Abstract
Immune checkpoint inhibitors (ICIs) block inhibitory molecules, such as cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), programmed cell death protein 1 (PD-1), or its ligand, programmed cell death protein ligand 1 (PD-L1) and enhance antitumor T-cell activity. ICIs provide clinical benefits in a percentage of patients with advanced cancers, but they are usually associated with a remarkable spectrum of immune-related adverse events (irAEs) (e.g., rash, colitis, hepatitis, pneumonitis, endocrine, cardiac and musculoskeletal dysfunctions). Particularly patients on combination therapy (e.g., anti-CTLA-4 plus anti-PD-1/PD-L1) experience some form of irAEs. Different mechanisms have been postulated to explain these adverse events. Host factors such as genotype, gut microbiome and pre-existing autoimmune disorders may affect the risk of adverse events. Fatal ICI-related irAEs are due to myocarditis, colitis or pneumonitis. irAEs usually occur within the first months after ICI initiation but can develop as early as after the first dose to years after ICI initiation. Most irAEs resolve pharmacologically, but some appear to be persistent. Glucocorticoids represent the mainstay of management of irAEs, but other immunosuppressive drugs can be used to mitigate refractory irAEs. In the absence of specific trials, several guidelines, based on data from retrospective studies and expert consensus, have been published to guide the management of ICI-related irAEs.
Keywords: cancer, cytotoxic T lymphocyte-associated protein (CTLA-4), immunotherapy, immune checkpoint inhibitor (ICI), immune-related adverse event (irAE), programmed cell death protein -1 (PD-1), PD-L1
Introduction
Immune checkpoint inhibitors (ICIs) have revolutionized the management of several advanced cancers (1, 2) and can result in durable responses in a percentage of patients (3–5). ICIs are monoclonal antibodies (mAbs) that block inhibitory molecules involved in regulation of immune system pathways, such as cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) (e.g., ipilimumab), programmed cell death protein 1 (PD-1) (e.g., nivolumab, pembrolizumab, cemiplimab), or its ligand programmed cell death protein ligand 1 (PD-L1) (e.g., atezolizumab, avelumab, durvalumab) in the tumor microenvironment, which leads to systemic immune cell activation ( Figure 1 ) (6, 7). Immune checkpoints constitute mechanisms of central relevance in the regulation of immune response to avoid autoimmunity and limit tissue damage (8, 9). Immune checkpoints can be exploited by cancer cells as mechanisms of immunoevasion and immunoresistance (10).
Figure 1.
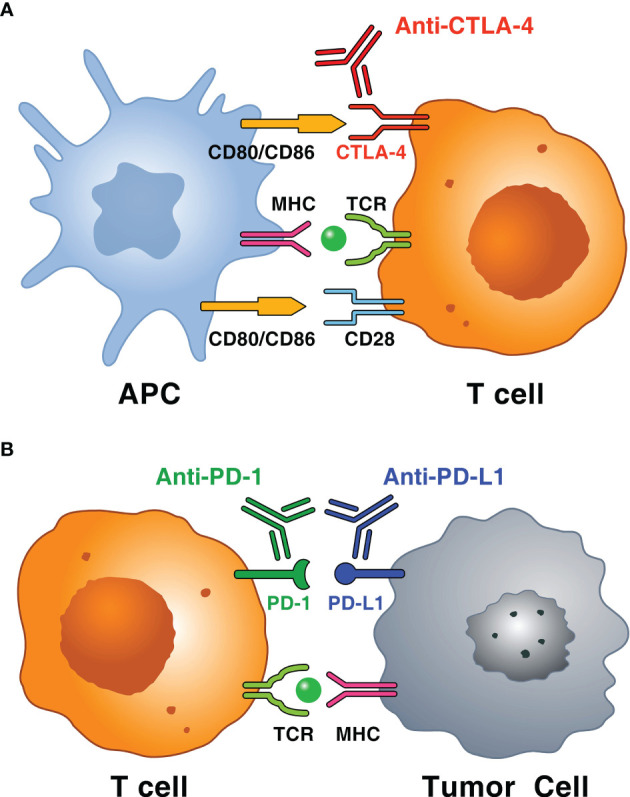
Schematic representation of immune mechanisms of immune checkpoints and immune checkpoint inhibitors (ICIs). (A) T cells, particularly CD4+ T cells in the lymph node, recognize tumor antigens in the context of MHC molecules or antigen-presenting cell (APC) and T cell receptor (TCR) on T cells. The interaction between CD80 (also known as B7-1) or CD86 (also known as B7-2) on APC and CD28 mediates T cell co-stimulation in conjunction with TCR signals. CTLA-4 on activated T cell interacts with both ligands (i.e., CD80 or CD86) with higher affinity and avidity than CD28 and, unlike CD28, sends an inhibitory signal to T cell. Monoclonal antibodies anti-CTLA-4 (i.e., ipilimumab) block this inhibitory pathway restoring T cell activity. (B) T cells, particularly cytotoxic CD8+ T cells, which recognize tumor antigens in the context of MHC class, result in the adaptive expression of PD-L1 on the surface of tumor cells. The interaction between PD-1 and PD-L1 negatively regulates the anti-tumor T cell response. This interaction is useful in preventing autoimmunity in physiological conditions, whereas cancer cells exploit this mechanism to escape from immune system upregulating PD-L1 expression. Anti-PD-1 (i.e., pembrolizumab, nivolumab and cemiplimab) and anti-PD-L1 mAbs (i.e., atezolizumab, avelumab and durvalumab) block this inhibitory pathway restoring T cell activity.
ICIs activate T cells and are often associated with a large spectrum of autoimmune responses, which are commonly referred to as “immune-related Adverse Events” (irAEs). The pleiotropic manifestations of irAEs can affect almost any organ (e.g., skin, colon, endocrine organs, joints, heart and lungs) and clinicians should be able to recognize and treat the heterogeneous manifestations of irAEs. Several comprehensive reviews have examined in detail the toxicity of ICIs affecting the skin (11–13), the gastrointestinal (14–16) and cardiovascular systems (17–24), the lung (25, 26), the endocrine organs (27–29), the joints (30, 31), the nervous (32) and the hematologic systems (33). In this review, we summarize the most recent observations and the complex pathophysiology and clinical characteristics of irAEs and their putative predictors and emerging therapies.
Incidence/Prevalence of IrAEs
Distinct immunological mechanisms underlie anti-CTLA-4 (ipilimumab) and anti-PD-1/PD-L1 checkpoint blockade (34). Therefore, it is not surprising that the incidence of any irAEs with these two groups of ICIs varies greatly. The pattern, incidence and severity of irAEs vary according to the type of ICI (anti-CTLA-4 or anti-PD-1/PD-L1) and the treatment schedule (monotherapy or combination therapy). It has been estimated that the incidence of irAEs in patients treated with anti-CTLA-4 mAb (ipilimumab) is higher than in those treated with anti-PD-1/PD-L1 mAbs (35). The highest incidence and the high-grade irAEs are usually associated with combination therapy of ipilimumab plus anti-PD-1/PD-L1 (36). In a large meta-analysis examining 16,485 patients, colitis and hypophysitis were more frequent with ipilimumab, while diabetes and pneumonitis were more frequent with anti-PD-1/PD-L1 (35). Colitis, hepatitis, pancreatitis, and ICI-associated diabetes are more likely to be high-grade (37).
Timing Of IrAEs
In melanoma patients treated with ipilimumab, the time of onset of skin-related irAEs is two to three weeks after ICI initiation, gastrointestinal and hepatic irAEs after six weeks, and endocrine irAEs after six to nine weeks (38, 39). Most high-grade irAEs resolve in two to five weeks with immunosuppression but some, such as arthritis, tend to persist (40). Endocrine irAEs (e.g., diabetes, thyroid dysfunctions) are usually irreversible and require prolonged hormone replacement therapy (39).
Fatal ICI-related irAEs tend to occur in the early phases of therapy and the incidence varies with the type of treatment. Fatalities are more common with combination therapy than with anti-PD-1/PD-L1 or anti-CTLA-4 (20). Fatality rates were approximately 39% for myocarditis and 5% for colitis (20).
There are some similarities between autoimmune manifestations of ICI-related irAEs and their spontaneous autoimmune counterparts but also several differences (41). For instance, ICI-induced diabetes can manifest with diabetic ketoacidosis, similar to T1D (42). The frequency of autoantibodies in ICI-induced diabetes is lower than in T1D (42, 43). ICI-induced hyperthyroidism is typically found at presentation and usually progresses to hypothyroidism (44, 45). ICI-induced colitis differs from inflammatory bowel disease (IBD) because is usually reversible (14).
Long-Term Adverse Effects of ICIs
ICIs have been successfully introduced in the treatment of various cancers only a few years ago. Therefore, there is limited experience on the long-term side effects of ICIs. Acute irAEs have thus far attracted major attention owing to their dramatic clinical presentation and need for urgent treatment. However, increasing evidences indicate that chronic irAEs are more prevalent than originally recognized (46, 47). Endocrinopathies (such as ICI-induced hypothyroidism and diabetes) and rheumatological toxicities (such as arthritis) are the most common chronic irAEs (48, 49). Endocrinopathies provide classical examples of irreversible damage of the relevant hormone-secreting cells. These syndromes are usually irreversible and require the use of lifelong exogenous hormone replacement therapy (45, 50). On the other hand, ICI-induced arthritis provides a classical example of smouldering inflammation in which ICIs trigger persistent subacute or chronic arthritis, closely mimicking that of rheumatoid arthritis (48, 51).
Several experimental studies have demonstrated that CTLA-4 and PD-1/PD-L1 axes are critical negative regulators of atherosclerosis (52–55). A recent retrospective study by Drobni et al. reported an association between ICIs with accelerated progression of atherosclerosis and cardiovascular events (56). They found increased atherosclerotic inflammatory activity 5 months after ICI therapy (57). Another retrospective study on 20 patients with melanoma found by positron emission tomography/computed tomography with 2-[18F] fluorodeoxyglucose (18F-FDG) that ICI therapy induced inflammatory activity in large arteries (57). The results of these two studies will certainly influence the approach to cardiovascular care for individuals receiving ICIs. Cardiac evaluation before initiation of ICI treatment should focus on long-term prevention rather than focusing only on early irAEs. Moreover, cancer trials should prospectively examine not only early but also late cardiovascular events (58).
Pathophysiology of IrAEs
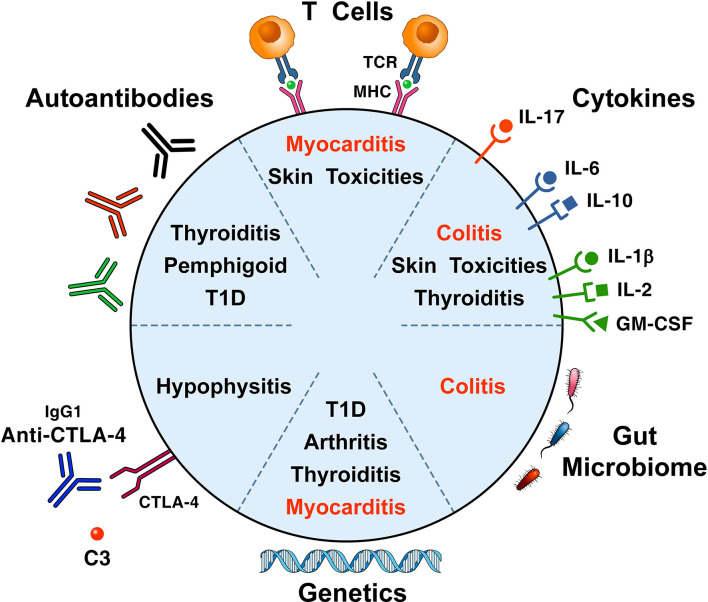
Several immunopathogenic mechanisms (i.e., cellular autoimmunity, autoantibodies, complement activation, cytokines/chemokines release, genetics and alterations of the gut microbiome) have been suggested to be involved in the development of ICI-related irAEs ( Figure 2 ).
Figure 2.
Proposed immunopathogenic mechanisms for the development of immune checkpoint-induced immune-related adverse events. A proposed mechanism postulates that self-antigens (e.g., heart and skeletal muscle antigens) activate T cell clones driving antitumor responses and organ-specific autoimmunity (24). Thyroid autoantibodies may be involved in patients who develop thyroid dysfunction (44, 59, 60), ICI-associated diabetes (42, 43), bullous pemphigoid (61), hypophysitis (62, 63), and myasthenia gravis (64). Cytokines/chemokines released from immune cells can cause immune-mediated tissue damage (12, 65, 66). The pivotal role of genetic factors in the development of ICI-associated irAEs, originally highlighted in mice (67, 68), has been confirmed in patients with arthritis (69), ICI-associated diabetes (42, 43, 70), and pruritus (71). There is growing evidence that gut microbiome may play a role in the development of experimental irAEs (72, 73) and of colitis in patients with melanoma (74, 75). irAEs contributing to most fatalities are presented in red.
Cellular Autoimmunity
The importance of T cells in the mechanisms of ICI-associated irAEs is supported by genetic loss-of-function studies in mice (67, 68). Autoreactive T cells can be activated by shared antigens between tumor and peripheral tissues. Shared T cell clones in the tumor, heart, and skeletal muscle were found in melanoma patients, who died from fatal myocarditis and myositis after treatment with anti-CTLA-4/PD-1 mAbs (76). The exact mechanism of cardiac toxicity remains unknown, but it has been suggested that shared antigens may drive both antitumor responses and organ-specific autoimmunity (24). Shared T cell antigens were found in the skin and tumor of lung cancer patients who developed skin toxicities (77). Similarly, vitiligo is common in melanoma patients treated with ICIs (78). Recently, Lozano and collaborators demonstrated that in melanoma patients treated with anti-PD-1 or anti-PD-1 and anti-CTLA-4 combination, two pretreatment factors in peripheral blood - activated CD4 memory T cell abundance and TCR diversity - were associated with severe irAEs development (79).
CTLA-4 is a modulator of Tregs (80) and these cells act as gatekeepers for the prevention of autoimmunity. The role of Tregs in ICI-induced irAEs deserves additional studies (81). It has been suggested that tissue-resident memory T cells (Trm) in tumor microenvironment can play a role in irAEs (82). Single-cell analysis of ICI-associated colitis patient samples found expansion of CTLA-4+ Treg cells and differentiation of CD8 Trm cells to cytotoxic effector cells (83).
Humoral Immunity
Anti-thyroglobulin (TG) and/or anti-thyroid peroxidase (TPO) autoantibodies are found in 13–70% of patients who develop ICI-related thyroid dysfunction (44, 59, 60, 84). Thyroid autoantibodies increase the risk of ICI-induced thyroid dysfunction (85–87) and β-cell autoantibodies are found in approximately 50% of patients with ICI-induced diabetes (42, 43, 88). Autoantibodies anti-BP180 can be found in the majority of patients with anti-PD-L1-associated bullous pemphigoid (61).
Human pituitary cells express CTLA-4 at both mRNA and protein levels and in an animal model the injection of anti-CTLA-4 antibodies induced lymphocytic infiltration and complement activation of the pituitary gland (62). Anti-pituitary antibodies were detected only in patients with ipilimumab-associated hypophysitis but not in those without hypophysitis (62). Autoantibodies against guanine nucleotide-binding protein G subunit alpha (GNAL) and integral membrane protein 2B (ITM2B) have been found associated with ICI-induced hypophysitis (63). These findings implicate both autoantibodies and T cell-mediated processes in ICI-associated pituitary destruction (89). Overexpression of pituitary CTLA-4 was reported in a patient with severe ipilimumab-associated hypophysitis (90). It has been suggested that hypophysitis is caused by complement activation from endogenous autoantibodies and/or exogenous IgG1 anti-CTLA-4 (ipilimumab) (62). IgG1, used in ipilimumab, activates the classic complement pathway explaining the elevated frequency of pituitary gland damage compared with its occurrence in patients treated with anti-PD-1/anti-PD-L-1 IgG4 antibodies (91, 92).
Anti-acetylcholine receptor antibodies can be found in approximately 50% of patients with ICI-induced myasthenia gravis (64). Patients with ICI-induced arthritis are commonly rheumatoid factor (RF) and cyclic citrullinated peptide (CCP) negative (69). Unfortunately, in the majority of these studies the presence of autoantibodies prior to ICI initiation has not been evaluated.
Cytokines and Chemokines
Cytokine release syndrome (CRS) is a systemic inflammatory disorder characterized by a massive release of cytokines (93). It can present with a variety of symptoms ranging from mild (e.g., fever, fatigue, nausea, rash) to life threating, sometime fatal. A recent analysis of WHO global pharmacovigilance database, found that ICI-related CRS can occur in ICI-treated patients (94). On similar ground, cytokines and chemokines are involved in different irAEs. Increased baseline IL-17 concentrations were temporally associated with subsequent development of ICI-associated colitis (65). Increased IL-6 and IL-10 concentrations were found in patients with skin irAEs (12). Increased concentrations of IL-1β, IL-2, and GM-CSF at baseline have been associated with ICI-related thyroid dysfunction (66). High concentrations of T cell chemotactic chemokines (i.e., CXCL9 and CXCL10) are associated with irAEs (95). A recent study in patients with melanoma treated with combined immune checkpoint blockade (CICB) targeting CTLA-4 and PD-1 who developed ≥ grade 3 colitis demonstrates an intestinal overexpression of IL1β and TNF compared to normal tissue (96).
Genetic Factors
Genetic factors influence the development and progression of several autoimmune disorders. The importance of genetic factors in the development of ICI-associated irAEs was originally highlighted by genetic loss-of-function studies in mice (67, 68). Thus, the possibility exists that genetic susceptibility may play a role in the pathogenesis of irAEs. Experimental studies have demonstrated that CTLA-4 and PD-1 deletion or inhibition can cause autoimmune myocarditis with lymphocytic infiltration of cytotoxic T-cells (67, 97–99). The majority (≅ 52%) of patients with ICI-related arthritis possess the RA-associated HLA-DR susceptibility allele (69). The majority of patients with ICI-related diabetes had at least one HLA-DR risk allele (70). HLA-DR4 predominance has been reported in patients with ICI-induced diabetes (42, 43). HLA-DRB1*04:05 has been associated with ICI-induced arthritis (69) and HLA-DRB1*11:01 with pruritis (71). A multicenter study found that a polygenic risk score (PRS) for thyroid disorders is associated with developing thyroid irAEs in patients with non-small cell lung cancer (NSCLC) treated with anti-PD-1 or anti-PD-1 and anti-CTLA-4 combination (84). In this study, thyroid irAEs were associated with better response to ICIs. Moreover, in a phase 3 randomized controlled trial (RCT), it was found that PRS for dermatological autoimmune diseases were associated with increased risk for skin irAEs and longer overall survival in bladder cancer patients treated with atezolizumab (100).
Microbiome
The multiple interactions among tumor microenvironment, microbiome, host factors, and response to ICI, and the development of ICI-associated irAEs are largely unknown (101). There is evidence that the gut microbiome might play a role in tumor response (102–104). For instance, gut microbiome modulates response to anti-PD-1 in melanoma patients (105) and epithelial tumors (103). Mice repleted with B. fragilis less likely developed irAEs after exposure to anti-CTLA-4 inhibitors (72). Moreover, microbiota-derived peptides from Bacteroides induced autoimmune myocarditis (73).
Melanoma patients treated with ipilimumab and with baseline gut microbiome enriched for Faecalibacterium and other Firmicutes had longer progression-free survival and overall survival (74). In a prospective study of melanoma treated with ipilimumab, patients with abundant Bacteroidetes phylum less likely developed ICI-induced colitis (75). A recent study found that gut microbiota signatures are associated with irAEs to CICB targeting CTLA-4 and PD-1 in melanoma patients and in experimental models (96). In this study, the rate of any grade of irAEs was high (93.5%) and 49% of patients experienced severe (≥ grade 3) irAEs. The alpha diversity of the gut microbiome in patients who did or did not develop severe irAEs was similar. However, Bacteroides intestinalis (B. intestinalis) were more abundant in patients with ≥ grade 3 irAEs versus those who did not. In melanoma patients who developed colitis there was an overexpression of mucosal IL1β and IL-17, but not TNF. These fascinating results were corroborated by results in experimental models in which mice, gavaged with different strains of B. intestinalis following gut sterilization with antibiotics, showed overexpression of Il1b. Moreover, fecal microbiota transplant (FMT) in antibiotic-treated animals using fecal material from human donors harboring high endogenous levels of B. intestinalis induced ileal overexpression of Il1b after administration of CICB. Collectively, these human and experimental studies highlight a contribution of commensal microbiota to intestinal damage associated with CICB.
Putative Biomarkers of Immune-Related Adverse Events
Several studies have or are evaluating the possibility of identifying biomarkers of irAEs associated with different types of ICIs. Table 1 lists genetic, clinical, immune, microbial and tumor biomarkers that have been linked to irAEs. In particular, some studies have identified an association between HLA and irAEs. The association of baseline antibodies (85–87, 109, 110), baseline cytokine levels (65, 66, 111, 112), and immune cell changes (79, 83, 113–116) suggests that humoral and cellular immunity play a role in some specific irAEs. In particular, activated CD4 memory T cell abundance and TCR diversity in peripheral blood are associated with severe irAEs development in patients with melanoma (79). The emerging results from parallel human and experimental models highlight a contribution of specific gut microbiota to the development of intestinal irAEs (74, 75, 96). It should be emphasized that the small size of these studies requires validation in larger cohorts of patients with different types of cancer.
Table 1.
Putative predictors of immune adverse events associated with immune checkpoint inhibitors.
| Genotype | HLA-DR4 association with ICI-associated diabetes in patients with a variety of cancers treated with anti-PD-1/PD-L1 (42). HLA-DR4 association with ICI-associated diabetes in patients treated with anti-PD-1/PD-L1 (43). HLA-DRB1*04:05 association with ICI-induced arthritis in patients with a variety of cancers (69). HLA-DRB1*11:01 association with ICI-induced pruritis and HLA-DQB1* 03:01 and colitis in patients with non-small lung cancer (NSCLC) or melanoma treated with anti-PD-1, anti-CTLA-4 or their combination (71). |
| Pre-existing autoimmune disease | irAEs are more frequent and occur sooner in patients with autoimmune disease treated with anti-PD-1 (106, 107). Pre-existing autoimmune disease associated with modest increases in hospitalization with irAEs in patients treated with ICIs (108). |
| Baseline autoantibodies | Thyroid autoantibodies (anti-TPO, anti-tg) at baseline increases the risk of thyroid dysfunction in patients treated with nivolumab or pembrolizumab (85–87, 109). Baseline autoantibody signatures, such as those targeting TNF-α signaling pathways may be predictive of irAEs in patients with melanoma treated with anti-CTLA-4, anti-PD-1 or their combination (110). Skin irAEs may be more frequent in patients with positive RF at baseline in patients with NSCLC treated with nivolumab or pembrolizumab (87). |
| Baseline cytokine levels | Baseline IL-17 serum levels may predict ICI-induced colitis in patients with melanoma treated with ipilimumab (65). Baseline IL-6 serum levels were associated with higher risk of toxicity in melanoma patients treated with ipilimumab (111). Cytokine toxicity score predictive of severe irAEs in patients with melanoma treated with ipilimumab, anti-PD-1 or their combination (112). Baseline serum levels of IL-1β, IL-2, and GM-CSF predict thyroid dysfunction in patients with a variety of cancers (66). |
| Immune cell changes | Reduction in circulating B cells, increase in CD2lo PD-1+ B cells and plasmablasts precede adverse events in patients with melanoma treated with ipilimumab, anti-PD-1 or their combination (113). Neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio may predict appearance of irAEs in patients with NSCLC treated with anti-PD-1/PD-L1 (114). High baseline absolute eosinophil count (AEC) (> 135/μl) correlates with the risk of irAEs in patients with melanoma, renal cell carcinoma, and NSCLC treated with anti-CTLA-4 (115, 116). High proliferative index in circulating effector and control memory CD8+ T lymphocytes at early time points in melanoma patients treated with CICB who developed ≥ grade 3 irAEs (96). Lower expression of surface CD28 and CD27 on circulating CD4+ and CD8+ effector T lymphocytes of melanoma patients treated with CICB who did not develop severe irAEs (96). Increased activated CD4 memory T cells and TCR diversity in peripheral blood are associated with severe irAEs in patients with melanoma treated with anti-PD-1 or anti-PD-1 and ipilimumab combination (79). |
| Microbiome |
Bacteroidetes phylum may be protective for development of colitis in melanoma patients treated with ipilimumab (75). Faecalibacterium may be predictive of colitis in melanoma patients treated with ipilimumab (74). Bacteroides intestinalis is associated with ≥ grade 3 colitis in patients with melanoma treated with combined ipilimumab and PD-1 blockade (96). |
| Tumor burden | High tumor burden is associated with higher risk of severe irAEs in patients with NSCLC (117). |
Clinical Manifestations of ICI Associated Autoimmunity
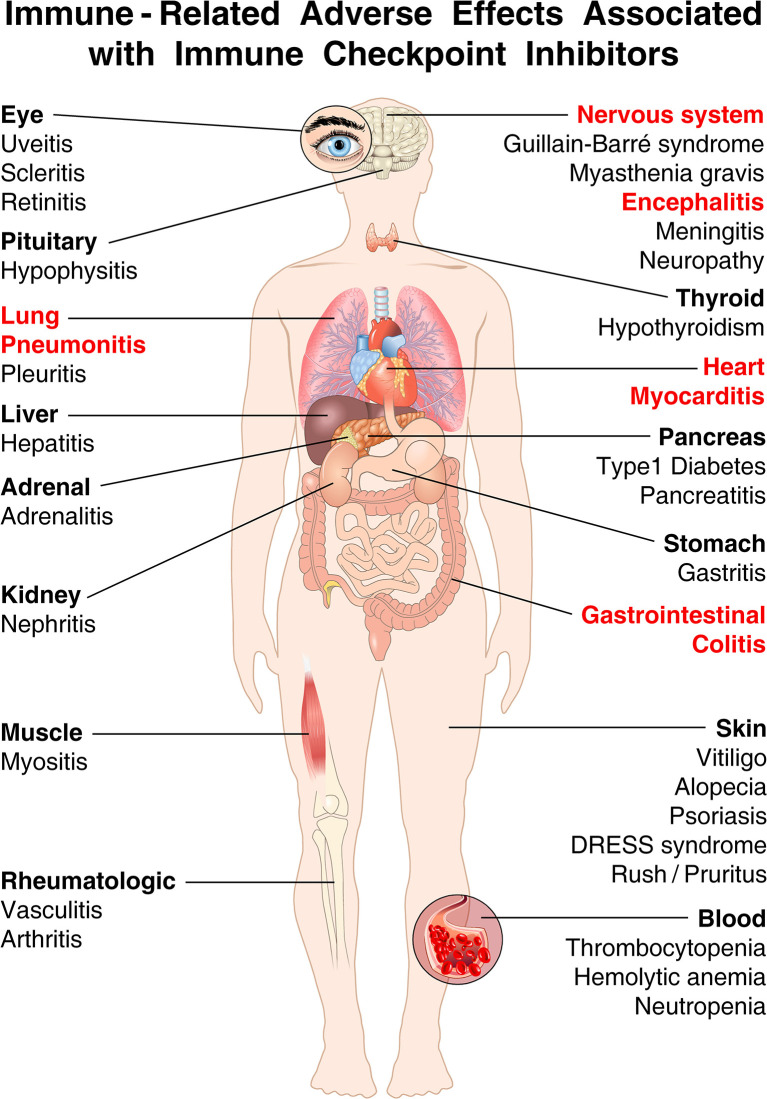
The results of a meta-analysis of 35 trials demonstrated the extreme heterogeneity of manifestations and severity of autoimmune complications of ICIs (35). Figure 3 illustrates that irAEs can affect nearly every organ in association with ICIs.
Figure 3.
The spectrum of organs affected by irAEs associated with immune checkpoint inhibitors (ICIs) is very broad. Shown are the most common immune-related adverse events (irAEs) that clinicians can encounter in cancer patients treated with ICIs. irAEs contributing to most fatalities are highlighted in red [modified from (19)]. ICI-associated diabetes is almost exclusively seen in patients treated with anti-PD-1/PD-L1 antibodies, and rarely with ipilimumab monotherapy (42, 118). By contrast, hypophysitis occurs more often in patients receiving ipilimumab (119). Colitis occurs more commonly with ipilimumab and with combined immune checkpoint blockade than with anti-PD-1/PD-L1 alone (112, 120). Endocrinopathies (such as hypothyroidism, hypophysitis, and adrenal insufficiency) and rheumatological disorders have the highest incidence of development into subacute/chronic toxicity (49). Endocrine toxicities, unlike many other irAEs, are not managed using high-dose glucocorticoids, which have no effect on either initial severity and resolution (121, 122). Although acute myocarditis was the first cardiovascular irAEs associated with ICIs (123), an important unanswered question relates to the long-term cardiovascular sequelae of ICIs (49, 56, 57).
Skin Rash
Skin manifestations during ICI therapy show the highest incidence of irAEs and appear higher in patients with melanoma than in patients with other malignancies (124). ICI-related skin manifestations include maculopapular, eczematous, psoriasiform, lichenoid, and bullous eruptions, frequently associated with pruritus (125). Vitiligo associated with anti-PD-1 therapy differs from canonical vitiligo because of a patchy distribution, occurring in sun-exposed areas, and lacking the Köebner phenomenon (126). Bullous skin disease tends to occur in patients treated with anti-PD-1/PD-L1 rather than ipilimumab (127). Rare cases of severe Stevens-Johnson syndrome/toxic epidermal necrolysis have been reported (128).
Most patients have mild skin reactions, while severe (grade 3-4) toxicities are found in a low proportion of patients (5.8% for the ipilimumab + nivolumab combination, even lower values for anti-PD-1 monotherapy), significantly lower than the toxicities of other sites (such as hepatic or gastrointestinal) (129). The management of skin toxicities needs specific treatment for the type and severity of the condition: topical glucocorticoids are effective in treating low-grade skin reactions, whereas high-grade events must be treated with systemic glucocorticoids.
Diarrhea and Colitis
Diarrhea is the most frequent manifestation of gastrointestinal toxicity from ICI and is one of the main causes of emergency department visits for patients treated with ICI (130). Colitis occurs more commonly with ipilimumab and with CICB than with anti-PD-1/PD-L1 mAbs alone (120, 131). No differences have been described in the incidence of diarrhea/colitis in different types of malignancy (132). The presence of ulcerative lesions appears to be related to a greater probability of glucocorticoid resistance and greater severity of diarrhea (133). Lesions primarily affect the distal colon, but lesions can occur in more proximal tracts. Colitis-associated autoantibodies are seldom present (134). Prophylactic budesonide is not effective against ipilimumab-induced colitis (135). Infliximab induces a shorter time to symptom resolution than glucocorticoids (136). Vedolizumab (an antibody directed against α4-β7 integrin), has also been used as glucocorticoid sparing agent (137). There is some evidence that early treatment with either infliximab or vedolizumab reduces symptom duration and glucocorticoid administration (138). Several clinical trials are evaluating the safety and efficacy of infliximab (NCT05034536, NCT03293784, NCT04407247) or certolizumab (NCT03293784) in clinical response of ICI-induced colitis.
Recent clinical and experimental models indicate that higher abundance of B. intestinalis is associated with high grade-colitis in melanoma patients treated with CICB (96). The gut microbiome appears to mediate intestinal toxicity via IL-1β and treatment of mice with an anti-IL-1R antagonist (anakinra) reduced intestinal inflammation. A clinical trial is evaluating the safety and efficacy of anakinra on irAEs and cytokine profiles in patients with different cancers (NCT04576429).
Hepatitis and Pancreatitis
Immune-mediated liver injury caused by ICIs can present with fatigue, fever, nausea, and jaundice. The severity of hepatic toxicity can be classified in relation to the increase in liver enzymes (AST and ALT) and bilirubinemia. The incidence of irAEs varies between 3% to 9% for anti-CTLA-4, and 0.7% to 1.8% for anti-PD-1/PD-L1 (139). CICB (ipilimumab + nivolumab) is associated with an incidence of any-grade hepatotoxicity of 29% and high-grade hepatotoxicity of 17% (39). Approximately 50% of the patients with ICI-associated liver injury have antinuclear antibodies and 19% have anti-smooth muscle antibodies (140). Pancreatitis can also occur in response to ICIs (141).
Thyroiditis
Thyroid dysfunction is the most common endocrinologic irAEs due to ICIs (27, 29, 45). The median time of onset is approximately 6 weeks after the start of immunotherapy. ICI-associated thyroid dysfunction is more common after anti-PD-1/PD-L1 antibodies or combination therapy than with ipilimumab alone (41, 142, 143). Patients receiving ICIs should undergo regular thyroid testing (i.e., TSH and T4). Patients usually experience a first transient phase of hyperthyroidism, followed by euthyroidism or hypothyroidism (45, 144). The presence of thyroid autoantibodies (anti-TPO and anti-TG) before ICI treatment increases the risk of thyroid dysfunction in patients treated with nivolumab or pembrolizumab (85–87, 109). The mechanism responsible for ICI-induced thyroid dysfunction is unclear. It has been hypothesized that polymorphic variants of the PDCD1 in some individuals might predispose them to an increased risk of thyroid dysfunction (145). It is also unknown whether thyroid autoantibodies are the cause of thyroid dysfunction or the result of an immunological response to thyroid antigens released during ICI-related thyroiditis (146). ICI therapy can be continued with close follow-up and monitoring of TSH and T4 in the context of thyroiditis. In the presence of more severe irAEs, ICI should be held until symptoms resolve. Glucocorticoids do not improve the clinical course of thyroid dysfunction (121).
Hypophysitis
Hypophysitis is a specific complication of ipilimumab treatment and rarely occurs in anti-PD-1/PD-L1-treated individuals (62, 91, 92). Most patients present with headache and/or fatigue (147, 148). Magnetic resonance imaging (MRI) of the brain highlights an expansion of the pituitary gland and/or infundibulum (147–149). Enlargement on MRI precedes the clinical diagnosis of ipilimumab-related hypophysitis (147). The pituitary gland decreases in size over 4-12 weeks leading to atrophy (147, 150, 151). Importantly, a normal MRI does not completely rule out hypophysitis, and therapy should be based on clinical symptoms and pituitary hormone levels (152). Glucocorticoids do not improve the degree or duration of hypophysitis (149). Hormone deficiencies are managed with the corresponding hormone replacement (149).
Diabetes
Although not very common (42), PD-1 pathway blockade can cause autoimmune ICI-induced diabetes mellitus, which usually (≅ 70%) presents as diabetic ketoacidosis (29, 50, 153–156). ICI-associated diabetes rarely occurs in patients treated with ipilimumab (42, 118). In these individuals, C-peptide levels are very low and approximately 50% of patients have T1D associated antibodies (anti-glutamic acid decarboxylase) (43). Human leukocyte antigen risk alleles (HLA-DR4, -DQ8, -DR3, and DR2) can be associated with high frequency of spontaneous T1D (157). In ICI-induced diabetes, there is a predominance of HLA-DR4, which is present in approximately 70% of patients. Other HLA alleles associated with high risk of spontaneous T1D are not overrepresented in ICI-associated diabetes (42, 43). ICI-induced diabetes tends to be permanent (50) and attempts with glucocorticoids administration showed no recovery (158).
Pneumonitis
Early clinical trials and meta-analyses suggested an incidence of ICI-associated pneumonitis of 3-5% (159–163); recent studies examining real-world populations suggest this could be as high as 13-19% (164–166). While the incidence of all-grade pneumonitis appears to be higher in the real-world population as opposed to clinical trials, the percentage of ≥ grade 3 pneumonitis appears to be relatively consistent across both populations (163–165, 167).
Immune-related pneumonia represents one of the main causes of death during treatment with anti-PD-1/PD-L1 alone and the fourth cause during combined treatment with ipilimumab plus anti-PD-1/PD-L1 (14% of total cases) after colitis, myocarditis and hepatitis (20). Anti-CTLA-4 treatment causes a lower incidence of immune-related pneumonia compared to treatment with anti-PD-1/PD-L1 alone (168). Immune-related pneumonia are usually associated with CICB (169). One-third of these patients are asymptomatic, whereas the others present with dyspnea and/or cough (159). Radiographic findings on chest computed tomography (CT) do not highlight specific characteristics (159, 170). Previous thoracic radiotherapy and previous lung disorders are predictors of pneumonitis associated with anti-PD-1 (171, 172). The differential diagnosis of these patients should be made after ruling out other causes of similar lung involvement. This issue is particularly relevant during the current outbreak of COVID-19 (173). Guidelines on the management of ICI-related pneumonitis have been published from ESMO (174) and ASCO/NCCN (175–177). A clinical trial is evaluating the safety and efficacy of infliximab versus intravenous immunoglobulin therapy (IVIG) in treating glucocorticoid-refractory pneumonitis (NCT04438382).
Arthritis
Arthritis is not very common (≅ 4%) in patients with ICIs (178). A systematic review encompassing 372 patients found that the time of onset of arthritis ranged from 1 day to 53 months (median time: 4 months) (51); 49% had polyarthritis, 17% oligoarthritis, 10% arthralgia, and 21% polymyalgia rheumatica (178–181). More than half of patients had a “rheumatoid arthritis-like” presentation (51). RF and anti-citrullinated peptide antibodies (ACPA) are present in ≅19% of patients (51, 180, 182). Treatment of irAEs should be guided by severity (183). Most patients can be managed with non-steroidal anti-inflammatory drugs or intra-articular glucocorticoid injections. More severe patients, especially those who received CICB, require systemic glucocorticoids (182). Arthritis often persists even after stopping ICIs and may require prolonged immunosuppression with biological disease-modifying anti-rheumatic drugs (DMARDs) (40). Diagnostic and management algorithms for rheumatoid irAEs have been recently proposed (183).
Myositis
ICI-related myositis can manifest in the form of acute or subacute myalgia or muscle weakness (183, 184). When concomitant myocarditis and myasthenia gravis-like symptoms (e.g., ptosis and oculomotor weakness) occur, fatality rates are relatively high (185). Muscle biopsy shows inflammation and myonecrosis (184). Myositis-associated antibodies (anti-TIF1-y, SRP, Ro52; PL-7, PL-12, or SRP) can be detected in ICI-associated myositis (186). Anti-striated muscle antibodies can be found even without clinical evidence of myasthenia gravis (187, 188). Glucocorticoids are the first-line therapy for ICI-associated myositis. Initial dosing can range from 0.5 mg/Kg prednisone daily up to 2,000 mg IV methylprednisolone (183). IVIG and plasmapheresis have been used in refractory cases (183, 184, 186).
Myocarditis
The true incidence of ICI-associated myocarditis remains uncertain. Early ICI-based cancer trials did not prospectively screen for myocarditis (189). Moreover, because the diagnosis of myocarditis can be difficult, cases could easily be missed. Recent reports suggest that the incidence of ICI-associated myocarditis is 0.27% to 1.14% (76, 190). Myocarditis is an infrequent, but often lethal complication of ICI therapy (76, 191). Elevated troponin level and abnormal electrocardiogram were found in the majority of these patients with ICI-associated myocarditis. Interestingly, half of these patients showed preserved ejection fraction (190). The clinical manifestation of ICI-associated myocarditis is variable. Fulminant cases characterized by early-onset have been described (19, 123, 192). In these cases, cardiac arrhythmias are common (23, 190). The association of skeletal myositis and myasthenia gravis following ICI therapy should orientate for myocarditis (185, 193). “Smouldering” cases of myocarditis have been also reported (194).
Diagnosis of ICI-associated myocarditis is challenging and includes a combination of biomarker tests (troponin), cardiac MRI, late gadolinium enhancement, and possibly biopsy (T cell infiltrate) (195). Major adverse cardiac events (MACE) can occur also in patients with preserved ejection fraction. A troponin T level ≥ 1.5 ng/mL was associated with a marked increase in MACE during follow-up (190). The precise mechanisms by which ICIs cause cardiotoxicity remain undefined. Existing data support T cell-mediated immunity as a major component in pathogenesis, but many fundamental questions remain (24). Early and aggressive treatment with high doses of glucocorticoids is critical (132, 190, 196). Treatment of ICI-associated myocarditis includes ICI discontinuation, supportive management, and glucocorticoids (175). Prednisone (0.5 to 2.0 mg/kg), followed by 4–6 week taper upon symptoms improvement, is recommended (24, 175, 197). Despite this treatment, mortality remains substantial, and individual case reports demonstrate successful treatment with alemtuzumab (anti-CD52 mAb) (198), or abatacept (a fusion protein composed of the extracellular domain of CTLA-4 and the Fc region of human IgG1) (1). Prospective clinical trials are needed to compare the safety and efficacy of different immunosuppressive therapies in ICI-associated myocarditis. Recent experimental data point to the hypothesis that anti-PD-1 therapy induces a smouldering disruption of cardiac immunity towards an inflammatory phenotype, with manifest consequences on cardiac function in the presence of a second hit, in the form of systemic stress induced by presence of a tumor (199). This also indicates the possibility that the inflammatory phenotype raises the risk for the development of myocarditis upon exposure to additional, yet unknown risk factors (200). Furthermore, treatment with anti-PD-1 may first produce only a latent inflammatory involvement associated to dysregulated cardiac metabolism that may progress to overt myocarditis in a subset of patients (200, 201).
Neurological
Neurological complications of ICIs (headache, myasthenia gravis, peripheral neuropathy, meningitis, and encephalitis) are uncommon (≅ 1%) (32, 77, 184, 202, 203). Myasthenia gravis (77) and encephalitis are more common with anti-PD-1 antibodies, whereas Guillain Barré and meningitis are more common with ipilimumab. MG associated with myositis and myocarditis has a poor prognosis (64, 203). Approximately 50% of MG patients have anti-acetylcholine receptor antibodies (64). Glucocorticoids and, in some patients, IVIG, are the mainstay of therapy (64).
Hematologic
In contrast to other anticancer therapies, hematological irAEs in patients treated with ICIs are uncommon. Neutropenia, autoimmune hemolytic anemia, and immune thrombocytopenia can occur rarely (≅ 5%) (204, 205). A positive direct antiglobulin test is present in the majority (≅ 60%) of patients with ICI-related autoimmune hemolytic anemia (206). Glucocorticoids are the first line of therapy; IVIG or rituximab can be considered in difficult cases. Neutropenic patients can be treated with G-CSF (204, 206).
Renal
Renal dysfunction is rare with ipilimumab and with anti-PD-1/PD-L1 therapies occurring in <1% of patients (207). The incidence is higher with combination of ipilimumab plus anti-PD-1/PD-L1 reaching approximately 4% (208, 209). Renal dysfunction is usually due to acute interstitial nephritis (210) or, more rarely, to glomerulonephritis (211).
Ocular
irAEs of the eye are rare and occur in <1% of patients treated with ICIs (212, 213). Uveitis can be a complication of ICI treatment (214). Few cases of Vogt-Koyanagi-Harada disease have been described in melanoma patients, which hinted a possible cross-reactivity between T lymphocytes targeting melanoma cells and the melanocytes of the eye (215). In the cases of ICI-associated sicca syndrome, oral manifestations are more common than the ocular ones (216). Anti-Ro (SS-A) and anti-La (SS-B) are usually negative (216).
Pre-Existing Autoimmune Disease and ICIs
Cancer patients with underlying autoimmune disease were initially excluded from ICI RCT (162, 217–219). Therefore, the prevalence and incidence of exacerbations of pre-existing autoimmune disorders was not immediately appreciated. Patients with autoimmune disease, however, represent 20 to 50 million people in the United States alone, and one study reported that approximately 13% of lung cancer patients had a concurrent diagnosis of autoimmune disease (220).
There is now evidence that irAEs are more frequent and occur faster in cancer patients with several autoimmune diseases (psoriasis, rheumatoid arthritis, IBD, systemic lupus erythematosus, vasculitis) (106–108). These findings suggest that a close monitoring is mandatory during early phases of treatment. The majority of autoimmune flares and irAEs can be managed without ICI discontinuation, but fatalities can occur (221). Conflicting results have been reported in studies concerning the risk of autoimmune flares or irAEs in patients with active versus inactive autoimmune disease (106, 108, 221, 222). Patients with underlying autoimmune disorders should be carefully managed by multidisciplinary teams.
IrAEs and Efficacy of ICIs
In one large, retrospective study of ipilimumab, the treatment outcomes were similar in patients with and without irAEs (223). Subsequent studies reported that melanoma patients who develop vitiligo or endocrine complications have better tumor response and improved survival (122, 175, 224–226). The results of randomized trials have shown that patients who discontinue ICIs due to toxicity respond better than patients without irAEs (227). Patients that developed thyroiditis after PD-1 or PD-L1 blockade had longer overall survival compared to the thyroid irAE negative group (60, 225). Prospective studies are needed to verify whether different irAEs are associated with improved tumor response to ICIs.
Management of IrAEs
No trials are evaluating the efficacy of different irAEs treatments. Therefore, management of irAEs are based on retrospective studies and expert consensus (11, 29, 31, 45, 174, 175, 228, 229). Low-grade irAEs usually do not need ICI discontinuation and immunosuppressive treatment. Higher grade irAEs may require both therapeutic strategies. Table 2 schematically summarizes the anti-inflammatory and immunosuppressive drugs routinely used to treat ICI-related irAEs.
Table 2.
Therapies used for immune adverse events associated with immune checkpoint inhibitors.
| Drug | Mechanism of action | Efficacy in reversing irAE | Effect on tumor response | Clinical trial |
|---|---|---|---|---|
| Glucocorticoids | Anti-inflammatory and immunosuppressive |
First line therapy for most ICI-induced irAEs Not effective for reversing endocrinopathies |
Some studies suggest that response rates and survival are not affected by low doses of glucocorticoids (223, 224, 230), whereas others (122, 231–235) show negative impact of high doses. In vitro experiments data suggest negative impact of high dose glucocorticoids on anti-tumor effects of T cells (236). |
|
| Infliximab | Anti-TNF-α mAb | Colitis (16). | Controversial results on the net effect of TNF-α inhibition on tumorigenesis (237–240). In murine models, prophylactic TNF-α inhibition eliminated ICI-induced colitis without affecting anti-tumor response (241). |
NCT05034536: Comparison of pembrolizumab + infliximab versus pembrolizumab + placebo in patients with melanoma. NCT03293784: Comparison of infliximab or certolizumab + nivolumab + ipilimumab in patients with melanoma. NCT04407247: Comparison of vedolizumab versus infliximab for clinical remission/response of ICI-associated diarrhea/colitis. |
Glucocorticoids are powerful anti-inflammatory and immunosuppressive drugs commonly employed as first-line treatment in patients with ICI-induced irAEs. The efficacy of glucocorticoids varies tremendously in the treatment of different types of irAEs. For instance, these compounds are effective in most cases but do not reverse hypophysitis, and high doses may worsen outcomes (122). By contrast, initial high-dose glucocorticoids were superior to intermediate and low-dose steroid in the treatment of ICI-associated myocarditis (242). Another important issue is the timing of initiation of glucocorticoid administration. In the above retrospective, observational study, early (≤ 24 hours) administration of glucocorticoids can also vary in different irAEs. For example, arthritis is also unique since inflammation often persists even after stopping ICIs and may require prolonged glucocorticoid treatment (40) and/or biological DMARDs (243).
Glucocorticoids are associated with potential multiple side effects and impact on anti-tumor response. Low doses of glucocorticoids used to treat irAEs do not affect the response rates and/or the survival of ICI-treated patients (223, 224, 230). A meta-analysis found that glucocorticoids do not negatively affect survival (230). However, glucocorticoids before or early during ICI treatment may negatively affect outcomes (231–234). Baseline treatment of lung cancer patients with anti-PD-1 and high doses (> 10 mg/day) of prednisone negatively affects outcomes compared to those treated with low dose glucocorticoids (231, 235). The side effects of glucocorticoids depend on the daily dose, the cumulative dose administered and possibly the type of underlying disease (244). Therefore, awareness of specific glucocorticoid-induced side effects is required.
irAEs refractory to glucocorticoids may require the administration of a mAb targeting TNF-α (i.e., infliximab) to treat certain irAEs such as colitis (16, 136, 237, 245) and pneumonitis (25, 175, 246). Preclinical studies suggest that prophylactic TNF-α inhibition eliminated ICI-induced colitis without affecting anti-tumor response (241). There is conflicting evidence on the effect of short-term TNF-α inhibition with infliximab to treat ICI-induced irAEs on overall survival in cancer patients (237–240). A comprehensive review has examined in detail the role of different cytokines in the pathophysiology of irAEs. In addition, the authors provided an in-depth analysis of strategies to uncouple the cytokine response that participates in ICI-associated irAEs (247).
Potential Therapies and Prevention of IrAEs
The increasing use of ICIs in a growing number of solid and hematologic cancers requires us to offer the best-targeted therapies of irAEs. Table 3 summarizes the potential therapies of irAEs.
Table 3.
Emerging and potential future therapies for immune adverse events associated with immune checkpoint inhibitors.
| Drug | Mechanism of action | Efficacy in reversing irAE | Effect on tumor response | Clinical trial |
|---|---|---|---|---|
| Vedolizumab | Anti-integrin α4β7 mAb | Colitis (137, 248). Prevention of autoimmune flares in patients with IBD (249). |
Favorable clinical outcomes (248, 249). |
NCT04407247: Comparison of vedolizumab versus infliximab for clinical response of ICI-induced diarrhea/colitis. NCT04797325: Comparison of vedolizumab versus prednisolone for clinical response of ICI-induced colitis. |
| Alemtuzumab | CD52 mAb | Myocarditis (198). | Unknown | |
| Abatacept | CTLA-4 agonist | Myocarditis (250). | Unknown | |
| Rituximab | Anti-CD20 mAb | Neurological complications (e.g., encephalitis and myasthenia gravis), bullous pemphigoid-like skin disease, renal vasculitis, hematological complications (12, 175, 204, 251–253). | Progression, partial and complete responses reported (251–253). | NCT03719131: Evaluation of rituximab on ICI-induced irAEs. |
| Tocilizumab | Anti-IL-6 receptor mAb | Pneumonitis, colitis, and pancreatitis (254). Inflammatory arthritis (255). |
Clinical improvement with trend towards worse survival with increased doses of tocilizumab (254). 1/3 patients maintained anti-tumor response (255). |
NCT03999749: Evaluation of tocilizumab on diarrhea and/or colitis and/or arthritis induced by ICIs. NCT04691817: Evaluation of tocilizumab on irAEs in patients with non-small lung cancer (NSCLC) treated with atezolizumab. |
| Secukinumab | Anti-IL-17A mAb | Psoriatic rash and colitis (256). Psoriasiform dermatological complication (257). |
Tumor progression occurred in one patient (256). No impact on tumor response (257). | |
| Anakinra | IL-1 receptor antagonist | Experimental intestinal inflammation associated with combined immune checkpoint blockade in mice (96). | Unknown |
NCT04576429: Evaluation of anakinra on irAEs and cytokine profile in patients with different cancers. |
| Fecal microbiota transplant | Possibly increased Tregs and decreased effector T cells (258). | Colitis (258). | Unknown |
NCT04038619: Phase I trial of fecal microbiota transplant (FMT) for ICI-induced colitis/diarrhea. NCT04163289: Prevention of irAEs using fecal microbiota transplant. NCT03819296: Prevention of gastrointestinal irAEs by FMT in patients with melanoma or genitourinary cancer. |
| Certolizumab | anti-TNF-α |
NCT03293784: Comparison of infliximab or certolizumab + nivolumab + ipilimumab in patients with melanoma. |
||
| Intravenous immunoglobulin (IVIG) |
NCT04438382: Comparison of infliximab versus IVIG in patients with pneumonitis. |
Understanding the pathophysiology of ICI-associated myocarditis and developing effective treatments is of great importance. The quest for novel therapies for glucocorticoid-resistant ICI myocarditis is a clinical unmet need. If symptoms and laboratory findings in ICI myocarditis do not regress upon high-dose glucocorticoids, other immunosuppressant agents [e.g. mycophenolate mofetil, methotrexate, calcineurin inhibitors, intravenous immunoglobulin (IVIG), anti-thymocyte globulin, rituximab and infliximab] may be considered for treatment of ICIs cardiotoxicity, but data are still controversial (201, 259, 260).
Alemtuzumab (anti-CD52 mAb) and abatacept, a protein consisting of the human CTLA-4 extracellular domain fused to the Fc portion of IgG, acting as a CTLA-4 agonist, have been employed for the treatment of single cases of glucocorticoid-refractory myocarditis (198, 250). Concerns with abatacept are possible infections and tumor progression. Abatacept has also been used to treat ICI-induced MG (261). Alemtuzumab causes T cell depletion and its impact on tumor growth remains unknown. Overall, the evidence available at present is insufficient to support any of the anecdotal, albeit reasonable, strategies outlined above and more evidence-based guidance in this critical care is urgently needed. While blocking TNF-α in heart failure (HF) has been proven contraindicated in symptomatic (NYHA III and IV) patients (262, 263), anti-TNF-α may be a promising approach to prevent the early stages of cardiotoxicity from anti-PD-1 immunotherapy (200).
Vedolizumab is a specific anti-integrin α4β7 antibody, used for the treatment of IBD (264–266). Preclinical studies have reported that vedolizumab induces remission in ICI-induced glucocorticoid-refractory colitis with good safety profiles (137, 248). Early treatment with vedolizumab is a potential treatment of ICI-associated colitis (138). Two clinical trials are evaluating the safety and efficacy of vedolizumab in the treatment of ICI-induced colitis (NCT04407247, NCT04797325).
Rituximab, a mAb targeting CD20, has been used in glucocorticoid-refractory encephalitis and myasthenia gravis (251, 252), bullous pemphigoid-like skin disease (12), renal vasculitis (253), and hematological complications (204).
Tocilizumab, a mAb anti-IL-6 receptor, has been used to treat ICI-associated arthritis (255) and glucocorticoid-refractory irAEs (254). The safety and efficacy of tocilizumab on ICI-associated irAEs are under evaluation in two clinical trials (NCT03999749, NCT04691817).
IL-17 blockade has been used in few patients with colon cancer and melanoma (256, 257). A recent study found that there is an upregulation of intestinal IL-1β in melanoma patients treated with CICB who developed high-grade colitis (96). In two mouse models, CICB was associated with intestinal inflammation characterized by upregulation of Il1b, but not Tnfa or Il6. Interestingly, mice concurrently treated with CICB and anakinra (anti-IL-1R) showed less intestinal inflammation. These parallel studies in humans and mice suggest that severe intestinal inflammation associated with CICB could be prevented by an IL-1R antagonist. A clinical trial is evaluating the safety and efficacy of anakinra on ICI-induced irAEs (NCT04576429).
Gut microbiome can influence efficacy of PD-1-based immunotherapy (103, 105). A clinical trial is prospectively analyzing the intestinal microbiome as predictor of ICI-associated irAEs (NCT04107311). Fecal microbiota transplant (FMT), which transfers an entire microbiome from a healthy donor to a recipient, is a therapeutic tool with several potential applications but numerous caveats (267). FMT may be a potential mechanism for treating ICI-induced colitis (258). FMT can be performed via an oral capsule containing fecal extracts (268–270), colonoscopy-guided transfer (269, 271) or enema (272, 273). Microbiome composition varies widely among healthy donors and can affects success rates (274, 275). FMT involves the risk for transmission of infectious agents via FMT (276). Regulatory authorities have released specific guidelines to offer FMT with safety at the time of COVID-19 pandemic (277). Two patients with refractory ICI-associated colitis were successfully treated with FMT (258). Clinical and experimental studies are needed to evaluate the efficacy of this approach as well as to provide further mechanistic insights. In summary, there is some evidence that microbiome manipulation could potentially impact cancer course and perhaps ICI-associated irAEs. Several clinical trials are evaluating the safety and efficacy of FMT on the prevention/treatment of intestinal irAEs (NCT04038619, NCT04163289, NCT03819296).
Conclusions and Perspectives
Emerging real-world data suggests the incidence of ICI-associated irAEs may be higher than previously found in clinical trials. This trend has the potential to increase further as the use of canonical ICIs (anti-CTLA-4 and anti-PD-1/PD-L1), alone or in combination, is increasing exponentially and nearly 50% of patients treated will experience some form of irAEs (278). Furthermore, the combination of first and/or second generation of ICIs (e.g., anti-TIGIT) or with anti-angiogenic agents (279) could open a new scenario of irAEs associated with novel forms of cancer immunotherapy.
Great efforts have been devoted to the identification of genetic, humoral and cellular biomarkers predictive of irAEs associated with ICIs. Although these putative biomarkers have not been incorporated into clinical practice, they have highlighted some novel aspects of irAE pathogenesis. For instance, recent human and experimental studies have highlighted the contribution of specific gut microbiota to intestinal damage caused by CICB in melanoma patients (96). This study also suggests that specific peripheral blood signatures are associated with a risk of developing toxicity after CICB. Of course, additional studies of larger cohorts of ICI-treated patients with different cancers will be needed to validate these findings.
Management of irAEs is essentially based on retrospective studies and expert consensus (11, 29, 31, 45, 174, 175, 228, 229). Glucocorticoids, commonly employed as first-line treatment of irAEs, do not affect certain toxicities, and high doses may negatively affect outcomes (122, 231–234). Awareness of glucocorticoid-induced side effects is required. A wide spectrum of emerging therapeutic options, including mAbs (anti-TNF-α, anti-IL-6, anti-CD20, anti-IL-1R) (12, 96, 241, 251, 252, 254) and FMT (96, 258) are under clinical and experimental investigations. There is currently no clinical evidence that irAEs can be pharmacologically prevented. However, there is experimental evidence that prophylactic administration anti-TNF-α (241) or anti-IL-1R (96) is associated with less intestinal inflammation caused by CICB in different tumor models.
Table 4 presents some of the outstanding pathophysiological and therapeutic questions that should be addressed to better understand the complexity of different irAEs. Due to the clinical complexity surrounding autoimmune disease development and management, Clinical Immunologists should be involved in the care of cancer patients before, during, and after checkpoint blockade. Perhaps, multidisciplinary irAE management teams, facilitating prompt diagnosis and treatment should be enlisted. Further research is needed to improve early diagnosis, understand immunological and genetic mechanisms, and develop management algorithms for these disorders.
Table 4.
Outstanding pathophysiological and therapeutic questions relevant to immune-related adverse events (irAEs) associated with immune checkpoint inhibitors.
|
Author Contributions
RP, GC, FC, CT, and GV wrote and edited the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported in part by grants from the CISI-Lab Project (University of Naples Federico II) and TIMING Project and Campania Bioscience (Regione Campania).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank the medical artist Fabrizio Fiorbianco for preparing figures and the administrative staff (Dr. Roberto Bifulco, Dr. Anna Ferraro and Dr. Maria Cristina Fucci), without whom it would not be possible to work as a team. The authors apologize to the many researchers who have contributed importantly to the field and whose work was not cited due to space constraints.
Abbreviations
ACPA, anti-citrullinated peptide antibodies; B. intestinalis, Bacteroides intestinalis; CT, chest computed tomography; CICB, combined immune checkpoint blockade; CCP, cyclic citrullinated peptide; CTLA-4, cytotoxic T-lymphocyte-associated protein 4; DMARDs, disease modifying anti-rheumatic drugs; FMT, fecal microbiota transplant; G-CSF, granulocyte colony-stimulating factor; GM-CSF, granulocyte-macrophage colony-stimulating factor; GNAL, guanine nucleotide-binding protein G subunit alpha; HLA, human leukocyte antigen; ICIs, immune checkpoint inhibitors; irAEs, immune-related adverse events; IBD, inflammatory bowel disease; ITM2B, integral membrane protein 2B; IVIG), intravenous immunoglobulin therapy; MRI, magnetic resonance imaging; MACE, major adverse cardiac events; mAbs, monoclonal antibodies; MCP-1, monocyte chemoattractant protein-1; NCI-CTCAE, national cancer institute common terminology criteria for adverse events; NSCLC, non-small cell lung cancer; PD-1, programmed cell death protein 1; PD-L1, programmed cell death protein ligand 1; PRS, polygenic risk score; RCT, randomized controlled trial; RF, rheumatoid factor; SS-A, Sjogren’s syndrome antibodies anti-Ro; SS-B, Sjogren’s syndrome antibodies anti-La; TG, thyroglobulin; TPO, thyroid peroxidase; Trm, tissue-resident memory T; 18F-FDG, tomography/computed tomography with 2-[18F] fluorodeoxyglucose; TNF-α, tumor necrosis factor-α; T1D, type 1 diabetes.
References
- 1. Wei SC, Duffy CR, Allison JP. Fundamental Mechanisms of Immune Checkpoint Blockade Therapy. Cancer Discov (2018) 8:1069–86. doi: 10.1158/2159-8290.CD-18-0367 [DOI] [PubMed] [Google Scholar]
- 2. Sharma P, Allison JP. Immune Checkpoint Targeting in Cancer Therapy: Toward Combination Strategies With Curative Potential. Cell (2015) 161:205–14. doi: 10.1016/j.cell.2015.03.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lebbe C, Weber JS, Maio M, Neyns B, Harmankaya K, Hamid O, et al. Survival Follow-Up and Ipilimumab Retreatment of Patients With Advanced Melanoma Who Received Ipilimumab in Prior Phase II Studies. Ann Oncol (2014) 25:2277–84. doi: 10.1093/annonc/mdu441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wolchok JD, Chiarion-Sileni V, Gonzalez R, Rutkowski P, Grob JJ, Cowey CL, et al. Overall Survival With Combined Nivolumab and Ipilimumab in Advanced Melanoma. N Engl J Med (2017) 377:1345–56. doi: 10.1056/Nejmoa1709684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Five-Year Survival and Correlates Among Patients With Advanced Melanoma, Renal Cell Carcinoma, or Non-Small Cell Lung Cancer Treated With Nivolumab. JAMA Oncol (2019) 5:1411–20. doi: 10.1001/jamaoncol.2019.2187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ribas A, Wolchok JD. Cancer Immunotherapy Using Checkpoint Blockade. Science (2018) 359:1350–5. doi: 10.1126/science.aar4060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fritz JM, Lenardo MJ. Development of Immune Checkpoint Therapy for Cancer. J Exp Med (2019) 216:1244–54. doi: 10.1084/jem.20182395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Topalian SL, Sharpe AH. Balance and Imbalance in the Immune System: Life on the Edge. Immunity (2014) 41:682–4. doi: 10.1016/j.immuni.2014.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ise W, Kohyama M, Nutsch KM, Lee HM, Suri A, Unanue ER, et al. CTLA-4 Suppresses the Pathogenicity of Self Antigen-Specific T Cells by Cell-Intrinsic and Cell-Extrinsic Mechanisms. Nat Immunol (2010) 11:129–35. doi: 10.1038/ni.1835ni.1835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Menzies AM, Johnson DB, Ramanujam S, Atkinson VG, Wong ANM, Park JJ, et al. Anti-PD-1 Therapy in Patients With Advanced Melanoma and Preexisting Autoimmune Disorders or Major Toxicity With Ipilimumab. Ann Oncol (2017) 28:368–76. doi: 10.1093/annonc/mdw443 [DOI] [PubMed] [Google Scholar]
- 11. Muntyanu A, Netchiporouk E, Gerstein W, Gniadecki R, Litvinov IV. Cutaneous Immune-Related Adverse Events (irAEs) to Immune Checkpoint Inhibitors: A Dermatology Perspective on Management. J Cutan Med Surg (2021) 25:59–76. doi: 10.1177/1203475420943260 [DOI] [PubMed] [Google Scholar]
- 12. Phillips GS, Wu J, Hellmann MD, Postow MA, Rizvi NA, Freites-Martinez A, et al. Treatment Outcomes of Immune-Related Cutaneous Adverse Events. J Clin Oncol (2019) 37:2746–58. doi: 10.1200/JCO.18.02141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sibaud V. Dermatologic Reactions to Immune Checkpoint Inhibitors: Skin Toxicities and Immunotherapy. Am J Clin Dermatol (2018) 19:345–61. doi: 10.1007/s40257-017-0336-3 [DOI] [PubMed] [Google Scholar]
- 14. Soularue E, Lepage P, Colombel JF, Coutzac C, Faleck D, Marthey L, et al. Enterocolitis Due to Immune Checkpoint Inhibitors: A Systematic Review. Gut (2018) 67:2056–67. doi: 10.1136/gutjnl-2018-316948 [DOI] [PubMed] [Google Scholar]
- 15. Siakavellas SI, Bamias G. Checkpoint Inhibitor Colitis: A New Model of Inflammatory Bowel Disease? Curr Opin Gastroenterol (2018) 34:377–83. doi: 10.1097/MOG.0000000000000482 [DOI] [PubMed] [Google Scholar]
- 16. Wang Y, Abu-Sbeih H, Mao E, Ali N, Ali FS, Qiao W, et al. Immune-Checkpoint Inhibitor-Induced Diarrhea and Colitis in Patients With Advanced Malignancies: Retrospective Review at MD Anderson. J Immunother Cancer (2018) 6:37. doi: 10.1186/s40425-018-0346-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Herrmann J. Adverse Cardiac Effects of Cancer Therapies: Cardiotoxicity and Arrhythmia. Nat Rev Cardiol (2020) 17:474–502. doi: 10.1038/s41569-020-0348-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Neilan TG, Rothenberg ML, Amiri-Kordestani L, Sullivan RJ, Steingart RM, Gregory W, et al. Myocarditis Associated With Immune Checkpoint Inhibitors: An Expert Consensus on Data Gaps and a Call to Action. Oncologist (2018) 23:874–8. doi: 10.1634/theoncologist.2018-0157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Varricchi G, Galdiero MR, Marone G, Criscuolo G, Triassi M, Bonaduce D, et al. Cardiotoxicity of Immune Checkpoint Inhibitors. ESMO Open (2017) 2:e000247. doi: 10.1136/esmoopen-2017-000247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang DY, Salem JE, Cohen JV, Chandra S, Menzer C, Ye F, et al. Fatal Toxic Effects Associated With Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis. JAMA Oncol (2018) 4:1721–8. doi: 10.1001/jamaoncol.2018.3923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Varricchi G, Galdiero MR, Tocchetti CG. Cardiac Toxicity of Immune Checkpoint Inhibitors: Cardio-Oncology Meets Immunology. Circulation (2017) 136:1989–92. doi: 10.1161/CIRCULATIONAHA.117.029626 [DOI] [PubMed] [Google Scholar]
- 22. Tocchetti CG, Galdiero MR, Varricchi G. Cardiac Toxicity in Patients Treated With Immune Checkpoint Inhibitors: It Is Now Time for Cardio-Immuno-Oncology. J Am Coll Cardiol (2018) 71:1765–7. doi: 10.1016/j.jacc.2018.02.038 [DOI] [PubMed] [Google Scholar]
- 23. Escudier M, Cautela J, Malissen N, Ancedy Y, Orabona M, Pinto J, et al. Clinical Features, Management, and Outcomes of Immune Checkpoint Inhibitor-Related Cardiotoxicity. Circulation (2017) 136:2085–7. doi: 10.1161/CIRCULATIONAHA.117.030571 [DOI] [PubMed] [Google Scholar]
- 24. Moslehi J, Lichtman AH, Sharpe AH, Galluzzi L, Kitsis RN. Immune Checkpoint Inhibitor-Associated Myocarditis: Manifestations and Mechanisms. J Clin Invest (2021) 131:e145186. doi: 10.1172/JCI145186145186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Reuss JE, Suresh K, Naidoo J. Checkpoint Inhibitor Pneumonitis: Mechanisms, Characteristics, Management Strategies, and Beyond. Curr Oncol Rep (2020) 22:56. doi: 10.1007/s11912-020-00920-z [DOI] [PubMed] [Google Scholar]
- 26. Su Q, Zhu EC, Wu JB, Li T, Hou YL, Wang DY, et al. Risk of Pneumonitis and Pneumonia Associated With Immune Checkpoint Inhibitors for Solid Tumors: A Systematic Review and Meta-Analysis. Front Immunol (2019) 10:108. doi: 10.3389/fimmu.2019.00108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Byun DJ, Wolchok JD, Rosenberg LM, Girotra M. Cancer Immunotherapy - Immune Checkpoint Blockade and Associated Endocrinopathies. Nat Rev Endocrinol (2017) 13:195–207. doi: 10.1038/nrendo.2016.205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chang LS, Barroso-Sousa R, Tolaney SM, Hodi FS, Kaiser UB, Min L. Endocrine Toxicity of Cancer Immunotherapy Targeting Immune Checkpoints. Endocr Rev (2019) 40:17–65. doi: 10.1210/er.2018-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ferrari SM, Fallahi P, Elia G, Ragusa F, Ruffilli I, Patrizio A, et al. Autoimmune Endocrine Dysfunctions Associated With Cancer Immunotherapies. Int J Mol Sci (2019) 20:2560. doi: 10.3390/ijms20102560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Calabrese LH, Calabrese C, Cappelli LC. Rheumatic Immune-Related Adverse Events From Cancer Immunotherapy. Nat Rev Rheumatol (2018) 14:569–79. doi: 10.1038/s41584-018-0074-9 [DOI] [PubMed] [Google Scholar]
- 31. Kostine M, Finckh A, Bingham CO, Visser K, Leipe J, Schulze-Koops H, et al. EULAR Points to Consider for the Diagnosis and Management of Rheumatic Immune-Related Adverse Events Due to Cancer Immunotherapy With Checkpoint Inhibitors. Ann Rheum Dis (2021) 80:36–48. doi: 10.1136/annrheumdis-2020-217139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Spain L, Tippu Z, Larkin JM, Carr A, Turajlic S. How We Treat Neurological Toxicity From Immune Checkpoint Inhibitors. ESMO Open (2019) 4:e000540. doi: 10.1136/esmoopen-2019-000540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Michot JM, Lazarovici J, Tieu A, Champiat S, Voisin AL, Ebbo M, et al. Haematological Immune-Related Adverse Events With Immune Checkpoint Inhibitors, How to Manage? Eur J Cancer (2019) 122:72–90. doi: 10.1016/j.ejca.2019.07.014 [DOI] [PubMed] [Google Scholar]
- 34. Wei SC, Levine JH, Cogdill AP, Zhao Y, Anang NAS, Andrews MC, et al. Distinct Cellular Mechanisms Underlie Anti-CTLA-4 and Anti-PD-1 Checkpoint Blockade. Cell (2017) 170:1120–1133 e1117. doi: 10.1016/j.cell.2017.07.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Arnaud-Coffin P, Maillet D, Gan HK, Stelmes JJ, You B, Dalle S, et al. A Systematic Review of Adverse Events in Randomized Trials Assessing Immune Checkpoint Inhibitors. Int J Cancer (2019) 145:639–48. doi: 10.1002/ijc.32132 [DOI] [PubMed] [Google Scholar]
- 36. Gu L, Khadaroo PA, Su H, Kong L, Chen L, Wang X, et al. The Safety and Tolerability of Combined Immune Checkpoint Inhibitors (Anti-PD-1/PD-L1 Plus Anti-CTLA-4): A Systematic Review and Meta-Analysis. BMC Cancer (2019) 19:559. doi: 10.1186/s12885-019-5785-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang Y, Zhou S, Yang F, Qi X, Wang X, Guan X, et al. Treatment-Related Adverse Events of PD-1 and PD-L1 Inhibitors in Clinical Trials: A Systematic Review and Meta-Analysis. JAMA Oncol (2019) 5:1008–19. doi: 10.1001/jamaoncol.2019.0393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Weber JS, Kahler KC, Hauschild A. Management of Immune-Related Adverse Events and Kinetics of Response With Ipilimumab. J Clin Oncol (2012) 30:2691–7. doi: 10.1200/JCO.2012.41.6750 [DOI] [PubMed] [Google Scholar]
- 39. Sznol M, Ferrucci PF, Hogg D, Atkins MB, Wolter P, Guidoboni M, et al. Pooled Analysis Safety Profile of Nivolumab and Ipilimumab Combination Therapy in Patients With Advanced Melanoma. J Clin Oncol (2017) 35:3815–22. doi: 10.1200/JCO.2016.72.1167 [DOI] [PubMed] [Google Scholar]
- 40. Braaten TJ, Brahmer JR, Forde PM, Le D, Lipson EJ, Naidoo J, et al. Immune Checkpoint Inhibitor-Induced Inflammatory Arthritis Persists After Immunotherapy Cessation. Ann Rheum Dis (2020) 79:332–8. doi: 10.1136/annrheumdis-2019-216109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chan KK, Bass AR. Autoimmune Complications of Immunotherapy: Pathophysiology and Management. BMJ (2020) 369:m736. doi: 10.1136/bmj.m736 [DOI] [PubMed] [Google Scholar]
- 42. Stamatouli AM, Quandt Z, Perdigoto AL, Clark PL, Kluger H, Weiss SA, et al. Collateral Damage: Insulin-Dependent Diabetes Induced With Checkpoint Inhibitors. Diabetes (2018) 67:1471–80. doi: 10.2337/dbi18-0002dbi18-0002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. de Filette JMK, Pen JJ, Decoster L, Vissers T, Bravenboer B, van der Auwera BJ, et al. Immune Checkpoint Inhibitors and Type 1 Diabetes Mellitus: A Case Report and Systematic Review. Eur J Endocrinol (2019) 181:363–74. doi: 10.1530/EJE-19-0291EJE-19-0291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. de Filette J, Jansen Y, Schreuer M, Everaert H, Velkeniers B, Neyns B, et al. Incidence of Thyroid-Related Adverse Events in Melanoma Patients Treated With Pembrolizumab. J Clin Endocrinol Metab (2016) 101:4431–9. doi: 10.1210/jc.2016-2300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Elia G, Ferrari SM, Galdiero MR, Ragusa F, Paparo SR, Ruffilli I, et al. New Insight in Endocrine-Related Adverse Events Associated to Immune Checkpoint Blockade. Best Pract Res Clin Endocrinol Metab (2020) 34:101370. doi: 10.1016/j.beem.2019.101370 [DOI] [PubMed] [Google Scholar]
- 46. Owen CN, Bai X, Quah T, Lo SN, Allayous C, Callaghan S, et al. Delayed Immune-Related Adverse Events With Anti-PD-1-Based Immunotherapy in Melanoma. Ann Oncol (2021) 32:917–25. doi: 10.1016/j.annonc.2021.03.204 [DOI] [PubMed] [Google Scholar]
- 47. Patrinely JR, Jr., Johnson R, Lawless AR, Bhave P, Sawyers A, Dimitrova M, et al. Chronic Immune-Related Adverse Events Following Adjuvant Anti-PD-1 Therapy for High-Risk Resected Melanoma. JAMA Oncol (2021) 7:744–8. doi: 10.1001/jamaoncol.2021.0051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cappelli LC, Bingham CO, 3rd. Spectrum and Impact of Checkpoint Inhibitor-Induced irAEs. Nat Rev Rheumatol (2021) 17:69–70. doi: 10.1038/s41584-020-00546-2 [DOI] [PubMed] [Google Scholar]
- 49. Johnson DB, Nebhan CA, Moslehi JJ, Balko JM. Immune-Checkpoint Inhibitors: Long-Term Implications of Toxicity. Nat Rev Clin Oncol (2022) 1–14. doi: 10.1038/s41571-022-00600-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. de Filette J, Andreescu CE, Cools F, Bravenboer B, Velkeniers B. A Systematic Review and Meta-Analysis of Endocrine-Related Adverse Events Associated With Immune Checkpoint Inhibitors. Horm Metab Res (2019) 51:145–56. doi: 10.1055/a-0843-3366 [DOI] [PubMed] [Google Scholar]
- 51. Ghosh N, Tiongson MD, Stewart C, Chan KK, Jivanelli B, Cappelli L, et al. Checkpoint Inhibitor-Associated Arthritis: A Systematic Review of Case Reports and Case Series. J Clin Rheumatol (2020) 27:e317-22. doi: 10.1097/RHU.0000000000001370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Fernandez DM, Rahman AH, Fernandez NF, Chudnovskiy A, Amir ED, Amadori L, et al. Single-Cell Immune Landscape of Human Atherosclerotic Plaques. Nat Med (2019) 25:1576–88. doi: 10.1038/s41591-019-0590-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Strauss L, Mahmoud MAA, Weaver JD, Tijaro-Ovalle NM, Christofides A, Wang Q, et al. Targeted Deletion of PD-1 in Myeloid Cells Induces Antitumor Immunity. Sci Immunol (2020) 5:eaay1863. doi: 10.1126/sciimmunol.aay18635/43/eaay1863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gotsman I, Grabie N, Dacosta R, Sukhova G, Sharpe A, Lichtman AH. Proatherogenic Immune Responses Are Regulated by the PD-1/PD-L Pathway in Mice. J Clin Invest (2007) 117:2974–82. doi: 10.1172/JCI31344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Poels K, van Leent MMT, Boutros C, Tissot H, Roy S, Meerwaldt AE, et al. Immune Checkpoint Inhibitor Therapy Aggravates T Cell-Driven Plaque Inflammation in Atherosclerosis. JACC CardioOncol (2020) 2:599–610. doi: 10.1016/j.jaccao.2020.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Drobni ZD, Alvi RM, Taron J, Zafar A, Murphy SP, Rambarat PK, et al. Association Between Immune Checkpoint Inhibitors With Cardiovascular Events and Atherosclerotic Plaque. Circulation (2020) 142:2299–311. doi: 10.1161/CIRCULATIONAHA.120.049981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Calabretta R, Hoeller C, Pichler V, Mitterhauser M, Karanikas G, Haug A, et al. Immune Checkpoint Inhibitor Therapy Induces Inflammatory Activity in Large Arteries. Circulation (2020) 142:2396–8. doi: 10.1161/CIRCULATIONAHA.120.048708 [DOI] [PubMed] [Google Scholar]
- 58. Minasian L, Dimond E, Davis M, Adhikari B, Fagerstrom R, Fabian C, et al. The Evolving Design of NIH-Funded Cardio-Oncology Studies to Address Cancer Treatment-Related Cardiovascular Toxicity. JACC CardioOncol (2019) 1:105–13. doi: 10.1016/j.jaccao.2019.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Mazarico I, Capel I, Gimenez-Palop O, Albert L, Berges I, Luchtenberg F, et al. Low Frequency of Positive Antithyroid Antibodies is Observed in Patients With Thyroid Dysfunction Related to Immune Check Point Inhibitors. J Endocrinol Invest (2019) 42:1443–50. doi: 10.1007/s40618-019-01058-x [DOI] [PubMed] [Google Scholar]
- 60. Yamauchi I, Yasoda A, Matsumoto S, Sakamori Y, Kim YH, Nomura M, et al. Incidence, Features, and Prognosis of Immune-Related Adverse Events Involving the Thyroid Gland Induced by Nivolumab. PloS One (2019) 14:e0216954. doi: 10.1371/journal.pone.0216954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Siegel J, Totonchy M, Damsky W, Berk-Krauss J, Castiglione F, Jr., Sznol M, et al. Bullous Disorders Associated With Anti-PD-1 and Anti-PD-L1 Therapy: A Retrospective Analysis Evaluating the Clinical and Histopathologic Features, Frequency, and Impact on Cancer Therapy. J Am Acad Dermatol (2018) 79:1081–8. doi: 10.1016/j.jaad.2018.07.008 [DOI] [PubMed] [Google Scholar]
- 62. Iwama S, De Remigis A, Callahan MK, Slovin SF, Wolchok JD, Caturegli P. Pituitary Expression of CTLA-4 Mediates Hypophysitis Secondary to Administration of CTLA-4 Blocking Antibody. Sci Transl Med (2014) 6:230ra245. doi: 10.1126/scitranslmed.3008002 [DOI] [PubMed] [Google Scholar]
- 63. Tahir SA, Gao J, Miura Y, Blando J, Tidwell RSS, Zhao H, et al. Autoimmune Antibodies Correlate With Immune Checkpoint Therapy-Induced Toxicities. Proc Natl Acad Sci USA (2019) 116:22246–51. doi: 10.1073/pnas.1908079116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Johansen A, Christensen SJ, Scheie D, Hojgaard JLS, Kondziella D. Neuromuscular Adverse Events Associated With Anti-PD-1 Monoclonal Antibodies: Systematic Review. Neurology (2019) 92:663–74. doi: 10.1212/WNL.0000000000007235 [DOI] [PubMed] [Google Scholar]
- 65. Tarhini AA, Zahoor H, Lin Y, Malhotra U, Sander C, Butterfield LH, et al. Baseline Circulating IL-17 Predicts Toxicity While TGF-Beta1 and IL-10 are Prognostic of Relapse in Ipilimumab Neoadjuvant Therapy of Melanoma. J Immunother Cancer (2015) 3:39. doi: 10.1186/s40425-015-0081-181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kurimoto C, Inaba H, Ariyasu H, Iwakura H, Ueda Y, Uraki S, et al. Predictive and Sensitive Biomarkers for Thyroid Dysfunctions During Treatment With Immune-Checkpoint Inhibitors. Cancer Sci (2020) 111:1468–77. doi: 10.1111/cas.14363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH. Loss of CTLA-4 Leads to Massive Lymphoproliferation and Fatal Multiorgan Tissue Destruction, Revealing a Critical Negative Regulatory Role of CTLA-4. Immunity (1995) 3:541–7. doi: 10.1016/1074-7613(95)90125-6 [DOI] [PubMed] [Google Scholar]
- 68. Nishimura H, Okazaki T, Tanaka Y, Nakatani K, Hara M, Matsumori A, et al. Autoimmune Dilated Cardiomyopathy in PD-1 Receptor-Deficient Mice. Science (2001) 291:319–22. doi: 10.1126/science.291.5502.319 [DOI] [PubMed] [Google Scholar]
- 69. Cappelli LC, Dorak MT, Bettinotti MP, Bingham CO, Shah AA. Association of HLA-DRB1 Shared Epitope Alleles and Immune Checkpoint Inhibitor-Induced Inflammatory Arthritis. Rheumatology (Oxford) (2019) 58:476–80. doi: 10.1093/rheumatology/key358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Akturk HK, Kahramangil D, Sarwal A, Hoffecker L, Murad MH, Michels AW. Immune Checkpoint Inhibitor-Induced Type 1 Diabetes: A Systematic Review and Meta-Analysis. Diabetes Med (2019) 36:1075–81. doi: 10.1111/dme.14050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Hasan Ali O, Berner F, Bomze D, Fassler M, Diem S, Cozzio A, et al. Human Leukocyte Antigen Variation is Associated With Adverse Events of Checkpoint Inhibitors. Eur J Cancer (2019) 107:8–14. doi: 10.1016/j.ejca.2018.11.009 [DOI] [PubMed] [Google Scholar]
- 72. Vetizou M, Pitt JM, Daillere R, Lepage P, Waldschmitt N, Flament C, et al. Anticancer Immunotherapy by CTLA-4 Blockade Relies on the Gut Microbiota. Science (2015) 350:1079–84. doi: 10.1126/science.aad1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Gil-Cruz C, Perez-Shibayama C, De Martin A, Ronchi F, van der Borght K, Niederer R, et al. Microbiota-Derived Peptide Mimics Drive Lethal Inflammatory Cardiomyopathy. Science (2019) 366:881–6. doi: 10.1126/science.aav3487 [DOI] [PubMed] [Google Scholar]
- 74. Chaput N, Lepage P, Coutzac C, Soularue E, Le Roux K, Monot C, et al. Baseline Gut Microbiota Predicts Clinical Response and Colitis in Metastatic Melanoma Patients Treated With Ipilimumab. Ann Oncol (2017) 28:1368–79. doi: 10.1093/annonc/mdx108 [DOI] [PubMed] [Google Scholar]
- 75. Dubin K, Callahan MK, Ren B, Khanin R, Viale A, Ling L, et al. Intestinal Microbiome Analyses Identify Melanoma Patients at Risk for Checkpoint-Blockade-Induced Colitis. Nat Commun (2016) 7:10391. doi: 10.1038/ncomms10391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Johnson DB, Balko JM, Compton ML, Chalkias S, Gorham J, Xu Y, et al. Fulminant Myocarditis With Combination Immune Checkpoint Blockade. N Engl J Med (2016) 375:1749–55. doi: 10.1056/NEJMoa1609214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Berner F, Bomze D, Diem S, Ali OH, Fassler M, Ring S, et al. Association of Checkpoint Inhibitor-Induced Toxic Effects With Shared Cancer and Tissue Antigens in Non-Small Cell Lung Cancer. JAMA Oncol (2019) 5:1043–7. doi: 10.1001/jamaoncol.2019.0402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Michot JM, Bigenwald C, Champiat S, Collins M, Carbonnel F, Postel-Vinay S, et al. Immune-Related Adverse Events With Immune Checkpoint Blockade: A Comprehensive Review. Eur J Cancer (2016) 54:139–48. doi: 10.1016/j.ejca.2015.11.016 [DOI] [PubMed] [Google Scholar]
- 79. Lozano AX, Chaudhuri AA, Nene A, Bacchiocchi A, Earland N, Vesely MD, et al. T Cell Characteristics Associated With Toxicity to Immune Checkpoint Blockade in Patients With Melanoma. Nat Med (2022) 28:353–62. doi: 10.1038/s41591-021-01623-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, et al. CTLA-4 Control Over Foxp3+ Regulatory T Cell Function. Science (2008) 322:271–5. doi: 10.1126/science.1160062 [DOI] [PubMed] [Google Scholar]
- 81. Liu Y, Zheng P. How Does an Anti-CTLA-4 Antibody Promote Cancer Immunity? Trends Immunol (2018) 39:953–6. doi: 10.1016/j.it.2018.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Blanc C, Hans S, Tran T, Granier C, Saldman A, Anson M, et al. Targeting Resident Memory T Cells for Cancer Immunotherapy. Front Immunol (2018) 9:1722. doi: 10.3389/fimmu.2018.01722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Luoma AM, Suo S, Williams HL, Sharova T, Sullivan K, Manos M, et al. Molecular Pathways of Colon Inflammation Induced by Cancer Immunotherapy. Cell (2020) 182:655–71.e622. doi: 10.1016/j.cell.2020.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Luo J, Martucci VL, Quandt Z, Groha S, Murray MH, Lovly CM, et al. Immunotherapy-Mediated Thyroid Dysfunction: Genetic Risk and Impact on Outcomes With PD-1 Blockade in Non-Small Cell Lung Cancer. Clin Cancer Res (2021) 27:5131–40. doi: 10.1158/1078-0432.CCR-21-0921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Maekura T, Naito M, Tahara M, Ikegami N, Kimura Y, Sonobe S, et al. Predictive Factors of Nivolumab-Induced Hypothyroidism in Patients With Non-Small Cell Lung Cancer. In Vivo (2017) 31:1035–9. doi: 10.21873/invivo.11166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Kimbara S, Fujiwara Y, Iwama S, Ohashi K, Kuchiba A, Arima H, et al. Association of Antithyroglobulin Antibodies With the Development of Thyroid Dysfunction Induced by Nivolumab. Cancer Sci (2018) 109:3583–90. doi: 10.1111/cas.13800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Toi Y, Sugawara S, Sugisaka J, Ono H, Kawashima Y, Aiba T, et al. Profiling Preexisting Antibodies in Patients Treated With Anti-PD-1 Therapy for Advanced Non-Small Cell Lung Cancer. JAMA Oncol (2019) 5:376–83. doi: 10.1001/jamaoncol.2018.5860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Bingley PJ. Clinical Applications of Diabetes Antibody Testing. J Clin Endocrinol Metab (2010) 95:25–33. doi: 10.1210/jc.2009-1365 [DOI] [PubMed] [Google Scholar]
- 89. Lin HH, Gutenberg A, Chen TY, Tsai NM, Lee CJ, Cheng YC, et al. In Situ Activation of Pituitary-Infiltrating T Lymphocytes in Autoimmune Hypophysitis. Sci Rep (2017) 7:43492. doi: 10.1038/srep43492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Caturegli P, Di Dalmazi G, Lombardi M, Grosso F, Larman HB, Larman T, et al. Hypophysitis Secondary to Cytotoxic T-Lymphocyte-Associated Protein 4 Blockade: Insights Into Pathogenesis From an Autopsy Series. Am J Pathol (2016) 186:3225–35. doi: 10.1016/j.ajpath.2016.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, et al. Safety and Activity of Anti-PD-L1 Antibody in Patients With Advanced Cancer. N Engl J Med (2012) 366:2455–65. doi: 10.1056/NEJMoa1200694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Robert C, Ribas A, Wolchok JD, Hodi FS, Hamid O, Kefford R, et al. Anti-Programmed-Death-Receptor-1 Treatment With Pembrolizumab in Ipilimumab-Refractory Advanced Melanoma: A Randomised Dose-Comparison Cohort of a Phase 1 Trial. Lancet (2014) 384:1109–17. doi: 10.1016/S0140-6736(14)60958-2 [DOI] [PubMed] [Google Scholar]
- 93. Godel P, Shimabukuro-Vornhagen A, von Bergwelt-Baildon M. Understanding Cytokine Release Syndrome. Intensive Care Med (2018) 44:371–3. doi: 10.1007/s00134-017-4943-5 [DOI] [PubMed] [Google Scholar]
- 94. Ceschi A, Noseda R, Palin K, Verhamme K. Immune Checkpoint Inhibitor-Related Cytokine Release Syndrome: Analysis of WHO Global Pharmacovigilance Database. Front Pharmacol (2020) 11:557. doi: 10.3389/fphar.2020.00557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Khan S, Khan SA, Luo X, Fattah FJ, Saltarski J, Gloria-McCutchen Y, et al. Immune Dysregulation in Cancer Patients Developing Immune-Related Adverse Events. Br J Cancer (2019) 120:63–8. doi: 10.1038/s41416-018-0155-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Andrews MC, Duong CPM, Gopalakrishnan V, Iebba V, Chen WS, Derosa L, et al. Gut Microbiota Signatures are Associated With Toxicity to Combined CTLA-4 and PD-1 Blockade. Nat Med (2021) 27:1432–41. doi: 10.1038/s41591-021-01406-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Lucas JA, Menke J, Rabacal WA, Schoen FJ, Sharpe AH, Kelley VR. Programmed Death Ligand 1 Regulates a Critical Checkpoint for Autoimmune Myocarditis and Pneumonitis in MRL Mice. J Immunol (2008) 181:2513–21. doi: 10.4049/jimmunol.181.4.2513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Okazaki T, Tanaka Y, Nishio R, Mitsuiye T, Mizoguchi A, Wang J, et al. Autoantibodies Against Cardiac Troponin I are Responsible for Dilated Cardiomyopathy in PD-1-Deficient Mice. Nat Med (2003) 9:1477–83. doi: 10.1038/nm955nm955 [DOI] [PubMed] [Google Scholar]
- 99. Tarrio ML, Grabie N, Bu DX, Sharpe AH, Lichtman AH. PD-1 Protects Against Inflammation and Myocyte Damage in T Cell-Mediated Myocarditis. J Immunol (2012) 188:4876–84. doi: 10.4049/jimmunol.1200389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Khan Z, Di Nucci F, Kwan A, Hammer C, Mariathasan S, Rouilly V, et al. Polygenic Risk for Skin Autoimmunity Impacts Immune Checkpoint Blockade in Bladder Cancer. Proc Natl Acad Sci USA (2020) 117:12288–94. doi: 10.1073/pnas.1922867117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Anderson R, Theron AJ, Rapoport BL. Immunopathogenesis of Immune Checkpoint Inhibitor-Related Adverse Events: Roles of the Intestinal Microbiome and Th17 Cells. Front Immunol (2019) 10:2254. doi: 10.3389/fimmu.2019.02254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Sivan A, Corrales L, Hubert N, Williams JB, Aquino-Michaels K, Earley ZM, et al. Commensal Bifidobacterium Promotes Antitumor Immunity and Facilitates Anti-PD-L1 Efficacy. Science (2015) 350:1084–9. doi: 10.1126/science.aac4255science.aac4255[pii [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillere R, et al. Gut Microbiome Influences Efficacy of PD-1-Based Immunotherapy Against Epithelial Tumors. Science (2018) 359:91–7. doi: 10.1126/science.aan3706 [DOI] [PubMed] [Google Scholar]
- 104. Matson V, Fessler J, Bao R, Chongsuwat T, Zha Y, Alegre ML, et al. The Commensal Microbiome is Associated With Anti-PD-1 Efficacy in Metastatic Melanoma Patients. Science (2018) 359:104–8. doi: 10.1126/science.aao3290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Gopalakrishnan V, Spencer CN, Nezi L, Reuben A, Andrews MC, Karpinets TV, et al. Gut Microbiome Modulates Response to Anti-PD-1 Immunotherapy in Melanoma Patients. Science (2018) 359:97–103. doi: 10.1126/science.aan4236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Haanen J, Ernstoff MS, Wang Y, Menzies AM, Puzanov I, Grivas P, et al. Autoimmune Diseases and Immune-Checkpoint Inhibitors for Cancer Therapy: Review of the Literature and Personalized Risk-Based Prevention Strategy. Ann Oncol (2020) 31:724–44. doi: 10.1016/j.annonc.2020.03.285 [DOI] [PubMed] [Google Scholar]
- 107. Danlos FX, Voisin AL, Dyevre V, Michot JM, Routier E, Taillade L, et al. Safety and Efficacy of Anti-Programmed Death 1 Antibodies in Patients With Cancer and Pre-Existing Autoimmune or Inflammatory Disease. Eur J Cancer (2018) 91:21–9. doi: 10.1016/j.ejca.2017.12.008 [DOI] [PubMed] [Google Scholar]
- 108. Kehl KL, Yang S, Awad MM, Palmer N, Kohane IS, Schrag D. Pre-Existing Autoimmune Disease and the Risk of Immune-Related Adverse Events Among Patients Receiving Checkpoint Inhibitors for Cancer. Cancer Immunol Immunother (2019) 68:917–26. doi: 10.1007/s00262-019-02321-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Kobayashi T, Iwama S, Yasuda Y, Okada N, Tsunekawa T, Onoue T, et al. Patients With Antithyroid Antibodies Are Prone To Develop Destructive Thyroiditis by Nivolumab: A Prospective Study. J Endocr Soc (2018) 2:241–51. doi: 10.1210/js.2017-00432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Gowen MF, Giles KM, Simpson D, Tchack J, Zhou H, Moran U, et al. Baseline Antibody Profiles Predict Toxicity in Melanoma Patients Treated With Immune Checkpoint Inhibitors. J Transl Med (2018) 16:82. doi: 10.1186/s12967-018-1452-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Valpione S, Pasquali S, Campana LG, Piccin L, Mocellin S, Pigozzo J, et al. Sex and Interleukin-6 are Prognostic Factors for Autoimmune Toxicity Following Treatment With Anti-CTLA4 Blockade. J Transl Med (2018) 16:94. doi: 10.1186/s12967-018-1467-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Lim SY, Lee JH, Gide TN, Menzies AM, Guminski A, Carlino MS, et al. Circulating Cytokines Predict Immune-Related Toxicity in Melanoma Patients Receiving Anti-PD-1-Based Immunotherapy. Clin Cancer Res (2019) 25:1557–63. doi: 10.1158/1078-0432.CCR-18-2795 [DOI] [PubMed] [Google Scholar]
- 113. Das R, Bar N, Ferreira M, Newman AM, Zhang L, Bailur JK, et al. Early B Cell Changes Predict Autoimmunity Following Combination Immune Checkpoint Blockade. J Clin Invest (2018) 128:715–20. doi: 10.1172/JCI9679896798[pii [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Pavan A, Calvetti L, Dal Maso A, Attili I, Del Bianco P, Pasello G, et al. Peripheral Blood Markers Identify Risk of Immune-Related Toxicity in Advanced Non-Small Cell Lung Cancer Treated With Immune-Checkpoint Inhibitors. Oncologist (2019) 24:1128–36. doi: 10.1634/theoncologist.2018- [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Giommoni E, Giorgione R, Paderi A, Pellegrini E, Gambale E, Marini A, et al. Eosinophil Count as Predictive Biomarker of Immune-Related Adverse Events (irAEs) in Immune Checkpoint Inhibitors (ICIs) Therapies in Oncological Patients. Immuno (2021) 1:253. doi: 10.3390/immuno1030017 [DOI] [Google Scholar]
- 116. Schindler K, Harmankaya K, Kuk D, Mangana J, Michielin O, HoellerReinhard Dummer C, et al. Abstract: Correlation of Absolute and Relative Eosinophil Counts With Immune-Related Adverse Events in Melanoma Patients Treated With Ipilimumab. J Clin Oncol (2014) 32:9096. doi: 10.1200/jco.2014.32.15_suppl.9096 [DOI] [Google Scholar]
- 117. Sakata Y, Kawamura K, Ichikado K, Shingu N, Yasuda Y, Eguchi Y, et al. The Association Between Tumor Burden and Severe Immune-Related Adverse Events in non-Small Cell Lung Cancer Patients Responding to Immune-Checkpoint Inhibitor Treatment. Lung Cancer (2019) 130:159–61. doi: 10.1016/j.lungcan.2019.02.011 [DOI] [PubMed] [Google Scholar]
- 118. Wright JJ, Salem JE, Johnson DB, Lebrun-Vignes B, Stamatouli A, Thomas JW, et al. Increased Reporting of Immune Checkpoint Inhibitor-Associated Diabetes. Diabetes Care (2018) 41:e150–1. doi: 10.2337/dc18-1465dc18-1465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Faje A, Reynolds K, Zubiri L, Lawrence D, Cohen JV, Sullivan RJ, et al. Hypophysitis Secondary to Nivolumab and Pembrolizumab is a Clinical Entity Distinct From Ipilimumab-Associated Hypophysitis. Eur J Endocrinol (2019) 181:211–9. doi: 10.1530/EJE-19-0238EJE-19-0238 [DOI] [PubMed] [Google Scholar]
- 120. Dougan M, Pietropaolo M. Time to Dissect the Autoimmune Etiology of Cancer Antibody Immunotherapy. J Clin Invest (2020) 130:51–61. doi: 10.1172/JCI131194131194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Ma C, Hodi FS, Giobbie-Hurder A, Wang X, Zhou J, Zhang A, et al. The Impact of High-Dose Glucocorticoids on the Outcome of Immune-Checkpoint Inhibitor-Related Thyroid Disorders. Cancer Immunol Res (2019) 7:1214–20. doi: 10.1158/2326-6066.CIR-18-0613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Faje AT, Lawrence D, Flaherty K, Freedman C, Fadden R, Rubin K, et al. High-Dose Glucocorticoids for the Treatment of Ipilimumab-Induced Hypophysitis is Associated With Reduced Survival in Patients With Melanoma. Cancer (2018) 124:3706–14. doi: 10.1002/cncr.31629 [DOI] [PubMed] [Google Scholar]
- 123. Moslehi JJ, Salem JE, Sosman JA, Lebrun-Vignes B, Johnson DB. Increased Reporting of Fatal Immune Checkpoint Inhibitor-Associated Myocarditis. Lancet (2018) 391:933. doi: 10.1016/S0140-6736(18)30533-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Eigentler TK, Hassel JC, Berking C, Aberle J, Bachmann O, Grunwald V, et al. Diagnosis, Monitoring and Management of Immune-Related Adverse Drug Reactions of Anti-PD-1 Antibody Therapy. Cancer Treat Rev (2016) 45:7–18. doi: 10.1016/j.ctrv.2016.02.003 [DOI] [PubMed] [Google Scholar]
- 125. Coleman E, Ko C, Dai F, Tomayko MM, Kluger H, Leventhal JS. Inflammatory Eruptions Associated With Immune Checkpoint Inhibitor Therapy: A Single-Institution Retrospective Analysis With Stratification of Reactions by Toxicity and Implications for Management. J Am Acad Dermatol (2019) 80:990–7. doi: 10.1016/j.jaad.2018.10.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Larsabal M, Marti A, Jacquemin C, Rambert J, Thiolat D, Dousset L, et al. Vitiligo-Like Lesions Occurring in Patients Receiving Anti-Programmed Cell Death-1 Therapies are Clinically and Biologically Distinct From Vitiligo. J Am Acad Dermatol (2017) 76:863–70. doi: 10.1016/j.jaad.2016.10.044 [DOI] [PubMed] [Google Scholar]
- 127. Aggarwal P. Disproportionality Analysis of Bullous Pemphigoid Adverse Events With PD-1 Inhibitors in the FDA Adverse Event Reporting System. Expert Opin Drug Saf (2019) 18:623–33. doi: 10.1080/14740338.2019.1619693 [DOI] [PubMed] [Google Scholar]
- 128. Lacouture M, Sibaud V. Toxic Side Effects of Targeted Therapies and Immunotherapies Affecting the Skin, Oral Mucosa, Hair, and Nails. Am J Clin Dermatol (2018) 19:31–9. doi: 10.1007/s40257-018-0384-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Soldatos TG, Dimitrakopoulou-Strauss A, Larribere L, Hassel JC, Sachpekidis C. Retrospective Side Effect Profiling of the Metastatic Melanoma Combination Therapy Ipilimumab-Nivolumab Using Adverse Event Data. Diagnostics (Basel) (2018) 8:76. doi: 10.3390/diagnostics8040076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. El Majzoub I, Qdaisat A, Thein KZ, Win MA, Han MM, Jacobson K, et al. Adverse Effects of Immune Checkpoint Therapy in Cancer Patients Visiting the Emergency Department of a Comprehensive Cancer Center. Ann Emerg Med (2019) 73:79–87. doi: 10.1016/j.annemergmed.2018.04.019 [DOI] [PubMed] [Google Scholar]
- 131. Beck KE, Blansfield JA, Tran KQ, Feldman AL, Hughes MS, Royal RE, et al. Enterocolitis in Patients With Cancer After Antibody Blockade of Cytotoxic T-Lymphocyte-Associated Antigen 4. J Clin Oncol (2006) 24:2283–9. doi: 10.1200/JCO.2005.04.5716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Wang DY, Ye F, Zhao S, Johnson DB. Incidence of Immune Checkpoint Inhibitor-Related Colitis in Solid Tumor Patients: A Systematic Review and Meta-Analysis. Oncoimmunology (2017) 6:e1344805. doi: 10.1080/2162402X.2017.1344805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Wang Y, Abu-Sbeih H, Mao E, Ali N, Qiao W, Trinh VA, et al. Endoscopic and Histologic Features of Immune Checkpoint Inhibitor-Related Colitis. Inflamm Bowel Dis (2018) 24:1695–705. doi: 10.1093/ibd/izy1044989161 [DOI] [PubMed] [Google Scholar]
- 134. Marthey L, Mateus C, Mussini C, Nachury M, Nancey S, Grange F, et al. Cancer Immunotherapy With Anti-CTLA-4 Monoclonal Antibodies Induces an Inflammatory Bowel Disease. J Crohns Colitis (2016) 10:395–401. doi: 10.1093/ecco-jcc/jjv227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Weber J, Thompson JA, Hamid O, Minor D, Amin A, Ron I, et al. A Randomized, Double-Blind, Placebo-Controlled, Phase II Study Comparing the Tolerability and Efficacy of Ipilimumab Administered With or Without Prophylactic Budesonide in Patients With Unresectable Stage III or IV Melanoma. Clin Cancer Res (2009) 15:5591–8. doi: 10.1158/1078-0432.CCR-09-1024 [DOI] [PubMed] [Google Scholar]
- 136. Johnson DH, Zobniw CM, Trinh VA, Ma J, Bassett RL, Jr., Abdel-Wahab N, et al. Infliximab Associated With Faster Symptom Resolution Compared With Corticosteroids Alone for the Management of Immune-Related Enterocolitis. J Immunother Cancer (2018) 6:103. doi: 10.1186/s40425-018-0412-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Bergqvist V, Hertervig E, Gedeon P, Kopljar M, Griph H, Kinhult S, et al. Vedolizumab Treatment for Immune Checkpoint Inhibitor-Induced Enterocolitis. Cancer Immunol Immunother (2017) 66:581–92. doi: 10.1007/s00262-017-1962-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Abu-Sbeih H, Ali FS, Wang X, Mallepally N, Chen E, Altan M, et al. Early Introduction of Selective Immunosuppressive Therapy Associated With Favorable Clinical Outcomes in Patients With Immune Checkpoint Inhibitor-Induced Colitis. J Immunother Cancer (2019) 7:93. doi: 10.1186/s40425-019-0577-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Suzman DL, Pelosof L, Rosenberg A, Avigan MI. Hepatotoxicity of Immune Checkpoint Inhibitors: An Evolving Picture of Risk Associated With a Vital Class of Immunotherapy Agents. Liver Int (2018) 38:976–87. doi: 10.1111/liv.13746 [DOI] [PubMed] [Google Scholar]
- 140. De Martin E, Michot JM, Papouin B, Champiat S, Mateus C, Lambotte O, et al. Characterization of Liver Injury Induced by Cancer Immunotherapy Using Immune Checkpoint Inhibitors. J Hepatol (2018) 68:1181–90. doi: 10.1016/j.jhep.2018.01.033 [DOI] [PubMed] [Google Scholar]
- 141. Abu-Sbeih H, Tang T, Lu Y, Thirumurthi S, Altan M, Jazaeri AA, et al. Clinical Characteristics and Outcomes of Immune Checkpoint Inhibitor-Induced Pancreatic Injury. J Immunother Cancer (2019) 7:31. doi: 10.1186/s40425-019-0502-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Boutros C, Tarhini A, Routier E, Lambotte O, Ladurie FL, Carbonnel F, et al. Safety Profiles of Anti-CTLA-4 and Anti-PD-1 Antibodies Alone and in Combination. Nat Rev Clin Oncol (2016) 13:473–86. doi: 10.1038/nrclinonc.2016.58 [DOI] [PubMed] [Google Scholar]
- 143. Varricchi G, Loffredo S, Marone G, Modestino L, Fallahi P, Ferrari SM, et al. The Immune Landscape of Thyroid Cancer in the Context of Immune Checkpoint Inhibition. Int J Mol Sci (2019) 20:3934. doi: 10.3390/ijms20163934ijms20163934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Iyer PC, Cabanillas ME, Waguespack SG, Hu MI, Thosani S, Lavis VR, et al. Immune-Related Thyroiditis With Immune Checkpoint Inhibitors. Thyroid (2018) 28:1243–51. doi: 10.1089/thy.2018.0116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Orlov S, Salari F, Kashat L, Walfish PG. Induction of Painless Thyroiditis in Patients Receiving Programmed Death 1 Receptor Immunotherapy for Metastatic Malignancies. J Clin Endocrinol Metab (2015) 100:1738–41. doi: 10.1210/jc.2014-4560 [DOI] [PubMed] [Google Scholar]
- 146. Delivanis DA, Gustafson MP, Bornschlegl S, Merten MM, Kottschade L, Withers S, et al. Pembrolizumab-Induced Thyroiditis: Comprehensive Clinical Review and Insights Into Underlying Involved Mechanisms. J Clin Endocrinol Metab (2017) 102:2770–80. doi: 10.1210/jc.2017-004483805504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Faje AT, Sullivan R, Lawrence D, Tritos NA, Fadden R, Klibanski A, et al. Ipilimumab-Induced Hypophysitis: A Detailed Longitudinal Analysis in a Large Cohort of Patients With Metastatic Melanoma. J Clin Endocrinol Metab (2014) 99:4078–85. doi: 10.1210/jc.2014-2306 [DOI] [PubMed] [Google Scholar]
- 148. Albarel F, Gaudy C, Castinetti F, Carre T, Morange I, Conte-Devolx B, et al. Long-Term Follow-Up of Ipilimumab-Induced Hypophysitis, a Common Adverse Event of the Anti-CTLA-4 Antibody in Melanoma. Eur J Endocrinol (2015) 172:195–204. doi: 10.1530/EJE-14-0845 [DOI] [PubMed] [Google Scholar]
- 149. Min L, Hodi FS, Giobbie-Hurder A, Ott PA, Luke JJ, Donahue H, et al. Systemic High-Dose Corticosteroid Treatment Does Not Improve the Outcome of Ipilimumab-Related Hypophysitis: A Retrospective Cohort Study. Clin Cancer Res (2015) 21:749–55. doi: 10.1158/1078-0432.CCR-14-2353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Carpenter KJ, Murtagh RD, Lilienfeld H, Weber J, Murtagh FR. Ipilimumab-Induced Hypophysitis: MR Imaging Findings. AJNR Am J Neuroradiol (2009) 30:1751–3. doi: 10.3174/ajnr.A1623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Juszczak A, Gupta A, Karavitaki N, Middleton MR, Grossman AB. Ipilimumab: A Novel Immunomodulating Therapy Causing Autoimmune Hypophysitis: A Case Report and Review. Eur J Endocrinol (2012) 167:1–5. doi: 10.1530/EJE-12-0167 [DOI] [PubMed] [Google Scholar]
- 152. Voskens CJ, Goldinger SM, Loquai C, Robert C, Kaehler KC, Berking C, et al. The Price of Tumor Control: An Analysis of Rare Side Effects of Anti-CTLA-4 Therapy in Metastatic Melanoma From the Ipilimumab Network. PloS One (2013) 8:e53745. doi: 10.1371/journal.pone.0053745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Kotwal A, Haddox C, Block M, Kudva YC. Immune Checkpoint Inhibitors: An Emerging Cause of Insulin-Dependent Diabetes. BMJ Open Diabetes Res Care (2019) 7:e000591. doi: 10.1136/bmjdrc-2018-000591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Gaudy C, Clevy C, Monestier S, Dubois N, Preau Y, Mallet S, et al. Anti-PD1 Pembrolizumab Can Induce Exceptional Fulminant Type 1 Diabetes. Diabetes Care (2015) 38:e182–183. doi: 10.2337/dc15-1331dc15-1331 [DOI] [PubMed] [Google Scholar]
- 155. Mellati M, Eaton KD, Brooks-Worrell BM, Hagopian WA, Martins R, Palmer JP, et al. Anti-PD-1 and Anti-PDL-1 Monoclonal Antibodies Causing Type 1 Diabetes. Diabetes Care (2015) 38:e137–138. doi: 10.2337/dc15-0889dc15-0889 [DOI] [PubMed] [Google Scholar]
- 156. Hughes J, Vudattu N, Sznol M, Gettinger S, Kluger H, Lupsa B, et al. Precipitation of Autoimmune Diabetes With Anti-PD-1 Immunotherapy. Diabetes Care (2015) 38:e55–57. doi: 10.2337/dc14-234938/4/e55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. James EA, Mallone R, Kent SC, DiLorenzo TP. T-Cell Epitopes and Neo-Epitopes in Type 1 Diabetes: A Comprehensive Update and Reappraisal. Diabetes (2020) 69:1311–35. doi: 10.2337/dbi19-002269/7/1311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Gauci ML, Laly P, Vidal-Trecan T, Baroudjian B, Gottlieb J, Madjlessi-Ezra N, et al. Autoimmune Diabetes Induced by PD-1 Inhibitor-Retrospective Analysis and Pathogenesis: A Case Report and Literature Review. Cancer Immunol Immunother (2017) 66:1399–410. doi: 10.1007/s00262-017-2033-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Naidoo J, Wang X, Woo KM, Iyriboz T, Halpenny D, Cunningham J, et al. Pneumonitis in Patients Treated With Anti-Programmed Death-1/Programmed Death Ligand 1 Therapy. J Clin Oncol (2017) 35:709–17. doi: 10.1200/JCO.2016.68.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, Activity, and Immune Correlates of Anti-PD-1 Antibody in Cancer. N Engl J Med (2012) 366:2443–54. doi: 10.1056/NEJMoa1200690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab Versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med (2015) 373:1627–39. doi: 10.1056/NEJMoa1507643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Brahmer J, Reckamp KL, Baas P, Crino L, Eberhardt WE, Poddubskaya E, et al. Nivolumab Versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med (2015) 373:123–35. doi: 10.1056/NEJMoa1504627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Nishino M, Giobbie-Hurder A, Hatabu H, Ramaiya NH, Hodi FS. Incidence of Programmed Cell Death 1 Inhibitor-Related Pneumonitis in Patients With Advanced Cancer: A Systematic Review and Meta-Analysis. JAMA Oncol (2016) 2:1607–16. doi: 10.1001/jamaoncol.2016.24532544610 [DOI] [PubMed] [Google Scholar]
- 164. Fukihara J, Sakamoto K, Koyama J, Ito T, Iwano S, Morise M, et al. Prognostic Impact and Risk Factors of Immune-Related Pneumonitis in Patients With Non-Small-Cell Lung Cancer Who Received Programmed Death 1 Inhibitors. Clin Lung Cancer (2019) 20:442–450 e444. doi: 10.1016/j.cllc.2019.07.006 [DOI] [PubMed] [Google Scholar]
- 165. Suresh K, Voong KR, Shankar B, Forde PM, Ettinger DS, Marrone KA, et al. Pneumonitis in Non-Small Cell Lung Cancer Patients Receiving Immune Checkpoint Immunotherapy: Incidence and Risk Factors. J Thorac Oncol (2018) 13:1930–9. doi: 10.1016/j.jtho.2018.08.2035 [DOI] [PubMed] [Google Scholar]
- 166. Cho JY, Kim J, Lee JS, Kim YJ, Kim SH, Lee YJ, et al. Characteristics, Incidence, and Risk Factors of Immune Checkpoint Inhibitor-Related Pneumonitis in Patients With non-Small Cell Lung Cancer. Lung Cancer (2018) 125:150–6. doi: 10.1016/j.lungcan.2018.09.015 [DOI] [PubMed] [Google Scholar]
- 167. Khunger M, Jain P, Rakshit S, Pasupuleti V, Hernandez AV, Stevenson J, et al. Safety and Efficacy of PD-1/PD-L1 Inhibitors in Treatment-Naive and Chemotherapy-Refractory Patients With Non-Small-Cell Lung Cancer: A Systematic Review and Meta-Analysis. Clin Lung Cancer (2018) 19:e335–48. doi: 10.1016/j.cllc.2018.01.002 [DOI] [PubMed] [Google Scholar]
- 168. Khoja L, Day D, Wei-Wu Chen T, Siu LL, Hansen AR. Tumour- and Class-Specific Patterns of Immune-Related Adverse Events of Immune Checkpoint Inhibitors: A Systematic Review. Ann Oncol (2017) 28:2377–85. doi: 10.1093/annonc/mdx286 [DOI] [PubMed] [Google Scholar]
- 169. Wu J, Hong D, Zhang X, Lu X, Miao J. PD-1 Inhibitors Increase the Incidence and Risk of Pneumonitis in Cancer Patients in a Dose-Independent Manner: A Meta-Analysis. Sci Rep (2017) 7:44173. doi: 10.1038/srep44173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Larsen BT, Chae JM, Dixit AS, Hartman TE, Peikert T, Roden AC. Clinical and Histopathologic Features of Immune Checkpoint Inhibitor-Related Pneumonitis. Am J Surg Pathol (2019) 43:1331–40. doi: 10.1097/PAS.0000000000001298 [DOI] [PubMed] [Google Scholar]
- 171. Cui P, Liu Z, Wang G, Ma J, Qian Y, Zhang F, et al. Risk Factors for Pneumonitis in Patients Treated With Anti-Programmed Death-1 Therapy: A Case-Control Study. Cancer Med (2018) 7:4115–20. doi: 10.1002/cam4.1579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Yamaguchi T, Shimizu J, Hasegawa T, Horio Y, Inaba Y, Yatabe Y, et al. Pre-Existing Pulmonary Fibrosis is a Risk Factor for Anti-PD-1-Related Pneumonitis in Patients With non-Small Cell Lung Cancer: A Retrospective Analysis. Lung Cancer (2018) 125:212–7. doi: 10.1016/j.lungcan.2018.10.001 [DOI] [PubMed] [Google Scholar]
- 173. Rossi E, Schinzari G, Tortora G. Pneumonitis From Immune Checkpoint Inhibitors and COVID-19: Current Concern in Cancer Treatment. J Immunother Cancer (2020) 8:e000952. doi: 10.1136/jitc-2020-000952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Haanen J, Carbonnel F, Robert C, Kerr KM, Peters S, Larkin J, et al. Management of Toxicities From Immunotherapy: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann Oncol (2017) 28:iv119–42. doi: 10.1093/annonc/mdx2253958159 [DOI] [PubMed] [Google Scholar]
- 175. Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM, et al. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol (2018) 36:1714–68. doi: 10.1200/JCO.2017.77.6385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Gomatou G, Tzilas V, Kotteas E, Syrigos K, Bouros D. Immune Checkpoint Inhibitor-Related Pneumonitis. Respiration (2020) 99:932–42. doi: 10.1159/000509941 [DOI] [PubMed] [Google Scholar]
- 177. Thompson JA, Schneider BJ, Brahmer J, Andrews S, Armand P, Bhatia S, et al. Management of Immunotherapy-Related Toxicities, Version 1.2019. J Natl Compr Canc Netw (2019) 17:255–89. doi: 10.6004/jnccn.2019.0013 [DOI] [PubMed] [Google Scholar]
- 178. Kostine M, Rouxel L, Barnetche T, Veillon R, Martin F, Dutriaux C, et al. Rheumatic Disorders Associated With Immune Checkpoint Inhibitors in Patients With Cancer-Clinical Aspects and Relationship With Tumour Response: A Single-Centre Prospective Cohort Study. Ann Rheum Dis (2018) 77:393–8. doi: 10.1136/annrheumdis-2017-212257 [DOI] [PubMed] [Google Scholar]
- 179. Cappelli LC, Gutierrez AK, Bingham CO, 3rd, Shah AA. Rheumatic and Musculoskeletal Immune-Related Adverse Events Due to Immune Checkpoint Inhibitors: A Systematic Review of the Literature. Arthritis Care Res (Hoboken) (2017) 69:1751–63. doi: 10.1002/acr.23177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Smith MH, Bass AR. Arthritis After Cancer Immunotherapy: Symptom Duration and Treatment Response. Arthritis Care Res (Hoboken) (2019) 71:362–6. doi: 10.1002/acr.23467 [DOI] [PubMed] [Google Scholar]
- 181. Calabrese C, Cappelli LC, Kostine M, Kirchner E, Braaten T, Calabrese L. Polymyalgia Rheumatica-Like Syndrome From Checkpoint Inhibitor Therapy: Case Series and Systematic Review of the Literature. RMD Open (2019) 5:e000906. doi: 10.1136/rmdopen-2019-000906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Cappelli LC, Brahmer JR, Forde PM, Le DT, Lipson EJ, Naidoo J, et al. Clinical Presentation of Immune Checkpoint Inhibitor-Induced Inflammatory Arthritis Differs by Immunotherapy Regimen. Semin Arthritis Rheum (2018) 48:553–7. doi: 10.1016/j.semarthrit.2018.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Cappelli LC, Bingham CO, 3rd. Expert Perspective: Immune Checkpoint Inhibitors and Rheumatologic Complications. Arthritis Rheumatol (2021) 73:553–65. doi: 10.1002/art.41587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Touat M, Maisonobe T, Knauss S, Ben Hadj Salem O, Hervier B, Aure K, et al. Immune Checkpoint Inhibitor-Related Myositis and Myocarditis in Patients With Cancer. Neurology (2018) 91:e985–94. doi: 10.1212/WNL.0000000000006124 [DOI] [PubMed] [Google Scholar]
- 185. Anquetil C, Salem JE, Lebrun-Vignes B, Johnson DB, Mammen AL, Stenzel W, et al. Immune Checkpoint Inhibitor-Associated Myositis: Expanding the Spectrum of Cardiac Complications of the Immunotherapy Revolution. Circulation (2018) 138:743–5. doi: 10.1161/CIRCULATIONAHA.118.035898 [DOI] [PubMed] [Google Scholar]
- 186. Moreira A, Loquai C, Pfohler C, Kahler KC, Knauss S, Heppt MV, et al. Myositis and Neuromuscular Side-Effects Induced by Immune Checkpoint Inhibitors. Eur J Cancer (2019) 106:12–23. doi: 10.1016/j.ejca.2018.09.033 [DOI] [PubMed] [Google Scholar]
- 187. Liewluck T, Kao JC, Mauermann ML. PD-1 Inhibitor-Associated Myopathies: Emerging Immune-Mediated Myopathies. J Immunother (2018) 41:208–11. doi: 10.1097/CJI.0000000000000196 [DOI] [PubMed] [Google Scholar]
- 188. Shah M, Tayar JH, Abdel-Wahab N, Suarez-Almazor ME. Myositis as an Adverse Event of Immune Checkpoint Blockade for Cancer Therapy. Semin Arthritis Rheum (2019) 48:736–40. doi: 10.1016/j.semarthrit.2018.05.006 [DOI] [PubMed] [Google Scholar]
- 189. Groarke JD, Cheng S, Moslehi J. Cancer-Drug Discovery and Cardiovascular Surveillance. N Engl J Med (2013) 369:1779–81. doi: 10.1056/NEJMp1313140 [DOI] [PubMed] [Google Scholar]
- 190. Mahmood SS, Fradley MG, Cohen JV, Nohria A, Reynolds KL, Heinzerling LM, et al. Myocarditis in Patients Treated With Immune Checkpoint Inhibitors. J Am Coll Cardiol (2018) 71:1755–64. doi: 10.1016/j.jacc.2018.02.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Hu JR, Florido R, Lipson EJ, Naidoo J, Ardehali R, Tocchetti CG, et al. Cardiovascular Toxicities Associated With Immune Checkpoint Inhibitors. Cardiovasc Res (2019) 115:854–68. doi: 10.1093/cvr/cvz0265304411[pii [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Salem JE, Manouchehri A, Moey M, Lebrun-Vignes B, Bastarache L, Pariente A, et al. Cardiovascular Toxicities Associated With Immune Checkpoint Inhibitors: An Observational, Retrospective, Pharmacovigilance Study. Lancet Oncol (2018) 19:1579–89. doi: 10.1016/S1470-2045(18)30608-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Allenbach Y, Anquetil C, Manouchehri A, Benveniste O, Lambotte O, Lebrun-Vignes B, et al. Immune Checkpoint Inhibitor-Induced Myositis, the Earliest and Most Lethal Complication Among Rheumatic and Musculoskeletal Toxicities. Autoimmun Rev (2020) 19:102586. doi: 10.1016/j.autrev.2020.102586 [DOI] [PubMed] [Google Scholar]
- 194. Norwood TG, Westbrook BC, Johnson DB, Litovsky SH, Terry NL, McKee SB, et al. Smoldering Myocarditis Following Immune Checkpoint Blockade. J Immunother Cancer (2017) 5:91. doi: 10.1186/s40425-017-0296-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Bonaca MP, Olenchock BA, Salem JE, Wiviott SD, Ederhy S, Cohen A, et al. Myocarditis in the Setting of Cancer Therapeutics: Proposed Case Definitions for Emerging Clinical Syndromes in Cardio-Oncology. Circulation (2019) 140:80–91. doi: 10.1161/CIRCULATIONAHA.118.034497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Mir H, Alhussein M, Alrashidi S, Alzayer H, Alshatti A, Valettas N, et al. Cardiac Complications Associated With Checkpoint Inhibition: A Systematic Review of the Literature in an Important Emerging Area. Can J Cardiol (2018) 34:1059–68. doi: 10.1016/j.cjca.2018.03.012 [DOI] [PubMed] [Google Scholar]
- 197. Puzanov I, Diab A, Abdallah K, Bingham CO, 3rd, Brogdon C, Dadu R, et al. Managing Toxicities Associated With Immune Checkpoint Inhibitors: Consensus Recommendations From the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer (2017) 5:95. doi: 10.1186/s40425-017-0300-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Esfahani K, Buhlaiga N, Thebault P, Lapointe R, Johnson NA, Miller WH., Jr. Alemtuzumab for Immune-Related Myocarditis Due to PD-1 Therapy. N Engl J Med (2019) 380:2375–6. doi: 10.1056/NEJMc1903064 [DOI] [PubMed] [Google Scholar]
- 199. Thackeray JT, Pietzsch S, Stapel B, Ricke-Hoch M, Lee CW, Bankstahl JP, et al. Insulin Supplementation Attenuates Cancer-Induced Cardiomyopathy and Slows Tumor Disease Progression. JCI Insight (2017) 2 e93098. doi: 10.1172/jci.insight.9309893098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Michel L, Helfrich I, Hendgen-Cotta UB, Mincu RI, Korste S, Mrotzek SM, et al. Targeting Early Stages of Cardiotoxicity From Anti-PD1 Immune Checkpoint Inhibitor Therapy. Eur Heart J (2022) 43:316–29. doi: 10.1093/eurheartj/ehab4306352226 [DOI] [PubMed] [Google Scholar]
- 201. Varricchi G, Galdiero MR, Tocchetti CG. Novel Actors on the Stage of Cardiac Dysfunction Induced by Anti-PD1 Oncological Treatments. Eur Heart J (2021) 43:330–2. doi: 10.1093/eurheartj/ehab5846359545[pii [DOI] [PubMed] [Google Scholar]
- 202. Cuzzubbo S, Javeri F, Tissier M, Roumi A, Barlog C, Doridam J, et al. Neurological Adverse Events Associated With Immune Checkpoint Inhibitors: Review of the Literature. Eur J Cancer (2017) 73:1–8. doi: 10.1016/j.ejca.2016.12.001 [DOI] [PubMed] [Google Scholar]
- 203. Johnson DB, Manouchehri A, Haugh AM, Quach HT, Balko JM, Lebrun-Vignes B, et al. Neurologic Toxicity Associated With Immune Checkpoint Inhibitors: A Pharmacovigilance Study. J Immunother Cancer (2019) 7:134. doi: 10.1186/s40425-019-0617-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Delanoy N, Michot JM, Comont T, Kramkimel N, Lazarovici J, Dupont R, et al. Haematological Immune-Related Adverse Events Induced by Anti-PD-1 or Anti-PD-L1 Immunotherapy: A Descriptive Observational Study. Lancet Haematol (2019) 6:e48–57. doi: 10.1016/S2352-3026(18)30175-3 [DOI] [PubMed] [Google Scholar]
- 205. Kramer R, Zaremba A, Moreira A, Ugurel S, Johnson DB, Hassel JC, et al. Hematological Immune Related Adverse Events After Treatment With Immune Checkpoint Inhibitors. Eur J Cancer (2021) 147:170–81. doi: 10.1016/j.ejca.2021.01.013 [DOI] [PubMed] [Google Scholar]
- 206. Leaf RK, Ferreri C, Rangachari D, Mier J, Witteles W, Ansstas G, et al. Clinical and Laboratory Features of Autoimmune Hemolytic Anemia Associated With Immune Checkpoint Inhibitors. Am J Hematol (2019) 94:563–74. doi: 10.1002/ajh.25448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Hofmann L, Forschner A, Loquai C, Goldinger SM, Zimmer L, Ugurel S, et al. Cutaneous, Gastrointestinal, Hepatic, Endocrine, and Renal Side-Effects of Anti-PD-1 Therapy. Eur J Cancer (2016) 60:190–209. doi: 10.1016/j.ejca.2016.02.025 [DOI] [PubMed] [Google Scholar]
- 208. Murakami N, Motwani S, Riella LV. Renal Complications of Immune Checkpoint Blockade. Curr Probl Cancer (2017) 41:100–10. doi: 10.1016/j.currproblcancer.2016.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Martins F, Sofiya L, Sykiotis GP, Lamine F, Maillard M, Fraga M, et al. Adverse Effects of Immune-Checkpoint Inhibitors: Epidemiology, Management and Surveillance. Nat Rev Clin Oncol (2019) 16:563–80. doi: 10.1038/s41571-019-0218-0 [DOI] [PubMed] [Google Scholar]
- 210. Cortazar FB, Marrone KA, Troxell ML, Ralto KM, Hoenig MP, Brahmer JR, et al. Clinicopathological Features of Acute Kidney Injury Associated With Immune Checkpoint Inhibitors. Kidney Int (2016) 90:638–47. doi: 10.1016/j.kint.2016.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Mamlouk O, Selamet U, Machado S, Abdelrahim M, Glass WF, Tchakarov A, et al. Nephrotoxicity of Immune Checkpoint Inhibitors Beyond Tubulointerstitial Nephritis: Single-Center Experience. J Immunother Cancer (2019) 7:2. doi: 10.1186/s40425-018-0478-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Antoun J, Titah C, Cochereau I. Ocular and Orbital Side-Effects of Checkpoint Inhibitors: A Review Article. Curr Opin Oncol (2016) 28:288–94. doi: 10.1097/CCO.0000000000000296 [DOI] [PubMed] [Google Scholar]
- 213. Zhou L, Wei X. Ocular Immune-Related Adverse Events Associated With Immune Checkpoint Inhibitors in Lung Cancer. Front Immunol (2021) 12:701951. doi: 10.3389/fimmu.2021.701951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Bitton K, Michot JM, Barreau E, Lambotte O, Haigh O, Marabelle A, et al. Prevalence and Clinical Patterns of Ocular Complications Associated With Anti-PD-1/PD-L1 Anticancer Immunotherapy. Am J Ophthalmol (2019) 202:109–17. doi: 10.1016/j.ajo.2019.02.012 [DOI] [PubMed] [Google Scholar]
- 215. Noble CW, Gangaputra SS, Thompson IA, Yuan A, Apolo AB, Lee JM, et al. Ocular Adverse Events Following Use of Immune Checkpoint Inhibitors for Metastatic Malignancies. Ocul Immunol Inflamm (2020) 28:854–9. doi: 10.1080/09273948.2019.1583347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Warner BM, Baer AN, Lipson EJ, Allen C, Hinrichs C, Rajan A, et al. Sicca Syndrome Associated With Immune Checkpoint Inhibitor Therapy. Oncologist (2019) 24:1259–69. doi: 10.1634/theoncologist.2018-0823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, et al. Pembrolizumab Versus Ipilimumab in Advanced Melanoma. N Engl J Med (2015) 372:2521–32. doi: 10.1056/NEJMoa1503093 [DOI] [PubMed] [Google Scholar]
- 218. Ferris RL, Blumenschein G, Jr., Fayette J, Guigay J, Colevas AD, Licitra L, et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N Engl J Med (2016) 375:1856–67. doi: 10.1056/NEJMoa1602252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Postow MA, Chesney J, Pavlick AC, Robert C, Grossmann K, McDermott D, et al. Nivolumab and Ipilimumab Versus Ipilimumab in Untreated Melanoma. N Engl J Med (2015) 372:2006–17. doi: 10.1056/NEJMoa1414428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Khan SA, Pruitt SL, Xuan L, Gerber DE. Prevalence of Autoimmune Disease Among Patients With Lung Cancer: Implications for Immunotherapy Treatment Options. JAMA Oncol (2016) 2:1507–8. doi: 10.1001/jamaoncol.2016.2238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Abdel-Wahab N, Shah M, Lopez-Olivo MA, Suarez-Almazor ME. Use of Immune Checkpoint Inhibitors in the Treatment of Patients With Cancer and Preexisting Autoimmune Disease: A Systematic Review. Ann Intern Med (2018) 168:121–30. doi: 10.7326/M17-20732667695 [DOI] [PubMed] [Google Scholar]
- 222. Tison A, Quere G, Misery L, Funck-Brentano E, Danlos FX, Routier E, et al. Safety and Efficacy of Immune Checkpoint Inhibitors in Patients With Cancer and Preexisting Autoimmune Disease: A Nationwide, Multicenter Cohort Study. Arthritis Rheumatol (2019) 71:2100–11. doi: 10.1002/art.41068 [DOI] [PubMed] [Google Scholar]
- 223. Horvat TZ, Adel NG, Dang TO, Momtaz P, Postow MA, Callahan MK, et al. Immune-Related Adverse Events, Need for Systemic Immunosuppression, and Effects on Survival and Time to Treatment Failure in Patients With Melanoma Treated With Ipilimumab at Memorial Sloan Kettering Cancer Center. J Clin Oncol (2015) 33:3193–8. doi: 10.1200/JCO.2015.60.8448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Weber JS, Hodi FS, Wolchok JD, Topalian SL, Schadendorf D, Larkin J, et al. Safety Profile of Nivolumab Monotherapy: A Pooled Analysis of Patients With Advanced Melanoma. J Clin Oncol (2017) 35:785–92. doi: 10.1200/JCO.2015.66.1389 [DOI] [PubMed] [Google Scholar]
- 225. Kotwal A, Kottschade L, Ryder M. PD-L1 Inhibitor-Induced Thyroiditis Is Associated With Better Overall Survival in Cancer Patients. Thyroid (2020) 30:177–84. doi: 10.1089/thy.2019.0250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Hua C, Boussemart L, Mateus C, Routier E, Boutros C, Cazenave H, et al. Association of Vitiligo With Tumor Response in Patients With Metastatic Melanoma Treated With Pembrolizumab. JAMA Dermatol (2016) 152:45–51. doi: 10.1001/jamadermatol.2015.2707 [DOI] [PubMed] [Google Scholar]
- 227. Larkin J, Hodi FS, Wolchok JD. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med (2015) 373:1270–1. doi: 10.1056/NEJMc1509660 [DOI] [PubMed] [Google Scholar]
- 228. Kociol RD, Cooper LT, Fang JC, Moslehi JJ, Pang PS, Sabe MA, et al. Recognition and Initial Management of Fulminant Myocarditis: A Scientific Statement From the American Heart Association. Circulation (2020) 141:e69–92. doi: 10.1161/CIR.0000000000000745 [DOI] [PubMed] [Google Scholar]
- 229. Ammirati E, Frigerio M, Adler ED, Basso C, Birnie DH, Brambatti M, et al. Management of Acute Myocarditis and Chronic Inflammatory Cardiomyopathy: An Expert Consensus Document. Circ Heart Fail (2020) 13:e007405. doi: 10.1161/CIRCHEARTFAILURE.120.007405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Petrelli F, Signorelli D, Ghidini M, Ghidini A, Pizzutilo EG, Ruggieri L, et al. Association of Steroids Use With Survival in Patients Treated With Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis. Cancers (Basel) (2020) 12:546. doi: 10.3390/cancers12030546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Arbour KC, Mezquita L, Long N, Rizvi H, Auclin E, Ni A, et al. Impact of Baseline Steroids on Efficacy of Programmed Cell Death-1 and Programmed Death-Ligand 1 Blockade in Patients With Non-Small-Cell Lung Cancer. J Clin Oncol (2018) 36:2872–8. doi: 10.1200/JCO.2018.79.0006 [DOI] [PubMed] [Google Scholar]
- 232. Scott SC, Pennell NA. Early Use of Systemic Corticosteroids in Patients With Advanced NSCLC Treated With Nivolumab. J Thorac Oncol (2018) 13:1771–5. doi: 10.1016/j.jtho.2018.06.004 [DOI] [PubMed] [Google Scholar]
- 233. Fuca G, Galli G, Poggi M, Lo Russo G, Proto C, Imbimbo M, et al. Modulation of Peripheral Blood Immune Cells by Early Use of Steroids and its Association With Clinical Outcomes in Patients With Metastatic non-Small Cell Lung Cancer Treated With Immune Checkpoint Inhibitors. ESMO Open (2019) 4:e000457. doi: 10.1136/esmoopen-2018-000457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Chasset F, Pages C, Biard L, Roux J, Sidina I, Madelaine I, et al. Single-Center Study Under a French Temporary Authorization for Use (TAU) Protocol for Ipilimumab in Metastatic Melanoma: Negative Impact of Baseline Corticosteroids. Eur J Dermatol (2015) 25:36–44. doi: 10.1684/ejd.2014.2471 [DOI] [PubMed] [Google Scholar]
- 235. Ricciuti B, Dahlberg SE, Adeni A, Sholl LM, Nishino M, Awad MM. Immune Checkpoint Inhibitor Outcomes for Patients With Non-Small-Cell Lung Cancer Receiving Baseline Corticosteroids for Palliative Versus Nonpalliative Indications. J Clin Oncol (2019) 37:1927–34. doi: 10.1200/JCO.19.00189 [DOI] [PubMed] [Google Scholar]
- 236. Draghi A, Borch TH, Radic HD, Chamberlain CA, Gokuldass A, Svane IM, et al. Differential Effects of Corticosteroids and Anti-TNF on Tumor-Specific Immune Responses: Implications for the Management of irAEs. Int J Cancer (2019) 145:1408–13. doi: 10.1002/ijc.32080 [DOI] [PubMed] [Google Scholar]
- 237. Verheijden RJ, May AM, Blank CU, Aarts MJB, van den Berkmortel F, van den Eertwegh AJM, et al. Association of Anti-TNF With Decreased Survival in Steroid Refractory Ipilimumab and Anti-PD1-Treated Patients in the Dutch Melanoma Treatment Registry. Clin Cancer Res (2020) 26:2268–74. doi: 10.1158/1078-0432.CCR-19-3322 [DOI] [PubMed] [Google Scholar]
- 238. Lesage C, Longvert C, Prey S, Maanaoui S, Dreno B, Machet L, et al. Incidence and Clinical Impact of Anti-TNFalpha Treatment of Severe Immune Checkpoint Inhibitor-Induced Colitis in Advanced Melanoma: The Mecolit Survey. J Immunother (2019) 42:175–9. doi: 10.1097/CJI.0000000000000268 [DOI] [PubMed] [Google Scholar]
- 239. Chen AY, Wolchok JD, Bass AR. TNF in the Era of Immune Checkpoint Inhibitors: Friend or Foe? Nat Rev Rheumatol (2021) 17:213–23. doi: 10.1038/s41584-021-00584-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Suijkerbuijk KPM, Verheijden RJ. TNF Inhibition for Immune Checkpoint Inhibitor-Induced irAEs: The Jury is Still Out. Nat Rev Rheumatol (2021) 17:505. doi: 10.1038/s41584-021-00640-z [DOI] [PubMed] [Google Scholar]
- 241. Perez-Ruiz E, Minute L, Otano I, Alvarez M, Ochoa MC, Belsue V, et al. Prophylactic TNF Blockade Uncouples Efficacy and Toxicity in Dual CTLA-4 and PD-1 Immunotherapy. Nature (2019) 569:428–32. doi: 10.1038/s41586-019-1162-y [DOI] [PubMed] [Google Scholar]
- 242. Zhang L, Zlotoff DA, Awadalla M, Mahmood SS, Nohria A, Hassan MZO, et al. Major Adverse Cardiovascular Events and the Timing and Dose of Corticosteroids in Immune Checkpoint Inhibitor-Associated Myocarditis. Circulation (2020) 141:2031–4. doi: 10.1161/CIRCULATIONAHA.119.044703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Galdiero MR, Morelli E, Triggianese P, Carucci L, Punziano A, Pinto A, et al. First Report of De Novo Nivolumab-Induced Oligoarthritis in a Young Man With Relapsing Classic Hodgkin Lymphoma. J Clin Rheumatol (2020) 27:S877–8. doi: 10.1097/RHU.0000000000001459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Shimba A, Ikuta K. Control of Immunity by Glucocorticoids in Health and Disease. Semin Immunopathol (2020) 42:669–80. doi: 10.1007/s00281-020-00827-8 [DOI] [PubMed] [Google Scholar]
- 245. Badran YR, Cohen JV, Brastianos PK, Parikh AR, Hong TS, Dougan M. Concurrent Therapy With Immune Checkpoint Inhibitors and TNFalpha Blockade in Patients With Gastrointestinal Immune-Related Adverse Events. J Immunother Cancer (2019) 7:226. doi: 10.1186/s40425-019-0711-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Kalisz KR, Ramaiya NH, Laukamp KR, Gupta A. Immune Checkpoint Inhibitor Therapy-Related Pneumonitis: Patterns and Management. Radiographics (2019) 39:1923–37. doi: 10.1148/rg.2019190036 [DOI] [PubMed] [Google Scholar]
- 247. Kang JH, Bluestone JA, Young A. Predicting and Preventing Immune Checkpoint Inhibitor Toxicity: Targeting Cytokines. Trends Immunol (2021) 42:293–311. doi: 10.1016/j.it.2021.02.006 [DOI] [PubMed] [Google Scholar]
- 248. Abu-Sbeih H, Ali FS, Alsaadi D, Jennings J, Luo W, Gong Z, et al. Outcomes of Vedolizumab Therapy in Patients With Immune Checkpoint Inhibitor-Induced Colitis: A Multi-Center Study. J Immunother Cancer (2018) 6:142. doi: 10.1186/s40425-018-0461-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Frohne CC, Llano EM, Perkovic A, Cohen RD, Luke JJ. Complete Response of Metastatic Melanoma in a Patient With Crohn’s Disease Simultaneously Receiving Anti-Alpha4beta7 and Anti-PD1 Antibodies. J Immunother Cancer (2019) 7:1. doi: 10.1186/s40425-018-0484-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250. Salem JE, Allenbach Y, Vozy A, Brechot N, Johnson DB, Moslehi JJ, et al. Abatacept for Severe Immune Checkpoint Inhibitor-Associated Myocarditis. N Engl J Med (2019) 380:2377–9. doi: 10.1056/NEJMc1901677 [DOI] [PubMed] [Google Scholar]
- 251. Ito M, Fujiwara S, Fujimoto D, Mori R, Yoshimura H, Hata A, et al. Rituximab for Nivolumab Plus Ipilimumab-Induced Encephalitis in a Small-Cell Lung Cancer Patient. Ann Oncol (2017) 28:2318–9. doi: 10.1093/annonc/mdx252 [DOI] [PubMed] [Google Scholar]
- 252. Crusz SM, Radunovic A, Shepherd S, Shah S, Newey V, Phillips M, et al. Rituximab in the Treatment of Pembrolizumab-Induced Myasthenia Gravis. Eur J Cancer (2018) 102:49–51. doi: 10.1016/j.ejca.2018.07.125 [DOI] [PubMed] [Google Scholar]
- 253. Mamlouk O, Lin JS, Abdelrahim M, Tchakarov AS, Glass WF, Selamet U, et al. Checkpoint Inhibitor-Related Renal Vasculitis and Use of Rituximab. J Immunother Cancer (2020) 8:e000750. doi: 10.1136/jitc-2020-000750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Stroud CR, Hegde A, Cherry C, Naqash AR, Sharma N, Addepalli S, et al. Tocilizumab for the Management of Immune Mediated Adverse Events Secondary to PD-1 Blockade. J Oncol Pharm Pract (2019) 25:551–7. doi: 10.1177/1078155217745144 [DOI] [PubMed] [Google Scholar]
- 255. Kim ST, Tayar J, Trinh VA, Suarez-Almazor M, Garcia S, Hwu P, et al. Successful Treatment of Arthritis Induced by Checkpoint Inhibitors With Tocilizumab: A Case Series. Ann Rheum Dis (2017) 76:2061–4. doi: 10.1136/annrheumdis-2017-211560 [DOI] [PubMed] [Google Scholar]
- 256. Esfahani K, Miller WH., Jr. Reversal of Autoimmune Toxicity and Loss of Tumor Response by Interleukin-17 Blockade. N Engl J Med (2017) 376:1989–91. doi: 10.1056/NEJMc1703047 [DOI] [PubMed] [Google Scholar]
- 257. Johnson D, Patel AB, Uemura MI, Trinh VA, Jackson N, Zobniw CM, et al. IL17A Blockade Successfully Treated Psoriasiform Dermatologic Toxicity From Immunotherapy. Cancer Immunol Res (2019) 7:860–5. doi: 10.1158/2326-6066.CIR-18-0682 [DOI] [PubMed] [Google Scholar]
- 258. Wang Y, Wiesnoski DH, Helmink BA, Gopalakrishnan V, Choi K, DuPont HL, et al. Fecal Microbiota Transplantation for Refractory Immune Checkpoint Inhibitor-Associated Colitis. Nat Med (2018) 24:1804–8. doi: 10.1038/s41591-018-0238-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Zhang RS, Padegimas A, Murphy KM, Evans PT, Peters CJ, Domenico CM, et al. Treatment of Corticosteroid Refractory Immune Checkpoint Inhibitor Myocarditis With Infliximab: A Case Series. Cardiooncology (2021) 7:13. doi: 10.1186/s40959-021-00095-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. Cautela J, Zeriouh S, Gaubert M, Bonello L, Laine M, Peyrol M, et al. Intensified Immunosuppressive Therapy in Patients With Immune Checkpoint Inhibitor-Induced Myocarditis. J Immunother Cancer (2020) 8:e001887. doi: 10.1136/jitc-2020-001887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. Kumar B, Ballas Z. Adverse Events Associated With Immune Checkpoint Blockade. N Engl J Med (2018) 378:1164. doi: 10.1056/NEJMc1801663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Mann DL. Innate Immunity and the Failing Heart: The Cytokine Hypothesis Revisited. Circ Res (2015) 116:1254–68. doi: 10.1161/CIRCRESAHA.116.302317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Frantz S, Falcao-Pires I, Balligand JL, Bauersachs J, Brutsaert D, Ciccarelli M, et al. The Innate Immune System in Chronic Cardiomyopathy: A European Society of Cardiology (ESC) Scientific Statement From the Working Group on Myocardial Function of the ESC. Eur J Heart Fail (2018) 20:445–59. doi: 10.1002/ejhf.1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Feagan BG, Rutgeerts P, Sands BE, Hanauer S, Colombel JF, Sandborn WJ, et al. Vedolizumab as Induction and Maintenance Therapy for Ulcerative Colitis. N Engl J Med (2013) 369:699–710. doi: 10.1056/NEJMoa1215734 [DOI] [PubMed] [Google Scholar]
- 265. Sandborn WJ, Feagan BG, Rutgeerts P, Hanauer S, Colombel JF, Sands BE, et al. Vedolizumab as Induction and Maintenance Therapy for Crohn’s Disease. N Engl J Med (2013) 369:711–21. doi: 10.1056/NEJMoa1215739 [DOI] [PubMed] [Google Scholar]
- 266. Sands BE, Peyrin-Biroulet L, Loftus EV, Jr., Danese S, Colombel JF, Toruner M, et al. Vedolizumab Versus Adalimumab for Moderate-To-Severe Ulcerative Colitis. N Engl J Med (2019) 381:1215–26. doi: 10.1056/NEJMoa1905725 [DOI] [PubMed] [Google Scholar]
- 267. Varricchi G, Poto R, Ianiro G, Punziano A, Marone G, Gasbarrini A, et al. Gut Microbiome and Common Variable Immunodeficiency: Few Certainties and Many Outstanding Questions. Front Immunol (2021) 12:712915. doi: 10.3389/fimmu.2021.712915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. Hirsch BE, Saraiya N, Poeth K, Schwartz RM, Epstein ME, Honig G. Effectiveness of Fecal-Derived Microbiota Transfer Using Orally Administered Capsules for Recurrent Clostridium Difficile Infection. BMC Infect Dis (2015) 15:191. doi: 10.1186/s12879-015-0930-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Kao D, Roach B, Silva M, Beck P, Rioux K, Kaplan GG, et al. Effect of Oral Capsule- vs Colonoscopy-Delivered Fecal Microbiota Transplantation on Recurrent Clostridium Difficile Infection: A Randomized Clinical Trial. JAMA (2017) 318:1985–93. doi: 10.1001/jama.2017.17077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270. Youngster I, Russell GH, Pindar C, Ziv-Baran T, Sauk J, Hohmann EL. Oral, Capsulized, Frozen Fecal Microbiota Transplantation for Relapsing Clostridium Difficile Infection. JAMA (2014) 312:1772–8. doi: 10.1001/jama.2014.13875 [DOI] [PubMed] [Google Scholar]
- 271. Allegretti JR, Korzenik JR, Hamilton MJ. Fecal Microbiota Transplantation via Colonoscopy for Recurrent C. Difficile Infection. J Vis Exp (2014) 52154. doi: 10.3791/52154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272. Silverman MS, Davis I, Pillai DR. Success of Self-Administered Home Fecal Transplantation for Chronic Clostridium Difficile Infection. Clin Gastroenterol Hepatol (2010) 8:471–3. doi: 10.1016/j.cgh.2010.01.007 [DOI] [PubMed] [Google Scholar]
- 273. Tariq R, Pardi DS, Bartlett MG, Khanna S. Low Cure Rates in Controlled Trials of Fecal Microbiota Transplantation for Recurrent Clostridium Difficile Infection: A Systematic Review and Meta-Analysis. Clin Infect Dis (2019) 68:1351–8. doi: 10.1093/cid/ciy7215078604 [DOI] [PubMed] [Google Scholar]
- 274. de Groot P, Scheithauer T, Bakker GJ, Prodan A, Levin E, Khan MT, et al. Donor Metabolic Characteristics Drive Effects of Faecal Microbiota Transplantation on Recipient Insulin Sensitivity, Energy Expenditure and Intestinal Transit Time. Gut (2020) 69:502–12. doi: 10.1136/gutjnl-2019-318320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275. Wilson BC, Vatanen T, Cutfield WS, O’Sullivan JM. The Super-Donor Phenomenon in Fecal Microbiota Transplantation. Front Cell Infect Microbiol (2019) 9:2. doi: 10.3389/fcimb.2019.00002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276. Marcella C, Cui B, Kelly CR, Ianiro G, Cammarota G, Zhang F. Systematic Review: The Global Incidence of Faecal Microbiota Transplantation-Related Adverse Events From 2000 to 2020. Aliment Pharmacol Ther (2021) 53:33–42. doi: 10.1111/apt.16148 [DOI] [PubMed] [Google Scholar]
- 277. Ianiro G, Mullish BH, Kelly CR, Kassam Z, Kuijper EJ, Ng SC, et al. Reorganisation of Faecal Microbiota Transplant Services During the COVID-19 Pandemic. Gut (2020) 69:1555–63. doi: 10.1136/gutjnl-2020-321829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278. Esfahani K, Elkrief A, Calabrese C, Lapointe R, Hudson M, Routy B, et al. Moving Towards Personalized Treatments of Immune-Related Adverse Events. Nat Rev Clin Oncol (2020) 17:504–15. doi: 10.1038/s41571-020-0352-8 [DOI] [PubMed] [Google Scholar]
- 279. Huinen ZR, Huijbers EJM, van Beijnum JR, Nowak-Sliwinska P, Griffioen AW. Anti-Angiogenic Agents - Overcoming Tumour Endothelial Cell Anergy and Improving Immunotherapy Outcomes. Nat Rev Clin Oncol (2021) 18:527–40. doi: 10.1038/s41571-021-00496-y [DOI] [PubMed] [Google Scholar]