Version Changes
Revised. Amendments from Version 1
Some changes have been introduced in the revised version of the manuscript as per the suggestion of the peer-reviewer. The methodology has been described in detail. Baseline details of Plasmodium falciparum and mixed infections had been removed from the manuscript to remove distraction from the main objective. Also, statistical tests to differentiate between baseline features of severe and non-severe malaria have been removed. Table 2 shows the PQ prescription patterns in a more succinct manner so Figure 1 is removed to avoid confusion. The paragraph on severe malaria has been removed, as suggested by the reviewers.
Abstract
Background: India is endemic for Plasmodium vivax (Pv) malaria. Despite a decrease in incidence, its elimination is hampered by recurrences. This study aimed to characterize recurrences in Pv malaria and study its association with primaquine (PQ) usage.
Methods: Symptomatic adult Pv patients were followed-up for up to 23 months for recurrences. The time to recurrence was compared by the PQ dosage they received using a log-rank test.
Results: Of the 294 malaria patients, 206 (70%) patients had Pv infection during the study period. A total of 20 (9.7%) recurrences were seen in 17 (8.2%) patients of Pv. The percentage of first-time recurrences were highest in the no PQ group (25%), followed by the weekly PQ group (20%), low dose daily PQ (8.2%) group, and high dose daily PQ group (3.1%).
Conclusions: Recurrence in Pv malaria is common, especially in those who receive an incorrect prescription of primaquine.
Keywords: Primaquine; relapse; severe malaria
Introduction
Malaria is a major global health problem, with around 228 million reported cases alone in 2018, most due to Plasmodium falciparum ( Pf). 1 Consequently, most reports on malaria concentrate on Pf. Traditionally, Pf has been described as the causative agent for severe malaria. However, recent reports have shown that malaria caused by Plasmodium vivax ( Pv) can also be severe. Although India represents a small percentage of the overall global malaria cases, it is responsible for nearly half of the total cases of Pv. 2 , 3 Despite a decline in the number of Malaria cases in India, the major roadblock to elimination is the tendency of Pv to relapse frequently, mainly when primaquine (PQ) is not prescribed or prescribed in sub-therapeutic dosage. 4 , 5 Therefore, the objective of the study was to calculate the incidence of recurrence in patients with Pv malaria and find the impact of PQ prescription practices on recurrence.
Methods
A prospective observational study was conducted at Kasturba Hospital, Manipal in Udupi district of Karnataka State, India, for two years, from October 2016 to August 2018. The study was commenced after taking approval from the Institute's Ethical Committee (IEC 636/2016). All patients of either sex above 18 years of age who presented during the study period with fever and had Pv malarial parasites on the quantitative buffy coat (QBC) or peripheral smear examination were included in the study after taking written informed consent. Those patients with Pf or mixed infections ( Pv and Pf) were excluded. The article was reported according to the STROBE guidelines and all the criteria in the STROBE checklist were met. The sample size was calculated as 206 cases of Pv, considering recurrence prevalence as 31.5%, 95% level of confidence and 6.5% precision. 6
They diagnosis of Pv was based on the results of peripheral smear. A detailed history (including comorbidities), physical examination, and laboratory parameters were noted in a predefined case study form. In addition, the worst value of the variables during hospitalization was recorded. The patients were classified as having severe disease if they met the criteria for severity laid down by World Health Organisation (WHO). 7 Since the study aimed to record the prescription practices of treating physicians, the study objectives were not disclosed to them to avoid bias. The diagnosed cases were treated by the treating team. Glucose-6 Phosphate dehydrogenase (G6PD) levels were requested by the treating physician’s discretion. The enzyme activity was quantified by the manual spectrophotometric kinetic 'gold standard' method in the institutional biochemistry laboratory. G6PD deficiency was defined as less than 30% of mean G6PD activity.
Chloroquine was used in all patients for the treatment of Pv malaria. In an ideal situation, G6PD levels should be done prior to initiation of primaquine. If the levels are within normal range, WHO recommends 0.5 mg/kg primaquine to prevent relapse in tropical areas. The national guidelines in India, however, recommend 0.25 mg/kg according to their last available guidance. If the levels are low, weekly primaquine is recommended for 8 weeks. 8 , 9
The treating physicians decided the dosage of antimalarials, including PQ. The details of treatment, supportive care hospitalization days and mortality during hospital stay were noted. The primary outcome was microbiologically-confirmed recurrence at the end of the study period. Individuals were followed up telephonically every two months until the end of the study period for the development of fever recurrence. Additionally, individuals were asked to report if the fever recurred and were classified as recurrence if they were microscopically proven to have malaria again.
Statistical analysis was performed using Statistical Package for the Social Sciences version 23.0 (SPSS, RRID:SCR_002865, http://www-01.ibm.com/software/uk/analytics/spss/ ). Continuous variables were summarized as mean with standard deviation (SD) or median with interquartile range (IQR) (in skewed data). Categorical variables were summarized as the frequency with proportion. Overall, patients with Pv were divided into four groups according to PQ dosage- no PQ, weekly PQ, low dose daily PQ (0.25 mg/kg/day), and high dose daily PQ (0.5 mg/kg/day). The number of recurrences in each group were calculated. A Kaplan-Meier survival plot was generated to determine the survival function of recurrences according to PQ categories until 23 months' follow-up duration. Log-rank test was used to compare the survival function. A p-value of less than 0.01 was considered significant.
Results
A total of 294 malaria cases were screened during the study period, of which 206 (70%) were Pv, 79 (27%) were Pf, and 9 (3%) were mixed ( pv+pf). A total of 29.6 % (87/294) cases had severe malaria. The proportion of severity, the requirement of supportive care, and mortality were comparable in both groups and summarized. The baseline clinical and laboratory features of patients with Pv and Pf malaria have been summarized in Table 1.
Table 1. Baseline clinical and laboratory features of patients with severe or non-severe vivax malaria.
| Plasmodium vivax (N=206) | Plasmodium falciparum (N=79) | |||||
|---|---|---|---|---|---|---|
| Non-severe (n=144) | Severe (n=62) | P-value * | Non-severe (n=56) | Severe (n=23) | P-value * | |
| Age (years) | 36.1±14.2 | 40.6±14.1 | 0.76 | 34.4±14.6 | 38.59±13.1 | 0.41 |
| Male gender | 121(84%) | 55 (88.5%) | 0.38 | 48 (85.7%) | 21 (95.5%) | 0.22 |
| Fever in days | 4 (3,7) | 4 (3,6) | 0.83 | 4 (3,6) | 6 (4,7) | 0.01 |
| Diabetes mellitus | 15 (10.45%) | 13 (21%) | 0.04 | 5 (9%) | 1 (4.3%) | 0.48 |
| Hypertension | 14 (9.72%) | 10 (16.1%) | 0.18 | 5 (9%) | 3 (13.04%) | 0.58 |
| Pulse rate (beats/min) | 88±14 | 92±16 | 0.22 | 88±11 | 87±12 | 0.47 |
| Respiratory rate (breaths/min) | 19±2 | 20±5 | 0.007 | 18±2 | 21±6 | 0.001 |
| ARDS | 5 (3.5%) | 0 | 0.001 | 3 (5.4%) | 0 | 0.005 |
| Systolic blood pressure (mmHg) | 120±14 | 114±20 | 0.001 | 121±17 | 113±15 | 0.8 |
| Diastolic blood pressure (mmHg) | 77±8 | 73±12 | 0.002 | 77±9 | 73±13 | 0.15 |
| Shock | 7 (3.4%) | 0 | <0.001 | 4 (7.1%) | 0 | 0.001 |
| Pallor | 5 (3.5%) | 6 (9.8%) | 0.07 | 5 (8.9%) | 4 (18.2%) | 0.28 |
| Icterus | 44 (30.6%) | 0 | <0.001 | 17 (30.4%) | 0 | <0.001 |
| Impaired consciousness | 3 (2.1%) | 0 | 0.009 | 1 (1.8%) | 0 | 0.108 |
| Convulsion | 1 (0.7%) | 0 | 0.136 | 1 (1.8%) | 0 | 0.108 |
| Metabolic acidosis | 3 (2.1%) | 0 | 0.010 | 1 (1.8%) | 0 | 0.108 |
| Renal failure | 10 (6.9%) | 0 | <0.001 | 3 (5.4%) | 0 | 0.005 |
| Splenomegaly | 17 (11.8%) | 14 (23%) | 0.04 | 11 (19.6%) | 8 (36.4%) | 0.15 |
| Hepatomegaly | 8 (5.6%) | 15 (24.6%) | <0.001 | 6 (10.7%) | 4 (18.2%) | 0.41 |
| Hemoglobin (g/dL) | 13.4 ± 1.9 | 12.8 ± 2.5 | 0.01 | 12.9 ± 2.1 | 12.2 ± 3.2 | 0.03 |
| Hematocrit (%) | 39.7 ± 5.6 | 37.8 ± 7.3 | 0.02 | 38 ± 6.5 | 35.7 ± 9 | 0.12 |
| Total Leukocyte count (cells/mm 3) | 5655 ± 2154 | 5813 ± 2978 | 0.008 | 5049 ± 1804 | 7632 ± 4516 | 0.001 |
| Platelet count (cells/mm 3) | 74500 (49250,113250) | 47000 (30750,79500) | 0.001 | 75000 (48500,136250) | 39000 (16000,96500) | 0.007 |
| Plasma Glucose (mg/dL) | 132±54 | 149±60.1 | 0.13 | 139±70.8 | 132±44.5 | 0.15 |
| Blood Urea (mg/dL) | 25 (20,31) | 32 (23,45.5) | <0.001 | 24 (19, 30) | 32 (22, 65) | 0.01 |
| Serum Creatinine (mg/dL) | 0.98±0.27 | 1.17±0.48 | <0.001 | 1.01±0.42 | 1.7±2.01 | 0.01 |
| Total Bilirubin (mg/dL) | 1.49 ± 0.62 | 3.8 ± 2.9 | <0.001 | 1.5 ± 0.6 | 6.8 ± 7.56 | <0.001 |
| Direct Bilirubin (mg/dL) | 0.6 ± 0.3 | 2.08 ± 2.42 | <0.001 | 0.6 ± 0.4 | 4.23 ± 5.16 | <0.001 |
| Aspartate transaminase (IU/L) | 33.5 (24,43) | 49 (30,65.5) | <0.001 | 36 (25, 58.5) | 47.5 (37.3, 96) | 0.02 |
| Alanine transaminase (IU/L) | 34 (22,53) | 43.5 (27.2,87.7) | 0.01 | 43 (24, 70) | 54.5 (31.7, 103.2) | 0.2 |
| Alkaline phosphatase (IU/L) | 75 (60,94) | 99 (76.3,144.7) | <0.001 | 93 (61, 115.8) | 122.5 (76.3, 181.5) | 0.02 |
Categorical variables are summarized as the frequency with proportion whereas continuous variables are summarized as either mean (±SD) or median (IQR). Chi-square or Fischer's exact test and Independent sample t-test or Mann Whitney U test were performed, p-value less than 0.05 shows the statistically significant difference and shown in bold font. ARDS: Acute Respiratory Distress Syndrome.
Of 206 Pv cases included in the study, there were 20 recurrences in 17 (8.5%) patients. The median time to follow-up was 388 (293–567) days. The median time to the first recurrence was 83 (66.5–242.5) days.
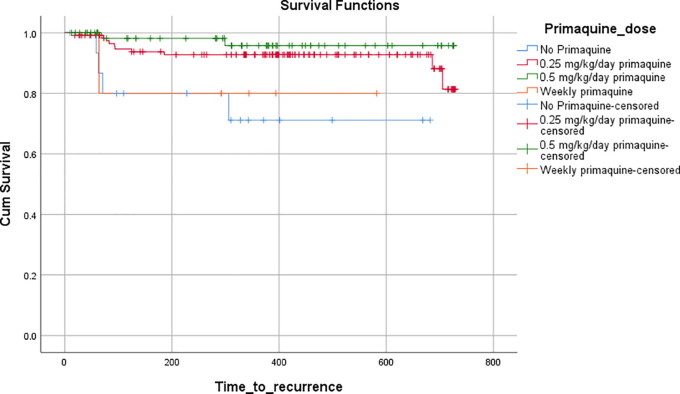
Of the 206 patients with Pv, G6PD levels could be done in 196 patients only, out of which nine patients were found to have low G6PD levels ( Table 2). No case of PQ-induced hemolysis was noted in our cohort. The dose of PQ was significantly associated with recurrences on the Chi-square test (p<0.001). The percentage of first-time recurrences were highest in the no PQ group (25%), followed by the weekly PQ group (20%), low dose daily PQ (8.2%) group, and high dose daily PQ group (3.1%) ( Table 2). A Kaplan-Meier curve was plotted to compare the median time to recurrence in each of the PQ-based groups, and the difference was found to be significant on the log-rank test (p=0.009) ( Figure 1).
Table 2. Recurrences in Plasmodium vivax cases stratified according to G6PD levels and primaquine prescription patterns.
| Primaquine (PQ) | G6PD levels low (n=9) | G6PD levels normal (n=187) | G6PD not done (n=10) | |||
|---|---|---|---|---|---|---|
| PQ dose | Total prescribed | Recurrences | Total prescribed | Recurrences | Total prescribed | Recurrences |
| No PQ | 1 | 0 | 10 | 2 | 5 | 2 |
| Weekly PQ | 5 | 1 | 0 | 0 | 0 | 0 |
| Daily PQ (0.25 mg/kg) | 3 | 1 | 114 | 8 | 4 | 1 |
| Daily PQ (0.5 mg/kg) | 0 | 0 | 63 | 2 | 1 | 0 |
PQ: Primaquine; G6PD: Glucose 6 Phosphate dehydrogenase.
Figure 1. Time to recurrence stratified according to primaquine dosing on Kaplan-Meier survival analysis.
Discussion
Udupi district has a population of 1,177,908 with an area of 3,582 sq. km and is located 13°32′ 24.43′′ N latitude and 74°52′26.78′′ E longitude, with typical tropical climatic conditions. The monsoon in this region starts in June and extends till October, with an average rainfall of more than 4000mm every year. The catchment area of our hospital encompasses both the rural and urban populations of coastal and interior Karnataka, Goa and Kerala. Pv is the largest infecting species in this region, followed by Pf. 10 , 11 The same trend is noted in other parts of India. 12 , 13
As expected, all but one recurrence were seen in patients with Pv. The percentage recurrence in Pv cases was close to 10%, which was considerably lower than recurrences reported in the previous series (24–38%). 14 , 15 Like a previous study, all recurrent cases had mild symptoms, presumably due to the development of acquired immunity from the previous episode. 16 The median time to recurrence was 83 days in our study, similar to previously published studies. 14 Those patients for whom PQ was not used had higher rates of recurrence.
We classified the patients according to the G6PD levels because in those patients where G6PD levels were not done, we couldn’t judge the correctness of the prescription choices. The idea was to show that a G6PD levels were not even offered to some patients. On top of that, many patients were given incorrect prescriptions despite G6PD levels indicating otherwise. Of the 16 patients for whom no PQ was used, only one patient had proven low levels of G6PD. This implies that PQ was not prescribed because of possible lack of awareness. This reflects the need to reinforce the fact that G6PD levels should be done in all patients with Pv and the prescriptions should be guided by the G6PD levels.
Since the recurrence rates were lowest in those wher primaquine was used as 0.5 mg/kg , patients with normal G6PD levels should receive 0.5 mg/kg prophylaxis. Even with a lower dose of PQ (0.25 mg/kg), the recurrences are lower when compared to those who were not given PQ. Similar results were observed in other studies as well. 17 Since the study was done in a tertiary care hospital where G6PD levels and specialist referrals are available, the study cannot be generalized to primary care settings. Similar widespread prescription audits are required all over the country to understand the practices and pattern of recurrences in patients with Pv.
Limitations of the study
Self-limiting intermittent recurrences that are asymptomatic could not be ruled out as symptom-based screening for recurrence was done. The genotyping of recurrences could not be done to discern relapse and reinfection. The possibility of non-compliance cannot be ruled out as PQ therapy was unsupervised. New Pv infections could be differentiated from relapses in this study. The study follow-up was of long duration so recurrences at 1-2 years may or may not be related to the PQ dose.
Conclusions
The study concludes that, Pv may be associated with recurrences, especially when PQ prescription practices are not aligned with international evidence based recommendations. G6PD levels should be ascertained in all patients with Pv malaria, and daily PQ prophylaxis should be given to those patients with normal G6PD levels. There is a need for improving prescription practices amongst primary care physicians through regular educational interventions.
Data availability
Data cannot be shared due to ethical and security concerns, however a de-identified dataset with all the details can be shared with reviewer or readers at reasonable request to corresponding author.
Author's contributions
All authors have read and approved the final manuscript. The requirements for authorship have been met, and each author believes that the manuscript represents honest work.
Divya Gandrala: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Writing-original draft preparation, Writing-Review & Editing
Nitin Gupta: Formal analysis, Validation, Writing-original draft preparation, Writing-Review & Editing
Alekhya Lavu: Data curation, Formal analysis, Investigation, Methodology
Vishnu Teja Nallapati: Writing-original draft preparation, Writing-Review & Editing
Vasudeva Guddattu: Formal analysis, Software, Writing-original draft preparation, Writing-Review & Editing
Kavitha Saravu: Conceptualization, Data curation, Formal analysis, Project administration, Supervision, Validation, Writing-original draft preparation, Writing-Review & Editing
Acknowledgements
Authors gratefully acknowledge the seed grant funding and publication support from Manipal Center for Infectious Diseases, Prasanna School of Public Health, Manipal Academy of Higher Education, Manipal, India.
Funding Statement
The author(s) declared that no grants were involved in supporting this work.
[version 2; peer review: 2 approved]
References
- 1. World Health Organization: World Malaria Report, World Health, vol. WHO/HTM/GM, no. December. 2019; p.238. ISBN 978 92 4 1564403.
- 2. Saravu K, Kumar R, Ashok H, et al. : Therapeutic Assessment of Chloroquine-Primaquine Combined Regimen in Adult Cohort of Plasmodium vivax Malaria from Primary Care Centres in Southwestern India. PLoS One. 2016 Jun 17;11(6):e0157666. 10.1371/journal.pone.0157666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rahi M, Sharma S, Das P, et al. : Connecting the dots to strengthen malaria elimination strategies in India: A Malaria Elimination Research Alliance - India initiative. Indian J. Med. Res. 2021 Jul;154(1):19–23. 10.4103/ijmr.IJMR_4370_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kumar R, Guddattu V, Saravu K: Therapeutic assessment of primaquine for radical cure of plasmodium vivax malaria at primary and tertiary care centres in Southwestern India. Korean J. Parasitol. 2016;54(6):733–742. 10.3347/kjp.2016.54.6.733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rishikesh K, Kamath A, Hande MH, et al. : Therapeutic assessment of chloroquine–primaquine combined regimen in adult cohort of Plasmodium vivax malaria from a tertiary care hospital in southwestern India. Malar. J. 2015;14(1):1–6. 10.1186/s12936-015-0824-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Douglas NM, Nosten F, Ashley EA, et al. : Plasmodium vivax recurrence following falciparum and mixed species malaria: risk factors and effect of antimalarial kinetics. Clin. Infect. Dis. 2011 Mar 1;52(5):612–620. 10.1093/cid/ciq249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. World Health Organization: WHO-Severe malaria. World Health Organization;2014. Reference Source [Google Scholar]
- 8. World Health Organization: Guidelines for the treatment of malaria. World Health Organization;2015 Aug 13. [Google Scholar]
- 9. Directorate of National Vector Borne Disease Control Programme: Guidelines for Diagnosis and Treatment of Malaria in India. 2014. [Google Scholar]
- 10. Saravu K, Docherla M, Vasudev A, et al. : Thrombocytopenia in vivax and falciparum malaria: an observational study of 131 patients in Karnataka, India. Ann. Trop. Med. Parasitol. 2011;105(8):593–598. 10.1179/2047773211Y.0000000013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Saravu K, Rishikesh K, Parikh CR: Risk Factors and Outcomes Stratified by Severity of Acute Kidney Injury in Malaria. PLoS One. 2014;9(3):e90419. 10.1371/journal.pone.0090419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nadkar MY, Huchche AM, Singh R, et al. : Clinical profile of severe Plasmodium vivax malaria in a tertiary care centre in Mumbai from June 2010-January 2011. J. Assoc. Physicians India. 2012 Oct;60:11–13. [PubMed] [Google Scholar]
- 13. Zubairi AB, Nizami S, Raza A, et al. : Severe Plasmodium vivax malaria in Pakistan. Emerg. Infect. Dis. 2013 Nov;19(11):1851–1854. 10.3201/eid1911.130495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zuluaga-Idárraga L, Blair S, Akinyi Okoth S, et al. : Prospective Study of Plasmodium vivax Malaria Recurrence after Radical Treatment with a Chloroquine-Primaquine Standard Regimen in Turbo, Colombia. Antimicrob. Agents Chemother. 2016 Jul 22;60(8):4610–4619. 10.1128/AAC.00186-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kim JR, Nandy A, Maji AK, et al. : Genotyping of Plasmodium vivax reveals both short and long latency relapse patterns in Kolkata. PLoS One. 2012;7(7):e39645. 10.1371/journal.pone.0039645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kochar DK, Saxena V, Singh N, et al. : Plasmodium vivax. Emerg. Infect. Dis. 2005;8(11):11–12. 10.1128/CDLI.8.5.891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ganguly S, Saha P, Guha SK, et al. : Recurrence Pattern of P. Vivax Malaria Following Treatment with Chloroquine Either Alone or in Combination with Primaquinein Urban Kolkata, India. Int. J. Recent Sci. Res. 2014;5(6):1046–1049. [Google Scholar]