Key Points
Question
What are the humoral immune response rates and risk factors associated with diminished response after COVID-19 vaccination in recipients of solid organ transplant?
Findings
In this systematic review and meta-analysis of 29 studies and 11 713 recipients of solid organ transplant, seroconversion rates increased with progressively increased numbers of mRNA COVID-19 vaccine doses. Older age, recent transplantation, deceased donor status, active use of antimetabolites, and recent exposure to antithymocyte globulin or rituximab were risk factors associated with diminished humoral immune response after receiving 2 doses of mRNA vaccines.
Meaning
These findings suggest that more efforts are needed to modulate the risk factors associated with reduced humoral responses among recipients of solid organ transplant.
This systematic review and meta-analysis assesses the current evidence on vaccine response and risk factors associated with diminished immune response in recipients of solid organ transplant.
Abstract
Importance
Recipients of solid organ transplant (SOT) experience decreased immunogenicity after COVID-19 vaccination.
Objective
To summarize current evidence on vaccine responses and identify risk factors for diminished humoral immune response in recipients of SOT.
Data Sources
A literature search was conducted from existence of database through December 15, 2021, using MEDLINE, Embase, Web of Science, Cochrane Library, and ClinicalTrials.gov.
Study Selection
Studies reporting humoral immune response of the COVID-19 vaccines in recipients of SOT were reviewed.
Data Extraction and Synthesis
Two reviewers independently extracted data from each eligible study. Descriptive statistics and a random-effects model were used. This report was prepared following the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline. Data were analyzed from December 2021 to February 2022.
Main Outcomes and Measures
The total numbers of positive immune responses and percentage across each vaccine platform were recorded. Pooled odds ratios (pORs) with 95% CIs were used to calculate the pooled effect estimates of risk factors for poor antibody response.
Results
A total of 83 studies were included for the systematic review, and 29 studies were included in the meta-analysis, representing 11 713 recipients of SOT. The weighted mean (range) of total positive humoral response for antispike antibodies after receipt of mRNA COVID-19 vaccine was 10.4% (0%-37.9%) for 1 dose, 44.9% (0%-79.1%) for 2 doses, and 63.1% (49.1%-69.1%) for 3 doses. In 2 studies, 50% of recipients of SOT with no or minimal antibody response after 3 doses of mRNA COVID-19 vaccine mounted an antibody response after a fourth dose. Among the factors associated with poor antibody response were older age (mean [SE] age difference between responders and nonresponders, 3.94 [1.1] years), deceased donor status (pOR, 0.66 [95% CI, 0.53-0.83]; I2 = 0%), antimetabolite use (pOR, 0.21 [95% CI, 0.14-0.29]; I2 = 70%), recent rituximab exposure (pOR, 0.21 [95% CI, 0.07-0.61]; I2 = 0%), and recent antithymocyte globulin exposure (pOR, 0.32 [95% CI, 0.15-0.71]; I2 = 0%).
Conclusions and Relevance
In this systematic review and meta-analysis, the rates of positive antibody response in solid organ transplant recipients remained low despite multiple doses of mRNA vaccines. These findings suggest that more efforts are needed to modulate the risk factors associated with reduced humoral responses and to study monoclonal antibody prophylaxis among recipients of SOT who are at high risk of diminished humoral response.
Introduction
Individuals with COVID-19 who have undergone solid organ transplant (SOT) experience higher mortality and prolonged viral shedding compared with the general population.1,2,3,4,5 Multiple vaccine platforms have been proven successful in reducing viral spread and preventing poor outcomes in the general population.6,7,8 Unfortunately, recipients of SOT were excluded from the initial licensing trials of these vaccines, and accumulating data have shown reduced immunogenicity among recipients of SOT.9,10,11,12,13,14
The US Food and Drug Administration (FDA) has approved the COVID-19 mRNA vaccines BNT162b2 (Pfizer-BioNTech) and mRNA-1273 (Moderna) and has granted emergency use authorization (EUA) for the adenoviral vector vaccine Ad26.COV2.S (Janssen).15,16,17,18 In response to emerging SARS-CoV-2 variants and evidence of a mortality benefit from booster or additional doses, the US Centers for Disease Control and Prevention (CDC) recommended a booster or additional dose after completion of the primary COVID-19 vaccination series for all adults who received BNT162b2, mRNA-1273, or Ad26.COV2.S.19,20,21 For patients who are immunocompromised, including recipients of SOT, the CDC recommended an additional primary shot (third dose of mRNA COVID-19 vaccine for those receiving BNT162b2 or a booster dose of mRNA-1273) and a subsequent dose (fourth dose of BNT162b2 or second booster dose of mRNA-1273 for those receiving mRNA COVID-19 vaccine or second dose for those receiving Ad26.COV2.S).22 Despite this strategy, there are concerns for inadequate protection and risks of breakthrough infections among recipients of SOT because of diminished immunogenicity. We conducted this systematic review and meta-analysis to summarize the current evidence on vaccine responses and identify risk factors associated with diminished humoral immune response among recipients of SOT.
Methods
Data Sources and Searches
A systematic search was conducted independently by 2 of us (N.C. and K.M.) in MEDLINE, Embase, Web of Science (Clarivate), Cochrane Library, and ClinicalTrials.gov databases for research available through December 15, 2021. Complete search terms are included in the eMethods in the Supplement. Studies from different databases were combined, and duplicates were excluded. We did not limit our search by language. We conducted the study according to Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline. This study was registered in the International Prospective Register of Systematic Reviews (PROSPERO) (CRD42021277109).
Study Selection and Quality Assessment
Two authors (N.C. and K.M.) independently reviewed all studies and selected studies that reported the immunogenicity of COVID-19 vaccines in recipients of SOT, described as study participants in the methods and results. We included clinical trials and observational studies consisting of prospective cohort, retrospective cohort, and case-control studies. We excluded studies of humoral immunity after COVID-19 infection in study participants. Corresponding authors were contacted for immunogenicity testing or vaccination information if needed. We used Google Translate (Alphabet) to translate non-English studies during title and abstract screening. The Newcastle-Ottawa scale was used for assessing the risk of bias of the studies (eTable 1 in the Supplement).23 Conflicts were resolved by mutual consensus between reviewers.
Data Extraction
The checklist for critical appraisal and data extraction for systematic reviews of prediction modeling studies was used.24 Our primary outcome was the seroconversion rate after COVID-19 vaccine administration. We extracted the numbers of responders and total participants to calculate the seroconversion rates. Responders were defined as participants whose humoral response met definitions and cutoffs of antibody testing in each primary study. The numbers of responders, total participants, and odds ratios (ORs) with 95% CIs of factors associated with vaccine response were extracted. If ORs were not available, crude numbers were extracted for OR calculation.
Statistical Analysis
Descriptive statistics were used to characterize humoral immune response, the primary outcome, for each COVID-19 vaccine platform and for each number of doses. We then performed a meta-analysis with Comprehensive Meta-Analysis software version 3.3 (Biostat) to identify risk factors associated with poor humoral immune response. To determine the factors associated with humoral immunogenicity, pooled ORs (pORs) with 95% CIs for binary variables and differences in means (with SEs) for continuous variables were calculated using meta-analysis with the random-effects model. If the study provided both adjusted and unadjusted ORs, we used adjusted ORs for calculations. If the primary study provided ORs of the factors associated with vaccine nonresponse, we used log transformation to calculate ORs associated with vaccine response of those specific factors. We performed sensitivity analyses using a leave-1-out method.25 Funnel plot and Egger regression were used to assess the publication bias.26 If the P value of Egger regression was P < .1, the publication bias was considered significant.27 Factors with concerns of publication bias were further adjusted by the Duval and Tweedie trim-and-fill method.28 We assessed the heterogeneity of effect size estimates of each study using the I2 statistic. The I2 statistic ranged from 0% to 100%, with I2less than 25% indicating low heterogeneity; I2 of 25% to 60%, moderate heterogeneity; and I2 greater than 60%, substantial heterogeneity.29 P values were 2-sided, and statistical significance was set at P = .05. Data were analyzed from December 2021 to February 2022.
Results
Study and Patient Characteristics
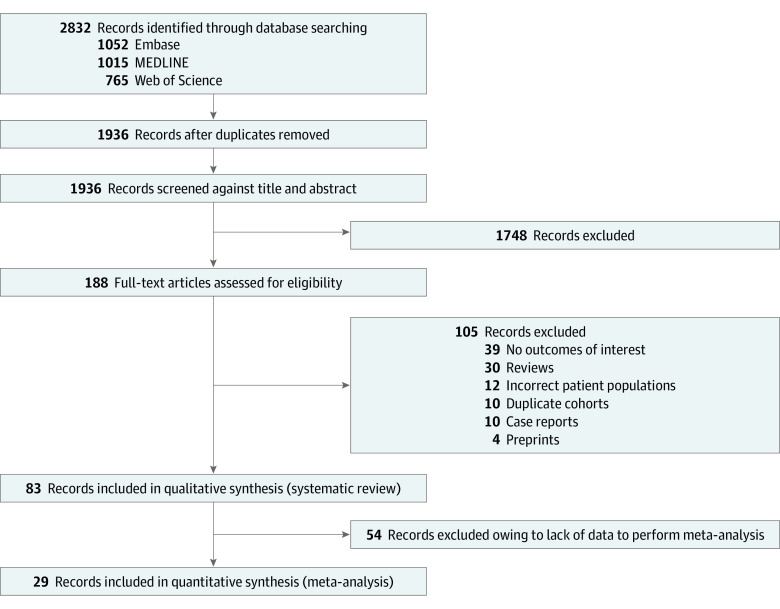
Our initial search generated 2832 studies; 896 studies were removed because they were duplicates, and 1748 studies were excluded by screening through the titles and abstracts. We performed full-study reviews on 188 articles. After review, 105 articles were excluded owing to being a review article, case report, preprint, incorrect patient population, or duplicate cohort or having no outcomes of interest. A total of 83 studies9,10,11,12,13,14,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106 were included in the systematic review, of which 29 studies were included in the meta-analysis (Figure 1). The characteristics of 83 included studies are described in eTable 2 in the Supplement. There were 11 713 study participants across all studies, including heart, lung, heart-lung, liver, kidney, pancreas, kidney-pancreas, and other combined transplantation. Grading of recommendation assessment, development and evaluation for potential factors associated with seroconversion was reported in eTable 3 in the Supplement.107
Figure 1. Study Selection Flowchart.
Humoral Immune Responses
mRNA Vaccines
A total of 83 studies of immunogenicity of the mRNA COVID-19 vaccines in study participants were identified. Of these, 18 studies reported antibody response after 1 dose, 54 studies after 2 doses, 11 studies after 3 doses, and 2 studies after 4 doses of the mRNA COVID-19 vaccines.
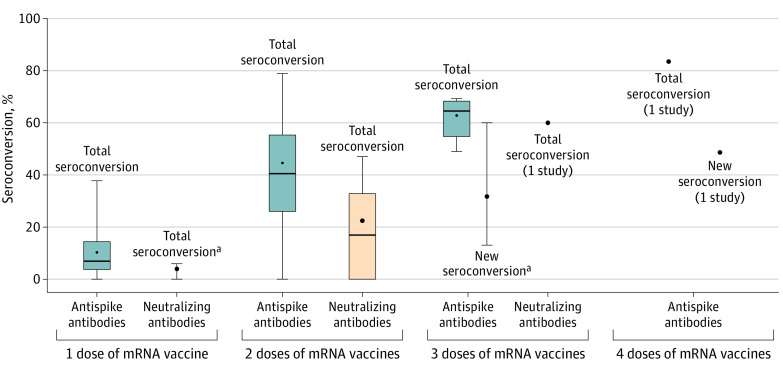
Among the studies analyzed, the weighted mean (range) seroconversion rate after 1 dose of mRNA vaccine was 10.4% (0%-37.9%) for antispike antibodies (18 studies12,33,35,37,40,41,42,44,48,51,52,55,57,69,71,76,103,105) and 4.1% (0%-5.9%) for neutralizing antibodies (2 studies12,69) (Figure 2). The mean (range) antibody testing time was 25.5 (21-28) days after the first dose.
Figure 2. Antibody Response of mRNA Vaccines.
Total seroconversion includes all seroconversion regardless of humeral immune response from the previous dose. New seroconversion only includes seroconversion from patients with no or minimal immune response from the previous dose. Dark lines indicates medians; dots, means; boxes, IQRs; whiskers, ranges.
aBox plot cannot be graphed because fewer than 5 studies were included.
The weighted mean (range) total seroconversion rate after 2 doses of mRNA COVID-19 vaccines was 44.9% (0%-79.1%) for antispike antibodies (53 studies10,11,12,13,14,32,35,36,38,40,41,42,43,45,46,47,48,49,50,51,52,53,54,55,56,57,60,61,62,63,64,65,66,67,69,70,71,74,79,80,82,84,86,88,91,92,93,94,95,97,102,103,105) and 22.6% (0%-47.5%) for neutralizing antibodies (8 studies12,49,62,65,67,69,71,95) (Figure 2). Among studies reporting seroconversion rates after 2 doses of mRNA COVID-19 vaccines, we reviewed the rates of positive antibody response by types of the mRNA COVID-19 vaccines. The BNT162b2 vaccine had a weighted mean (range) seroconversion rate of 44% (range 0%-79.1%) for antispike antibodies (36 studies11,13,35,40,41,42,43,45,46,47,49,50,51,52,53,54,56,57,60,62,63,64,65,66,67,69,70,80,84,86,88,91,92,93,102,105) and 15.3% (0%-35%) for neutralizing antibodies (5 studies49,62,65,67,69). The mRNA-1273 vaccine demonstrated a weighted mean (range) seroconversion rate of 51.4% (29.9%-76.2%) for antispike antibodies (7 studies10,12,32,48,79,103,105) and a mean of 26.9% for neutralizing antibodies (1 study12). The mean (range) antibody testing time after the second dose was 31.9 (8-81) days for all vaccines, 33.8 (8-81) days for the BNT162b2 vaccine, and 25.2 (14-28) days for the mRNA-1273 vaccine. Humoral immune response rates after 2 doses of mRNA vaccines by the different testing modalities are summarized in eTable 4 in the Supplement.
Three doses of mRNA vaccines showed higher total seroconversion rates (including all seroconversion regardless of humoral immune response from the second dose) with a weighted mean (range) of 63.1% (49.1%-69.1%) for antispike antibodies (8 studies31,41,52,56,72,78,81,85,99) and a mean of 60% for neutralizing antibodies (1 study81) (Figure 2). Two studies reported new seroconversion (ie, only study participants with no or minimal immune response after the second dose), with the weighted mean (range) seropositivity rate of 32% (13.3%-60%) for antispike antibodies (Figure 2). The mean (range) antibody testing time was 26.3 (14-30) days after the third dose. A study by Schrezenmeier et al104 reported a 36% antispike antibodies response rate and a 35% neutralizing antibodies response rate after 2 doses of BNT162b2 followed by either 1 dose of BNT162b2 or AZD1222 (University of Oxford and Vaccitech).
A study by Alejo et al30 reported high positive antibody response rates after 4 doses of mRNA vaccines, with a mean response rate of 83.3% for antispike antibodies; however, the study also included study participants with positive antibody response after the third dose. A study by Kamar et al98 included only study participants with negative or low positive antibody response after the third dose, and reported a seropositivity rate of 48.7% after 4 doses of the BNT162b2 vaccine for antispike antibodies.
Other Vaccine Platforms
For study participants vaccinated with the viral-vectored vaccine platform, Boyarsky et al9 reported that 16.7% had positive antispike antibodies after 1 dose of Ad26.COV2.S, and Prendecki et al86 reported 43.6% had positive antispike antibodies after 2 doses of AZD1222. Masset et al100 reported that either 2 doses of AZD1222 followed by 1 dose of mRNA vaccine or 1 dose of AZD1222 followed by 2 doses of mRNA vaccine resulted in antispike antibody seroconversion in 75% of participants. Among inactivated COVID-19 vaccine platforms, only CoronaVac (Sinovac Biotech) has been studied in recipients of kidney transplants, and the seroconversion rate for antispike antibodies was 15.2% after 1 does (1 study58) and 40.8% (range, 18.8%-43%) after 2 doses.58,89 There were no studies available for other vaccine platforms response in recipients of SOT at the time of our data search. Humoral immune response rates by the COVID-19 vaccine types and doses are summarized in eTable 5 in the Supplement.
Factors Associated With Reduced Humoral Immune Responses After 2 Doses of mRNA Vaccines
Host Characteristics
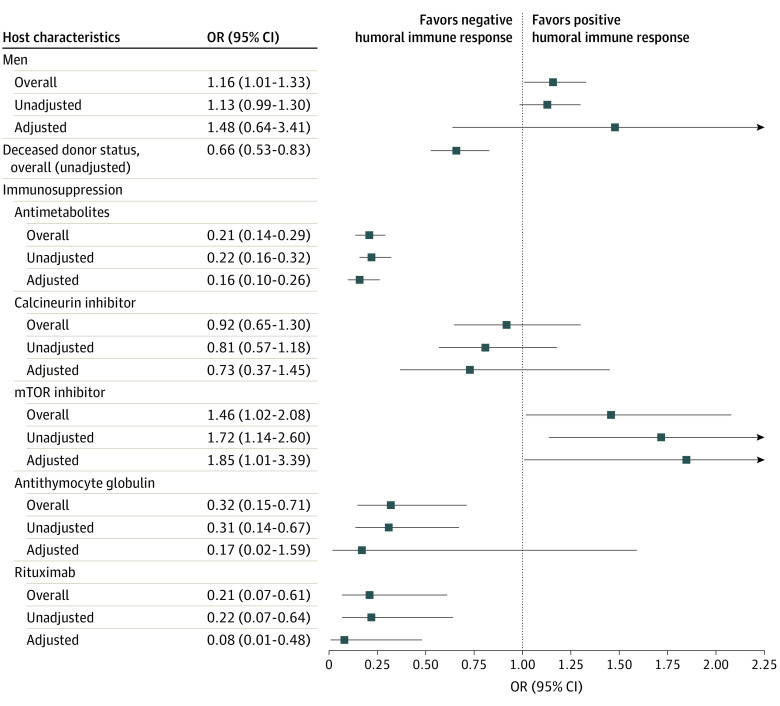
Increased age was associated with lower seroconversion rates. The pooled difference in means (SE) of 10 studies showed study participants with antibody response were 3.94 (1.1) years younger than those without antibody response (P = .001)45,46,47,49,53,56,59,63,66,103 (Table; eFigure 1 in the Supplement). Male sex was associated with higher seroconversion rates (pOR, 1.16 [95% CI, 1.01-1.33]; P = .04; I2 = 0%) (26 studies32,45,46,47,49,50,53,56,57,59,62,63,64,66,70,74,79,80,82,84,86,88,92,93,94,103) (Table, Figure 3; eFigure 1 in the Supplement). Body mass index (BMI) and absolute lymphocyte count were not associated with differences in antibody response based on differences of means that were not statistically significant (Table; eFigure 1 in the Supplement).
Table. Summary of Factors Associated With Immunogenicity After 2 Doses of mRNA Vaccines.
| Risk factor | Positive humoral immune response, pOR (95% CI) | Pooled difference in positive humoral immune response, mean (SE) | Studies, No. | Certainty of evidence (GRADE) | Comments |
|---|---|---|---|---|---|
| Host characteristics | |||||
| Age | NA | –3.94 (1.1) | 10 | Very low | None |
| Male | 1.16 (1.01-1.33) | NA | 26 | Low | pOR of male sex lost significance after removing 1 of several studies from analysis |
| BMI | NA | 0.15 (0.23) | 9 | Very low | None |
| Lymphocyte count | NA | 0.16 (0.13) | 4 | Very low | None |
| Transplant characteristics | |||||
| Time from transplant, y | NA | 2.12 (0.71) | 9 | Low | None |
| Deceased donor status | 0.66 (0.53-0.83) | NA | 10 | Moderate | None |
| Maintenance IS | |||||
| Antimetabolites | 0.21 (0.14-0.29) | NA | 25 | Low | pOR of mTOR inhibitors lost significance after removing 1 of several studies from analysis; it also lost significance after accounting for publication bias. |
| Calcineurin inhibitors | 0.92 (0.65-1.30) | NA | 17 | Low | None |
| mTOR inhibitors | 1.46 (1.02-2.08) | NA | 21 | Very low | None |
| Augmented IS in 12 mo | |||||
| Antithymocyte globulin | 0.32 (0.15-0.71) | NA | 5 | Very low | pORs of rituximab exposure lost significance after removing Haskin et al47 study from the analysis |
| Rituximab | 0.21 (0.07-0.61) | NA | 5 | Moderate | None |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); IS, immunosuppression; mTOR, mammalian (mechanistic) target of rapamycin; NA, not applicable; pORs, pooled odds ratios.
Figure 3. Overall, Unadjusted, and Adjusted Pooled Odds Ratios (ORs) Accounting for Confounders.
mTOR indicates mammalian (mechanistic) target of rapamycin.
Transplant Characteristics
Receipt of a deceased donor organ was associated with lower seroconversion rates compared with living donor status (pOR, 0.66 [95% CI, 0.53-0.83]; P < .001; I2 = 0%) (10 studies32,45,47,49,56,59,66,84,94,103) (Table, Figure 3; eFigure 1 in the Supplement). Time from transplantation to vaccination was associated with seroconversion rate. The pooled difference in means (SE) from 9 studies45,46,47,49,53,56,59,63,66 showed study participants with positive antibody response had 2.17 (0.71) years longer from transplantation to vaccination compared with those without antibody response (P = .002) (Table; eFigure 1 in the Supplement).
Immunosuppression
A total of 25 studies32,45,46,49,50,53,56,57,59,62,63,64,66,70,74,79,80,82,88,91,92,93,94,95,103 reported on antimetabolite use at the time of vaccine administration, which was associated with lower seroconversion rates (pOR, 0.21 [95% CI, 0.14-0.29]; P < .001; I2 = 70%) (Table, Figure 3; eFigure 1 in the Supplement). A total of 21 studies10,32,45,46,49,50,53,56,57,62,63,64,66,70,74,80,82,84,88,93,103 reported active use of mammalian (mechanistic) target of rapamycin (mTOR) inhibitors, which was associated with higher seroconversion rates (pOR, 1.46 [95% CI, 1.02-2.08]; P = .04; I2 = 42%) (Table, Figure 3; eFigure 1 in the Supplement). Furthermore, 17 studies32,45,46,49,50,53,56,57,63,64,74,79,80,82,84,92,103 reported calcineurin inhibitor (CNI) use at the time of vaccine administration, which was not associated with antibody response (pOR, 0.92 [95% CI, 0.65-1.30]; P = .64; I2 = 21%) (Table, Figure 3; eFigure 1 in the Supplement).
Both rituximab exposure45,47,66,74,103 and antithymocyte globulin (ATG) exposure10,45,47,66,74 within 12 months of vaccination were associated with lower seroconversion rates (rituximab: pOR, 0.21 [95% CI, 0.07-0.61]; P = .005; I2 = 0%; ATG: pOR, 0.32 [95% CI, 0.15-0.71]; P = .005; I2 = 0%) (Table, Figure 3; eFigure 1 in the Supplement). All data extraction of potential risk factors are summarized in eTable 6 and eTable 7 in the Supplement.
Sensitivity Analysis and Publication Bias
Results of sensitivity analysis are reported in eFigure 2 in the Supplement. The pORs of male sex and seroconversion lost significance after removing 1 of the following studies: Cholankeril et al,92 Davidov et al,93 Ducloux et al,80 Haskin et al,47 Kantauskaite et al,82 Masset et al,56 Rozen-Zvi et al,66 Sanders et al,103 or Villanego et al.74 The pORs of rituximab exposure and seroconversion lost significance after removing Haskin et al47 from the analysis. The pORs of mTOR inhibitor use and seroconversion lost significance after removing any 1 of the following studies: Benotmane et al,32 Cucchiari et al,10 Grupper et al,45 Kantauskaite et al,82 Korth et al,53 Peled et al,62 Rabinowich et al,63 Rashidi-Alavijeh et al,64 Sanders et al,103 or Villanego et al.74
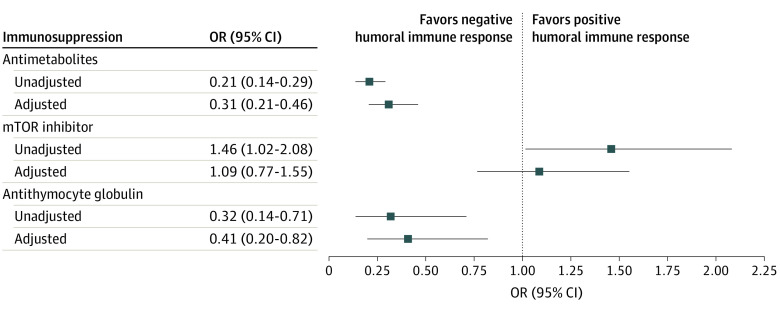
We found evidence of publication bias for age, BMI, lymphocyte count, antimetabolite use, mTOR inhibitor use, and ATG exposure (eFigure 3 in the Supplement). After accounting for publication bias by the Duval and Tweedie trim-and-fill method, antimetabolites (adjusted pOR, 0.31 [95% CI, 0.21-0.46]) and ATG (adjusted pOR, 0.41 [95% CI, 0.20, 0.82]) remained significantly associated with lower seroconversion rates (Figure 4; and eFigure 4 in the Supplement). After adjusting, the association of mTOR inhibitors was no longer significant (adjusted pOR, 1.09 [95% CI, 0.77-1.55]) (Figure 4; and eFigure 4 in the Supplement). The adjusted pooled difference in means of age remained significant, while those of BMI and lymphocyte count remained not statistically significant (eFigure 4 in the Supplement).
Figure 4. Unadjusted and Adjusted Pooled Odds Ratios (ORs) Accounting for Publication Bias.
mTOR indicates mammalian (mechanistic) target of rapamycin; OR, odds ratio.
Discussion
This systematic review and meta-analysis summarizes the cumulative evidence of immunogenicity of COVID-19 vaccines and risk factors associated with poor humoral response in recipients of SOT. Despite receiving multiple doses of mRNA vaccines, approximately 20% to 40% of recipients of SOT did not mount an antibody response. Although the correlation between positive humoral immune response with the clinical efficacy of COVID-19 vaccines in recipients of SOT has yet to be determined, the rates of humoral immune response in these populations after 3 or 4 doses of the mRNA COVID-19 vaccines are lower than those found in the general populations of the phase III clinical trials,7,8,108 and the rates of COVID-19 vaccine breakthrough infection in recipients of SOT are higher than those of the general public.39,109 COVID-19 breakthrough infection rates before the emergence of the Omicron variant in recipients of SOT who had received at least 2 doses of an mRNA vaccine or 1 dose of an adenovirus vaccine varied from 0.23% to 2.5%, as reported among transplant centers in the US. The breakthrough infection rate was up to 5% among recipients of kidney transplant treated with belatacept in a French cohort study,39 which is much higher than the general public.110,111
Our study has identified several risk factors associated with the lower seroconversion rate after 2 doses of mRNA COVID-19 vaccines in recipients of SOT. Recipients of SOT who were older, had recent transplants, or received deceased donor organ transplants had lower seroconversion rates. Unfortunately, we cannot determine specific cutoffs of age or time after transplantation associated with poor antibody response based on our study design. Similarly, recipients of SOT who were actively using antimetabolite immunosuppression (eg, mycophenolate mofetil, mycophenolic acid, or azathioprine) or who had recent exposure to rituximab or ATG within 12 months had lower seroconversion rates. We hypothesize that lower seroconversion with these agents could be caused by direct suppression of B-lymphocyte function or suppression of T-lymphocyte-dependent B-lymphocyte activation.112,113,114 Currently, there are ongoing clinical trials in Israel (NCT04961229), the US (NCT04969263), and the Netherlands (NCT05030974) to evaluate immunogenicity in solid organ transplant recipients after modulation of immunosuppression.
Despite higher seroconversion rates with progressively higher numbers of vaccine doses in recipients of SOT, the durability of the antibody response to repeated vaccination and the clinical outcomes in infection rates, disease severity, and mortality remain unknown.81 As of December 20, 2021, the US FDA has issued an EUA for tixagevimab-cilgavimab, a combination of long-acting monoclonal antibodies (mAb), for preexposure prophylaxis in patients who are immunocompromised, including recipients of SOT.115,116 Although the EUA states that mAb preexposure prophylaxis should not be considered an alternative to vaccination, there is inherent tension between the strategy of active immunization through vaccination and passive immunization with mAbs. Long-acting mAb preexposure prophylaxis is a valuable resource to add protection for recipients of SOT who have received all available and recommended doses of COVID-19 vaccines but who have demonstrated poor humoral immunity or who have the risks factors associated with lower seroconversion rates identified in our study. But for recipients of SOT who have not received all available doses of COVID-19 vaccines, there are now 2 options: to proceed with vaccination according to the recommended schedule or to postpone vaccination and pursue mAbs. The risk factors identified in this study for lower seroconversion rates after 2 vaccine doses may also help to inform transplant professionals who must advise their patients on time-sensitive decisions about active vs passive immunization by weighing the likelihood of benefit from vaccination compared with the likelihood of benefit from mAbs.
Additional unanswered questions include the optimal number of doses for a primary vaccination series, particularly in recipients of SOT with risk factors associated with lower seroconversion rates. Additionally, the role of antibody testing to determine strategies for additional doses or mAbs, the appropriate antibody cutoff level or other tests to ascertain immunity, and the most just allocation of scarce vaccine supply toward first doses or additional and booster doses at the global level are questions worthy of investigation and discussion.
Limitation
This study has some limitations. One limitation of COVID-19 vaccine immunogenicity research in recipients of SOT to date is the overrepresentation of the mRNA platform. Many recipients of SOT, particularly those living outside the US, may not have been fully vaccinated against COVID-19 or may not have access to mRNA vaccines owing to vaccine scarcity. Second, several techniques of SARS-CoV-2 antibody testing were used in the studies, and there is no criterion standard at this time. Third, the correlation between humoral immune response and the clinical efficacy of COVID-19 vaccines in recipients of SOT remains unclear. Fourth, most study participants were recipients of kidney transplant, with relatively fewer other organ transplant types. Fifth, the data regarding other vaccine platforms, including the heterologous prime-boost strategy, in recipients of SOT are extremely limited.
Conclusions
In this systematic review and meta-analysis of 29 studies and 11 713 recipients of SOT, seroconversion rates among recipients of SOT vaccinated with mRNA vaccines were higher with successive doses but remained lower than those among the general population. The availability of long-acting mAbs for preexposure prophylaxis presented an additional option for solid organ transplant recipients who have been vaccinated but may still be at inordinate risk for COVID-19, and mAbs are an additional consideration for recipients of SOT who have not yet been vaccinated and who may have multiple risk factors associated with a lower immune response to vaccination.
eMethods. Search Strategies
eTable 1. Newcastle-Ottawa Quality Assessment Scale of Included Studies in Meta-Analysis
eTable 2. Study Characteristics
eTable 3. Grading of Recommendation Assessment, Development and Evaluation (GRADE) for Potential Factors Associated With Diminished Humoral Immune Response
eTable 4. Humoral Immune Response Rates After 2 Doses of mRNA Vaccines by the Different Testing
eTable 5. Humoral Immune Response by the COVID-19 Vaccine Types and Doses
eTable 6. Participants and the Effect Estimates of Potential Risk Factors for Diminished Humoral Immune Response in Each Study
eTable 7. Continuous Data of Potential Risk Factors for Diminished Humoral Immune Response
eFigure 1. Forest Plots of Studied Risk Factors
eFigure 2. Sensitivity Analysis of Studied Risk Factors
eFigure 3. Publication Bias of Studied Risk Factors
eFigure 4. Funnel Plots and Adjusted Effect Estimates Accounting for Publication Bias
eReferences.
References
- 1.Boyarsky BJ, Po-Yu Chiang T, Werbel WA, et al. Early impact of COVID-19 on transplant center practices and policies in the United States. Am J Transplant. 2020;20(7):1809-1818. doi: 10.1111/ajt.15915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pereira MR, Mohan S, Cohen DJ, et al. COVID-19 in solid organ transplant recipients: initial report from the US epicenter. Am J Transplant. 2020;20(7):1800-1808. doi: 10.1111/ajt.15941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bossini N, Alberici F, Delbarba E, et al. ; Brescia Renal COVID task force . Kidney transplant patients with SARS-CoV-2 infection: the Brescia Renal COVID task force experience. Am J Transplant. 2020;20(11):3019-3029. doi: 10.1111/ajt.16176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cravedi P, Mothi SS, Azzi Y, et al. COVID-19 and kidney transplantation: results from the TANGO International Transplant Consortium. Am J Transplant. 2020;20(11):3140-3148. doi: 10.1111/ajt.16185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benotmane I, Risch S, Doderer-Lang C, Caillard S, Fafi-Kremer S. Long-term shedding of viable SARS-CoV-2 in kidney transplant recipients with COVID-19. Am J Transplant. 2021;21(8):2871-2875. doi: 10.1111/ajt.16636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Polack FP, Thomas SJ, Kitchin N, et al. ; C4591001 Clinical Trial Group . Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383(27):2603-2615. doi: 10.1056/NEJMoa2034577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baden LR, El Sahly HM, Essink B, et al. ; COVE Study Group . Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403-416. doi: 10.1056/NEJMoa2035389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sadoff J, Gray G, Vandebosch A, et al. ; ENSEMBLE Study Group . Safety and efficacy of single-dose Ad26.COV2.S vaccine against COVID-19. N Engl J Med. 2021;384(23):2187-2201. doi: 10.1056/NEJMoa2101544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boyarsky BJ, Chiang TPY, Ou MT, et al. Antibody response to the Janssen COVID-19 vaccine in solid organ transplant recipients. Transplantation. 2021;105(8):e82-e83. doi: 10.1097/TP.0000000000003850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cucchiari D, Egri N, Bodro M, et al. Cellular and humoral response after MRNA-1273 SARS-CoV-2 vaccine in kidney transplant recipients. Am J Transplant. 2021;21(8):2727-2739. doi: 10.1111/ajt.16701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Danthu C, Hantz S, Dahlem A, et al. Humoral response after SARS-CoV-2 mRNA vaccination in a cohort of hemodialysis patients and kidney transplant recipients. J Am Soc Nephrol. 2021;32(9):2153-2158. doi: 10.1681/ASN.2021040490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hall VG, Ferreira VH, Ierullo M, et al. Humoral and cellular immune response and safety of two-dose SARS-CoV-2 mRNA-1273 vaccine in solid organ transplant recipients. Am J Transplant. 2021;21(12):3980-3989. doi: 10.1111/ajt.16766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Havlin J, Svorcova M, Dvorackova E, et al. Immunogenicity of BNT162b2 mRNA COVID-19 vaccine and SARS-CoV-2 infection in lung transplant recipients. J Heart Lung Transplant. 2021;40(8):754-758. doi: 10.1016/j.healun.2021.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Narasimhan M, Mahimainathan L, Clark AE, et al. Serological response in lung transplant recipients after two doses of SARS-CoV-2 mRNA vaccines. Vaccines (Basel). 2021;9(7):708. doi: 10.3390/vaccines9070708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Comrinaty (purple cap). Package insert. US Food and Drug Administration; 2021. Accessed March 9, 2022. https://www.fda.gov/media/151707/download
- 16.Comirnaty (gray cap). Package insert. US Food and Drug Administration; 2021. Accessed March 9, 2022. https://www.fda.gov/media/154834/download
- 17.SPIKEVAX. Package insert. US Food and Drug Administration; 2022. Accessed March 9, 2022. https://www.fda.gov/media/155675/download
- 18.US Food and Drug Administration . Janssen COVID-19 vaccine. Accessed October 6, 2021. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/janssen-covid-19-vaccine
- 19.Arbel R, Hammerman A, Sergienko R, et al. BNT162b2 vaccine booster and mortality due to COVID-19. N Engl J Med. 2021;385(26):2413-2420. doi: 10.1056/NEJMoa2115624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.CDC endorses ACIP’s updated COVID-19 vaccine recommendations. News release. Centers for Disease Control and Prevention . December 16, 2021. Accessed December 24, 2021. https://www.cdc.gov/media/releases/2021/s1216-covid-19-vaccines.html
- 21.COVID-19 booster shot. News release. Centers for Disease Control and Prevention . December 9, 2021. Accessed December 24, 2021. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/booster-shot.html
- 22.COVID-19 vaccines for moderately or severely immunocompromised people. News release. Centers for Disease Control and Prevention . February 11, 2020. Accessed December 25, 2021. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/immuno.html
- 23.Wells G, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Accessed October 6, 2021. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- 24.Moons KGM, de Groot JAH, Bouwmeester W, et al. Critical appraisal and data extraction for systematic reviews of prediction modelling studies: the CHARMS checklist. PLoS Med. 2014;11(10):e1001744. doi: 10.1371/journal.pmed.1001744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patsopoulos NA, Evangelou E, Ioannidis JPA. Sensitivity of between-study heterogeneity in meta-analysis: proposed metrics and empirical evaluation. Int J Epidemiol. 2008;37(5):1148-1157. doi: 10.1093/ije/dyn065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sterne JA, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol. 2001;54(10):1046-1055. doi: 10.1016/S0895-4356(01)00377-8 [DOI] [PubMed] [Google Scholar]
- 27.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629-634. doi: 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455-463. doi: 10.1111/j.0006-341X.2000.00455.x [DOI] [PubMed] [Google Scholar]
- 29.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557-560. doi: 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alejo JL, Mitchell J, Chiang TPY, et al. Antibody response to a fourth dose of a SARS-CoV-2 vaccine in solid organ transplant recipients: a case series. Transplantation. 2021;105(12):e280-e281. doi: 10.1097/TP.0000000000003934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Benotmane I, Gautier G, Perrin P, et al. Antibody response after a third dose of the mRNA-1273 SARS-CoV-2 vaccine in kidney transplant recipients with minimal serologic response to 2 doses. JAMA. 2021;26(11):1063-1065. doi: 10.1001/jama.2021.12339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Benotmane I, Gautier-Vargas G, Cognard N, et al. Low immunization rates among kidney transplant recipients who received 2 doses of the mRNA-1273 SARS-CoV-2 vaccine. Kidney Int. 2021;99(6):1498-1500. doi: 10.1016/j.kint.2021.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Benotmane I, Gautier-Vargas G, Cognard N, et al. Weak anti-SARS-CoV-2 antibody response after the first injection of an mRNA COVID-19 vaccine in kidney transplant recipients. Kidney Int. 2021;99(6):1487-1489. doi: 10.1016/j.kint.2021.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bertrand D, Hamzaoui M, Lemée V, et al. Antibody and T cell response to SARS-CoV-2 messenger RNA BNT162b2 vaccine in kidney transplant recipients and hemodialysis patients. J Am Soc Nephrol. 2021;32(9):2147-2152. doi: 10.1681/ASN.2021040480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bertrand D, Hanoy M, Edet S, et al. Antibody response to SARS-CoV-2 mRNA BNT162b2 vaccine in kidney transplant recipients and in-centre and satellite centre haemodialysis patients. Clin Kidney J. 2021;14(9):2127-2128. doi: 10.1093/ckj/sfab100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boyarsky BJ, Werbel WA, Avery RK, et al. Antibody response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA. 2021;325(21):2204-2206. doi: 10.1001/jama.2021.7489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boyarsky BJ, Werbel WA, Avery RK, et al. Immunogenicity of a single dose of SARS-CoV-2 messenger RNA vaccine in solid organ transplant recipients. JAMA. 2021;325(17):1784-1786. doi: 10.1001/jama.2021.4385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cao J, Liu X, Muthukumar A, Gagan J, Jones P, Zu Y. Poor humoral response in solid organ transplant recipients following complete mRNA SARS-CoV-2 vaccination. Clin Chem. 2021;68(1):251-253. doi: 10.1093/clinchem/hvab149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chavarot N, Morel A, Leruez-Ville M, et al. Weak antibody response to three doses of mRNA vaccine in kidney transplant recipients treated with belatacept. Am J Transplant. 2021;21(12):4043-4051. doi: 10.1111/ajt.16814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chavarot N, Ouedrani A, Marion O, et al. Poor anti–SARS-CoV-2 humoral and T-cell responses after 2 injections of mRNA vaccine in kidney transplant recipients treated with belatacept. Transplantation. 2021;105(9):e94-e95. doi: 10.1097/TP.0000000000003784 [DOI] [PubMed] [Google Scholar]
- 41.Del Bello A, Abravanel F, Marion O, et al. Efficiency of a boost with a third dose of anti-SARS-CoV-2 messenger RNA-based vaccines in solid organ transplant recipients. Am J Transplant. 2022;22(1):322-323. doi: 10.1111/ajt.16775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Firket L, Descy J, Seidel L, et al. Serological response to mRNA SARS-CoV-2 BNT162b2 vaccine in kidney transplant recipients depends on prior exposure to SARS-CoV-2. Am J Transplant. 2021;21(11):3806-3807. doi: 10.1111/ajt.16726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Georgery H, Devresse A, Yombi JC, et al. Disappointing immunization rate after 2 doses of the BNT162b2 vaccine in a Belgian cohort of kidney transplant recipients. Transplantation. 2021;105(12):e283-e284. doi: 10.1097/TP.0000000000003861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Georgery H, Devresse A, Yombi JC, et al. Very low immunization rate in kidney transplant recipients after one dose of the BNT162b2 vaccine: beware not to lower the guard! Transplantation. 2021;105(10):e148-e149. doi: 10.1097/TP.0000000000003818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grupper A, Rabinowich L, Schwartz D, et al. Reduced humoral response to mRNA SARS-CoV-2 BNT162b2 vaccine in kidney transplant recipients without prior exposure to the virus. Am J Transplant. 2021;21(8):2719-2726. doi: 10.1111/ajt.16615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guarino M, Cossiga V, Esposito I, Furno A, Morisco F. Effectiveness of SARS-CoV-2 vaccination in liver transplanted patients: the debate is open! J Hepatol. 2022;76(1):237-239. doi: 10.1016/j.jhep.2021.07.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Haskin O, Ashkenazi-Hoffnung L, Ziv N, et al. Serological response to the BNT162b2 COVID-19 mRNA vaccine in adolescent and young adult kidney transplant recipients. Transplantation. 2021;105(11):e226-e233. doi: 10.1097/TP.0000000000003922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Herrera S, Colmenero J, Pascal M, et al. Cellular and humoral immune response after mRNA-1273 SARS-CoV-2 vaccine in liver and heart transplant recipients. Am J Transplant. 2021;21(12):3971-3979. doi: 10.1111/ajt.16768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hod T, Ben-David A, Olmer L, et al. Humoral response of renal transplant recipients to the BNT162b2 SARS-CoV-2 mRNA vaccine using both RBD IgG and neutralizing antibodies. Transplantation. 2021;105(11):e234-e243. doi: 10.1097/TP.0000000000003889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Holden IK, Bistrup C, Nilsson AC, et al. Immunogenicity of SARS-CoV-2 mRNA vaccine in solid organ transplant recipients. J Intern Med. 2021;290(6):1264-1267. doi: 10.1111/joim.13361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Itzhaki Ben Zadok O, Shaul AA, Ben-Avraham B, et al. Immunogenicity of the BNT162b2 mRNA vaccine in heart transplant recipients—a prospective cohort study. Eur J Heart Fail. 2021;23(9):1555-1559. doi: 10.1002/ejhf.2199 [DOI] [PubMed] [Google Scholar]
- 52.Kamar N, Abravanel F, Marion O, Couat C, Izopet J, Del Bello A. Three doses of an mRNA COVID-19 vaccine in solid-organ transplant recipients. N Engl J Med. 2021;385(7):661-662. doi: 10.1056/NEJMc2108861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Korth J, Jahn M, Dorsch O, et al. Impaired humoral response in renal transplant recipients to SARS-CoV-2 vaccination with BNT162b2 (Pfizer-BioNTech). Viruses. 2021;13(5):756. doi: 10.3390/v13050756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marinaki S, Adamopoulos S, Degiannis D, et al. Immunogenicity of SARS-CoV-2 BNT162b2 vaccine in solid organ transplant recipients. Am J Transplant. 2021;21(8):2913-2915. doi: 10.1111/ajt.16607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Marion O, Del Bello A, Abravanel F, et al. Safety and immunogenicity of anti–SARS-CoV-2 messenger RNA vaccines in recipients of solid organ transplants. Ann Intern Med. 2021;174(9):1336-1338. doi: 10.7326/M21-1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Masset C, Kerleau C, Garandeau C, et al. A third injection of the BNT162b2 mRNA COVID-19 vaccine in kidney transplant recipients improves the humoral immune response. Kidney Int. 2021;100(5):1132-1135. doi: 10.1016/j.kint.2021.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mazzola A, Todesco E, Drouin S, et al. Poor antibody response after two doses of SARS-CoV-2 vaccine in transplant recipients. Clin Infect Dis. 2021;ciab580. doi: 10.1093/cid/ciab580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Medina-Pestana J, Cristelli MP, Viana LA, et al. Clinical impact, reactogenicity, and immunogenicity after the first CoronaVac dose in kidney transplant recipients. Transplantation. 2022;106(1):e95-e97. doi: 10.1097/TP.0000000000003901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Midtvedt K, Tran T, Parker K, et al. Low immunization rate in kidney transplant recipients also after dose 2 of the BNT162b2 vaccine: continue to keep your guard up! Transplantation. 2021;105(8):e80-e81. doi: 10.1097/TP.0000000000003856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Miele M, Busà R, Russelli G, et al. Impaired anti–SARS-CoV-2 humoral and cellular immune response induced by Pfizer-BioNTech BNT162b2 mRNA vaccine in solid organ transplanted patients. Am J Transplant. 2021;21(8):2919-2921. doi: 10.1111/ajt.16702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Noble J, Langello A, Bouchut W, Lupo J, Lombardo D, Rostaing L. Immune response post–SARS-CoV-2 mRNA vaccination in kidney transplant recipients receiving belatacept. Transplantation. 2021;105(11):e259-e260. doi: 10.1097/TP.0000000000003923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Peled Y, Ram E, Lavee J, et al. BNT162b2 vaccination in heart transplant recipients: clinical experience and antibody response. J Heart Lung Transplant. 2021;40(8):759-762. doi: 10.1016/j.healun.2021.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rabinowich L, Grupper A, Baruch R, et al. Low immunogenicity to SARS-CoV-2 vaccination among liver transplant recipients. J Hepatol. 2021;75(2):435-438. doi: 10.1016/j.jhep.2021.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rashidi-Alavijeh J, Frey A, Passenberg M, et al. Humoral response to SARS-Cov-2 vaccination in liver transplant recipients—a single-center experience. Vaccines (Basel). 2021;9(7):738. doi: 10.3390/vaccines9070738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rincon-Arevalo H, Choi M, Stefanski AL, et al. Impaired humoral immunity to SARS-CoV-2 BNT162b2 vaccine in kidney transplant recipients and dialysis patients. Sci Immunol. 2021;6(60):eabj1031. doi: 10.1126/sciimmunol.abj1031 [DOI] [PubMed] [Google Scholar]
- 66.Rozen-Zvi B, Yahav D, Agur T, et al. Antibody response to SARS-CoV-2 mRNA vaccine among kidney transplant recipients: a prospective cohort study. Clin Microbiol Infect. 2021;27(8):1173.e1-1173.e4. doi: 10.1016/j.cmi.2021.04.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sattler A, Schrezenmeier E, Weber UA, et al. Impaired humoral and cellular immunity after SARS-CoV-2 BNT162b2 (tozinameran) prime-boost vaccination in kidney transplant recipients. J Clin Invest. 2021;131(14):150175. doi: 10.1172/JCI150175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schmidt T, Klemis V, Schub D, et al. Cellular immunity predominates over humoral immunity after homologous and heterologous mRNA and vector-based COVID-19 vaccine regimens in solid organ transplant recipients. Am J Transplant. 2021;21(12):3990-4002. doi: 10.1111/ajt.16818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schramm R, Costard-Jäckle A, Rivinius R, et al. Poor humoral and T-cell response to two-dose SARS-CoV-2 messenger RNA vaccine BNT162b2 in cardiothoracic transplant recipients. Clin Res Cardiol. 2021;110(8):1142-1149. doi: 10.1007/s00392-021-01880-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shostak Y, Shafran N, Heching M, et al. Early humoral response among lung transplant recipients vaccinated with BNT162b2 vaccine. Lancet Respir Med. 2021;9(6):e52-e53. doi: 10.1016/S2213-2600(21)00184-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stumpf J, Siepmann T, Lindner T, et al. Humoral and cellular immunity to SARS-CoV-2 vaccination in renal transplant versus dialysis patients: a prospective, multicenter observational study using mRNA-1273 or BNT162b2 mRNA vaccine. Lancet Reg Health Eur. 2021;9(101777707):100178. doi: 10.1016/j.lanepe.2021.100178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stumpf J, Tonnus W, Paliege A, et al. Cellular and humoral immune responses after 3 doses of BNT162b2 mRNA SARS-CoV-2 vaccine in kidney transplant. Transplantation. 2021;105(11):e267-e269. doi: 10.1097/TP.0000000000003903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Thuluvath PJ, Robarts P, Chauhan M. Analysis of antibody responses after COVID-19 vaccination in liver transplant recipients and those with chronic liver diseases. J Hepatol. 2021;75(6):1434-1439. doi: 10.1016/j.jhep.2021.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Villanego F, Cazorla JM, Vigara LA, et al. Protecting kidney transplant recipients against SARS-CoV-2 infection: a third dose of vaccine is necessary now. Am J Transplant. 2021;(100968638). doi: 10.1111/ajt.16829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Werbel WA, Boyarsky BJ, Ou MT, et al. Safety and immunogenicity of a third dose of SARS-CoV-2 vaccine in solid organ transplant recipients: a case series. Ann Intern Med. 2021;174(9):1330-1332. doi: 10.7326/L21-0282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yi SG, Knight RJ, Graviss EA, et al. Kidney transplant recipients rarely show an early antibody response following the first COVID-19 vaccine administration. Transplantation. 2021;105(7):e72-e73. doi: 10.1097/TP.0000000000003764 [DOI] [PubMed] [Google Scholar]
- 77.Azzi Y, Raees H, Wang T, et al. Risk factors associated with poor response to COVID-19 vaccination in kidney transplant recipients. Kidney Int. 2021;100(5):1127-1128. doi: 10.1016/j.kint.2021.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bertrand D, Hamzaoui M, Lemée V, et al. Antibody and T-cell response to a third dose of SARS-CoV-2 mRNA BNT162b2 vaccine in kidney transplant recipients. Kidney Int. 2021;100(6):1337-1340. doi: 10.1016/j.kint.2021.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Crespo M, Barrilado-Jackson A, Padilla E, et al. ; Mariscovid Research Group . Negative immune responses to two-dose mRNA COVID-19 vaccines in renal allograft recipients assessed with simple antibody and interferon gamma release assay cellular monitoring. Am J Transplant. 2022;22(3):786-800. doi: 10.1111/ajt.16854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ducloux D, Colladant M, Chabannes M, Bamoulid J, Courivaud C. Factors associated with humoral response after BNT162b2 mRNA COVID-19 vaccination in kidney transplant patients. Clin Kidney J. 2021;14(10):2270-2272. doi: 10.1093/ckj/sfab125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hall VG, Ferreira VH, Ku T, et al. Randomized Trial of a Third Dose of mRNA-1273 Vaccine in Transplant Recipients. N Engl J Med. 2021;385(13):1244-1246. doi: 10.1056/NEJMc2111462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kantauskaite M, Müller L, Kolb T, et al. Intensity of mycophenolate mofetil treatment is associated with an impaired immune response to SARS-CoV-2 vaccination in kidney transplant recipients. Am J Transplant. 2022;22(2):634-639. doi: 10.1111/ajt.16851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Middleton RJ, Gorton J, O’Riordan E, Knight S, Kalra PA, Poulikakos D. Impact of shielding and first dose of COVID-19 vaccination in kidney transplant recipients. Nephron. 2022;146(1):64-66. doi: 10.1159/000518631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pedersen RM, Bang LL, Tornby DS, et al. The SARS-CoV-2–neutralizing capacity of kidney transplant recipients 4 weeks after receiving a second dose of the BNT162b2 vaccine. Kidney Int. 2021;100(5):1129-1131. doi: 10.1016/j.kint.2021.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Peled Y, Ram E, Lavee J, et al. Third dose of the BNT162b2 vaccine in heart transplant recipients: Immunogenicity and clinical experience. J Heart Lung Transplant. 2022;41(2):148-157. doi: 10.1016/j.healun.2021.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Prendecki M, Thomson T, Clarke CL, et al. ; Imperial Renal COVID-19 vaccine study group in collaboration with the OCTAVE Study Consortium . Immunological responses to SARS-CoV-2 vaccines in kidney transplant recipients. Lancet. 2021;398(10310):1482-1484. doi: 10.1016/S0140-6736(21)02096-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ruether DF, Schaub GM, Duengelhoef PM, et al. SARS-CoV2–specific humoral and T-cell immune response after second vaccination in liver cirrhosis and transplant patients. Clin Gastroenterol Hepatol. 2022;20(1):162-172.e9. doi: 10.1016/j.cgh.2021.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Russo G, Lai Q, Poli L, et al. SARS-COV-2 vaccination with BNT162B2 in renal transplant patients: risk factors for impaired response and immunological implications. Clin Transplant. 2022;36(1):e14495. doi: 10.1111/ctr.14495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Eren Sadioğlu R, Demir E, Evren E, et al. Antibody response to two doses of inactivated SARS-CoV-2 vaccine (CoronaVac) in kidney transplant recipients. Transpl Infect Dis. 2021;23(6):e13740. doi: 10.1111/tid.13740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Westhoff TH, Seibert FS, Anft M, et al. A third vaccine dose substantially improves humoral and cellular SARS-CoV-2 immunity in renal transplant recipients with primary humoral nonresponse. Kidney Int. 2021;100(5):1135-1136. doi: 10.1016/j.kint.2021.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bergman P, Blennow O, Hansson L, et al. ; COVAXID-collaborator group (shown separately) . Safety and efficacy of the mRNA BNT162b2 vaccine against SARS-CoV-2 in five groups of immunocompromised patients and healthy controls in a prospective open-label clinical trial. EBioMedicine. 2021;74:103705. doi: 10.1016/j.ebiom.2021.103705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cholankeril G, Al-Hillan A, Tarlow B, et al. Clinical factors associated with lack of serological response to SARS-CoV-2 messenger RNA vaccine in liver transplantation recipients. Liver Transpl. 2022;28(1):123-126. doi: 10.1002/lt.26351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Davidov Y, Tsaraf K, Cohen-Ezra O, et al. Immunogenicity and adverse effects of the 2-dose BNT162b2 messenger RNA vaccine among liver transplantation recipients. Liver Transpl. 2022;28(2):215-223. doi: 10.1002/lt.26366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dębska-Ślizień A, Ślizień Z, Muchlado M, et al. Predictors of humoral response to mRNA COVID-19 vaccines in kidney transplant recipients: a longitudinal study—the COViNEPH Project. Vaccines (Basel). 2021;9(10):1165. doi: 10.3390/vaccines9101165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.D’Offizi G, Agrati C, Visco-Comandini U, et al. Coordinated cellular and humoral immune responses after two-dose SARS-CoV2 mRNA vaccination in liver transplant recipients. Liver Int. 2022;42(1):180-186. doi: 10.1111/liv.15089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Havlin J, Skotnicova A, Dvorackova E, et al. Impaired humoral response to third dose of BNT162b2 mRNA COVID-19 vaccine despite detectable spike protein–specific T cells in lung transplant recipients. Transplantation. 2022;106(3):e183-e184. doi: 10.1097/TP.0000000000004021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hoffman TW, Meek B, Rijkers GT, van Kessel DA. Poor serologic response to 2 doses of an mRNA-based SARS-CoV-2 vaccine in lung transplant recipients. Transplantation. 2022;106(1):e103-e104. doi: 10.1097/TP.0000000000003966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kamar N, Abravanel F, Marion O, et al. Assessment of 4 doses of SARS-CoV-2 messenger RNA–based vaccine in recipients of a solid organ transplant. JAMA Netw Open. 2021;4(11):e2136030. doi: 10.1001/jamanetworkopen.2021.36030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Massa F, Cremoni M, Gérard A, et al. Safety and cross-variant immunogenicity of a three-dose COVID-19 mRNA vaccine regimen in kidney transplant recipients. EBioMedicine. 2021;73:103679. doi: 10.1016/j.ebiom.2021.103679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Masset C, Ville S, Garandeau C, et al. Observations on improving COVID-19 vaccination responses in kidney transplant recipients: heterologous vaccination and immunosuppression modulation. Kidney Int. 2022;101(3):642-645. doi: 10.1016/j.kint.2021.11.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Quiroga B, Soler MJ, Ortiz A, et al. Safety and immediate humoral response of COVID-19 vaccines in chronic kidney disease patients: the SENCOVAC study. Nephrol Dial Transplant. 2021;gfab313. doi: 10.1093/ndt/gfab313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rahav G, Lustig Y, Lavee J, et al. BNT162b2 mRNA COVID-19 vaccination in immunocompromised patients: a prospective cohort study. EClinicalMedicine. 2021;41:101158. doi: 10.1016/j.eclinm.2021.101158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sanders JF, Bemelman FJ, Messchendorp AL, et al. ; RECOVAC Collaborators . The RECOVAC immune-response study: the immunogenicity, tolerability, and safety of COVID-19 vaccination in patients with chronic kidney disease, on dialysis, or living with a kidney transplant. Transplantation. Published online November 9, 2021. doi: 10.1097/TP.0000000000003983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Schrezenmeier E, Rincon-Arevalo H, Stefanski AL, et al. B and T cell responses after a third dose of SARS-CoV-2 vaccine in kidney transplant recipients. J Am Soc Nephrol. 2021;32(12):3027-3033. doi: 10.1681/ASN.2021070966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wijtvliet VPWM, Ariën KK, Abrams S, et al. mRNA-1273 vaccine (Moderna): a better option than BNT162b2 (Pfizer) in kidney transplant recipients and dialysis patients? Nephrol Dial Transplant. 2021;gfab352. doi: 10.1093/ndt/gfab352 [DOI] [PubMed] [Google Scholar]
- 106.Kumar D, Ferreira VH, Hall VG, et al. Neutralization of SARS-CoV-2 variants in transplant recipients after two and three doses of mRNA-1273 Vaccine : secondary analysis of a randomized trial. Ann Intern Med. 2022;175(2):226-233. doi: 10.7326/M21-3480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Huguet A, Hayden JA, Stinson J, et al. Judging the quality of evidence in reviews of prognostic factor research: adapting the GRADE framework. Syst Rev. 2013;2:71. doi: 10.1186/2046-4053-2-71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sahin U, Muik A, Vogler I, et al. BNT162b2 vaccine induces neutralizing antibodies and poly-specific T cells in humans. Nature. 2021;595(7868):572-577. doi: 10.1038/s41586-021-03653-6 [DOI] [PubMed] [Google Scholar]
- 109.Tenforde MW, Patel MM, Ginde AA, et al. ; Influenza and Other Viruses in the Acutely Ill (IVY) Network . Effectiveness of SARS-CoV-2 mRNA vaccines for preventing COVID-19 hospitalizations in the United States. Clin Infect Dis. 2021;ciab687. doi: 10.1093/cid/ciab687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Anjan S, Natori Y, Fernandez Betances AA, et al. Breakthrough COVID-19 infections after mRNA vaccination in solid organ transplant recipients in Miami, Florida. Transplantation. 2021;105(10):e139-e141. doi: 10.1097/TP.0000000000003902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Qin CX, Moore LW, Anjan S, et al. Risk of breakthrough SARS-CoV-2 infections in adult transplant recipients. Transplantation. 2021;105(11):e265-e266. doi: 10.1097/TP.0000000000003907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Allison AC, Eugui EM. Mycophenolate mofetil and its mechanisms of action. Immunopharmacology. 2000;47(2-3):85-118. doi: 10.1016/S0162-3109(00)00188-0 [DOI] [PubMed] [Google Scholar]
- 113.Boross P, Leusen JHW. Mechanisms of action of CD20 antibodies. Am J Cancer Res. 2012;2(6):676-690. [PMC free article] [PubMed] [Google Scholar]
- 114.Mohty M. Mechanisms of action of antithymocyte globulin: T-cell depletion and beyond. Leukemia. 2007;21(7):1387-1394. doi: 10.1038/sj.leu.2404683 [DOI] [PubMed] [Google Scholar]
- 115.US Food and Drug Administration . Fact sheet for healthcare providers: emergency use authorization for evusheld (tixagevimab co-packaged with cilgavimab). Accessed March 9, 2022. https://www.fda.gov/media/154701/download
- 116.Coronavirus (COVID-19) update: FDA authorizes new long-acting monoclonal antibodies for pre-exposure prevention of COVID-19 in certain individuals. News release. US Food and Drug Administration . December 8, 2021. Accessed December 25, 2021. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-new-long-acting-monoclonal-antibodies-pre-exposure
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Search Strategies
eTable 1. Newcastle-Ottawa Quality Assessment Scale of Included Studies in Meta-Analysis
eTable 2. Study Characteristics
eTable 3. Grading of Recommendation Assessment, Development and Evaluation (GRADE) for Potential Factors Associated With Diminished Humoral Immune Response
eTable 4. Humoral Immune Response Rates After 2 Doses of mRNA Vaccines by the Different Testing
eTable 5. Humoral Immune Response by the COVID-19 Vaccine Types and Doses
eTable 6. Participants and the Effect Estimates of Potential Risk Factors for Diminished Humoral Immune Response in Each Study
eTable 7. Continuous Data of Potential Risk Factors for Diminished Humoral Immune Response
eFigure 1. Forest Plots of Studied Risk Factors
eFigure 2. Sensitivity Analysis of Studied Risk Factors
eFigure 3. Publication Bias of Studied Risk Factors
eFigure 4. Funnel Plots and Adjusted Effect Estimates Accounting for Publication Bias
eReferences.