Abstract
Human T-lymphotropic viruses 1 and 2 (HTLV-1 and HTLV-2) are retroviruses that originated on the African continent and dispersed throughout other continents through human migratory flows. This study describes the prevalence of HTLV-1 and HTLV-2 infection in residents of 11 quilombo remnant communities in the state of Pará, Brazil, and the associated risk factors. A total of 859 individuals (334 men and 525 women), aged between 7 and 91 years, participated in the study. All subjects answered a questionnaire with questions on sociodemographic characteristics and on risk factors associated with HTLV infection, and blood samples were collected and separated into plasma and leukocytes. An immunoenzymatic assay (ELISA; Murex HTLV-I+II, DiaSorin, Dartford, UK) was used as a screening test, and positive samples were subjected to line immunoassay confirmatory tests (Inno-LIA HTLV I/II Score FUJIREBIO) and DNA extraction for subsequent real-time PCR to differentiate the viral type. Four of the 859 individuals were seropositive for HTLV. HTLV-1 infection was confirmed in one individual from the Itamoari community (0.92%), and HTLV-2 infection was confirmed in two individuals from São Benedito (3.17%) and in one individual from Arimandeua (2.22%). Blood transfusion was the only risk factor associated with HTLV infection in this study. This study reports the occurrence of HTLV-1 and HTLV-2 in quilombo remnant communities in the state of Pará. Considering the African origin of the virus and its introduction into Brazil from the slave trade, the continued evaluation of quilombola communities in the state of Pará is essential to better characterize the distribution of infections in these populations and to create public health policies for the control of the spread of the virus and associated diseases.
Keywords: HTLV-1/2, Amazon, quilombos, epidemiology, vulnerable population
Introduction
Human T-lymphotropic viruses 1 (HTLV-1) and 2 (HTLV-2) were the first isolated retroviruses to infect humans (1, 2). They are viruses with known tropism for T lymphocytes and belong to the family Retroviridae, subfamily Orthoretrovirinae, and genus Deltaretrovirus (3–5). Evidence suggests an African origin for both viruses from independent events of interspecies transmission of the simian T-lymphotropic virus (STLV-1 and STLV-2) from non-human primates to humans (6–8). HTLV-1 has been associated with several diseases, particularly a neoplasm, i.e., adult T cell leukemia/lymphoma (ATLL), and an inflammatory neurodegenerative disease, i.e., HTLV-1-associated myelopathy (HAM) (9–11). In addition, HTLV-1 is also associated with ocular (uveitis) (12, 13), rheumatologic (14) and pulmonary (15, 16) diseases, among other pathologies (17–19). HTLV-2, in turn, does not have clinical manifestations necessarily associated with infection; however, clinical symptoms have been described in some patients (20, 21).
The main forms of HTLV-1/2 transmission are unprotected sex (22, 23), blood transfusion (24), sharing syringes and needles (25), and vertical transmission, in particular, prolonged breastfeeding (26, 27). HTLV-1 is widely distributed in geographic areas such as sub-Saharan Africa, Japan, Melanesia, the Caribbean, and South America (28–31). HTLV-2 has a more restricted distribution to pygmy populations in Africa and to indigenous peoples of the Americas, especially in the Brazilian Amazon region (7, 8, 32–36).
The entry of HTLV-1 into Brazil must have occurred along the east coast of the country, between the 16th and 19th centuries, through the African slave trade (37, 38). HTLV-2 may have followed the oldest human migratory flows that occurred thousands of years ago from the Asian continent via the Bering Strait, reaching North America and moving toward South America and, thus, Brazil (35, 37, 39). In Brazil, the viruses are distributed throughout the territory, and the main foci of infection are found in the North and Northeast regions (40). In the North region of the country, in the state of Pará, HTLV-1, and HTLV-2 have been described in several population strata, including urban, rural, and riverine populations, blood donors, and pregnant women, in addition to the presence of coinfections in people living with HIV (PLHIV), intravenous drug users, and isolated populations such as indigenous and quilombola peoples (25, 41–47). During the colonial period in Brazil, enslaved blacks in search of freedom fled coffee and sugarcane farms to the forest interior, where they formed isolated societies called quilombos. In the state of Pará, there are indications that quilombos formed in the 18th century, of which many were formed on the banks of rivers, such as the Gurupi, Tocantins, and Trombetas rivers (48). Many quilombo remnant communities lack basic sanitation, which promotes the transmission of diseases and fosters precarious health conditions; thus, this is one of the most vulnerable populations in Brazil (49–51). Infections by HTLV-1 and HTLV-2 have already been described in quilombo remnant communities in the Marajó Archipelago (43), but there are no studies on these viruses in other quilombos in the state of Pará. Thus, the aim of this study was to describe the prevalence of HTLV-1 and HTLV-2 and the main risk factors in 11 quilombola communities in the state of Pará.
Materials and Methods
Study Population and Sample Collection
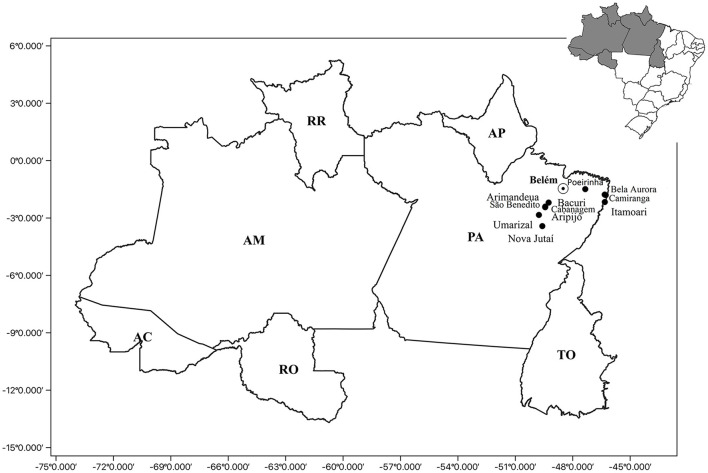
A total of 11 quilombola communities located in the state of Pará (northern Brazil) were visited from September 2020 to April 2021, and information was collected from 859 individuals. The following communities were studied: Poeirinha (n = 20), Umarizal Beira (n = 303), Arimandeua (n = 45), Aripijó (n = 31), Bacuri (n = 10), Cabanagem (n = 17), São Benedito (n = 63), Bela Aurora (n = 35), Camiranga (n = 89), Itamoari (n = 109), and Nova Jutaí (n = 137) (Figure 1).
Figure 1.
Geographic location of the quilombolas communities: Poeirinha, municipality of Bonito; Bela Aurora, Camiranga and Itamoari, municipality of Cachoeira do Piriá; Arimandeua, Aripijó Bacuri, Cabanagem, and São Benedito, municipality of Cametá; Umarizal Beira, municipality of Baião; and Nova Jutaí, municipality of Breu Branco. PA, Para State; Belém, capital of the Para State; AP, Amapá State; TO, Tocantins State; RR, Roramia State; AM, Amazonas State; AC, Acre State; RO, Rondônia State.
All study participants signed an informed consent form and answered a questionnaire with questions about sociodemographic aspects (age, sex, marital status, education, and family income) and behavioral risk factors for exposure to HTLV (“tattoos,” “use of illicit drugs,” “blood transfusion,” “piercings,” “sexually active,” “use of condoms,” and “previous diagnosis of STI,” among others). Individuals under 18 years old signed an informed assent form, and their legal guardians signed an informed consent form.
From all participants, peripheral blood samples (4 mL) were collected in vacuum tubes containing ethylenediaminetetraacetic acid (EDTA) as an anticoagulant. The samples were then subjected to centrifugation to separate the plasma and leukocyte fractions, followed by storage at −20°C until use.
Ethical Aspects
This study was approved by the National Research Ethics Committee (CONEP, acronym in Portuguese) (CAAE: 27290619.2.0000.0018), in compliance with resolution 466/12 of the Ministry of Health, which regulates research involving human beings. All subjects who voluntarily agreed to participate in the study signed an informed consent form.
Serological Analysis
Immunoenzymatic assays (Murex HTLV-I+II, DiaSorin, Dartford, UK) were used to detect anti-HTLV-1/2 antibodies in the tested samples following the manufacturer's instructions. Samples with positive results were subjected to line immunoassays (INNO-LIA® HTLV I/II Score, Fujirebio, Japan) and real-time PCR (Applied Biosystems Step One Plus Real Time PCR) to confirm infection and differentiate the viral type, respectively.
Real-Time PCR
To perform real-time PCR, DNA was extracted from the seropositive samples using a QiaAmp DNA mini kit (Qiagen, Germany) following the manufacturer's protocol. Molecular confirmation of infection was performed using a multiplex real-time PCR for three target sequences: the albumin gene (141 bp), as an endogenous control; the pol gene (186 bp) of HTLV-1; and the tax-2 gene (75 bp) of HTLV-2. The primer sequences used were 5′-ccctacaatccaaccagctcag-3′ (HTLV-1F), 5′-gtggtgaagctgccatcgggtttt-3′ (HTLV-1R), 5′-cgattgtgtacaggccgattg-3′ (HTLV-2F), 5′-caggagggcatgtcgatgtag-3′ (HTLV-2R), 5′-gctgtcatctcttgtgggctgt-3′ (F Albumin), 5′-aaactcatgggagctgctggtt-3′ (R Albumin); the probe sequences used were JOE-5′-ctttactgacaaacccgacctacccatgga-3′-BHQ (HTLV-1), FAM-5′-tgtcccgtctcaggtggtctatgttcca-3′-MGB (HTLV-2), and NED-5′-cctgtcatgcccacacaaatctc-3′-MGB (albumin). The reaction components, based on the TaqMan® Universal system, were as follows: 12.5 μL of Master Mix, 6.0 μL of water, 1.0 μL of Assay-by-Design (primer and probe set) and 0.5 μL of DNA in a final volume of 20 μL. For cycling, the protocol was as follows: 50°C for 2 min; 95°C for 10 min; and 50 cycles of 90°C for 50 s and 60°C for 1 min.
Statistical Analyses
The collected information was stored in a database using Epi-Info7.2 software. The socioeconomic aspects, behavioral factors, and risk factors for exposure to HTLV-1 and HTLV-2 are described as frequencies and percentages. The confidence interval (95% CI) was used to estimate overall and community prevalence. Fisher's exact test was used to evaluate the association of behavioral risk factors with the presence/absence of HTLV-1 or HTLV-2. The significance level adopted was 5%. Statistical analyses were performed using the programs BioEstat 5.3, GraphPad Prism 7.0 for Windows, and MINITAB Release 14 for Windows.
Results
The median age of the individuals was 39 years (IIQ: 32); 61.1% (n = 525) of the individuals were women, and 38.9% (n = 334) were men. A total of 49.9% (n = 429) of the individuals said they were married/living with a partner. Most of these individuals declared themselves black (60.5%; n = 520) and brown (33.5%; n = 288). Regarding education, 40.6% (n = 349) were literate, 16.5% (142) had completed elementary school, and 28.4% (n = 244) had completed secondary education. A total of 46.6% (n = 400) live on <1 (1) minimum wage, and 47.1% (n = 405) live on one (2) to three (3) minimum wages (Table 1).
Table 1.
Sociodemographic profile of the study participants.
| Sociodemographic variable | N = 859 | % |
|---|---|---|
| Sex | ||
| Female | 525 | 61.1 |
| Male | 334 | 38.9 |
| Color | ||
| Yellow | 9 | 1.0 |
| White | 42 | 4.9 |
| Black | 520 | 60.5 |
| Brown | 288 | 33.5 |
| Marital status | ||
| Married/living with partner | 429 | 49.9 |
| Separated | 59 | 6.9 |
| Single | 311 | 36.2 |
| Widowed | 41 | 4.8 |
| Not reported | 19 | 2.2 |
| Education | ||
| Illiterate | 22 | 2.6 |
| Literate | 349 | 40.6 |
| Elementary education | 142 | 16.5 |
| Secondary education | 244 | 28.4 |
| Higher education | 63 | 7.3 |
| Graduate | 15 | 1.7 |
| Not reported | 24 | 2.8 |
| Income | ||
| <1 | 400 | 46.6 |
| 1–3 | 405 | 47.1 |
| >3 | 7 | 0.8 |
| Not reported | 47 | 5.5 |
| Age (years) | ||
| 07–11 | 45 | 5.2 |
| 12–18 | 108 | 12.6 |
| 19–29 | 141 | 16.4 |
| 30–59 | 424 | 49.4 |
| 60 or 60+ | 136 | 15.8 |
| Not reported | 5 | 0.6 |
HTLV-1/2 infection was detected in four individuals (0.4%, 95% CI: 0.13–1.19). Table 2 provides the distribution of infection by community, with 3.2% (2/63) of those tested in São Benedito (95% CI: 0.38–11) and 2.2% (1/45) of those tested in Arimandeua (95% CI: 0.05–11) being positive for HTLV-2 infection and 0.9% (1/109) of those tested in Itamoari (95% CI: 0.02–5.00) being positive for HTLV-1 infection; all of the communities are located in the northeastern region of the state of Pará. HTLV-1 infection was identified in a young, 24-year-old male. Two males and one female were diagnosed with HTLV-2; all three were over 60 years of age.
Table 2.
Prevalence of HTLV-1 and HTLV-2 in the communities based on the tests used.
| Population | N | ELISA | Inno-Lia | Real-time PCR | ||
|---|---|---|---|---|---|---|
| HTLV-1 (%) | HTLV-2 (%) | HTLV-1 (%) | HTLV-2 (%) | |||
| Baião | ||||||
| Umarizal Beira | 303 | 00 | – | – | – | – |
| Breu Branco | ||||||
| Nova Jutaí | 137 | 00 | – | – | – | – |
| Bonito | ||||||
| Poeirinha | 20 | 00 | – | – | – | – |
| Cachoeira do Piriá | ||||||
| Bela Aurora | 35 | 00 | – | – | – | – |
| Camiranga | 89 | 00 | – | – | – | – |
| Itamoari | 109 | 01 | 01 (0.9) | – | 01 (0.9) | – |
| Cametá | ||||||
| Arimandeua | 45 | 01 | – | 01 (2.2) | – | 01 (2.2) |
| Aripijó | 31 | 00 | – | – | – | – |
| Bacuri | 10 | 00 | – | – | – | – |
| Cabanagem | 17 | 00 | – | – | – | – |
| São Benedito | 63 | 02 | – | 02 (3.2) | – | 02 (3.2) |
| Total | 859 | 04 | 01 (0.1) | 03 (0.3) | 01 (0.1) | 03 (0.3) |
Only the individual diagnosed with HTLV-1 infection reported having a tattoo (25%). Three individuals (75%) denied having used illicit drugs, and one did not report anything. All of them denied having piercings, a previous diagnosis of an STI, or having more than one sexual partner. Two (50%) of those infected reported having received blood transfusions. Half (2) of the diagnosed individuals were sexually active and used condoms during sexual intercourse. Three (75%) of the individuals were breastfed, and one individual did not know (Table 3). According to Fisher's exact test, there was a significant association between blood transfusion (p = 0.0176) and HTLV-1/2 infection, with no association with any of the other factors investigated (Table 4).
Table 3.
Sociodemographic and behavioral characteristics of individuals with confirmed HTLV-1 and HTLV-2 infection.
| Characteristics | Infected individuals | |||
|---|---|---|---|---|
| SBEN-002 | SBEN-019 | ARI-007B | ITM-040 | |
| Sex | F | M | M | M |
| Age | 64 | 67 | 67 | 23 |
| Color | Black | Black | Brown | Brown |
| Marital status | Single | Single | Married/lives with partner | Single |
| Education | IE | IE | IE | IE |
| Income (number of minimum wages) | 1 | >1 | 1 | >1 |
| Tattoo(s) | N | N | N | Y |
| Piercing(s) | N | N | N | N |
| Blood transfusion | Y | N | N | Y |
| Breastfed | Y | NR | Y | Y |
| Sexually active | N | N | Y | Y |
| Age at 1st sexual relationship | NR | NR | 18 | NR |
| Sex for money | N | N | N | NR |
| Condoms | N | N | Y | Y |
| Number of partners | NR | 1 | 1 | 1 |
| Diagnosis of an STI | N | N | N | N |
| Type of HTLV | HTLV-2 | HTLV-2 | HTLV-2 | HTLV-1 |
F, female; M, male; IE, incomplete elementary school; IM, incomplete middle school; N, no; Y, yes; NR, not reported.
Table 4.
Association of HTLV-1/2 infection with behavioral risk factors.
| Positive | Negative | Grand total | P | |
|---|---|---|---|---|
| n = 4 | n = 855 | n = 859 | ||
| N (%) | N (%) | N (%) | ||
| Tattoo(s) | ||||
| Yes | 1 (25) | 51 (6) | 52 (6.1) | |
| No | 3 (75) | 778 (91) | 781 (90.9) | 0.2276 |
| Not reported | 26 (3) | 26 (3) | ||
| Illicit drugs | ||||
| Yes | 0 (0) | 34 (4) | 34 (4) | |
| No | 3 (75) | 756 (88.4) | 759 (88.4) | 1.000 |
| Not reported | 1 (25) | 65 (7.6) | 66 (7.7) | |
| Blood transfusion | ||||
| Yes | 2 (50) | 44 (5.1) | 46 (5.4) | |
| No | 2 (50) | 763 (89.2) | 765 (89.1) | 0.0176 |
| Not informed | 48 (5.6) | 48 (5.6) | ||
| Piercing(s) | ||||
| Yes | 0 (0) | 21 (2.5) | 21 (2.4) | |
| No | 4 (100) | 781 (91.3) | 785 (91.4) | 1.000 |
| Not reported | 53 (6.2) | 53 (6.2) | ||
| Breastfed | ||||
| Yes | 3 (75) | 774 (90.5) | 777 (90.5) | |
| No | 0 (0) | 27 (3.2) | 27 (3.1) | 1.000 |
| Not reported | 1 (25) | 54 (6.3) | 55 (6.4) | |
| Sexually active | ||||
| Yes | 2 (50) | 637 (78.6) | 639 (78.5) | |
| No | 2 (50) | 159 (19.6) | 161 (19.8) | 0.1823 |
| Not reported | 0 (0) | 14 (1.7) | 14 (1.7) | |
| Number of sexual partners (week) | ||||
| 1 | 2 (100) | 532 (83.5) | 534 (83.6) | |
| ≥2 | 0 (0) | 23 (3.6) | 23 (3.6) | 0.1000 |
| Not reported | 82 (2.9) | 82 (2.8) | ||
| Condoms | ||||
| Yes | 2 (50) | 262 (32.3) | 264 (32.4) | |
| No | 2 (50) | 459 (56.7) | 461 (56.6) | 0.6279 |
| Not reported | 0 (0) | 89 (11) | 89 (10.9) | |
| Diagnosis of an STI | ||||
| Yes | 0 (0) | 32 (3.7) | 32 (3.7) | |
| No | 4 (100) | 677 (79.2) | 681 (79.3) | 1.000 |
| Does not know | 0 (0) | 34 (4) | 34 (4) | |
| Not reported | 0 (0) | 112 (13.1) | 112 (13) | |
Discussion
Herein, we report the occurrence of anti-HTLV-1/2 antibodies in four of the 859 individuals tested, with confirmed HTLV-1 infection in only one resident of the Itamoari community and confirmed HTLV-2 infection in two communities—Arimandeua and São Benedito. This is the first time that HTLV-1 and HTLV-2 have been identified in these communities, and no previous studies have been conducted in these communities.
Vallinoto et al. (43) found that the prevalence rate of HTLV-1 in quilombolas of the Marajó Archipelago (Pará) ranged from 1.0% in the Ponta de Pedras community to 2.06% in Santana do Arari; these numbers are close to those found in this study. Regarding the prevalence of HTLV-2, the rates found in this study were also similar to those described in Santana do Arari (1.06%). Regarding other Afro-descendant populations studied in Brazil, the prevalence of HTLV-1 in the present study was similar to that found in quilombo remnant communities in Central Brazil (0.5%) (52).
The prevalence of HTLV-2 in the present study was lower than that reported by other studies involving traditional populations of Pará, such as indigenous populations. The prevalence in three Kayapó villages in the Xikrin territory was 29%, ranging from 21 to 38% among the villages (41). In turn, the prevalence data for this study are consistent with the data for the population of Belém, the capital of Pará, and for blood donors in this same state (42, 44).
Itamoari is a community located on the Pará side of the Gurupi River. The Gurupi River and the Turiaçu River (State of Maranhão) form a region called Turiaçu. The port in this region served as an entrance for a large number of slave through illegal slave trade during the 19th century (48). In this sense, the occurrence of HTLV-1 in the Itamoari community may be related to the African origin of the virus, and its entry into Brazil may have been by the slave trade from the African continent, considering that cases occur in isolated communities.
There are historical reports (48) that slaves sometimes found indigenous tribes during their escape into forests and lived with residents in locations highly endemic for HTLV-2 (48). Thus, the detection of HTLV-2 in São Benedito and Arimandeua can be attributed to the contact between indigenous populations and slaves.
History of an STI, multiple sexual partners, and non-use of condoms are risk factors that have been associated with a higher risk of HTLV-1/2 infection; however, in the present study, no associations were found (23, 25, 53). Nevertheless, two individuals infected with HTLV-2 reported not using condoms because they were no longer sexually active, leading to the hypothesis or inference that they did not use condoms in previous relationships. None of the four infected had any history of an STI or reported having more than one sexual partner.
Blood transfusion was a risk factor for HTLV-1/2 infection in this study as well as in other studies (54, 55). In November 1993, the Brazilian Ministry of Health filed Ordinance No. 1,376, through which blood donation candidates are subjected to clinical screening, including HTLV screening, to ensure their safety and that of recipients (56). Of the two individuals who reported receiving blood transfusions, the resident of São Benedito reported that this procedure was performed in 1994, while the other did not provide the date on which the transfusion was received. Because clinical screening was established shortly before the woman with HTLV-2 was transfused, there is no certainty as to whether she received screened blood, and thus, the transfusion may have been a source of infection. However, the association with blood transfusion could be a spurious result if we consider the small number of HTLV-1/2 positive subjects found in the present study, which could be considered a limitation of our study.
All three HTLV-2-infected patients have one surname in common; thus, it is possible that there is some degree of kinship between them due to the isolation of these communities. In addition, the 67-year-old woman who was infected reported that she had 11 pregnancies and breastfed all of her children for more than 6 months. However, her children could not be found to be tested, and thus, it is not known whether there was vertical transmission of the virus or if her infection occurred after her pregnancies. A new visit to the community is being arranged to investigate intrafamily transmission.
Although most of those infected with HTLV-1 are asymptomatic, this virus is associated with clinical manifestations that can lead to severe symptoms and death, and there is no cure or treatment available for the infection, which is neglected, not only in Brazil but also in several regions of the world (57). Therefore, HTLV-1/2 infection is a public health problem, especially in vulnerable populations (40, 57).
Although many Brazilian population segments are in a situation of vulnerability, there is a higher prevalence of black populations among the vulnerable populations, including those living in quilombo remnant communities (49, 58). These populations are vulnerable because of health determinants of individual control (behaviors) and collective control (low health conditions and poor infrastructure). Thus, measures such as (i) providing prenatal screening, (ii) providing screening for infection in different quilombola communities, (iii) performing confirmatory tests, (iv) increasing the awareness of the population about the virus and its modes of transmission, (v) encouraging the use of condoms, and (vi) providing counseling for infected pregnant women about the risk of transmission to the child through breastfeeding are means that can mitigate the transmission of HTLV in these communities; these actions promote health promotion/prevention from the perspective of comprehensive care, which is in line with the national policy of comprehensive health for the black population (59).
Conclusions
Considering the occurrence of HTLV-1/2 infection in populations associated with the African origin of the virus and its introduction into Brazil from the slave trade, the continuous evaluation of quilombola communities in the state of Pará and other locations in the Brazilian Amazon is needed to better characterize the distribution (prevalence) of infection in these populations to better formulate public health policies to control the spread of the virus and associated diseases.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Human Research Ethics Committee of the Health Sciences Institute of the Federal University of Pará (CAAE: 55699316.6.0000.0018). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
AV, JG, ES, IC, and GC-C conceived and designed the study. WB, LR, KP, FL, IA, CL, and AL assisted in subject recruitment and sample collection. BS, FL, WB, IA, and CL performed the serological and molecular analyses. SL and WB performed statistical analyses. WB, GC-C, IC, and AV wrote the manuscript. All authors read and approved the final manuscript.
Funding
This study was supported by the National Council for Scientific and Technological Development (CNPq; # 301869/2017-0; 442522/2019-3) and the Federal University of Pará(PAPQ-2022).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank all the individuals who agreed to participate in this study.
References
- 1.Poiesz BJ, Ruscetti FW, Gazdar AF, Bunn PA, Minna JD, Gallo RC. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci U S A. (1980) 77:7415–9. 10.1073/pnas.77.12.7415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kalyanaraman VS, Sarngadharan MG, Robert-Guroff M, Miyoshi I, Golde D, Gallo RC. A new subtype of human T-cell leukemia virus (HTLV-II) associated with a T-cell variant of hairy cell leukemia. Science. (1982) 218:571–3. 10.1126/science.6981847 [DOI] [PubMed] [Google Scholar]
- 3.Ijichi S, Ramundo MB, Takahashi H, Hall WW. In vivo cellular tropism of human T cell leukemia virus type II (HTLV-II). J Exp Med. (1992) 176:293–6. 10.1084/jem.176.1.293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Richardson JH, Edwards AJ, Cruickshank JK, Rudge P, Dalgleish AG. In vivo cellular tropism of human T-cell leukemia virus type 1. J Virol. (1990) 64:5682–7. 10.1128/jvi.64.11.5682-5687.1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.International Committee on Taxonomy of Viruses . Taxonomy History (ICTV): Primate T-Lymphotropic Virus 1 (2019). Available online at: https://talk.ictvonline.org/taxonomy/p/taxonomy-history?taxnode_id=202004999 (acessed October 18, 2019).
- 6.Koralnik IJ, Boeri E, Saxinger WC, Monico AL, Fullen J, Gessain A, et al. Phylogenetic associations of human and simian T-cell leukemia/lymphotropic virus type I strains: evidence for interspecies transmission. J Virol. (1994) 68:2693–707. 10.1128/jvi.68.4.2693-2707.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gessain A, Mauclère P, Froment A, Biglione M, Le Hesran JY, Tekaia F, et al. Isolation and molecular characterization of a human T-cell lymphotropic virus type II (HTLV-II), subtype B, from a healthy Pygmy living in a remote area of Cameroon: an ancient origin for HTLV-II in Africa. Proc Natl Acad Sci U S A. (1995) 92:4041–5. 10.1073/pnas.92.9.4041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vandamme AM, Salemi M, Van Brussel M, Liu HF, Van Laethem K, Van Ranst M, et al. African origin of human T-lymphotropic virus type 2 (HTLV-2) supported by a potential new HTLV-2d subtype in Congolese Bambuti Efe Pygmies. J Virol. (1998) 72:4327–40. 10.1128/JVI.72.5.4327-4340.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoshida M, Miyoshi I, Hinuma Y. Isolation and characterization of retrovirus from cell lines of human adult T-cell leukemia and its implication in the disease. Proc Natl Acad Sci U S A. (1982) 79:2031–5. 10.1073/pnas.79.6.2031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Osame M, Usuku K, Izumo S, Ijichi N, Amitani H, Igata A, et al. HTLV-I associated myelopathy, a new clinical entity. Lancet. (1986) 1:1031–2. 10.1016/S0140-6736(86)91298-5 [DOI] [PubMed] [Google Scholar]
- 11.Ijichi S, Eiraku N, Osame M, Izumo S, Kubota R, Maruyama I, et al. Activated T lymphocytes in cerebrospinal fluid of patients with HTLV-I-associated myelopathy (HAM/TSP). J Neuroimmunol. (1989) 25:251–4. 10.1016/0165-5728(89)90143-4 [DOI] [PubMed] [Google Scholar]
- 12.Chew R, Henderson T, Aujla J, Whist E, Einsiedel L. Turning a blind eye: HTLV-1-associated uveitis in Indigenous adults from Central Australia. Int Ophthalmol. (2018) 38:2159–62. 10.1007/s10792-017-0659-3 [DOI] [PubMed] [Google Scholar]
- 13.Mochizuki M, Watanabe T, Yamaguchi K, Tajima K, Yoshimura K, Nakashima S, et al. Uveitis associated with human T lymphotropic virus type I: seroepidemiologic, clinical, and virologic studies. J Infect Dis. (1992) 166:943–4. 10.1093/infdis/166.4.943 [DOI] [PubMed] [Google Scholar]
- 14.Nishioka K, Maruyama I, Sato K, Kitajima I, Nakajima Y, Osame M. Chronic inflammatory arthropathy associated with HTLV-I. Lancet. (1989) 1:441. 10.1016/S0140-6736(89)90038-X [DOI] [PubMed] [Google Scholar]
- 15.Einsiedel L, Chiong F, Jersmann H, Taylor GP. Human T-cell leukaemia virus type 1 associated pulmonary disease: clinical and pathological features of an under-recognised complication of HTLV-1 infection. Retrovirology. (2021) 18:5. 10.1186/s12977-020-00543-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Einsiedel L, Fernandes L, Spelman T, Steinfort D, Gotuzzo E. Bronchiectasis is associated with human T-lymphotropic virus 1 infection in an Indigenous Australian population. Clin Infect Dis. (2012) 54:43–50. 10.1093/cid/cir766 [DOI] [PubMed] [Google Scholar]
- 17.Schierhout G, McGregor S, Gessain A, Einsiedel L, Martinello M, Kaldor J. Association between HTLV-1 infection and adverse health outcomes: a systematic review and meta-analysis of epidemiological studies. Lancet Infect Dis. (2020) 20:133–43. 10.1016/S1473-3099(19)30402-5 [DOI] [PubMed] [Google Scholar]
- 18.Pinheiro SR, Lana-Peixoto MA, Proietti AB, Oréfice F, Lima-Martins MV, Proietti FA. HTLV-I associated uveitis, myelopathy, rheumatoid arthritis and Sjögren's syndrome. Arq Neuropsiquiatr. (1995) 53:777–81. 10.1590/S0004-282X1995000500011 [DOI] [PubMed] [Google Scholar]
- 19.Vernant JC, Buisson G, Magdeleine J, De Thore J, Jouannelle A, Neisson-Vernant C, et al. T-lymphocyte alveolitis, tropical spastic paresis, and Sjögren syndrome. Lancet. (1988) 1:177. 10.1016/S0140-6736(88)92744-4 [DOI] [PubMed] [Google Scholar]
- 20.Hjelle B, Appenzeller O, Mills R, Alexander S, Torrez-Martinez N, et al. Chronic neurodegenerative disease associated with HTLV-II infection. Lancet. (1992) 339:645–6. 10.1016/0140-6736(92)90797-7 [DOI] [PubMed] [Google Scholar]
- 21.Jacobson S, Lehky T, Nishimura M, Robinson S, McFarlin DE, Dhib-Jalbut S. Isolation of HTLV-II from a patient with chronic, progressive neurological disease clinically indistinguishable from HTLV-I-associated myelopathy/tropical spastic paraparesis. Ann Neurol. (1993) 33:392–6. 10.1002/ana.410330411 [DOI] [PubMed] [Google Scholar]
- 22.Paiva A, Casseb J. Sexual transmission of human T-cell lymphotropic virus type 1. Rev Soc Bras Med Trop. (2014) 47:265–74. 10.1590/0037-8682-0232-2013 [DOI] [PubMed] [Google Scholar]
- 23.Murphy EL, Figueroa JP, Gibbs WN, Brathwaite A, Holding-Cobham M, Waters D, et al. Sexual transmission of human T-lymphotropic virus type I (HTLV-I). Ann Intern Med. (1989) 111:555–60. 10.7326/0003-4819-111-7-555 [DOI] [PubMed] [Google Scholar]
- 24.Okochi K, Sato H, Hinuma Y. A retrospective study on transmission of adult T cell leukemia virus by blood transfusion: seroconversion in recipients. Vox Sang. (1984) 46:245–53. 10.1159/000466190 [DOI] [PubMed] [Google Scholar]
- 25.Oliveira-Filho AB, Araújo APS, Souza APC, Gomes CM, Silva-Oliveira GC, Martins LC, et al. Human T-lymphotropic virus 1 and 2 among people who used illicit drugs in the state of Pará, northern Brazil. Sci Rep. (2019) 9:14750. 10.1038/s41598-019-51383-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ando Y, Nakano S, Saito K, Shimamoto I, Ichijo M, Toyama T, et al. Transmission of adult T-cell leukemia retrovirus (HTLV-I) from mother to child: comparison of bottle- with breast-fed babies. Jpn J Cancer Res. (1987) 78:322–4. [PubMed] [Google Scholar]
- 27.Wiktor SZ, Pate EJ, Rosenberg PS, Barnett M, Palmer P, Medeiros D, et al. Mother-to-child transmission of human T-cell lymphotropic virus type I associated with prolonged breast-feeding. J Hum Virol. (1997) 1:37–44. [PubMed] [Google Scholar]
- 28.Gessain A, Cassar O. Epidemiological aspects and world distribution of HTLV-1 infection. Front Microbiol. (2012) 3:388. 10.3389/fmicb.2012.00388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Einsiedel LJ, Pham H, Woodman RJ, Pepperill C, Taylor KA. The prevalence and clinical associations of HTLV-1 infection in a remote Indigenous community. Med J Aust. (2016) 205:305–9. 10.5694/mja16.00285 [DOI] [PubMed] [Google Scholar]
- 30.Sagara Y, Iwanaga M, Morita M, Sagara Y, Nakamura H, Hirayama H, et al. Fine-scale geographic clustering pattern of human T-cell leukemia virus type 1 infection among blood donors in Kyushu-Okinawa, Japan. J Med Virol. (2018) 90:1658–65. 10.1002/jmv.25239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramassamy JL, Cassar O, Toumbiri M, Diané A, Idam Mamimandjiami A, Bengone C, et al. High prevalence of human T-cell leukemia virus type-1b genotype among blood donors in Gabon, Central Africa. Transfusion. (2020) 60:1483–91. 10.1111/trf.15838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mauclère P, Afonso PV, Meertens L, Plancoulaine S, Calattini S, Froment A, et al. HTLV-2B strains, similar to those found in several Amerindian tribes, are endemic in central African Bakola Pygmies. J Infect Dis. (2011) 203:1316–23. 10.1093/infdis/jir031 [DOI] [PubMed] [Google Scholar]
- 33.Leon-Ponte M, Noya O, Bianco N, Echeverría de Perez G. Highly endemic human T-lymphotropic virus type II (HTLV-II) infection in a Venezuelan Guahibo Amerindian group. J Acquir Immune Defic Syndr Hum Retrovirol. (1996) 13:281–6. 10.1097/00042560-199611010-00011 [DOI] [PubMed] [Google Scholar]
- 34.Lowis GW, Sheremata WA, Wickman PR, Dube S, Dube K, Poiesz BJ. HTLV-II riskfactors in Native Americans in Florida. Neuroepidemiology. (1999) 18:37–47. 10.1159/000026194 [DOI] [PubMed] [Google Scholar]
- 35.Vallinoto AC, Ishak MO, Azevedo VN, Vicente AC, Otsuki K, Hall WW, et al. Molecular epidemiology of human T-lymphotropic virus type II infection in Amerindian and urban populations of the Amazon region of Brazil. Hum Biol. (2002) 74:633–44. 10.1353/hub.2002.0059 [DOI] [PubMed] [Google Scholar]
- 36.Ishak R, Harrington WJ Jr, Azevedo VN, Eiraku N, Ishak MO, Guerreiro JF, et al. Identification of human T cell lymphotropic virus type IIa infection in the Kayapo, an indigenous population of Brazil. AIDS Res Hum Retroviruses. (1995) 11:813–21. 10.1089/aid.1995.11.813 [DOI] [PubMed] [Google Scholar]
- 37.Ishak R, Guimarães Ishak MO, Azevedo VN, Machado LFA, Vallinoto IMC, Queiroz MAF et al. HTLV in South America: origins of a silent ancient human infection. Virus Evol. (2020) 6:veaa053. 10.1093/ve/veaa053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aleluia MM, Mello MAG, Alcântara LCJ, Rego FFA, Santos LPS, Galvão-Castro B, et al. The origin of HTLV-1 in southern Bahia by phylogenetic, mtDNA and β-globin analysis. Virol Rep. (2015) 5:63–74. 10.1016/j.virep.2015.05.002 [DOI] [Google Scholar]
- 39.Switzer WM, Black FL, Pieniazek D, Biggar RJ, Lal RB, Heneine W. Endemicity and phylogeny of the human T cell lymphotropic virus type II subtype A from the Kayapo Indians of Brazil: evidence for limited regional dissemination. AIDS Res Hum Retroviruses. (1996) 12:635–40. 10.1089/aid.1996.12.635 [DOI] [PubMed] [Google Scholar]
- 40.Brasil . Prevalência da Infecção por HTLV-1/2no Brasil. Brasília: Ministério da Saúde. Boletim Epidemiológico. Secretaria de Vigilância em Saúde (2020). [Google Scholar]
- 41.Braço ILJ, de Sá KSG, Waqasi M, Queiroz MAF, da Silva ANR, Cayres-Vallinoto IMV, et al. High prevalence of human T-lymphotropic virus 2 (HTLV-2) infection in villages of the Xikrin tribe (Kayapo), Brazilian Amazon region. BMC Infect Dis. (2019) 19:459. 10.1186/s12879-019-4041-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Catalan-Soares B, Carneiro-Proietti AB, de F, Proietti FA. Heterogeneous geographic distribution of human T-cell lymphotropic viruses I andII (HTLV-I/II): serological screening prevalence rates in blood donorsfrom large urban areas in Brazil. Cad Saude Publica. (2005) 21:926–31. 10.1590/S0102-311X2005000300027 [DOI] [PubMed] [Google Scholar]
- 43.Vallinoto ACR, Pontes GS, Muto NS, Lopes IGL, Machado LFA, Azevedo VN, et al. Identification of human T-cell lymphotropic virus infection in a semi-isolated Afro-Brazilianquilombo located in the Marajó Island (Pará, Brazil). Mem Inst Oswaldo Cruz. (2006) 101:103–5. 10.1590/S0074-02762006000100020 [DOI] [PubMed] [Google Scholar]
- 44.Silva IC, Pinheiro BT, Nobre AFS, Coelho JL, Pereira CCC, Ferreira LSC, et al. Moderate endemicity of the human T-lymphotropic virus infection in the metropolitan region of Belém, Pará, Brazil. Rev Bras Epidemiol. (2018) 21:e180018. 10.1590/1980-549720180018 [DOI] [PubMed] [Google Scholar]
- 45.Guerra AB, Siravenha LQ, Laurentino RV, Feitosa RNM, Azevedo VN, Vallinoto ACR, et al. Seroprevalence of HIV, HTLV, CMV, HBV and rubella virus infections in pregnant adolescents who received care in the city of Belém, Pará, Northern Brazil. BMC Pregnan Childbirth. (2018) 18:169. 10.1186/s12884-018-1753-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ferreira LSC, Costa JHG, Costa CA, Melo MFC, Andrade ML, Martins LC, et al. Soroprevalência do vírus linfotrópico de células T humanas em comunidades ribeirinhas da região nordeste do Estado do Pará, Brasil. Rev Pan-Amaz Saude. (2010) 1:103–8. 10.5123/S2176-62232010000300014 [DOI] [Google Scholar]
- 47.Alencar SP, Souza MC, Fonseca RRS, Menezes CR, Azevedo VN, Ribeiro ALR, et al. Prevalence and molecular epidemiology of human T-lymphotropic virus (HTLV) infection in people living with HIV/AIDS in the Pará State, Amazon Region of Brazil. Front Microbiol. (2020) 11:572381. 10.3389/fmicb.2020.572381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Salles C. O Negro no Pará: Sob o Regime da Escravidão. Rio de Janeiro: Fundação Getúlio Vargas, Belém: UFPA; (1971). 336 p. [Google Scholar]
- 49.Santos RP, França SAS, Arede ANF, Ramos EMLS. Condições Habitacionais e de Saúde da Comunidade Remanescente de Quilombo Mangueiras, Ilha Do Marajó, Pará, Brasil. RESMA. (2020) 10:43–59. Available online at: https://periodicos.ufms.br/index.php/sameamb/article/view/9145 [Google Scholar]
- 50.Melo MFT, Silva HP. Doenças Crônicas e os Determinantes Sociais da Saúde em Comunidades Quilombolas do Pará, Amazônia, Brasil. Revista ABPN. (2015) 7:168–89. Available online at: http://www.abpnrevista.org.br/revista/index.php/revistaabpn1/article/view/103 [Google Scholar]
- 51.Tavares RB, Silva HP. Educação Em Saúde E Ambiente Em Comunidades Quilombolas Do Pará/Brasil. Margens. (2016) 8:131–44. 10.18542/rmi.v8i11.3246 [DOI] [Google Scholar]
- 52.Nascimento LB, Carneiro MAS, Teles SA, Lopes CLR, Reis RS, et al. Prevalência da infecção pelo HTLV-1, em remanescentes de quilombos no Brasil Central. Rev Soc Bras Med Trop. (2009) 42:657–60. 10.1590/S0037-86822009000600009 [DOI] [PubMed] [Google Scholar]
- 53.Gotuzzo E, Sánchez J, Escamilla J, Carrillo C, Phillips IA, Moreyra L, et al. Human T cell lymphotropic virus type I infection among female sex workers in Peru. J Infect Dis. (1994) 169:754–9. 10.1093/infdis/169.4.754 [DOI] [PubMed] [Google Scholar]
- 54.Hedayati-Moghaddam MR, Tehranian F, Bayati M. Human T-Lymphotropic Virus Type I (HTLV-1) infection among Iranian blood donors: first case-control study on the risk factors. Viruses. (2015) 7:5736–45. 10.3390/v7112904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Santos RF, Conceição GC, Martins MS, Kraychete A, Penalva MA, Carvalho EM, et al. Prevalence and risk factors for Human T-Lymphotropic Virus Type 1 (HTLV-1) among maintenance hemodialysis patients. BMC Nephrol. (2017) 18:64. 10.1186/s12882-017-0484-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brasil . Portaria 1376 de 19/11/1993. Brasília: Ministério da Saúde; (1993). [Google Scholar]
- 57.Rosadas C, Menezes MLB, Galvão-Castro B, Assone T, Miranda AE, Aragón MG, et al. Blocking HTLV-1/2 silent transmission in Brazil: Current public health policies and proposal for additional strategies. PLoS Negl Trop Dis. (2021) 15:e0009717. 10.1371/journal.pntd.0009717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mota AN, Maciel ES, Quaresma FRP, Araújo FA, Sousa LVA, et al. Um olhar para a vulnerabilidade: análise da ausência de acesso à saúde pelos quilombolas no Brasil. J Hum Growth. (2021) 31:302–9. 10.36311/jhgd.v31.11404 [DOI] [Google Scholar]
- 59.Brasil . Política Nacional de Saúde Integral da População Negra: Uma Política do SUS. Brasília: Ministério da Saúde; (2009). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.