Abstract
Staphylococcus aureus Mu50, which has reduced susceptibility to vancomycin, has a remarkably thickened cell wall with an increased proportion of glutamine nonamidated muropeptides. In addition, Mu50 had enhanced glutamine synthetase and l-glutamine d-fructose-6-phosphate aminotransferase activities, which are involved in the cell-wall peptidoglycan synthesis pathway. Furthermore, significantly increased levels of incorporation of 14C-labeled d-glucose into the cell wall was observed in Mu50. Unlike a femC mutant S. aureus strain, increased levels of production of nonamidated muropeptides in Mu50 was not caused by lower levels of glutamine synthetase activity but was considered to be due to the glutamine depletion caused by increased glucose utilization by the cell to biosynthesize increased amounts of peptidoglycan. After the cells were allowed to synthesize cell wall in the absence or presence of glucose and glutamine, cells with different cell-wall thicknesses and with cell walls with different levels of cross-linking were prepared, and susceptibility testing of these cells demonstrated a strong correlation between the cell-wall thickness and the degree of vancomycin resistance. Affinity trapping of vancomycin molecules by the cell wall and clogging of the outer layers of peptidoglycan by bound vancomycin molecules were considered to be the mechanism of vancomycin resistance of Mu50. The reduced cross-linking and the increased affinity of binding to vancomycin of the Mu50 cell wall presumably caused by the increased proportion of nonamidated muropeptides may also contribute to the resistance to some extent.
Since the detection of Staphylococcus aureus clinical strain Mu50, which has reduced susceptibility to vancomycin (designated glycopeptide-intermediate S. aureus [31] or vancomycin-resistant S. aureus [11, 12]), there has been great interest in the mechanism by which vancomycin resistance is expressed in this strain. No enterococcal van genes have been found in the strain (9, 32). Our previous studies with Mu50 and Mu3, the presumed heterotypic precursor strain of strain Mu50, showed that both strains share several phenotypes associated with accelerated cell-wall synthesis and turnover. These include enhanced incorporation of N-acetylglucosamine (GlcNAc) into the cell wall, increased cytoplasmic pool size of the murein monomer precursor (UDP-N-acetylmuramyl-pentapeptide), enhanced autolysis, increased rate of cell-wall turnover as measured by the release of radioactivity from GlcNAc-labeled cells, and increased levels of production of penicillin-binding protein 2 (PBP 2) and PBP 2′ compared to the levels produced by vancomycin-susceptible strains (7–9, 13–15, 31). Compared to Mu3, however, Mu50 possesses about twofold increased cell-wall thickness and an increased amount of glutamine nonamidated muropeptides in the cell wall, in addition to the characteristics common between the two strains. The unusual muropeptide is different from the normal muropeptide in that the iso-d-glutamate residue in the pentapeptide stem (–l-Ala–d-Glu–l-Lys–d-Ala–d-Ala) remains nonamidated (8).
Production of such abnormal muropeptides has been known as a characteristic of the femC mutant BB589, a methicillin-susceptible mutant derived from a methicillin-resistant S. aureus (MRSA) strain, in which a Tn551 is inserted in the vicinity of the glutamine synthetase (GS) repressor gene (glnR), which exerts a polar effect on the transcription of the GS synthetase gene (glnA) (5, 23). The resultant reduction in the GS activity in BB589 leads to the reduced intracellular pool size of glutamine. Since glutamine serves as the NH4+ donor in the amidation reaction of the iso-d-glutamate in the stem pentapeptide of the murein monomer precursor, the decrease in the amount of glutamine causes an increase in the proportion of nonamidated muropeptides in the cell wall of the femC mutant (23). This study was conducted to explore the mechanism for the thickened cell wall and the increase in the levels of nonamidated muropeptides and their possible role in the vancomycin resistance expressed by Mu50.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
All the strains used in this study and their relevant characteristics are described in Table 1. Strain BB255 (NCTC 8325), strain BB270 (NCTC 8325 transduced with the mec determinant), and femC mutant strain BB589 derived from BB270 were kindly provided by B. Berger-Bachi (5). Strain Mu50ω was isolated in 1997 from the same patient from whom Mu50 was isolated. After his discharge, the patient experienced three episodes of relapse of a surgical site wound infection and was rehospitalized in Juntendo Hospital for treatment. On his third hospitalization (1 year after the first episode), an abscess under the sternum, just beneath the original surgical incision site, grew multiple MRSA colonies on agar plates. They (10 colonies were tested) had identical pulsed-field gel electrophoresis patterns, and the vancomycin MICs (8 mg/liter) for all except one of the colonies were the same as the MIC for Mu50. The colony that was the exception was small in size, and the vancomycin MIC for the colony was 0.5 mg/liter, although it had a pulsed-field gel electrophoresis pattern identical to that of Mu50. This strain was considered a probable spontaneous revertant derivative of Mu50 and was designated Mu50ω. All cultures were grown in brain heart infusion (BHI) broth (Difco, Detroit, Mich.) at 37°C with aeration unless indicated otherwise. For each experiment, an overnight culture was diluted 100-fold in prewarmed fresh BHI broth and was further incubated with aeration to ensure exponential growth conditions before sampling. Cell growth was monitored by measuring the optical density of the culture at 578 nm (OD578) with a spectrophotometer (Pharmacia LKB Biotechnology, Inc., Uppsala, Sweden).
TABLE 1.
S. aureus strains used in this study
| Strain | Relevant feature of the strain | Reference |
|---|---|---|
| Mu50 | Reduced susceptibility to vancomycin (MIC, 8 μg/ml) | 11 |
| Mu3 | Reduced susceptibility to vancomycin (heterogeneousa) | 11 |
| Mu50ω | Reduced susceptibility to vancomycin (heterogeneousa) | This study |
| N315 | Susceptible to vancomycin, pre-MRSA | 7 |
| H1 | Susceptible to vancomycin, MRSA | 11 |
| FDA209P | Susceptible to vancomycin, MSSAb type strain | 11 |
| BB255 | Equivalent to NCTC8325, MSSA | 11 |
| BB270 | NCTC8325 transduced with mec determinant | 5 |
| BB589 | femC mutant derived from BB270 | 5 |
Contains subpopulation of cells for which the MIC is 8 μg/ml or greater.
MSSA, methicillin-susceptible S. aureus.
Nucleotide sequencing.
DNA fragments of 2.7 kb and with the entire glnA and glnR genes and the surrounding region were obtained by PCR amplification with genomic DNA extracted from strains Mu50, Mu3, N315, and NCTC 8325 as templates with the synthetic primers 5′-GATCAAAGACCTTCAATTCC-3′ and 5′-AAAGTGGTCAAGTGAAATCC-3′ (5). After purification with the QIAquik PCR purification kit (QIAGEN, Hilden, Germany), the PCR products were sequenced with a set of serial synthetic oligonucleotide primers (designed on the basis of the reported nucleotide sequence [5]) with a dye-labeled terminator–Taq DNA polymerase cycle sequencing kit (Applied Biosystems Inc., Foster City, Calif.). The sequence was read on a 373A automated fluorescent DNA sequencing system (Perkin-Elmer, Foster City, Calif.). Alignment of the sequences and estimation of the degree of homology with the reported sequence were performed with Genetyx-Mac (Software Development Co., Ltd., Tokyo, Japan) programs.
Incorporation of 14C-labeled d-glucose into the cell wall.
The test bacterial strains were cultivated at 37°C for 18 h in BHI broth. The culture was diluted 20-fold with fresh prewarmed BHI broth and was further cultivated until an OD578 of 0.7 was reached by monitoring the density with a U320 spectrophotometer (Hitachi Inc., Tokyo, Japan). The cells were then pelleted from the 8-ml portion of the culture by centrifugation at 4,000 × g for 10 min. The pellets were washed once with RMg− (the modified resting medium [RM] in which glucose was omitted from the reported ingredients) (8, 27) and resuspended in 8 ml of the same medium. The cell suspension was divided into two 4-ml portions: one for the glucose incorporation experiment and another for measurement of cell density. One 4-ml portion of the suspension was added to 20 μl of 1 M d-glucose (Wako Pure Chemical Industries, Ltd., Osaka, Japan) solution and 4 μl of 14C-labeled d-glucose (3,700 MBq/ml; NEC-043X GLUCOSE, d-[1-14C]−; DuPont NEN, NEN Life Science Products, Boston, Mass.) and then incubated at 37°C with gentle shaking. After 0, 15, 30, 60, and 120 min of incubation, a 0.5-ml portion of the cell suspension was taken and transferred to a microcentrifuge tube containing 0.1 ml of 1 M d-glucose to inhibit further incorporation of the radioactive glucose. Then, 0.5 ml of 10% trichloroacetic acid was immediately added to disrupt the cells, followed by centrifugation at 12,000 × g for 15 min. The pellets were suspended in 1 ml of 4% sodium dodecyl sulfate (SDS), and the mixture was incubated at 90°C for 30 min to further dissolve the protein. The samples were then centrifuged, and the pellets were washed several times with distilled water until the SDS was completely removed. The pellet was then resuspended in 1 ml of 40% (wt/vol) aqueous hydrofluoric acid, and the mixture was incubated for 18 h at 4°C to remove teichoic acids. After centrifugation at 12,000 × g for 5 min, the supernatant was discarded. The crude cell-wall material was washed once with 0.1% SDS and twice with distilled water and was resuspended in 0.5 ml of distilled water. The suspension was then mixed with 5 ml of Aquasol2 (Packard Inc., Meriden, Ill.), and the radioactivity was counted with an LS3801 liquid scintillation counter (Beckman Instruments Inc., Palo Alto, Calif.). In the experiment conducted to test the influence of glutamine, the addition of 14C-labeled d-glucose to the cell suspension was preceded by incubation of the cell suspension in RMg− with various concentrations of l-glutamine at 37°C for 30 min. The experiment was performed in duplicate on three independent occasions, and the results are shown as the mean value ± the standard deviation (SD).
Preparation of crude cell extracts and assay of Glms.
The overnight culture in BHI medium was diluted 100-fold in 25 ml of prewarmed BHI medium and was further cultivated at 37°C with shaking to an OD578 of 0.7. The cells were harvested and washed twice with cold 20 mM TE (Tris-EDTA) buffer (pH 7.6). The crude extracts were obtained by digestion with 0.1% lysostaphin (Sigma Chemical Co., St. Louis, Mo.) in 0.2 ml of TE buffer at 37°C for 10 min, followed by the addition of 0.1 ml of 0.2% DNase (Sigma Chemical Co.). The l-glutamine d-fructose-6-phosphate (Fru-6-P) aminotransferase (GlmS) activity in the extract was assayed by the method described by Zalkin (34). One unit of enzyme activity was defined as the activity that catalyzed the synthesis of 1.0 μmol of glucosamine-6-phosphate (GlcN-6-P) per hour. Specific activity was described as the number of units per milligram of protein. The protein concentration was determined by using the BCA protein assay reagent (Pierce, Rockford, Ill.), with bovine serum albumin used as the standard. Determination of the activity in the sample was done in triplicate on three independent occasions, and the results are shown as means ± SDs.
GS assay.
The GS assay was carried out with cells in the mid-exponential growth phase harvested at an OD578 of 0.7. GS activity was measured in hexadecyltrimethylammonium bromide (CTAB)-permeabilized cells on the basis of the formation of γ-glutamylhydroxamate in both the biosynthetic and the transferase reactions (3). Before the cells were harvested, 10 ml of CTAB (1 mg/ml) was added to 100 ml of the cultures, and the cultures were shaken for 5 min at 37°C. The cells were harvested, washed with 10 ml of 0.05 M imidazole (pH 7.0)–0.2 mM EDTA–0.2 mg of dithiothreitol per ml, and resuspended in 0.5 ml of 0.05 M imidazole (pH 7.0)–0.5 M EDTA–0.5 mg of dithiothreitol per ml. A total of 50 μl of the suspension was added to 450 μl of each of the two GS assay mixtures: 50 mM glutamate, 40 mM hydroxylamine, 100 mM MgCl2, 18 mM ATP, and 0.05 M imidazole for the biosynthetic reaction and 25 mM Tris-HCl (pH 7.5), 25 mM imidazole, 50 mM NH2OH, 40 mM glutamine, 2 mM ADP, 0.33 mM MnCl2, and 20 mM sodium arsenate for the transferase reaction. The reaction was run at 37°C for 30 min, at which time 1 ml of stop solution (0.37 M FeCl3, 0.67 N HCl, 0.2 M trichloroacetic acid) was added, and the assay mixture was placed on ice for 5 min. The cells were removed by centrifugation at 12,000 × g for 10 min, and the absorbance (OD540) of the supernatant was measured. A unit of GS activity was defined as the activity that formed 1 nmol of γ-glutamylhydroxamate per min. Specific activity was described as the number of units per milligram of protein. Protein was measured as described above for the GlmS assay. Determination of the activity of the sample was done in triplicate on three independent occasions, and the results are expressed as means ± SDs.
Influences of d-glucose, l-glutamine, and GlcNAc on cell-wall thickness, peptidoglycan composition, and regrowth capability of Mu50 in the presence of vancomycin.
Mu50 cells were cultivated in BHI broth to an OD578 of 0.7 (about 8.9 × 108 CFU/ml) and were washed twice with RMg− at 4,000 × g for 10 min, and they were then further cultivated in RMg− containing 30 mM d-glucose and/or l-glutamine or GlcNAc for 2 h. Then the cell-wall thickness, the peptidoglycan composition, and the ability of the cells to regrow in the presence of vancomycin were analyzed. The cell-wall thickness was examined by transmission electron microscopy (see below). The preparation and composition analysis of peptidoglycan were performed as described previously (7, 8). For the evaluation of vancomycin resistance, the cells were spun down and the pellets were resuspended in prewarmed BHI medium containing 30 μg of vancomycin per ml and were then cultivated in a photo-recording incubator (TN-261; ADVANTEC, Tokyo, Japan) at 37°C, with the OD600 of the culture monitored every 2 min. At various times, sterile filtrates of the culture were prepared and subjected to bioassay for determination of the vancomycin concentration (see below).
Binding of vancomycin by purified peptidoglycan.
The assay for detection of the binding of vancomycin to peptidoglycan was carried out by using high-pressure liquid chromatography (HPLC), and the number of vancomycin molecules bound to 1 mg of peptidoglycan was calculated as described previously (8). The yield of purified peptidoglycan from Mu50 cells in RM was 20 to 23 mg from 2 × 1011 cells (ca. 1 mg from 1 × 1010 cells). The experiment was performed in triplicate on two independent occasions, and the results are expressed as the means ± SDs.
Vancomycin bioassay.
A microbiological assay (bioassay) for determination of the vancomycin concentration in culture medium was carried out by the paper disk diffusion method with Bacillus subtilis ATCC 6633 as the indicator organism and L broth (Takara, Biomedical Group, Tokyo, Japan) containing 0.5% agar (Difco, Becton Dickinson, Cockeysville, Md.) as the test medium. A fresh suspension of the indicator strain was adjusted to an OD578 of 0.3 in Trypticase soy broth and was mixed with 0.5% Luria-Bertani agar (cooled to 45°C) at 1:1,000 (vol/vol) volume. Then, 22-mm-thick agar plates were prepared by pouring the mixture and solidified. Paper disks (diameter, 8 mm; ADVANTEC; Toyo Roshi Kaisha, Ltd., Tokyo, Japan) impregnated with 0.05 ml of serial dilutions of vancomycin of known concentration and of sterile filtrates of the culture were aseptically placed on the inoculated plates, and the plates were incubated overnight. Then, the diameters of the inhibition zones around the paper disks were measured with a micrometer. A standard curve was constructed to correlate the zone size with vancomycin concentrations of 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 15, 20, 25, and 30 mg/liter. The vancomycin concentrations in the test samples were determined by fitting the mean zone of inhibition to the standard curve. The zone diameter for each test sample was determined as a mean of six inhibition zones in two agar plates (three disks per plate).
Transmission electron microscopy.
Preparation and examination of S. aureus cells by transmission electron microscopy were performed as described previously (7). Morphometric evaluation of cell-wall thickness was performed by using photographs of images obtained at a final magnification of ×30,000. A transparent grid made up of 20 radial lines arranged regularly at angles of 18° was then placed on the center of each cell examined to measure the interaction zones between the lines and the wall at a minimum of 10 different points. The thicknesses of the cell walls of 30 cells of each strain with nearly equatorially cut surfaces were measured, and the results were expressed as the means ± SDs.
Statistical analysis of data.
The statistical significance of the data was evaluated by Student's t test.
RESULTS
Analysis of vancomycin-resistant subpopulations of strains used in this study.
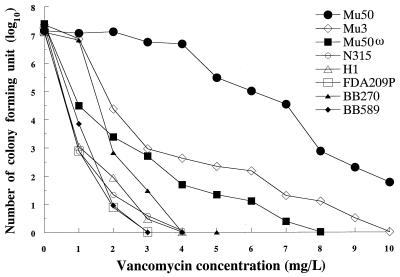
Figure 1 illustrates the results of population analysis of the strains used in this study. The vancomycin-susceptible apparent revertant strain Mu50ω showed a typical heterogeneous-type population curve like that of Mu3. There were fewer resistant subpopulations of Mu50ω, however, compared to the number of resistant subpopulations of Mu3. BB589 is the femC mutant strain derived from BB270. BB589 produces an increased proportion of glutamine nonamidated muropeptides in the cell wall (5). The population curves for both strains showed a pattern of vancomycin susceptibility. However, BB270 had a significantly larger population of cells than Mu50ω capable of growth in the presence of 1 mg of vancomycin per liter (Fig. 1).
FIG. 1.
Analysis of vancomycin-resistant subpopulations of S. aureus strains used in this study. The population patterns of Mu50, Mu3, H1, and FDA209P have been described previously (11). Strain Mu50ω also showed a pattern of heterogeneous-type resistance to vancomycin, as did Mu3. femC mutant strain BB589 as well as its parent strain, BB270, exhibited vancomycin-susceptible population curves, as did H1 and FDA209P.
Increased incorporation of 14C-labeled d-glucose into cell wall of Mu50.
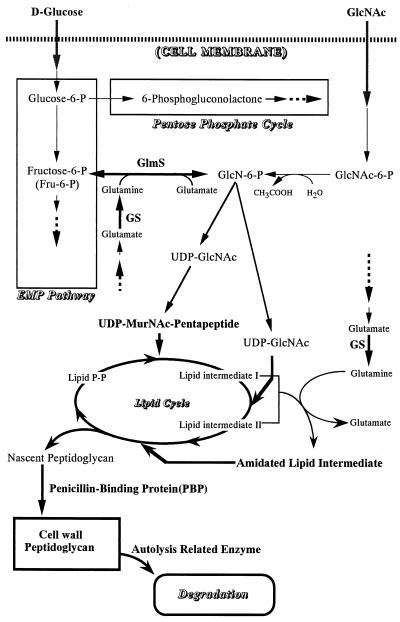
Bacterial cells are known to produce cell-wall peptidoglycan by two metabolic pathways (Fig. 2). One pathway starts with the uptake of GlcNAc from outside the cell, which then is converted to GlcN-6-P. This pathway has been shown to be enhanced almost equally in Mu3 and Mu50 compared to the pathways in vancomycin-susceptible S. aureus strains (7, 9). However, Mu50 has a much thicker cell wall than Mu3, as observed by electron microscopy (7). Therefore, we explored the possibility that the other pathway of cell-wall synthesis was also enhanced in Mu50. The pathway involves the initial steps of the Embden-Meyerhof pathway (from the uptake of glucose to the generation of Fru-6-P) and then digresses to the generation of GlcN-6-P (Fig. 2).
FIG. 2.
Metabolic pathways for the synthesis of cell-wall peptidoglycan. Two major biosynthetic pathways supply UDP-GlcNAc, the cardinal precursor metabolite for cell-wall peptidoglycan. Two enzymes involved in the cell-wall synthesis pathways require glutamine as the NH4+ donor for the amidation reaction catalyzed by the enzymes: GlmS catalyzes the conversion of Fru-6-P to GlcN-6-P, and a putative enzyme catalyzes the amidation of iso-glutamate of the murein monomer precursor (in the form of lipid I or II). GS helps the activities of these enzymes by supplying glutamine by assimilating ammonia into glutamate.
Figure 3A shows the time course of incorporation of 14C-labeled d-glucose into the cell wall of a fixed number (8.5 × 108 CFU) of bacterial cells. The incorporation of 14C-labeled d-glucose into the cell wall increased linearly during the 120 min for all strains tested. The total radioactivities of cell walls measured at 120 min for strains Mu50, Mu3, H1, Mu50ω, and N315 were 2.6, 1.9, 1.7, 1.7, and 1.1 times higher, respectively, than that for FDA209P (fixed as 1) (P values for Mu50 versus the other strains including Mu3 were <0.001 for all comparisons). Therefore, Mu50 used more glucose for cell-wall synthesis than the other strains including Mu3. The utilization of glucose in this pathway is mediated by GlmS, the enzyme that converts Fru-6-P into GlcN-6-P, by using glutamine as the ammonium donor (Fig. 2). Therefore, the increased enzyme activity inevitably consumes more glutamine. As reported previously for a femC mutant strain (5), glutamine deficiency is the cause of the increased amount of nonamidated muropeptides in the cell wall, a characteristic feature of Mu50 as well as of the femC mutant (8). In the femC mutant, glutamine depletion is caused by inactivation of GS activity. Therefore, we proceeded to measure the GlmS activity as well as the GS activity of Mu50 in comparison with those of the control strains.
FIG. 3.
Rate of cell-wall synthesis and GS and GlmS activities of Mu50. (A) The rates of 14C-labeled d-glucose incorporation into the cell wall among the test strains were compared. The time course of incorporation of 14C-labeled d-glucose into the cell walls in RMg− was evaluated. Symbols: closed triangle, Mu50; small closed circle, Mu3; large open triangle, H1; small open triangle, Mu50ω; large closed circle, N315; open circle, FDA209P. (B) GlmS and GS activities in Mu50 and other test strains. Bacteria in the logarithmic growth phase were harvested for measurement of the enzyme activities as described in Materials and Methods. GS was tested for both its biosynthetic (glutamine synthetase) and its transferase (glutamyltransferase) activities. ND, not done. (C) Effect of l-glutamine on rate of glucose incorporation. The rates of incorporation of 14C-labeled d-glucose into the cell walls of test strains in medium containing various concentrations of l-glutamine were compared. The bacterial culture in BHI medium was harvested at an OD578 of 0.7, washed twice with RMg−, and incubated in RMg− with various concentration of l-glutamine 30 min before the addition of radioactive glucose. The values shown are those obtained at 20 min in the incorporation assay. Note that in the case of Mu50, more than twice the amount of glucose was incorporated in the presence of 10 mM glutamine.
Increased GlmS and GS activities in Mu50 and Mu3.
Figure 3B compares the GlmS activities of the crude cell extract of Mu50 and the control strains harvested in the exponential growth phase (OD578, 0.7). Mu50 and Mu3 had increased GlmS activities compared to those for the other control strains (P < 0.01). Mu50 had the highest activity, which was 1.3 times greater than that of Mu3 (P < 0.01) and 1.6 to 1.8 times greater than those of H1, Mu50ω, and FDA209P (P < 0.01).
Figure 3B also shows the GS activity of Mu50 in comparison with those of the control strains. It was noticeable that the activities were higher for Mu50 and Mu3 than for the control strains FDA209P and H1 (P values <0.05 to <0.001 for pairwise comparisons). This showed that the observed increase in the levels of glutamine nonamidated muropeptides in Mu50 was not caused by a decreased GS activity, as opposed to the case for femC mutant BB589 (5), which had reduced GS activity compared to that of its parent strain, BB270 (Fig. 3B). In contrast to BB270, strain BB589 had notably enhanced GlmS activity.
In agreement with the intact GS activity in Mu50 and Mu3, the nucleotide sequences of PCR-amplified DNAs encompassing glnR and glnA (which encode GS) of these strains were identical to those of vancomycin-susceptible strain N315 (a representative of Japanese MRSA clonotype II-A, to which the former strains belong [17]). The nucleotide sequence was different from those of BB255 and BB270 (5) at 4 of 2,718 total nucleotides. However, the deduced amino acid sequences of GlnR and GlnA showed no alteration except for a single amino acid substitution in GlnA: glutamic acid (Glu) at the ninth amino acid position of GlnA of strain BB270 was replaced by aspartic acid (Asp) in the GlnA sequences of Mu50, Mu3, and N315 (24).
If the glutamine consumption of Mu50 was due to the increased activity of GlmS, supplementation of the culture medium with glutamine should further enhance the utilization of glucose as a cell-wall precursor metabolite. As shown in Fig. 3C, addition of l-glutamine had a significant enhancement effect on the incorporation of 14C-labeled d-glucose into the cell wall of Mu50 in a dose-dependent manner (P < 0.005 when different glutamine concentrations are compared). The enhancement effect was also observed with the control strains including Mu3, but the degrees of enhancement of the strains were significantly lower than that observed with Mu50 (P < 0.05 to <0.001). The degree of enhancement was well correlated with the GlmS activities of the strains tested (Fig. 3B and C) (the correlation coefficient was 0.973 when the GlmS activities were correlated with the rates of increase in [14C]glucose incorporation by the presence of 10 mM glutamine).
Effect of glucose and glutamine in medium on regrowth capability of Mu50 in presence of vancomycin.
To evaluate the influence of nutrients such as glucose, glutamine, and GlcNAc on cell-wall synthesis and vancomycin resistance, we used RM, which lacks most of the amino acids essential for cell growth and yet which supports cell-wall synthesis.
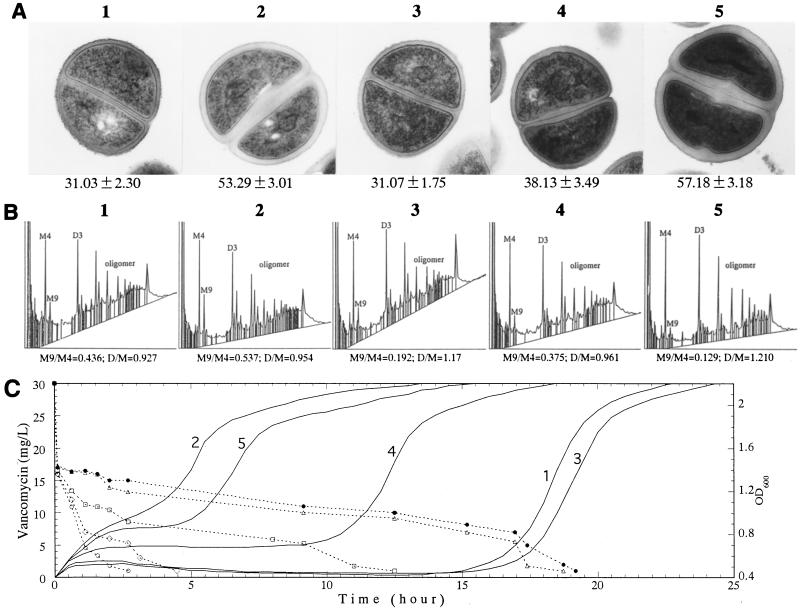
Figure 4 shows transmission electron micrographs, cell-wall composition, and level of vancomycin resistance (defined as time to regrowth [TRG] in the presence of vancomycin) of Mu50 after its cell wall was synthesized in RM with different nutrient compositions: RMg−, RM (RMg− with 30 mM d-glucose), RMg− with 30 mM l-glutamine, RMg− with 30 mM GlcNAc, and RM and 30 mM l-glutamine. As shown in Fig. 4A, the addition of d-glucose to RMg− made the cell wall of Mu50 nearly twice as thick as the cell wall of Mu50 grown in RMg− (31.03 ± 2.30 versus 53.29 ± 3.01 nm [P < 0.001]). Addition of GlcNAc produced a cell wall with an intermediate thickness (38.13 ± 3.49 nm [P < 0.001 compared with that in RMg−]). The cell wall became thickest when d-glucose and l-glutamine were added together, while no thickening effect was observed by the addition of l-glutamine only. Figure 4B shows the results of HPLC analysis of the muramidase-digested muropeptides of cell walls synthesized under various conditions. The M9 peak, which corresponds to the glutamine nonamidated murein monomer (8), was significantly decreased when l-glutamine was added to the medium (indicated by the decrease in the ratio of the area of the M9 peak divided by that of the M4 peak [M9/M4 ratio]). As expected, the level of cross-linking recovered with the decrease in the amount of nonamidated murein monomer, as reflected in the increase in the ratio of the sum of the area of dimer peaks divided by that of the area of monomer peaks [D/M ratio]). The increase in the level of cross-linking of peptidoglycan reduces the number of free d-alanyl–d-alanine residues in the cell wall (8, 23). To confirm this, we measured the vancomycin-binding capacity of purified peptidoglycan synthesized in RM and in RM plus 30 mM glutamine. A unit weight of peptidoglycan synthesized in RM bound about two times more vancomycin molecules than that synthesized in RM plus 30 mM glutamine: (2.069 ± 0.208) × 1017 versus (1.072 ± 0.077) × 1017 molecules/mg of peptidoglycan (P < 0.001).
FIG. 4.
Correlation between the cell-wall thickness, cell-wall structure, and TRG in the presence of vancomycin in Mu50. Mu50 was grown in BHI medium to an OD600 of 0.7, washed twice with RMg− and then incubated at 37°C for 2 h with shaking in either one of the following media: RMg− (panels 1), RM (RMg− plus 30 mM d-glucose) (panels 2), RMg− plus 30 mM l-glutamine (panels 3), RMg− plus 30 mM GlcNAc (panels 4), and RM plus 30 mM l-glutamine (panels 5). The cells were analyzed for cell-wall thickness by transmission electron microscopy (A), cell-wall composition by HPLC (B), and the level of resistance by observing the cell growth in BHI medium containing 30 μg of vancomycin (solid line in panel C) per ml. The vancomycin concentration in the medium was determined by bioassay, as described in Materials and Methods, by using the sterile filtrates of the culture at various times after the initiation of culture (broken line in panel C). Magnification in panel A, ×22,500. M9/M4 and D/M in panel B indicate the ratio of the peak area of the glutamine-nonamidated monomer (M9 peak) versus that of the amidated monomer (M4 peak) and the ratio of the total area of dimer peaks versus that of monomer peaks (8), respectively. The numbers used to label the panels and lines in panels A, B, and C correspond to one another. The values given under each picture in panel A are the means and SDs of the cell wall thickness (in nanometers). In panel C, the symbols for the broken lines (vancomycin concentration) correspond to the numerals for the solid lines (cell growth), as follows: ▵, 1; ○, 2; ●, 3; □, 4; ◊, 5.
After the cell wall synthesis in various RMs, the TRG was assessed in BHI broth with 30 mg of vancomycin per liter (Fig. 4C). The time course of the decrease in the vancomycin concentration in the culture media is also shown in Fig. 4C (indicated by broken lines). Cells with thicker cell walls had shorter TRGs as they started growing earlier (compare the lines labeled 2 and 5, line 4, and lines 1 and 3 in Fig. 4C). On the other hand, when the cells with similar cell-wall thicknesses were compared, the cells with higher M9/M4 ratios and decreased D/M ratios had slightly shorter TRGs (compare line 2 and line 5 and compare line 1 and line 3 in Fig. 4C). Although this difference was small, the orders of growth of the cells represented on lines 2 and 5 in Fig. 4C were reproducible in three separate experiments. (In the case of the cells represented on lines 1 and 3, they grew in the order shown in Fig. 4C two times in three experiments and grew simultaneously in one experiment.) When the cells represented on lines 2 and 5 in Fig. 4C were compared, it was noteworthy that the former started to grow earlier than the latter, even though the latter had a relatively thicker cell wall than the former (see Fig. 4A, panels 2 and 5).
Vancomycin concentrations dropped sharply within 20 min after the cells were inoculated. However, not much difference in the extent of the decrease among the cells with various cell-wall thicknesses or cross-linkages was seen (Fig. 4C). The difference in the TRGs of these cells correlated better with the level of vancomycin consumption in the subsequent 1 to 3 h (Fig. 4C).
Comparison of TRG for Mu50 with those for other strains after cell-wall synthesis in RM as well as in conventional medium.
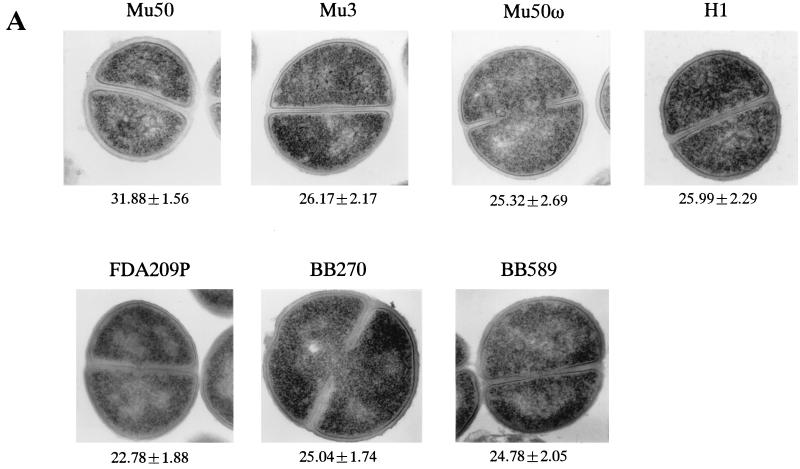
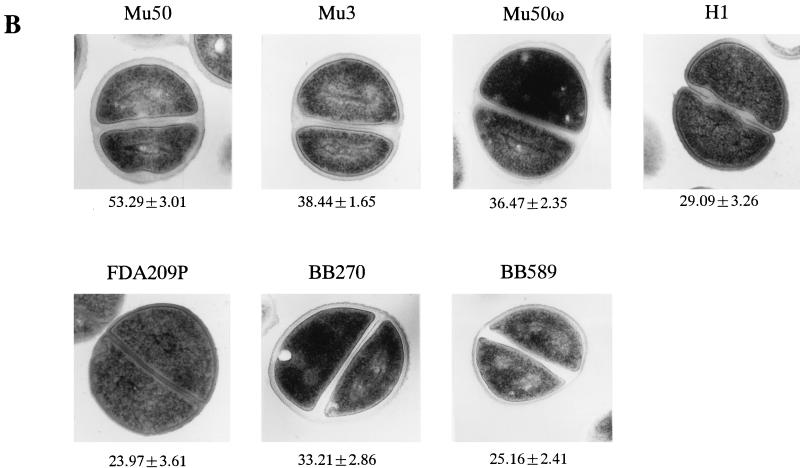
To see if the shortening of the TRG after cell-wall synthesis in RM was specific to Mu50, we tested other strains under the same experimental conditions. Figure 5 shows the representative electron microscopic morphologies of Mu50 and control cells after their cell walls were synthesized either in BHI medium (Fig. 5A) or in RM (Fig. 5B). In BHI medium, Mu50 had a thicker cell wall than each of the other strains, and the difference was statistically significant (P < 0.0001). On the other hand, BB589 had the thinnest cell wall, but the differences in the cell-wall thicknesses among Mu3, Mu50ω, BB270, BB589, and H1 were not significant (P > 0.05).
FIG. 5.
Comparison of cell-wall thickness and TRG in the presence of vancomycin among the test strains after cultivation (or incubation) in BHI medium and RM. (A and B) Comparison of cell-wall thicknesses of the test strains cultivated in BHI medium (A) and after further incubation in RM (B). The values under each panel are mean ± SD thicknesses (in nanometers). The bacterial cultures at an OD600 of 0.7 in BHI medium were divided into two portions. One of them was directly subjected to electron microscopy (A). The other portion was washed twice with RMg−, further incubated in RM at 37°C for 2 h, and then subjected to electron microscopic examination (B). (C and D) TRG was compared among the cell preparations shown in panels A (C) and B (D). (C) Vancomycin was added to a final concentration of 30 mg/liter when the culture (inoculated with the cells shown in p1anel A at an initial concentration of 106 cells/ml) reached an OD600 of 0.4. (D) Cells incubated in RM were spun down and resuspended in BHI medium containing vancomycin at 30 mg/liter.
Unlike in BHI medium, the differences in the cell-wall thicknesses of the different strains were significant after incubation in RM (P < 0.0001 for all comparisons except for the combination of BB589 and FDA209P, for which the P value was 0.14) (Fig. 5B). With all strains, the cell-wall thickness was greater in RM than in BHI medium, with ratios of increases in the cell-wall thickness of 1.675 ± 0.116 times for Mu50, 1.478 ± 0.127 for Mu3, 1.450 ± 0.121 for Mu50ω, 1.33 ± 0.16 for BB270, 1.121 ± 0.101 for H1, 1.02 ± 0.12, and 1.056 ± 0.158 for FDA209P. These values were statistically significantly different for all combinations (P < 0.001) except Mu3 and Mu50ω, H1 and FDA209P, and BB589 and FDA209P (P > 0.05).
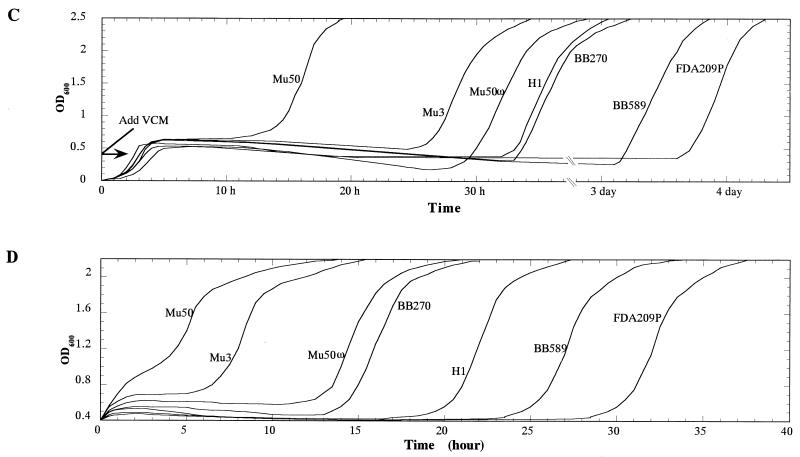
Figure 5C and D illustrate the growth curves of the strains in vancomycin-containing BHI medium after they were either grown in BHI medium (Fig. 5C) or incubated in RM (Fig. 5D). As shown in Fig. 5C, Mu50 grew much earlier than the other strains, and FDA209P grew much later than the other strains, which corresponded well to the statistically significant different cell-wall thickness groupings for these strains (see above and Fig. 5A). It was noteworthy that only Mu50 started growing within 15 h. This was comparable to the timing of growth of Mu50 which had been incubated in RMg− (Fig. 5). In contrast, it took 3 days before FDA209P started to grow under these experimental conditions (Fig. 5C). When the strains were incubated in RM, their TRGs were shortened (Fig. 5D). The TRG was inversely correlated with the cell-wall thickness of the strains, as measured by electron microscopy (Fig. 5B). It was noticed that the rate of shortening of the TRG of Mu3 was much greater than those of the other strains tested (except for FDA209P).
DISCUSSION
The nutrient dependence was a remarkable feature of the reduced vancomycin susceptibility of Mu50, and this also held true for the other strains tested, although to lesser degrees (Fig. 4 and 5). According to our study, the media with large amounts of cell-wall-component amino acids and glucose seemed to better support the vancomycin resistance of S. aureus cells. In this regard, it is noteworthy that the vancomycin MIC for Mu50 measured in Mueller-Hinton broth (MHB) is raised to the level measured in BHI broth when MHB is supplemented with 20% horse serum (9). In the same way, the population susceptibility profiles of Mu50 and Mu3 were subject to change depending on the medium used for analysis. This may be why a recent study of Aeschlimann et al. (1) failed to reproduce the resistance level of Mu50 that we reported since those investigators used Mueller-Hinton agar instead of BHI agar for population analysis (1).
Figure 2 illustrates the flow of the peptidoglycan synthetic pathway in S. aureus (10, 18, 20, 22, 25, 26). We reported that the uptake of GlcNAc is enhanced in both Mu50 and Mu3 (7, 8, 13–16). More than 95% of the GlcNAc taken up is incorporated into the cell wall via UDP-GlcNAc, the central precursor metabolite of cell-wall peptidoglycan synthesis (2, 4, 33; L. Cui, unpublished observation). This suggests that the accelerated peptidoglycan synthesis is a salient common feature of strains Mu50 and Mu3. In this study, we showed that, in addition to the increased uptake of GlcNAc, another pathway that supplies UDP-GlcNAc was enhanced in Mu50, i.e., the GlmS pathway, which digresses from the Embden-Meyerhof pathway at the Fru-6-P step to generate GlcN-6-P (Fig. 2). This agrees with the view that Mu50 produces more peptidoglycan than Mu3 and likely explains the characteristic cell-wall thickening of Mu50 observed by transmission electron microscopy (Fig. 5).
Other evidence supportive of the enhanced GlmS pathway is the characteristically high proportion of nonamidated muropeptides in the cell wall of Mu50 (7, 8). GlmS requires glutamine as the ammonium source to convert Fru-6-P into GlcN-6-P (4, 6) (Fig. 2). Increased GlmS activity would require more glutamine, and if GlmS is overactivated in disproportion to the supply of glutamine, the intracellular glutamine pool would likely fall short. Overactivation of GlmS seemed to be present since the addition of glutamine to the culture medium of Mu50 significantly increased the cell-wall incorporation of radioactive glucose to a level not observed in other strains including Mu3 (Fig. 3).
It has been reported that the amidation enzyme of glutamate residues of murein monomer precursors requires glutamine as the NH4+ donor (23, 28). If the intracellular glutamine pool became low because of the increased GlmS activity, the activity of the amidation enzyme would be dampened, leaving more murein monomers nonamidated at the iso-glutamate residue (28, 30). In fact, the application of an excess amount of glutamine was shown here to substantially decrease the proportion of nonamidated muropeptides in Mu50 (Fig. 4B).
The appearance of nonamidated muropeptide has characteristically been associated with femC mutant strain BB589, derived from a homogeneously methicillin-resistant strain (5). The mutant is depleted of glutamine because of a reduction in the level of GS activity, which resulted from the insertion of a transposon adjacent to the glnA gene, which encodes GS (5). In Mu50, the glnA gene and the surrounding region were found to be intact, and the GS activity of the strain was increased, as opposed to the case with the femC mutant (Fig. 3B). This agrees with the view that the glutamine depletion in Mu50 was caused by the increased consumption of glutamine because of the enhanced activity of GlmS and that the increased GS activity may be explained as a compensatory reaction in the face of the glutamine deficiency status of the cell.
We have proposed that activated cell-wall synthesis is responsible for the increased vancomycin resistance in Mu50 (7, 14–16). In the first part of the current study, we found that the degree of cell-wall synthesis was influenced by nutrients in the culture medium. We next proceeded to evaluate the level of resistance expressed by Mu50 cells by determining the TRG after their cell walls were synthesized in different RMs without cell growth. By incubating cells in RMs with and without supplemented nutrients, we could prepare Mu50 cell samples with different cell-wall thicknesses and different D/M and M9/M4 ratios (Fig. 4A and B). We also evaluated how quickly vancomycin was consumed and when cells started growth after each sample was inoculated into BHI medium containing a high concentration of vancomycin (Fig. 4C). It was found that the thicker the cell wall, the shorter the TRG. Thus, the cell-wall thickness seemed to be an important factor in the level of vancomycin resistance of Mu50.
Cell growth started when the vancomycin concentration of the medium decreased below 5 to 7 mg/liter (Fig. 4C). The drop in the vancomycin concentration observed within 2 h of cell inoculation seems to be the result of entrapment of vancomycin molecules by the cell wall, which we proposed as one of the mechanisms of vancomycin resistance, that is, affinity trapping (14–16). As expected, the thicker the cell wall, the greater the level of consumption of vancomycin molecules, which prevented the vancomycin molecules from reaching the cell-wall synthesis machinery. A similar observation of the consumption of vancomycin from the culture medium has been reported by others with an in vitro mutant S. aureus strain with an increased level of vancomycin resistance (29).
Curiously, the initial steep drop in the vancomycin concentration observed within 20 min of incubation was almost uniform among the cells with different cell-wall sizes and compositions; no correlation between the degree of consumption and the cell-wall thicknesses of the test cells was seen (Fig. 4C). About 13 μg of vancomycin was consumed within 20 min by a 1-ml culture of Mu50 cells which contained 8.9 × 108 cells. Calculations indicate that one cell consumed about 0.6 × 107 vancomycin molecules. Since 1 mg of purified peptidoglycan obtained from 1010 cells of Mu50 in RM binds 2.1 × 1017 vancomycin molecules, the peptidoglycan of a single cell can theoretically bind 2.1 × 107 molecules of vancomycin, which is 3.5 times greater than the amount determined experimentally as described above. The S. aureus cell wall comprises about 20 layers of peptidoglycan (14). In the case of Mu50, 30 to 40 layers of peptidoglycan may be present, and it seems that only about 10 outer layers of peptidoglycan of the Mu50 cell was engaged in the initial consumption of vancomycin. In this case, the mesh structure of the outer layers of peptidoglycan might have been clogged with bound vancomycin molecules, preventing further consumption of vancomycin by the inner layers of peptidoglycan. If these outermost layers were old layers of peptidoglycan that had been produced before incubation in RM, it would be reasonable that there would be no difference in the vancomycin-binding capacities among all the test cells. For this to be the case, the outer layers should remain unshed from the surface of the cells during incubation in RM. We have observed that autolysis of the cells is nearly completely suppressed during incubation in RM (L. Cui, unpublished observation), and this might be correlated with the presumed suppression of cell-wall shedding mediated by the cell-wall lytic enzymes. However, this remains hypothetical at the moment, and more detailed studies for clarification of this point are ongoing.
The subsequent decrease in the vancomycin concentration within 1 to 3 h after the initiation of the culture correlated well with the cell-wall thickness of each test cell (Fig. 4). The profile of vancomycin consumption during this period seems to be composed of the following factors. First, the thickness of the cell wall seems to work as a barrier to vancomycin molecules trying to reach the cytoplasmic membrane. If the cell wall were thicker, fewer vancomycin molecules would be able to reach the cytoplasmic membrane. Thus, the degree of impact of vancomycin on cell-wall synthesis would be smaller for cells with thicker cell walls. Second, during cultivation in BHI medium, cell wall newly synthesized in RM may be exposed to the medium due to the shedding of old layers. Finally, the new cell wall, the septum, would come into contact with vancomycin in the culture medium due to the splitting of the cells, and more consumption of vancomycin would ensue.
It was remarkable that even FDA209P could eventually grow in the medium that (initially) contained 30 mg of vancomycin per liter after 3 days (Fig. 4). The grown cells were not resistant mutants: the vancomycin MIC for the cells was the same as that for the original FDA209P strain (L. Cui, unpublished observation). This indicates that vancomycin could not kill the susceptible S. aureus cells appreciably and that the concentration of vancomycin above the MIC could not completely inhibit cell-wall synthesis of the cells. Therefore, it seems that the important difference between Mu50 and vancomycin-susceptible S. aureus resides in the greater rate of cell-wall synthesis of the former and, as a result, in the greater rate of vancomycin consumption from the medium by Mu50 than by vancomycin-susceptible S. aureus.
Heterotypic strain Mu3 came between Mu50 and susceptible strains in terms of the TRG. The significant shortening of the TRG when Mu3 was incubated in RM instead of BHI broth indicates that Mu3 may well express a significantly increased level of vancomycin resistance if high concentrations of cell-wall nutrients were provided in the environment. As reported previously, heterotypic strains are difficult to detect by conventional susceptibility tests (11). Therefore, measurement of the TRG after incubation in RM could be used as a novel detection assay for Mu3-like strains.
As demonstrated in this study and also as demonstrated previously (8), a unit weight of purified peptidoglycan with a high nonamidated muropeptide content consumes more vancomycin molecules than a unit weight of peptidoglycan with a low nonamidated muropeptide content. Besides causing the under-cross-linking of peptidoglycan, the nonamidated muropeptide is considered to contribute directly to the increased level of consumption of vancomycin due to its greater affinity of binding to vancomycin than that of its amidated counterpart (8). Besides increased consumption of vancomycin, the under-cross-linked cell wall may also protect the cells by enhancing clogging through the binding of more vancomycin molecules per unit volume of the peptidoglycan outer layers. It is noteworthy in this regard that a significant decrease in the cell-wall cross-linkage was also observed in a vancomycin-resistant in vitro mutant strain (29). However, the under-cross-linkage of cell-wall peptidoglycan does not seem to make the cell resistant to vancomycin if it is not accompanied by a thickening of the cell wall, as demonstrated in this study with femC mutant strain BB589 (Fig. 1). In this case, the cell had a thin cell wall presumably because glutamine depletion hampered the cell-wall synthesis pathway driven by GlmS (Fig. 2). The increased GlmS activity demonstrated in BB589 (Fig. 3B) seems to be the result of a putative regulatory response to glutamine depletion to recover dampened cell-wall synthesis.
In conclusion, this study strongly indicates that cell-wall thickness is the major contributor to the vancomycin resistance of Mu50. In addition, the increased production of nonamidated muropeptides (21, 30) may also contribute positively to vancomycin resistance by increasing the efficiency of affinity trapping and clogging of the mesh of the peptidoglycan outer layers. These phenotypic characteristics seem to be simply explained by the accelerated cell-wall-synthesis system of Mu50. The search for the regulatory genes whose alteration would lead to the acceleration of cell-wall synthesis in Mu50 is under way (19).
ACKNOWLEDGMENTS
We thank Yoko Inaba, Keiko Okuma, and Nanae Aritaka for technical assistance and Katsuhiro Sato, Juntendo Laboratory, for help with electronic microscopy.
This study was supported by a Monbusho Specifically Designated Research Promotion and by a nonrestricted research grant from Merck & Co., Inc., Rahway, N.J.
REFERENCES
- 1.Aeschlimann J R, Hershberger E, Rybak M J. Analysis of vancomycin population susceptibility profiles, killing activity, and postantibiotic effect against vancomycin-intermediate Staphylococcus aureus. Antimicrob Agents Chemother. 1999;43:1914–1918. doi: 10.1128/aac.43.8.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bearne S L. Active site-directed inactivation of Escherichia coli glucosamine-6-phosphate synthase. Determination of the fructose 6-phosphate binding constant using a carbohydrate-based inactivator. J Biol Chem. 1996;271:3052–3057. [PubMed] [Google Scholar]
- 3.Fisher S H, Sonenshein A L. Bacillus subtilis glutamine synthetase mutants pleiotropically altered in glucose catabolite repression. J Bacteriol. 1984;157:612–621. doi: 10.1128/jb.157.2.612-621.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ghosh S, Blumenthal H J, Davidson E, Roseman S. Glucosamine metabolism. V. Enzymatic synthesis of glucosamine-6-phosphate. J Biol Chem. 1960;235:1265–1273. [PubMed] [Google Scholar]
- 5.Gustafson J, Strassle A, Hachler H, Kayser F H, Berger-Bachi B. The femC locus of Staphylococcus aureus required for methicillin resistance includes the glutamine synthetase operon. J Bacteriol. 1994;176:1460–1467. doi: 10.1128/jb.176.5.1460-1467.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Halpern Y S. Genetics of amino acid transport in bacteria. Annu Rev Genet. 1974;8:103–133. doi: 10.1146/annurev.ge.08.120174.000535. [DOI] [PubMed] [Google Scholar]
- 7.Hanaki H, Kuwahara-Arai K, Boyle-Vavra S, Daum R S, Labischinski H, Hiramatsu K. Activated cell-wall synthesis is associated with vancomycin resistance in methicillin-resistant Staphylococcus aureus clinical strains Mu3 and Mu50. J Antimicrob Chemother. 1998;42:199–209. doi: 10.1093/jac/42.2.199. [DOI] [PubMed] [Google Scholar]
- 8.Hanaki H, Labischinski H, Inaba Y, Kondo N, Murakami H, Hiramatsu K. Increase in glutamine-non-amidated muropeptides in the peptidoglycan of vancomycin-resistant Staphylococcus aureus strain Mu50. J Antimicrob Chemother. 1998;42:315–320. doi: 10.1093/jac/42.3.315. [DOI] [PubMed] [Google Scholar]
- 9.Hanaki H, Hiramatsu K. Evaluation of reduced vancomycin susceptibility of MRSA strain Mu50 with various conditions of antibiotic susceptibility test. J Antibiot (Tokyo) 1997;50:794–798. [PubMed] [Google Scholar]
- 10.Helling R B. Pathway choice in glutamate synthesis in Escherichia coli. J Bacteriol. 1998;180:4571–4575. doi: 10.1128/jb.180.17.4571-4575.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hiramatsu K, Aritaka N, Hanaki H, Kawasaki S, Hosoda Y, Hori S, Fukuchi Y, Kobayashi I. Dissemination in Japanese hospitals of strains of Staphylococcus aureus heterogeneously resistant to vancomycin. Lancet. 1997;350:1670–1673. doi: 10.1016/S0140-6736(97)07324-8. [DOI] [PubMed] [Google Scholar]
- 12.Hiramatsu K, Hanaki H, Ino T, Yabuta K, Oguri T, Tenover F C. Methicillin-resistant Staphylococcus aureus clinical strain with reduced vancomycin susceptibility. J Antimicrob Chemother. 1997;40:135–136. doi: 10.1093/jac/40.1.135. [DOI] [PubMed] [Google Scholar]
- 13.Hiramatsu K. The emergence of Staphylococcus aureus with reduced susceptibility to vancomycin in Japan. Am J Med. 1998;104(Suppl. 5A):7S–10S. doi: 10.1016/s0002-9343(98)00149-1. [DOI] [PubMed] [Google Scholar]
- 14.Hiramatsu K. Vancomycin resistance in Staphylococci. Drug Resist Updates. 1998;1:135–150. doi: 10.1016/s1368-7646(98)80029-0. [DOI] [PubMed] [Google Scholar]
- 15.Hiramatsu K, Ito T, Hanaki H. Mechanisms of methicillin and vancomycin resistance in Staphylococcus aureus. Bailliere's Clin Infect Dis. 1999;5:211–242. [Google Scholar]
- 16.Hiramatsu K, Hanaki H. Glycopeptide resistance in staphylococci. Curr Opin Infect Dis. 1998;11:653–658. doi: 10.1097/00001432-199812000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Hiramatsu K, Kondo N, Ito T. Genetic basis for the molecular epidemiology of MRSA. J Infect Chemother. 1996;2:117–129. doi: 10.3412/jsb.52.417. [DOI] [PubMed] [Google Scholar]
- 18.Imada A, Nozaki Y, Kawashima F, Yoneda M. Regulation of glucosamine utilization in Staphylococcus aureus and Escherichia coli. J Gen Microbiol. 1997;100:329–337. doi: 10.1099/00221287-100-2-329. [DOI] [PubMed] [Google Scholar]
- 19.Kuroda M, Kuwahara-Arai K, Hiramatsu K. Identification of the up- and down-regulated genes in vancomycin-resistant Staphylococcus aureus strains Mu3 and Mu50 by cDNA differential hybridization method. Biochem Biophys Res Commun. 2000;269:485–490. doi: 10.1006/bbrc.2000.2277. [DOI] [PubMed] [Google Scholar]
- 20.Linnett P E, Strominger J L. Amidation and cross-linking of the enzymatically synthesized peptidoglycan of Bacillus stearothermophilus. J Biol Chem. 1974;249:2489–2496. [PubMed] [Google Scholar]
- 21.Nakel M, Ghuysen J M, Kandler O. Wall peptidoglycan in Aerococcus viridans strains 201 Evans and ATCC 11563 and in Gaffkya homari strain ATCC 10400. Biochemistry. 1971;10:2170–2175. doi: 10.1021/bi00787a033. [DOI] [PubMed] [Google Scholar]
- 22.Neidhardt F C, Ingraham J L, Schachter M. Physiology of the bacterial cell: a molecular approach. Sunderland, Mass: Sinauer Associates, Inc.; 1990. Assembly and polymerization: the bacterial interior. [Google Scholar]
- 23.Ornelas-Soares A, de Lencastre H, de Jonge B, Gage D, Chang Y S, Tomasz A. The peptidoglycan composition of a Staphylococcus aureus mutant selected for reduced methicillin resistance. Biol Chem. 1993;268:26268–26272. [PubMed] [Google Scholar]
- 24.Pearson W R, Lipman D J. Improved tools for biological sequence comparison. Proc Natl Acad Sci USA. 1988;85:2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Plumbridge J. Co-ordinated regulation of amino sugar biosynthesis and degradation: the NagC repressor acts as both an activator and a repressor for the transcription of the glmUS operon and requires two separated NagC binding sites. EMBO J. 1995;15:3958–3965. doi: 10.1002/j.1460-2075.1995.tb00067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reynolds P E, Somner E A. Comparison of the target sites and mechanisms of action of glycopeptide and lipoglycodepsipeptide antibiotics. Drugs Exp Clin Res. 1990;16:385–389. [PubMed] [Google Scholar]
- 27.Shockman G D, Conover M J, Kolb J J, Riley L S, Toennies G. Nutritional requirements for bacterial cell wall synthesis. J Bacteriol. 1961;81:44–50. doi: 10.1128/jb.81.1.44-50.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Siewert G, Strominger J L. Biosynthesis of the peptidoglycan of bacterial cell walls. XI. Formation of the isoglutamine amide group in the cell walls of Staphylococcus aureus. J Biol Chem. 1968;243:783–790. [PubMed] [Google Scholar]
- 29.Siradzki K, Tomasz A. Inhibition of cell wall turnover and autolysis by vancomycin in a highly vancomycin-resistant mutant of Staphylococcus aureus. J Bacteriol. 1997;179:2557–2566. doi: 10.1128/jb.179.8.2557-2566.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stranden A M, Roos M, Berger-Bachi B. Glutamine synthetase and heteroresistance in methicillin-resistant Staphylococcus aureus. Microb Drug Resist. 1996;2:201–207. doi: 10.1089/mdr.1996.2.201. [DOI] [PubMed] [Google Scholar]
- 31.Tanaka M, Wada N, Kurosaka S M, Chiba M, Sato K, Hiramatsu K. In-vitro activity of DU-6859a against methicillin-resistant Staphylococcus aureus isolates with reduced susceptibilities to vancomycin. Antimicrob Chemother. 1998;42:552–553. doi: 10.1093/jac/42.4.552. [DOI] [PubMed] [Google Scholar]
- 32.Tenover F C, Lancaster M V, Hill B C, Steward C D, Stocker S A, Hancock G A, O'Hara C M, McAllister S K, Clark N C, Hiramatsu K. Characterization of staphylococci with reduced susceptibilities to vancomycin and other glycopeptides. J Clin Microbiol. 1998;36:1020–1027. doi: 10.1128/jcm.36.4.1020-1027.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wong W, Young F E, Chatterjee A N, Young F E, Tuazon C U. Regulation of bacterial cell walls: turnover of cell wall in Staphylococcus aureus. J Bacteriol. 1974;120:837–843. doi: 10.1128/jb.120.2.837-843.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zalkin H. Glucosamine-6-phosphate synthase. Methods Enzymol. 1985;113:278–281. doi: 10.1016/s0076-6879(85)13038-7. [DOI] [PubMed] [Google Scholar]