Abstract
Oral infections with the pathogenic yeast Candida albicans are one of the most frequent and earliest opportunistic infections in human immunodeficiency virus-infected patients. The widespread use of azole antifungal drugs has led to the development of drug-resistant isolates. Several molecular mechanisms that contribute to drug resistance have been identified, including increased mRNA levels for two types of efflux pump genes: the ATP binding cassette transporter CDRs (CDR1 and CDR2) and the major facilitator MDR1. Using Northern blot analyses, the expression patterns of these genes have been determined during logarithmic and stationary phases of cell growth and during growth in different carbon sources in a set of matched susceptible and fluconazole-resistant isolates that have been characterized previously. MDR1, CDR1, and CDR2 are expressed early during logarithmic growth, CDR4 is expressed late during logarithmic growth, and CDR1 is preferentially expressed in stationary-phase cells. There is a small decrease in expression of these genes when the cells are grown in carbon sources other than glucose. While increased mRNA levels of efflux pump genes are commonly associated with azole resistance, the causes of increased mRNA levels have not yet been resolved. Southern blot analysis demonstrates that the increased mRNA levels in these isolates are not the result of gene amplification. Nuclear run-on assays show that MDR1 and CDR mRNAs are transcriptionally overexpressed in the resistant isolate, suggesting that the antifungal drug resistance in this series is associated with the promoter and trans-acting factors of the CDR1, CDR2, and MDR1 genes.
Candida albicans is a pathogenic yeast that causes oral, vaginal, and systemic infections (reviewed in reference 28). These infections are usually treated with antifungal drugs, including the polyene amphotericin B and the azoles, such as fluconazole. Azole-resistant strains of C. albicans are an increasing problem in human immunodeficiency virus-infected patients and other immunosuppressed individuals (37). One recent study estimates that up to a third of all AIDS patients retain an azole-resistant C. albicans isolate orally (17). Recently, there have been reports of azole-resistant Candida infections in other immunosuppressed patients (21, 22, 24, 27). Factors that contribute to the development of clinical resistance in patients are numerous and include the extent of immunosuppression, the level of exposure to azole drugs, and intrinsic properties of the fungus, including drug susceptibility (37).
Several molecular mechanisms that contribute to C. albicans azole resistance have been identified (reviewed in reference 37). The fungistatic azoles, such as fluconazole, work by competitive inhibition of lanosterol demethylase, the product of ERG11 and an important enzyme in the ergosterol biosynthetic pathway. Ergosterol is an essential component and the major sterol of the fungal cell membrane. Alterations in this pathway that contribute to resistance include point mutations and increased expression of ERG11 and possible genetic alterations in other genes in the biosynthetic pathway for ergosterol. Azole resistance has also been correlated with increased export of azoles from the cell, usually associated with the increased expression of efflux pumps. Increased mRNA levels of the efflux pump gene family CDR (members of the ATP binding cassette transporter superfamily) and MDR1 (a major facilitator) have correlated with increased resistance. At least seven CDR genes have been identified (CDR1 to CDR7) (C. albicans information web page [http://alces.med.umn.edu/Candia.html]) although to date only CDR1 and CDR2 have been associated with azole resistance (2, 7, 32, 33). A series of 17 isolates from a human immunodeficiency virus-infected patient has previously been shown to exhibit many of the resistance mechanisms described above (35–38). Azole resistance developed gradually in this series. Several resistance mechanisms were identified in the series. The timing of the occurrence of each of these resistance mechanisms correlated with an incremental increase in the MIC, a standard measure of the resistant phenotype of the cells (25).
The correlation between resistance and increased mRNA levels of efflux pumps and genetic alterations of ERG11 has been well-documented in several different C. albicans series. These increases have usually been investigated during mid-logarithmic growth of the culture in media containing glucose (as in references 35 and 36). Recently, one study has demonstrated changes in CDR1 mRNA levels during cell growth (15). Under standard growth conditions (i.e., 30°C in rich or minimal medium with glucose as a carbon source), yeast cells such as Saccharomyces cerevisiae or C. albicans undergo several phases of growth (reviewed in references 14 and 34). After an initial lag phase, the cells begin a rapid growth phase (logarithmic growth) in which glucose fermentation is the major source of ATP production and cells divide exponentially. As cells exhaust the glucose in the medium, they undergo a diauxic shift and begin preparation for the use of other carbon sources (e.g., ethanol). Finally, cell growth slows as the culture reaches stationary phase, in which cell growth arrests due to depletion of available carbon sources.
The mRNA levels of genes linked with azole resistance have not been defined throughout these phases of growth. The diauxic shift of C. albicans has only been investigated as it relates to mannitol catabolism (26). If gene expression associated with azole resistance is variable during particular growth phases, this may have a large impact on azole susceptibility in the distinct growth environments of oral, vaginal, and systemic candidiasis during both colonization and infection.
As mentioned above, increased mRNA levels of ERG11, CDR1, and MDR1 have been correlated with azole resistance. However, it has not been determined by what mechanism(s) these mRNAs are increased. Eukaryotic cells generally employ several techniques to increase mRNA levels. One common method is gene amplification, whereby a gene is copied several times. Normal transcription rates of each gene copy result in a greater total mRNA product. Alternatively, the transcription of a gene can be increased by altering the levels of trans-acting factors that interact with the gene promoter or by mutations in the promoter. mRNA levels can also be increased by transcribing a gene at a normal rate but increasing the half-life of the mRNA, generally accomplished by a mutation in the 3′ end of the gene that affects the degradation of the mRNA. Further, nuclear export, 5′ capping, polyadenylation, and RNA splicing can all affect mRNA levels. The standard method for detecting increased mRNA transcription is a nuclear run-on assay (8, 9, 23). In this assay, cells are permeabilized and radioactively labeled UTP is added. The labeled UTP then enters the nucleus, where polymerases that are actively transcribing expressed genes incorporate the labeled nucleotide into nascent RNA chains. Total RNA, which includes the labeled nascent transcripts, is prepared and hybridized to specific gene probes. The radioactive signal detected is a measure of the level of active transcription of the gene. This technique has been successfully performed in a variety of eukaryotic cells, including S. cerevisiae (3, 5), and we have adapted the procedure for use in C. albicans.
In this study, levels of the ERG11, CDR, and MDR1 mRNAs were determined in a susceptible isolate and a fluconazole-resistant isolate from a single strain taken from an AIDS patient. The levels of expression were monitored throughout the course of cell growth (logarithmic, diauxic, and stationary) and during growth in different carbon sources. In addition, Southern blot analyses were used to rule out gene amplification of the efflux pumps and nuclear run-on analysis was used to determine if increased transcription is a cause of the increased mRNA levels of ERG11, CDR, and MDR1 observed in this series.
MATERIALS AND METHODS
Strains and growth of cultures.
The C. albicans isolates used in this study include a susceptible isolate (isolate 1, designated 2-76) and a resistant isolate (isolate 17, designated 12-99) from a series of 17 oral isolates from a single AIDS patient (30). Cultures were routinely inoculated from single colonies. The isolates were grown at 30°C in YEPD (10 g of yeast extract, 20 g of peptone, and 20 g of dextrose per liter) or on YEPD agar plates (10 g of yeast extract, 20 g of peptone, 20 g of dextrose, and 15 g of agar per liter), stored at 4°C, and subcultured weekly or stored at −80°C in YEPD containing 10% glycerol. MICs of fluconazole were determined using the NCCLS broth microdilution method (25). All reagents were purchased from Fisher Scientific (Pittsburgh, Pa.) or Sigma Chemical Co. (St. Louis, Mo.) unless otherwise specified.
DNA extraction and Southern analysis.
Genomic DNAs from the susceptible and resistant isolates were prepared as described (13). Restriction enzyme digestions and Southern blot analyses were performed using standard techniques (19, 31).
RNA manipulations for Northern analysis. (i) Logarithmic growth.
Cells from 24-h cultures grown in YEPD were inoculated in 200 ml of YEPD to a starting concentration of 2 × 104 cells/ml. The cultures were grown overnight at 30°C with agitation. Total RNAs were prepared from cultures of the susceptible and resistant isolates at an optical density at 600 nm (OD600) of 0.1 and at each subsequent doubling time (roughly every 90 min) up to an OD600 of 6.4.
(ii) Late-logarithmic phase, diauxic shift, and stationary phase.
Cells from 24-h YEPD cultures were inoculated into 50 ml of YEPD to a starting concentration of 2 × 104 cells/ml. The cultures were grown at 30°C with agitation. RNA was prepared when the culture reached an OD600 of 6.4 and at 3 and 8 days of growth.
(iii) Carbon source.
Cells from 24-h stationary-phase YEPD cultures were transferred to three different media: YEP (10 g of yeast extract, 20 g of peptone per liter)–2% glucose (equivalent to YEPD), YEP–3% glycerol, and YEP–3% sodium acetate at an initial concentration of 5 × 106 cells/ml. The cultures were grown at 30°C with agitation, and RNAs were prepared when the culture reached an OD600 of approximately 1.0.
Total RNA preparation, gel electrophoresis, Northern blotting, oligonucleotide labeling with polynucleotide kinase, and random priming for radioactive probe preparation were performed according to standard published methods (19, 31).
Nuclear run-on analysis.
The nuclear run-on was performed using previously described methods (5) with the following modifications. Cells were grown at 30°C in YEPD with agitation until the culture reached an OD600 of 1.0. An aliquot of 3 × 107 cells was mixed with a smaller volume of crushed ice in a prechilled round-bottom 15-ml polypropylene tube. The cells were centrifuged for 5 min at 4,000 × g and resuspended in 5 ml of cold TMN (10 mM Tris, 100 mM NaCl, 5 mM MgCl2, pH 7.4). The cells were again centrifuged for 5 min at 4,000 × g and resuspended in 0.95 ml of ice-cold water. Fifty microliters of 10% N-lauroyl sarcosine (Sigma) was added and the mixture was incubated at 4°C for 15 min to permeabilize the cells. The cells were transferred to an Eppendorf tube and centrifuged at 4°C and 6,000 rpm for 2 min (Eppendorf centrifuge model 54 15C; Brinkman Instruments, Westbury, N.Y.). The cells were resuspended in 60 μl of the following freshly made ice-cold reaction mixture: 50 mM Tris (pH 7.9), 100 mM KCl, 5 mM MgCl2, 1 mM MnCl2, 2 mM dithiothreitol, 0.5 mM rATP, 0.25 mM rGTP, 0.25 mM rCTP, 1 U of RNase inhibitor (Boehringer Mannheim, Indianapolis, Ind.) per μl, 10 mM phosphocreatine, 1.2 μg of creatine phosphokinase per μl and 2 μCi of UTP per μl (3,000 Ci/mM). The mixture was incubated at room temperature for 12 min. To stop the reaction, 5 μl of Escherichia coli tRNA (50 mg/ml for carrier RNA) (Boehringer Mannheim), 6 μl of 1 mM CaCl2, and 1 μl of RQ1 DNase (Promega, Madison, Wis.) were added and the mixture was incubated at 37°C for 15 min. (Unlike in the published protocol, α-amanitin was not used to stop the reaction.) One hundred and thirty microliters of RNA buffer (0.05 M Tris, 0.1 M EDTA, 0.1 M NaCl, pH 8.0), 10 μl of 10% sodium dodecyl sulfate, and 4 μl of proteinase K (10 mg/ml) were added, and the mixture was incubated at 37°C for 30 min. To prepare RNA, 200 μl of buffered phenol (pH 8.0) and 200 μg of acid-washed glass beads (Sigma) were added and the tube was vortexed for 5 min. The resulting slurry was centrifuged at 14,000 rpm for 5 min in the microcentrifuge. The aqueous phase was transferred to a new tube on ice at 4°C. Another 200 μl of RNA buffer was added to the remaining slurry, and the tube was again vortexed and centrifuged to extract any remaining RNA. The aqueous phases were pooled, and 400 μl of buffered phenol (pH 8.0) was added. The mixture was vortexed for 1 min and centrifuged at 14,000 rpm for 5 min in the microcentrifuge. The aqueous phase was transferred to a new tube, and the RNA was precipitated with 2.5 M NH4-acetate and an equal volume of isopropanol. The mixture was stored overnight at −20°C. To pellet the RNA, the mixture was centrifuged at 14,000 rpm for 10 min in the microcentrifuge. The isopropanol was removed, and the pellet was resuspended in 0.75 ml of Trizol (GIBCO BRL, Grand Island, N.Y.). The total RNA was then prepared according to the manufacturer's specifications. This double extraction of RNA was used to ensure that there was a minimum of DNA contamination.
Gene fragments and gene probes.
Plasmids containing gene inserts (all within the coding regions) of ERG11, CDR1, MDR1, and ACT1 were used as gene targets against the labeled nuclear run-on probe. The gene fragments were prepared by PCR amplification of a section of the coding region of the gene. The sections that were amplified and cloned were as follows (numbers represent positions in the GenBank sequences; GenBank accession numbers and references are given in parentheses): ERG11: position 164 to 1589 (X13296 [16]), MDR1: position 2885 to 3754 (X53823 [6]), CDR1: position 1210 to 2016 (X77589 [29]), and ACT1: position 1714 to 2515 (X16377, [18]). ACT1 is the gene encoding the actin gene and is used as a control in most of the experiments in this study. In addition to these gene fragments, a 1,045-bp PvuII fragment of the pCR-Script Amp SK(+) plasmid (position 2416 to 550 [including position 1]; Stratagene, La Jolla, Calif.) was used to control for binding of labeled nuclear RNA to nonspecific DNA targets. These plasmids were digested so that the gene insert was separated from the vector DNA and electrophoresed on a 0.8% agarose gel at 80 V for 3 h. Southern blotting, hybridization, and washing were performed at 60°C using previously described methods (19, 31).
Gene fragments and oligonucleotides were used as probes for Northern blots. Oligonucleotides were prepared to be complementary to the mRNA for each of the genes. The probe for ACT1 was a 50-mer, positions 2478 to 2527 (X16377 [18]). The probes for ERG11 and MDR1 were the gene fragments listed in the previous paragraph. The oligonucleotides for the CDR genes are as follows: CDR1: short, position 1211 to 1229, and long, position 1211 to 1260 (X77589 [29]); CDR2: short, position 902 to 920, and long, position 902 to 951 (U63812 [32]); CDR3: position 651 to 680 (U89714 [2]); and CDR4: position 501 to 530 (AF044921 [7]). The short CDR1 and CDR2 oligonucleotides were used as probes for Fig. 1. The long CDR1 and CDR2 oligonucleotides were used as probes for Fig. 2 to 4.
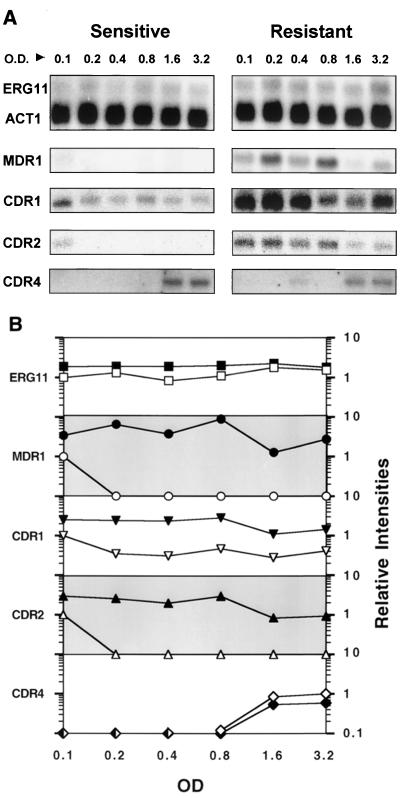
FIG. 1.
(A) Northern analysis of gene expression during logarithmic growth. Total RNA was prepared from the susceptible and resistant isolates at an initial OD600 of 0.1 and at each doubling time (roughly every 90 min) until the cells reached an OD600 of 3.2. Northern blots of these RNAs were hybridized with gene probes for ERG11, MDR1, CDR1, CDR2, and CDR4 (see Materials and Methods). Each of these blots was simultaneously probed with an oligonucleotide for ACT1. Only the ERG11 actin control is shown here. (B) Relative intensities of gene expression during logarithmic growth. The intensities of mRNA levels from panel A were quantified using a Storm phosphorimager. The intensities were standardized for each RNA in the series using the ACT1 control for each blot. These standardized levels were then normalized to the standardized level of the susceptible isolate at the start of growth, at an OD600 of 0.1. The exceptions were the CDR4 signals, which were not apparent until later in the growth of the culture and which were normalized to the standardized level of the susceptible isolate at an OD600 of 3.2. For each gene, the resistant isolate is represented by filled symbols and the susceptible isolate is represented by open symbols. The relative intensities for each gene are presented on a logarithmic scale with a range of 0.1 to 10 (y axis, labeled on right side of graph).
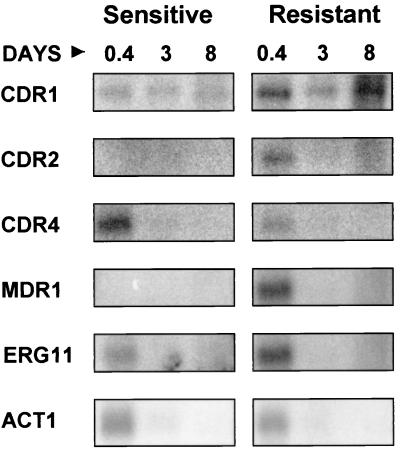
FIG. 2.
Northern analysis of gene expression in stationary phase. Total RNA was prepared from the susceptible and resistant isolates at a cell concentration of 6.4 OD600 and at 3 and 8 days. Northern blots of these RNAs were hybridized with gene probes for ERG11, MDR1, CDR1, CDR2, and CDR4 (see Materials and Methods). RNAs were loaded so that the visible rRNA bands were approximately equivalent. A control hybridization using housekeeping genes ACT1 or TEF3 was not possible using these RNAs (see Results).
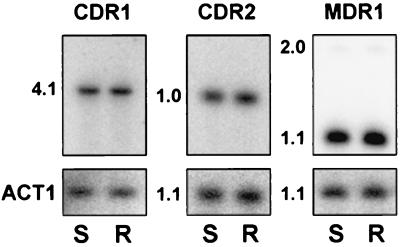
FIG. 4.
Southern analysis of gene amplification for CDR1, CDR2, and MDR1. Southern blots were prepared for total genomic DNA from the susceptible (S) and resistant (R) isolates, which were digested with HincII and hybridized with gene-specific probes. Sizes of the hybridizing bands are presented in kilobases. The MDR1 probe recognizes two bands, 2.0 and 1.1 kb, because a HincII restriction site is located near one end of the MDR1 probe. Each blot was also hybridized with ACT1 as a loading control. The MDR1, CDR2, and ACT1 restriction fragments are similar but not identical in size and could easily be distinguished from each other on the blots.
RESULTS
Time course Northern blot analysis.
A time course Northern blot analysis was performed to understand mRNA expression of genes associated with azole resistance throughout cell growth. A susceptible isolate (isolate 1) (MIC = 1.0 μg/ml) and a resistant isolate (isolate 17) (MIC ≥64 μg/ml) were grown at 30°C in YEPD, and total RNA was prepared from the culture at an OD600 of 0.1 and each subsequent doubling time to an OD600 of 6.4. Total RNA was also prepared from the isolates at 3 days and 8 days of growth. Using these RNAs, Northern blot analysis was performed to examine mRNA levels throughout the course of cell growth for the efflux pumps CDR1 to CDR4 and MDR1 and also the target gene for fluconazole ERG11. For all Northern blot analyses, RNAs were loaded onto the agarose gel so that the visible rRNA bands were equivalent in amount in all lanes, which ensures equivalent loading of RNAs. Loading based on total RNA concentration can give uneven amounts of RNA because of unequal recovery of small RNAs, including small rRNAs and degraded RNAs, as well as aggregation of RNAs in aqueous solution. We have found that loading based on visible rRNAs is the most accurate. The ACT1 gene was used as a control, as ACT1 is expected to be constitutively expressed under most of the growth conditions.
mRNA levels of ERG11 and MDR1 during logarithmic growth.
As shown in Fig. 1A, ACT1 mRNA levels are constant throughout logarithmic growth (usually to an OD600 of 6.4 [see below]), consistent with the equivalent loading of all gel lanes based on amounts of rRNA. Figure 1A also shows the levels of ERG11 (the target enzyme of the azole drugs) during this time course. ERG11 mRNA expression consistently shows a small increase in the resistant isolate compared to the susceptible isolate after standardization for ACT1 (Fig. 1B). The ERG11 overexpression in the resistant isolate varied from 1.2- to 2.3-fold, which is consistent with our previous report of overexpression at mid-log growth (35). The mRNA levels observed in both growth series (susceptible and resistant isolates) remained roughly equivalent from early to late logarithmic growth.
mRNA levels for the major facilitator efflux pump MDR1 (Fig. 1) were measured from early to late logarithmic growth. In the susceptible isolate, an MDR1 mRNA signal was only detectable early in logarithmic growth at an OD600 of 0.1. In the resistant isolate expression of MDR1 varied considerably. This variation was observed in repeated RNA preparations (data not shown), suggesting that MDR1 mRNA may have a short half-life. At an OD600 of 0.1, the overexpression of MDR1 in the resistant isolate was approximately threefold higher than that in the susceptible isolate. Overexpression of MDR1 in the resistant isolate was at least 30-fold higher than that in the susceptible isolate for the rest of the time points, again consistent with our previous report for mid-logarithmic growth (35).
mRNA levels of the CDR genes during logarithmic growth.
mRNA levels of the ATP binding cassette transporter genes CDR1 to CDR4 were measured from early to late logarithmic growth (OD600s of 0.1 to 3.2) in both the susceptible and resistant isolates using gene-specific oligonucleotides (Fig. 1). mRNA for the CDR1 gene was detected throughout the time course for both the susceptible and resistant isolates. CDR1 was overexpressed in the resistant isolate compared to the susceptible isolate at every time point, with levels of overexpression varying from 2.5 to 7.6. There was an increase in CDR1 expression in the susceptible isolate at the start of the series—an OD600 of 0.1 (Fig. 1). There was also a small decrease in CDR1 expression in late log phase in both the susceptible and resistant isolates.
mRNA for CDR2 was detected in the susceptible isolate at an OD600 of 0.1 but was not detected at later time points, similar to the MDR1 pattern of expression. In the resistant isolate, CDR2 expression was consistent from early to mid-log growth but decreased as the cells reached late log phase. Expression of CDR3 was not detected at any time point for either the susceptible or resistant isolates (data not shown). CDR4 mRNA was observed only during late log-phase growth. The mRNA levels of CDR4 were repeatedly higher in the susceptible isolate than in the resistant isolate. At OD600s of 1.6 and 3.2, the CDR4 mRNA levels of the susceptible isolate were 1.6- to 1.7-fold higher than the CDR4 mRNA levels of the resistant isolate.
mRNA levels during late log phase and stationary phase.
mRNA levels were also studied during late log phase (an OD600 of 6.4), after 3 days of growth (post-diauxic shift phase), and after 8 days of growth (stationary phase) using Northern analysis (Fig. 2). Post-diauxic shift phase and stationary phase were determined by repeated monitoring of the growth of the culture and assessing shifts to slower growth (diauxic shift) and eventually no growth (stationary phase). The results cannot be quantified as mRNA is partially degraded when prepared at these later time points, due to the nature of the cells. Low-molecular-weight RNA (less than 200 bp) is consistently observed in ethidium bromide-stained gels of RNA prepared at these later time points (data not shown). In addition, rRNA levels and mRNA levels do not always correlate in these growth phases (reference 34 and data not shown). An adequate control for mRNA levels between samples is not possible because the housekeeping genes ACT1 and TEF3 are degraded or down-regulated after cell growth reaches an OD600 of 6.4 (Fig. 2 and data not shown). RNA levels were monitored by ethidium bromide staining of the rRNA bands in agarose gels, sufficient to obtain qualitative comparisons of the time points but not sufficient to present quantitative results. rRNA bands were equivalent in each lane of the gels analyzed.
For all genes tested, the hybridization patterns of the RNAs prepared at an OD600 of 6.4 (0.4 day) were similar to the patterns from RNAs prepared at an OD600 of 3.2 (compare Fig. 1A, OD600 3.2, with Fig. 2, day 0.4). For CDR2, CDR4, MDR1, ERG11, and ACT1, mRNA levels were greatly reduced at 3 and 8 days. In Fig. 2, these mRNAs were not detectable at 3 and 8 days. In comparable experiments with higher levels of sensitivity, low levels of the mRNAs are detected at these times (data not shown). The exact timing of this reduction in the mRNA signal for these genes is variable. It has been observed to occur at day 3 or day 4 depending on the experiment (data not shown). This reduction in expression most likely corresponds to the diauxic shift, where the glucose in the culture is exhausted and cells shift to alternative carbon sources.
mRNA for the CDR1 gene was detectable at 3 and 8 days at levels that are comparable to the levels seen at an OD600 of 6.4, suggesting that CDR1 is expressed preferentially in stationary phase cells or not degraded as rapidly or as thoroughly as the other mRNAs.
mRNA levels during growth in different carbon sources.
Since the diauxic shift is a shift from the utilization of one carbon source to another, it was of interest to determine the expression patterns of these genes during growth in different carbon sources. Glycerol and acetate were chosen as nonfermentable carbon sources. The susceptible and resistant isolates were grown to an OD600 of 1.0 in glucose, glycerol, or acetate. Northern blots of total RNA were probed with the individual genes CDR1, CDR2, MDR1, and ERG11. The signals from the Northern blots were quantified, standardized using ACT1 mRNA expression as a control, and normalized to the signal for the gene in the resistant isolate grown in glucose (Fig. 3). In the resistant isolate, there was a consistent reduction in signal for each of the genes when the cells were grown in glycerol or acetate relative to growth in glucose. However, the resistant isolate consistently overexpresses these genes compared to the susceptible isolate. The largest change in gene expression was a 60% reduction in the MDR1 signal for the resistant isolate when the isolate was grown in glycerol or acetate. The expression patterns seen with different carbon sources at an OD600 of 1 do not explain the expression patterns seen during 3 and 8 days of growth of the cells (Fig. 2). Despite the reduction in gene expression in these different carbon sources (Fig. 3), no significant changes in MICs were observed when cells were grown in defined media containing these different carbon sources (data not shown).
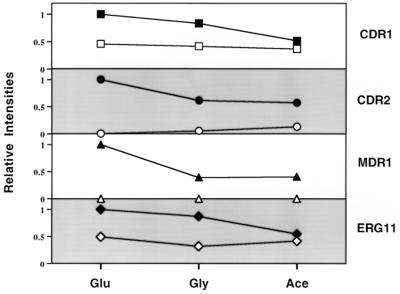
FIG. 3.
Relative intensities of gene expression during growth in different carbon sources. Northern blots were prepared with total RNA from the susceptible and resistant isolates at an OD600 of 1.0 in YEP medium containing glucose (Glu), glycerol (Gly) or acetate (Ace). The blots of these RNAs were hybridized with gene probes for CDR1, CDR2, MDR1, ERG11, and ACT1 (see Materials and Methods). The signals from each blot were quantified using a Storm phosphorimager. The intensities were standardized for each RNA in the series using the corresponding ACT1 signal for the same lane. The standardized levels were then normalized to the standardized level of the resistant isolate grown in glucose, which was assigned a value of 1. For each gene (labeled on the right side of the graph), the resistant isolate is represented by filled symbols and the susceptible isolate is represented by open symbols. The relative intensities for each gene are presented on a linear scale with a range of 0 to 1.2 (y axis, labeled on left side of graph). The lines connecting similar data points are presented for interpretation of the figure, not to imply a temporal or stepwise progression.
Lack of gene amplification for efflux pumps.
In drug-resistant eukaryotes overexpression of a resistance gene is often associated with gene amplification (4). For this series it has been previously documented that there is no gene amplification of ERG11 (35). To test for gene amplification of the efflux pumps, Southern blots of genomic DNA from the susceptible isolate and the resistant isolate were hybridized with MDR1, CDR1 and CDR2 (Fig. 4). The blots were also hybridized with ACT1 as a control for DNA loading. In this series, the MDR1, CDR1, and CDR2 genes were not amplified in the resistant isolate compared to the susceptible isolate. This eliminates gene amplification as an explanation for increased mRNA levels of these genes.
Nuclear run-on analysis of susceptible and resistant isolates.
Levels of cellular mRNA can be altered by several methods including, but not limited to, increased transcription of a gene, mRNA transport from the nucleus, and mRNA degradation. To test for elevated transcription levels of CDR, MDR1, and ERG11 a nuclear run-on assay was performed. Susceptible and resistant cells at an OD600 of 1.0 were permeabilized with a detergent, and radioactively labeled UTP was added. The labeled UTP enters the cell and the nucleus, where it is incorporated by transcriptionally active polymerases into nascent RNAs. These labeled nascent RNAs were then used as a probe against Southern blots with MDR1, CDR, and ERG11 gene fragments as targets. The size of gene fragment required for this analysis (greater than 300 bp) precludes the use of gene-specific fragments for the CDR gene family, since the fragments can cross-hybridize to several CDR genes. Therefore, a single gene fragment from CDR1 that cross-hybridizes with CDR2-4 (data not shown) was used in this analysis.
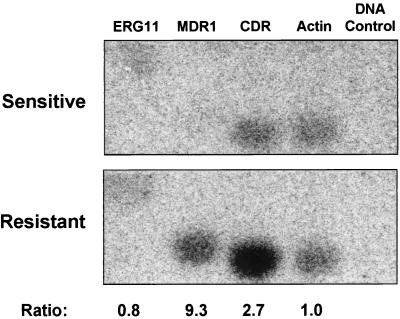
As seen in Fig. 5, the resistant isolate demonstrated increased transcription rates for the efflux pumps CDR and MDR1 relative to the susceptible isolate. The CDR transcription rate was increased by 2.7-fold and the MDR1 rate was increased by at least 9.3-fold in the resistant isolate compared to the susceptible isolate. The MDR1 signal for the susceptible isolate is indistinguishable from background. Therefore, the increased transcription seen for MDR1 in the resistant isolate (9.3-fold) is a minimum estimate of the contribution due to transcription. No difference was detected for ERG11 between the susceptible and resistant isolates. These results demonstrate that at least part of the resistance phenotype of isolate 17 is due to increased transcription of the efflux pump genes.
FIG. 5.
Nuclear run-on analysis for the susceptible and resistant isolates. Southern blots were prepared for DNA (10 μg/sample) from within the coding regions of the gene targets ERG11, MDR1, CDR, ACT1, and a DNA plasmid control (see Materials and Methods). The blots were probed with labeled nuclear run-on RNA (see Materials and Methods). Signal intensities of nuclear RNA were quantified using a Storm phosphorimager and standardized to the actin intensities. DNA from a pBluescript SK plasmid was used to control for the nonspecific binding of nuclear RNA to random DNA fragments. The standardized intensities for the susceptible isolate are 0.64, 0.15, 1.38, and 1 for ERG11, MDR1, CDR, and ACT1, respectively. The standardized intensities for the resistant isolate are 0.49, 1.42, 3.64, and 1. The ratio between resistant and susceptible nuclear RNA standardized intensities is given at the bottom of the figure for each gene. Background levels were observed for the DNA controls and for the MDR1 signal from the susceptible isolate. Since the MDR1 signal for the susceptible isolate is indistinguishable from background, the MDR1 ratio is a minimal estimate.
DISCUSSION
This series of experiments has documented altered mRNA levels for several genes associated with azole resistance (ERG11, MDR1, CDR1, CDR2, and CDR4) during logarithmic, diauxic, and stationary phase growth in this series of isolates (summarized in Fig. 6). mRNA levels for ERG11, MDR1, CDR1, and CDR2 are consistently higher in the resistant isolate compared to the susceptible isolate at each time point during cell growth, while overall mRNA levels vary depending upon the stage of cell growth. Two experiments address the possible molecular mechanisms that result in these increased mRNA levels in the resistant isolate: a genomic Southern blot analysis revealed that the genes for CDR1, CDR2, and MDR1 are not amplified, and nuclear run-on analysis demonstrated that one mechanism for increased mRNA levels of MDR1 and CDR is increased mRNA transcription.
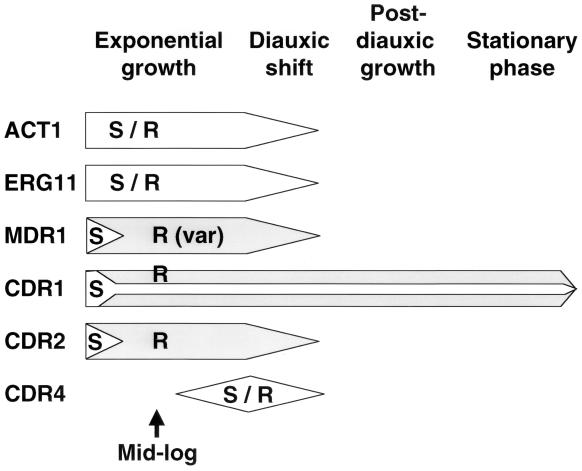
FIG. 6.
Schematic of gene expression during cell growth. The expression patterns of six genes are shown during four different phases of cell growth. The timing of expression is shown horizontally in the figure. S represents gene expression in susceptible cells, R (shaded area) represents gene expression in resistant cells, and S/R represents equivalent expression in both cell types. The height of the arrow is a qualitative representation of the amount of expression at each time point for each gene. Approximate OD600s or length for each phase would be as follows: exponential growth, OD600 of 0.1 to 3.2; diauxic shift, OD600 of approximately 6.4; post-diauxic shift, 3 to 6 days; and stationary phase, 8 days. The format for this figure was adapted from a review of stationary phase growth in S. cerevisiae by Werner-Washburne et al. (34).
The Northern analysis shown in Fig. 1 revealed that the levels of ERG11 mRNA were slightly increased in the resistant isolate throughout logarithmic growth, and expression in both the susceptible and resistant isolates was constant during growth, correlating with actin mRNA levels. MDR1 mRNA levels in the susceptible isolate were only detectable in early log phase growth, while MDR1 mRNA in the resistant isolate was detected at high levels throughout growth. However, the level of MDR1 mRNA in the resistant isolate varies widely during growth. This variability is likely due to a short half-life of the mRNA, such that variations in the preparation of total RNA from the cells can result in different levels of MDR1 mRNA despite constant levels of ACT1, which is expected to have a relatively long half-life. This variability during growth has been observed in several independent time courses (data not shown). Recently, several mutant alleles of MDR1 were shown to express mRNA at varying levels and were shown to be inducible under several different growth conditions (10).
Figure 1 also demonstrates the differential expression of CDR1, CDR2, and CDR4 between the susceptible isolate and resistant isolate, as detected by oligonucleotide probes specific for each of the genes. In the susceptible isolate, both CDR1 and CDR2 show expression at early log growth. While CDR2 expression is only detected in early log growth, CDR1 shows expression throughout the logarithmic growth phase. This is consistent with a previous publication that described the expression of CDR1 during growth of the cells (15). mRNA for CDR4 is only detected in late log-phase cells, and mRNA for CDR3 was not detected, which is consistent with its description as a phase-specific gene (2). The expression of efflux pumps early in logarithmic growth occurs in cells that have been grown for 16 h from a very small inoculum (Fig. 1). Thus, pump expression is not residual from stationary phase growth or a cellular response to fresh medium, since the cells have been growing for 16 h. Expression may be related to quorum sensing in bacteria (reviewed in reference 11, to different metabolic needs during different stages of growth, or small regulators such as MARS (morphogenic autoregulatory substance) of C. albicans that represses hyphal growth at high cell concentrations (12).
In this series, increased mRNA levels of CDR are present in the resistant isolate at mid-log growth. These mRNA levels at mid-log growth have been previously described (35). The increased mRNA levels can be attributed to the cumulative expression of CDR1 and CDR2. In the resistant isolate, CDR1 and CDR2 showed increased mRNA levels throughout cell growth, with a slight decrease as the cells reached late log phase. CDR4 mRNA is again expressed only in late log phase cells, but the mRNA levels are reduced in the resistant isolate compared to those in the susceptible isolate (Fig. 1 and 2). This is surprising, as it is the first description of an efflux pump that is down-regulated as cells develop a resistant phenotype. The data suggest that both CDR1 and CDR2 may contribute to the final azole-resistant phenotype of this series but that CDR3 and CDR4 do not contribute to resistance, at least in vitro. Expression patterns for CDR5 to CDR7 have not been tested, so it is possible that these and other CDR genes may also contribute to the drug-resistant phenotype seen in the resistant isolate. These observations are consistent with other studies (2, 7, 32, 33), which find that CDR1 and CDR2 are the only members of the CDR gene family to date that correlate with azole drug resistance.
In Fig. 1, the levels of overexpression of ERG11, MDR1, and CDR in the resistant isolate in mid-logarithmic growth are substantial but are not as large as previously reported (35). There are several possibilities for this. The RNAs for the previous publication were prepared at an OD of 1, while the RNAs in the present study were prepared at ODs of 0.8 and 1.6. It is possible that overexpression in the resistant isolate is gradually lost over time or during storage at −80°C. It is also possible that growth in culture modifies the expression of both the susceptible and resistant isolates. There is some indication that the MIC for the susceptible isolate has increased over time, which might reflect increased expression of the pumps in this isolate. The MIC for the resistant isolate continues to remain above the upper limit of the assay.
In vivo, C. albicans is likely to grow under a variety of conditions, which do not always include a rich medium containing glucose. Therefore, it was important to examine gene expression after the diauxic shift and during stationary phase. Most of the genes studied (ACT1, ERG11, MDR1, CDR2, and CDR4) were repressed or down-regulated by 3 days of growth (post-diauxic shift) and were not detectable at 8 days of growth (stationary phase) in both susceptible and resistant cells (Fig. 2). The surprising finding was that mRNA was detected for the CDR1 gene at both 3 and 8 days in both the susceptible and resistant isolates. This may be the result of persistent transcription or selective protection from degradation of the CDR1 message. This suggests that CDR1 pump expression is important for the continued survival of both susceptible and resistant cells under these conditions, perhaps by removing toxins, which would accumulate during long-term growth, from the cell. The overexpression of CDR1 in the resistant isolate continues at these late time points, suggesting that the resistant phenotype persists throughout the growth of the cells. Attempts to monitor the exact phase of cell growth at these time points, using genes expected to be expressed in these phases such as SUR1 (C. albicans information Web page), were unsuccessful (data not shown).
Since the diauxic shift represents a shift in growth from glucose to a nonfermentable carbon source, gene expression was monitored on glucose, glycerol, and acetate. As seen in Fig. 3, there was a modest reduction in gene expression in both the susceptible and resistant isolates for CDR1, CDR2, MDR1, and ERG11 when the cells were grown on the alternate carbon sources. Despite these changes in pump and target enzyme expression, no change in MIC was observed when cells were grown in these carbon sources (data not shown), suggesting that these minor reductions in gene expression do not significantly impact drug susceptibility.
Southern analysis indicated that gene amplification was not a cause of the elevated mRNA levels of CDR1, CDR2, or MDR1 in this series (Fig. 4). Previous data have shown the same for ERG11 in this series (35) and for CDR1 in a second series of isolates (21). To date, there is only one example of gene amplification associated with azole resistance: a chromosome duplication in Candida glabrata (20).
Nuclear run-on analysis demonstrated that CDR and MDR1 mRNAs were transcribed at higher rates, 2.6-fold and 9.3-fold, respectively, in the resistant isolate than in the susceptible isolate (Fig. 5). No increase in transcription for ERG11 was observed. Previous Northern blot analysis data of RNA prepared at an OD600 of 1.0 showed increased mRNA levels of CDR and MDR1 to be 5-fold and 25-fold, respectively (35). The transcription rates of the genes are consistent with the Northern blot analysis. The level of sensitivity of the nuclear run-ons is lower than that of Northern analysis, since the run-ons have not been able to consistently detect genes with a low level of expression, such as TEF3, a housekeeping gene. Therefore, the nuclear run-ons underestimate the contribution of transcription to overexpression (data not shown). This may explain the differences between Northern blot quantification of mRNA and transcription rates as determined by nuclear run-on experiments. A generalized conclusion is that the azole resistance phenotype observed in this series is due in part to transcriptional overexpression of efflux pump mRNAs. While the term “overexpression” has been previously applied to clinical isolates, the nuclear run-on data presented above are the first to actually monitor transcription rather than increased mRNA levels that can be the result of several cellular processes.
The CDR probe used for this analysis cross-hybridized with many members of the CDR gene family, and it is not known specifically which CDR mRNA have increased transcription. It should be noted that these experiments do not rule out other mechanisms of elevating mRNA levels, such as increasing mRNA half-lives. In addition, increased levels of mRNA do not always lead to an increased expression of protein or increased enzymatic activity. Further analysis is necessary to clarify these issues.
As shown above, mRNA levels of ERG11, MDR1, CDR1, and CDR2, which have all been correlated with azole resistance in C. albicans, rely on the exact growth phase of the cells. Nuclear run-on analysis has demonstrated that at least one reason for the observed increases of mRNA is an increase in mRNA transcription. However, cell growth and expression of these genes was conducted in vitro. Little is known about the growth environments and growth stages of C. albicans in vivo. The growth stages may vary greatly in vivo in oral (both pseudomembranous and erythematous), vaginal, and systemic infections. Growth may also vary in biofilms compared to growth in a flask (1). Depending upon the type of infection, the yeast may exist in several different states of growth (i.e., hyphal or pseudohyphal), and the cells may be growing exponentially or in a phase resembling the stationary phase. These distinct phases of growth are likely to influence the expression of resistance genes. This is likely to have major implications for azole drug resistance and drug therapy.
The lack of gene amplification and the increases in transcription observed for the efflux pumps direct attention to the promoter and associated trans-acting factors as important mechanisms of azole resistance, at least in this series of isolates. Clearly, a thorough characterization of the molecular mechanisms of gene transcription will be important for a greater understanding of in vivo azole resistance in C. albicans.
ACKNOWLEDGMENTS
We thank Spencer Redding (University of Texas Health Science Center at San Antonio) for the use of his isolates. We thank Simone Sanchez for assistance with the Southern blot analysis. We thank Kieren Marr for helpful comments and discussions and the other members of our laboratory for their support and comments on the manuscript.
This research was supported by NIH NIDR grant RO1 DE-11367. T.C.W. is supported by a New Investigator Award from the M. J. Murdock Charitable Trust and is the recipient of a New Investigator Award in Molecular Pathogenic Mycology from the Burroughs Wellcome Fund.
REFERENCES
- 1.Baillie G S, Douglas L J. Effect of growth rate on resistance of Candida albicans biofilms to antifungal agents. Antimicrob Agents Chemother. 1998;42:1900–1905. doi: 10.1128/aac.42.8.1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balan I, Alarco A M, Raymond M. The Candida albicans CDR3 gene codes for an opaque-phase ABC transporter. J Bacteriol. 1997;179:7210–7218. doi: 10.1128/jb.179.23.7210-7218.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blau J, Xiao H, McCracken S, O'Hare P, Greenblatt J, Bentley D. Three functional classes of transcriptional activation domain. Mol Cell Biol. 1996;16:2044–2055. doi: 10.1128/mcb.16.5.2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borst P. Genetic mechanisms of drug resistance. A review. Acta Oncol. 1991;30:87–105. doi: 10.3109/02841869109091819. [DOI] [PubMed] [Google Scholar]
- 5.Elion E A, Warner J R. An RNA polymerase I enhancer in Saccharomyces cerevisiae. Mol Cell Biol. 1986;6:2089–2097. doi: 10.1128/mcb.6.6.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fling M E, Kopf J, Tamarkin A, Gorman J A, Smith H A, Koltin Y. Analysis of a Candida albicans gene that encodes a novel mechanism for resistance to benomyl and methotrexate. Mol Gen Genet. 1991;227:318–329. doi: 10.1007/BF00259685. [DOI] [PubMed] [Google Scholar]
- 7.Franz R, Michel S, Morschhauser J. A fourth gene from the Candida albicans CDR family of ABC transporters. Gene. 1998;220:1–2. doi: 10.1016/s0378-1119(98)00412-0. [DOI] [PubMed] [Google Scholar]
- 8.Greenberg M E, Ziff E B. Stimulation of 3T3 cells induces transcription of the c-fos proto-oncogene. Nature. 1984;311:433–438. doi: 10.1038/311433a0. [DOI] [PubMed] [Google Scholar]
- 9.Groudine M, Peretz M, Weintraub H. Transcriptional regulation of hemoglobin switching in chicken embryos. Mol Cell Biol. 1981;1:281–288. doi: 10.1128/mcb.1.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gupta V, Kohli A, Krishnamurthy S, Puri N, Aalamgeer S A, Panwar S, Prasad R. Identification of polymorphic mutant alleles of CaMDR1, a major facilitator of Candida albicans which confers multidrug resistance, and its in vitro transcriptional activation. Curr Genet. 1998;34:192–199. doi: 10.1007/s002940050385. [DOI] [PubMed] [Google Scholar]
- 11.Hastings J W, Greenberg E P. Quorum sensing: the explanation of a curious phenomenon reveals a common characteristic of bacteria. J Bacteriol. 1999;181:2667–2668. doi: 10.1128/jb.181.9.2667-2668.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hazen K C, Cutler J E. Isolation and purification of morphogenic autoregulatory substance produced by Candida albicans. J Biochem (Tokyo) 1983;94:777–783. doi: 10.1093/oxfordjournals.jbchem.a134419. [DOI] [PubMed] [Google Scholar]
- 13.Hoffman C S, Winston F. A ten-minute DNA preparation from yeast efficiently releases autonomous plasmids for transformation of Escherichia coli. Gene. 1987;57:267–272. doi: 10.1016/0378-1119(87)90131-4. [DOI] [PubMed] [Google Scholar]
- 14.Johnston M, Carlson M. Regulation of carbon and phosphate utilization. In: Jones E W, Pringle J R, Broach J R, editors. The molecular and cellular biology of the yeast Saccharomyces. Vol. 2. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1992. pp. 193–281. [Google Scholar]
- 15.Krishnamurthy S, Gupta V, Prasad R, Panwar S L, Prasad R. Expression of CDR1, a multidrug resistance gene of Candida albicans: transcriptional activation by heat shock, drugs and human steroid hormones. FEMS Microbiol Lett. 1998;160:191–197. doi: 10.1111/j.1574-6968.1998.tb12910.x. [DOI] [PubMed] [Google Scholar]
- 16.Lai M H, Kirsch D R. Nucleotide sequence of cytochrome P450 L1A1 (lanosterol 14 alpha-demethylase) from Candida albicans. Nucleic Acids Res. 1989;17:804. doi: 10.1093/nar/17.2.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Law D, Moore C B, Wardle H M, Ganguli L A, Keaney M G, Denning D W. High prevalence of antifungal resistance in Candida spp. from patients with AIDS. J Antimicrob Chemother. 1994;34:659–686. doi: 10.1093/jac/34.5.659. [DOI] [PubMed] [Google Scholar]
- 18.Losberger C, Ernst J F. Sequence of the Candida albicans gene encoding actin. Nucleic Acids Res. 1989;17:9488. doi: 10.1093/nar/17.22.9488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maniatis T, Fritsch E F, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1982. [Google Scholar]
- 20.Marichal P, Vanden Bossche H, Odds F C, Nobels G, Warnock D W, Timmerman V, Van Broeckhoven C, Fay S, Larsen P M. Molecular-biological characterization of an azole-resistant Candida glabrata isolate. Antimicrob Agents Chemother. 1997;41:2229–2237. doi: 10.1128/aac.41.10.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marr K A, Lyons C N, Rustad T R, Bowden R A, White T C. Rapid, transient fluconazole resistance in Candida albicans is associated with increased mRNA levels of CDR. Antimicrob Agents Chemother. 1998;42:2584–2589. doi: 10.1128/aac.42.10.2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marr K A, White T C, van Burik J A H, Bowden R A. Development of fluconazole resistance in Candida albicans causing disseminated infection in a patient undergoing marrow transplantation. Clin Infect Dis. 1997;25:908–910. doi: 10.1086/515553. [DOI] [PubMed] [Google Scholar]
- 23.Marzluff W F, Huang R C C. Transcription of RNA in isolated nuclei. In: Hames B D, Higgins S J, editors. Transcription and translation: a practical approach. Oxford, England: IRL Press; 1984. pp. 89–129. [Google Scholar]
- 24.Mori T, Matsumura M, Kanamaru Y, Miyano S, Hishikawa T, Irie S, Oshimi K, Saikawa T, Oguri T. Myelofibrosis complicated by infection due to Candida albicans: emergence of resistance to antifungal agents during therapy. Clin Infect Dis. 1997;25:1470–1471. doi: 10.1086/516987. [DOI] [PubMed] [Google Scholar]
- 25.National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard. NCCLS document M27-A. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 26.Niimi M, Tokunaga M, Nakayama H. Regulation of mannitol catabolism in Candida albicans: evidence for cyclic AMP-independent glucose effect. J Med Vet Mycol. 1986;24:211–217. doi: 10.1080/02681218680000311. [DOI] [PubMed] [Google Scholar]
- 27.Nolte F S, Parkinson T, Falconer D J, Dix S, Williams J, Gilmore C, Geller R, Wingard J R. Isolation and characterization of fluconazole- and amphotericin B-resistant Candida albicans from blood of two patients with leukemia. Antimicrob Agents Chemother. 1997;41:196–199. doi: 10.1128/aac.41.1.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Odds F C. Candida and Candidosis: a review and bibliography. London, England: Bailliere Tindall; 1988. [Google Scholar]
- 29.Prasad R, De W P, Goffeau A, Balzi E. Molecular cloning and characterization of a novel gene of Candida albicans, CDR1, conferring multiple resistance to drugs and antifungals. Curr Genet. 1995;27:320–329. doi: 10.1007/BF00352101. [DOI] [PubMed] [Google Scholar]
- 30.Redding S, Smith J, Farinacci G, Rinaldi M, Fothergill A, Rhine C J, Pfaller M. Resistance of Candida albicans to fluconazole during treatment of oropharyngeal candidiasis in a patient with AIDS: documentation by in vitro susceptibility testing and DNA subtype analysis. Clin Infect Dis. 1994;18:240–242. doi: 10.1093/clinids/18.2.240. [DOI] [PubMed] [Google Scholar]
- 31.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 32.Sanglard D, Ischer F, Monod M, Bille J. Cloning of Candida albicans genes conferring resistance to azole antifungal agents: characterization of CDR2, a new multidrug ABC transporter gene. Microbiology. 1997;143:405–416. doi: 10.1099/00221287-143-2-405. [DOI] [PubMed] [Google Scholar]
- 33.Sanglard D, Kuchler K, Ischer F, Pagani J L, Monod M, Bille J. Mechanisms of resistance to azole antifungal agents in Candida albicans isolates from AIDS patients involve specific multidrug transporters. Antimicrob Agents Chemother. 1995;39:2378–2386. doi: 10.1128/aac.39.11.2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Werner-Washburne M, Braun E, Johnston G C, Singer R A. Stationary phase in the yeast Saccharomyces cerevisiae. Microbiol Rev. 1993;57:383–401. doi: 10.1128/mr.57.2.383-401.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.White T C. Increased mRNA levels of ERG16, CDR, and MDR1 correlate with increases in azole resistance in Candida albicans isolates from a patient infected with human immunodeficiency virus. Antimicrob Agents Chemother. 1997;41:1482–1487. doi: 10.1128/aac.41.7.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.White T C. The presence of an R467K amino acid substitution and loss of allelic variation correlate with an azole-resistant lanosterol 14 alpha demethylase in Candida albicans. Antimicrob Agents Chemother. 1997;41:1488–1494. doi: 10.1128/aac.41.7.1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.White T C, Marr K A, Bowden R A. Clinical, cellular, and molecular factors that contribute to antifungal drug resistance. Clin Microbiol Rev. 1998;11:382–402. doi: 10.1128/cmr.11.2.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.White T C, Pfaller M A, Rinaldi R G, Smith J, Redding S W. Stable azole drug resistance associated with a substrain of Candida albicans from an HIV-infected patient. Oral Dis. 1997;3:S102–S109. doi: 10.1111/j.1601-0825.1997.tb00336.x. [DOI] [PubMed] [Google Scholar]