Abstract
Background:
Race/ethnicity and low English proficiency healthcare disparities are well established in the United States. We sought to determine if there are race/ethnicity differences in anti-obesity medication (AOM) prescription rates among youth with severe obesity treated in a pediatric weight management clinic and if, among youth from non-primary English speaking families, there are differences in prescriptions between those using interpreters during visits versus not.
Methods:
We reviewed electronic health records of 2- to 18-year-olds with severe obesity seen from 2012 to 2021. Race/ethnicity was self-report, and AOMs included topiramate, stimulants (e.g. phentermine, lisdexamfetamine), naltrexone (±bupropion), glucagon-like peptide-1 agonists, and orlistat. We used general linear regression models with log-link to compare incidence rate ratios (IRRs) within the first 1 and 3 years of being followed, controlling for age, percent of the 95th BMI percentile (%BMIp95), number of obesity-related comorbidities (e.g. insulin resistance, hypertension), median household income, and interpreter use. We repeated similar analyses among youth from non-primary English speaking families, comparing those using interpreters versus not.
Results:
1,725 youth (mean age 11.5 years; %BMIp95 142%; 53% non-Hispanic White, 20% Hispanic/Latino, 16% non-Hispanic black; 6% used interpreters) were seen, of which 15% were prescribed AOMs within 1 year. The IRR for prescriptions was lower among Hispanic/Latino compared to non-Hispanic White youth at one (IRR 0.70; CI: 0.49–1.00; p = 0.047) but not 3 years. No other statistically significant differences by race/ethnicity were found. Among non-primary English speaking families, the IRR for prescriptions was higher at 1 year (IRR 2.49; CI: 1.32–4.70; p = 0.005) in those using interpreters versus not.
Conclusions:
Among youth seen in a pediatric weight management clinic, AOM prescription incidence rates were lower in Hispanics/Latinos compared to non-Hispanic Whites. Interpreter use was associated with higher prescription incidence rates among non-primary English speakers. Interventions to achieve equity in AOM prescriptions may help mitigate disparities in pediatric obesity.
Keywords: anti-obesity agents, healthcare disparities, limited english proficiency, obesity, pediatric obesity
Introduction
The presence of healthcare disparities by race/ethnicity in the United States is well established. There are many potential mechanisms underlying these disparities including, but not limited to, provider unconscious or implicit bias, differences in socioeconomic status, cultural barriers around the use of medications and other interventions, and patient-provider communication difficulties resulting from limited English language proficiency.1,2 Due to such factors, optimal treatment options may not be offered, may be delayed, or may be accepted disproportionately, and rates of these treatments may differ by race/ethnicity.
As for whether or not race/ethnicity disparities exist in terms of medication prescriptions, evidence is overall mixed with some studies showing disparities and others not. For example, studies have shown that Hispanic/Latino and non-Hispanic Black adults with coronary artery disease, or who are at risk for coronary artery disease, are less likely to be on treatment with lipid-lowering medications compared to non-Hispanic Whites.3,4 However, studies have also shown no difference by race/ethnicity in terms of medications prescribed for the indications of type 2 diabetes mellitus in adults and asthma in children.5,6
Specifically in terms of anti-obesity medication (AOM) prescriptions, few studies have evaluated whether or not race/ethnicity disparities exist. In a study by Lewis et al. 7 in which 5,400 racially/ethnically and geographically diverse US adults with overweight and obesity were surveyed, the prevalence of patients receiving information from their primary care providers about AOMs did not significantly differ among patients who self-identified as non-Hispanic White, non-Hispanic Black, Hispanic/Latino, or Asian. In another study exploring AOM prescriptions in adults as determined by a review of patient and pharmacy electronic health record (EHR) data, Saxon et al. found that, among approximately 2.3 million patients eligible to receive AOMs, the prevalence of those who did receive AOMs was similar among non-Hispanic White (1.3%) and Hispanic/Latino (1.2%), and higher among non-Hispanic Blacks (2.1%). 8 To our knowledge, no studies have examined whether rates of AOM prescriptions differ by race/ethnicity among children and adolescents receiving care through pediatric weight management clinics. This may be due to the overall lower prevalence of AOM prescriptions among youth, a population for which, when these are prescribed, they are often done so ‘off-label’.9,10
Given the mixed evidence on whether or not the prevalence of prescriptions differ by race/ethnicity, combined with the lack of such studies as it pertains to AOMs in youth, we sought to compare AOM prescription rates by race/ethnicity among youth followed in a large pediatric weight management clinic. Secondarily, as limited English language proficiency may be a notable driver of healthcare disparities 11 we also sought to determine if, among youth from families in whom English is not the primary language spoken, there are differences in AOM prescription rates between those using an interpreter during pediatric weight management clinic visits and those not.
Methods and materials
Study design and participants
This was a retrospective cohort study performed through EHR data review of children and adolescents seen in a large Midwestern, academic health center-based pediatric weight management clinic from January 1, 2012 through March 1, 2021. Patients seen in our pediatric weight management clinics are either referred from primary care providers or specialists (e.g. gastroenterology where they are seen for transaminitis concerning for non-alcoholic fatty liver disease, psychiatry where they are noted to have obesity in addition to psychiatric diagnoses), or are self-referred.
Participants included were those who did not opt out of having their EHR reviewed for the purposes of research via the Consent for Services form that all patients receiving care through our University medical system complete. Additional inclusion criteria included ages 2 to 18 years when first seen in the pediatric weight management clinic and having severe obesity, defined as a body mass index (BMI) of ⩾ 1.2 times the 95th age- and sex-adjusted percentile and/or BMI ⩾ 35 kg/m2, per standard definition. 12 Exclusion criteria included the following: no data on race/ethnicity available, Hawaiian/Pacific Islander (n = 6), interpreter used for American Sign Language (n = 5), a documented eating disorder, or already prescribed AOMs prior to the initial pediatric weight management clinic appointment. Specifically, we excluded Hawaiian/Pacific Islanders and those using interpreters for American Sign Language due to low sample sizes limiting data interpretation, and those with a documented eating disorder as this could be a contraindication for AOMs if still active, which could not be reliably determined from EHR review. This study was approved by the University of Minnesota Institutional Review Board.
Variables
Race/ethnicity was determined by self-report and categorized as non-Hispanic White, non-Hispanic Black, Hispanic/Latino, Asian, American Indian/Alaska Native, and mixed race/ethnicity. Primary and secondary languages spoken by the patient/family, as well as use of interpreters during pediatric weight management clinic visits, were also captured through EHR review. We were unable to determine if interpreter use during visits was via telephone, video, or in-person.
AOMs prescribed, determined by EHR review which tracks all medications prescribed by providers, included topiramate, phentermine, psychostimulants (e.g. lisdexamfetamine), naltrexone (± bupropion), glucagon-like peptide-1 receptor agonists ( e.g. liraglutide), and orlistat. All of these medications are prescribed in our and other pediatric weight management clinics primarily for the indication of obesity and eating behaviors associated with its development, such as hyperphagia and binge eating. 13 Most of these medications are prescribed ‘off label’. Specifically, topiramate, psychostimulants, and naltrexone (± bupropion) are not Food and Drug Administration (FDA)-approved for weight loss for patients of any age. Orlistat is FDA-approved for adolescents ⩾ 12 years old, and phentermine is only FDA-approved for short-term use (⩽12 weeks) in adolescents > 16 years of age. While liraglutide 3.0 mg daily is now FDA-approved for weight loss in adolescents ⩾ 12 years old, it was not FDA-approved for this indication until December 2020 which is after the time that 1 and 3 years data were available for our study and, therefore, was ‘off label’ during the study duration.
We did not include metformin in our primary analysis because, while this medication is also prescribed in pediatric weight management clinics, it is often done so for indications aside from ‘primary’ and ‘typical’ weight management purposes (e.g. insulin resistance/type 2 diabetes mellitus, polycystic ovarian syndrome, curbing weight gain associated with atypical antipsychotic medications), and the primary indication for the metformin prescription could not be reliably determined through EHR review. However, we did perform a subsequent secondary analysis including metformin with other AOMs.
Additional co-variates, selected based upon their relationships to our primary predictor (race/ethnicity) and outcome (AOM prescriptions) included baseline age, degree of obesity, number of obesity-related comorbidities, and area-level socioeconomic status. Degree of obesity was determined by percent of the 95th BMI percentile (%BMIp95), a commonly used metric in pediatric weight management clinics. 14 Obesity-related comorbidities included diabetes/insulin resistance, lipid abnormalities (e.g. hypercholesterolemia, hypertriglyceridemia, low HDL), obstructive sleep apnea, fatty liver disease, and hypertension as reported in patient problem lists (list of search terms available in Supplemental Table 1). Median household income based upon ZIP code was used as a surrogate area-based socioeconomic status indicator. 15 This was determined for each patient by matching their ZIP code of residence at the time of the initial pediatric weight management clinic visit with 5-year estimate data (2014-2018) from the American Community Survey as available in the National Historical Geographical Information System. 16
Statistical methods
Baseline characteristics are presented as descriptive summaries including mean and standard deviation for continuous variables and frequency with percent for categorical variables. Prescription rates were calculated using incidence rate ratios (IRRs). For the primary analysis evaluating race/ethnicity differences, we used Poisson models (i.e. generalized linear models with log-link) to compare IRRs of being prescribed AOMs within the first one and three years of being followed in the pediatric weight management clinic. These models adjusted for age, %BMIp95, number of obesity-related comorbidities, median household income based on residence ZIP code, and interpreter use. Data beyond 3 years was not analyzed given sample size limitations and the overall rarity of being prescribed an AOM for the first time after 3 years, and being prescribed AOMs was treated as a binary outcome. All models used robust variance estimation and included an offset for the log number of pediatric weight management clinic visits within 1 and 3 years, allowing us to consider that patients may be more likely to be prescribed AOMs if they are seen more compared to less often in clinic.
For the secondary analysis evaluating if, among youth from families in whom English was not the primary language spoken there are differences in AOM prescription rates between those using an interpreter and those not, we used a similar technique; however, we controlled for race/ethnicity. Similarly, we compared youth from primary English speaking families to those from non-primary English speaking families who used interpreters during study visits. Statistical significance was based on a type 1 error rate of 0.05 (p < 0.05). All statistical analyses were performed using R version 3.6.0 (R Foundation for Statistical Computing).
Results
Participant characteristics
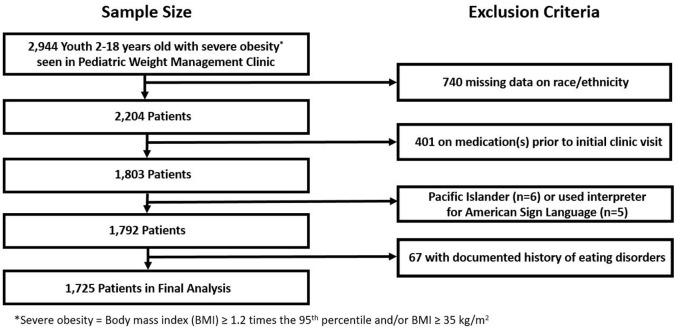
Descriptive statistics for the entire study population of youth seen in a pediatric weight management clinic, and the subset of youth from non-primary English speaking families, are shown in Tables 1 and 2, respectively. Overall, the percentage of youth seen in our pediatric weight management clinics who opted out of having their EHR reviewed for research purposes was 1.1%, ranging from 0.1% to 0.5% for each race/ethnicity. Of 2,944 youth who had (1) EHR data available, (2) severe obesity, (3) were not on AOMs prior to their initial appointment, and (4) did not have additional exclusionary criteria (e.g. missing data on race/ethnicity), 1,725 were included in our analysis (see Figure 1). Just over half self-identified as non-Hispanic White, while 20.4% self-identified as Hispanic/Latino and 15.7% as non-Hispanic Black. Nearly half of the sample was male and the mean age was 11.5 years. The mean %BMIp95 was in the class three pediatric obesity category (defined as BMI ⩾ 140% of the 95th percentile) 12 for all races/ethnicities. Most patients had 0 to 1 obesity-related comorbidities (all had between zero and four), the most common being diabetes/insulin resistance with a prevalence above 40% in Hispanics/Latinos and Asians and below 20% in non-Hispanic Whites. 6.0% of all youth used interpreters during pediatric weight management clinic visits. Among youth from non-primary English speaking families, more than two-thirds were Hispanic/Latino, and one-third used interpreters during pediatric weight management clinic visits. Confidence intervals for all model covariates are shown in Supplementary Table 2.
Table 1.
Descriptive statistics for 1,725 youth seen in a pediatric weight management clinic from January 2012 to March 2021, by race/ethnicity.
| Overall (n = 1,725) | Non-Hispanic White (n = 921) | Non-Hispanic Black (n = 271) | Hispanic/Latino (n = 352) | Asian (n = 99) | American Indian/Alaska Native (n = 29) | Mixed (n = 53) | |
|---|---|---|---|---|---|---|---|
| Sex (% male, N) | 48.5% (837) | 46.6% (429) | 39.1% (106) | 55.4% (195) | 61.6% (61) | 65.5% (19) | 50.9% (27) |
| Use of interpreter (%, N) | 6.0% (104) | 0.8% (7) | 1.8% (5) | 22.4% (79) | 13.1% (13) | 0.0% (0) | 0.0% (0) |
| Anthropometrics | |||||||
| Age, years (mean, SD) | 11.5 ± 3.8 | 11.9 ± 3.9 | 11.6 ± 3.3 | 10.9 ± 3.6 | 9.7 ± 4.1 | 11.4 ± 4.6 | 11.0 ± 3.7 |
| Weight, kg (mean, SD) | 82.2 ± 32.7 | 85.2 ± 33.1 | 86.3 ± 31.1 | 75.2 ± 30.0 | 65.5 ± 30.6 | 89.0 ± 40.1 | 83.3 ± 33.6 |
| BMI, kg/m2(mean, SD) | 34.2 ± 6.8 | 34.5 ± 6.9 | 35.5 ± 6.8 | 33.0 ± 6.4 | 31.8 ± 6.4 | 35.1 ± 6.6 | 35.2 ± 7.1 |
| %BMIp95 | 142 ± 18.6 | 141 ± 17.6 | 147 ± 21.3 | 141 ± 17.1 | 142 ± 20.3 | 148 ± 20.1 | 150 ± 20.9 |
| Co-morbidities (%, N) | |||||||
| Diabetes/insulin resistance | 25.7% (444) | 15.7% (145) | 31.0% (84) | 40.9% (144) | 42.4% (42) | 37.9% (11) | 34.0% (18) |
| Lipid abnormalities | 15.4% (265) | 16.2% (149) | 9.2% (25) | 19.3% (68) | 15.2% (15) | 20.7% (6) | 3.8% (2) |
| Obstructive sleep apnea | 3.1% (53) | 2.2% (20) | 4.8% (13) | 3.7% (13) | 5.1% (5) | 3.4% (1) | 1.9% (1) |
| Fatty liver disease | 1.8% (31) | 1.4% (13) | 0.0% (0) | 4.0% (14) | 1.0% (1) | 3.4% (1) | 3.8% (2) |
| Hypertension | 0.9% (16) | 0.8% (7) | 4.8% (13) | 1.7% (6) | 1.0% (1) | 3.4% (1) | 0.0% (0) |
| Total # Co-morbidities (%, N) | |||||||
| 0 | 64.4% (1111) | 72.1% (664) | 62.4% (169) | 51.1% (180) | 47.5% (47) | 62.1% (18) | 62.3% (33) |
| 1 | 25.4% (439) | 20.1% (185) | 29.9% (81) | 31.8% (112) | 41.4% (41) | 10.3% (3) | 32.1% (17) |
| 2 | 9.0% (156) | 7.3% (67) | 7.7% (21) | 13.6% (48) | 10.1% (10) | 24.1% (7) | 5.7% (3) |
| 3 | 1.0% (18) | 0.5% (5) | 0.0% (0) | 3.1% (11) | 1.0% (1) | 3.4% (1) | 0.0% (0) |
| 4 | 0.1% (1) | 0.0% (0) | 0.0% (0) | 0.3% (1) | 0.0% (0) | 0.0% (0) | 0.0% (0) |
| Median Household Income (mean, SD) | $72,818 ± 27,295 | $80,732 ± 26,481 | $63,774 ± 25,422 | $64,666 ± 24,642 | $64,900 ± 29,496 | $50,987 ± 25,402 | $62,397 ± 19,257 |
| Prescribed AOMs (not including metformin) | |||||||
| Within 1 year (%, N) | 15.0% (259) | 16.3% (150) | 15.5% (42) | 13.6% (48) | 9.1% (9) | 6.9% (2) | 15.1% (8) |
| Within 3 years (%, N) | 18.6% (321) | 19.5% (180) | 19.2% (52) | 18.2% (64) | 12.1% (12) | 10.3% (3) | 18.9% (10) |
| Prescribed AOMs (including metformin) | |||||||
| Within 1 year (%, N) | 17.6% (304) | 18.2% (168) | 18.1% (49) | 18.2% (64) | 10.1% (10) | 6.9% (2) | 20.8% (11) |
| Within 3 years (%, N) | 21.9% (378) | 21.9% (202) | 22.1% (60) | 23.6% (83) | 15.2% (15) | 13.8% (4) | 26.4% (14) |
| Pediatric Weight Management Clinic Visits | |||||||
| Only 1 Visit within First Year (%, N) | 37.9% (654) | 35.3% (325) | 45.0% (122) | 34.7% (122) | 57.6% (57) | 37.9% (11) | 32.1% (17) |
| # Visits in First Year (mean, SD) | 2.8 ± 2.1 | 2.9 ± 2.2 | 2.4 ± 1.9 | 2.9 ± 2.2 | 2.0 ± 1.5 | 2.2 ± 1.4 | 3.1 ± 2.2 |
| Only 1 Visit within 3 Years (%, N) | 34.3% (592) | 32.2% (297) | 41.7% (113) | 31.2% (110) | 52.5% (52) | 27.6% (8) | 22.6% (12) |
| # Visits within 3 Years (mean, SD) | 3.9 ± 4.0 | 4.1 ± 4.1 | 3.4 ± 4.0 | 4.2 ± 4.3 | 2.7 ± 3.2 | 3.0 ± 2.2 | 4.2 ± 3.5 |
AOM, Anti-obesity medication; BMI, body mass index; %BMIp95, percent of the 95th BMI percentile.
Table 2.
Descriptive statistics for 300 youth from non-primary English speaking families seen in a pediatric weight management clinic from January 2012 to March 2021 comparing those using interpreters versus not.
| Overall (n = 300) | Used Interpreter During PWMC Visits (n = 100) | No Interpreter Used During PWMC Visits (n = 200) | |
|---|---|---|---|
| Sex (% male, N) | 58.7% (176) | 65.0% (65) | 55.5% (111) |
| Race/Ethnicity | |||
| Non-Hispanic White | 5.0% (15) | 7.0% (7) | 4.0% (8) |
| Non-Hispanic Black | 6.7% (20) | 5.0% (5) | 7.5% (15) |
| Hispanic/Latino | 69.3%(208) | 75.0% (75) | 66.5% (133) |
| Asian | 19.0% (57) | 13.0% (13) | 22.0% (44) |
| American Indian/Alaska Native | 0.0% (0) | 0.0% (0) | 0.0% (0) |
| Mixed | 0.0% (0) | 0.0% (0) | 0.0% (0) |
| Anthropometrics | |||
| Age, years (mean, SD) | 10.2 ± 3.6 | 10.5 ± 3.5 | 10.0 ± 3.7 |
| Weight, kg (mean, SD) | 70.1 ± 29.2 | 73.1 ± 28.8 | 68.7 ± 29.4 |
| BMI, kg/m2(mean, SD) | 32.2 ± 6.1 | 33.0 ± 6.2 | 31.8 ± 6.1 |
| %BMIp95 | 141 ± 17.9 | 144 ± 20.5 | 140 ± 16.3 |
| Co-Morbidities (%, N) | |||
| Diabetes/insulin resistance | 41.3% (124) | 38.0% (38) | 43.0% (86) |
| Lipid abnormalities | 19.7% (59) | 23.0% (23) | 18.0% (36) |
| Obstructive sleep apnea | 4.7% (14) | 6.0% (6) | 4.0% (8) |
| Fatty liver disease | 3.0% (9) | 3.0% (3) | 3.0% (6) |
| Hypertension | 1.7% (5) | 4.0% (4) | 0.5% (1) |
| Total # Co-Morbidities (%, N) | |||
| 0 | 50.7% (152) | 52.0% (52) | 50.0% (100) |
| 1 | 32.3% (97) | 26.0% (26) | 35.5% (71) |
| 2 | 13.3% (40) | 18.0% (18) | 11.0% (22) |
| 3 | 3.3% (10) | 4.0% (4) | 3.0% (6) |
| 4 | 03% (1) | 0.0% (0) | 0.5% (1) |
| Median household income (mean, SD) | $58,118 ± 21,034 | $56,112 ± 19,356 | $59,121 ± 21,803 |
| Prescribed AOMs (not including metformin) | |||
| Within 1 year (%, N) | 11.7% (35) | 22.0% (22) | 6.5% (13) |
| Within 3 years (%, N) | 15.0% (45) | 26.0% (26) | 9.5% (19) |
| Prescribed AOMs (including metformin) | |||
| Within 1 year (%, N) | 15.3% (46) | 26.0% (26) | 10.0% (20) |
| Within 3 years (%, N) | 20.3% (61) | 34.0% (34) | 13.5% (27) |
| Pediatric weight management clinic visits | |||
| Only 1 visit within first year (%, N) | 39.0% (117) | 29.0% (29) | 44.0% (88) |
| # Visits in first year (mean, SD) | 35.3% (106) | 24.0% (24) | 41.0% (82) |
| Only 1 visit within 3 years (%, N) | 2.75 ± 2.0 | 3.4 ± 2.4 | 2.4 ± 1.8 |
| # Visits within 3 years (mean, SD) | 3.9 ± 3.9 | 5.5 ± 4.8 | 3.0 ± 3.1 |
AOM, Anti-obesity medication; BMI, body mass index; %BMIp95, percent of the 95th BMI percentile.
Figure 1.
Schematic of study population.
Anti-obesity medication prescriptions
15% were prescribed at least one AOM within the first year and 18.6% within three years, with the highest prevalence in non-Hispanic Whites and lowest in American Indian/Alaska Native at both times points. The most commonly prescribed AOM among all races/ethnicities was topiramate (13.9% of all patients), followed by psychostimulants and phentermine (3.5% and 2.8% of all patients, respectively). Other AOMs prescribed included naltrexone, glucagon-like peptide-1 receptor agonists, and orlistat (<1% of all patients). Metformin was prescribed in 3.9% of all patients, and represented a higher prevalence of prescriptions in Hispanic/Latinos (23%) compared to other races/ethnicities (e.g. 15% in non-Hispanic Blacks, 12% in non-Hispanic Whites).
Table 3 shows adjusted incidence rate ratios of AOM prescriptions within the first one and three years of being followed in the pediatric weight management clinic by race/ethnicity, after adjusting for age, %BMIp95, number of obesity-related comorbidities, median household income based on ZIP code, and interpreter use. Hispanic/Latino patients were prescribed AOMs at a statistically significantly lower rate (p = 0.047) compared to non-Hispanic Whites within the first year; however, rates were not statistically different by three years. While point estimates for AOM prescription rates were lower in Asian and American Indian/Alaska Native patients at one and three years compared to non-Hispanic Whites, these relationships were not statistically significant. Higher total number of comorbidities and higher %BMIp95 were associated with higher rates of being prescribed AOMs at 1 and 3 years, and using an interpreter was associated with a higher rate of being prescribed an AOM within 1 year (see Model 1, Supplemental Table 2). In a sensitivity analysis including prescriptions for metformin with other AOMs, adjusted incident rate ratios between Hispanic/Latino and non-Hispanic Whites at one year were no longer statistically significant (IRR: 0.83; CI: 0.61–1.14 p = 0.251).
Table 3.
Incidence rate ratios of anti-obesity medication prescription(s) within one and three years of being followed in a pediatric weight management clinic by race/ethnicity.
| IRR for anti-obesity medication prescription(s) within 1 year (95%CI) a | p-valueb | IRR for anti-obesity medication prescription(s) within 3 years (95%CI) a | p-value b | |
|---|---|---|---|---|
| Race/ethnicity | ||||
| Non-Hispanic White | Ref. | – | Ref. | – |
| Non-Hispanic Black | 1.13 (0.80, 1.59) | 0.477 | 1.16 (0.83, 1.61) | 0.383 |
| Hispanic/Latino | 0.69 (0.48, 1.00) | 0.047 | 0.80 (0.59, 1.09) | 0.161 |
| Asian | 0.65 (0.34, 1.25) | 0.200 | 0.64 (0.34, 1.21) | 0.174 |
| American Indian/Alaskan Native | 0.51 (0.13, 2.02) | 0.339 | 0.57 (0.18, 1.80) | 0.334 |
| Mixed | 0.86 (0.43, 1.72) | 0.677 | 0.81 (0.41, 1.60) | 0.545 |
IRR, incidence rate ratio.
Adjusted for age, percent of the 95th body mass index percentile, number of obesity-related comorbidities, median household income based on residence ZIP code, and interpreter use.
Values in bold = statistically significant (p < 0.05).
Among youth from non-primary English speaking families, the prevalence of being prescribed at least one AOM was higher in those who used an interpreter compared to those who did not, both within one (22% versus 7%) and three (26% versus 10%) years of being followed in the pediatric weight management clinic. After adjusting for age, %BMIp95, number of obesity-related comorbidities, median household income based on ZIP code, and race/ethnicity, the incidence rate ratio of being prescribed AOMs within the first year was 2.5 times higher in those using interpreters compared to those not (p = 0.005; see Table 4). All adjustment variables were not statistically significant at 1 or 3 years (see Model 2, Supplemental Table 2). Point estimates showed that this trend continued out to 3 years, however, was no longer statistically significant (IRR 1.68; p = 0.077; see Table 4). In a sensitivity analysis including prescriptions for metformin with other AOMs, point estimates were overall similar, with adjusted incidence rates two times higher within 1 year (p = 0.010) and 1.7 times higher within 3 years (p = 0.045) of being followed in the PWMC among those using interpreters compared to those not.
Table 4.
Incidence rate ratios of anti-obesity medication prescription(s) within one and three years of being followed in a pediatric weigh management clinic among youth from non-primary English speaking families.
| IRR for Anti-Obesity Medication Prescription(s) within 1 year (95%CI) a | p-value b | IRR for Anti-Obesity Medication Prescription(s) within 3 years (95%CI) a | p-value b | |
|---|---|---|---|---|
| Use of Interpreter vs No Use of Interpreter among non-Primary English Speaking Families | 2.49 (1.32, 4.70) | 0.005 | 1.68 (0.95, 2.97) | 0.077 |
| From Primary English Speaking Families vs Non-Primary English Speaking Families Using Interpreters | 1.31 (0.77, 2.21) | 0.320 | 0.95 (0.57, 1.57) | 0.833 |
IRR, incidence rate ratio.
Adjusted for age, percent of the 95th body mass index percentile, number of obesity-related comorbidities, median household income based on residence ZIP code, and race/ethnicity.
Values in bold = statistically significant (p < 0.05).
Finally, after adjusting for covariates, the rate of being prescribed AOMs among youth from primary English speaking families was not statistically significantly different compared to non-primary English speaking families who used interpreters at both 1 and 3 years (p = 0.320 and p = 0.833, respectively; see Table 4).
Discussion
In this large retrospective cohort study performed through EHR review, we found that after adjusting for age, degree of obesity, number of obesity-related comorbidities, median household income of residence ZIP code, and interpreter use, AOM prescription rates in youth with severe obesity were lower among Hispanics/Latinos compared to non-Hispanic Whites within the first year of being followed in a PWMC. We did not find statistically significant race/ethnicity differences in AOM prescription rates when non-Hispanic Whites were compared to non-Hispanic Blacks, Asian, American Indian/Alaska Native, and mixed race/ethnicity youth. Further, among youth from non-primary English language speaking families, those using an interpreter during pediatric weight management clinic visits were prescribed AOMs at 2.5 times the rate within the first year compared to those not using an interpreter, a relationship that persisted out to 3 years but was not statistically significant at that time point.
The reasons why race/ethnic disparities exist in terms of prescriptions for some medications continue to remain poorly understood, and may not be fully explained by socioeconomic status, presence of comorbidities, or disease severity. 17 It may be that a combination of factors explain prescription disparities, and reasons may differ among various races/ethnicities. For example, providers may have unconscious or implicit biases that affect decision-making around prescribing certain medications to particular individuals. 18 In time-pressed situations, as is often the case in clinical settings, studies have shown that provider communications are more likely to be influenced by unconscious bias.19,20 Thus, it is possible that a provider’s unconscious biases may influence him or her into believing that patients of certain races/ethnicities are less likely to afford, understand, or accept the use of particular medicines or classes of medications. This may be especially pertinent when it comes to ‘off-label’ indications, which pharmaceutical companies are prohibited from advertising or distributing information about and which are often not covered by insurance plans, as is often the case for AOMs prescribed in pediatric or adult weight management clinics. 21
It is also possible that a patient and/or family may be offered a medication by a provider, and choose not to initiate it. Studies have shown that one’s belief about the necessity of medication differs among cultural groups, an effect that has been seen across several conditions including diabetes, depression, and hypertension. 2 Indeed, an individual’s or family’s belief about a disease and approach to management, including the use of complementary and alternative treatments, is influenced by history, culture, and family experiences, all of which are complexities usually beyond the general ‘risks and benefits’ conversations between providers, patients, and family members. 2
Moreover, access to interventions may vary by race/ethnicity. For instance, while AOMs are thought to be overall underutilized in youth, 10 it may be that this underutilization affects some races/ethnicities disproportionately. Indeed, Hispanic/Latino and non-Hispanic Black youth with obesity are often treated for this disease through primary care clinics, while AOMs are prescribed far more often through specialized multidisciplinary pediatric weight management clinics. 22 This particular concern does not apply to our study as we examined youth followed in an academic health center-based pediatric weight management clinic. That said, when AOMs are prescribed, their cost may be a greater issue among those from lower socioeconomic backgrounds, which disproportionately affects certain races/ethnicities including Hispanics/Latinos and non-Hispanic Blacks. 22 While some private insurance companies cover FDA-approved AOMs, few provide coverage through Medicaid programs. 23
In our study, we found lower AOM prescription rates among Hispanics/Latinos compared to non-Hispanic Whites, a finding that appears to align with studies examining prescription use for other diseases. For example, in a study examining trends in the diagnosis of attention-deficit/hyperactivity disorder and stimulant use in preschool children, Davis et al. 24 found that Hispanic/Latino children were prescribed stimulants at a lower rate compared to their non-Hispanic White counterparts. In another study exploring ‘off-label’ antidepressant prescriptions using Medical Expenditure Panel Survey data, Lim and Jung found that Hispanics/Latinos filled significantly fewer prescriptions compared to non-Hispanic Whites. 25 Moreover, Lê Cook et al. 26 found that Hispanic/Latino youth with psychological impairment were less often prescribed psychotropic medication for this indication compared to non-Hispanic White youth.
It is notable that, when metformin was included with other AOMs, we found that prescription rates within the first year of being followed in a pediatric weight management clinic were no longer statistically significantly different between Hispanic/Latino and non-Hispanic White youth. This may be driven by a higher prevalence of obesity-related comorbidities for which metformin is often prescribed in Hispanics/Latinos compared to non-Hispanic Whites.27–30 In our study, metformin was prescribed nearly twice as often, and the prevalence of diabetes/insulin resistance (an indication for metformin use) was more than double, in Hispanic/Latino compared to non-Hispanic White youth. Therefore, it may have been that metformin was prescribed more often in Hispanics/Latinos not primarily for weight loss, but rather for other indications more prevalent in this population. We were unable to determine from the EHR the exact rationales as to why providers may have prescribed metformin for particular patients. While we considered examining if the prevalence of all obesity-related comorbidities for which metformin is prescribed were statistically higher among Hispanics/Latinos which could explain this finding, EHR problem lists are often incomplete and, therefore, we could not make this comparison with a high degree of certainty. 31 Indeed, we found that only about one-third of youth in our study had any obesity-related comorbidities listed in the EHR, a prevalence far lower than longitudinal cohort studies of youth with severe obesity suggest. For example, Freedman et al. 32 found that 85% of adolescents with severe obesity already have at least one cardiovascular risk factor.
Aside from the finding that AOM prescription rates were lower among Hispanics/Latinos compared to non-Hispanic Whites within the first year of being followed in a pediatric weight management clinic, we did not find any additional statistically significant race/ethnicity differences. Indeed, previous cross-sectional studies in adults have suggested that the prevalence of being provided information on or prescribed AOMs may not differ by race/ethnicity.7,8 Therefore, it is possible that, in terms of AOM prescriptions fewer race/ethnic disparities overall may exist. We must also consider that this study only examined patients being followed in an academic health center based-pediatric weight management clinic, where providers receive specific training on the use and costs of AOMs, and prescribe these medications more frequently. Therefore, it is possible that providers specifically seeing patients in a multidisciplinary pediatric weight management clinic may feel more comfortable prescribing these medications to any patient regardless of race/ethnicity. Finally, topiramate was by far the most commonly prescribed AOM in our pediatric weight management clinic. Topiramate is FDA-approved for children down to the age of 2 years old for the management of epilepsy, and a generic formulation has been FDA-approved since 2009. Therefore, unconscious biases as they specifically relate to affordability and prescribing this medication ‘off-label’ as an AOM to children may be decreased compared to other AOMs, many of which are more expensive and may not have been FDA-approved for any indication in certain youth.
We also found that, among youth from families in whom English was not the primary language spoken, use of an interpreter during pediatric weight management clinic visits was associated with higher AOM prescription rates compared to non-interpreter use. It may be that providers feel less comfortable prescribing certain medications in youth from families with limited English proficiency who do not use interpreters, especially in situations where medications are being used ‘off-label’ as is common in pediatric weight management clinics. Communications difficulties with patients and families with limited English proficiency may decrease a provider’s ability to educate patients on a medication’s benefits and risks.2,33 Further, studies have shown that communication difficulties may improve when interpreters are utilized in patients and families with limited English proficiency.34–36 Our results appear to support this, as we did not find statistically significant difference in AOM prescription rates when youth from primary English speaking families were compared to those from non-Primary English families who used interpreters.
A significant strength of this study is our large multiracial/multiethnic cohort of youth followed in a pediatric weight management clinic over the span of nearly a decade. However, our results must be interpreted within the context of a number of limitations. First, as this was an observational study, while we could determine associations we were unable to determine causality. Therefore, our results should be considered preliminary, and future prospective cohort studies are needed to confirm these findings. Second, this study was performed through review of EHR data, which may be incomplete as this relies on providers correctly and accurately entering all information in the EHR. 31 Further, the amount of missingness in race/ethnicity data available in the EHR could have biased the results if the data were not missing at random.
Moreover, there were smaller sample sizes for some races/ethnicities, notably American Indian/Alaska Native and Mixed race/ethnicity. For these populations, results may have differed if larger sample sizes were available (i.e. it is possible to have meaningful rate ratios that are not accompanied by statistical significance). For example, in our study the IRR comparing AOM prescriptions within the first year for Asians was similar to Hispanic/Latinos; however, was not statistically significant. Finally, results from this study came from a single academic health center-based pediatric weight management clinic located in the Midwestern portion of the US and, therefore, it is unclear how generalizable our findings are to other pediatric weight management clinics in the US or worldwide.
In conclusion, we found that, among youth with severe obesity followed in a pediatric weight management clinic, AOM prescription rates were lower in Hispanic/Latino compared to non-Hispanic White youth within the first year of being followed after accounting for age, degree of obesity, number of obesity-related comorbidities, median household income based on ZIP code, and interpreter use. Among youth from families in whom English was not the primary language spoken, use of an interpreter during pediatric weight management clinic visits was associated with higher AOM prescription rates. These findings highlight the importance of continuing to examine contributors to healthcare disparities in pediatric weight management clinics in order to improve care delivery among youth with severe obesity, and the need for further prospective cohort studies in this area. Interventions to achieve equity in AOM prescription rates, including reducing barriers to interpreter use, using protocols for AOM prescriptions, and provider implicit bias training, among others, may help mitigate disparities in pediatric severe obesity.
Supplemental Material
Supplemental material, sj-docx-1-tae-10.1177_20420188221090009 for Anti-obesity medication prescriptions by race/ethnicity and use of an interpreter in a pediatric weight management clinic by Eric M. Bomberg, Elise F. Palzer, Kyle D. Rudser, Aaron S. Kelly, Carolyn T. Bramante, Hilary K. Seligman, Favour Noni and Claudia K. Fox in Therapeutic Advances in Endocrinology and Metabolism
Supplemental material, sj-docx-2-tae-10.1177_20420188221090009 for Anti-obesity medication prescriptions by race/ethnicity and use of an interpreter in a pediatric weight management clinic by Eric M. Bomberg, Elise F. Palzer, Kyle D. Rudser, Aaron S. Kelly, Carolyn T. Bramante, Hilary K. Seligman, Favour Noni and Claudia K. Fox in Therapeutic Advances in Endocrinology and Metabolism
Footnotes
Author contributions: Eric M. Bomberg: Conceptualization; Funding acquisition; Investigation; Methodology; Supervision; Writing – original draft; Writing – review & editing
Elise F. Palzer: Formal analysis; Methodology; Validation; Writing – review & editing
Kyle D. Rudser: Formal analysis; Investigation; Methodology; Supervision; Validation; Writing – review & editing
Aaron S. Kelly: Conceptualization; Methodology; Supervision; Writing – review & editing
Carolyn T. Bramante: Conceptualization; Investigation; Methodology; Writing – review & editing
Hilary K. Seligman: Conceptualization; Methodology; Writing – review & editing
Favor Noni: Conceptualization; Methodology; Writing – review & editing
Claudia K. Fox: Conceptualization; Methodology; Supervision; Writing – review & editing
Conflict of interest statement: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: E.M.B. is a site principal investigator and co-investigator for Novo Nordisk. A.S.K. serves as an unpaid consultant for Novo Nordisk, Vivus, and Eli Lilly, and receives donated drug and placebo from Vivus for a National Institutes of Diabetes and Digestive and Kidney Diseases (NIDDK)-funded clinical trial. C.T.B. is funded by the NIH National Center for Advancing Translational Sciences, grants KL2TR002494 and UL1TR002494. C.K.F. is a site principal investigator and co-investigator for Novo Nordisk. All other authors have no other relevant disclosures. Research reported in this publication was supported by the NIDDK of the National Institutes of Health under Award Number K23DK125668. Additional support was provided by the Minnesota Population Center, which is funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (Grant P2 C HD041023), and award number UL1TR002494 from the National Institutes of Health’s National Center for Advancing Translational Sciences. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Eric M. Bomberg  https://orcid.org/0000-0002-8037-4314
https://orcid.org/0000-0002-8037-4314
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Eric M. Bomberg, Center for Pediatric Obesity Medicine, Department of Pediatrics, Medical School, University of Minnesota, 717 Delaware Street SE, Room 370, Minneapolis, MN 55414, USA; Department of Pediatrics, Medical School, University of Minnesota, Minneapolis, MN, USA.
Elise F. Palzer, Division of Biostatistics, School of Public Health, University of Minnesota, Minneapolis, MN, USA
Kyle D. Rudser, Center for Pediatric Obesity Medicine, Department of Pediatrics, Medical School, University of Minnesota, Minneapolis, MN, USA Division of Biostatistics, School of Public Health, University of Minnesota, Minneapolis, MN, USA.
Aaron S. Kelly, Center for Pediatric Obesity Medicine, Department of Pediatrics, Medical School, University of Minnesota, Minneapolis, MN, USA Department of Pediatrics, Medical School, University of Minnesota, Minneapolis, MN, USA.
Carolyn T. Bramante, Center for Pediatric Obesity Medicine, Department of Pediatrics, Medical School, University of Minnesota, Minneapolis, MN, USA Department of Medicine, Medical School, University of Minnesota, Minneapolis, MN, USA.
Hilary K. Seligman, Department of Medicine, University of California San Francisco, San Francisco, CA, USA
Favour Noni, University of Minnesota, Minneapolis, MN, USA.
Claudia K. Fox, Center for Pediatric Obesity Medicine, Department of Pediatrics, Medical School, University of Minnesota, Minneapolis, MN, USA Department of Pediatrics, Medical School, University of Minnesota, Minneapolis, MN, USA.
References
- 1. Crowley R. Racial and ethnic disparities in health care, updated 2010: a position paper of the American College of Physicians, https://www.acponline.org/system/files/documents/advocacy/current_policy_papers/assets/racial_disparities.pdf (2010, accessed 1 July 2021). [DOI] [PubMed]
- 2. McQuaid EL, Landier W. Cultural issues in medication adherence: disparities and directions. J Gen Int Med 2017; 33: 200–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Goff DC, Bertoni AG, Kramer H, et al. Dyslipidemia prevalence, treatment, and control in the Multi-Ethnic Study of Atherosclerosis (MESA): gender, ethnicity, and coronary artery calcium. Circulation 2006; 113: 647–656. [DOI] [PubMed] [Google Scholar]
- 4. Pauff BR, Jiroutek MR, Holland MA, et al. Statin prescribing patterns: an analysis of data from patients with diabetes in the National Hospital Ambulatory Medical Survey Outpatient Department and National Ambulatory Medical Care Survey Databases, 2005–2010. Clin Therap 2015; 37: 1329–1339. [DOI] [PubMed] [Google Scholar]
- 5. Goonesekera SD, Yang MH, Hall SA, et al. Racial ethnic differences in type 2 diabetes treatment patterns and glycaemic control in the Boston Area Community Health Survey. BMJ Open 2015; 5: e007375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Andrews AL, Teufel RJ, 2nd, Basco WT, Jr. Low rates of controller medication initiation and outpatient follow-up after emergency department visits for asthma. J Pediatr 2012; 160: 325–330. [DOI] [PubMed] [Google Scholar]
- 7. Lewis KL, Gudzune KA, Fischer H, et al. Racial and ethnic minority patients report different weight-related care experiences than non-Hispanic Whites. Prev Med Rep 2016; 4: 296–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Saxon DR, Iwamoto SJ, Mettenbrink CJ, et al. Antiobesity medication use in 2.2 million adults across eight large health care organizations: 2009-2015. Obesity (Silver Spring) 2019; 27: 1975–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fox CK, Gross AC, Bomberg EM, et al. Severe obesity in the pediatric population: current concepts in clinical care. Curr Obes Rep 2019; 8: 201–209. [DOI] [PubMed] [Google Scholar]
- 10. San Giovanni CB, Sweeney B, Skelton JA, et al. Aversion to off-label prescribing in clinical pediatric weight management: the quintessential double standard. J Clin Endocrinol Metab 2021; 106: 2103–2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Thornton JD, Pham K, Engelberg RA, et al. Families with limited English proficiency receive less information and support in interpreted intensive care unit family conferences. Crit Care Med 2009; 37: 89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kelly AS, Barlow SE, Rao G, et al. Severe obesity in children and adolescents: identification, associated health risks, and treatment approaches: a scientific statement from the American Heart Association. Circulation 2013; 128: 1689–1712. [DOI] [PubMed] [Google Scholar]
- 13. Singhal V, Sella AC, Malhotra S. Pharmacotherapy in pediatric obesity: current evidence and landscape. Curr Opin Endocrinol 2021; 28: 55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Freedman DS, Butte NF, Taveras EM, et al. BMI z-scores are a poor indicator of adiposity among 2- to 19-year-olds with very high BMIs, NHANES 1999-2000 to 2013-2014. Obesity (Silver Spring) 2017; 25: 739–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Berkowitz SA, Traore CY, Singer DE, et al. Evaluating area-based socioeconomic status indicators for monitoring disparities within health care systems: results from a primary network. Health Serv Res 2015; 50: 398–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Manson S, Schroeder J, Van Riper D, et al. IPUMS national historical geographic information system: version 14.0 [Database]. Minneapolis, MN: IPUMS, 2019. [Google Scholar]
- 17. Hall-Lipsey EA, Chisholm-Burns MA. Pharmacotherapeutic disparities: racial, ethnic, and sex variations in medication treatment. Am J Health Syst Pharm 2010; 67: 462–468. [DOI] [PubMed] [Google Scholar]
- 18. Marcelin JR, Siraj DS, Victor R, et al. The impact of unconscious bias in healthcare: how to recognize and mitigate it. J Infect Dis 2019; 220(Suppl. 2): S62–S73. [DOI] [PubMed] [Google Scholar]
- 19. Hall WJ, Chapman MV, Lee KM, et al. Implicit racial/ethnic bias among health care professionals and its influence on health care outcomes: a systematic review. Am J Public Health 2015; 105: e60–e76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chapman EN, Kaatz A, Carnes M. Physicians and implicit bias: how doctors may unwittingly perpetuate health care disparities. J Gen Intern Med 2013; 28: 1504–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kang S, Socal MP, Bai Ge, Anderson GF. Off-label coverage of high-cost drugs by independent patient assistant programs. J Gen Intern Med 2020; 36: 555–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Johnson VR, Acholonu NO, Dolan AC, et al. Racial disparities in obesity treatment among children and adolescents. Curr Obes Rep 2021; 10: 342–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gomez G, Stanford FC. US health policy and prescription drug coverage of FDA-approved medications for the treatment of obesity. Int J Obes (Lond) 2018; 42: 495–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Davis DW, Feygin Y, Creel L, et al. Longitudinal trends in the diagnosis of attention-deficit/hyperactivity disorder and stimulant use in preschool children on medicaid. J Pediatr 2019; 207: 185–191. [DOI] [PubMed] [Google Scholar]
- 25. Lim D, Jung J. Racial-ethnic differences in off-label antidepressant use, by insurance type. Psychiatr Serv 2017; 68: 1271–1279. [DOI] [PubMed] [Google Scholar]
- 26. Lê Cook B, Carson NJ, Kafali EN, et al. Examining psychotropic medication use among youth in the U.S. Gen Hosp Psychiatry 2017; 45: 32–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cheng JY, Kanaya AM, Araneta MG, et al. Prevalence of diabetes by race and ethnicity in the United States, 2011-2016. JAMA 2019; 322: 2389–2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wolf WM, Wattick RA, Kinkade ON, et al. Geographical prevalence of polycystic ovary syndrome as determined by region and race/ethnicity. Int J Environ Res Public Health 2018; 15: 2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lee JM, Okumura MJ, Davis MM, et al. Prevalence and determinants of insulin resistance among U.S. adolescents. Diabetes Care 2006; 29: 2427–2432. [DOI] [PubMed] [Google Scholar]
- 30. Ioannou GN, Bryson CL, Boyko EJ. Prevalence and trends of insulin resistance, impaired fasting glucose, and diabetes. J Diabetes Complications 2007; 21: 363–370. [DOI] [PubMed] [Google Scholar]
- 31. Gianfrancesco MA, Tamang S, Yazdany J, et al. Potential biases in machine learning algorithms using electronic health record data. JAMA Int Med 2018; 178: 1544–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Freedman DS, Mei Z, Srinivasan SR, et al. Cardiovascular risk factors and excess adiposity among overweight children and adolescents: the Bogalusa Heart Study. J Pediatr 2007; 150: 12–17. [DOI] [PubMed] [Google Scholar]
- 33. Karliner LS, Perez-Stable EJ, Gildengorin G. The language divide. The importance of training and use of interpreters for outpatient practice. J Gen Intern Med 2004; 19: 175–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jacobs EA, Lauderdale DS, Meltzer D, et al. Impact of interpreter services on delivery of health care to limited-English-proficient patients. J Gen Intern Med 2001; 16: 468–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jacobs EA, Shepard DS, Suaya JA, et al. Overcoming language barriers in health care: costs and benefits of interpreter services. Am J Public Health 2004; 94: 866–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Njeru JW, Boehm DH, Jacobson DJ, et al. Diabetes outcome and process measures among patients who require language interpreter services in Minnesota primary care practices. J Community Health 2017; 42: 819–825. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tae-10.1177_20420188221090009 for Anti-obesity medication prescriptions by race/ethnicity and use of an interpreter in a pediatric weight management clinic by Eric M. Bomberg, Elise F. Palzer, Kyle D. Rudser, Aaron S. Kelly, Carolyn T. Bramante, Hilary K. Seligman, Favour Noni and Claudia K. Fox in Therapeutic Advances in Endocrinology and Metabolism
Supplemental material, sj-docx-2-tae-10.1177_20420188221090009 for Anti-obesity medication prescriptions by race/ethnicity and use of an interpreter in a pediatric weight management clinic by Eric M. Bomberg, Elise F. Palzer, Kyle D. Rudser, Aaron S. Kelly, Carolyn T. Bramante, Hilary K. Seligman, Favour Noni and Claudia K. Fox in Therapeutic Advances in Endocrinology and Metabolism