Abstract
Surgical septal myectomy is the preferred treatment option for patients with medically intractable obstructive hypertrophic cardiomyopathy. Extended transaortic septal myectomy is a widely performed surgical procedure for patients with subaortic obstruction. The transapical approach may provide an alternative surgical option in less common phenotypes, such as apical hypertrophy or long-segmental septal hypertrophy. In this report, we describe a case of a procedure performed to achieve left ventricular enlargement procedure using a combined transaortic and transapical dual approach in a patient with diffuse-type hypertrophic cardiomyopathy with apical aneurysm and mid-cavity obstruction.
Keywords: Hypertrophic obstructive cardiomyopathy, Left heart ventricle, Case report
Case report
A 73-year-old man with a history of diabetes mellitus, hypertension, and asthma was hospitalized for aggravating dyspnea. The patient suffered from resting chest pain and New York Heart Association (NYHA) functional class IV dyspnea since 6 months before his visit. Electrocardiography showed atrial fibrillation with a slow ventricular response. Echocardiography revealed diffuse left ventricular (LV) wall thickening with flow accelerations at the LV mid-cavity (Fig. 1A). The peak velocity of mid-ventricular flow acceleration was 2.7–3.1 m/sec, suggesting mid-LV-cavity obliteration. Mild thickening of the mitral leaflets and posterior annular calcification were observed, but there was no definite septal contact or systolic anterior motion of the anterior mitral leaflet. Mitral regurgitation was trivial and the sub-valvular apparatus showed no significant abnormality. The coronary angiogram was near-normal; therefore, the aneurysmal change of LV apex implied a burn-out state of the LV myocardium. Preoperative computed tomography showed diffuse-type hypertrophic cardiomyopathy (HCMP) with a small LV cavity and obliteration during the systolic phase (Fig. 2). Despite appropriate medical therapy, intractable heart failure persisted with atrial fibrillation.
Fig. 1.
Preoperative echocardiography revealed diffuse left ventricular (LV) wall thickening and mid-LV-cavity obliteration (A). Postoperative echocardiography showed relieved LV outflow tract obstruction and an enlarged LV cavity (B).
Fig. 2.
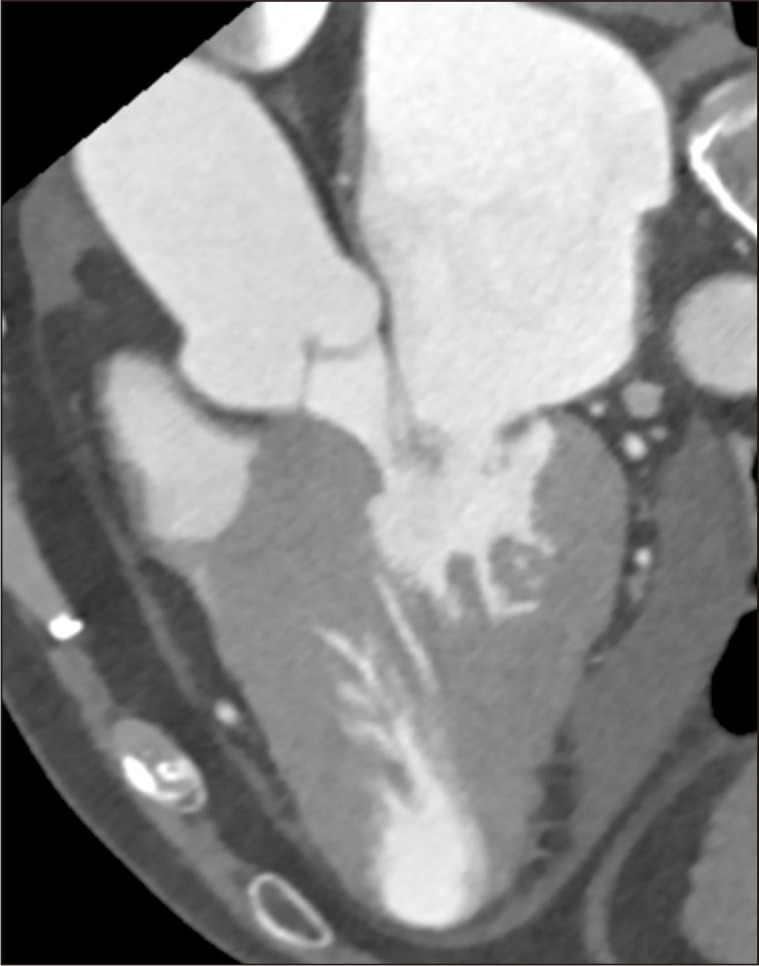
Preoperative computed tomography demonstrates diffuse-type hypertrophic cardiomyopathy with a small left ventricular cavity.
An elective operation for LV cavity enlargement with septal myectomy through a dual approach (transaortic and transapical) was planned. A Cox maze procedure for atrial fibrillation was also considered. A median sternotomy approach was performed and cardiopulmonary bypass was established via bicaval and ascending aortic cannulation. After antegrade administration of cardioplegic solution through the aortic root, left-sided surgical ablation for atrial fibrillation and left atrial appendage resection were performed. The transapical approach was made at the aneurysmal site, and myectomy (removing 42 g of tissue) was performed (Fig. 3). LV apical incision was repaired with a double-layered polytetrafluoroethylene felt strip. The total pump time and aortic clamping time were 189 minutes and 160 minutes, respectively. Intraoperative transesophageal echocardiography showed relieved mid-cavity obstruction and an enlarged LV.
Fig. 3.
Myectomy was performed, with the removal of a total of 42 g of tissue: 15 g via the transaortic approach and 27 g via the transapical approach.
The patient was transferred to the intensive care unit with a minimal dose of inotropic infusion. Immediate postoperative electrocardiography showed junctional bradycardia with a ventricular rate ranging from 50 to 60 beats per minute. Extubation was done on postoperative day 1, and he was transferred to the general ward on postoperative day 2. The patient’s postoperative course was uneventful. Postoperative echocardiography was performed on postoperative day 4 and revealed relieved LV outflow tract obstruction and mid-cavity obliteration (Fig. 1B). The LV internal diameter in systole (LVIDs) and diastole (LVIDd) had increased from 15 to 31 mm and from 35 to 48 mm, respectively. The LV end-systolic volume (ESV) and diastolic volume (EDV) increased from 27 to 44 mL and from 74 to 87 mL, respectively. Atrial contraction was observed after the Cox maze procedure and a normal sinus rhythm, with a pattern of left bundle branch block, was observed on 24-hour postoperative Holter monitoring. The patient’s symptoms were also relieved and he was discharged on postoperative day 16 after medical management for heart failure.
The patient visited the outpatient department until postoperative 9 months and was referred to a local cardiovascular clinic. The last follow-up echocardiogram was at postoperative 6 months with an LVIDs of 34 mm, an LVIDd of 48 mm, an ESV of 37 mL, and an EDV of 78 mL. Electrocardiography showed a normal sinus rhythm with a rate of 92 beats per minute.
The patient provided formal informed consent for publication of his clinical information.
Discussion
The first surgical procedure for obstructive HCMP was performed by Goodwin and colleagues in 1958, and the operative technique has subsequently evolved. Surgical myectomy remains the primary treatment option for severely symptomatic patients with obstructive HCMP [1]. There is a wide spectrum of HCMP subtypes, and several cardiac surgeons modified the myectomy procedure to extend more distally for complex HCMP [2]. Complex HCMP is defined as any case of HCMP not confined to the subaortic region, such as long-segmental HCMP, apical HCMP, or diffuse-type HCMP combined with midventricular obstruction (MVO) [3].
In cases of HCMP with subaortic obstruction or LV outflow tract obstruction, basal septal myectomy through the transaortic approach provides excellent long-term outcomes and a low operative mortality rate. A recent study of apical myectomy in severely symptomatic patients with apical HCMP showed that transapical ventricular myectomy improved diastolic function and increased the LV EDV [4]. Combined transaortic and transapical septal myectomy also could be an effective approach for septal HCMP with MVO [5].
There are no detailed studies on the surgical treatment of diffuse-type HCMP with a thickened LV free wall and a small LV volume. In this case, we performed an LV enlargement procedure in a patient with diffuse-type complex HCMP. LV wall thickening was present not only in the interventricular septum, but also in the LV free wall. Flow acceleration at the LV mid-cavity on echocardiography suggested MVO, and a concomitant aneurysmal change on the apex was also present. LV cavity enlargement with surgical myectomy through a dual transaortic and transapical approach was performed. The patient’s symptoms were relieved from NYHA functional class IV to class II, and the LV EDV increased from 74 to 87 mL.
Severely limiting symptoms, low cardiac output, and LV diastolic dysfunction in patients with diffuse-type HCMP are mainly caused by a small LV volume and diffusely thickened myocardium. For the surgical treatment of diffuse-type HCMP, surgeons should consider not only the relief of LV outflow tract obstruction, but also the enlargement of the LV cavity to increase the LV EDV, stroke volume, and diastolic function. In this case, successful surgical myectomy of diffuse-type HCMP via a combined transaortic and transapical dual approach was performed. The patient was observed for only 9 months after the operation. To verify whether an LV enlargement procedure could be an alternative surgical option in diffuse-type HCMP, other cases of this procedure with long-term follow-up would be needed.
Footnotes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
References
- 1.Maron BJ. Controversies in cardiovascular medicine: surgical myectomy remains the primary treatment option for severely symptomatic patients with obstructive hypertrophic cardiomyopathy. Circulation. 2007;116:196–206. doi: 10.1161/CIRCULATIONAHA.107.691378. [DOI] [PubMed] [Google Scholar]
- 2.Schaff HV, Brown ML, Dearani JA, et al. Apical myectomy: a new surgical technique for management of severely symptomatic patients with apical hypertrophic cardiomyopathy. J Thorac Cardiovasc Surg. 2010;139:634–40. doi: 10.1016/j.jtcvs.2009.07.079. [DOI] [PubMed] [Google Scholar]
- 3.Nguyen A, Schaff HV, Miranda WR, Tajik AJ. The first operation for apical hypertrophic cardiomyopathy-Dr Dwight McGoon, 1972. Ann Thorac Surg. 2017;104:e133–6. doi: 10.1016/j.athoracsur.2017.02.053. [DOI] [PubMed] [Google Scholar]
- 4.Minami Y, Kajimoto K, Terajima Y, et al. Clinical implications of midventricular obstruction in patients with hypertrophic cardiomyopathy. J Am Coll Cardiol. 2011;57:2346–55. doi: 10.1016/j.jacc.2011.02.033. [DOI] [PubMed] [Google Scholar]
- 5.Hang D, Schaff HV, Ommen SR, Dearani JA, Nishimura RA. Combined transaortic and transapical approach to septal myectomy in patients with complex hypertrophic cardiomyopathy. J Thorac Cardiovasc Surg. 2018;155:2096–102. doi: 10.1016/j.jtcvs.2017.10.054. [DOI] [PubMed] [Google Scholar]




