; (2) spinal cord: Dmax <45 Gy; and (3) heart: V40 <30% and Dmean <30 Gy.
Follow-Up and Statistical Analysis
For posttreatment follow-up, enhanced CT and upper gastrointestinal endoscopy were reevaluated 1 month after treatment and every 3 months after that. Progression-free survival (PFS) was defined as the period between the date of initial treatment until disease progression or recurrence or death. Overall survival (OS) was defined as the period from initial therapy to censor or death. OS and PFS rates were evaluated using the Kaplan–Meier method with the log-rank test. Continuous variables were compared with the Student’s t-test, while categorical variables were compared with Fisher’s exact or Pearson’s χ2 test. We performed multivariate Cox regression analysis to identify clinical variables independently associated with PFS and OS, and factors with p < 0.05 in the univariate Cox regression analysis were included. Statistical analysis was undertaken using R version 4.0.2 software, and p-values <0.05 were considered statistically significant.
To minimize potential selection bias and confounders, propensity score matching (PSM) was used to control for differences in baseline characteristics. Using a caliper of width equal to 0.2 without replacement, patients in the entire cohort were matched at a 1:1 ratio to simulate random allocation. Covariates entered into the propensity model included body mass index, ECOG score, TNM stage, number of metastatic sites, absolute neutrophil count, and albumin level. All baseline covariates were balanced in the locally advanced disease and metastatic disease subgroups. Therefore, PSM was not performed in the subgroup analysis.
Results
Patient Characteristics
A total of 194 patients with advanced ESCC were deemed eligible and assessed. Among them, 97 patients (50%) were locally advanced, and 97 patients (50%) had distant metastasis. The majority of patients with distant metastasis had a low systemic tumor burden. Seventy-seven (79.4%) patients had only one metastatic site (40.2%, 16.9%, 14.3%, 14.3%, and 14.3% of these patients presented with metastasis in the non-regional lymph node, lung, liver, bone, and others, respectively), and 14 (14.4%) patients had two metastatic sites. Merely 6 (6.2%) patients had at least three or more metastatic sites. A total of 101 patients (52.1%) received CRT, and 93 patients (47.9%) received CT alone. The median cycles of chemotherapy for the entire cohort were 3 (1–6 cycles). Before propensity score matching, patients in the CT-alone group had significantly worse baseline characteristics compared to those in the CRT group, with a lower body mass index (mean 20.3 vs. 21.5 kg/m2, p = 0.01), poorer physical performance (ECOG score 2: 8.6% vs. 0%, p = 0.011), greater tumor burden (stage IVb: 65.6% vs. 35.6%, p = 0.000, and distant metastatic sites ≥3: 6.4% vs. 0%, p = 0.001), lower absolute neutrophil count (mean 5.9 vs. 5.1 × 109/l, p = 0.022), and lower albumin level (mean 35.5 vs. 36.7 g/l, p = 0.047). After matching, 63 well-paired patients were selected. There were no significant differences between the CRT group and the CT-alone group in baseline characteristics after PSM, as shown in Table 1.
Table 1.
Patient characteristics before and after PSM in the CRT and CT alone groups.
| Before PSM | After PSM | ||||||
|---|---|---|---|---|---|---|---|
| Factor | Level | CT (n = 93) | CRT (n = 101) | p | CT (n = 63) | CRT (n = 63) | p |
| Gender | Male | 86 | 91 | 0.741 | 58 | 57 | 1.000 |
| Female | 7 | 10 | 5 | 6 | |||
| Age (yrs, mean ± SD) | 57.4 ( ± 0.9) | 56.1 ( ± 0.9) | 0.298 | 56.3 ( ± 1.0) | 55.0 ( ± 1.1) | 0.149 | |
| BMI (m2/kg, mean ± SD) | 20.3 ( ± 0.3) | 21.5 ( ± 0.3) | 0.010 | 20.8 ( ± 0.4) | 21.0 ( ± 0.3) | 0.687 | |
| ECOG | 0 | 8 | 10 | 0.011 | 5 | 3 | 0.715 |
| 1 | 77 | 91 | 58 | 60 | |||
| 2 | 8 | 0 | |||||
| Smoking | No | 25 | 38 | 0.149 | 17 | 23 | 0.339 |
| Yes | 68 | 63 | 46 | 40 | |||
| Drinking | No | 25 | 32 | 0.565 | 17 | 22 | 0.441 |
| Yes | 68 | 69 | 46 | 41 | |||
| Family history | No | 89 | 92 | 0.320 | 61 | 59 | 0.676 |
| Yes | 4 | 9 | 2 | 4 | |||
| T stage | 2 | 6 | 4 | 1.146 | 3 | 1 | 0.457 |
| 3 | 26 | 18 | 17 | 14 | |||
| 4 | 61 | 79 | 43 | 48 | |||
| N stage | 1 | 31 | 36 | 1.140 | 21 | 24 | 0.310 |
| 2 | 40 | 52 | 30 | 33 | |||
| 3 | 22 | 13 | 12 | 6 | |||
| Number of metastatic sites | 0 | 32 | 65 | 0.001 | 30 | 35 | 0.669 |
| 1 | 48 | 29 | 28 | 24 | |||
| 2 | 7 | 7 | 5 | 4 | |||
| ≥3 | 6 | 0 | 0 | 0 | |||
| TNM stage | IVa | 32 | 65 | 0.000 | 30 | 35 | 1.000 |
| IVb | 61 | 36 | 33 | 28 | |||
| Tumor location | Up | 20 | 29 | 0.114 | 11 | 16 | 0.271 |
| Middle | 46 | 57 | 34 | 36 | |||
| Down | 27 | 14 | 16 | 11 | |||
| Multiple lesions | 2 | 1 | 2 | 0 | |||
| HBG (g/L, mean ± SD) | 123.1 ( ± 1.6) | 123.3 ( ± 1.8) | 0.920 | 122.1 ( ± 1.9) | 124.3 ( ± 2.2) | 0.450 | |
| PLT (109/L, mean ± SD) | 318.9 ( ± 11.1) | 302.8 ( ± 8.3) | 0.248 | 300.2 ( ± 12.4) | 318.8 ( ± 12.2) | 0.288 | |
| NEU (109/L, mean ± SD) | 5.9 ( ± 0.3) | 5.1 ( ± 0.2) | 0.022 | 5.4 ( ± 0.3) | 5.2 ( ± 0.3) | 0.691 | |
| LYMPH (109/L, mean ± SD) | 1.9 ( ± 0.1) | 2.0 ( ± 0.2) | 0.573 | 2.0 ( ± 0.2) | 1.8 ( ± 0.1) | 0.338 | |
| ALB (g/L, mean ± SD) | 35.5 ( ± 0.5) | 36.7 ( ± 0.4) | 0.047 | 36.8 ( ± 0.6) | 37.7 ( ± 0.6) | 0.248 | |
| AST (U/L, mean ± SD) | 17.1 ( ± 1.1) | 17.2 ( ± 1.0) | 0.973 | 18.1 ( ± 1.7) | 17.2 ( ± 1.4) | 0.670 | |
| ALT (U/L, mean ± SD) | 25.2 ( ± 1.6) | 22.7 ( ± 0.8) | 0.159 | 26.4 ( ± 2.4) | 22.3 ( ± 1.0) | 0.118 | |
| Urea (mmol/L, mean ± SD) | 4.8 ( ± 0.2) | 4.7 ( ± 0.0) | 0.786 | 4.7 ( ± 0.2) | 4.5 ( ± 0.2) | 0.517 | |
| Creatinine (μmol/L, mean ± SD) | 78.3 ( ± 2.5) | 76.9 ( ± 1.4) | 0.630 | 78.1 ( ± 3.1) | 76.1 ( ± 1.8) | 0.589 | |
| Chemotherapy cycle | ≤3 | 62 | 57 | 0.144 | 43 | 35 | 0.142 |
| >3 | 31 | 44 | 20 | 28 | |||
BMI, body mass index; ECOG score, Eastern Cooperative Oncology Group; WBC, white blood cell counts; HGB, hemoglobin level; PLT, blood platelet count; NEU, absolute neutrophil count; LYMPH, absolute lymphocyte count; ALB, albumin level; ALT, alanine aminotransferase level; AST, aspartate aminotransferase level.
Overall Survival and Progression-Free Survival
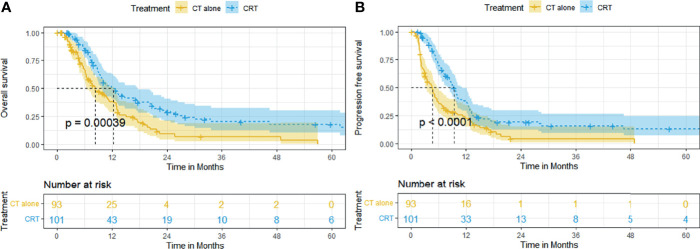
The median follow-up time was 32.2 months. At the end date of follow-up, 136 (70.1%) patients died and 58 (29.9%) patients were right-censored. Before matching, the median OS and rates of OS at 6, 12, 24, and 60 months were superior in the CRT group to that in the CT group (12.2 months [95% CI, 9.0–15.3], 84.1%, 50.8%, 29.0%, 17.9% vs. 8.2 months [95% CI, 5.4–11.1], 66.0%, 38.3%, 9.1%, 0%, p = 0.00039, Figure 1A). The median PFS and rates of PFS at 6, 12, 24, and 60 months were also superior in the CRT group to that in the CT group (9.4 months [95% CI, 8.0–10.8], 69.5%, 38.1%, 19.2%, 13.1% vs. 4.7 months [95% CI, 3.5–5.9], 36.6%, 22.4%, 4.1%, 0%, p < 0.0001, Figure 1B).
Figure 1.
Kaplan–Meier curves of survival in patients with advanced ESCC treated with CRT and CT before PSM: (A) overall survival before PSM; (B) progression-free survival before PSM.
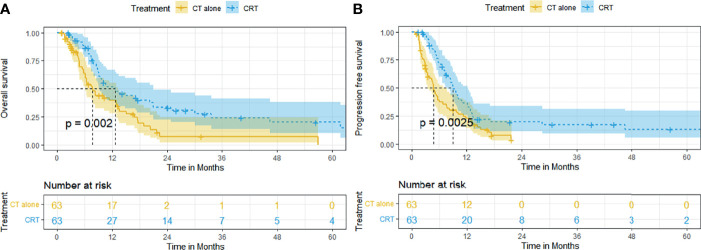
Adjusting for all baseline factors also demonstrated significant differences between the CRT group and CT group in PFS and OS. The median OS and rates of OS at 6, 12, 24, and 60 months remained superior in the CRT group to that in the CT group (12.5 months [95% CI, 7.1–18.0], 85.9%, 47.4%, 23.4%, 17.3% vs. 7.6 months [95% CI, 5.4–9.8], 63.6%, 39.4%, 7.3%, 0%, p = 0.002, Figure 2A). The median PFS and rates of PFS at 6, 12, 24, and 60 months also remained superior in the CRT group to that in the CT group (9.0 months [95% CI, 7.6–10.5], 70.9%, 36.5%, 19.7%, 12.9% vs. 4.8 months [95% CI,4.0–5.6], 39.1%, 22.7%, 3.8%, 3.8%, p = 0.0025, Figure 2B).
Figure 2.
Kaplan–Meier curves of survival in patients with advanced ESCC treated with CRT and CT after PSM: (A) overall survival after PSM; (B) progression-free survival after PSM.
Subgroup Analysis of LocallyAdvanced Disease
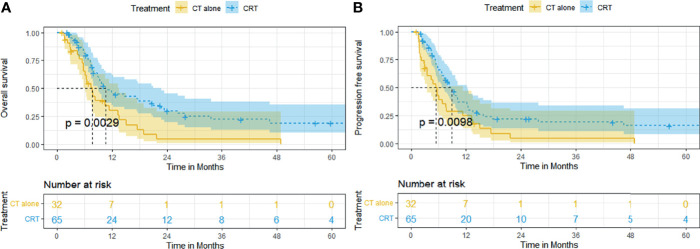
In patients with locally advanced ESCC, the survival outcome of the CRT group was significantly better than that of the CT group. The median OS and rates of OS at 6, 12, 24, and 60 months were superior in the CRT group to that in the CT group (10.5 months [95% CI, 7.3–13.7], 79.8%, 46.6%, 29.8%, 18.9% vs. 7.6 months [95% CI, 5.9–9.3], 65.4%, 30.3%, 4.3%, 0%, p = 0.0029, Figure 3A). The median PFS and rates of PFS at 6, 12, 24, and 60 months were also superior in the CRT group to that in the CT group (8.9 months [95% CI, 6.9–10.9], 65.7%, 37.3%, 22.0%, 16.0% vs. 5.4 months [95% CI, 3.8–7.0], 42.8%, 25.0%, 4.5, 0%, p = 0.0098, Figure 3B).
Figure 3.
Kaplan–Meier curves of survival in patients with locally advanced ESCC treated with CRT and CT: (A) overall survival; (B) progression-free survival.
Subgroup Analysis of Metastatic Disease
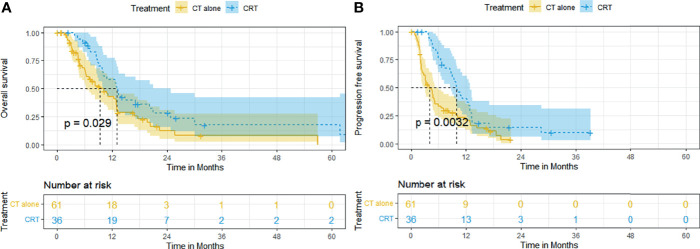
CRT also conferred survival benefit to ESCC patients with distant metastasis. The median OS and rates of OS at 6, 12, 24, and 60 months were superior in the CRT group to that in the CT group (12.9 months [95% CI, 10.2–15.7], 91.4%, 58.0%, 28.1%, 17.6% vs. 9.3 months [95% CI, 5.7–13.0], 66.6%, 42.8%, 12.2%, 8.2%, p = 0.029, Figure 4A). The median PFS and rates of PFS at 6, 12, 24, and 60 months were also superior in the CRT group to that in the CT group (9.9 months [95% CI, 7.9–11.9], 76.5%, 39.9%, 14.7%, 9.8% vs. 4.0 months [95% CI, 2.4–5.7], 33.4%, 20.8%, 3.7%, 3.7%, p = 0.0032, Figure 4B).
Figure 4.
Kaplan–Meier curves of survival in patients with metastatic ESCC treated with CRT and CT: (A) overall survival; (B) progression-free survival.
Survival Analyses on Patients With Locally Advanced and Metastatic Disease
In the entire cohort, both OS (p = 0.75, Supplementary Figure S1A) and PFS (p = 0.1, Supplementary Figure S1B) did not differ significantly between patients with locally advanced disease and metastatic disease. Similarly, subgroup analysis based on treatment also showed no significant difference in OS (CRT subgroup: p = 0.97, Supplementary Figure S2A, CT-alone subgroup: p = 0.28, Supplementary Figure S2B) and PFS (CRT subgroup: p = 0.97, Supplementary Figure S3A, CT-alone subgroup: p = 0.5, Supplementary Figure S3B) between patients with locally advanced disease and metastatic disease.
Univariate and Multivariate Analysis for Prognostic Factors
Univariable and multivariable Cox analyses for OS and PFS of the entire cohort before PSM are shown in Supplementary Table S1. On multivariable analysis of the whole cohort before matching, additional RT, albumin levels, absolute neutrophil count, and chemotherapy cycle were independent prognostic factors for both OS (p = 0.041, p = 0.000, p = 0.001, and p = 0.002, respectively) and PFS (p = 0.007, p = 0.02, p = 0.02, and p = 0.000, respectively). At the same time, the N stage and number of metastatic independently predicted only PFS (p = 0.015 and p = 0.007, respectively).
Treatment Toxicities
Early toxicities that occurred in CRT and CT-alone cohorts were assessed according to the National Cancer Institute Common Toxicity Criteria for Adverse Events, version 4.0 (CTCAE 4.0) (17). The most common adverse events (grades 1–2) of the entire cohort were dysphagia, fatigue, anorexia, nausea, vomit, and hematological toxicities.
Fourteen (22.2%) patients had grade ≥3 radiation esophagitis in the matched CRT group. One (1.6%) patient developed grade 3 radiation pneumonitis 4 months after RT. One (1.6%) patient developed grade 5 upper GI bleeding, and one (1.6%) patient developed grade 3 upper esophageal fistula, both at 3 months after RT. No patient experienced grade ≥3 radiation dermatitis. Twenty-three (36.5%) patients had grade ≥3 leukopenia, 21 (33.3%) had grade ≥3 neutropenia, 8 (12.7%) had grade ≥3 anemia, and 4 (6.4%) had grade ≥3 thrombocytopenia.
In the matched CT-alone group, esophageal fistula occurred in 1 (1.6%) patient after one cycle of CT. Two patients (3.2%) developed grade 3 and 5 upper gastrointestinal bleeding after 2 and 3 cycles of CT, respectively. Four (6.3%) patients had grade ≥3 leukopenia, 6 (9.5%) had grade ≥3 neutropenia, 6 (9.5%) had grade ≥3 anemia, and no patient had grade ≥3 thrombocytopenia.
Patients receiving CRT had a significantly higher incidence of grade ≥3 leukopenia (p = 0.000), neutropenia (p = 0.000), and thrombocytopenia (p = 0.006).
Discussion
The current study showed that combined definitive dose RT (≥50.4) to the primary tumor with chemotherapy resulted in better OS and PFS than chemotherapy alone in advanced ESCC, even in the presence of metastatic disease, with manageable toxicities. In terms of metastatic EC, extended survival after definitive CRT has been reported by several previous studies. A prospective randomized phase 2 study demonstrated that the CRT was associated with significantly improved median PFS (9.3 vs. 4.7 months, p = 0.021) and median OS (18.3 vs. 10.2 months, p = 0.001) than CT alone (18). Moreno et al. (19) also suggested that additional RT could derive better survival compared to CT alone with extended 2- and 5-year OS of 6.4% and 2.7%, respectively (p <.001). In a large cohort of 12,683 patients with metastatic EC, Guttmann et al. (13) reported that definitive-dose (≥50.4 Gy) CRT was associated with superior survival compared to CT alone (median OS 8.3 vs. 11.3 months). As shown in Table 2, the clinical survival outcomes of the metastatic population in this study were highly consistent with those of previous studies (13, 18–21).
Table 2.
Definitive radiotherapy combined with chemotherapy versus chemotherapy alone for metastatic esophageal cancer.
| Authors | Study design | Number of cases | Chemotherapy cycles | RT prescription, Gy | OS | Median PFS (m) | Median OS (m) | ||
|---|---|---|---|---|---|---|---|---|---|
| 1 y (%) | 2 y (%) | ||||||||
| Li et al. (18) | Prospective | CT | 30 | Mean 3.6 | NA | 46.6 | 26.7 | 4.7 | 10.2 |
| CRT | 30 | Mean 3.8 | Median 54.7 (range:50–61) | 73.3 | 43.3 | 9.3 | 18.3 | ||
| Guttmann et al. (13) | Retrospective | CT | 7229 | NA | NA | 34 | 12 | NA | 8.4 |
| CRT | 2409 | NA | >50.4 | 47 | 19 | NA | 11.2 | ||
| Moreno et al. (19) | Retrospective | CT | NA | NA | NA | NA | 12.4 | NA | NA |
| CRT | NA | NA | 40–60 | NA | 18.8 | NA | NA | ||
| Lyu et al. (20) | Retrospective | CT | 86 | 31.4%>2 | NA | 43 | 14 | 6 | 11 |
| CRT | 55 | 36.4%>2 | Median 56.4 (range: 50–66) | 58 | 25.5 | 8 | 14 | ||
| Xu et al. (21) | Retrospective | Non-RT | 327 | NA | NA | NA | NA | NA | 6 |
| RT | 327 | NA | NA | NA | NA | NA | 10 | ||
| Present study | Retrospective | CT | 61 | Median 3 | NA | 42.8 | 12.2 | 4.0 | 9.3 |
| CRT | 36 | Median 3 | Median 60 (range: 50–66) | 58.0 | 28.1 | 9.9 | 12.9 | ||
NA, not applicable.
In the current study, most (93.8%) patients with metastatic disease had only one or two metastatic sites. We found no statistical difference in survival results between the locally advanced disease and metastatic disease. These results highlight that for advanced ESCC patients with low systemic tumor load, survival is most threatened by the failure of local control of the primary tumor. On the one hand, additional RT for primary tumor can effectively shrink the primary tumor and reduce dysphagia resulting from esophageal stricture. By increasing oral nutritional intake, RT may improve response rates, performance status, and long-term survival (22, 23). In the current study, multivariate analysis revealed that albumin level (p = 0.000) before treatment was an independent prognostic factor for OS, further illustrating the importance of the nutritional state for patients with advanced EC. On the other hand, aggressive RT for primary tumor can reduce life-threatening events, including airway stenosis either by external compression or by direct tumor growth into the airways, fistula, perforation, and massive bleeding. It is reported that external beam RT could provide significantly more effective relief of pain and tumor-related complications for metastatic EC compared to esophageal stent placement (5).
Based on modern radiotherapy techniques, definitive RT (≥50.4) to the primary tumor may confer more significant survival benefits than palliative RT (≤50.4 Gy) in patients with advanced EC. In the palliative setting, low-dose radiotherapy of less than 50.4 Gy is commonly used to relieve symptoms such as dysphagia, pain, and bleeding (5). However, the toxicity resulting from low-dose radiotherapy with chemotherapy may overweight the clinical benefit it confers. In a phase 3 randomized controlled trial, concurrent palliative RT (20 Gy in 5 fractions or 30 Gy in 10 fractions) did not derive additional benefit on survival for advanced EC patients with self-expanding metal stent placement (median OS: 19.7 weeks with usual care vs. 18.9 weeks with EBRT, p = 0.07) (24). Another multicenter randomized controlled trial (TROG 03.01) also indicated that palliative CRT (30–35 Gy in 10–15 fractions) failed to significantly relieve dysphagia and prolong survival (median OS: 6.9 vs. 6.7 months, p = 0.88) compared to RT alone, with increased toxicity (grade 3–4 acute toxicity: 36% vs. 16%, p = 0.0017) (25). Guttmann et al. (13) reported that compared to CT alone, definitive-dose (≥50.4 Gy) CRT was associated with superior survival (median OS 11.2 vs. 8.4 months, p ≤ 0.001), while palliative dose (<50.4 Gy) CRT was associated with slightly inferior outcomes (median OS: 7.6 vs. 8.4 months, p = 0.004).
In the current study, patients in the definitive CRT group received a high radiation dose of 56–66 Gy, with most patients receiving radiation dose ≥60 Gy (97 in 101, 96%). The precise dose of definitive RT remains controversial. The landmark RTOG94-05 trial (26) failed to demonstrate the superiority of high-dose (64.8 Gy) over conventional-dose (50.4) concurrent CRT, providing a theoretical basis for the standard RT paradigm for EC in Europe and America (10). According to this study, high-dose RT was not able to increase survival time (median OS: 13.0 vs. 18.1 months, p > 0.05) and regional control (56% vs. 52%, p > 0.05), but rather it seemed to increase the toxicity and higher treatment-related mortality rate. Notably, patients with squamous cell carcinoma account for the vast majority of the participants in this trial. Differently, radiation doses above 60 Gy are more frequently adopted in Asia. Several studies proposed that high-dose concurrent CRT of ≥60Gy could improve clinical outcomes compared with standard dose (50–54 Gy) in EC (27, 28), especially ESCC. A pooled analysis reported that in cisplatin-based definitive concurrent CRT, high-dose RT (≥60 Gy) was associated with significantly higher local regional recurrent rates (22% vs. 30%, p = 0.01) and distant failure rates (13% vs. 25%, p < 0.000) compared with conventional-dose RT (50–54 Gy) in ESCC patients (27). However, according to the ARTDECO study, an increase in RT dose to 61.6 Gy did not result in better local control over 50.4 Gy for both adenocarcinoma and squamous cell carcinoma (29). The optimal radiation dose of definitive CRT for EC merits further investigation, especially in a metastatic setting.
Safety findings in the current study were consistent with the known safety profile of CRT and CT alone (30–32). Significantly higher incidences of grade ≥3 hematological toxicities were observed in patients treated with CRT. What is more, additional definitive RT has led to severe radiation-related toxicities such as radiation esophagitis in certain patients, but with an acceptable incidence rate (14/63, 22.3%). Advancement of modern RT techniques, such as intensity-modulated RT (IMRT), volume modulated arc therapy (VMAT), and image-guided RT (IGRT), has improved the safety of definitive RT with precise radiation delivery while reducing the dose to organ at risk. Therefore, standard CRT may be a better option in well-selected metastatic ESCC patients who are in good general condition and had low burden of distant metastases. It should be considered after patients are fully informed of the risk benefits.
In the rapid development of immunotherapeutic strategies, local radiotherapy may play a more significant role in metastatic EC. Anti-programmed death 1 (PD-1)/programmed death ligand-1 (PD-L1) therapies are currently the research hotspot and have demonstrated durable antitumor activity in patients with advanced EC (32–34). According to the recently published ESCORT-1st randomized clinical trial, camrelizumab combined with chemotherapy significantly improved OS (15.3 vs. 12.0 months, p = 0.001) and PFS (6.9 vs. 5.6 months, p <; 0.001) compared with chemotherapy alone as first-line treatment in patients with advanced ESCC (14). As previously demonstrated in various cancers (such as non-small cell lung cancer (NSCLC) and metastatic melanoma), the combination of radiotherapy and immune checkpoint inhibitors could promote systemic antitumor immunity and abscopal effect (35, 36). This novel approach also represents an effective therapeutic option in advanced EC, and pertinent clinical trials are currently ongoing (37). A phase 3 study (KEYNOTE-975) of definitive CRT plus pembrolizumab in advanced EC is now in the recruiting phase (NCT04210115). The dual primary endpoints are OS and event-free survival, which is highly anticipated (38).
The present study had several limitations. Firstly, propensity score matching was used to reduce selection bias in this study. However, this led to the selection of patients and thereby decreased the sample size. Secondly, due to the retrospective nature of this study, data on quality of life were not available to us. Thirdly, we did not consider the changes in objective factors during the long-term period, such as increased applications of PET-CT, radiotherapy techniques, and chemotherapy regimens.
In conclusion, additional definitive-dose radiotherapy was associated with improved clinical outcomes in locally advanced and metastatic ESCC. Therefore, more consideration should be given to its application in the metastatic setting.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by the Institutional Ethics Committee of Guangxi Medical University Cancer Hospital. The ethics committee waived the requirement of written informed consent for participation.
Author Contributions
Data curation: L-QL, Y-DW, and W-DZ, W-WM. Formal analysis: L-QL and Q-GF. Writing—original draft: L-QL and Q-GF. Writing—review and editing: T-SS. Funding acquisition: T-SS. All authors contributed to the article and approved the submitted version.
Funding
This research was supported in part by the Guangxi Natural Science Foundation (CN) (2020GXNSFAA297171), China International Medical Foundation-Tumor Precise Radiotherapy Spark Program (2019-N-11-01), Guangxi Medical University Training Program for Distinguished Young Scholars, and Guangxi BaGui Scholars’ Special Fund.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.824206/full#supplementary-material
References
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin (2018) 68(6):394–424. doi: 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2. Tanaka T, Fujita H, Matono S, Nagano T, Nishimura K, Murata K, et al. Outcomes of Multimodality Therapy for Stage IVB Esophageal Cancer With Distant Organ Metastasis (M1-Org). Dis Esophagus (2010) 23(8):646–51. doi: 10.1111/j.1442-2050.2010.01069.x [DOI] [PubMed] [Google Scholar]
- 3. Jeene PM, Vermeulen BD, Rozema T, Braam PM, Lips I, Muller K, et al. Short-Course External Beam Radiotherapy Versus Brachytherapy for Palliation of Dysphagia in Esophageal Cancer: A Matched Comparison of Two Prospective Trials. J Thorac Oncol (2020) 15(8):1361–8. doi: 10.1016/j.jtho.2020.04.032 [DOI] [PubMed] [Google Scholar]
- 4. Deressa BT, Tigeneh W, Bogale N, Buwenge M, Morganti AG, Farina E. Short-Course 2-Dimensional Radiation Therapy in the Palliative Treatment of Esophageal Cancer in a Developing Country: A Phase II Study (Sharon Project). Int J Radiat Oncol Biol Phys (2020) 106(1):67–72. doi: 10.1016/j.ijrobp.2019.10.004 [DOI] [PubMed] [Google Scholar]
- 5. Martin EJ, Bruggeman AR, Nalawade VV, Sarkar RR, Qiao EM, Rose BS, et al. Palliative Radiotherapy Versus Esophageal Stent Placement in the Management of Patients With Metastatic Esophageal Cancer. J Natl Compr Canc Netw (2020) 18(5):569–74. doi: 10.6004/jnccn.2019.7524 [DOI] [PubMed] [Google Scholar]
- 6. Cooper JS, Guo MD, Herskovic A, Macdonald JS, Martenson JA, Jr, Al-Sarraf M, et al. Chemoradiotherapy of Locally Advanced Esophageal Cancer: Long-Term Follow-Up of a Prospective Randomized Trial (RTOG 85-01). Radiation Therapy Oncology Group. JAMA (1999) 281(17):1623–7. doi: 10.1001/jama.281.17.1623 [DOI] [PubMed] [Google Scholar]
- 7. Stahl M, Wilke H, Lehmann N, Stuschke M. Long-Term Results of a Phase III Study Investigating Chemoradiation With and Without Surgery in Locally Advanced Squamous Cell Carcinoma (LA-SCC) of the Esophagus. J Clin Oncol (2008) 26(15):431–6. doi: 10.1200/jco.2008.26.15_suppl.4530 [DOI] [Google Scholar]
- 8. Bedenne L, Michel P, Bouché O, Milan C, Binquet C. Chemoradiation Followed by Surgery Compared With Chemoradiation Alone in Squamous Cancer of the Esophagus: FFCD 9102. J Clin Oncol (2007) 25(10):1160–8. doi: 10.1200/JCO.2005.04.7118 [DOI] [PubMed] [Google Scholar]
- 9. Shah MA, Kennedy EB, Catenacci DV, Deighton DC, Goodman KA, Malhotra NK, et al. Treatment of Locally Advanced Esophageal Carcinoma: ASCO Guideline. J Clin Oncol (2020) 38(23):2677–94. doi: 10.1200/JCO.20.00866 [DOI] [PubMed] [Google Scholar]
- 10. Ajani JA, D’Amico TA, Bentrem DJ, Chao J, Corvera C, Das P, et al. Esophageal and Esophagogastric Junction Cancers, Version 2.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw (2019) 17(7):855–83. doi: 10.6004/jnccn.2019.0033 [DOI] [PubMed] [Google Scholar]
- 11. Chen K, Fan Q, Fang W, Fu J, Han Y, Hu B, et al. Guidelines of Chinese Society of Chinical Oncology(CSCO):Esophageal Cancer. Available at: http://meeting.csco.org.cn/MUser/M/1?returnurl=http://www.csco.org.cn/cn/index.aspx.
- 12. Muro K, Lordick F, Tsushima T, Pentheroudakis G, Baba E, Lu Z, et al. Pan-Asian Adapted ESMO Clinical Practice Guidelines for the Management of Patients With Metastatic Oesophageal Cancer: A JSMO-ESMO Initiative Endorsed by CSCO, KSMO, MOS, SSO and TOS. Ann Oncol (2019) 30(1):34–43. doi: 10.1093/annonc/mdy498 [DOI] [PubMed] [Google Scholar]
- 13. Guttmann DM, Mitra N, Bekelman J, Metz JM, Plastaras J, Feng W, et al. Improved Overall Survival With Aggressive Primary Tumor Radiotherapy for Patients With Metastatic Esophageal Cancer. J Thorac Oncol (2017) 12(7):1131–42. doi: 10.1016/j.jtho.2017.03.026 [DOI] [PubMed] [Google Scholar]
- 14. Luo H, Lu J, Bai Y, Mao T, Wang J, Fan Q, et al. Effect of Camrelizumab vs Placebo Added to Chemotherapy on Survival and Progression-Free Survival in Patients With Advanced or Metastatic Esophageal Squamous Cell Carcinoma: The ESCORT-1st Randomized Clinical Trial. JAMA (2021) 326(10):916–25. doi: 10.1001/jama.2021.12836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Forde PM, Kelly RJ. Chemotherapeutic and Targeted Strategies for Locally Advanced and Metastatic Esophageal Cancer. J Thorac Oncol (2013) 8(6):673–84. doi: 10.1097/JTO.0b013e31828b5172 [DOI] [PubMed] [Google Scholar]
- 16. Rice TW, Ishwaran H, Blackstone EH, Hofstetter WL, Kelsen DP, Apperson-Hansen C, et al. Recommendations for Clinical Staging (cTNM) of Cancer of the Esophagus and Esophagogastric Junction for the 8th Edition AJCC/UICC Staging Manuals. Dis Esophagus (2016) 29(8):913–9. doi: 10.1111/dote.12540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. National Cancer Institute . Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0. US Department of Health and Human Services (2009) no. 09-5410. Available at: https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/CTCAE_4.03_2010-06-1_QuickReference_5x7.pdf. [Google Scholar]
- 18. Li T, Lv J, Li F, Diao P, Wang J, Li C, et al. Prospective Randomized Phase 2 Study of Concurrent Chemoradiation Therapy (CCRT) Versus Chemotherapy Alone in Stage IV Esophageal Squamous Cell Carcinoma (ESCC). Int J Radiat Oncol Biol Phys (2016) 96(2):4050. doi: 10.1016/j.ijrobp.2016.06.020 [DOI] [Google Scholar]
- 19. Moreno AC, Zhang N, Giordano S, Komaki RU, Liao Z, Nguyen QN, et al. Comparative Effectiveness of Chemotherapy Alone Versus Chemotherapy and Radiation Therapy for Patients With Stage IV Esophageal Cancer. Int J Radiat Oncol Biol Phys (2017) 99(2):E172–E3. doi: 10.1016/j.ijrobp.2017.06.1014 [DOI] [Google Scholar]
- 20. Lyu J, Li T, Wang Q, Li F, Diao P, Liu L, et al. Outcomes of Concurrent Chemoradiotherapy Versus Chemotherapy Alone for Stage IV Esophageal Squamous Cell Carcinoma: A Retrospective Controlled Study. Radiat Oncol (2018) 13(1):233. doi: 10.1186/s13014-018-1183-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Xu J, Lu D, Zhang L, Li J, Sun G. Palliative Resection or Radiation of Primary Tumor Prolonged Survival for Metastatic Esophageal Cancer. Cancer Med (2019) 8(17):7253–64. doi: 10.1002/cam4.2609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mendes J, Alves P, Amaral TF. Comparison of Nutritional Status Assessment Parameters in Predicting Length of Hospital Stay in Cancer Patients. Clin Nutr (2014) 33(3):466–70. doi: 10.1016/j.clnu.2013.06.016 [DOI] [PubMed] [Google Scholar]
- 23. Cotogni P, Pedrazzoli P, De Waele E, Aprile G, Farina G, Stragliotto S, et al. Nutritional Therapy in Cancer Patients Receiving Chemoradiotherapy: Should We Need Stronger Recommendations to Act for Improving Outcomes? J Cancer (2019) 10(18):4318–25. doi: 10.7150/jca.31611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Adamson D, Byrne A, Porter C, Blazeby J, Griffiths G, Nelson A, et al. Palliative Radiotherapy After Oesophageal Cancer Stenting (ROCS): A Multicentre, Open-Label, Phase 3 Randomised Controlled Trial. Lancet Gastroenterol Hepatol (2021) 6(4):292–303. doi: 10.1016/S2468-1253(21)00004-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Penniment MG, De Ieso PB, Harvey JA, Stephens S, Au HJ, O’Callaghan CJ, et al. Palliative Chemoradiotherapy Versus Radiotherapy Alone for Dysphagia in Advanced Oesophageal Cancer: A Multicentre Randomised Controlled Trial (TROG 03.01). Lancet Gastroenterol Hepatol (2018) 3(2):114–24. doi: 10.1016/S2468-1253(17)30363-1 [DOI] [PubMed] [Google Scholar]
- 26. Minsky BD, Pajak TF, Ginsberg RJ, Pisansky TM, Martenson J, Komaki R, et al. INT 0123 (Radiation Therapy Oncology Group 94-05) Phase III Trial of Combined-Modality Therapy for Esophageal Cancer: High-Dose Versus Standard-Dose Radiation Therapy. J Clin Oncol (2002) 20(5):1167–74. doi: 10.1200/JCO.2002.20.5.1167 [DOI] [PubMed] [Google Scholar]
- 27. Song T, Liang X, Fang M, Wu S. High-Dose Versus Conventional-Dose Irradiation in Cisplatin-Based Definitive Concurrent Chemoradiotherapy for Esophageal Cancer: A Systematic Review and Pooled Analysis. Expert Rev Anticancer Ther (2015) 15(10):1157–69. doi: 10.1586/14737140.2015.1074041 [DOI] [PubMed] [Google Scholar]
- 28. Luo HS, Huang HC, Lin LX. Effect of Modern High-Dose Versus Standard-Dose Radiation in Definitive Concurrent Chemo-Radiotherapy on Outcome of Esophageal Squamous Cell Cancer: A Meta-Analysis. Radiat Oncol (2019) 14(1):178. doi: 10.1186/s13014-019-1386-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hulshof M, Geijsen ED, Rozema T, Oppedijk V, Buijsen J, Neelis KJ, et al. Randomized Study on Dose Escalation in Definitive Chemoradiation for Patients With Locally Advanced Esophageal Cancer (ARTDECO Study). J Clin Oncol (2021) 39(25):2816–24. doi: 10.1200/JCO.20.03697 [DOI] [PubMed] [Google Scholar]
- 30. Hironaka S, Komori A, Machida R, Ito Y, Takeuchi H, Ogawa G, et al. The Association of Primary Tumor Site With Acute Adverse Event and Efficacy of Definitive Chemoradiotherapy for Cstage II/III Esophageal Cancer: An Exploratory Analysis of JCOG0909. Esophagus (2020) 17(4):417–24. doi: 10.1007/s10388-020-00741-w [DOI] [PubMed] [Google Scholar]
- 31. Song T, Zhang X, Fang M, Zhao R, Wu S. Long-Term Results of Definitive Concurrent Chemoradiotherapy Using Paclitaxel Plus Oxaliplatin in Unresectable Locally Advanced Esophageal Cancer: A Prospective Phase II Trial. Cancer Med (2016) 5(12):3371–7. doi: 10.1002/cam4.897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kojima T, Shah MA, Muro K, Francois E, Adenis A, Hsu CH, et al. Randomized Phase III KEYNOTE-181 Study of Pembrolizumab Versus Chemotherapy in Advanced Esophageal Cancer. J Clin Oncol (2020) 38(35):4138–48. doi: 10.1200/JCO.20.01888 [DOI] [PubMed] [Google Scholar]
- 33. Kato K, Shah MA, Enzinger P, Bennouna J, Shen L, Adenis A, et al. KEYNOTE-590: Phase III Study of First-Line Chemotherapy With or Without Pembrolizumab for Advanced Esophageal Cancer. Future Oncol (2019) 15(10):1057–66. doi: 10.2217/fon-2018-0609 [DOI] [PubMed] [Google Scholar]
- 34. Doi T, Piha-Paul SA, Jalal SI, Saraf S, Lunceford J, Koshiji M, et al. Safety and Antitumor Activity of the Anti-Programmed Death-1 Antibody Pembrolizumab in Patients With Advanced Esophageal Carcinoma. J Clin Oncol (2018) 36(1):61–7. doi: 10.1200/JCO.2017.74.9846 [DOI] [PubMed] [Google Scholar]
- 35. Liu Y, Dong Y, Kong L, Shi F, Zhu H, Yu J. Abscopal Effect of Radiotherapy Combined With Immune Checkpoint Inhibitors. J Hematol Oncol (2018) 11(1):104. doi: 10.1186/s13045-018-0647-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Grassberger C, Ellsworth SG, Wilks MQ, Keane FK, Loeffler JS. Assessing the Interactions Between Radiotherapy and Antitumour Immunity. Nat Rev Clin Oncol (2019) 16(12):729–45. doi: 10.1038/s41571-019-0238-9 [DOI] [PubMed] [Google Scholar]
- 37. Sardaro A, Ferrari C, Carbonara R, Altini C, Lavelli V, Rubini G. Synergism Between Immunotherapy and Radiotherapy in Esophageal Cancer: An Overview of Current Knowledge and Future Perspectives. Cancer Biother Radiopharm (2021) 36(2):123–32. doi: 10.1089/cbr.2020.3643 [DOI] [PubMed] [Google Scholar]
- 38. Shah MA, Bennouna J, Doi T, Shen L, Kato K, Adenis A, et al. KEYNOTE-975 Study Design: A Phase III Study of Definitive Chemoradiotherapy Plus Pembrolizumab in Patients With Esophageal Carcinoma. Future Oncol (2021) 17(10):1143–53. doi: 10.2217/fon-2020-0969 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.