Abstract
Background
Riociguat is effective in delaying the time to clinical worsening (TCW) in patients with groups 1 and 4 pulmonary hypertension.
Research Question
Is riociguat more effective than placebo in prolonging TCW in sarcoidosis-associated pulmonary hypertension (SAPH)?
Study Design and Methods
This was a double-blind placebo-controlled trial. Patients with SAPH confirmed by right heart catheterization were randomized 1:1 to riociguat or placebo. Patients underwent 6-min walk distance (6MWD) and spirometry testing every 8 weeks. The primary end point was TCW, which was defined by the time to the first of the following: (1) all-cause mortality, (2) need for hospitalization because of worsening cardiopulmonary status attributable to progression of disease, (3) > 50 m decrease in the 6MWD test, or (4) worsening of World Health Organization functional class.
Results
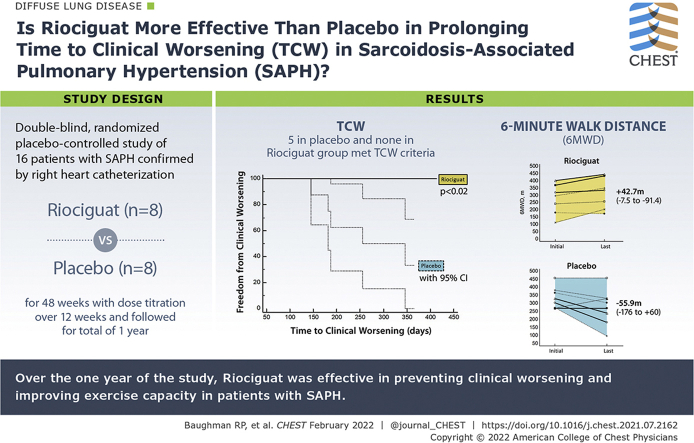
A total of 16 patients were randomized to riociguat (n = 8) or placebo (n = 8). No difference was found in pulmonary artery mean, pulmonary vascular resistance, initial 6MWD, or FVC between the two groups. Five of eight patients who received placebo met TCW criteria, whereas none of the patients who received riociguat experienced a qualifying event. By log-rank analysis, patients who received riociguat were in the study for a significantly longer period (χ 2 = 6.259; P = .0124). The 6MWD decreased in the placebo group (median, –55.9 m; range, –176.8 to 60 m), but rose in the riociguat group (median, +42.7 m; range, –7.5 to +91.4 m; P = .0149), with a placebo-corrected difference of 94 m (P < .01). Four of eight patients who received riociguat, but only 1 of 8 patients who received placebo, showed a > 30-m improvement in 6MWD (P > .05). No significant adverse events associated with riociguat occurred.
Interpretation
Over the 1 year of the study, riociguat was effective in preventing clinical worsening and improving exercise capacity in patients with SAPH.
Trial Registry
ClinicalTrials.gov; No.: NCT02625558; URL: www.clinicaltrials.gov
Key Words: 6-min walk distance, pulmonary fibrosis, riociguat, sarcoidosis-associated pulmonary hypertension
Abbreviations: 6MWD, 6-min walk distance; 6MWT, 6-min walk test; FAS, Fatigue Assessment Scale; IRB, institutional review board; KSQ, King’s Sarcoidosis Questionnaire; PA, pulmonary artery; PAH, pulmonary arterial hypertension; PRO, patient-reported outcome; SAPH, sarcoidosis-associated pulmonary hypertension; SF-36, 36-item Short Form Health Survey; TCW, time to clinical worsening; WHO, World Health Organization
Graphical Abstract
Take-home Points.
Study Question: Is riociguat more effective than placebo in prolonging time to clinical worsening (TCW) in sarcoidosis-associated pulmonary hypertension (SAPH)?
Results: Patients treated with placebo showed a significantly shorter TCW and were more likely to show worsening of the 6-min walk distance than those treated with riociguat.
Interpretation: The use of riociguat was associated with stabilization to improvement in 6-min walk distance and delay in TCW in SAPH.
Sarcoidosis-associated pulmonary hypertension (SAPH) often results in significant morbidity and mortality,1 with up to one-half of patients with SAPH dying within 5 years of diagnosis.2,3 Previous case series and open-label studies have demonstrated the effectiveness of various agents to treat precapillary pulmonary hypertension in sarcoidosis.4,5 However, very few randomized controlled therapy trials for SAPH have been conducted. A double-blind placebo-controlled trial of bosentan demonstrated significant hemodynamic improvement of SAPH, but no change in 6-min walk distance (6MWD).6
In less robust studies of SAPH, a significant improvement in 6MWD is uncommon.7,8 The use of the 6MWD test result as a primary end point has fallen into disfavor in World Health Organization (WHO) group 1 pulmonary hypertension (PH), despite many of the available PH medications being approved based on the results of this test.9 The time to clinical worsening (TCW), which has been constituted variably by a categorical decline in the 6MWD (usually of 15%), cardiopulmonary hospitalization, lung transplantation, or death, has emerged as a more robust end point in PH clinical trials. In a study of inhaled treprostinil for PH resulting from interstitial lung disease, the secondary end point of TCW, consisting of the same four components, was met. This underscores that TCW may be a viable end point in studies of PH resulting from parenchymal lung disease, such as SAPH.10 To date, no placebo-controlled trial has evaluated treatment of SAPH using TCW as either a primary or a secondary end point.
Riociguat is a soluble guanylate cyclase stimulator that has been shown to be a successful treatment for WHO group 1 pulmonary arterial hypertension (PAH) and WHO group 4 chronic thromboembolic pulmonary hypertension.11,12 Unfortunately, riociguat did not meet with success when studied in PH resulting from interstitial lung disease, with the Riociguat for idiopathic interstitial pneumonia-associated pulmonary hypertension (RISE-IIP) study being stopped early because of observed increased harm in the active treatment arm.13 This information became available only during the recruitment phase of a current double-blind, placebo-controlled trial of riociguat vs placebo for SAPH. After the RISE-IIP study results were known, a brief study pause in the current study ensued, but given the poor prognosis of SAPH and the exclusion of patients with significant emphysema, the trial was resumed.
Methods
This was a multicenter trial of patients older than 18 years with a diagnosis of sarcoidosis14 and SAPH confirmed by right heart catheterization. For this study, the diagnosis of SAPH required a pulmonary artery (PA) pressure mean of ≥ 25 mm Hg and a PA occlusion pressure of ≤ 15 mm Hg,15 in accordance with the reports from the PH 5th World Symposium.16 Patients with mean PA pressure of 21 to 24 mm Hg were not included in this study. Patients with known postcapillary or pulmonary venoocclusive disease were excluded from the study. Other major exclusion criteria from the study included those patients with an FVC of < 50% predicted or an FEV1 to FVC ratio of < 50%, presence of more emphysema than fibrosis,17 or the presence of a mycetoma. Exclusionary medications included specific (eg, sildenafil or tadalafil) or nonspecific phosphodiesterase inhibitors or any other investigational drug. Patients were allowed to receive concurrent therapy with an endothelin receptor antagonist. The protocol was approved by the local institutional review board (IRB) for each institution (University of Cincinnati IRB Identifier: 2014-7130; INOVA IRB Identifier: 15-1899; Temple WIRB IRB Identifier: 1249182; and Cleveland Clinic IRB Identifier: FLA18-096), and patients provided written informed consent. The study was registered at ClinicalTrials.gov as NCT02625558.
e-Table 1 provides the schedule of events and variables captured during the trial. An initial high-resolution CT scan was performed to assess for aspergillomas and to determine the extent of fibrosis and emphysema.17 Patients underwent a 6-min walk test (6MWT) and spirometry every 8 weeks. Baseline lung function, including FVC, FEV1, FEV1 to FVC ratio, and single-breath diffusing capacity for carbon monoxide were measured, with the percent predicted calculated, correcting for ethnicity.18,19 A standardized protocol for the 6MWT was implemented at all institutions.20 The 6MWT recorded variables including the distance walked in 6 min, the level of oxygenation, and level of dyspnea using a 10-point dyspnea scale (Borg score) before and immediately after the test. At the baseline test, patients used the same amount of supplemental oxygen they were using for exercise. This level of supplemental oxygen was noted and used for all subsequent 6MWTs unless deemed clinically unacceptable. All medications for sarcoidosis (including prednisone and methotrexate) were kept constant through the study unless modified by the treating physician for clinical cause, with due reasons being noted.
Quality of life was determined by several scores: the 36-item Short Form Health Survey (SF-36) components for physical health, mental health, and total score21; the disease-specific King’s Sarcoidosis Questionnaire (KSQ), including the domains for general health and lung disease22; and a sarcoidosis-specific Fatigue Assessment Scale (FAS).23 The SF-36 and KSQ are total scales of 0 to 100. The higher the value, the better the quality of life. The FAS is a score from 10 to 50, with a lower score indicating less fatigue.
Patients were block randomized at each institution to receive either riociguat or placebo at 1:1 for the 48 weeks of the study. Patients received the first dose of 0.5 mg riociguat or placebo in the clinic and were monitored for hypotension. Study medication was titrated every 2 weeks over 12 weeks to a maximum of 2.5 mg riociguat three times daily or placebo.
The primary end point of the study was TCW, as defined by the time to the first of the following predefined criteria: (1) all-cause mortality, (2) need for hospitalization because of worsening cardiopulmonary status attributable to progression of disease, (3) > 50-m decrease in the 6MWD test, or (4) worsening of WHO functional class. The > 50-m decrease in 6MWD was not verified with a repeat 6MWT, which is similar procedurally to the registration trials of riociguat in PAH and chronic thromboembolic pulmonary hypertension.10, 11, 12 Patients who met TCW or did not comply with medication underwent an end-of-study visit. Changes in 6MWD, FVC, and quality of life were calculated by subtracting the initial value from that at the end-of-study visit.
Our original plan called for studying at least 40 patients. This was based on data from an ongoing registry for sarcoidosis for pulmonary hypertension2 in which we found that one-quarter of patients experienced a severe worsening event within 6 months. By assuming that the rate of clinical worsening events would be 50% after 48 weeks for the placebo group, we calculated that we would be able to detect a 60% reduction in the rate of clinical worsening events with riociguat in a 1-year study (χ 2 = 3.857; P = .0495).
Statistical Analysis
Analysis was performed using MedCalc software. Differences in proportions between groups were calculated using Fisher exact test for baseline characteristics. Comparisons between groups were made with either the independent or paired Student t test, as appropriate. The primary end point, TCW, was calculated using Kaplan-Meier survival analysis, and comparison between survival curves was made using log-rank analysis. A P value of less than .05 was considered significant.
Results
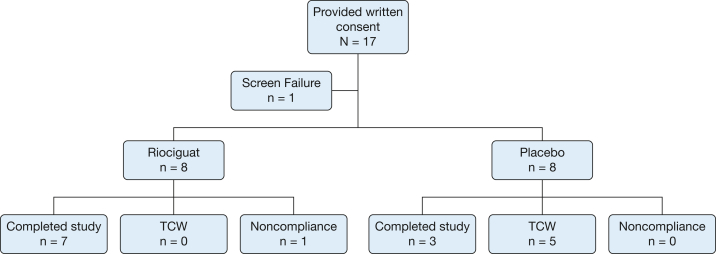
A total of 17 patients from four centers provided written consent for participation in the study. One patient was a screening failure, and the remaining 16 patients (eight in each arm) were randomized (Fig 1).24 All 16 patients were receiving riociguat at a dose of 2.5 mg three times daily or the placebo equivalent by week 12. One patient in the riociguat arm did not comply, was withdrawn from the study after 3 months of therapy, and completed the end-of-study visit. Table 1 summarizes the clinical features of the 16 patients who received at least one dose of therapy.25 No significant differences were found in the initial features between the two groups except that the patients who received placebo were older. Most patients demonstrated pulmonary fibrosis on chest radiographs. All but one patient who received the placebo were being treated with antiinflammatory therapy for sarcoidosis with no significant differences in the regimens between the two groups.
Figure 1.
Consolidated Standards of Reporting Trials24 flow diagram showing patients enrolled in study. Of 27 patients considered for study, 17 were believed to be potential candidates and provided written consent for further testing. TCW = time to clinical worsening.
Table 1.
Clinical Features of Patients at the Time of Enrollment
| Variable | Riociguat | Placebo |
|---|---|---|
| Female to male ratio | 6:2 | 8:0 |
| Race or ethnicity | ||
| Black | 7 | 6 |
| White | 0 | 2 |
| Hispanic | 1 | 0 |
| Age, y | 52 ± 7.0 | 64 ± 6.3a |
| Most recent RHC findings at time of study entry | ||
| PA, mm Hg | ||
| Systolic | 51 ± 4.8 | 49 ± 7.6 |
| Diastolic | 24 ± 4.2 | 19 ± 4.5 |
| Mean | 35 ± 5.1 | 31 ± 5.3 |
| Wedge | 12 ± 4.1 | 10 ± 3.6 |
| Pulmonary vascular resistance, Woods units | 4.4 ± 1.19 | 5.1 ± 2.10 |
| Pulmonary function at enrollment | ||
| FVC, L | 1.98 ± 0.522 | 1.86 ± 0.422 |
| FVC % predicted | 63.8 ± 13.94 | 72.8 ± 13.80 |
| FEV1, L | 1.30 ± 0.491 | 1.23 ± 0.212 |
| FEV1 to FVC ratio, % | 65.7 ± 15.30 | 68.8 ± 16.02 |
| Dlco, mL/min/mm Hg | 10.5 ± 2.73 | 9.1 ± 3.91 |
| Dlco % predicted | 40.6 ± 14.07 | 35.9 ± 9.63 |
| 6MWD, m | 271 ± 95.8 | 332 ± 66.7 |
| Chest radiograph Scadding stage25 | ||
| 1 | 0 | 0 |
| 2 | 1 | 2 |
| 3 | 0 | 1 |
| 4 | 7 | 5 |
| No. of patients receiving individual concurrent antiinflammatory therapy | ||
| Prednisone | ||
| No. | 7 | 5 |
| Daily dose, mg | 10 (5-10) | 10 (5-30) |
| Methotrexate | 2 | 1 |
| Azathioprine | 2 | 0 |
| Mycophenolate mofetil | 1 | 0 |
| Leflunomide | 1 | 0 |
| Hydroxychloroquine | 1 | 2 |
| No therapy | 0 | 1 |
Data are presented as No., median (range), or mean ± SD. 6MWD = 6-min walk distance; Dlco = diffusing capacity for carbon monoxide; PA = pulmonary artery.
Significantly higher than riociguat, P < .02.
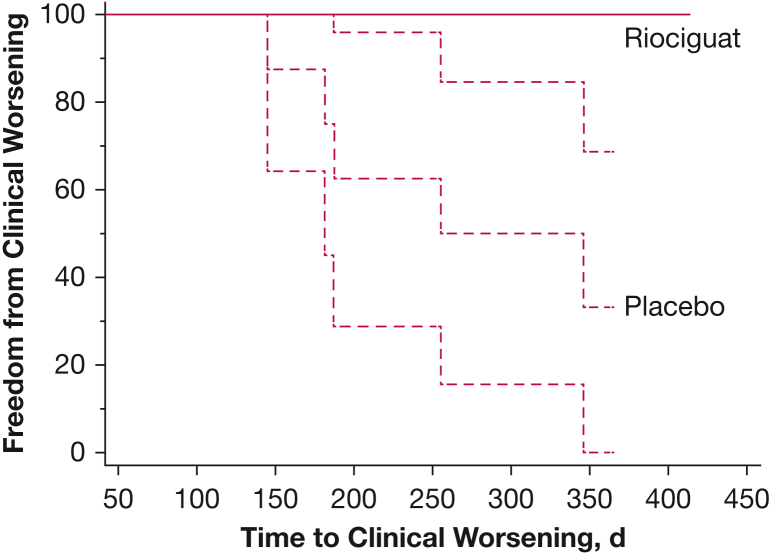
Five of eight patients who received placebo met TCW criteria, whereas none of the patients who received riociguat experienced a qualifying event. In all cases, the TCW was met by a > 50 m drop in 6MWD. Four of the patients who received placebo showed a > 15% 6MWD drop, with one patient showing a 13.5% drop. No other criteria for clinical worsening were met by any patient after the initial 50-m decrease in 6MWD. By log-rank analysis, patients who received riociguat were in the study for a significantly longer period (Fig 2) (null hypothesis rejected; χ 2 = 6.259; P = .0124).
Figure 2.
Line graph showing time to clinical worsening for riociguat vs placebo (P < .02). The 95% CIs are shown for each curve. For riociguat, the 95% CI for the curve is no different from the calculated mean value.
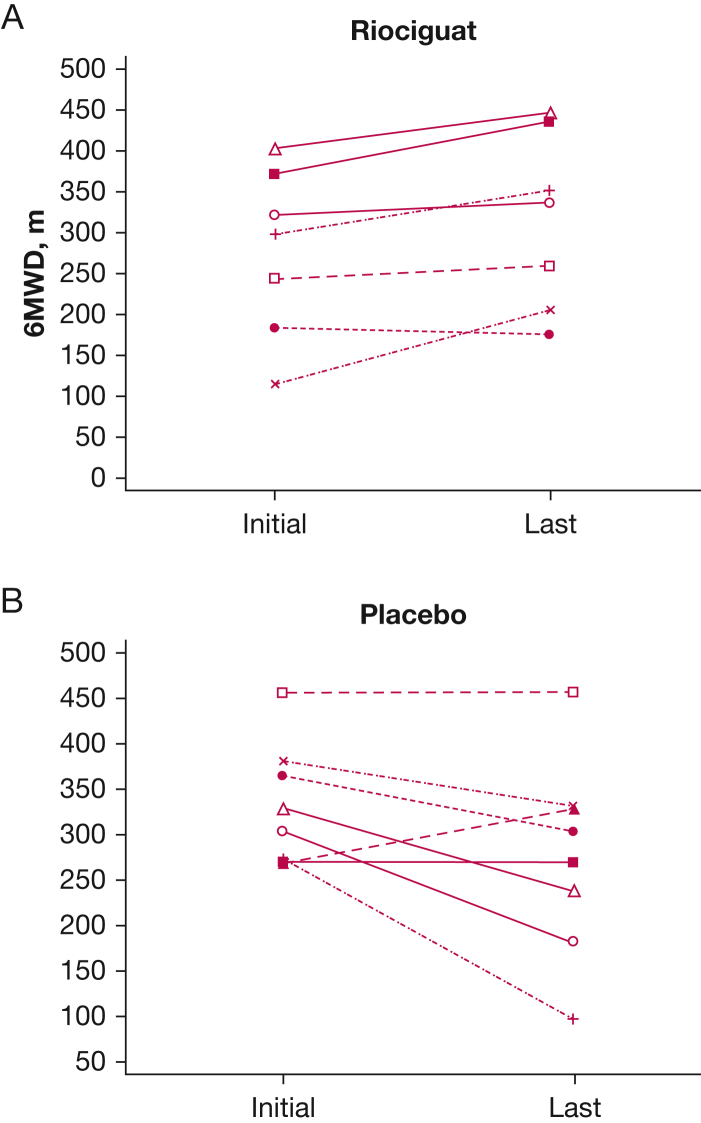
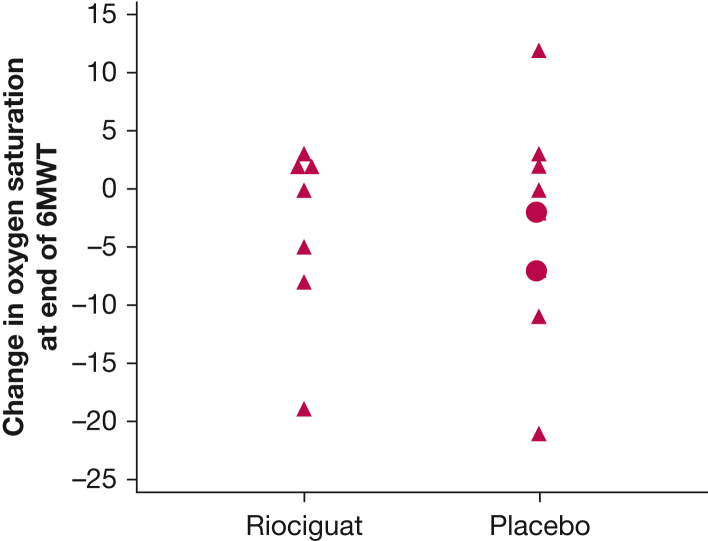
For the riociguat group, a significant increase was found in the 6MWD (39 ± 34.0 m; P < .025) (Fig 3A), whereas the placebo group showed a nonsignificant decrement in 6MWD (–55 + 75.5 m; P > .05) (Fig 3B). The change in 6MWD was significantly different between the riociguat and placebo groups, with a placebo-corrected difference of 94 m (P < .01) (Table 2). In addition, four of eight patients who received riociguat but only 1 of 8 patients who received placebo showed a > 30-m improvement in 6MWD (P > .05). As directed by the protocol, 13 of 15 patients completing the end-of-study 6MWT demonstrated the same supplemental oxygen needs as they did at the initial 6MWT. Two patients who received placebo required significantly higher rates of supplemental oxygen by the end of the study, and it was not believed to be clinically safe to retest them at the initial supplemental oxygen flow rate. No significant differences were found between the two groups regarding the degree of desaturation from rest to the end of the 6MWT (Fig 4). The two patients who received placebo and received increased oxygen supplementation at the end of study are indicated as circles rather than triangles in the figure (unchanged oxygen flow rates). Also, no significant difference was found in the Borg scale or in changes in FVC between the two groups.
Figure 3.
A, B, Lines graphs showing 6MWD initially and at end of study for riociguat (A) (P < .025) and placebo (B) (P > .05). End-of-study visit for riociguat was more than 330 days for all but one patient who dropped out at 98 days. End-of-study visit for patients who received placebo ranged from 145 to 365 days, with five patients at less than 330 days. 6MWD = 6-min walk distance.
Table 2.
Changes in 6MWD and Spirometry Results
| Variable | Riociguat |
Placebo |
||
|---|---|---|---|---|
| Median | 25%-75% Quartile | Median | 25%-75% Quartile | |
| Change in 6MWD, ma | 42.7 | 15.200-61.850 | –55.9 | –105.950 to 0.000 |
| Change in Borg score | 0 | 0.000-0.750 | 1 | –1.000 to 2.500 |
| Change in percent saturation (after walk minus before walk saturation) | 0 | –7.250 to 2.000 | –1 | –9.000 to 2.500 |
| Change in change of percent saturation at end of 6MWT (absolute change in percent saturation) | –4 | –8.500 to 1.250 | –2.5 | –6.500 to 0.500 |
| Change in FVC, mL | –0.17 | –0.190 to 0.0275 | –0.005 | –0.0900 to 0.140 |
| Change in FVC, % predicted | –6 | –6.750 to 0.750 | 0 | –2.000 to 6.000 |
6MWD = 6-min walk distance; 6MWT = 6-min walk test.
Differs from Rio, P < .01.
Figure 4.
Graph showing the level of desaturation at the end of the 6MWT at the end-of-study visit. Only two patients did not receive same level of supplemental oxygen at end of the study visit compared with the initial visit. Both were receiving placebo, and their values are indicated as circles, rather than the triangles used for all others. No significant difference was found in the desaturation between the riociguat and placebo groups. 6MWT = 6-min walk test.
Initial values and changes in quality of life were assessed (Table 3). No significant differences were found between treatment groups for the initial values or change in these measurements between the last completed questionnaire vs initial questionnaire. Also, no correlation was found between the change in these quality-of-life instruments and the change in 6MWD (data not shown).
Table 3.
Initial Quality-of-Life Findings and Changes in Quality-of-Life Findings
| Variable | Riociguat |
Placebo |
||
|---|---|---|---|---|
| Median | 25%-75% Quartile | Median | 25%-75% Quartile | |
| Initial | ||||
| KSQ GH | 54 | 45-62 | 61 | 56-66 |
| KSQ lung | 45 | 41-49 | 49 | 47-51 |
| SF-36 PH | 58 | 20-65 | 52 | 33-59 |
| SF-36 MH | 61 | 30-81 | 70 | 36-78 |
| SF-36 total | 67 | 26-76 | 66 | 31-74 |
| FAS | 26 | 16-35 | 24 | 20-28 |
| Change | ||||
| KSQ GH | –8 | –14 to –7 | –1 | –6 to 4 |
| KSQ lung | 2 | –2 to 4 | –1 | –4 to 1 |
| SF-36 PH | –1 | –6 to 4 | 8 | –6 to 14 |
| SF-36 MH | 0 | –2 to 4 | 3 | –12 to 10 |
| SF-36 total | –1 | –2 to 3 | 4 | –8 to 7 |
| FAS | 0 | –4 to 2 | 0 | –2 to 5 |
FAS = fatigue assessment scale; GH = general health; KSQ = King’s Sarcoidosis Questionnaire; MH = mental health; PH = physical health; SF-36 = 36-item Short Form Health Survey.
All 15 patients who completed the study were receiving the target dose of 2.5 mg three times daily (or placebo). Two patients (both receiving placebo) who were receiving a maintenance endothelin receptor antagonist throughout the study (one was receiving ambrisentan and the other was receiving macitentan). No significant changes were found in antiinflammatory therapy for any of the patients. No significant adverse events associated with either riociguat or placebo therapy occurred.
Discussion
In this double-blind, placebo-controlled trial, we found that 48 weeks of riociguat therapy was associated with a significant delay in TCW compared with placebo. Our TCW used predefined criteria, including death, transplantation, hospitalization for progression of disease, or decrease in 6 MWD of > 50 m. In this study, all the TCW events were heralded by a > 50-m decrease in 6MWD.
The approval of most medications for the treatment of PAH have relied on surrogate markers as end points, including pulmonary vascular hemodynamics, 6-min walk distance, and time to clinical worsening.26,27 Although survival has not been the end point in PAH clinical trials, it is clear that the use of pulmonary hypertension medications has resulted in improved survival in patients with PAH.28 For SAPH, several open-label trials demonstrated improvement in pulmonary hemodynamics after 3 months or more of therapy.3,4,29 A few SAPH trials have demonstrated an improvement in 6MWD,4,30 whereas several other studies have not.3,6,8,29 In this study, we were able to demonstrate a substantial difference in the 6MWD between the two groups favoring treatment with riociguat. In general, the prior studies failing to demonstrate improvement in 6MWD evaluated the patient after fewer than 6 months of therapy, whereas in the current study, patients were studied for 1 year. In addition, prior studies have been inclusive of patients with more severe fibrosis, whereas in this study, those patients with an FVC of less than 50% predicted were excluded, based on a prior study of SAPH.4
One prior randomized controlled trial of bosentan in SAPH did demonstrate a significant improvement in mean PA pressure and pulmonary vascular resistance for bosentan-treated patients vs those who received a placebo.6 However, no significant change was found in the 6MWD for either group during the 16 weeks of the study. The conundrum about the best clinical outcome measure has led to the adoption of time to clinical worsening as the major end point in several pivotal trials for treating WHO group 1 pulmonary hypertension.31, 32, 33 In WHO group 1 studies, the placebo treated patients reached a TCW in more than one-third of cases by 1 year.31,32 For SAPH, we and others observed a 10% mortality or need for transplantation by 1 year.2,3 In addition, other components of a composite end point, such as decrease in 6MWD and need for hospitalization, often are seen.
The current study was designed to determine whether riociguat was associated with a significant delay in TCW worsening in SAPH. None of the patients receiving riociguat reached the predefined TCW, whereas five of eight patients receiving placebo met TCW during the 48 weeks of the study (Fig 2). In all five patients, the TCW was result of a > 50-m decrease in the 6MWD. The choice of 50-m fall in 6MWD as a significant worsening of disease was based on prior literature.34, 35, 36 Others have used a 15% or more decrease in 6MWD as criteria for TCW.10,11 This threshold was met in four patients; the fifth patient showed a 13% decrease in 6MWD. Why none of the other components of this end point were not met in our study is a little uncertain. Because the decrease in 6MWD was first of these four possible outcomes, it is conceivable that other events will occur later. However, none of these occurred in the time frame of this study. Another important finding was that many of the patients receiving riociguat therapy showed a significant improvement in 6MWD, which raises the possibility that for future SAPH trials, a composite of time to clinical change perhaps should be used, because this encompasses both deterioration and improvement.
Riociguat has been shown to exert significant positive effects on pulmonary hemodynamics and 6MWD and to reduce TCW for WHO groups 1 and 4 patients.11,12 Continued response to riociguat has been documented in long-term follow-up studies.37 Perhaps the closest population to the present SAPH cohort in whom riociguat was studied are patients with PH resulting from interstitial lung disease who were studied in a large trial (RISE-IIP). Unfortunately, riociguat was found to be associated with increased serious adverse events including mortality, which resulted in the study being stopped early.13 A post hoc analysis found that the unfavorable response to riociguat was associated strongly with significant emphysematous changes on high-resolution CT scan.17 The phenotype of pulmonary hypertension in combined pulmonary fibrosis and emphysema has been found to have a worse prognosis than pulmonary hypertension with pulmonary fibrosis alone.38,39 It is noteworthy that in the current trial, we excluded patients with significant emphysema. Although the combination of severe emphysema and fibrosis can occur in sarcoidosis,40 it is relatively rare.41 This is in part because most sarcoidosis patients are lifetime nonsmokers.42
Pulmonary vasodilators in patients with any form of parenchymal lung disease may worsen hypoxia, leading to worsening of outcomes.13,43 In SAPH, desaturation often worsens over time.6 In the current study, we found that worsening desaturation with exercise occurred for both groups. However, only two patients required significant increases in the rate of supplemental oxygen during the course of the study, both of whom were receiving the placebo.
The study has some limitations, most notably the small sample size. Study enrollment was hindered by concerns regarding use of riociguat in fibrotic lung disease.10 Other factors included the exclusion of patients receiving a phosphodiesterate-5 inhibitor and as well as those with FVC of < 50% predicted. In addition, study enrollment was halted during the COVID-19 pandemic. Therefore, we performed an interim analysis and found that we had reached the primary end point. The fact that the study met its end point despite not fulfilling the initial recruitment goal could be explained by it being the longest duration placebo-controlled for SAPH with an event rate that was higher than anticipated. Although we did find a significant difference in the TCW end point, this was exclusively through one component of the four-component composite. In addition, this was not accompanied by any change in the patient-reported outcomes (PROs) from the SF-36, KSQ, or FAS quality-of-life measurements. The initial values for the KSQ, SF-36, and FAS are similar to that have been reported previously in other patient populations with sarcoidosis.44,45 All three of these instruments have been shown to be useful in assessing changes in the quality of life for other aspects of sarcoidosis.44, 45, 46, 47 However, prior studies of SAPH also have failed to demonstrate improvement in PROs.6,7 In PAH clinical trials, PROs have not been demonstrated to improve in most patients, barring one recent large clinical trial.32,48 General PROs may not be useful in demonstrating improved quality of life for patients with PH, and although a pulmonary hypertension-specific PRO has been developed,49 it was not available for this study.50 Indeed, a SAPH-specific PRO tool may be an area for future research. We did not perform repeat right heart catheterizations in the patients as part of the study. We believed that it was more important to retain patients in this longer-term study to detect a change in clinical outcome and that repeat hemodynamic studies would limit patient participation. In addition, the vasodilatory effects of riociguat have been well documented. It is noteworthy, too, that repeat right heart catheterizations were not implemented in the studies that resulted in riociguat being approved for PAH and chronic thromboembolic pulmonary hypertension. The patients were randomized in a blinded fashion, but in this small study, the patients who received placebo were significantly older. Although this might have influenced baseline walk distances, it is unlikely to have played a significant role in the change in the 6MWD. Nonetheless, we can not rule out that this may have influenced our results, although considerable overlap in ages was present between the two groups.
Interpretation
In conclusion, this small 1-year double-blind placebo-controlled randomized controlled trial of riociguat in SAPH demonstrated significant improvement in 6MWD and a prolonged TCW for riociguat compared with placebo. This supports the implementation of a larger phase 3 clinical trial of riociguat for this specific indication with a number of lessons to be learned from this small study in the design of any such future program.
Acknowledgments
Author contributions: R. P. B. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis, including and especially any adverse effects. R. P. B., O. A. S., R. G., P. J. E., J. I. S., E. E. L., F. F. R., J. Z., and S. D. N. contributed substantially to the study design, data analysis and interpretation, and the writing of the manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: R. P. B. reports that he has had grant support from Bayer, Genentech, aTyr, Mallinckrodt, Novartis, Celgene, and Actelion. He has been a consultant for Bellephron, Actelion, and Mallinckrodt. He is on the speaker’s board for Boehringer Ingelheim, United Therapeutics, and Mallinckrodt. O. A. S. has been a consultant for and is on the speaker’s bureau for J&J, Actelion, Bayer, and United Therapeutics. P. J. E. has been consultant for Bayer, Actelion, and United Therapeutics. E. E. L. reports that she has had grant support from Bayer, Genentech, Mallinckrodt, Novartis, Celgene, and Actelion. F. F. R. reports grant support from Acceleron, Janssen PH, Bayer, and Mallinckrodt; is a consultant for Acceleron, Janssen PH, Bayer, Mallinckrodt, and United Therapeutics; and is a speaker for Janssen PH, Bayer, Mallinckrodt, and United Therapeutics. S. D. N. is a consultant for Altavant, Boehringer-Ingelheim, Bellerophon, Bayer, Roche-Genentech, Merck, Third Pole, United Therapeutics, and Galapagos. He is on the speakers bureau for Boehringer-Ingelheim, Roche-Genentech, and United Therapeutics. None declared (R. G., J. I. S., J. Z.).
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Additional information: The e-Table can be found in the Supplemental Materials section of the online article.
Footnotes
FUNDING/SUPPORT: This study was supported by an investigational grant from Bayer Pharmaceuticals and the National Institutes of Health [Grant 2UL1TR001425-05A1].
Supplementary Data
References
- 1.Baughman R.P., Engel P.J., Taylor L., et al. Survival in sarcoidosis associated pulmonary hypertension: the importance of hemodynamic evaluation. Chest. 2010;138:1078–1085. doi: 10.1378/chest.09-2002. [DOI] [PubMed] [Google Scholar]
- 2.Shlobin OA, Kouranos V, Barnett SD, et al. Survival of patients with sarcoidosis associated pulmonary hypertension: the impact of baseline hemodynamics, pulmonary function, and six minute walk test in a multi-national registry cohort. Eur Respir J. 2020;55(5):1901747. doi: 10.1183/13993003.01747-2019. [DOI] [PubMed] [Google Scholar]
- 3.Boucly A., Cottin V., Nunes H., et al. Management and long-term outcomes of sarcoidosis-associated pulmonary hypertension. Eur Respir J. 2017;50(4) doi: 10.1183/13993003.00465-2017. 50-54-2017. [DOI] [PubMed] [Google Scholar]
- 4.Barnett C.F., Bonura E.J., Nathan S.D., et al. Treatment of sarcoidosis-associated pulmonary hypertension: a two-center experience. Chest. 2009;135:1455–1461. doi: 10.1378/chest.08-1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonham C.A., Oldham J.M., Gomberg-Maitland M., et al. Prostacyclin and oral vasodilator therapy in sarcoidosis-associated pulmonary hypertension: a retrospective case series. Chest. 2015;148(4):1055–1062. doi: 10.1378/chest.14-2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baughman R.P., Culver D.A., Cordova F.C., et al. Bosentan for sarcoidosis associated pulmonary hypertension: a double-blind placebo controlled randomized trial. Chest. 2014;145:810–817. doi: 10.1378/chest.13-1766. [DOI] [PubMed] [Google Scholar]
- 7.Baughman R.P., Judson M.A., Lower E.E., et al. Inhaled iloprost for sarcoidosis associated pulmonary hypertension. Sarcoidosis Vasc Diffuse Lung Dis. 2009;26:110–120. [PubMed] [Google Scholar]
- 8.Judson M.A., Highland K.B., Kwon S., et al. Ambrisentan for sarcoidosis associated pulmonary hypertension. Sarcoidosis Vasc Diffuse Lung Dis. 2011;28(2):139–145. [PubMed] [Google Scholar]
- 9.Sitbon O., Gomberg-Maitland M., Granton J., et al. Clinical trial design and new therapies for pulmonary arterial hypertension. Eur Respir J. 2019;53(1):1801908–1802018. doi: 10.1183/13993003.01908-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Waxman A., Restrepo-Jaramillo R., Thenappan T., et al. Inhaled treprostinil in pulmonary hypertension due to interstitial lung disease. N Engl J Med. 2021;384(4):325–334. doi: 10.1056/NEJMoa2008470. [DOI] [PubMed] [Google Scholar]
- 11.Ghofrani H.A., Galie N., Grimminger F., et al. Riociguat for the treatment of pulmonary arterial hypertension. N Engl J Med. 2013;369(4):330–340. doi: 10.1056/NEJMoa1209655. [DOI] [PubMed] [Google Scholar]
- 12.Ghofrani H.A., D’Armini A.M., Grimminger F., et al. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension. N Engl J Med. 2013;369(4):319–329. doi: 10.1056/NEJMoa1209657. [DOI] [PubMed] [Google Scholar]
- 13.Nathan S.D., Behr J., Collard H.R., et al. Riociguat for idiopathic interstitial pneumonia-associated pulmonary hypertension (RISE-IIP): a randomised, placebo-controlled phase 2b study. Lancet Respir Med. 2019;7(9):780–790. doi: 10.1016/S2213-2600(19)30250-4. [DOI] [PubMed] [Google Scholar]
- 14.Hunninghake G.W., Costabel U., Ando M., et al. ATS/ERS/WASOG statement on sarcoidosis. American Thoracic Society/European Respiratory Society/World Association of Sarcoidosis and other Granulomatous Disorders. Sarcoidosis Vasc Diffuse Lung Dis. 1999;16(Sep):149–173. [PubMed] [Google Scholar]
- 15.Baughman R.P., Shlobin O.A., Wells A.U., et al. Clinical features of sarcoidosis associated pulmonary hypertension: results of a multi-national registry. Respir Med. 2018;139:72–78. doi: 10.1016/j.rmed.2018.04.015. [DOI] [PubMed] [Google Scholar]
- 16.Rosenkranz S. Pulmonary hypertension 2015: current definitions, terminology, and novel treatment options. Clin Res Cardiol. 2015;104(3):197–207. doi: 10.1007/s00392-014-0765-4. [DOI] [PubMed] [Google Scholar]
- 17.Nathan S.D., Cottin V., Behr J., et al. Impact of lung morphology on clinical outcomes with riociguat in patients with pulmonary hypertension and idiopathic interstitial pneumonia: a post hoc subgroup analysis of the RISE-IIP study. J Heart Lung Transplant. 2021;40(6):494–503. doi: 10.1016/j.healun.2021.02.006. [DOI] [PubMed] [Google Scholar]
- 18.Hankinson J.L., Odencrantz J.R., Fedan K.B. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159(1):179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 19.Crapo R.O., Morris A.H. Standardized single breath normal values for carbon monoxide diffusing capacity. Am Rev Respir Dis. 1981;123(2):185–189. doi: 10.1164/arrd.1981.123.2.185. [DOI] [PubMed] [Google Scholar]
- 20.Baughman R.P., Sparkman B.K., Lower E.E. Six-minute walk test and health status assessment in sarcoidosis. Chest. 2007;132(1):207–213. doi: 10.1378/chest.06-2822. [DOI] [PubMed] [Google Scholar]
- 21.McHorney C.A., Ware J.E., Jr., Raczek A.E. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993;31(3):247–263. doi: 10.1097/00005650-199303000-00006. [DOI] [PubMed] [Google Scholar]
- 22.Patel A.S., Siegert R.J., Creamer D., et al. The development and validation of the King’s Sarcoidosis Questionnaire for the assessment of health status. Thorax. 2013;68(1):57–65. doi: 10.1136/thoraxjnl-2012-201962. [DOI] [PubMed] [Google Scholar]
- 23.de Vries J., Michielsen H., van Heck G.L., et al. Measuring fatigue in sarcoidosis: the Fatigue Assessment Scale (FAS) Br J Health Psychol. 2004;9(pt 3):279–291. doi: 10.1348/1359107041557048. [DOI] [PubMed] [Google Scholar]
- 24.Hopewell S., Clarke M., Moher D., et al. CONSORT for reporting randomized controlled trials in journal and conference abstracts: explanation and elaboration. PLoS Med. 2008;5(1):e20. doi: 10.1371/journal.pmed.0050020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scadding J.G. Prognosis of intrathoracic sarcoidosis in England. Br Med J. 1961;4:1165–1172. doi: 10.1136/bmj.2.5261.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McLaughlin V.V., Badesch D.B., Delcroix M., et al. End points and clinical trial design in pulmonary arterial hypertension. J Am Coll Cardiol. 2009;54(1 suppl):S97–S107. doi: 10.1016/j.jacc.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 27.Galie N., Simonneau G., Barst R.J., et al. Clinical worsening in trials of pulmonary arterial hypertension: results and implications. Curr Opin Pulm Med. 2010;16(suppl 1):S11–S19. doi: 10.1097/01.mcp.0000370206.61003.7e. [DOI] [PubMed] [Google Scholar]
- 28.McLaughlin V.V., Sitbon O., Badesch D.B., et al. Survival with first-line bosentan in patients with primary pulmonary hypertension. Eur Respir J. 2005;25(2):244–249. doi: 10.1183/09031936.05.00054804. [DOI] [PubMed] [Google Scholar]
- 29.Milman N., Burton C.M., Iversen M., et al. Pulmonary hypertension in end-stage pulmonary sarcoidosis: therapeutic effect of sildenafil? J Heart Lung Transplant. 2008;27(3):329–334. doi: 10.1016/j.healun.2007.11.576. [DOI] [PubMed] [Google Scholar]
- 30.Keir G.J., Walsh S.L., Gatzoulis M.A., et al. Treatment of sarcoidosis-associated pulmonary hypertension: a single centre retrospective experience using targeted therapies. Sarcoidosis Vasc Diffuse Lung Dis. 2014;31(2):82–90. [PubMed] [Google Scholar]
- 31.Galie N., Barbera J.A., Frost A.E., et al. Initial use of ambrisentan plus tadalafil in pulmonary arterial hypertension. N Engl J Med. 2015;373(9):834–844. doi: 10.1056/NEJMoa1413687. [DOI] [PubMed] [Google Scholar]
- 32.Pulido T., Adzerikho I., Channick R.N., et al. Macitentan and morbidity and mortality in pulmonary arterial hypertension. N Engl J Med. 2013;369(9):809–818. doi: 10.1056/NEJMoa1213917. [DOI] [PubMed] [Google Scholar]
- 33.Baughman R.P., Drent M., Culver D.A., et al. Endpoints for clinical trials of sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2012;29:90–98. [PubMed] [Google Scholar]
- 34.Gabler N.B., French B., Strom B.L., et al. Validation of 6-minute walk distance as a surrogate end point in pulmonary arterial hypertension trials. Circulation. 2012;126(3):349–356. doi: 10.1161/CIRCULATIONAHA.112.105890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McGlinchey N., Peacock A.J. Endpoints in PAH clinical trials in the era of combination therapy: how do we decide whether something is working without going bankrupt? Drug Discov Today. 2014;19(8):1236–1240. doi: 10.1016/j.drudis.2014.04.020. [DOI] [PubMed] [Google Scholar]
- 36.King T.E., Jr., Bradford W.Z., Castro-Bernardini S., et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2014;370(22):2083–2092. doi: 10.1056/NEJMoa1402582. [DOI] [PubMed] [Google Scholar]
- 37.Ghofrani H.A., Grimminger F., Gruning E., et al. Predictors of long-term outcomes in patients treated with riociguat for pulmonary arterial hypertension: data from the PATENT-2 open-label, randomised, long-term extension trial. Lancet Respir Med. 2016;4(5):361–371. doi: 10.1016/S2213-2600(16)30019-4. [DOI] [PubMed] [Google Scholar]
- 38.Cottin V., Le P.J., Prevot G., et al. Pulmonary hypertension in patients with combined pulmonary fibrosis and emphysema syndrome. Eur Respir J. 2010;35(1):105–111. doi: 10.1183/09031936.00038709. [DOI] [PubMed] [Google Scholar]
- 39.Mejia M., Carrillo G., Rojas-Serrano J., et al. Idiopathic pulmonary fibrosis and emphysema: decreased survival associated with severe pulmonary arterial hypertension. Chest. 2009;136(1):10–15. doi: 10.1378/chest.08-2306. [DOI] [PubMed] [Google Scholar]
- 40.Zhang C., Chan K.M., Schmidt L.A., et al. Histopathology of explanted lungs from patients with a diagnosis of pulmonary sarcoidosis. Chest. 2016;149(2):499–507. doi: 10.1378/chest.15-0615. [DOI] [PubMed] [Google Scholar]
- 41.Hennebicque A.S., Nunes H., Brillet P.Y., et al. CT findings in severe thoracic sarcoidosis. Eur Radiol. 2005;15(1):23–30. doi: 10.1007/s00330-004-2480-4. [DOI] [PubMed] [Google Scholar]
- 42.Newman L.S., Rose C.S., Bresnitz E.A., et al. A case control etiologic study of sarcoidosis: environmental and occupational risk factors. Am J Respir Crit Care Med. 2004;170(12):1324–1330. doi: 10.1164/rccm.200402-249OC. [DOI] [PubMed] [Google Scholar]
- 43.Raghu G., Behr J., Brown K.K., et al. Treatment of idiopathic pulmonary fibrosis with ambrisentan: a parallel, randomized trial. Ann Intern Med. 2013;158(9):641–649. doi: 10.7326/0003-4819-158-9-201305070-00003. [DOI] [PubMed] [Google Scholar]
- 44.Baughman R.P., Judson M.A., Beaumont J.L., et al. Evaluating the minimal clinically important difference of the King’s Sarcoidosis Questionnaire (KSQ) in a multi-center, prospective study. Ann Am Thorac Soc. 2020;18:477–485. doi: 10.1513/AnnalsATS.202006-607OC. [DOI] [PubMed] [Google Scholar]
- 45.Lo K.H., Donohue J., Judson M.A., et al. The St. George’s Respiratory Questionnaire in pulmonary sarcoidosis. Lung. 2020;198(6):917–924. doi: 10.1007/s00408-020-00394-7. [DOI] [PubMed] [Google Scholar]
- 46.de Kleijn W.P., De V.J., Wijnen P.A., et al. Minimal (clinically) important differences for the Fatigue Assessment Scale in sarcoidosis. Respir Med. 2011;105(9):1388–1395. doi: 10.1016/j.rmed.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 47.Baughman R.P., Sweiss N., Keijsers R., et al. Repository corticotropin for chronic pulmonary sarcoidosis. Lung. 2017;195(3):313–322. doi: 10.1007/s00408-017-9994-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mehta S., Sastry B.K.S., Souza R., et al. Macitentan improves health-related quality of life for patients with pulmonary arterial hypertension: results from the randomized controlled SERAPHIN trial. Chest. 2017;151(1):106–118. doi: 10.1016/j.chest.2016.08.1473. [DOI] [PubMed] [Google Scholar]
- 49.McCollister D., Shaffer S., Badesch D.B., et al. Development of the Pulmonary Arterial Hypertension-Symptoms and Impact (PAH-SYMPACT®) questionnaire: a new patient-reported outcome instrument for PAH. Respir Res. 2016;17(1):72. doi: 10.1186/s12931-016-0388-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen H., Taichman D.B., Doyle R.L. Health-related quality of life and patient-reported outcomes in pulmonary arterial hypertension. Proc Am Thorac Soc. 2008;5(5):623–630. doi: 10.1513/pats.200802-020SK. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.