Key Points
Question
Is prenatal exposure to maternal social disadvantage and psychosocial stress associated with global and relative infant brain volumes at birth?
Findings
In this longitudinal, observational cohort study of 280 mother-infant dyads, prenatal exposure to greater maternal social disadvantage, but not psychosocial stress, was associated with statistically significant reductions in white matter, cortical gray matter, and subcortical gray matter volumes and cortical folding at birth after accounting for maternal health and diet.
Meaning
These findings suggest that prenatal exposure to social disadvantage is associated with global reductions in brain volumes and folding in the first weeks of life.
This cohort study of mother-infant dyads examines whether maternal social disadvantage and psychosocial stress are associated with neonatal brain volumes at birth.
Abstract
Importance
Exposure to early-life adversity alters the structural development of key brain regions underlying neurodevelopmental impairments. The association between prenatal exposure to adversity and brain structure at birth remains poorly understood.
Objective
To examine whether prenatal exposure to maternal social disadvantage and psychosocial stress is associated with neonatal global and regional brain volumes and cortical folding.
Design, Setting, and Participants
This prospective, longitudinal cohort study included 399 mother-infant dyads of sociodemographically diverse mothers recruited in the first or early second trimester of pregnancy and their infants, who underwent brain magnetic resonance imaging in the first weeks of life. Mothers were recruited from local obstetric clinics in St Louis, Missouri from September 1, 2017, to February 28, 2020.
Exposures
Maternal social disadvantage and psychosocial stress in pregnancy.
Main Outcomes and Measures
Confirmatory factor analyses were used to create latent constructs of maternal social disadvantage (income-to-needs ratio, Area Deprivation Index, Healthy Eating Index, educational level, and insurance status) and psychosocial stress (Perceived Stress Scale, Edinburgh Postnatal Depression Scale, Everyday Discrimination Scale, and Stress and Adversity Inventory). Neonatal cortical and subcortical gray matter, white matter, cerebellum, hippocampus, and amygdala volumes were generated using semiautomated, age-specific, segmentation pipelines.
Results
A total of 280 mothers (mean [SD] age, 29.1 [5.3] years; 170 [60.7%] Black or African American, 100 [35.7%] White, and 10 [3.6%] other race or ethnicity) and their healthy, term-born infants (149 [53.2%] male; mean [SD] infant gestational age, 38.6 [1.0] weeks) were included in the analysis. After covariate adjustment and multiple comparisons correction, greater social disadvantage was associated with reduced cortical gray matter (unstandardized β = −2.0; 95% CI, −3.5 to −0.5; P = .01), subcortical gray matter (unstandardized β = −0.4; 95% CI, −0.7 to −0.2; P = .003), and white matter (unstandardized β = −5.5; 95% CI, −7.8 to −3.3; P < .001) volumes and cortical folding (unstandardized β = −0.03; 95% CI, −0.04 to −0.01; P < .001). Psychosocial stress showed no association with brain metrics. Although social disadvantage accounted for an additional 2.3% of the variance of the left hippocampus (unstandardized β = −0.03; 95% CI, −0.05 to −0.01), 2.3% of the right hippocampus (unstandardized β = −0.03; 95% CI, −0.05 to −0.01), 3.1% of the left amygdala (unstandardized β = −0.02; 95% CI, −0.03 to −0.01), and 2.9% of the right amygdala (unstandardized β = −0.02; 95% CI, −0.03 to −0.01), no regional effects were found after accounting for total brain volume.
Conclusions and Relevance
In this baseline assessment of an ongoing cohort study, prenatal social disadvantage was associated with global reductions in brain volumes and cortical folding at birth. No regional specificity for the hippocampus or amygdala was detected. Results highlight that associations between poverty and brain development begin in utero and are evident early in life. These findings emphasize that preventive interventions that support fetal brain development should address parental socioeconomic hardships.
Introduction
Childhood exposure to early-life adversity (ELA), such as poverty, parental psychopathology, and psychosocial or physiological stress, is a well-described risk factor for adverse neurodevelopmental, socioemotional, and health outcomes.1,2,3,4,5 The pathways by which ELA is biologically embedded are complex and incompletely understood, with hypotheses centered on the effects of material deprivation, environmental exposures, and stressful psychosocial experiences on the hypothalamic-pituitary-adrenal (HPA) axis and systemic inflammation.3,6,7,8 Human and animal studies2,7,9,10,11 posit altered structural brain development as a key mechanism by which ELA contributes to poor outcomes. Magnetic resonance imaging (MRI) studies12,13,14,15,16,17 suggest that poverty in early childhood is associated with reduced cortical gray and white matter, hippocampus, and amygdala volumes at school age. In turn, reduced cortical and hippocampal volumes in childhood mediate associations between ELA (eg, poverty and family stress) and cognitive and behavioral impairments.16,17,18,19 Despite clear and compelling links between ELA and childhood neurodevelopment,1,2,3,4 much less is known about its prenatal effects.
The prenatal period is a particularly vulnerable stage of brain development,20,21 containing most neurogenesis and neuronal migration, with ongoing synaptogenesis, pruning, and myelination throughout the second and third trimesters.22 A small but growing body of literature demonstrates lasting consequences of prenatal exposure to ELA on childhood outcomes, including cognitive delays and externalizing disorders.23,24,25 However, few studies have explored the association between prenatal ELA and brain outcomes at birth, and cumulative or dimensional models have rarely been applied.26 The extant prenatal literature has largely conducted parallel lines of research concentrating on specific factors, including maternal alcohol or other substance use, health conditions, or psychosocial stress (ie, mood or affect problems, stress, and trauma).27 Few studies have examined prenatal exposure to poverty or multiple other factors,26,27 despite their overlapping findings.28
To date, studies29,30,31 investigating maternal perinatal psychosocial stress in association with neonatal brain volumes in healthy infants have focused on the hippocampus and amygdala, with differential findings for offspring sex, exposures, and the timing of those exposures. Maternal depression and/or stress during pregnancy were associated with altered hippocampus, amygdala, and cerebellum volumes and cortical folding in utero and shortly after birth.30,31,32,33 These studies reported negative associations between maternal psychosocial stress and income,29,31 but they represented populations of higher socioeconomic status (SES) and/or did not consistently control for SES.32,33 Although studies of early childhood SES also demonstrate consistent associations with hippocampus volume,2,8 limited fetal and neonatal MRI investigations have found an association between lower parental SES in pregnancy and global metrics, including altered cortical gray matter volumes,34,35,36 increased gyrification,34 and decreased white matter, deep gray matter, cerebellum, and brainstem volumes.34,35,36 Independent of maternal educational level, maternal smoking and psychiatric history in pregnancy have been found to explain variability in neonatal brain volumes.36
Given the US rates of childhood poverty (16%)37 and maternal perinatal mood disorders (14% for depression and 11%-20% for anxiety),38 prenatal ELA likely affects a significant proportion of the population. Furthermore, pregnant women with low incomes are at disproportionately greater risk of psychiatric disorders39,40 and stress during pregnancy.41 Consequently, it is essential to evaluate the contributions of psychosocial stress and poverty to in utero brain development in order to design preventive strategies.
We addressed this critical gap by quantifying prenatal exposures to latent constructs of maternal psychosocial stress (depression, stress, and lifetime interpersonal traumas or stressors) and social disadvantage (broad measure of SES and related factors) along with maternal health, tobacco use, and marijuana exposure in healthy, term-born infants. We investigated the associations between these factors and neonatal brain volumes at birth (global measures of cortical and subcortical gray matter, white matter, and cerebellar volume and cortical folding) along with 2 structures of interest (amygdala and hippocampus). On the basis of existing literature, we hypothesized that greater maternal social disadvantage and psychosocial stress would each be independently associated with lower neonatal brain volumes and reduced cortical folding. Given the sensitivity of subcortical structures to HPA axis activation,8,42,43 we expected to observe regionally specific susceptibility of the hippocampus and amygdala to social disadvantage and psychosocial stress exposure.
Methods
Study Design and Population
In this longitudinal, observational, multiwave, multimethod collaboration, a cohort of pregnant women who participated in a large-scale study of preterm birth44 within the Washington University in St Louis March of Dimes Prematurity Research Center were recruited from September 1, 2017, to February 28, 2020. Women from the parent study (n = 663) were invited to participate in this investigation (see Luby et al45 for cohort details) with the following exclusion criteria: multiple gestation, infections known to cause congenital disease (eg, syphilis), and/or alcohol or drug use other than tobacco and marijuana. A total of 395 eligible participating mothers completed assessments during each trimester of pregnancy and at delivery. Medical data from mothers and their 399 singleton offspring (4 mothers had 2 singleton births during the recruitment period) were collected from questionnaires and medical record review. In order to assess the contributions of racial and ethnic discrimination and inequities, pregnant mothers’ self-reported race and ethnicity were extracted from the medical record. The following options were provided for race: American Indian/Alaskan Native, Asian, Black or African American, Native Hawaiian/Pacific Islander, White, unknown, or other (free text), and the following options for ethnicity: Hispanic/Latina, non-Hispanic/Latina, or unknown/not applicable. Neonatal brain MRI was performed in the first weeks of life only on infants born before the COVID-19 pandemic. Exclusion criteria included premature birth (<37 weeks’ gestation), neonatal intensive care unit admission for more than 7 days, birth weight less than 2000 g, or evidence of brain injury on MRI. After exclusion and data quality criteria were applied, 280 mother-infant dyads were included in current analysis (eFigure in the Supplement). Study procedures were reviewed and approved by the Washington University Institutional Review Board. Written informed consent was obtained for each participant, with written parental informed consent for each infant. The study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline for cohort studies.46
Measures
Maternal Social Disadvantage and Psychosocial Stress
Confirmatory factor analysis was used to derive 2 latent maternal social disadvantage and maternal psychosocial stress constructs.45 The following maternal measures were included in the social disadvantage construct: health insurance status (grouped by private insurance or public or no insurance), highest educational level, income-to-needs ratio47 in each trimester, national Area Deprivation Index percentile at birth,48 and Healthy Eating Index.49 The following maternal psychological measures were included in the psychosocial stress construct: Perceived Stress Scale50 and Edinburgh Postnatal Depression Scale (EPDS)51 in each trimester, Stress and Adversity Inventory,52 and Everyday Discrimination Scale (eMethods in the Supplement).53
Maternal Comorbidities and Exposures
A maternal medical risk score was calculated for each participant using questionnaires and medical record review.54 This validated index55 is a sum of weighted comorbidities, including advanced age, cardiac disease, and preeclampsia, with higher scores predicting increased risk of severe morbidity or mortality. Frequency of tobacco and marijuana use (none, some, or heavy) (Table 1) was self-reported on questionnaires at each trimester. At the discretion of the treating clinician, a subset of mothers underwent urine drug screens during prenatal clinical care. Marijuana exposure (any vs none) was, therefore, based on self-report and/or a urine drug screen result positive for tetrahydrocannabinol metabolites. Because maternal prepregnancy body mass index, marijuana exposure, and tobacco use are not included in the maternal medical risk index, they were independently evaluated as covariates of interest.
Table 1. Social Background and Infant Clinical Characteristics of the Sample.
| Characteristic | Data (N = 280) |
|---|---|
| Maternal age, mean (SD) [range], y | 29.1 (5.3) [18.7 to 41.8] |
| Maternal race and ethnicity (self-identified), No. (%) | |
| Black/African American | 170 (60.7) |
| White | 100 (35.7) |
| Othera | 10 (3.6) |
| Maternal medical risk score, median (IQR) [range] | 1.0 (0.0 to 2.0) [0 to 8] |
| Self-reported maternal tobacco use, No. (%) | |
| Heavy use (≥6 cigarettes daily) | 16 (5.7) |
| Some use (<6 cigarettes daily) | 20 (7.1) |
| None | 244 (87.1) |
| Any maternal marijuana exposure, No. (%) | 74 (26.4) |
| Positive urine drug screen result, No. (%)b | 59 (21.1) |
| Self-reported maternal marijuana use, No. (%) | |
| Daily use | 21 (7.5) |
| Some use (less than daily) | 15 (5.4) |
| None | 244 (87.1) |
| Insurance, No. (%) | |
| Medicaid or Medicare | 105 (37.6) |
| Individual or group health insurance | 144 (51.4) |
| Uninsured | 31 (11.0) |
| Married mothers, No. (%) | 99 (35.4) |
| Maternal educational level (n = 272), No. (%) | |
| Did not complete high school | 28 (10.3) |
| Finished high school or GED | 68 (25.0) |
| Some college or vocational school | 83 (30.5) |
| College degree (4 y) | 34 (12.5) |
| Graduate degree | 59 (21.7) |
| Income-to-needs ratio, median (IQR) [range] | |
| Trimester | |
| First (n = 271) | 1.25 (0.89 to 3.80) [0.43 to 12.15] |
| Second (n = 216) | 1.65 (0.91 to 5.17) [0.38 to 12.15] |
| Third (n = 238) | 1.46 (0.89 to 5.17) [0.35 to 11.83] |
| Area Deprivation Index score, mean (SD) [range] | 68.2 (24.9) [1 to 100] |
| Healthy Eating Index score (n = 223), mean (SD) [range] | 58.8 (10.0) [33.0 to 80.7] |
| Social disadvantage, mean (SD) [range] | –0.04 (0.97) [–2.2 to 1.5] |
| Perceived Stress Scale score, mean (SD) [range] | |
| Trimester | |
| First (n = 276) | 13.1 (7.2) [0 to 35] |
| Second (n = 215) | 12.9 (7.5) [0 to 36] |
| Third (n = 234) | 12.5 (7.3) [0 to 37] |
| Edinburgh Postpartum Depression Scale score, median (IQR) [range] | |
| Trimester | |
| First (n = 278) | 4.0 (1.0 to 7.0) [0 to 25] |
| Second (n = 235) | 3.0 (1.0 to 7.0) [0 to 20] |
| Third (n = 239) | 3.0 (1.0 to 6.0) [0 to 25] |
| STRAIN (n = 263), median (IQR) [range] | |
| Stressful event count | 6.0 (3.0 to 11.0) [0 to 30] |
| Weighted severity | 15.0 (7.0 to 29.0) [0 to 99] |
| Everyday Discrimination Scale score (n = 261), median (IQR) [range]c | 1.0 (1.0 to 1.8) [1 to 6] |
| Psychosocial stress, mean (SD) [range] | –.11 (.88) [–1.7 to 3.7] |
| Infant gestational age, mean (SD) [range], wk | 38.6 (1.0) [37 to 41] |
| Postmenstrual age at MRI, mean (SD) [range], wk | 41.7 (1.3) [38 to 45] |
| Infant sex (male), No. (%) | 149 (53.2) |
| Infant birth weight, mean (SD) [range], g | 3257.7 (487.7) [2200 to 4627] |
Abbreviation: STRAIN, Stress and Adversity Inventory.
Other includes Asian (n = 5), Latina (n = 3), Middle Eastern (n = 1), and Asian and White (n = 1).
A total of 119 mothers (42.5%) had urine drug screen data during pregnancy.
Everyday Discrimination Scale was scored for experiences of racial discrimination only (otherwise coded as 0).
MRI Data Collection, Preprocessing, and Volumetric Measures
All MRIs were performed within the first weeks of life without sedation during natural sleep. Magnetic resonance imaging data were collected using a Prisma 3T scanner and 64-channel head coil (Siemens). Infants (n = 10) without high-quality (ie, low motion) structural data as determined by an imaging scientist (D.A.) and pediatric neurologist (C.D.S.) were excluded. The Melbourne Children’s Regional Infant Brain Atlas Surface segmentation and surface extraction toolkit was used to generate segmentations into white and gray matter, cerebellum, brainstem, and subcortical gray matter and surface-based cortical parcellations from preprocessed T2-weighted images.56,57 See the eMethods in the Supplement for sequence parameters, preprocessing, and analysis procedures.
Brain volumes of interest included total cortical and subcortical gray matter, white matter, and cerebellum, in addition to right and left hippocampi and amygdalae. Total raw volumes for all structures were analyzed, along with standardized regional volumes for the hippocampi and amygdalae generated by dividing by total brain volume, as is common in neonatal neuroimaging studies.30,33 Cortical folding was measured using the total Gyrification Index (GI), a ratio of the cortical surface area divided by the cortical hull surface area.58
Statistical Analysis
Analyses were performed using SPSS software, version 28 (IBM Corporation). Potential covariates were explored using Pearson correlation and 2-tailed, unpaired t tests. Maternal tobacco use, infant sex, birth weight, and postmenstrual age (PMA) at MRI were associated with brain volumes of interest (eTable 1 in the Supplement). These covariates and social disadvantage and psychosocial stress factor scores were included as independent variables in hierarchical linear regression analyses, each with brain volumes or cortical folding as the dependent variable. For each volume of interest, the first step accounted for maternal tobacco use (no use = 0), infant sex (female = 0), birth weight, and PMA at MRI. The social disadvantage and psychosocial stress factors were entered simultaneously in the second step of the model to determine the unique, independent proportion of variance (change in R2) explained in brain volume and folding outcomes over and above covariate factors. Regression models were checked for linearity, homoscedasticity, and absence of multicollinearity, and the residuals approximated a normal distribution. Results for primary outcomes were corrected for multiple comparisons using the Benjamini-Hochberg false discovery rate procedure.59 P values and false discovery rate–adjusted P values <.05 were considered to be statistically significant.
Results
Infant Characteristics
A total of 280 mothers (mean [SD] age, 29.1 [5.3] years; 170 [60.7%] Black/African American, 100 [35.7%] White, and 10 [3.6%] of other race or ethnicity) and their healthy, term-born infants (149 [53.2%] male; mean [SD] infant gestational age, 38.6 [1.0] weeks) were included in the study (Table 1). Male infants had a larger mean (SD) birth weight (3316 [470] g) than female infants (3191 [500] g) (P = .03) (eTable 2 in the Supplement). No sex differences were found for PMA at MRI, social disadvantage, and psychosocial stress (eTable 2 in the Supplement). At the time of MRI, infants had a mean (SD) PMA of 42.0 (1.3) weeks, which was slightly younger than infants excluded because of low-quality or missing MRI data. No other differences were found between the 2 groups (eTable 3 in the Supplement).
Prenatal Life Adversity
Table 1 summarizes the prenatal life adversity characteristics of the sample, including the latent constructs of maternal social disadvantage and psychosocial stress. A total of 136 mothers (48.6%) in the cohort had public insurance or no health insurance. Median income-to-needs ratios at each trimester ranged from 1.25 to 1.65 (minimum, 0.38; maximum, 12.15). The median EPDS scores at each trimester ranged from 3.0 to 4.0 (minimum, 0; maximum, 25). Social disadvantage was correlated with more maternal psychosocial stress (r = 0.43, P < .001). Differences between this full-term cohort and the full sample (from which the factors were derived)45 were predominantly driven by infants born prematurely (eTable 4 in the Supplement).
MRI Measures
Brain Volumes
Table 2 summarizes the second, final step of the hierarchical linear regression results (full results in eTable 5 in the Supplement). In step 1, female sex, lower birth weight, and younger PMA at MRI were associated with smaller cortical (sex: β = 0.23, P < .001; birth weight: β = 0.29, P < .001; and PMA at MRI: β = 0.54, P < .001) and subcortical gray matter (sex: β = 0.23, P < .001; birth weight: β = 0.25, P < .001; and PMA at MRI: β = 0.54, P < .001), white matter (sex: β = 0.28, P < .001; birth weight: β = 0.27, P < .001; and PMA at MRI: β = 0.22, P < .001), and cerebellar (sex: β = 0.23, P < .001; birth weight: β = 0.21, P < .001; and PMA at MRI: β = 0.62, P < .001) volumes (eTable 5 in the Supplement). Tobacco use was associated with reduced subcortical gray (β = −0.11, P = .01) and white matter (β = −0.12, P = .02) (eTable 5 in the Supplement). In step 2, greater social disadvantage was associated with reduced volumes across all tissue types (Table 2 and Figure 1), except for the cerebellum (eTable 5 in the Supplement). Social disadvantage accounted for an additional 1.6% of the variance for total cortical gray matter (unstandardized β = –2.0; 95% CI, –3.5 to –0.5), 2.6% for subcortical gray matter (unstandardized β = –0.4; 95% CI, –0.7 to –0.2), and 7% for white matter (unstandardized β = –5.5; 95% CI, –7.8 to –3.3) (eTable 5 in the Supplement). The contribution of psychosocial stress was not significant (Table 2). A similar pattern of results was found for total brain volume (eTable 6 in the Supplement). Post hoc analyses showed similar results for the left and right hemispheric cortical gray matter, cerebral white matter, and cerebellar hemispheres (eTable 7 in the Supplement).
Table 2. Summary of Final Step in Hierarchical Linear Regression Assessing the Association of Maternal Social Disadvantage and Psychosocial Stress With Structural MRI Measures at Birtha .
| Variable | Standardized β | P value | Q valueb |
|---|---|---|---|
| Total cortical gray matter (R2 = .56, P < .001) | |||
| Sex | 0.24 | <.001 | <.001 |
| Birth weight | 0.24 | <.001 | <.001 |
| PMA at MRI | 0.52 | <.001 | <.001 |
| Tobacco use | −0.03 | .52 | .69 |
| Social disadvantage | −0.13 | .008 | .01 |
| Psychosocial stress | −0.02 | .59 | .64 |
| Total subcortical gray matter (R2 = .56, P < .001) | |||
| Sex | 0.24 | <.001 | <.001 |
| Birth weight | 0.20 | <.001 | <.001 |
| PMA at MRI | 0.52 | <.001 | <.001 |
| Tobacco use | −0.06 | .17 | .67 |
| Social disadvantage | −0.16 | .002 | .003 |
| Psychosocial stress | −0.05 | .30 | .60 |
| Total white matter (R2 = .36, P < .001) | |||
| Sex | 0.29 | <.001 | <.001 |
| Birth weight | 0.18 | <.001 | .001 |
| PMA at MRI | 0.19 | <.001 | <.001 |
| Tobacco use | −0.05 | .34 | .69 |
| Social disadvantage | −0.28 | <.001 | <.001 |
| Psychosocial stress | −0.03 | .64 | .64 |
| Left hippocampus (R2 = .22, P < .001) | |||
| Sex | 0.16 | .003 | .008 |
| Birth weight | 0.11 | .06 | .06 |
| PMA at MRI | 0.29 | <.001 | <.001 |
| Tobacco use | −0.03 | .57 | .70 |
| Social disadvantage | −0.18 | .007 | .01 |
| Psychosocial stress | 0.02 | .75 | .93 |
| Right hippocampus (R2 = .22, P < .001) | |||
| Sex | 0.14 | .01 | .02 |
| Birth weight | 0.14 | .01 | .02 |
| PMA at MRI | 0.26 | <.001 | <.001 |
| Tobacco use | −0.06 | .29 | .58 |
| Social disadvantage | −0.18 | .007 | .01 |
| Psychosocial stress | 0.01 | .82 | .93 |
| Left amygdala (R2 = .41, P < .001) | |||
| Sex | 0.30 | <.001 | <.001 |
| Birth weight | 0.13 | .01 | .02 |
| PMA at MRI | 0.37 | <.001 | <.001 |
| Tobacco use | −0.08 | .09 | .58 |
| Social disadvantage | −0.20 | <.001 | .003 |
| Psychosocial stress | 0.005 | .92 | .93 |
| Right amygdala (R2 = .42, P < .001) | |||
| Sex | 0.28 | <.001 | <.001 |
| Birth weight | 0.17 | <.001 | .003 |
| PMA at MRI | 0.38 | <.001 | <.001 |
| Tobacco use | −0.06 | .25 | .58 |
| Social disadvantage | −0.19 | <.001 | .003 |
| Psychosocial stress | 0.005 | .93 | .93 |
| Gyrification index (R2 = .31, P < .001) | |||
| Sex | 0.12 | .03 | .03 |
| Birth weight | 0.10 | .07 | .07 |
| PMA at MRI | 0.40 | <.001 | <.001 |
| Tobacco use | 0.10 | .07 | .07 |
| Social disadvantage | −0.26 | <.001 | <.001 |
| Psychosocial stress | 0.04 | .46 | .46 |
Abbreviations: MRI, magnetic resonance imaging; PMA, postmenstrual age.
Results for all steps of hierarchical linear regression are given in full in eTable 5 in the Supplement.
Q values represent P values after correction for multiple comparisons using the Benjamini-Hochberg false discovery rate procedure.
Figure 1. Correlation Between Total Brain Volume and Maternal Social Disadvantage Factor .
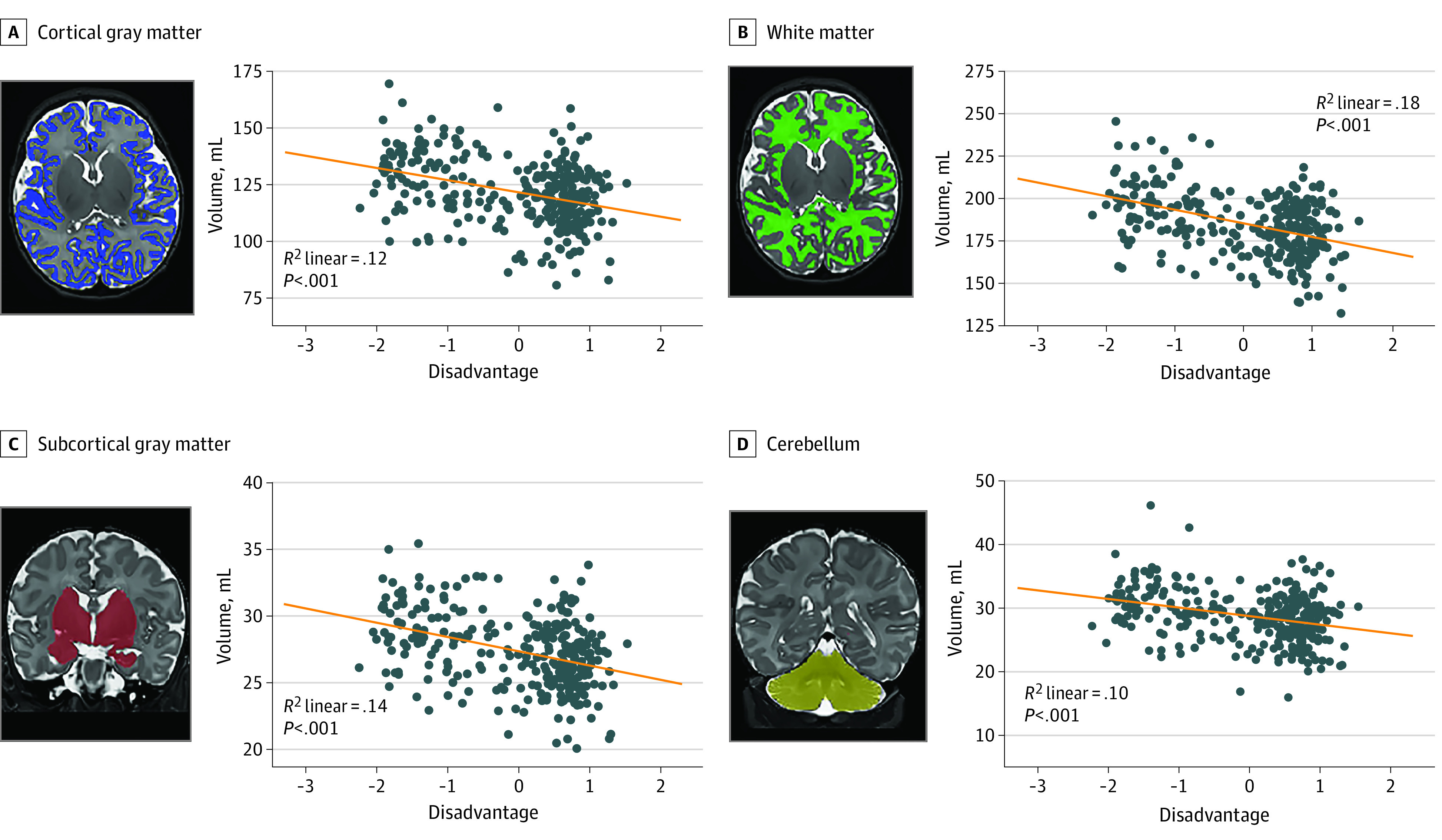
Correlation and P values are included for line of best fit. Automated volumetric segmentation for each tissue type is overlaid on T2-weighted image for a representative infant. X-axis indicates maternal social disadvantage.
Hippocampus and Amygdala
In step 1, female sex, lower birth weight, and younger PMA at MRI were associated with smaller right hippocampus (sex: β = 0.13, P = .02; birth weight: β = 0.20, P < .001; and PMA at MRI: β = 0.28, P < .001), left hippocampus (sex: β = 0.15, P = .006; birth weight: β = 0.16, P = .005; and PMA at MRI: β = 0.31, P < .001), right amygdala (sex: β = 0.27, P < .001; birth weight: β = 0.23, P < .001; and PMA at MRI: β = 0.40, P < .001) volumes, and left amygdala (sex: β = 0.29, P < .001; birth weight: β = 0.18, P < .001; and PMA at MRI: β = 0.39, P < .001) volumes (eTable 5 in the Supplement). Tobacco use was associated with reduced amygdalae volumes bilaterally (left amygdala: β = −0.13, P = .007; right amygdala: β = −0.11, P = .03) (eTable 5 in the Supplement). In step 2, greater social disadvantage was associated with reduced volumes for subcortical regions of interest and accounted for an additional 2.3% to 3.1% of the variance (Table 2 and Figure 2). Social disadvantage accounted for an additional 2.3% of the variance of the left hippocampus (unstandardized β = –0.03; 95% CI, –0.05 to –0.01), 2.3% of the right hippocampus (unstandardized β = –0.03; 95% CI, –0.05 to –0.01), 3.1% of the left amygdala (unstandardized β = –0.02; 95% CI, –0.03 to –0.01), and 2.9% of the right amygdala (unstandardized β = –0.02; 95% CI, –0.03 to –0.01) (eTable 5 in the Supplement). The contribution of psychosocial stress was not significant (Table 2). After standardization of hippocampal and amygdalae volumes using total brain volume, no significant associations were found with any covariates, social disadvantage, or psychosocial stress (eTable 5 in the Supplement).
Figure 2. Correlation Between Regional Brain Volume and Maternal Social Disadvantage Factor .
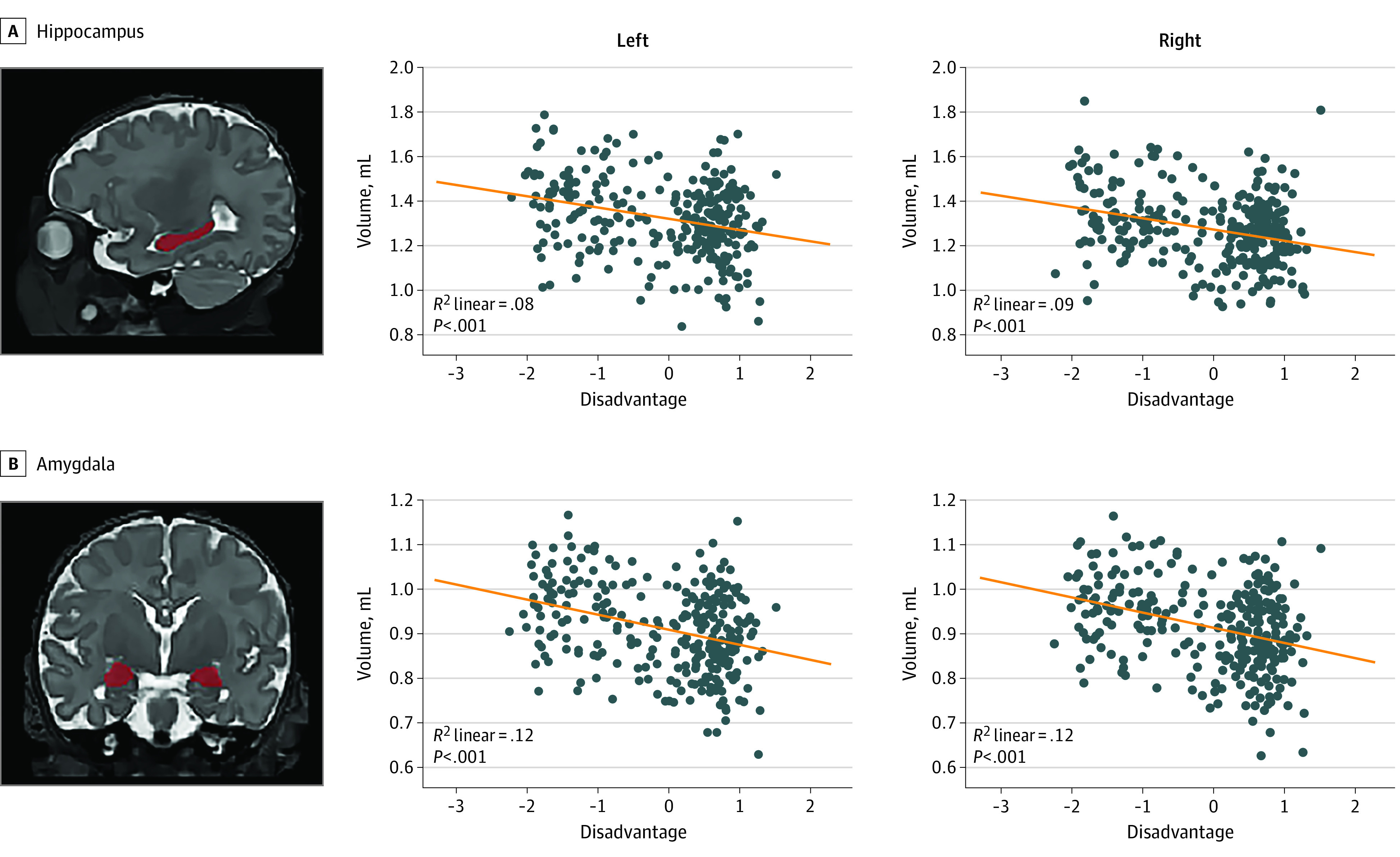
Correlation and P values are included for line of best fit. Automated volumetric segmentation for each structure is overlaid on T2-weighted image for a representative infant. Note similar results across hemispheres. X-axis indicates maternal social disadvantage.
Cortical Folding
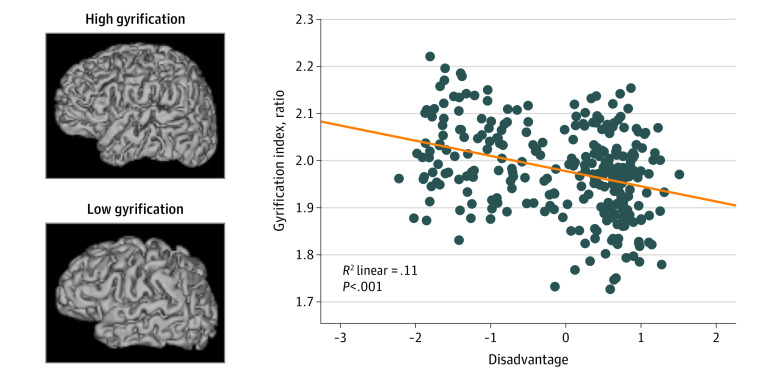
In step 1, female sex, smaller birth weight, and younger PMA at MRI were associated with diminished GI (sex: β = 0.10, P = .05; birth weight: β = 0.17, P = .002; PMA at MRI: β = 0.43, P < .001) (eTable 5 in the Supplement). In step 2, higher social disadvantage was associated with reduced GI (β = −0.26, P < .001) and accounted for an additional 4.8% of the variance (unstandardized β = –0.03; 95% CI, –0.04 to –0.01) (Table 2 and Figure 3). Tobacco use and psychosocial stress were not significantly associated with cortical folding.
Figure 3. Correlation Between the Gyrification Index and Maternal Social Disadvantage Factor.
Cortical surfaces for representative infants with high vs low gyrification index are included for reference. X-axis indicates maternal social disadvantage.
Discussion
This cohort study is one of the largest investigations of the fetal origins of health and disease beginning in the first trimester of gestation using comprehensive, multidimensional measures of maternal social disadvantage and psychosocial stress to assess associations with brain morphometry at birth. In healthy, term-born infants, prenatal exposure to social disadvantage demonstrated inverse associations with all brain tissue types, including reduced cortical and subcortical gray and white matter and decreased cortical folding in the first weeks of life. After accounting for global differences in brain volume, no regionally specific associations were found between social disadvantage or psychosocial stress and the hippocampus and amygdala. In our cohort, exposure to greater social disadvantage in utero appeared to play a greater role in brain structural development than maternal psychosocial stress.
We provide evidence of the association of prenatal exposure to social disadvantage with differences in global brain structural development at birth. Results persisted after accounting for infant birth weight, which also is associated with SES.60 Likely because of rigorous covariate control, effect sizes were small but consistent with reports in other samples of infants35 and children.16 Furthermore, findings are consistent with cross-sectional studies that found that a lower income-to-needs ratio was associated with reduced total cortical and subcortical gray matter in infants at 5 weeks35 and 5 months of age.13 Findings also align with work that reported regional and widespread reductions in cortical folding associated with lower SES among older children.14,61 Of note, we extend prior work13,14,25,61 to show that the associations between poverty and reduced brain volumes begin in utero and are evident in the first weeks of life. Social disadvantage was most strongly associated with reduced white matter volume, explaining 7% of the variance. This finding highlights the timing of prenatal exposure to poverty and the vulnerability of white matter as myelination occurs rapidly beginning at 28 to 29 weeks of gestation.62,63 During fetal development, oligodendrocyte progenitor cells and subplate neurons are sensitive to oxidative stress, which may have cascading effects on pruning and/or crossing fibers and subsequent white matter volume at birth.22,64
Although greater social disadvantage during pregnancy was associated with global reductions in infant brain volume and cortical folding, the amygdalae and hippocampi were not preferentially associated with social disadvantage or psychosocial stress. Differences between our findings and studies reporting on the effects of poverty on these subcortical structures may be attributed to prior works13,32,35 relying on single measures of SES, assessing brain development at later time points, and/or including higher SES samples. We interpret current study findings as evidence of a more widespread alteration in brain growth and development in the setting of exposure to significant, multifactorial socioeconomic disadvantage in utero.
This study addresses the independent contributions of maternal SES and psychosocial stress during pregnancy on offspring brain morphometry at birth.65 Consistent with other findings,66 our measure of social disadvantage correlated with psychosocial stress during pregnancy. However, prenatal exposure to social disadvantage was associated with brain volumes and cortical folding, whereas psychosocial stress was not significant. Current results could reflect the fact that participants were oversampled for mothers with greater social disadvantage. We also assessed multiple aspects of social adversity, which when examined together are likely more impactful.67,68 We anticipate our results will be generalizable to other socioeconomically diverse (but otherwise relatively healthy) US populations. Results may not generalize to populations that face different kinds of adversity or those with higher SES.
Although the precise mechanism remains unclear, postnatal ELA studies11,69 posit that long-term deprivation of resources and/or psychosocial stress overstimulate the HPA axis and the immune system, leading to altered brain-behavior outcomes. Fetal sensitivity to glucocorticoids is a leading hypothesis to explain the regional effects of prenatal ELA on the hippocampus, amygdala, and prefrontal cortex.42,43,70,71,72 In addition, changes in maternal immune activation incited by prenatal ELA may contribute globally to brain development in utero via several mechanisms, including increased synaptic pruning, altered neurotransmitter profiles, impaired placental delivery of neurotrophic factors, and placental epigenetic programming.73,74
Through the above mechanisms, including changes in cortisol production and systemic inflammation, poverty and psychosocial stress likely have overlapping effects on the developing brain.3,11,75,76 Additional contributing factors for mothers living in poverty may include specific macronutrient and micronutrient deficiencies77 and direct neurotoxic and indirect neuroinflammatory effects of household, outdoor, and water pollutants, such as lead78 or air pollution.79 Future directions to elucidate causal mechanisms of neurodevelopmental and socioemotional impairments include examining specific maternal factors, such as inflammatory cytokines and cortisol,42,80 in the context of maternal psychological stress, SES, and related nutritional and environmental exposures. There is further work to be done to clearly establish links between prenatal ELA, brain morphometry findings, and childhood outcomes.81,82
Limitations
Our findings should be interpreted in light of some study limitations. First, we assessed maternal depression with the EPDS. Although the EPDS is a validated measure, the lack of a semistructured interview may have led to symptom underreporting. Second, this study did not assess other environmental exposures, such as lead and air pollution, which may be linked with poverty and subsequent brain development. Third, we did not investigate the role of race in this analysis because of the collinearity between race and social disadvantage.45 This sample reflects the clear link between racial inequities and social disadvantage in the US and provides justification for including a measure of racial discrimination.
Conclusions
In this cohort study, we examined the independent roles of maternal social disadvantage and psychosocial stress during pregnancy and found global associations between social disadvantage and neonatal brain volumetric and folding measures. No association was found between brain volumes and psychosocial stress. Of note, results highlight that associations between poverty and neurodevelopment begin in utero and are evident in the first weeks of life. These findings may inform future randomized clinical trials of poverty reduction and family-based interventions to address the material and psychosocial needs of expectant parents and improve neonatal brain outcomes at birth.83
eFigure. Participant Flow From Study Enrollment to Inclusion in Current Analysis
eMethods. Supplemental Methods
eTable 1. Identification of Covariates of Interest Associated With Neonatal Volumetric MRI Measures at Birth (N=280)
eTable 2. Identification of Potential Covariates of Interest Associated With Infant Sex (N=280)
eTable 3. Comparison of Full-term Infants Excluded Due to Missing/Low-Quality MRI Data
eTable 4. Comparison of Full Cohort and Infants Excluded (Largely Due to Prematurity) From the Current Study
eTable 5. Full Results of Hierarchical Linear Regression Linking Maternal Social Disadvantage and Psychosocial Stress With Structural MRI Measures at Birth (N=280)
eTable 6. Hierarchical Linear Regression Linking Maternal Social Disadvantage and Psychosocial Stress with Total Brain Volumes (TBV) at Birth (N=280)
eTable 7. Hierarchical Linear Regression Exploring Hemispheric Effects of Maternal Social Disadvantage and Psychosocial Stress (N=280)
References
- 1.Barrero-Castillero A, Morton SU, Nelson CA III, Smith VC. Psychosocial stress and adversity: effects from the perinatal period to adulthood. Neoreviews. 2019;20(12):e686-e696. doi: 10.1542/neo.20-12-e686 [DOI] [PubMed] [Google Scholar]
- 2.Noble KG, Giebler MA. The neuroscience of socioeconomic inequality. Curr Opin Behav Sci. 2020;36:23-28. doi: 10.1016/j.cobeha.2020.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller GE, Chen E, Parker KJ. Psychological stress in childhood and susceptibility to the chronic diseases of aging: moving toward a model of behavioral and biological mechanisms. Psychol Bull. 2011;137(6):959-997. doi: 10.1037/a0024768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Evans GW. The environment of childhood poverty. Am Psychol. 2004;59(2):77-92. doi: 10.1037/0003-066X.59.2.77 [DOI] [PubMed] [Google Scholar]
- 5.Luby JL, Barch D, Whalen D, Tillman R, Belden A. Association between early life adversity and risk for poor emotional and physical health in adolescence: a putative mechanistic neurodevelopmental pathway. JAMA Pediatr. 2017;171(12):1168-1175. doi: 10.1001/jamapediatrics.2017.3009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McLaughlin KA, Weissman D, Bitrán D. Childhood adversity and neural development: a systematic review. Annu Rev Dev Psychol. 2019;1(1):277-312. doi: 10.1146/annurev-devpsych-121318-084950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tooley UA, Bassett DS, Mackey AP. Environmental influences on the pace of brain development. Nat Rev Neurosci. 2021;22(6):372-384. doi: 10.1038/s41583-021-00457-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson SB, Riis JL, Noble KG. State of the art review: poverty and the developing brain. Pediatrics. 2016;137(4):e20153075. doi: 10.1542/peds.2015-3075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Merz EC, Wiltshire CA, Noble KG. Socioeconomic inequality and the developing brain: spotlight on language and executive function. Child Dev Perspect. 2019;13(1):15-20. doi: 10.1111/cdep.12305 [DOI] [Google Scholar]
- 10.Perry RE, Finegood ED, Braren SH, et al. ; Family Life Project Key Investigators . Developing a neurobehavioral animal model of poverty: drawing cross-species connections between environments of scarcity-adversity, parenting quality, and infant outcome. Dev Psychopathol. 2019;31(2):399-418. doi: 10.1017/S095457941800007X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hantsoo L, Kornfield S, Anguera MC, Epperson CN. Inflammation: a proposed intermediary between maternal stress and offspring neuropsychiatric risk. Biol Psychiatry. 2019;85(2):97-106. doi: 10.1016/j.biopsych.2018.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luby J, Belden A, Botteron K, et al. The effects of poverty on childhood brain development: the mediating effect of caregiving and stressful life events. JAMA Pediatr. 2013;167(12):1135-1142. doi: 10.1001/jamapediatrics.2013.3139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanson JL, Hair N, Shen DG, et al. Family poverty affects the rate of human infant brain growth. PLoS One. 2013;8(12):e80954. doi: 10.1371/journal.pone.0080954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jednoróg K, Altarelli I, Monzalvo K, et al. The influence of socioeconomic status on children’s brain structure. PLoS One. 2012;7(8):e42486. doi: 10.1371/journal.pone.0042486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Noble KG, Houston SM, Brito NH, et al. Family income, parental education and brain structure in children and adolescents. Nat Neurosci. 2015;18(5):773-778. doi: 10.1038/nn.3983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taylor RL, Cooper SR, Jackson JJ, Barch DM. Assessment of neighborhood poverty, cognitive function, and prefrontal and hippocampal volumes in children. JAMA Netw Open. 2020;3(11):e2023774. doi: 10.1001/jamanetworkopen.2020.23774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hair NL, Hanson JL, Wolfe BL, Pollak SD. Association of child poverty, brain development, and academic achievement. JAMA Pediatr. 2015;169(9):822-829. doi: 10.1001/jamapediatrics.2015.1475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hanson JL, Nacewicz BM, Sutterer MJ, et al. Behavioral problems after early life stress: contributions of the hippocampus and amygdala. Biol Psychiatry. 2015;77(4):314-323. doi: 10.1016/j.biopsych.2014.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xerxa Y, Delaney SW, Rescorla LA, et al. Association of poor family functioning from pregnancy onward with preadolescent behavior and subcortical brain development. JAMA Psychiatry. 2021;78(1):29-37. doi: 10.1001/jamapsychiatry.2020.2862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luby JL, Baram TZ, Rogers CE, Barch DM. Neurodevelopmental optimization after early-life adversity: cross-species studies to elucidate sensitive periods and brain mechanisms to inform early intervention. Trends Neurosci. 2020;43(10):744-751. doi: 10.1016/j.tins.2020.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buss C, Entringer S, Wadhwa PD. Fetal programming of brain development: intrauterine stress and susceptibility to psychopathology. Sci Signal. 2012;5(245):10.1126/scisignal.2003406. doi: 10.1126/scisignal.2003406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tau GZ, Peterson BS. Normal development of brain circuits. Neuropsychopharmacology. 2010;35(1):147-168. doi: 10.1038/npp.2009.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Larson CP. Poverty during pregnancy: its effects on child health outcomes. Paediatr Child Health. 2007;12(8):673-677. doi: 10.1093/pch/12.8.673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Glover V. Maternal depression, anxiety and stress during pregnancy and child outcome; what needs to be done. Best Pract Res Clin Obstet Gynaecol. 2014;28(1):25-35. doi: 10.1016/j.bpobgyn.2013.08.017 [DOI] [PubMed] [Google Scholar]
- 25.O’Donnell KJ, Meaney MJ. Fetal origins of mental health: the developmental origins of health and disease hypothesis. Am J Psychiatry. 2017;174(4):319-328. doi: 10.1176/appi.ajp.2016.16020138 [DOI] [PubMed] [Google Scholar]
- 26.Lebel CA, McMorris CA, Kar P, et al. Characterizing adverse prenatal and postnatal experiences in children. Birth Defects Res. 2019;111(12):848-858. doi: 10.1002/bdr2.1464 [DOI] [PubMed] [Google Scholar]
- 27.Pulli EP, Kumpulainen V, Kasurinen JH, et al. Prenatal exposures and infant brain: Review of magnetic resonance imaging studies and a population description analysis. Hum Brain Mapp. 2019;40(6):1987-2000. doi: 10.1002/hbm.24480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Biggeri M, Cuesta JA. An integrated framework for child poverty and well-being measurement: reconciling theories. Child Indic Res. 2021;14(2):821-846. doi: 10.1007/s12187-020-09774-0 [DOI] [Google Scholar]
- 29.Qiu A, Rifkin-Graboi A, Chen H, et al. Maternal anxiety and infants’ hippocampal development: timing matters. Transl Psychiatry. 2013;3(9):e306-e306. doi: 10.1038/tp.2013.79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lehtola SJ, Tuulari JJ, Scheinin NM, et al. Newborn amygdalar volumes are associated with maternal prenatal psychological distress in a sex-dependent way. Neuroimage Clin. 2020;28:102380. doi: 10.1016/j.nicl.2020.102380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang C, Shen M, Guillaume B, et al. FKBP5 Moderates the association between antenatal maternal depressive symptoms and neonatal brain morphology. Neuropsychopharmacology. 2018;43(3):564-570. doi: 10.1038/npp.2017.232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu Y, Lu YC, Jacobs M, et al. Association of prenatal maternal psychological distress with fetal brain growth, metabolism, and cortical maturation. JAMA Netw Open. 2020;3(1):e1919940. doi: 10.1001/jamanetworkopen.2019.19940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu Y, Kapse K, Jacobs M, et al. Association of maternal psychological distress with in utero brain development in fetuses with congenital heart disease. JAMA Pediatr. 2020;174(3):e195316. doi: 10.1001/jamapediatrics.2019.5316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lu YC, Kapse K, Andersen N, et al. Association between socioeconomic status and in utero fetal brain development. JAMA Netw Open. 2021;4(3):e213526. doi: 10.1001/jamanetworkopen.2021.3526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Betancourt LM, Avants B, Farah MJ, et al. Effect of socioeconomic status (SES) disparity on neural development in female African-American infants at age 1 month. Dev Sci. 2016;19(6):947-956. doi: 10.1111/desc.12344 [DOI] [PubMed] [Google Scholar]
- 36.Knickmeyer RC, Xia K, Lu Z, et al. Impact of demographic and obstetric factors on infant brain volumes: a population neuroscience study. Cereb Cortex. 2017;27(12):5616-5625. doi: 10.1093/cercor/bhw331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.US Census Bureau . Income, Poverty and Health Insurance Coverage in the United States: 2020. 2021. Accessed February 1, 2022. https://www.census.gov/newsroom/press-releases/2021/income-poverty-health-insurance-coverage.html
- 38.Kendig S, Keats JP, Hoffman MC, et al. Consensus bundle on maternal mental health: perinatal depression and anxiety. Obstet Gynecol. 2017;129(3):422-430. doi: 10.1097/AOG.0000000000001902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rich-Edwards JW, Kleinman K, Abrams A, et al. Sociodemographic predictors of antenatal and postpartum depressive symptoms among women in a medical group practice. J Epidemiol Community Health. 2006;60(3):221-227. doi: 10.1136/jech.2005.039370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cena L, Mirabella F, Palumbo G, Gigantesco A, Trainini A, Stefana A. Prevalence of maternal antenatal and postnatal depression and their association with sociodemographic and socioeconomic factors: a multicentre study in Italy. J Affect Disord. 2021;279:217-221. doi: 10.1016/j.jad.2020.09.136 [DOI] [PubMed] [Google Scholar]
- 41.Herbell K, Zauszniewski JA, Williams E. Stress and depressive symptoms among demographically diverse American pregnant women. Issues Ment Health Nurs. 2020;41(1):73-82. doi: 10.1080/01612840.2019.1662145 [DOI] [PubMed] [Google Scholar]
- 42.Buss C, Davis EP, Shahbaba B, Pruessner JC, Head K, Sandman CA. Maternal cortisol over the course of pregnancy and subsequent child amygdala and hippocampus volumes and affective problems. Proc Natl Acad Sci U S A. 2012;109(20):E1312-E1319. doi: 10.1073/pnas.1201295109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McEwen BS, Nasca C, Gray JD. Stress effects on neuronal structure: hippocampus, amygdala, and prefrontal cortex. Neuropsychopharmacology. 2016;41(1):3-23. doi: 10.1038/npp.2015.171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stout MJ, Chubiz J, Raghuraman N, et al. A multidisciplinary prematurity research cohort study. medRxiv. Preprint posted online September 29, 2021. doi: 10.1101/2021.09.28.21264264 [DOI] [PMC free article] [PubMed]
- 45.Luby JL, Barch DM, Warner B, et al. Modeling prenatal adversity/advantage: effects on birth weight. medRxiv. Preprint posted online December 17, 2021. doi: 10.1101/2021.12.16.21267938 [DOI] [Google Scholar]
- 46.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative . The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453-1457. doi: 10.1016/S0140-6736(07)61602-X [DOI] [PubMed] [Google Scholar]
- 47.Brooks-Gunn J, Klebanov PK, Liaw F. The learning, physical, and emotional environment of the home in the context of poverty: the infant health and development program. Child Youth Serv Rev. 1995;17(1-2):251-276. doi: 10.1016/0190-7409(95)00011-Z [DOI] [Google Scholar]
- 48.Kind AJH, Buckingham WR. Making neighborhood-disadvantage metrics accessible—The Neighborhood Atlas. N Engl J Med. 2018;378(26):2456-2458. doi: 10.1056/NEJMp1802313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Krebs-Smith SM, Pannucci TE, Subar AF, et al. Update of the Healthy Eating Index: HEI-2015. J Acad Nutr Diet. 2018;118(9):1591-1602. doi: 10.1016/j.jand.2018.05.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385-396. doi: 10.2307/2136404 [DOI] [PubMed] [Google Scholar]
- 51.Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression: development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150(6):782-786. doi: 10.1192/bjp.150.6.782 [DOI] [PubMed] [Google Scholar]
- 52.Slavich GM, Shields GS. Assessing Lifetime Stress Exposure Using the Stress and Adversity Inventory for Adults (Adult STRAIN): an overview and initial validation. Psychosom Med. 2018;80(1):17-27. doi: 10.1097/PSY.0000000000000534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Williams DR, Yu Y, Jackson JS, Anderson NB. Racial differences in physical and mental health: socio-economic status, stress and discrimination. J Health Psychol. 1997;2(3):335-351. doi: 10.1177/135910539700200305 [DOI] [PubMed] [Google Scholar]
- 54.Bateman BT, Mhyre JM, Hernandez-Diaz S, et al. Development of a comorbidity index for use in obstetric patients. Obstet Gynecol. 2013;122(5):957-965. doi: 10.1097/AOG.0b013e3182a603bb [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Metcalfe A, Lix LM, Johnson JA, et al. Validation of an obstetric comorbidity index in an external population. BJOG. 2015;122(13):1748-1755. doi: 10.1111/1471-0528.13254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Alexander B, Murray AL, Loh WY, et al. A new neonatal cortical and subcortical brain atlas: the Melbourne Children’s Regional Infant Brain (M-CRIB) atlas. Neuroimage. 2017;147:841-851. doi: 10.1016/j.neuroimage.2016.09.068 [DOI] [PubMed] [Google Scholar]
- 57.Adamson CL, Alexander B, Ball G, et al. Parcellation of the Cortex Using Surface-Based Melbourne Children’s Regional Infant Brain Atlases (M-CRIB-S). bioRxiv. Preprint posted online September 4, 2019. doi: 10.1038/s41598-020-61326-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shimony JS, Smyser CD, Wideman G, et al. Comparison of cortical folding measures for evaluation of developing human brain. Neuroimage. 2016;125:780-790. doi: 10.1016/j.neuroimage.2015.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B. 1995;57(1):289-300. doi: 10.1111/j.2517-6161.1995.tb02031.x [DOI] [Google Scholar]
- 60.Martinson ML, Reichman NE. Socioeconomic inequalities in low birth weight in the United States, the United Kingdom, Canada, and Australia. Am J Public Health. 2016;106(4):748-754. doi: 10.2105/AJPH.2015.303007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gonzalez MR, Palmer CE, Uban KA, Jernigan TL, Thompson WK, Sowell ER. Positive economic, psychosocial, and physiological ecologies predict brain structure and cognitive performance in 9-10-year-old children. Front Hum Neurosci. 2020;14:578822. doi: 10.3389/fnhum.2020.578822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hüppi PS, Warfield S, Kikinis R, et al. Quantitative magnetic resonance imaging of brain development in premature and mature newborns. Ann Neurol. 1998;43(2):224-235. doi: 10.1002/ana.410430213 [DOI] [PubMed] [Google Scholar]
- 63.Gao W, Lin W, Chen Y, et al. Temporal and spatial development of axonal maturation and myelination of white matter in the developing brain. AJNR Am J Neuroradiol. 2009;30(2):290-296. doi: 10.3174/ajnr.A1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Volpe JJ. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol. 2009;8(1):110-124. doi: 10.1016/S1474-4422(08)70294-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Silveira PP, Pokhvisneva I, Parent C, et al. Cumulative prenatal exposure to adversity reveals associations with a broad range of neurodevelopmental outcomes that are moderated by a novel, biologically informed polygenetic score based on the serotonin transporter solute carrier family C6, member 4 (SLC6A4) gene expression. Dev Psychopathol. 2017;29(5):1601-1617. doi: 10.1017/S0954579417001262 [DOI] [PubMed] [Google Scholar]
- 66.Verbeek T, Bockting CLH, Beijers C, Meijer JL, van Pampus MG, Burger H. Low socioeconomic status increases effects of negative life events on antenatal anxiety and depression. Women Birth. 2019;32(1):e138-e143. doi: 10.1016/j.wombi.2018.05.005 [DOI] [PubMed] [Google Scholar]
- 67.Manley BJ, Roberts RS, Doyle LW, et al. ; Caffeine for Apnea of Prematurity (CAP) Trial Investigators; Caffeine for Apnea of Prematurity CAP Trial Investigators . Social variables predict gains in cognitive scores across the preschool years in children with birth weights 500 to 1250 grams. J Pediatr. 2015;166(4):870-6.e1, 2. doi: 10.1016/j.jpeds.2014.12.016 [DOI] [PubMed] [Google Scholar]
- 68.Jensen SKG, Berens AE, Nelson CA III. Effects of poverty on interacting biological systems underlying child development. Lancet Child Adolesc Health. 2017;1(3):225-239. doi: 10.1016/S2352-4642(17)30024-X [DOI] [PubMed] [Google Scholar]
- 69.Perry RE, Braren SH, Opendak M, et al. ; Family Life Project Key Investigators . Elevated infant cortisol is necessary but not sufficient for transmission of environmental risk to infant social development: cross-species evidence of mother-infant physiological social transmission. Dev Psychopathol. 2020;32(5):1696-1714. doi: 10.1017/S0954579420001455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lindsay KL, Buss C, Wadhwa PD, Entringer S. The interplay between nutrition and stress in pregnancy: implications for fetal programming of brain development. Biol Psychiatry. 2019;85(2):135-149. doi: 10.1016/j.biopsych.2018.06.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Muhammad A, Carroll C, Kolb B. Stress during development alters dendritic morphology in the nucleus accumbens and prefrontal cortex. Neuroscience. 2012;216:103-109. doi: 10.1016/j.neuroscience.2012.04.041 [DOI] [PubMed] [Google Scholar]
- 72.Lucassen PJ, Naninck EFG, van Goudoever JB, Fitzsimons C, Joels M, Korosi A. Perinatal programming of adult hippocampal structure and function; emerging roles of stress, nutrition and epigenetics. Trends Neurosci. 2013;36(11):621-631. doi: 10.1016/j.tins.2013.08.002 [DOI] [PubMed] [Google Scholar]
- 73.Beijers R, Buitelaar JK, de Weerth C. Mechanisms underlying the effects of prenatal psychosocial stress on child outcomes: beyond the HPA axis. Eur Child Adolesc Psychiatry. 2014;23(10):943-956. doi: 10.1007/s00787-014-0566-3 [DOI] [PubMed] [Google Scholar]
- 74.Knuesel I, Chicha L, Britschgi M, et al. Maternal immune activation and abnormal brain development across CNS disorders. Nat Rev Neurol. 2014;10(11):643-660. doi: 10.1038/nrneurol.2014.187 [DOI] [PubMed] [Google Scholar]
- 75.Barrington WE, Stafford M, Hamer M, Beresford SAA, Koepsell T, Steptoe A. Neighborhood socioeconomic deprivation, perceived neighborhood factors, and cortisol responses to induced stress among healthy adults. Health Place. 2014;27:120-126. doi: 10.1016/j.healthplace.2014.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Marques AH, O’Connor TG, Roth C, Susser E, Bjørke-Monsen AL. The influence of maternal prenatal and early childhood nutrition and maternal prenatal stress on offspring immune system development and neurodevelopmental disorders. Front Neurosci. 2013;7:120. doi: 10.3389/fnins.2013.00120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cortés-Albornoz MC, García-Guáqueta DP, Velez-van-Meerbeke A, Talero-Gutiérrez C. Maternal nutrition and neurodevelopment: a scoping review. Nutrients. 2021;13(10):3530. doi: 10.3390/nu13103530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ahmad F, Liu P. (Ascorb)ing Pb neurotoxicity in the developing brain. Antioxidants (Basel). 2020;9(12):1311. doi: 10.3390/antiox9121311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Herting MM, Younan D, Campbell CE, Chen JC. Outdoor air pollution and brain structure and function from across childhood to young adulthood: a methodological review of brain MRI studies. Front Public Health. 2019;7:332. doi: 10.3389/fpubh.2019.00332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Graham AM, Rasmussen JM, Rudolph MD, et al. Maternal systemic interleukin-6 during pregnancy is associated with newborn amygdala phenotypes and subsequent behavior at 2 years of age. Biol Psychiatry. 2018;83(2):109-119. doi: 10.1016/j.biopsych.2017.05.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vargas T, Damme KSF, Mittal VA. Neighborhood deprivation, prefrontal morphology and neurocognition in late childhood to early adolescence. Neuroimage. 2020;220:117086. doi: 10.1016/j.neuroimage.2020.117086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Overfeld J, Entringer S, Rasmussen JM, et al. Neonatal hippocampal volume moderates the effects of early postnatal enrichment on cognitive development. Dev Cogn Neurosci. 2020;45:100820. doi: 10.1016/j.dcn.2020.100820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Blair C, Raver CC. Poverty, stress, and brain development: new directions for prevention and intervention. Acad Pediatr. 2016;16(3)(suppl):S30-S36. doi: 10.1016/j.acap.2016.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. Participant Flow From Study Enrollment to Inclusion in Current Analysis
eMethods. Supplemental Methods
eTable 1. Identification of Covariates of Interest Associated With Neonatal Volumetric MRI Measures at Birth (N=280)
eTable 2. Identification of Potential Covariates of Interest Associated With Infant Sex (N=280)
eTable 3. Comparison of Full-term Infants Excluded Due to Missing/Low-Quality MRI Data
eTable 4. Comparison of Full Cohort and Infants Excluded (Largely Due to Prematurity) From the Current Study
eTable 5. Full Results of Hierarchical Linear Regression Linking Maternal Social Disadvantage and Psychosocial Stress With Structural MRI Measures at Birth (N=280)
eTable 6. Hierarchical Linear Regression Linking Maternal Social Disadvantage and Psychosocial Stress with Total Brain Volumes (TBV) at Birth (N=280)
eTable 7. Hierarchical Linear Regression Exploring Hemispheric Effects of Maternal Social Disadvantage and Psychosocial Stress (N=280)