Abstract
Objective
To analyze the published studies that investigated the physical function, activities of daily living and health-related quality of life in COVID-19 survivors.
Design
Systematic review.
Methods
We searched MEDLINE/PubMed, Scopus, SciELO, and Cochrane Library for studies that evaluated the physical function, activities of daily living and health-related quality of life after COVID-19 from the earliest date available to July 2021. Two independent reviewers screened and selected the studies. The Newcastle Ottawa Scale was used to evaluate methodological quality.
Results
We included 35 studies in this systematic review. Of the 35 studies included, 28 were cohort, and 7 cross-sectional studies The studies demonstrated that COVID-19 survivors had reduced levels of physical function, activities of daily living, and health-related quality of life. Furthermore, incomplete recovery of physical function, and performance in activities of daily living were observed 1 to 6 months post-infection.
Discussion
Physical disability and reduction in health-related quality of life is a common condition in post-COVID-19 and impairments may persist up to 1 to 6 months. Researchers and clinicians can use these findings to understand the potential disabilities and rehabilitation needs of people recovering from the COVID-19.
Keywords: COVID-19, post-acute COVID-19 syndrome, international classification of functioning, disability and health, quality of life
Background
Coronavirus disease (COVID-19) is a highly infectious respiratory infection disease, which leads to dysfunction of respiratory, physical, and psychological performance of patients.1,2 COVID-19 infection significantly increased mortality risk and the burden of disability in most survivors, regardless of symptom severity at onset.1–3
New evidence on the COVID-19 pandemic is being published daily.4 Knowledge about COVID-19, including its presentation and treatment, is changing very rapidly, and guidelines are quickly being created and updated.5 As COVID-19 is a multisystemic disease, many cases will require a total rehabilitation effort by the multidisciplinary team to enable recovery.6
Considering the increasing number of COVID-19 cases and the significant proportion of people who are hospitalized and require ICU care for the management of infection, COVID-19 is also likely to have an important impact on the functionality and quality of life of survivors.7,8 However, due to short duration of the SARS-CoV-2 pandemic, only a limited and scattered body of scientific evidence is available on physical function and health-related quality of life consequences of COVID-19.7,8
Another important aspect in patients with COVID-19 who are hospitalized is that long periods of movement restrictions and the own hospitalization can reduce physical function, because of reduced muscle strength, joint mobility, and respiratory capacity, among others. In consequence, there are losses in performing daily living activities, reducing the patient's autonomy and independence and, negatively impacting their quality of life.9,10 A better understand of functional repercussions of COVID-19 can be of help in designing and implementing strategies and interventions focused on the recovery of disability that can improve the quality of life of this population.9,10
Recently Pizarro-Pennarolli et al.,11 published a systematic review to understand the impact of COVID-19 on activities of daily living performance of adult patients and to describe the common scales used to assess performance of activities of daily living on patients post-COVID-19. In the nine studies included, all demonstrated reduced performance in activities of daily living revealing a vital worsening of functional ability in activities of daily living performance and consequently loss of independence in COVID-19 patients after the acute phase of infection.11 Despite this, as far as we know, there is no published systematic review that analyzed the impact of COVID-19 on the physical function, and health-related quality of life of survivors. The aim of this systematic review is to analyze the published studies on physical function, activities of daily living and health-related quality of life in COVID-19 survivors.
Methods
This review was developed in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.12
Eligibility criteria
We included published peer-reviewed studies that investigated the physical function, activities of daily living and health-related quality of life after COVID-19. Eligible studies should meet the following criteria: (a) included adult patients post-COVID-19 infection for the first time; (b) studies that assessed physical function, activities of daily living and/or health-related quality of life in COVID-19 survivors. Review studies, guidelines and case studies were excluded. In addition, studies in which patients were re-infected and diagnosed by covid-19 were excluded.
Database and search strategy
We searched for references on MEDLINE/PubMed, SciELO, Scopus and the Cochrane Library without language restrictions. Additionally, we search for studies in google scholar. The electronic databases were searched from inception to July 21st, 2022. A standard protocol for this search was developed and whenever possible, controlled vocabulary (Mesh term for MEDLINE/PubMed) was used. Key words and their synonymous were used to sensitize the search.
The strategy developed by Higgins and Green13 was used for the identification of studies in MEDLINE/PubMed. To identify studies in other database we adopted a search strategy using similar terms. Two groups of keywords were used for the preparation of the search strategy: participants and outcomes. The full search strategy can be found in Electronic Supplementary File 1 for independent replication.
We checked the references used in articles included in this systematic review to identify other potentially eligible studies. Authors were contacted by e-mail to obtain information for ongoing studies, confirmation about data and any additional information considered relevant for the analysis.
Data collection and analysis
Potential studies for the systematic review were initially selected according to their titles and abstracts and the pre-defined search strategy. Selection of studies records from databases and other resources will be uploaded to a database created by EndNote X7.8 software (Clarivate, Philadelphia, PA). Two authors independently evaluated the abstracts of each study. If at least one of the authors considered the study eligible for the systematic review, the full text was obtained for complete assessment. Two reviewers independently assessed the full text of selected articles to verify if they met the inclusion criteria.
Two authors independently extracted data from the published reports using standard data extraction forms adapted from Higgins and Green.13 Characteristics of study population, follow-up period, rates of missing data, outcome measures, and results were reviewed. In case of any disagreement, authors discussed the reasons for their decisions and a consensual decision was made. If necessary, further information was requested by e-mail to the corresponding authors of specific publications.
Risk of bias in included studies
The quality of studies included in this systematic review was scored by two researchers using the Newcastle Ottawa Scale (NOS) (with a score ranging from 0 to 9 points). The NOS is a review tool for evaluating risk of bias in observational studies. The scale consists of four domains of risk of bias assessment; (i) selection bias; (ii) performance bias; (iii) detection bias and; (iv) information bias.14
Results
Description of selected studies
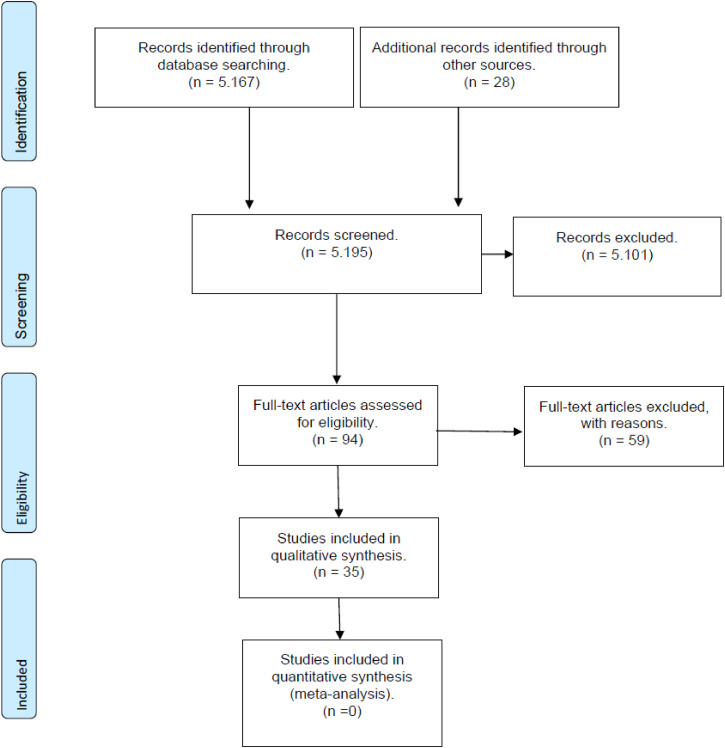
The initial search led to the identification of 5195 abstracts, from which 95 studies were considered as potentially relevant and were retrieved for detailed analysis. Initially the 5101 studies were excluded after reading the title and abstracts, for not meeting the eligibility criteria. After a complete reading of 94 articles, 59 were excluded (reasons presented in the flowchart), resulting in 35 studies15–49 that met the eligibility criteria. Figure 1 shows the flow diagram of studies in this review according to PRISMA guidelines.
Figure 1.
Search and selection of studies for systematic review according to PRISMA.
Of the 35 studies included in this review, 28 were cohort, and 7 cross-sectional studies. Twelve studies evaluated only physical function,15–17,19–21,33,37,43,45,47,48 two studies evaluate only activities of daily living24,49 and eighth only the health-related quality of life.23,29,30,32,39,40,42,46 Five studies evaluated physical function and activities of daily living.27,28,36,38,41 Five studies evaluated physical function and health-related quality of life18,22,25,26,34 two studies evaluated physical function, activities of daily living and health-related quality of life,31,35 and one study evaluated daily living activities and health-related quality of life.44 For each study, design, sample size, sex, outcomes measures and key findings were extracted (Table 1).
Table 1.
Characteristics of the participants and outcome measure of the studies included in the systematic review.
| Author/year | Study | Patient's phase, (N analyzed; age mean; % gender) | Physical Function: Outcome Measure | Activities of daily living: outcome measure | Quality of life/Outcome Measure | Follow-up |
|---|---|---|---|---|---|---|
| Baricich et al. 2021 | Cross-sectional | Post covid-19 (N = 204; age 57.9; 60% male) | Physical performance: SPPB Functional capacity: 2-MWT and 1-MSTST |
NA | NA | 3 to 6 months after discharge |
| Blanco et al. 2021 | Cohort | Post covid-19 (N = 100; age 54.8; 64% male) | Pulmonary functions: FEV1, FVC, VEF1/CVF, DLCO, CPT Functional capacity: 6-MWT |
NA | NA | 45 days after symptoms |
| Bellan et al. 2021 | Cohort | Post covid-19 (N = 238; age 61; 59.7% male) | Physical performance: SPPB Functional capacity: 2-min walk test Pulmonary functions: FEV1, FVC, DLCO |
NA | NA | 3 to 4 months after discharge |
| Cao et al. 2021 | Cohort | Post covid-19 (N = 81; age 45; 58% male) | Pulmonary functions: FEV1, FVC, MVV Functional capacity: 6-MWT |
NA | SF-36 | 1 to 3 months after discharge |
| Cortés-Telles et al. 2021 | Cohort | Post covid-19 (N = 186; age 47; 61% male) | Pulmonary functions: FEV, FVC, TCL Functional capacity: 6-MWT |
NA | NA | 30 and 90 after discharge |
| Debeaumot et al. 2021 | Cohort | Post covid-19 (N = 23; age 59; 52% male) | Pulmonary functions: VO2peak; mMRC | NA | NA | 6 months after discharge |
| Guler et al. 2021 | Cohort | Post covid-19 (N = 113; age 56.6; 67% male) | Functional capacity: 6-MWT Respiratory muscle strength: PImax and PEmax Pulmonary functions: FEV1, FVC, DLCO, CPT |
NA | NA | 128 days after symptoms |
| Huang et al. 2021 | Cohort | Post covid-19 (N = 1.733; age 57; 52% male) | Pulmonary functions: FEV, FVC, TCL Functional capacity: 6-MWT |
NA | EQ-5D-5L | 6 months after discharge |
| Iqbal et al. 2021 | Cross-sectional | Post covid-19 (N = 158; age 40.1; 55.1% female) | NA | NA | EQ-5D-5L | 20 to 90 days after symptoms |
| Leite et al. 2021 | Cross-sectional | Post covid-19 (N = 1966; age 71.8; 56.1% female) | NA | IADL and BI | NA | 1 months after discharge |
| Parker et al. 2021 | Cohort | Post covid-19 (N = 36; age 52.5; 64% male) | Muscle strength: HGS Pulmonary functions: FEV1, FVC, DLCO, KCO |
NA | SF-36 | 2 months after discharge |
| PHOSP-COVID et al. 2021 | Cohort | Post covid-19 (N = 1077; age 58; 35.7% female) | Pulmonary functions: FEV1, FVC, TLCO, KCO Functional capacity: ISWT Fatigue: FACIT |
NA | EQ-5D-5L | 2 and 7 months after discharge |
| Piquet et al. 2021 | Cohort | Post covid-19 (N = 100; age 66; 66% male) | Muscle strength:HGS Functional capacity:STS |
BI | NA | 14 days after symptoms |
| Puchner et al. 2021 | Cohort | Post covid-19 (N = 23; age 57; 70% male) | Muscle strength: MIP Pulmonary functions: FEV, FVC, TCL Functional capacity: 6-MWT |
BI | NA | 20 to 70 days after symptoms |
| Qu et al. 2021 | Cohort | Post covid-19(N = 311; age 47.5; 50% male) | NA | NA | SF-36 | 3 months after discharge |
| Rass et al. 2021 | Cohort | Post covid-19 (N = 135; age 56; 61% male) | NA | NA | SF-36 | 1 to 3 months after symptoms |
| Taboada et al. 2021 | Cohort | Post covid-19 (N = 91; age 65.5; 64.8% male) | NA | PCFS | EQ-5D-3L | 6 months after discharge |
| Todt et al. 2021 | Cohort | Post covid-19 (N = 239; age 48.9; 59.8% male) | NA | NA | EQ-5D (EQ-5D-3L) | 1 to 3 months after discharge |
| Townsend et al. 2021 | Cross-sectional study | Post covid-19 (N = 79; age 40.2; 72.2 female) | Functional capacity: 6-MWT | NA | NA | 75 days after symptoms |
| Van Gassel et al. 2021 | Cohort | Post covid-19 (N = 46; age 53.6; 69.6% male) | Muscle strength:HGS Functional capacity: 6-MWT |
NA | EQ-5D (EQ-5D) | 3 months after discharge |
| Zampogna et al. 2021 | Cohort | Post covid-19 (N = 56; age 69.4; 69.5% male) | Muscle strength: MRCm Functional capacity: 6-MWT and 1STS |
BI | EQ-5D-5L | 3 months after discharge |
| Wiertz et al. 2021 | Cross-sectional | Post covid-19 (N = 60; age 55.9; 75% male) | Muscle strength: MRCm and HGS | BI | NA | 3 months after discharge |
| Wu et al. 2021 | Cohort | Post covid-19 (N = 54; age 53.6; 59.5% male) | Pulmonary functions: VEF1, CVF, VEF1/CVF, CPT, DCLO | NA | NA | 6 months after discharge |
| Belli et al. 2020 | Cohort | Post covid-19 (N = 103; age 73.9; 51.5 male) | Physical performance: SPPB Functional capacity: 1STS | BI | NA | 15 days after symptoms |
| Carfi et al. 2020 | Cohort | Post covid-19 (N = 143; age 56.6; 62.9 male) | NA | NA | Euro-Qol | 60 days after symptoms |
| Chen et al. 2020 | Cross-sectional | Post covid-19 (N = 361; age 47.2; 51.5% male) | NA | NA | SF-36 | 3 months after discharge |
| Curci et al. 2020 | Cross-sectional | Post covid-19 (N = 32; age 72.6; 68.8% male) | Functional capacity: 6-MWT | BI | NA | 2 months after discharge |
| Garrigues et al. 2020 | Cohort | Post covid-19 (N = 120; age 63.2; 62.5% male) | NA | NA | EQ-5D-5L | 100 days after symptoms |
| Hewitt et al. 2020 | Cohort | Post covid-19 (N = 1564; age 74; 57.7% male) | Frailty: CFS | NA | NA | 3 months after discharge |
| Jacobs et al. 2020 | Cohort | Post covid-19 (N = 183; age 57; 61.5% male) | NA | PROMIS® Scale | PROMIS® Scale | 35 days after symptoms |
| Liang et al. 2020 | Cohort | Post covid-19 (N = 76, age 41.3; 72% female) | Pulmonary functions: VEF1, CVF, VEF1/CVF, CPT, DCLO | NA | NA | 3 months after discharge |
| Valent et al. 2020 | Cohort | Post covid-19 (N = 19, age 62, 71% male) | NA | NA | EQ-5D-3L and SF-36 | 3 months after discharge |
| Vilches-Moraga et al. 2020 | Cohort | Post covid-19 (N = 1.67; age 71; 44.4% female) | Frailty: CFS | NA | NA | 4 months after discharge |
| Zhao et al. 2020 | Cohort | Post covid-19 (N = 55; age 47.7; 58.2% male) | Pulmonary functions: FEV, FVC, TLC, DLCO | NA | NA | 3 months after discharge |
| Zhu et al. 2020 | Cohort | Post covid-19 (N = 432; age 49; 49% female) | NA | IADL and BI | NA | 3 months after discharge |
N: Included; NR: not reported; NA: not analyzed; HFNC: high-flow nasal cannula for oxygen therapy, NIV: non-invasive ventilation, IMV: invasive mechanical ventilation; ICU: intensive treatment unit; WHO: World Health Organization; H7N9: avian influenza A virus; MERS: Middle East respiratory syndrome; BI: Barthel of activity of daily life; Bd: Bathel dyspnoea; SBC: Single Breath Counting; SPPB: Short Physical Performance Battery; MRCm: Medical Research Council Muscular; 1STS: One Minute Sit to Stand; 6MWT: six minute walk test; EuroQoL-VAS: Euro Quality of Life with visual analog scale; (BI) Barthel Index; LLN: limite inferior da faixa normal; EQ-5D-5L: EuroQol five-dimension five-level; ISWT: shuttle walk test; FACIT: Fatigue Scale; NRS: Numeric rating scale; IADL: Lawton scale; 1STS: One Minute Sit to Stand; SBC: Single Breath Counting; CFS: Clinical frailty scale; SPPB: Short Physical Performance Battery of 0–6 points; CFQ-11 CFS: Chalder Fatigue Scale; VAS: visual analog scale to evaluate physical and mental fatigue; PROMIS®: Patient-Reported Outcomes Measurement Information System; HGS: Hand grip strength; MIP: maximal inspiratory pressure; STS:10 sit-to-stands; ST: standardized questionnaire; EuroQol: visual analog scale; SPPB: Short Physical Performance Battery; 2MWT: two-minute walk test; 6MWD: The 6-min walk distances; PR: physical role; RE: emotional role; SF: social functioning; mMRC; Medical Research Council dyspnea; 1-MSTST: 1-min sit-to-stand test; PCFS: Functional Status scale.
COVID-19-related outcomes on physical function, activities of daily living, and health-related quality of life in included studies are listed in Table 2. All studies included in this review showed a decline in physical function: peripheral and respiratory muscle strength assessed by handgrip strength and Medical Research Council Muscular, and maximal inspiratory pressure/maximal expiratory pressure, Pulmonary functions (forced vital capacity and forced expiratory volume in 1 s) assessed by spirometry, and measurement of carbon monoxide diffusing capacity. Physical performance was assessed by 6-min walk test and shuttle walk test, 1 min sit to stand, 10 sit-to-stands, Short Physical Performance Battery, 2-min walk test, clinical frailty scale and fatigue assessed by Chalder Fatigue Scale, Fatigue Scale, and visual analog scale to evaluate physical fatigue.
Table 2.
Results of the studies included in the systematic review.
| Author/year | Physical function | Activities of daily living | Quality of life | ||
|---|---|---|---|---|---|
| Peripheral and/or respiratory muscle strength | Pulmonary functions | Physical performance | |||
| Baricich et al. 2021 | NA | NA | 32% patients whit physical impairment in one of our second line tests, the 2-MWT and 1-MSTST. 14% whit an SPPB score ≤10, indicating impairment the lower limb strength and resistance. |
NA | NA |
| Blanco et al. 2021 | NA | Lung function was normal, except for DLCO < 80% was associated with severe disease in the SARS-CoV-2 group during their hospital stays. | No differences were observed after analyzing 6-MWT | NA | NA |
| Bellan et al. 2021 | NA | DCLO was reduced to less than 80% of the estimated value in 51.6% patients and less than 60% in 15.5% patients. | 2-min walk test revealed a subtler impairment in 75 patients (31.5%). 22.3% patients were found to have limited mobility based on SPPB test results. |
NA | NA |
| Cao et al. 2021 | NA | Patients manifested abnormal pulmonary function in the different disease severity subgroups. | The 6-MWT for male patients and female patients, which was significantly lower than in healthy controls. | NA | The SF-36 scores were significantly impaired in the PR, RE and SF domains. |
| Cortés-Telles et al. 2021 | NA | Patients with persistent dyspnoea had significantly lower FVC, FEV and DLCO, compared in the non-dyspnoea group. | Patients with persistent dyspnoea had lower predicted 6-MWT and SpO2 lower compared to non-dyspnoea patients. | NA | NA |
| Debeaumot et al. 2021 | NA | VO2 peak reduction in 87% hospitalized patients and dyspnea was significantly associated with a reduction in physical fitness. | NA | NA | NA |
| Guler et al. 2021 | Respiratory muscle strength did not differ in both groups. | FEV1, FVC, DLCO, CPT were significantly lower in patients severe/critic. | 6-MWT was 120 m lower in the severe/critical disease group. | NA | NA |
| Huang et al. 2021 | NA | More severely ill patients had increased risk of pulmonary diffusion abnormality. | More severely ill patients presented a short distance of 6-MWT. | NA | More severely ill patients more had mobility problems, pain or discomfort and anxiety. |
| Iqbal et al. 2021 | NA | NA | NA | NA | The severity of COVID-19 was significantly impacted in 5 dimensions of the EQ-5D-5L |
| Leite et al. 2021 | NA | NA | NA | Independence for ADLs, AIVDs was lower in the group (ICU) than in the ward group | .NA |
| Parker et al. 2021 | Observed significant physical weakness in critically ill patients and reduced handgrip strength | Pulmonary function tests identified a mild restrictive defect. | NA | NA | Scores were reduced in all domains of SF-36. |
| PHOSP-COVID et al. 2021 | NA | There was a higher proportion of individuals with a TLCO < 80% predicted | The percent predicted ISWT distance was lower in WHO category 7-9 | NA | EQ5D-5L VAS 0-100 worse than at hospital admission. |
| Piquet et al. 2021 | At admission, there was marked motor weakness and with a mean grip strength at 80% of normal values. | NA | Patients had at admission frequency of sitting down to stand upright were decreased. | Barthel at admission was thus notably low with 5% of the patients having even lost all autonomy for daily activities. | NA |
| Puchner et al. 2021 | Patients start of rehabilitation with reduced respiratory muscle strength (MIP 54 cmH2O) | FEV1, FVC, TCL, DCLO in 74% of all subjects reduced. | Patients start of rehabilitation with decrease in the 6-MWT 323 m. | Patients start of rehabilitation with limitation ADL 83/100. | NA |
| Qu et al. 2021 | NA | NA | NA | NA | The HRQoL of COVID-19, except for the general health dimension, was significantly lower than normal. |
| Rass et al. 2021 | NA | NA | NA | NA | SF-36 was impaired in 31% of patients. |
| Taboada et al. 2021 | NA | NA | NA | 38%patients had lowered two grades in the PCFS, and 45% patients whit persistent functional limitations (grades 2–4 in the PCFS). | Decrease in the quality of life was observed among 67%patients. |
| Todt et al. 2021 | NA | NA | NA | NA | Patients overall worsening of EQ-5D-3L this affected all 5 domains, but especially pain, anxiety and depression. |
| Townsend et al. 2021 | NA | NA | The distance covered was not associated with initial disease severity, was associated with frailty and length of stay. | NA | NA |
| Van Gassel et al. 2021 | Patients with impaired physical performance had more muscle weakness. Handgrip strength was corresponding to 81% of predicted. | Reduced lung diffusing capacity and a median DLCO of 62% of predicted | 6-MWT was below 80% of predicted in 48% of patients. | NA | EQ-5D (EQ-5D) was significantly lower in patients with impaired 6-MWT. |
| Zampogna et al. 2021 | MRCm strength test for quadriceps and biceps, were reduced for groups. | NA | 5.4% covered a mean distance of 423.7, around 70% of the predicted value. | All 56 patients showed a disability with Bi. | All 56 patients showed a reduced EuroQoL-VAS. |
| Wiertz et al. 2021 | 72.7% muscle weakness was present in all major muscle group. | NA | NA | Patients presented with limitation Barthel Index whit mean of 10.5 | NA |
| Wu et al. 2021 | NA | 41.5%had pulmonary dysfunction and 32.1% impairment DLCO < 80% of the predicted value | NA | NA | NA |
| Belli et al. 2020 | NA | NA | 74.4% of the patients below percentile 2.5 of the reference value the 1-min STS test | 67% of the patients scored poorly (≤60 points) on the Barthel index. | NA |
| Carfi et al. 2020 | NA | NA | NA | NA | Worse quality of life was observed in 44.1% of patients. |
| Chen et al. 2020 | NA | NA | NA | NA | Significant difference in HRQoL in patients with COVID-19 and small scores for FP, FS, and PR. |
| Curci et al. 2020 | NA | NA | 6-MWT was feasible in 18.8% patients with a mean distance of 45.0 ± 100.6 meters. | BI was 45.2, in patients in need of higher FiO2 (≥40%) showing lower values: 39.6 vs. 53.3. | NA |
| Garrigues et al. 2020 | NA | NA | NA | NA | In both groups, the EQ-5D (mobility, self-care, pain, anxiety, habitual activity) was changed. |
| Hewitt et al. 2020 | The prevalence of frailty (CFS 5–8) was 49.4% and this frailty was associated with both early death and longer hospital stay. | NA | NA | NA | NA |
| Jacobs et al. 2020 | NA | NA | NA | ADL declined with increased physical effort, such as climbing stairs, lifting and carrying, and walking fast. | Patients presented whit a lower for overall health and quality of life. |
| Liang et al. 2020 | NA | Some 42% patients with FEV1, FEV1/FVC and DLCO decreasing. | NA | NA | NA |
| Valent et al. 2020 | NA | NA | NA | NA | All survivors scored poorly across all SF-36 domains and questionnaire EQ-5D-3 L |
| Vilches-Moraga et al. 2020 | Frailty was associated with an increase in care needs compared to patients without frailty. | NA | NA | NA | NA |
| Zhao et al. 2020 | NA | Lung function abnormalities were detected in 25.4% patients. DLCO anomalies was the most common symptom appeared. | NA | NA | NA |
| Zhu et al. 2020 | NA | NA | NA | NA | ADL dependency was present in 16.44%. Age was an additional independent risk factor for IADL limitations and ADL dependance. |
NA: not analyzed; BI: Barthel of activity of daily life; Bd: Bathel dyspnoea; SBC: Single Breath Counting; SPPB: Short Physical Performance Battery; MRCm: Medical Research Council Muscular; 1STS: One Minute Sit to Stand; 6MWT: six minute walk test; EuroQoL-VAS: Euro Quality of Life with visual analog scale; (BI) Barthel Index; LLN: limite inferior da faixa normal; EQ-5D-5L: EuroQol five-dimension five-level; ISWT: shuttle walk test; FACIT: Fatigue Scale; NRS: Numeric rating scale; IADL: Lawton scale; 1STS: One Minute Sit to Stand; SBC: Single Breath Counting; CFS: Clinical frailty scale; SPPB: Short Physical Performance Battery of 0–6 points; CFQ-11 CFS: Chalder Fatigue Scale; VAS: visual analog scale to evaluate physical and mental fatigue; PROMIS®: Patient-Reported Outcomes Measurement Information System; HGS: Hand grip strength; MIP: maximal inspiratory pressure; STS:10 sit-to-stands; ST: standardized questionnaire; EuroQol: visual analog scale; SPPB: Short Physical Performance Battery; 2MWT: two-minute walk test; 6MWD: The 6-min walk distances; PR: physical role; RE: emotional role; SF: social functioning; mMRC; Medical Research Council dyspnea; 1-MSTST: 1-min sit-to-stand test; PCFS: Functional Status scale.
The findings revealed a decline in daily living activities of assessed by Barthel of activity of daily life, Lawton scale, and PROMIS Item Bank v1.0—Dyspnea Functional Limitations–Short Form 10a, and post-COVID-19 Functional Status scale. Additionally, a decline in health-related quality of life also was observed. Euro Quality of Life with visual analog scale, EuroQol five-dimension five-level, Patient-Reported Outcomes Measurement Information System (PROMIS1Scale v1.2—Global Health), and Short-Form 36-item questionnaire were used.
The NOS was applied to score and classify the quality of these studies, as displayed in Table 3. According to NOS 13 (37.14%) of the 35 studies were classified as “Good studies”, 19 (54.28%) as “Satisfactory Studies” and 3 (8.58%) as “Unsatisfactory Studies” quality ranking. The minimum and maximum scores were, respectively, 3 and 8 points. The items with lower proportion of compliance were adequacy of follow-up of cohorts, comparability of cohorts based on the design or analysis, and selection exposed cohort.
Table 3.
Newcastle-Ottawa scale of studies included in the systematic review.
| Study | Selection | Comparability | Outcome | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Representativeness | Selection exposed cohort | Ascertainment | Result not present at start of the study | Comparability for confounders | Assessment of outcome | Follow-up duration | Adequacy of follow-up | Total | |
| *Baricich et al. 2021 | * | * | * | * | * | * | - | 6 | |
| Blanco et al. 2021 | * | * | * | * | * | 5 | |||
| Bellan et al. 2021 | * | * | * | * | * | * | 6 | ||
| Cao et al. 2021 | * | ** | * | * | * | 6 | |||
| Cortés-Telles et al. 2021 | * | * | * | 3 | |||||
| Debeaumot et al. 2021 | * | * | * | * | * | * | * | 7 | |
| Guler et al. 2020 | * | * | * | * | * | * | * | 7 | |
| Huang et al. 2021 | * | * | * | * | ** | * | * | 8 | |
| *Iqbal et al. 2021 | * | * | * | * | * | - | 5 | ||
| *Leite et al. 2021 | * | * | * | * | * | - | 5 | ||
| Parker et al. 2021 | * | * | * | * | 4 | ||||
| PHOSP-COVID et al. 2021 | * | ** | * | * | * | * | 7 | ||
| Piquet et al. 2021 | * | * | * | * | * | * | 6 | ||
| Puchner et al. 2021 | * | * | * | ** | * | * | 7 | ||
| Qu et al. 2021 | * | ** | * | * | * | * | 7 | ||
| Rass et al. 2021 | * | * | * | * | * | 5 | |||
| Taboada et al. 2021 | * | * | * | * | * | * | 6 | ||
| Todt et al. 2021 | * | * | * | * | * | * | * | 6 | |
| *Townsend et al. 2021 | * | * | * | ** | * | * | - | 7 | |
| Van Gassel et al. 2021 | * | * | * | * | * | * | 6 | ||
| Zampogna et al. 2021 | * | * | * | * | * | * | * | 7 | |
| *Wiertz et al. 2021 | ** | ** | - | 4 | |||||
| Wu et al. 2021 | * | * | * | * | * | * | 6 | ||
| Belli et al. 2020 | * | * | * | * | * | 5 | |||
| Carfi et al. 2020 | * | * | ** | * | * | 6 | |||
| *Chen et al. 2020 | * | * | * | * | * | * | - | 6 | |
| *Curci et al. 2020 | * | ** | * | * | - | 5 | |||
| Garrigues et al. 2020 | * | * | * | * | * | 5 | |||
| Hewitt et al. 2020 | * | * | * | ** | * | * | 7 | ||
| Jacobs et al. 2020 | * | * | * | * | * | * | * | 7 | |
| Liang et al. 2020 | * | * | * | * | * | * | * | 7 | |
| Valent et al. 2020 | * | * | * | * | * | 5 | |||
| Vilches-Moraga et al. 2020 | * | * | * | ** | * | * | 7 | ||
| Zhao et al. 2020 | * | ** | * | * | * | 6 | |||
| Zhu et al. 2020 | * | ** | * | * | * | * | * | 8 | |
*Newcastle-Ottawa scale adapted for cross-sectional studies, item adequacy of monitoring does not score; Zero star the item is not registered in the article; Very good studies: 9 to 10 points; Good studies: 7-8 points; Satisfactory Studies: 5-6 points; Unsatisfactory Studies: 0 to 4 points; Cross-Sectional Studies: a study can receive a maximum of one star for each numbered item in the Selection and Exhibition categories. A maximum of two stars can be given for Comparability; Cohort Studies: A study can receive a maximum of one star for each numbered item in the Selection and Result categories. A maximum of two stars can be given for comparability.
Discussion
This systematic review indicate that COVID-19 survivors can have a reduction in physical function, individuals’ ability to perform activities of daily living and their health-related quality of life 1 to 6 months post-infection. Our systematic review expands the knowledge about the impact of COVID-19 on the activity of daily living and additionally includes other important outcomes such as physical function and health-related quality of life. Thus, this systematic review is important because it analyzes the impact of COVID-19 on important outcomes for COVID-19 survivors. the eligibility of physical function, activities of daily living and health-related quality as outcomes in this systematic review is important because they are related to prognosis in patients with chronic diseases.50–52
Post-acute COVID-19 is defined as persistent symptoms and/or delayed or long-term complications beyond 4 weeks from the onset of symptoms. There are increasing reports of persistent and prolonged effects after acute COVID-19.10 Thus, this systematic review is important because it analyzes the impact of COVID-19 on pulmonary function tests, functional and exercise capacity, fatigue, and health-related quality. Nalbandian et al.,10 reinforces the need for systematic studies of sequelae after recovery from acute COVID-19 with a focus on developing new evidence-based multidisciplinary team approaches to care for these patients and to inform research priorities. Furthermore, decreased levels of physical function, physical performance and exercise capacity are associated with an increased risk of mortality in the general population and in people with chronic diseases.50–54
Our results show that there is a reduction in physical and pulmonary function tests in COVID-19 survivors. Specifically, post-infection COVID-19 patients showed impaired lung function, muscle strength, exercise capacity and persistent fatigue. Patients also had reduced performance in functional tests such as Short Physical Performance Battery Test, 2-min walking test, and 1-min sit-to-stand test. Our findings are consistent with those of recent reviews, in which acute post-COVID-19 patients suffer from changes in respiratory function, fatigue, muscle weakness and disability.55–58 Welch et al.59 point out that declines in function/quantity of muscle mass in six months can result in acute sarcopenia, which has affected patients with COVID-19. According to Iqbal et al.,60 persistent respiratory symptoms are consistent with findings from previous outbreaks of SARS-CoV that demonstrated a restrictive pattern of lung function metrics consistent with the resultant muscle weakness six to eight weeks following hospital discharge. They also reported that the persistence of functional symptoms could be exacerbated in the context of social distancing and isolation.60
Our results also showed that there is a reduction in activities of daily living in COVID-19 survivors. In a recent systematic review, Pizarro-Pennarolli et al.,11 identified nine studies that assessed performance of activities of daily living using six different scales: Barthel Index, Activities of Daily Living Score, Functional Independence Measure, Composite Functional Score, Modified Rankin Scale, and EQ-5D-5L. In accordance with our results, they reported that in all included studies, the findings revealed a decline in activities of daily living performance after COVID-19 infection regardless of the scale applied.11 For Belli et al.,38 this generates loss of autonomy and independence for the patient, which can negatively impact their quality of life.38
Clemente-Suárez et al.61 reported that the COVID-19 negatively influences motor behavior, levels of regular exercise, nutritional patterns, and psychological status.61 In addition, immobilization during acute illness also can cause loss of physical functions with impact on activities of daily living. These factors may be associated with physical and functional decline and reduced performance in activities of daily living. Thus, further research is needed to determine the factors associated with reduced functioning and activities of daily living, as well as to identify the best rehabilitation strategy to improve functioning and activities of daily living after COVID-19.
Quality of life is a particularly important outcome in studies involving COVID-19 survivors. Our results also showed that there is a reduction in health-related quality of life in COVID-19 survivors. Valent et al.,46 reported that physical and psychological sequelae may be more frequent in COVID-19 patients because of restriction of visitation, and constraints on social as well as rehabilitation supports due to the risk of transmission. Iqbal et al.,23 describes that, after recovery, the COVID-19 survivors often experience discrimination and prejudice because of the community's irrational fear that they are still contagious. Thus, it is important to dispel any myths reinforcing the idea that a COVID-19 survivor is still contagious after recovery to help reduce the stigma and allow rapid reintegration of the individual into society.23
Considering the global scale of this pandemic, it is expected that the healthcare needs for patients with sequelae of COVID-19 will continue to increase for the future. A comprehensive understanding of patient care needs beyond the acute phase is necessary and will help in the development of infrastructure and healthcare for improved quality of life and physical health of survivors of COVID-19 in the long term.
Prioritization of follow-up care may be considered for those at high risk of post-acute COVID-19, including those who had severe illness during acute COVID-19 and/or required care in an ICU, and those with the highest burden of persistent symptoms.10 Nalbandian et al.10 reported that it is clear that care for patients with COVID-19 does not complete at the time of hospital discharge, and interdisciplinary cooperation is needed for comprehensive care of these patients in the outpatient setting.10 Rehabilitation programs after early hospitalization can minimize functional losses and improve quality of life. Sheehy et al.,9 suggest that a thorough assessment and an individualized, progressive treatment plan which focuses on functioning, and return to participation in society will help each patient to maximize their functioning and quality of life. Careful consideration on the rehabilitation environment will ensure that all patients recover as completely as possible.9 Thus, the available findings in this systematic review can be used to understand the potential disabilities and rehabilitation needs of people recovering from the COVID-19.
Despite the important findings presented, the results of this systematic review are limited by the lack of high-quality, larger, multicentric and long-term studies. Results were limited by heterogeneity of studies, insufficient standardization, and absence of control for confounders in individual studies. A main limitation of the included studies is the small sample sizes. In addition, included studies reported a mixture of cohort sampling (hospitalized, community, and mixed), different follow-up timepoints, and data collection measures, which likely contributed to the detected heterogeneity.
Conclusion
Physical disability and reduction in health-related quality of life is common in COVID-19 survivors 1 to 6 months post-infection. These results highlight the need for a long-term follow-up of those patients and for rehabilitation programs. Rehabilitation studies are warranted, especially low-cost strategies, to prevent and treat functional declines.
Supplemental Material
Supplemental material, sj-pdf-1-chi-10.1177_17423953221089309 for A systematic review on physical function, activities of daily living and health-related quality of life in COVID-19 survivors by Katna de Oliveira Almeida, Iura Gonzalez Nogueira Alves, Rodrigo Santos de Queiroz, Marcela Rodrigues de Castro, Vinicius Afonso Gomes, Fabiane Costa Santos Fontoura, Carlos Brites and Mansueto Gomes Neto in Chronic Illness
Footnotes
Contributorship: KA, MGN CB, IA, VF, FF and MS conceived the study. KA, RQ and MS researched literature. KA, MC, CB, MGN was involved in protocol development. KA, RQ, VG CB, IA, and MGN was involved in data analysis. KA, FF, VG, MC, CB, and MGN wrote the first draft of the manuscript. All authors reviewed and edited the manuscript and approved the final version of the manuscript.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical approval: Not applicable, because this article does not contain any studies with human or animal subjects.
Guarantor: MGN
Informed consent: Not applicable, because this article does not contain any studies with human or animal subjects.
Trial registration: Not applicable, because this article does not contain any clinical trials.
ORCID iDs: Katna de Oliveira Almeida https://orcid.org/0000-0002-6190-7189
Iura Gonzalez Nogueira Alves https://orcid.org/0000-0003-2455-3788
Mansueto Gomes Neto https://orcid.org/0000-0002-0717-9694
Supplemental material: Supplemental material for this article is available online.
References
- 1.De Sire A, Andrenelli E, Negrini Fet al. et al. International multiprofessional steering committee of cochrane rehabilitation REH-COVER action. Rehabilitation and COVID-19: a rapid living systematic review by Cochrane rehabilitation field updated as of December 31st, 2020 and synthesis of the scientific literature of 2020. Eur J Phys Rehabil Med 2021. DOI: 10.23736/S1973-9087.21.06870 [DOI] [PubMed] [Google Scholar]
- 2.Kiekens C, Boldrini P, Andreoli A, et al. Rehabilitation and respiratory management in the acute and early post-acute phase. “Instant paper from the field” on rehabilitation answers to the COVID-19 emergency. Eur J Phys Rehabil Med 2020; 56: 323–326. [DOI] [PubMed] [Google Scholar]
- 3.Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E, et al. Clinical, laboratory and imaging features of COVID-19: a systematic review and meta-analysis. Travel Med Infect Dis 2020; 34: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palayew A, Norgaard O, Safreed-Harmon Ket al. et al. Pandemic publishing poses a new COVID-19 challenge. Nat Hum Behav 2020; 4: 666–669. [DOI] [PubMed] [Google Scholar]
- 5.Borges do Nascimento IJ, Cacic N, Abdulazeem HM, et al. Novel coronavirus infection (COVID-19) in humans: a scoping review and meta-analysis. J Clin Med 2020; 9: 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barker-Davies RM, O’Sullivan O, Senaratne KPP, et al. The Stanford Hall consensus statement for post-COVID-19 rehabilitation. Br J Sports Med 2020; 54: 949–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rooney S, Webster A, Paul L. Systematic review of changes and recovery in physical function and fitness after severe acute respiratory syndrome-related coronavirus infection: implications for COVID-19 rehabilitation. Phys Ther 2020; 100: 1717–1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simpson R, Robinson L. Rehabilitation following critical illness in people with COVID-19 infection. Am J Phys Med Rehabil 2020; 99: 470–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sheehy LM. Considerations for postacute rehabilitation for survivors of COVID-19. JMIR Public Health Surveill 2020; 6: e19462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nalbandian A, Sehgal K, Gupta A, et al. Post-acute COVID-19 syndrome. Nat Med 2021; 27: 601–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pizarro-Pennarolli C, Sánchez-Rojas C, Torres-Castro R, et al. Assessment of activities of daily living in patients post COVID-19: a systematic review. PeerJ 2021; 9: e11026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Br Med J 2009; 339: b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Higgins JPT, Thomas J, Chandler J, et al. (eds). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021, www.training.cochrane.org/handbook (accessed 20 October 2021).
- 14.Wells GASB, O’Connell D, Peterson J, et al. The Newcastle -Ottawa scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses. 2011, http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed 12 March 2021).
- 15.Baricich A, Borg MB, Cuneo D, et al. Midterm functional sequelae and implications in rehabilitation after COVID-19: a cross-sectional study. Eur J Phys Rehabil Med 2021; 57: 199–207. [DOI] [PubMed] [Google Scholar]
- 16.Bellan M, Soddu D, Balbo PE, et al. Respiratory and psychophysical sequelae among patients with COVID-19 four months after hospital discharge. JAMA Netw Open 2021; 4: e2036142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blanco JR, Cobos-Ceballos MJ, Navarro F, et al. Pulmonary long-term consequences of COVID-19 infections after hospital discharge. Clin Microbiol Infect 2021; 27: 892–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cao J, Zheng X, Wei W, et al. Three-month outcomes of recovered COVID-19 patients: prospective observational study. Ther Adv Respir Dis 2021; 15: 17534666211009410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cortés-Telles A, López-Romero S, Figueroa-Hurtado E, et al. Pulmonary function and functional capacity in COVID-19 survivors with persistent dyspnoea. Respir Physiol Neurobiol 2021; 288: 103644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Debeaumont D, Boujibar F, Ferrand-Devouge E, et al. Cardiopulmonary exercise testing to assess persistent symptoms at 6 months in people with COVID-19 who survived hospitalization—a pilot study. Phys Ther 2021: pzab099. DOI: 10.1093/ptj/pzab099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guler SA, Ebner L, Aubry-Beigelman C, et al. Pulmonary function and radiological features 4 months after COVID-19: first results from the national prospective observational Swiss COVID-19 lung study. Eur Respir J 2021; 57: 2003690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang C, Huang L, Wang Y, et al. 6-month Consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet 2021; 397: 220–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iqbal A, Iqbal K, Arshad Ali S, et al. The COVID-19 sequelae: a cross-sectional evaluation of post-recovery symptoms and the need for rehabilitation of COVID-19 survivors. Cureus 2021; 13: e13080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leite VF, Rampim DB, Jorge VCet al. et al. Persistent symptoms and disability after COVID-19 hospitalization: data from a comprehensive telerehabilitation program. Arch Phys Med Rehabil 2021; 102: 1308–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parker AJ, Humbir A, Tiwary P, et al. Recovery after critical illness in COVID-19 ICU survivors. Br J Anaesth 2021; 126: e217–e219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.PHOSP-COVID Collaborative Group, et al. Physical, cognitive and mental health impacts of COVID-19 following hospitalisation—a multi-centre prospective cohort study. medRxiv preprint. DOI: 10.1101/2021.03.22.21254057; this version posted March 24, 2021. [DOI]
- 27.Piquet V, Luczak C, Seiler F, Monaury J, Martini A, Ward AB, et al. COVID rehabilitation study group. Do patients with COVID-19 benefit from rehabilitation? Functional outcomes of the first 100 patients in a COVID-19 rehabilitation unit. Arch Phys Med Rehabil 2021; 102: 1067–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Puchner B, Sahanic S, Kirchmair R, et al. Beneficial effects of multi-disciplinary rehabilitation in postacute COVID-19: an observational cohort study. Eur J Phys Rehabil Med 2021; 57: 189–198. [DOI] [PubMed] [Google Scholar]
- 29.Qu G, Zhen Q, Wang W, et al. Health-related quality of life of COVID-19 patients after discharge: a multicenter follow-up study. J Clin Nurs 2021; 30: 1742–1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rass V, Beer R, Schiefecker AJ, et al. Neurological outcome and quality of life 3 months after COVID-19: a prospective observational cohort study. Eur J Neurol 2021. DOI: 10.1111/ene.14803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taboada M, Moreno E, Cariñena A, et al. Quality of life, functional status, and persistent symptoms after intensive care of COVID-19 patients. Br J Anaesth 2021; 126: e110–e113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Todt BC, Szlejf C, Duim E, et al. Clinical outcomes and quality of life of COVID-19 survivors: a follow-up of 3 months post hospital discharge. Respir Med 2021; 184: 106453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Townsend L, Dowds J, O’Brien K, et al. Persistent poor health post-COVID-19 is not associated with respiratory complications or initial disease severity. Ann Am Thorac Soc 2021. DOI: 10.1513/AnnalsATS.202009-1175OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Gassel RJJ, Bels J, Remij L, et al. Functional outcomes and their association with physical performance in mechanically ventilated coronavirus disease 2019 survivors at 3 months following hospital discharge: a cohort study. Crit Care Med 2021. DOI: 10.1097/CCM.0000000000005089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zampogna E, Migliori GB, Centis R, et al. Functional impairment during post-acute COVID-19 phase: preliminary finding in 56 patients. Pulmonology 2021: S2531-0437(20)30268-3. DOI: 10.1016/j.pulmoe.2020.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wiertz CMH, Vints WAJ, Maas GJCM, et al. COVID-19: patient characteristics in the first phase of post-intensive care rehabilitation. Arch Rehabil Res Clin Transl 2021: 100108. DOI: 10.1016/j.arrct.2021.100108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu Q, Zhong L, Li H, et al. A follow-up study of lung function and chest computed tomography at 6 months after discharge in patients with coronavirus disease 2019. Can Respir J 2021; 2021: 6692409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Belli S, Balbi B, Prince I, et al. Low physical functioning and impaired performance of activities of daily life in COVID-19 patients who survived hospitalisation. Eur Respir J 2020; 56: 2002096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carfì A, Bernabei R, Landi F, et al. Persistent symptoms in patients after acute COVID-19. JAMA 2020; 324: 603–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen KY, Li T, Gong FHet al. et al. Predictors of health-related quality of life and influencing factors for COVID-19 patients, a follow-up at one month. Front Psychiatry 2020; 11: 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Curci C, Pisano F, Bonacci E, et al. Early rehabilitation in post-acute COVID-19 patients: data from an Italian COVID-19 rehabilitation unit and proposal of a treatment protocol. Eur J Phys Rehabil Med 2020; 56: 633–641. [DOI] [PubMed] [Google Scholar]
- 42.Garrigues E, Janvier P, Kherabi Y, et al. Post-discharge persistent symptoms and health-related quality of life after hospitalization for COVID-19. J Infect 2020; 81: e4–e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hewitt J, Carter B, Vilches-Moraga A, , et al. The effect of frailty on survival in patients with COVID-19 (COPE): a multicentre, European, observational cohort study. Lancet Public Health 2020; 5: e444–e451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jacobs LG, Gourna Paleoudis E, Lesky-Di Bari D, et al. Persistence of symptoms and quality of life at 35 days after hospitalization for COVID-19 infection. PLoS One 2020; 15: e0243882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liang L, Yang B, Jiang Net al. et al. Three-month follow-up study of survivors of coronavirus disease 2019 after discharge. J Korean Med Sci 2020; 35: e418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Valent A, Dudoignon E, Ressaire Qet al. et al. Three-month quality of life in survivors of ARDS due to COVID-19: a preliminary report from a French academic centre. Anaesth Crit Care Pain Med 2020; 39: 740–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vilches-Moraga A, Price A, Braude Pet al. et al. Increased care at discharge from COVID-19: the association between pre-admission frailty and increased care needs after hospital discharge; a multicentre European observational cohort study. BMC Med 2020; 18: 08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhao YM, Shang YM, Song WB, et al. Follow-up study of the pulmonary function and related physiological characteristics of COVID-19 survivors three months after recovery. E Clinical Medicine 2020; 25: 100463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhu S, Gao Q, Yang L, et al. Prevalence and risk factors of disability and anxiety in a retrospective cohort of 432 survivors of coronavirus disease-2019 (COVID-19) from China. PLoS One 2020; 15: e0243883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Metter EJ, Talbot LA, Schrager Met al. et al. Skeletal muscle strength as a predictor of all-cause mortality in healthy men. J Gerontol A Biol Sci Med Sci 2002; 57: B359–B365. [DOI] [PubMed] [Google Scholar]
- 51.Imboden MT, Harber MP, Whaley MHet al. et al. Cardiorespiratory fitness and mortality in healthy men and women. J Am Coll Cardiol 2018; 72: 2283–2292. [DOI] [PubMed] [Google Scholar]
- 52.Dickstein K, Cohen-Solal A, Filippatos G, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the task force for the diagnosis and treatment of acute and chronic heart failure 2008 of the European society of cardiology. Developed in collaboration with the heart failure association of the ESC (HFA) and endorsed by the European society of intensive care medicine (ESICM). Eur Heart J 2008; 29: 2388–2442. [DOI] [PubMed] [Google Scholar]
- 53.Blair SN, Wei M, Lee CD. Cardiorespiratory fitness determined by exercise heart rate as a predictor of mortality in the aerobics center longitudinal study. J Sports Sci 1998; 16(Suppl): S47–S55. [DOI] [PubMed] [Google Scholar]
- 54.Lee CD, Blair SN. Cardiorespiratory fitness and stroke mortality in men. Med Sci Sports Exerc 2002; 34: 592–595. [DOI] [PubMed] [Google Scholar]
- 55.Ali AM, Kunugi H. Skeletal muscle damage in COVID-19: a call for action. Medicina (Kaunas) 2021; 57: 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mihalick VL, Canada JM, Arena Ret al. et al. Cardiopulmonary exercise testing during the COVID-19 pandemic. Prog Cardiovasc Dis 2021: S0033-0620(21)00049-9. DOI: 10.1016/j.pcad.2021.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Torres-Castro R, Vasconcello-Castillo L, Alsina-Restoy X, et al. Respiratory function in patients post-infection by COVID-19: a systematic review and meta-analysis. Pulmonology 2020: S2531-0437(20)30245-2. DOI: 10.1016/j.pulmoe.2020.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Boutou AK, Georgopoulou A, Pitsiou Get al. et al. Changes in the respiratory function of COVID-19 survivors during follow-up: a novel respiratory disorder on the rise? Int J Clin Pract 2021: e14301. DOI: 10.1111/ijcp.14301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Welch C, Greig C, Masud Tet al. et al. COVID-19 and acute sarcopenia. Aging Dis 2020; 11: 1345–1351. PMID: 33269092; PMCID: PMC7673845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Iqbal FM, Lam K, Sounderajah Vet al. et al. Characteristics and predictors of acute and chronic post-COVID syndrome: a systematic review and meta-analysis. E Clinical Medicine 2021; 36: 100899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Clemente-Suárez VJ, Beltrán-Velasco AI, Ramos-Campo DJ, et al. Physical activity and COVID-19. The basis for an efficient intervention in times of COVID-19 pandemic. Physiol Behav 2022; 244: 113667. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-chi-10.1177_17423953221089309 for A systematic review on physical function, activities of daily living and health-related quality of life in COVID-19 survivors by Katna de Oliveira Almeida, Iura Gonzalez Nogueira Alves, Rodrigo Santos de Queiroz, Marcela Rodrigues de Castro, Vinicius Afonso Gomes, Fabiane Costa Santos Fontoura, Carlos Brites and Mansueto Gomes Neto in Chronic Illness