Abstract
Pulmonary arterial hypertension (PAH) is a rare disease associated with abnormally elevated pulmonary pressures and right heart failure resulting in high morbidity and mortality. Although the prognosis for patients with PAH has improved with the introduction of pulmonary vasodilators, disease progression remains a major problem. Given that available therapies are inadequate for preventing small-vessel loss and obstruction, there is active interest in identifying drugs capable of targeting angiogenesis and mechanisms involved in the regulation of cell growth and fibrosis. Among the mechanisms linked to PAH pathogenesis, preclinical studies have identified promising compounds that are currently being tested in clinical trials. These drugs target seven of the major mechanisms associated with PAH pathogenesis: bone morphogenetic protein signaling, tyrosine kinase receptors, estrogen metabolism, extracellular matrix, angiogenesis, epigenetics, and serotonin metabolism. In this review, we discuss the preclinical studies that led to prioritization of these mechanisms, and discuss completed and ongoing phase 2/3 trials using novel interventions such as sotatercept, anastrozole, rodatristat ethyl, tyrosine kinase inhibitors, and endothelial progenitor cells, among others. We anticipate that the next generation of compounds will build on the success of the current standard of care and improve clinical outcomes and quality of life for patients with PAH.
Key Words: clinical trials, pathogenesis, pulmonary hypertension, therapeutics
Abbreviations: 6MWT, 6-min walk test; BET, bromodomain and extra-terminal domain; BMP, bone morphogenetic protein; BMPR, bone morphogenetic protein receptor; BRD4, bromodomain-containing protein 4; eNOS, endothelial nitric oxide synthase; EPC, endothelial progenitor cell; NO, nitric oxide; PAH, pulmonary arterial hypertension; PASMC, pulmonary artery smooth muscle cell; PDGF, platelet-derived growth factor; PH, pulmonary hypertension; PVR, pulmonary vascular resistance; RV, right ventricular; RVSP, right ventricular systolic pressure
Pulmonary arterial hypertension (PAH) is an incurable disease associated with abnormally elevated pulmonary pressures and right heart failure resulting in premature death. PAH is defined by a mean pulmonary artery pressure > 20 mm Hg, a pulmonary artery wedge pressure < 15 mm Hg, and a pulmonary vascular resistance (PVR) ≥ 3 Wood units by right heart catheterization.1,2 The annual incidence of PAH is estimated at 7 to 10 individuals per million people, with a prevalence of up to 50 cases per million. Current treatments are based on the premise that an imbalance of vasoactive mediators results in inappropriate pulmonary vasoconstriction that can be addressed by the use of drugs with vasodilatory properties. PAH therapies target three key pathways and, depending on their activity, are classified into four distinct classes: endothelin receptor antagonists, phosphodiesterase type 5 inhibitors, soluble guanylate cyclase stimulators, or prostacyclin analogs. However, despite the availability of 14 US Food and Drug Administration-approved drugs, PAH remains a progressive disease; the 5-year survival for patients with PAH is approximately 57%, and lung transplantation remains the only treatment option for end-stage disease.3 Given that available therapies are inadequate to halt disease progression, there is widespread consensus that the next generation of PAH-specific therapies should target the genetic and molecular mechanisms behind small-vessel loss and obstructive vascular remodeling.
Tremendous advances have been made in understanding the biological basis of PAH. As a result, PAH is now recognized as a disease state dominated by a complex interplay of dysfunctional angiogenesis, smooth muscle hypertrophy, inappropriate inflammation, metabolic derangements, and many other processes working in concert to create a phenotype of vessel loss and obstruction.4 With the availability of omics technologies, human tissue biobanks, molecular tools, and multiple in vivo models, it is now possible to carry out comprehensive preclinical studies to validate mechanisms of disease and to screen drugs to gauge their clinical potential. This translational approach has accelerated the rate at which compounds move to clinical trials to test whether their addition to the standard of care can reduce morbidity and improve quality of life for patients with PAH.
In this review, we focus on drugs that are currently in phase 1-3 clinical trials and discuss the preclinical studies that provide the rationale for their use in PAH. These drugs target five of the major mechanisms linked to PAH pathogenesis: bone morphogenetic protein (BMP) signaling, tyrosine kinase receptors, estrogen metabolism, extracellular matrix, and angiogenesis. For readers interested in expanding their knowledge on PAH pathobiology and preclinical studies beyond the topics discussed here, we recommend excellent translational reviews by Dunmore et al,5 Gajecki et al,6 and Xiao et al.7
Tyrosine Kinase Receptor Inhibition
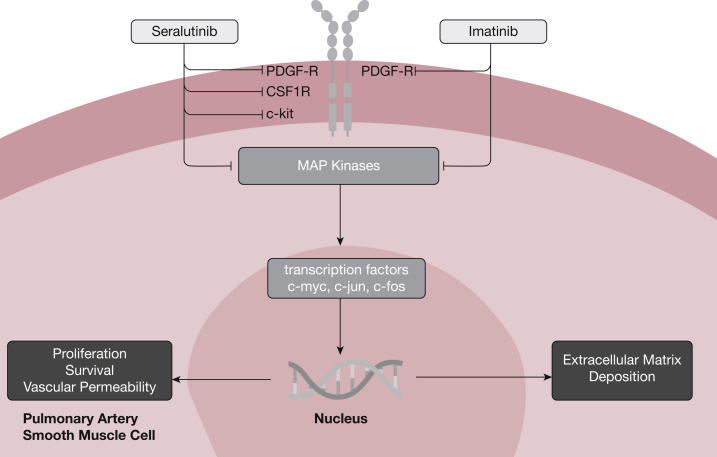
Vascular remodeling in PAH is characterized by the presence of highly proliferative and apoptosis-resistant endothelial and smooth muscle cells within the pulmonary arterial wall.4 This aberrant behavior is partly driven by inappropriate growth factor signaling activity resulting from the increased expression of several growth factors, including platelet-derived growth factor (PDGF), epidermal growth factor, and vascular endothelial growth factor, and their corresponding receptors.8, 9, 10 These growth factors signal through tyrosine kinase receptors, a class of receptors involved in mediating cell-to-cell communication and regulating a wide range of complex biological functions, including cell growth, motility, differentiation, and metabolism (Fig 1). In 2005, Ghofrani and colleagues11 reported their experience in treating a 61-year-old man with end-stage PAH, receiving standard triple combination therapy, with imatinib mesylate, an oral tyrosine kinase receptor inhibitor approved for use in the treatment of chronic myeloid leukemia. Over the course of 3 months, the patient’s clinical condition improved along with evidence of improved hemodynamics and right ventricular function. The same group also carried out preclinical studies using two animal models of PAH and demonstrated that treatment with imatinib mesylate could reverse established pulmonary vascular remodeling and improve right ventricular function,12 thus providing a solid rationale for pursuing clinical trials with imatinib mesylate and other tyrosine kinase receptor inhibitors for PAH.
Figure 1.
Tyrosine kinase inhibitor signaling, inhibition, and relevant pathways to pulmonary arterial hypertension pathogenesis. CSF1R = colony-stimulating factor 1 receptor; MAP = mitogen-activated protein; PDGF-R = platelet-derived growth factor receptor.
Since the first report by Ghofrani and colleagues, clinical trials of imatinib mesylate in patients with PAH have produced the full gamut of effects, ranging from beneficial to harmful. The IMPRES [Imatinib (QTI571) in Pulmonary Arterial Hypertension] study was a stage 3 multinational, multicenter, double-blind, placebo-controlled trial evaluating the safety and efficacy of imatinib.13 The trial was designed to evaluate the primary outcome of 6-min walk distance at 24 weeks, with an extension period of 144 weeks. It included patients in World Health Organization (WHO) functional classes II to IV, receiving two or more therapies for PAH, and targeted a dose of 400 mg once daily from an initial dose of 200 mg. After 24 weeks, a mean difference of 32 m in 6-min walk test (6MWT) distance was achieved between the imatinib and placebo groups (P = .002), as well as a 31.8% decrease in PVR, but no change in first clinical worsening event.13 Interestingly, the benefits trended toward being more dramatic for patients already taking three agents for PAH. Unfortunately, imatinib mesylate was poorly tolerated by the experimental group with 27% of patients discontinuing because of adverse effects, consistent with trials involving the use of imatinib mesylate for other diseases. Most concerning, eight patients in the imatinib group receiving concomitant systemic anticoagulation experienced a subdural hematoma compared with none in the placebo group. Given the competing safety concerns but dramatic hemodynamic improvements in certain patient groups from imatinib therapy, a new phase 2 clinical trial, Positioning Imatinib for Pulmonary Arterial Hypertension (PIPAH), is currently recruiting.14 This trial will look to determine a tolerable dose of imatinib mesylate by administering daily doses ranging from 100 to 400 mg and evaluating discontinuation of the drug for more than 5 consecutive days due to side effects as a primary end point for 12 months, in addition to change in PVR at 24-month follow-up. The trial will exclude patients receiving anticoagulation, looking to mitigate the occurrence of subdural hematoma as seen in IMPRES. Besides oral formulations, imatinib can also be aerosolized and delivered by inhalation, an approach that could bypass many of the systemic side effects associated with the oral drug.15 This version of imatinib (AER-901; Aerami Therapeutics) is currently being prepared for phase 1 studies to start in 2021.
Another approach being investigated is the development of more specific tyrosine kinase inhibitors that can bind to the PDGF receptors, a major driver of smooth muscle cell proliferation, motility, and survival in PAH.9 Seralutinib (GB002; Gossamer Bio) is a highly potent inhaled tyrosine kinase inhibitor that specifically targets the colony-stimulating factor 1 receptor (CSF1R), c-Kit, and PDGF receptors and also increases bone morphogenetic protein receptor type 2 (BMPR2) signaling activity in the pulmonary circulation. Compared with imatinib mesylate, inhaled seralutinib has shown 10-fold greater potency for PDGFα/β receptor inhibition while achieving sustained lung concentrations with minimal systemic exposure. Seralutinib is being manufactured as a dry powder inhaler to be used twice daily and has moved to a phase 2 clinical trial in patients with PAH with the Torrey Study,16 a 24-week double-blind placebo-controlled study in which the primary end point will be change in PVR and the secondary end point will be change in 6MWT distance.
Modulators of BMP Signaling
Heterozygous mutations in the gene encoding BMPR2 are the most common genetic causes of PAH, accounting for 53% to 86% of familial cases and 14% to 35% of idiopathic cases.17, 18, 19, 20 Within this signaling pathway, large whole-genome sequencing studies of heritable PAH have shown evidence for additional causal mutations—including a mutation in GDF2 (which encodes bone morphogenetic protein 9 [BMP9]).21 This was later independently replicated in a whole-exome sequenced Chinese PAH cohort, and found to account for 6.7% of idiopathic PAH cases.22 BMPR2 forms a signaling complex with activin receptor-like kinase 1 (ALK-1) and signals specifically in response to BMP9 and BMP10 in microvascular endothelial cells.23 GDF2 mutations result in BMP9 loss of function as well as reduced circulating levels of both BMP9 and BMP10.24 BMP9 circulates in serum at detectable levels and acts as a vascular quiescence factor, blocking angiogenesis in vitro and in vivo, making it a candidate for therapeutically targeting the dysfunctional pulmonary endothelium.25,26 BMP9 prevents apoptosis and angiogenesis while promoting monolayer integrity in pulmonary arterial endothelial cells. In animal studies, systemic administration of BMP9 prevented and reversed established PAH in the BMPR2 mutant knock-in mouse, monocrotaline rat, and Sugen-hypoxia rat models of PAH by enhancing endothelial BMPR2 signaling.27 On the basis of these preclinical studies, United Kingdom-based biotechnology company Morphogen-IX (now part of Centessa) announced in 2018 their intention to develop MGX292—an engineered variant of BMP9—as a therapeutic candidate for PAH. Clinical trials are expected to begin in 2021.
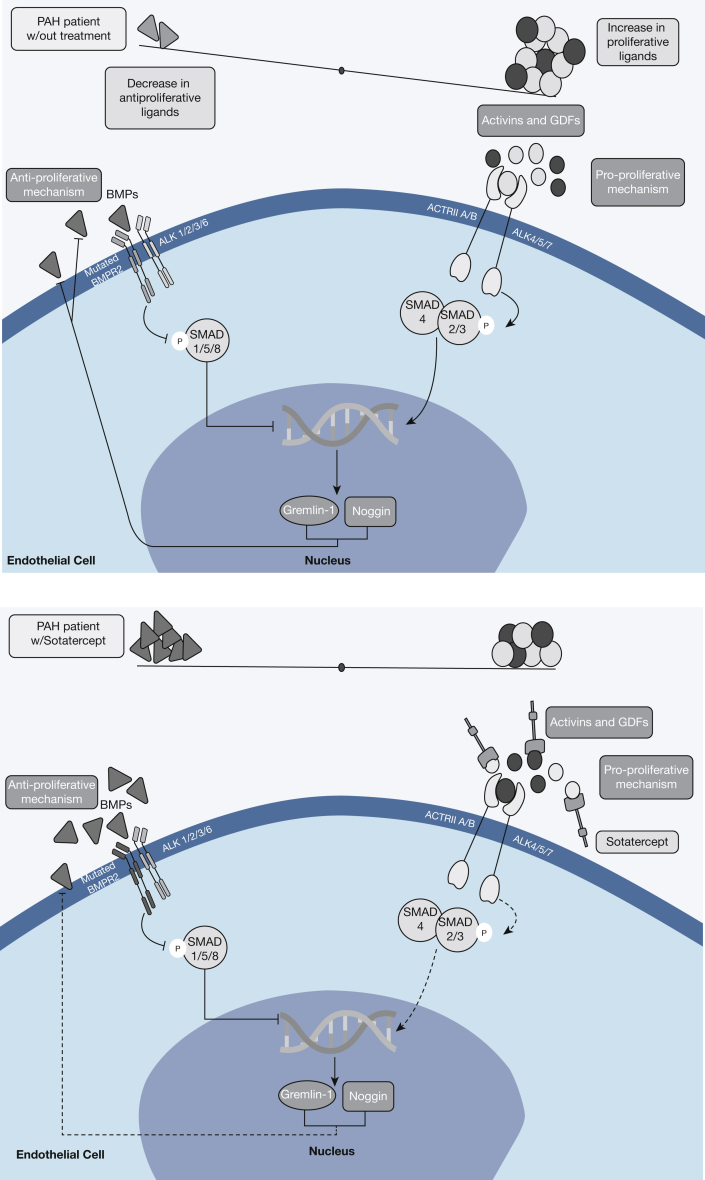
Other components of the BMPR2 signaling pathway have presented themselves as appealing targets for new therapies for PAH. A consequence of BMPR2 insufficiency is loss of downstream SMAD1/5/8 activation in favor of SMAD2/3/4 activation by the activin receptor type IIA (ACTRIIA). The overall result is that BMPR2/SMAD1/5/8 antiproliferative signaling is reduced, favoring pulmonary vascular remodeling driven by augmented ACTRIIA/SMAD2/3.28 Sotatercept (ACE-011) is a fusion protein of activin receptor type IIA linked to the Fc domain of human IgG1. It functions by sequestering free activins, the ligands for ACTRIIA/B, and thus serves to rebalance SMAD signaling, and proliferative behavior (Fig 2). These hypothesized changes were observed in both cultured endothelial and smooth muscle cells as well as in animal model pathologic vascular lesions associated with PAH. When combined with sildenafil in rat models, the effect of combination therapy was superior to sildenafil monotherapy in improving right ventricular hemodynamics and hypertrophy.28 In light of these encouraging preclinical data, phase 2 clinical trials were started in 2018 to test the efficacy of sotatercept for improving hemodynamics (PULSAR)29 and exercise capacity (SPECTRA)30 in patients with PAH. Of these two trials, the results of PULSAR were recently reported and are discussed in detail below.31
Figure 2.
Sotatercept mechanism of action in the context of BMP/SMAD signaling. ACTRII A/B = activin receptor type IIA/B; ALK = activin receptor-like kinase; BMP = bone morphogenetic protein; BMPR2 = bone morphogenetic protein receptor type 2; GDF = growth differentiation factor; PAH = pulmonary arterial hypertension.
PULSAR (A Study of Sotatercept for the Treatment of Pulmonary Arterial Hypertension) was a phase 2, double-blind, randomized, placebo-controlled, parallel-group study of sotatercept plus standard of care vs placebo plus standard of care in patients in WHO group 1 functional class II or III. A total of 106 adults receiving background PAH therapy were randomized to receive subcutaneous sotatercept (0.3 or 0.7 mg/kg body weight every 3 weeks) for 24 weeks, with the primary end point being the change in PVR from baseline and the secondary end point being changes in 6MWT distance and N-terminal pro-B-type natriuretic peptide (NT-proBNP), a surrogate marker of right ventricular dysfunction.32 Compared with the placebo group, sotatercept-treated patients demonstrated significant improvements in PVR (–145.8 dyn·s·cm–5 in the 0.3-mg group and –241 dyn·s·cm–5 in the 0.7-mg group), 6MWT distance (29.4 m in the 0.3-mg group and 21.4 m in the 0.7-mg group), and NT-proBNP. It is pertinent to point out that these changes were seen in the context of background monotherapy (9%-10% of patients), double combination therapy (33%-34% of patients), and triple combination therapy (56%-57% of patients) including prostacyclin infusion. In addition, a dose-dependent response (0.3 vs 0.7 mg/kg) was not observed. Adverse events were reported in 81% to 91% of patients receiving either dose of sotatercept, which included headache, diarrhea, and edema. Specific to sotatercept, the most common adverse event was thrombocytopenia without bleeding complications. In addition, it was recently reported that telangiectasias developed in approximately 10% of patients taking sotatercept. Increases in hemoglobin were seen in one patient (3%) in the 0.3-mg group and in seven patients (17%) in the 0.7-mg group, which resulted in withdrawal from the trial per protocol. In light of these promising results, sotatercept is moving to phase 3 clinical trials (STELLAR),33 which will evaluate sotatercept plus standard of care vs placebo plus standard of care with the primary end point of 6MWT distance at 24 weeks.
Besides the development of novel compounds, another strategy that has helped accelerate drug discovery and clinical trials in PAH is the repurposing of existing drugs being used for other indications.34 Through high-throughput screening of small-molecule chemical compound libraries, Spiekerkoetter and colleagues35 identified tacrolimus, a calcineurin inhibitor used for immunosuppression in solid-organ transplant patients, as an activator of BMPR2 signaling. Independent of the cause of BMPR2 insufficiency, low-dose tacrolimus can increase BMPR2-dependent SMAD1/5 activation and transcriptional activity by removing the inhibitory effect of the FK-binding protein 2 (FKBP12) on the BMPR2 receptor. In preclinical studies, low-dose tacrolimus led to increased expression of BMPR2 target genes (ID1 and apelin) in pulmonary endothelial cells with BMPR2 insufficiency and reversal of pulmonary vascular remodeling in two rat models of PAH and in a murine model of endothelial-specific BMPR2 knockout. Following these studies, the investigators reported their experience with compassionate use of low-dose tacrolimus in three patients with end-stage PAH receiving background therapy. In all three patients, tacrolimus treatment reduced symptomatic burden and improved exercise capacity in the context of increased expression of BMPR2 mRNA and several target genes in peripheral blood mononuclear cells.36 More recently, results of a 16-week phase 2a randomized controlled trial of tacrolimus at three different target serum levels (< 2, 2-3, and 3-5 ng/mL) were reported.37 Compared with placebo (n = 6), there were no significant differences in 6MWT and right ventricular (RV) function in the 14 patients with PAH treated with tacrolimus. Interestingly, individuals who demonstrated an increase in BMPR2 expression also tended to display greater improvement in 6MWT and RV function by echocardiography. Although the study results were negative, it must be recognized that the small patient number and heterogeneity of response across the study populations were significant limitations to the analysis. Despite these results, a phase 2 study is being planned to provide a definite answer on the usefulness of tacrolimus for the treatment of PAH.
Estrogen Modulators
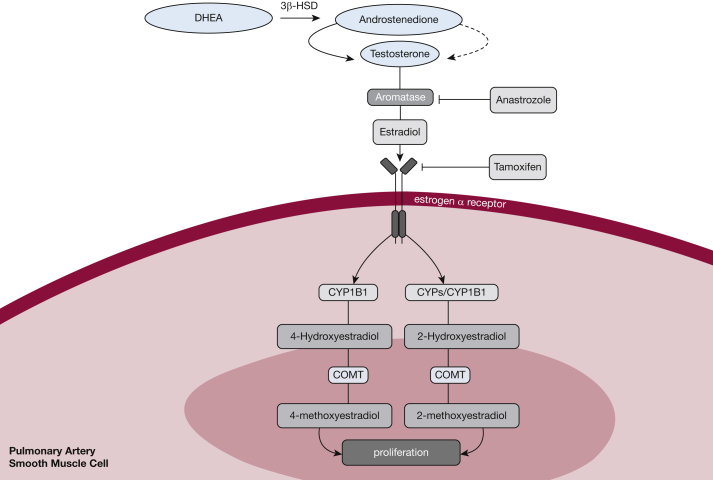
Elucidating the role of estrogen in PAH pathogenesis has been of great interest given the high incidence of PAH in women (3-4:1) and evidence that certain estrogen metabolites can predispose to PAH by promoting cell growth and pulmonary vascular remodeling. The link between estrogen and PAH is not limited to women because men with higher circulating levels of estradiol and lower dehydroepiandrosterone have been shown to have a greater risk of PAH and more severe right ventricular dysfunction.38,39 Human cell culture and animal models have demonstrated that estrogen signaling decreases BMPR2 expression.40 Also in cell culture, estrogen receptor α is highly expressed in female pulmonary artery smooth muscle cells (PASMCs) (Fig 3). Signaling via this receptor increases PASMC proliferation, whereas estrogen receptor α antagonism increased BMPR2 expression—and in a mouse model reversed pulmonary hypertension (PH).41 Although the true role of estrogen in PAH remains a hotly debated topic (see Lahm et al,42 Morris et al,43 and Tofovic and Jackson44 for more detailed reviews), investigators have posited that inhibition of estrogen production and signaling could serve as a rationale for novel PAH therapies.
Figure 3.
Estrogen signaling pathway with therapeutic targets of repurposed drugs for pulmonary arterial hypertension therapy. COMT = catechol-O-methyltransferase; CYPs = cytochrome P450 enzymes; CYP1B1 = cytochrome P450 family 1, subfamily B, member 1; DHEA = dehydroepiandrosterone; 3β-HSD = 3β-hydroxysteroid dehydrogenase.
As part of the efforts to test this hypothesis, clinical trials are being conducted with repurposed antiestrogen drugs currently in use for the treatment of breast cancer: anastrozole and tamoxifen. Anastrozole is an inhibitor of aromatase, an enzyme that catalyzes the formation of estradiol from testosterone. In preclinical studies, female mice treated with anastrozole and exposed to chronic hypoxia demonstrated reduced pulmonary vascular remodeling, lower right ventricular systolic pressure (RVSP), and decreased right ventricular hypertrophy compared with male mice.45 Together with fulvestrant, a selective estrogen receptor degrader, anastrozole was shown to increase insulin sensitivity and peroxisome proliferator-activated receptor γ (PPARγ) and CD36 in BMPR2 mutant mice, and reduced the percentage of muscularized pulmonary vessels and RVSP.46 On the basis of these preliminary data, a randomized double-blind placebo-controlled clinical study was carried out to test the safety and efficacy of anastrozole (1 mg/d) in 18 male and female patients with PAH. Over the course of 12 weeks, anastrozole significantly decreased 17β-estradiol levels and increased 6MWT distance by 26 m compared with placebo. However, there was no improvement in RV function and quality of life, and this trial did not assess invasive hemodynamics. Importantly, there were no differences in the occurrence of adverse side effects between anastrozole- and placebo-treated patients. PHANTOM (Pulmonary Hypertension and Anastrozole)47 is a multicenter double-blind, placebo-controlled phase 2 randomized clinical trial evaluating the safety and side effects of anastrozole in patients with PAH receiving background therapy for a period of 12 months. The 6-month change in 6MWT distance will be evaluated as the primary end point, in addition to several secondary end points including time to clinical worsening, RV function, bone mineral density, among others.
Another approach being tested in a single-center, double-blind randomized, placebo-controlled phase 2 trial is the efficacy and safety of the estrogen receptor blocker tamoxifen in PAH.48 This ongoing study is recruiting patients diagnosed with various forms of PAH, in functional class 1 to 3, and receiving background therapy who will be randomized to receive either tamoxifen (20 mg, 3 times/d) or placebo for 24 weeks with the primary end point being change in tricuspid annular plane systolic excursion measurement, and changes in 6MWT distance and quality of life as secondary end points. It is important to recognize that there are complexities of estrogen signaling and the “estrogen paradox” that these pharmacologic investigations do not necessarily address, but they are important steps to gain further insight into estrogen signaling as a therapeutic target in PAH.
Elafin
Increased elastase activity and fragmented elastin are common pathologic features of vascular lesions in patients and animal models of PAH.49 Elastase activity is a driver of pulmonary vascular remodeling by degrading the extracellular matrix and releasing elastin peptides and growth factors that promote cell proliferation, survival, and motility.50 Elafin is a potent antimicrobial and antiinflammatory agent that can be produced by many cell types, including cells of the pulmonary vasculature.51 It inhibits elastases such as neutrophil elastases, decreases the activity of matrix metalloproteinases, and prevents the degradation of elastin and extracellular matrix, thus helping to maintain the vascular structure. In animal models of PH, heightened elastase activity was observed in wild-type mice exposed to hypoxia, leading to elevated matrix metalloproteinase-9 as well as increased muscularity and loss of distal arteries in these mice. However, these changes were not seen in elafin-overexpressing hypoxic mice, supporting a protective role in the pulmonary circulation.52 When treated with elafin, pulmonary artery endothelial cells derived from patients with PAH demonstrate improved tube formation, as well as increased downstream BMP signaling.53 Elafin administration reverses vascular remodeling and decreases RVSP in the Sugen-hypoxia rat model of PH.53 Interestingly, the elafin-treated rats demonstrated increased expression of apelin, a transcriptional target of BMPR2-mediated signaling, suggesting that elafin could also serve to amplify the BMPR2 signaling cascade.
Given the evidence favoring a potential therapeutic role for elafin in PAH, a phase 1, staggered multiple-ascending dose clinical trial to evaluate the safety, tolerability, pharmacokinetics, and pharmacodynamics of elafin in healthy adult subjects54 has been completed. Ascending doses of elafin were administered subcutaneously daily for 7 days in normal healthy subjects monitored over a 28-day time period to document incidence of treatment-emergent adverse events (primary outcome) and pharmacokinetic/pharmacodynamic and immunogenicity parameters in blood (secondary outcome). A phase 2 proof-of-concept clinical trial in severe PAH is currently being planned.
Endothelial Progenitor Cells/Endothelial Nitric Oxide Synthase
Endothelial cell injury and apoptosis are two of the major pathologic findings in PAH tissues, resulting from a complex combination of underlying genetic and molecular mechanisms.55 Endothelial cells are central to the regulation of pulmonary vascular tone through the production and release of nitric oxide (NO), a potent vasodilator and a key player in the regulation of vascular homeostasis. Evidence of reduced NO production has served as the rationale for the use of phosphodiesterase type 5 inhibitors and soluble guanylate cyclase stimulators in the treatment of PAH.2,56 The basis of NO deficiency in PAH is reduced synthesis by endothelial nitric oxide synthase (eNOS), an enzyme that is subject to complex regulation by transcriptional and posttranslational mechanisms. Studies have shown that eNOS expression is significantly reduced in PAH endothelial cells and that restoration of eNOS could serve as the basis for novel therapeutics.7,57 One approach to deliver eNOS to the pulmonary circulation of patients with PAH is through autologous administration of patient-derived endothelial progenitor cells transfected with the eNOS gene (EPC-eNOS).
EPCs are bone marrow-derived cells that serve a key role in promoting endothelial homeostasis and angiogenesis in the context of injury.58 The rationale for the use of EPCs in PAH is based on multiple studies that have shown the capacity of these cells to repair damaged vessels by restoring endothelial function and angiogenesis through vessel repair and secretion of proangiogenic factors.59,60 Two studies by Zhao and colleagues61,62 demonstrated that administration of EPCs transduced with eNOS in monocrotaline-treated rats not only improved hemodynamics and RV function but also improved vascular remodeling and regenerated lost pulmonary arteries. These preclinical studies prompted the investigators to carry out a phase 1 dose escalation study (Pulmonary Hypertension and Angiogenic Cell Therapy [PHACeT])63 on seven patients receiving conventional therapy for severe PAH, using autologous EPCs transduced with eNOS ex vivo followed by patient administration. Although there were no sustained hemodynamic improvements over the 3-month period, 6MWT distance significantly improved at 1, 3, and 6 months.64 It is important to point out that, although the treatment was tolerated by most of the patients, one patient with a history of recurrent presyncope and multiple hospitalizations for right heart failure died of cardiac arrest soon after receiving the cell infusion. It should be noted that, when this patient was autopsied, there was no evidence of emboli; however, interstitial fibrosis and honeycombing were revealed, raising questions regarding the clinical classification of this patient.
On the basis of these promising results, the SAPPHIRE (Study of Angiogenic Cell Therapy for Progressive Pulmonary Hypertension: Intervention with Repeat Dosing of eNOS-enhanced EPCs)65 trial was initiated in 2016 to test the efficacy of EPC-eNOS administration for improvement of exercise capacity and hemodynamics in patients with PAH who are in functional classes 2 to 4 and receiving conventional therapy. This is a three-arm phase 2 randomized double-blind placebo-controlled trial in which the primary outcome measure will be change in 6MWT distance over a 6-month period and secondary outcomes will be changes in RV function, hemodynamics, and number of deaths/clinical worsening.
Apabetalone
One of the major areas of research interest in PAH has been elucidating the role of epigenetics and DNA damage in the disruption of gene expression and phenotypic changes found in PAH cells.66,67 Bromodomain-containing protein 4 (BRD4), a bromodomain and extra-terminal domain (BET) protein family member, is a protein that binds acetylated histone tails, and regulates the transcription of genes involved in the pathogenesis of PAH.68 In cancer biology, these histone interactions promote the oncogenic activity of genes such as BCL2, c-Myc, and many others that work to promote cell survival and apoptosis resistance.69 Specific to PAH, BRD4 has been shown to be significantly upregulated in human-derived PAH PASMCs, as well as in lung extracts and distal pulmonary arteries. In a multicenter preclinical study, investigators tested the potential of oral apabetalone, a clinically available BET inhibitor that binds specifically to the protein domain of BET proteins responsible for recognizing the N-terminal tails of histones, to improve cellular responses and cardiopulmonary function in four animal models of PAH.70 In a series of elegant and comprehensive studies, the investigators showed that treatment with apabetalone was capable of reversing abnormal responses in PAH pulmonary endothelial cells and smooth muscles cells in vitro. Moreover, oral administration of apabetalone improves hemodynamics and right ventricular function in all four animal models, including a rat model of pulmonary arterial banding. On the basis of these exciting results, the 16-week Apabetalone for Pulmonary Arterial Hypertension: a Pilot Study (APPRoAcH-p)71 was initiated in 2018 to test the safety and hemodynamic effects of oral apabetalone in patients with PAH who are in functional class 2 or 3 and receiving conventional therapy.
Bardoxolone Methyl
Bardoxolone methyl is a promising potential therapy for PAH which decreases oxidative stress and NF-kB activation. Mechanistically, bardoxolone activates nuclear factor erythroid 2-related factor 2 (Nrf2), which reduces reactive oxygen species via promotion of antioxidant gene transcription, repletion of ATP reserves, and more efficient oxidative phosphorylation.72 In addition, when activated, Nrf2 acts in multiple ways to decrease NF-κB transcriptional activity.73 The LARIAT phase 2 clinical trial demonstrated interim improvement in the connective tissue disease-associated pulmonary arterial hypertension (CTD-PAH) patient subset.74,75 This has led to two follow-up clinical trials, a phase 3 clinical trial CATALYST and RANGER, which have both been terminated because of the dangers of in-person clinic visits in the setting of the COVID-19 pandemic.76,77 Further studies are needed to identify the therapeutic role of bardoxolone in CTD-PAH, and possibly other forms of PAH as well.
Rodatristat Ethyl
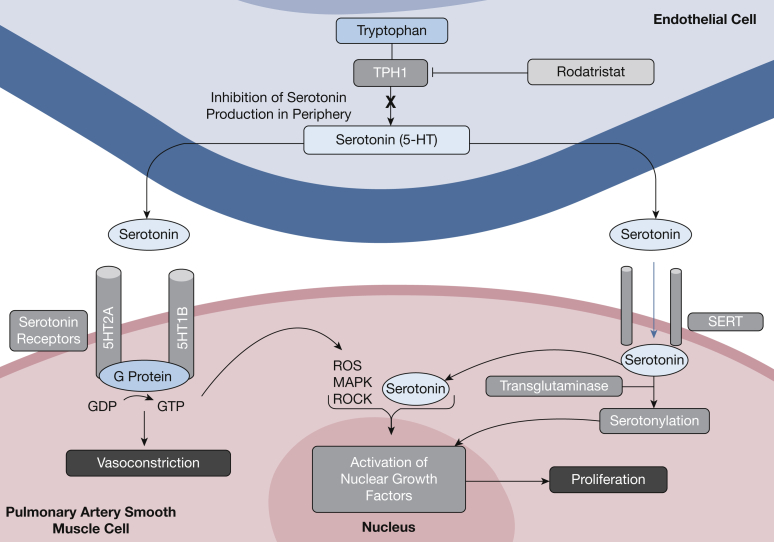
Serotonin is a neurotransmitter of the CNS that is synthesized from l-tryptophan through tryptophan hydroxylase, the enzyme responsible for peripheral serotonin generation. Compared with healthy individuals, patients with PAH have a significantly higher concentration of free serotonin in the blood, associated with increased pulmonary vasoconstriction and obstructive remodeling via excess smooth muscle cell proliferation (Fig 4). Rodatristat ethyl is an orally bioavailable, direct and reversible tryptophan hydroxylase inhibitor that suppresses peripheral serotonin production without CNS effects, as the drug does not cross the blood-brain barrier.78 Rodatristat ethyl for PAH is being evaluated in ELEVATE 2,79 an ongoing placebo-controlled, randomized controlled clinical trial designed to test rodatristat ethyl at 300 mg and 600 mg twice daily for a period of 24 weeks.80 The primary end point is to measure the change in pulmonary vascular resistance from baseline, at the time of initiation, to week 24 of treatment through right heart catheterization. Secondary end points measured from initiation to week 24 of treatment include, but are not limited to, measuring the cardiac index, mean pulmonary artery pressure, and RV fractional area change. The combination of rodatristat’s novel mechanism of action, peripheral specificity, and good tolerability make it an exciting new prospective treatment for the management of PAH.
Figure 4.
The role of serotonin in pathologic pulmonary arterial hypertension cell behavior and target site of rodatristat ethyl. MAPK = mitogen-activated protein kinase; ROCK = Rho-associated kinase; ROS = reactive oxygen species; SERT = serotonin transporter; TPH1 = tryptophan hydroxylase 1.
Conclusion
This review has focused on active clinical trials with a strong foundation in preclinical science and that target genetic and molecular mechanisms associated with PAH progression (Table 1). The ultimate goal is to demonstrate that this novel treatment paradigm can increase the benefits of the current standard of care while slowing or halting disease progression. Besides the compounds discussed, there are many more at various stages of development that are expected to move to clinical trials soon. Although there is no shortage of novel drug targets of potential therapeutic interest, the main challenge will be to prioritize candidates based on expected benefits, cost-effectiveness, and side effect profile. As we enter the age of precision medicine, it is expected that health care providers will have the opportunity to offer more treatment options and have a more sustained impact on the quality of life and clinical evolution of their patients with PAH.
Table 1.
Overview of Novel Therapeutics in Pulmonary Arterial Hypertension Under Investigation
| Treatment | Mechanism | Clinical Trial | Status or Results |
|---|---|---|---|
| Imatinib | Tyrosine kinase inhibitor; multiple targets to attenuate pro-proliferative, promigratory, and apoptosis-resistant cell behavior (not currently approved for the treatment of PAH) | IMPRES: Imatinib (QTI571) in Pulmonary Arterial Hypertension (NCT00902174) PIPAH: Positioning Imatinib for Pulmonary Arterial Hypertension (NCT04416750) |
Phase 3: Published13 Phase 2: Active; recruiting |
| Seralutinib | Tyrosine kinase inhibitor; multiple targets to attenuate pro-proliferative, promigratory, and apoptosis-resistant cell behavior with increased specificity for CSF1R and c-KIT inhibition with BMPR2 modulation | GB002 in Adult Subjects With Pulmonary Arterial Hypertension (NCT04456998) | Phase 2: Active; recruiting |
| BMP9 | Direct BMPR2 agonist | Announced, not yet enrolling | Not yet enrolling |
| Sotatercept | Activin sequestration and rebalancing of SMAD signaling leading to decreased pro-proliferative behavior | PULSAR: A Study of Sotatercept for the Treatment of Pulmonary Arterial Hypertension (NCT03496207) SPECTRA: A Study of Sotatercept for the Treatment of Pulmonary Arterial Hypertension (NCT03738150) STELLAR: A Study of Sotatercept for the Treatment of Pulmonary Arterial Hypertension (NCT04576988) |
Phase 2: Published31 Phase 2: Active; not recruiting Phase 3: Active; not recruiting |
| Tacrolimus | Calcineurin inhibitor; increased BMPR2-dependent SMAD 1/5 activation and transcriptional activity | Single-Center Randomized Controlled Phase II Study of Safety and Efficacy of FK-506 (Tacrolimus) in Pulmonary Arterial Hypertension (NCT01647945) | Phase 2a: Published37 Phase 2: Planned; not recruiting |
| Anastrozole | Aromatase inhibitor | PHANTOM: Pulmonary Hypertension and Anastrozole (NCT03229499) | Phase 2: Active; not recruiting |
| Tamoxifen | Estrogen receptor blocker | T3PAH: Tamoxifen Therapy to Treat Pulmonary Arterial Hypertension (NCT03528902) | Phase 2: Active; recruiting |
| Elafin | Serine elastase inhibitor, which maintains extracellular matrix integrity | Subcutaneous Elafin in Healthy Subjects (NCT03522935) | Phase 1: Completed |
| Endothelial progenitor cells | Direct administration of endothelial nitric oxide synthase (eNOS)-enhanced endothelial cells | PHACeT: Pulmonary Hypertension: Assessment of Cell Therapy (NCT00469027) SAPPHIRE: Study of Angiogenic Cell Therapy for Progressive Pulmonary Hypertension: Intervention With Repeat Dosing of eNOS-Enhanced EPCs (NCT03001414) |
Phase 1: Published64 Phase 2: Active; recruiting |
| Apabetalone | Bromodomain-containing protein 4 (BRD4) inhibitor | APPRoAcH-p: Apabetalone for Pulmonary Arterial Hypertension: A Pilot Study (NCT03655704) | Phase 1: Active; not recruiting |
| Rodatristat ethyl | Tryptophan hydroxylase inhibitor, which decreases peripheral serotonin production | ELEVATE 2: A Study of Rodatristat Ethyl in Patients With Pulmonary Arterial Hypertension (NCT04712669) | Phase 2: Active; recruiting |
BMP9 = bone morphogenetic protein type 9; BMPR2 = bone morphogenetic protein receptor type 2; CSF1R = colony-stimulating factor 1 receptor; EPC = endothelial progenitor cell; PAH = pulmonary arterial hypertension.
Acknowledgments
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: V. A. de J. P. has served as advisor for Acceleron, Bayer, and United Therapeutics. None declared (D. F. C., S. A., A. C., N. A., R. V., H. P., R. T. Z.).
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Footnotes
FUNDING/SUPPORT: This work was supported by the National Institutes of Health (grants R01 HL134776, R01 HL159886-01, and R01 HL139664 to V. A. de J. P.).
References
- 1.Condon D.F., Nickel N.P., Anderson R., Mirza S., de Jesus Perez V.A. The 6th World Symposium on Pulmonary Hypertension: what’s old is new. F1000Res. 2019;8:F1000. doi: 10.12688/f1000research.18811.1. Faculty Rev-888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thomas C.A., Anderson R.J., Condon D.F., de Jesus Perez V.A. Diagnosis and management of pulmonary hypertension in the modern era: insights from the 6th World Symposium. Pulm Ther. 2020;6(1):9–22. doi: 10.1007/s41030-019-00105-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Badesch D.B., Raskob G.E., Elliott C.G., et al. Pulmonary arterial hypertension: baseline characteristics from the REVEAL Registry. Chest. 2010;137(2):376–387. doi: 10.1378/chest.09-1140. [DOI] [PubMed] [Google Scholar]
- 4.Humbert M., Guignabert C., Bonnet S., et al. Pathology and pathobiology of pulmonary hypertension: state of the art and research perspectives. Eur Respir J. 2019;53(1):1801887. doi: 10.1183/13993003.01887-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dunmore B.J., Jones R.J., Toshner M.R., Upton P.D., Morrell N.W. Approaches to treat pulmonary arterial hypertension by targeting BMPR2: from cell membrane to nucleus. Cardiovasc Res. 2021;117(11):2309–2325. doi: 10.1093/cvr/cvaa350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gajecki D., Gawrys J., Szahidewicz-Krupska E., Doroszko A. Novel molecular mechanisms of pulmonary hypertension: a search for biomarkers and novel drug targets-from bench to bed site. Oxid Med Cell Longev. 2020;2020:7265487. doi: 10.1155/2020/7265487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xiao Y., Chen P.P., Zhou R.L., Zhang Y., Tian Z., Zhang S.Y. Pathological mechanisms and potential therapeutic targets of pulmonary arterial hypertension: a review. Aging Dis. 2020;11(6):1623–1639. doi: 10.14336/AD.2020.0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eddahibi S., Humbert M., Sediame S., et al. Imbalance between platelet vascular endothelial growth factor and platelet-derived growth factor in pulmonary hypertension: effect of prostacyclin therapy. Am J Respir Crit Care Med. 2000;162(4 Pt 1):1493–1499. doi: 10.1164/ajrccm.162.4.2003124. [DOI] [PubMed] [Google Scholar]
- 9.Humbert M., Monti G., Fartoukh M., et al. Platelet-derived growth factor expression in primary pulmonary hypertension: comparison of HIV seropositive and HIV seronegative patients. Eur Respir J. 1998;11(3):554–559. [PubMed] [Google Scholar]
- 10.Izikki M., Guignabert C., Fadel E., et al. Endothelial-derived FGF2 contributes to the progression of pulmonary hypertension in humans and rodents. J Clin Invest. 2009;119(3):512–523. doi: 10.1172/JCI35070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghofrani H.A., Seeger W., Grimminger F. Imatinib for the treatment of pulmonary arterial hypertension. N Engl J Med. 2005;353(13):1412–1413. doi: 10.1056/NEJMc051946. [DOI] [PubMed] [Google Scholar]
- 12.Schermuly R.T., Dony E., Ghofrani H.A., et al. Reversal of experimental pulmonary hypertension by PDGF inhibition. J Clin Invest. 2005;115(10):2811–2821. doi: 10.1172/JCI24838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frost A.E., Barst R.J., Hoeper M.M., et al. Long-term safety and efficacy of imatinib in pulmonary arterial hypertension. J Heart Lung Transplant. 2015;34(11):1366–1375. doi: 10.1016/j.healun.2015.05.025. [DOI] [PubMed] [Google Scholar]
- 14.National Institutes of Health Clinical Center . National Institutes of Health; 2020. Positioning imatinib for pulmonary arterial hypertension (PIPAH). NCT04416750. ClinicalTrials.gov.https://clinicaltrials.gov/ct2/show/NCT04416750 Updated September 20, 2021. [Google Scholar]
- 15.Pitsiou G., Zarogoulidis P., Petridis D., et al. Inhaled tyrosine kinase inhibitors for pulmonary hypertension: a possible future treatment. Drug Des Devel Ther. 2014;8:1753–1763. doi: 10.2147/DDDT.S70277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Institutes of Health Clinical Center . National Institutes of Health; 2020. GB002 in adult subject with pulmonary arterial hypertension (PAH). NCT04456998. ClinicalTrials.gov.https://clinicaltrials.gov/ct2/show/NCT04456998 Updated October 8, 2021. [Google Scholar]
- 17.Morrell N.W., Aldred M.A., Chung W.K., et al. Genetics and genomics of pulmonary arterial hypertension. Eur Respir J. 2019;53(1):1801899. doi: 10.1183/13993003.01899-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andruska A., Spiekerkoetter E. Consequences of BMPR2 deficiency in the pulmonary vasculature and beyond: contributions to pulmonary arterial hypertension. Int J Mol Sci. 2018;19(9):2499. doi: 10.3390/ijms19092499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lyu Z.C., Wang L., Lin J.H., et al. The features of rare pathogenic BMPR2 variants in pulmonary arterial hypertension: comparison between patients and reference population. Int J Cardiol. 2020;318:138–143. doi: 10.1016/j.ijcard.2020.06.068. [DOI] [PubMed] [Google Scholar]
- 20.Theilmann A.L., Hawke L.G., Hilton L.R., et al. Endothelial BMPR2 loss drives a proliferative response to BMP (bone morphogenetic protein) 9 via prolonged canonical signaling. Arterioscler Thromb Vasc Biol. 2020;40(11):2605–2618. doi: 10.1161/ATVBAHA.119.313357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Graf S., Haimel M., Bleda M., et al. Identification of rare sequence variation underlying heritable pulmonary arterial hypertension. Nat Commun. 2018;9(1):1416. doi: 10.1038/s41467-018-03672-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang X.J., Lian T.Y., Jiang X., et al. Germline BMP9 mutation causes idiopathic pulmonary arterial hypertension. Eur Respir J. 2019;53(3):1801609. doi: 10.1183/13993003.01609-2018. [DOI] [PubMed] [Google Scholar]
- 23.David L., Mallet C., Mazerbourg S., Feige J.J., Bailly S. Identification of BMP9 and BMP10 as functional activators of the orphan activin receptor-like kinase 1 (ALK1) in endothelial cells. Blood. 2007;109(5):1953–1961. doi: 10.1182/blood-2006-07-034124. [DOI] [PubMed] [Google Scholar]
- 24.Hodgson J., Swietlik E.M., Salmon R.M., et al. Characterization of GDF2 mutations and levels of BMP9 and BMP10 in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2020;201(5):575–585. doi: 10.1164/rccm.201906-1141OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.David L., Mallet C., Keramidas M., et al. Bone morphogenetic protein-9 is a circulating vascular quiescence factor. Circ Res. 2008;102(8):914–922. doi: 10.1161/CIRCRESAHA.107.165530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scharpfenecker M., van Dinther M., Liu Z., et al. BMP-9 signals via ALK1 and inhibits bFGF-induced endothelial cell proliferation and VEGF-stimulated angiogenesis. J Cell Sci. 2007;120(Pt 6):964–972. doi: 10.1242/jcs.002949. [DOI] [PubMed] [Google Scholar]
- 27.Long L., Ormiston M.L., Yang X., et al. Selective enhancement of endothelial BMPR-II with BMP9 reverses pulmonary arterial hypertension. Nat Med. 2015;21(7):777–785. doi: 10.1038/nm.3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yung L.M., Yang P., Joshi S., et al. ACTRIIA-Fc rebalances activin/GDF versus BMP signaling in pulmonary hypertension. Sci Transl Med. 2020;12(543) doi: 10.1126/scitranslmed.aaz5660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.National Institutes of Health Clinical Center . National Institutes of Health; 2018. A study of sotatercept for the treatment of pulmonary arterial hypertension (PAH) (PULSAR). NCT03496207. ClinicalTrials.gov.https://clinicaltrials.gov/ct2/show/NCT03496207 Updated May 5, 2021. [Google Scholar]
- 30.National Institutes of Health Clinical Center . National Institutes of Health; 2018. A study of sotatercept for the treatment of pulmonary arterial hypertension (SPECTRA). NCT03738150. ClinicalTrials.gov.https://clinicaltrials.gov/ct2/show/NCT03738150 Updated November 13, 2021. [Google Scholar]
- 31.Humbert M., McLaughlin V., Gibbs J.S.R., et al. Sotatercept for the treatment of pulmonary arterial hypertension. N Engl J Med. 2021;384(13):1204–1215. doi: 10.1056/NEJMoa2024277. [DOI] [PubMed] [Google Scholar]
- 32.Lewis R.A., Durrington C., Condliffe R., Kiely D.G. BNP/NT-proBNP in pulmonary arterial hypertension: time for point-of-care testing? Eur Respir Rev. 2020;29(156):200009. doi: 10.1183/16000617.0009-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.National Institutes of Health Clinical Center . National Institutes of Health; 2020. A study of sotatercept for the treatment of pulmonary arterial hypertension (STELLAR). NCT04576988. ClinicalTrials.gov.https://clinicaltrials.gov/ct2/show/NCT04576988 Updated September 13, 2021. [Google Scholar]
- 34.Prins K.W., Thenappan T., Weir E.K., Kalra R., Pritzker M., Archer S.L. Repurposing medications for treatment of pulmonary arterial hypertension: what’s old is new again. J Am Heart Assoc. 2019;8(1) doi: 10.1161/JAHA.118.011343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spiekerkoetter E., Tian X., Cai J., et al. FK506 activates BMPR2, rescues endothelial dysfunction, and reverses pulmonary hypertension. J Clin Invest. 2013;123(8):3600–3613. doi: 10.1172/JCI65592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spiekerkoetter E., Sung Y.K., Sudheendra D., et al. Low-dose FK506 (tacrolimus) in end-stage pulmonary arterial hypertension. Am J Respir Crit Care Med. 2015;192(2):254–257. doi: 10.1164/rccm.201411-2061LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spiekerkoetter E., Sung Y.K., Sudheendra D., et al. Randomised placebo-controlled safety and tolerability trial of FK506 (tacrolimus) for pulmonary arterial hypertension. Eur Respir J. 2017;50(3):1602449. doi: 10.1183/13993003.02449-2016. [DOI] [PubMed] [Google Scholar]
- 38.Ventetuolo C.E., Baird G.L., Barr R.G., et al. Higher estradiol and lower dehydroepiandrosterone-sulfate levels are associated with pulmonary arterial hypertension in men. Am J Respir Crit Care Med. 2016;193(10):1168–1175. doi: 10.1164/rccm.201509-1785OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ventetuolo C.E., Mitra N., Wan F., et al. Oestradiol metabolism and androgen receptor genotypes are associated with right ventricular function. Eur Respir J. 2016;47(2):553–563. doi: 10.1183/13993003.01083-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Austin E.D., Hamid R., Hemnes A.R., et al. BMPR2 expression is suppressed by signaling through the estrogen receptor. Biol Sex Differ. 2012;3(1):6. doi: 10.1186/2042-6410-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wright A.F., Ewart M.A., Mair K., et al. Oestrogen receptor alpha in pulmonary hypertension. Cardiovasc Res. 2015;106(2):206–216. doi: 10.1093/cvr/cvv106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lahm T., Tuder R.M., Petrache I. Progress in solving the sex hormone paradox in pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2014;307(1):L7–L26. doi: 10.1152/ajplung.00337.2013. [DOI] [PubMed] [Google Scholar]
- 43.Morris H., Denver N., Gaw R., Labazi H., Mair K., MacLean M.R. Sex differences in pulmonary hypertension. Clin Chest Med. 2021;42(1):217–228. doi: 10.1016/j.ccm.2020.10.005. [DOI] [PubMed] [Google Scholar]
- 44.Tofovic S.P., Jackson E.K. Estradiol metabolism: crossroads in pulmonary arterial hypertension. Int J Mol Sci. 2019;21(1):116. doi: 10.3390/ijms21010116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mair K.M., Wright A.F., Duggan N., et al. Sex-dependent influence of endogenous estrogen in pulmonary hypertension. Am J Respir Crit Care Med. 2014;190(4):456–467. doi: 10.1164/rccm.201403-0483OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen X., Austin E.D., Talati M., et al. Oestrogen inhibition reverses pulmonary arterial hypertension and associated metabolic defects. Eur Respir J. 2017;50(2):1602337. doi: 10.1183/13993003.02337-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.National Institutes of Health Clinical Center . National Institutes of Health; 2017. Pulmonary hypertension and anastrozole trial (PHANTOM). NCT03229499. ClinicalTrials.gov.https://clinicaltrials.gov/ct2/show/NCT03229499 Updated August 3, 2020. [Google Scholar]
- 48.National Institutes of Health Clinical Center . National Institutes of Health; 2018. Tamoxifen therapy to treat pulmonary arterial hypertension (T2PAH). NCT03528902. ClinicalTrials.gov.https://clinicaltrials.gov/ct2/show/NCT03528902 Updated February 18, 2021. [Google Scholar]
- 49.Todorovich-Hunter L., Johnson D.J., Ranger P., Keeley F.W., Rabinovitch M. Altered elastin and collagen synthesis associated with progressive pulmonary hypertension induced by monocrotaline: a biochemical and ultrastructural study. Lab Invest. 1988;58(2):184–195. [PubMed] [Google Scholar]
- 50.Thompson K., Rabinovitch M. Exogenous leukocyte and endogenous elastases can mediate mitogenic activity in pulmonary artery smooth muscle cells by release of extracellular-matrix bound basic fibroblast growth factor. J Cell Physiol. 1996;166(3):495–505. doi: 10.1002/(SICI)1097-4652(199603)166:3<495::AID-JCP4>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 51.Marischen L., Wesch D., Schroder J.M., Wiedow O., Kabelitz D. Human gammadelta T cells produce the protease inhibitor and antimicrobial peptide elafin. Scand J Immunol. 2009;70(6):547–552. doi: 10.1111/j.1365-3083.2009.02337.x. [DOI] [PubMed] [Google Scholar]
- 52.Zaidi S.H., You X.M., Ciura S., Husain M., Rabinovitch M. Overexpression of the serine elastase inhibitor elafin protects transgenic mice from hypoxic pulmonary hypertension. Circulation. 2002;105(4):516–521. doi: 10.1161/hc0402.102866. [DOI] [PubMed] [Google Scholar]
- 53.Nickel N.P., Spiekerkoetter E., Gu M., et al. Elafin reverses pulmonary hypertension via caveolin-1-dependent bone morphogenetic protein signaling. Am J Respir Crit Care Med. 2015;191(11):1273–1286. doi: 10.1164/rccm.201412-2291OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.National Institutes of Health Clinical Center . National Institutes of Health; 2018. Subcutaneous elafin in healthy subjects. NCT03522935. ClinicalTrials.gov.https://clinicaltrials.gov/ct2/show/NCT03522935 Updated April 28, 2021. [Google Scholar]
- 55.Evans C.E., Cober N.D., Dai Z., Stewart D.J., Zhao Y.Y. Endothelial cells in the pathogenesis of pulmonary arterial hypertension. Eur Respir J. 2021;58(3):2003957. doi: 10.1183/13993003.03957-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tonelli A.R., Haserodt S., Aytekin M., Dweik R.A. Nitric oxide deficiency in pulmonary hypertension: pathobiology and implications for therapy. Pulm Circ. 2013;3(1):20–30. doi: 10.4103/2045-8932.109911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lazar Z., Meszaros M., Bikov A. The nitric oxide pathway in pulmonary arterial hypertension: pathomechanism, biomarkers and drug targets. Curr Med Chem. 2020;27(42):7168–7188. doi: 10.2174/0929867327666200522215047. [DOI] [PubMed] [Google Scholar]
- 58.Asahara T., Murohara T., Sullivan A., et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275(5302):964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 59.Fadini G.P., Avogaro A., Ferraccioli G., Agostini C. Endothelial progenitors in pulmonary hypertension: new pathophysiology and therapeutic implications. Eur Respir J. 2010;35(2):418–425. doi: 10.1183/09031936.00112809. [DOI] [PubMed] [Google Scholar]
- 60.Asosingh K., Aldred M.A., Vasanji A., et al. Circulating angiogenic precursors in idiopathic pulmonary arterial hypertension. Am J Pathol. 2008;172(3):615–627. doi: 10.2353/ajpath.2008.070705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhao Y.D., Courtman D.W., Deng Y., et al. Rescue of monocrotaline-induced pulmonary arterial hypertension using bone marrow-derived endothelial-like progenitor cells: efficacy of combined cell and eNOS gene therapy in established disease. Circ Res. 2005;96(4):442–450. doi: 10.1161/01.RES.0000157672.70560.7b. [DOI] [PubMed] [Google Scholar]
- 62.Zhao Y.D., Courtman D.W., Ng D.S., et al. Microvascular regeneration in established pulmonary hypertension by angiogenic gene transfer. Am J Respir Cell Mol Biol. 2006;35(2):182–189. doi: 10.1165/rcmb.2005-0115OC. [DOI] [PubMed] [Google Scholar]
- 63.National Institutes of Health Clinical Center . National Institutes of Health; 2007. Pulmonary hypertension: assessment of cell therapy (PHACeT). NCT00469027. ClinicalTrials.gov.https://clinicaltrials.gov/ct2/show/NCT00469027 Updated October 17, 2016. [Google Scholar]
- 64.Granton J., Langleben D., Kutryk M.B., et al. Endothelial NO-synthase gene-enhanced progenitor cell therapy for pulmonary arterial hypertension: the PHACeT trial. Circ Res. 2015;117(7):645–654. doi: 10.1161/CIRCRESAHA.114.305951. [DOI] [PubMed] [Google Scholar]
- 65.National Institutes of Health Clinical Center . National Institutes of Health; 2016. Study of angiogenic cell therapy for progressive pulmonary hypertension: intervention with repeat dosing of eNOS-enhanced EPCs (SAPPHIRE). NCT03001414. ClinicalTrials.gov.https://clinicaltrials.gov/ct2/show/NCT03001414 Updated July 13, 2021. [Google Scholar]
- 66.Gamen E., Seeger W., Pullamsetti S.S. The emerging role of epigenetics in pulmonary hypertension. Eur Respir J. 2016;48(3):903–917. doi: 10.1183/13993003.01714-2015. [DOI] [PubMed] [Google Scholar]
- 67.Olschewski A., Berghausen E.M., Eichstaedt C.A., et al. Pathobiology, pathology and genetics of pulmonary hypertension: update from the Cologne Consensus Conference 2018. Int J Cardiol. 2018;272S:4–10. doi: 10.1016/j.ijcard.2018.09.070. [DOI] [PubMed] [Google Scholar]
- 68.Meloche J., Potus F., Vaillancourt M., et al. Bromodomain-containing protein 4: the epigenetic origin of pulmonary arterial hypertension. Circ Res. 2015;117(6):525–535. doi: 10.1161/CIRCRESAHA.115.307004. [DOI] [PubMed] [Google Scholar]
- 69.Belkina A.C., Denis G.V. BET domain co-regulators in obesity, inflammation and cancer. Nat Rev Cancer. 2012;12(7):465–477. doi: 10.1038/nrc3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Van der Feen D.E., Kurakula K., Tremblay E., et al. Multicenter preclinical validation of BET inhibition for the treatment of pulmonary arterial hypertension. Am J Respir Crit Care Med. 2019;200(7):910–920. doi: 10.1164/rccm.201812-2275OC. [DOI] [PubMed] [Google Scholar]
- 71.National Institutes of Health Clinical Center . National Institutes of Health; 2018. Apabetalone for pulmonary arterial hypertension: a pilot study (APPRoAcH-p). NCT03655704. ClinicalTrials.gov.https://clinicaltrials.gov/ct2/show/NCT03655704 Updated August 30, 2021. [Google Scholar]
- 72.Holmstrom K.M., Baird L., Zhang Y., et al. Nrf2 impacts cellular bioenergetics by controlling substrate availability for mitochondrial respiration. Biol Open. 2013;2(8):761–770. doi: 10.1242/bio.20134853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ahmed S.M., Luo L., Namani A., Wang X.J., Tang X. Nrf2 signaling pathway: pivotal roles in inflammation. Biochim Biophys Acta Mol Basis Dis. 2017;1863(2):585–597. doi: 10.1016/j.bbadis.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 74.Oudiz R.J., Meyer C., Chin M., et al. Results of interim analysis of the efficacy and safety of bardoxolone methyl in patients with pulmonary arterial hypertension associated with connective tissue disease (CTD) (the LARIAT study) [abstract] Am J Respir Crit Care Med. 2017;195:A6896. [Google Scholar]
- 75.National Institutes of Health Clinical Center . National Institutes of Health; 2014. Bardoxolone methyl evaluation in patients with pulmonary hypertension (PH)—LARIAT. NCT02036970. ClinicalTrials.gov.https://clinicaltrials.gov/ct2/show/NCT02036970 Last updated July 23, 2021. [Google Scholar]
- 76.National Institutes of Health Clinical Center . National Institutes of Health; 2016. Bardoxolone methyl in patients with connective tissue disease-associated pulmonary arterial hypertension—CATALYST. NCT02657356. ClinicalTrials.gov.https://clinicaltrials.gov/ct2/show/NCT02657356 Updated May 3, 2021. [Google Scholar]
- 77.National Institutes of Health Clinical Center . National Institutes of Health; 2017. Extended access program to assess long-term safety of bardoxolone methyl in patients with pulmonary hypertension RANGER (RANGER). NCT03068130. ClinicalTrials.gov.https://clinicaltrials.gov/ct2/show/NCT03068130 Updated September 28, 2021. [Google Scholar]
- 78.Bader M. Inhibition of serotonin synthesis: a novel therapeutic paradigm. Pharmacol Ther. 2020;205:107423. doi: 10.1016/j.pharmthera.2019.107423. [DOI] [PubMed] [Google Scholar]
- 79.National Institutes of Health Clinical Center . National Institutes of Health; 2021. A study of rodatristat ethyl in patients with pulmonary arterial hypertension (ELEVATE 2). NCT04712669. ClinicalTrials.gov.https://clinicaltrials.gov/ct2/show/NCT04712669 Updated August 20, 2021. [Google Scholar]
- 80.Johnson B., Palacios M., Zhou J.J., Schmith V.D., Wring S. A pharmacokinetic/pharmacodynamic based rationale for dose selection of the TPH inhibitor rodatristat ethyl in ELEVATE-2: a phase 2b study in pulmonary arterial hypertension [abstract] Am J Respir Crit Care Med. 2021;2003:A3604. [Google Scholar]