Abstract
The azanucleotide decitabine is used in the treatment of acute myeloid leukemia (AML). Studies have shown conflicting results with 10-day regimens used in previously untreated AML patients. Additionally, there is little data on 10-day decitabine regimens in the relapsed setting. This study investigated outcomes of 108 adult patients with AML in the upfront and relapsed setting treated with a 10-day decitabine regimen. In the upfront group, the overall response rate (ORR, CR+CRi) was 36.1% and the median overall survival (OS) was 6.6 months, while the relapsed/refractory group had an ORR of 25% with an OS of 4.8 months. When analyzed with respect to cytogenetics, the upfront group featured an ORR of 28.1% with an OS of 9.4 months in the intermediate cytogenetic cohort compared to a 40.5% ORR and an OS of 5.4 months in the unfavorable cytogenetic cohort. An analysis of the relapsed/refractory group demonstrated an ORR of 26.3% with an OS of 7.9 months for intermediate cytogenetics versus 25.0% with an OS of 1.8 months in the unfavorable cohort. While these response rates are similar to previously published data, the median OS appears shorter.
1. INTRODUCTION
Acute myeloid leukemia (AML) is a clonal disorder of myeloid hematopoiesis that results in accumulation of immature myeloid precursors, leading to progressive marrow failure and death.1,2 Unfortunately, despite years of advancement in prognostication and treatment, disease recurrence and resistance to therapy are common. Decitabine (Dacogen; 5-aza-2’-deoxycytidine) is a novel hypomethylating agent that was initially approved by the FDA for treatment of myelodysplastic syndrome (MDS) and its use has since expanded to the treatment of AML.3 Extramedullary toxicity of the hypomethylating agents is thought to be less than that seen with traditional intensive cytotoxic chemotherapy and is one reason why induction with decitabine with or without venetoclax has become an attractive option for patients that are ineligible to receive anthracycline-based therapy. Decitabine is activated by nucleotide kinases to the active metabolite 5-aza-2’-deoxycytidine-5’-triphosphate, which is incorporated into replicating DNA and inhibits cytosine methylation, resulting in genome-wide hypomethylation.4 The antineoplastic effect of decitabine is largely thought to be due to inhibition of DNA methyltransferases which are subsequently degraded, manifesting as downstream DNA hypomethylation and activation of genes affecting pathways responsible for cellular differentiation and/or apoptosis.5,6
For patients that are deemed ineligible for intensive cytotoxic chemotherapy, widespread use of hypomethylating agents has become routine. Historically, decitabine was administered initially in a 3-day schedule, and studies that have investigated the efficacy of longer regimens of decitabine have undergone scrutiny. For example, a recent study suggested that the efficacy and safety of 5-day and 10-day regimens were not significantly different, but these findings have undergone criticism for being insufficiently powered.7–9 Furthermore, studies that have investigated the efficacy of decitabine in a 10-day course repeated in 28-day cycles have shown complete response (CR) rates varying from 30–47%.10–13 In a study conducted by Ritchie et al., a 10-day regimen of decitabine administered at 20 mg/m2 in 52 newly diagnosed patients resulted in a CR of 40%, even in those who had adverse prognostic features, with higher CR rates being reported by Blum et al. at 47%.10,11 The median overall survival (OS) is 6.3–12.7 months in the upfront setting,10,13,14 with variability attributable to age and/or performance status, compared to a similar median OS of 7.7 months for a 5-day course of decitabine in newly diagnosed AML.15
While recent years have imparted considerable advancements in treatment of AML, data still remain scarce for patients with relapsed or primary refractory AML.16 Currently, the only treatments available for those unable to receive intensive salvage therapy are either low-intensity therapy or best supportive care.17 There still remains no definite consensus for treatment of relapsed or refractory disease, especially for patients with advanced age. Regardless, investigation has shed light on the efficacy of hypomethylating agents in this setting, with prior studies noting a 10-day course of decitabine featuring a CR rate of 15.7% with a median OS of 177 days (5.8 months).11 The majority of the available data on the efficacy of 10-day decitabine is from prospective clinical trials and data from real-world experience outside of the eligibility criteria of a clinical trial are lacking. Therefore, the purpose of this study was to describe the outcomes of AML patients treated with 10-day decitabine in the upfront and relapsed settings.
2. MATERIALS AND METHODS
2.1. ELIGIBILITY
The Institutional Review Board of Wake Forest Baptist Medical Center approved the protocol. Eligibility criteria included all patients who received a 10-day decitabine regimen while hospitalized or infused in outpatient clinics from January 2000 to December 2016 for AML in the upfront or relapsed setting. Patients were excluded if the response data were unavailable. AML was defined using World Health Organization criteria, with a minimum of one bone marrow biopsy revealing at least 20% or greater myeloblasts.18,19 Patients were treated with 10-day cycles of decitabine 20 mg/m2 intravenously approximately every 28 days until repeat bone marrow biopsies showed less than 5% myeloblasts or up to 4 cycles. Patients that survived induction were then transitioned to 5-day decitabine maintenance given every 28 days until progression of disease or unacceptable toxicity.
2.2. OBJECTIVES
The primary outcome measure for the study was the overall response rate, which included both CR and CRi as per Donher et. al.20 The secondary outcomes evaluated were overall survival, 30 and 60-day mortality, number of cycles received, and toxicity data.
2.3. DATA COLLECTION AND RESPONSE CRITERIA
Age, demographic data, comorbid conditions, cytogenetics, and pre-treatment laboratory data were collected for all patients. Dates of bone marrow biopsies, performed at the discretion of the treating provider, were obtained and included. Toxicity data captured included neutropenic fever, renal toxicity, nausea and vomiting. Adverse events were retrospectively graded by NCI CTCAE version 5.0. Additionally, if the patient received a stem cell transplant (SCT), the dates and conditioning regimen were documented. CR and CRi rates, disposition, and dates of death were collected. The Charlson comorbidity index scores and hematopoietic cell transplantation-specific comorbidity index scores were obtained for all patients.21,22
Response criteria were reported and categorized according to the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia and in Myelodysplastic Syndromes.23,24 Included categories were complete remission and complete remission with incomplete count recovery. CR was defined as the presence of <5% blasts in the bone marrow aspirate with a neutrophil count of ≥1×109/L and ≥100×109/L platelets in the peripheral blood measured on the day of the bone marrow or between negative bone marrow biopsies with no evidence of extramedullary disease as documented by the treating oncologist. CRi was defined as <5% blasts in the bone marrow aspirate without extramedullary disease but not meeting criteria for CR.
3. STATISTICAL ANALYSIS
Descriptive statistics were used to analyze the results to characterize the study groups. Fischer’s exact test was used to compare categorical groups with determination of the effect size with odds ratios. The confidence intervals (CI) for the odds ratios were computed with the Baptista-Pike method. The median overall survival was determined from the data using Kaplan-Meier survival analyses with a corresponding log-rank (Mantel-Cox) test. Therapy-related AML, MDS, comorbidities, and total risk scores were summarized using medians and stratified ranges. Additionally, toxicity data for the 10-day decitabine regimen will be presented. The analyses were performed separately for both upfront AML and relapsed/refractory AML. All p-values are two-sided, with statistical significance evaluated at the 0.05 alpha level. Data analysis was performed in GraphPad Prism 8 for Macintosh.
4. RESULTS
4.1. PATIENTS
A total of 108 patients were identified. Baseline characteristics of the patients are found in Table 1. The median age was 71 years and the range was 42 to 88. Of these, 97 patients (89.8%) were 60 years or older and there were 42 patients (38.9%) aged 75 years or older. There were 66 males (61.1%) and 42 females (38.9%). The group had a median of 3 comorbidities. In the total group, 39 patients (36.1%) had a prior cancer diagnosis and 26 (24.1%) were treated with prior chemotherapy or radiation, with the remainder treated with surgery. Thirty-three patients had a history of MDS (30.6%), and of these 33 patients, 13 (39.4%) were treated with hypomethylating agents prior to 10-day decitabine induction (Table 1).
Table 1:
Baseline Characteristics of Patients
| Characteristic | All Patients (N=108) | Upfront Setting (N=72) | Relapsed/Refractory Setting (N=36) | Relapse After Upfront Decitabine Induction (N=8) |
|---|---|---|---|---|
| Male sex – no. (%) | 66 (61.1) | 49 (68.1) | 17 (47.2) | 5 (62.5) |
| Age at diagnosis – yr. | ||||
| Median | 71 | 74 | 67.5 | 76.5 |
| Range | 42–88 | 44–88 | 42–84 | 72–85 |
| Median WBC | 3.1 × 109/L | 3.0 × 109/L | 3.4 × 109/L | 2.1 × 109/L |
| Median hemoglobin | 9.2 g/dL | 9.0 g/dL | 9.5 g/dL | 9.1 g/dL |
| Median platelets | 49 × 109/L | 54 × 109/L | 40 × 109/L | 52 × 109/L |
| Cytogenetic risk groupa - no. (%) | ||||
| Favorable | 4 (3.7) | 3 (4.2) | 1 (2.8) | 0(0) |
| Intermediate | 51 (47.2) | 32 (44.4) | 19 (52.8) | 2 (25.0) |
| Unfavorable | 53 (49.1) | 37 (51.4) | 16 (44.4) | 6 (75.0) |
| Therapy-related AML - no. (%) | 26 (24.1) | 24 (33.3) | 2 (5.6) | 1 (12.5) |
| Prior MDS - no. (%) | 33 (30.6) | 25 (34.7) | 4 (50.0) | |
| Total number of comorbidities | ||||
| Median | 3 | 3 | 2 | 3 |
| Range | 0–6 | 0–6 | 0–5 | 0–5 |
| Charlson comorbidity index scoreb | ||||
| Median | 1 | 1 | 1 | 1 |
| Range | 0–10 | 0–10 | 0–6 | 0–3 |
| HCT-CI comorbidity scorec | ||||
| Median | 3 | 3 | 3 | 2 |
| Range | 0–9 | 0–9 | 0–8 | 0–5 |
| Stem cell transplant - no. (%) | 7 (6.5) | 3 (4.2) | 4 (11.1) | 0 (0) |
| Total 10-day cycles of decitabine | ||||
| Median | - | 2 | 2 | 2 |
| Range | - | 1–8 | 1–7 | 1–4 |
| Total 5-day cycles of maintenance decitabine after 10-day induction | ||||
| Median | - | 6 | 0 | 0 |
| Range | - | 0–66 | 0–20 | 0–4 |
The cytogenetic risk groups were stratified according to ELN criteria. Intermediate-I and intermediate-II groups were combined into a single intermediate group.
The Charlson comorbidity index score is a weighted index that predicts the risk of death within one year of hospitalization.21
The hematopoietic cell transplantation-specific comorbidity index (HCT-CI) was developed to identify comorbidities associated with stem cell transplantation and to assess risk prior to allogeneic transplant.20
The 72 patients treated in the upfront setting received a median of two 10-day induction cycles of decitabine followed by a median of 6 maintenance cycles after remission was achieved. The median age was 74 years, 30 patients (41.7%) had prior cancer, and 24 (33.3%) received previous chemotherapy, radiation, or both. Thirty-seven patients (51.4%) had unfavorable cytogenetics, 32 patients (44.4%) had intermediate cytogenetics, and three (4.2%) had favorable cytogenetics (Table 1). The median Charlson comorbidity index score in this group was 1, with the most common medical comorbidities being uncomplicated diabetes mellitus (20.8 %), chronic obstructive pulmonary disease (18.1%), and chronic kidney disease (15.3%). Eight patients had relapsed disease while on maintenance 5-day decitabine and underwent re-escalation back to 10-day cycles. These eight patients were analyzed separately from the 36 patients with relapsed/refractory disease following other chemotherapy regimens.
The relapsed/refractory group of 36 patients treated with decitabine salvage received a median of two 10-day induction cycles. The median age of this group was 67.5 years, nine patients (25.0%) had prior cancer and two (5.6%) received chemotherapy. Sixteen patients (44.4%) had unfavorable cytogenetics, 19 (52.8%) had intermediate cytogenetics, and one (2.8%) had favorable cytogenetics. The median Charlson comorbidity index score in this group was 1, with the most common medical comorbidities being uncomplicated diabetes mellitus (19.4%), cerebrovascular disease (11.1%), and chronic obstructive pulmonary disease (11.1%). In the relapsed group of eight patients that were re-escalated to 10-day decitabine, the median age was 76.5 years. One patient (12.5%) had prior cancer and received chemotherapy. Six patients (75.0%) had unfavorable cytogenetics and two (25.0%) had intermediate cytogenetics.
4.2. TOXICITY AND ADVERSE EVENTS
Of the 72 patients undergoing decitabine induction, 34 (47.2%) experienced neutropenic fever, 11 (15.3%) had an acute kidney injury, and five (6.9%) were found to have significant nausea with emesis. In the cohort of 36 relapsed/refractory patients, 20 (55.6%) had neutropenic fever, 7 patients had renal toxicity (19.4%), and five (13.9%) had grade 1 or higher nausea with emesis. Antimicrobial prophylaxis at our institution is in accord with national practices and includes a fluroquinolone, acyclovir, and either posaconazole or fluconazole.
Overall, 9 patients of 108 (8.3%) required an ICU stay during hospitalization, 8 (11.1%) in the upfront setting and 1 (2.8%) in the relapsed/refractory setting. Among the 72 patients with previously untreated AML, there were 4 deaths within 30 days (5.6%), in the relapsed/refractory group of 36 patients 3 deaths occurred in the first 30 days (8.3%), and no deaths in the group of eight patients that underwent re-escalation to 10-day decitabine. There were 14 deaths within 60 days in the group of 72 previously untreated patients for a rate of 19.4%, nine deaths in the relapsed/refractory group of 36 patients for a rate of 25.0%, and five deaths in the group of eight patients (62.5%) that underwent decitabine re-escalation (Table 2).
Table 2:
Response to Decitabine and Survival
| Characteristic | Upfront Setting (N=72) | Relapsed/Refractory Setting (N=36) | Relapse After Upfront Decitabine Induction (N=8) |
|---|---|---|---|
| Response - no. (%) | |||
| Complete remission (CR) | 12 (16.7) | 3 (8.3) | 0 (0) |
| Complete remission with incomplete count recovery (CRi) | 14 (19.4) | 6 (16.7) | 0 (0) |
| Overall response rate (CR+CRi) | 26 (36.1) | 9 (25.0) | 0 (0) |
| Median overall survival (OS) - days (mo.) | 198 (6.6) | 143 (4.8) | 50 (1.7) |
| 30-day mortality - no. (%)a | 4 (5.6) | 3 (8.3) | 0 (0) |
| 60-day mortality - no. (%)b | 14 (19.4) | 9 (25.0) | 5 (62.5) |
| Response with Respect to Cytogenetics | |||
| Characteristic | Upfront Setting, Intermediate Cytogenetics (N=32) |
Relapsed/Refractory Setting, Intermediate Cytogenetics (N=19) | |
| Response - no. (%) | |||
| CR, intermediate cytogenetics | 4 (12.5) | 2 (10.5) | |
| CRi, intermediate cytogenetics | 5 (15.6) | 3 (15.8) | |
| Overall response rate (CR+CRi), intermediate cytogenetics | 9 (28.1) | 5 (26.3) | |
| Median OS, intermediate cytogenetics - days (mo.) | 282 (9.4) | 238 (7.9) | |
| Characteristic | Upfront Setting, Unfavorable Cytogenetics (N=37) | Relapsed/Refractory Setting, Unfavorable Cytogenetics (N=16) | |
| Response - no. (%) | |||
| CR, unfavorable cytogenetics | 6 (16.2) | 1 (6.3) | |
| CRi, unfavorable cytogenetics | 9 (24.3) | 3 (18.8) | |
| Overall response rate (CR+CRi), unfavorable cytogenetics | 15 (40.5) | 4 (25.0) | |
| Median OS, unfavorable cytogenetics - days (mo.) | 161 (5.4) | 53 (1.8) | |
Of the 30-day mortality group, 71 upfront patients were evaluable for mortality.
Of the 60-day mortality group, 70 upfront patients were evaluable for mortality.
4.3. RESPONSE
Of the 72 previously untreated patients, 12 (16.7%) achieved CR. An additional 14 (19.4%) achieved CRi for an overall response rate of 36.1%. Of the 26 patients that achieved a CR or CRi, 25 received maintenance therapy. One did not go on to receive maintenance therapy due to death shortly after induction. An additional ten patients received maintenance therapy despite not achieving either CR or CRi. The median number of 5-day cycles of maintenance therapy was 6. The median overall survival in the upfront setting across all cytogenetic risk categories was 198 days or 6.6 months. When the 72 patients in the upfront setting were stratified with respect to cytogenetics, the overall response rate was 28.1% with intermediate cytogenetics compared to 40.5% with unfavorable cytogenetics. However, the median OS was 282 days (9.4 months) in the intermediate cytogenetic category and 161 days (5.4 months) in the unfavorable cytogenetic category (Table 2).
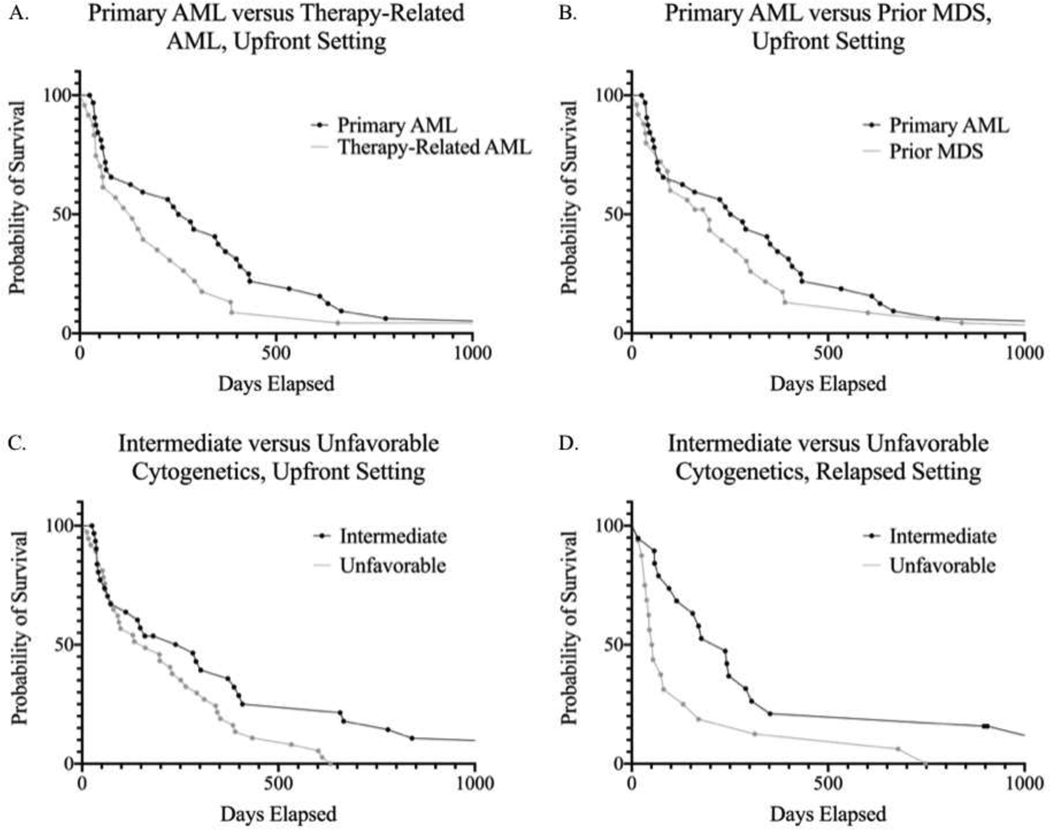
In the relapsed or refractory setting, CR was achieved in 3 patients out of 36 (8.3%) and CRi was achieved in 6 (16.7%), for a total overall response rate of 25.0%. The median overall survival in this setting was 143 days (4.8 months). Like the upfront group, the analysis of the relapsed/refractory group was stratified with respect to cytogenetics. The overall response rate for the intermediate cytogenetic category was 26.3% compared to the unfavorable cytogenetic category at 25.0%. However, a substantial survival difference was seen between these two groups, with the median OS in the intermediate group at 238 days (7.9 months) compared to the unfavorable group at 53 days (1.8 months) (Figure 1D).
FIGURES A-D:
Figure A demonstrates a Kaplan-Meier survival curve featuring the 33 patients with primary AML after upfront decitabine induction versus the 24 patients with therapy-related AML (median OS: 266.5 days vs 133 days, respectively). While there appeared to be improved survival in primary AML, especially in the first year, a log-rank test did not show statistical significance (p-value: 0.1879). Figure B is a Kaplan-Meier survival curve revealing no significant difference between 33 patients with primary AML in the upfront setting and 25 patients with MDS treated with upfront decitabine (p-value: 0.3282, median OS: 266.5 days vs 197 days, respectively). Figure C shows a Kaplan-Meier curve revealing a survival difference between 32 patients with intermediate cytogenetics and 37 patients with unfavorable cytogenetics in the upfront setting, which was statistically significant (p-value: 0.0333, median OS: 282 days vs 161 days, respectively). Figure D shows a Kaplan-Meier curve showing another statistically significant difference between 19 patients with intermediate cytogenetics and 16 patients with unfavorable cytogenetics, this time in the relapsed and refractory setting (p-value: 0.0153, median OS: 238 days vs 53 days, respectively).
In the group of eight patients with relapsed disease previously treated with 10-day decitabine, none achieved CR or CRi (0% response rate). The median overall survival was 50 days or 1.7 months.
The Kaplan-Meier survival curves were generated for select cohorts and are demonstrated in Figures 1A – D. Log-rank tests were performed on all four survival curve comparisons generated. While there appeared to be initial improved survival in patients with primary AML compared to therapy-related AML in the upfront setting, it failed to achieve statistical significance. Similarly, there was no statistical significance observed between patients with primary AML and those with prior MDS. Upfront disease was analyzed with respect to intermediate and unfavorable cytogenetics, which showed a statistically significant difference between the two groups in the same setting (p-value: 0.0333). Similar findings were seen in the relapsed setting, where there was a statistically significant trend toward improved survival in the intermediate cytogenetics risk category when compared to the unfavorable risk category (p-value: 0.0153).
5. DISCUSSION
This study presents the findings of a retrospective, single-institution investigation involving three separate cohorts of patients with AML. The results differ from other single-institution studies with regards to the response rate of 10-day decitabine, particularly in the upfront setting with a CR rate of 16.7% and an ORR of 36.1%. In a pooled meta-analysis of 718 patients with AML treated with 3, 5, and 10-day schedules of decitabine, the CR rates were 17% (95% CI: 13–21%) for the 5-day regimen, which contrasts to a CR rate of 45% with the 10-day regimen (95% CI: 37–54%).25 A subgroup analysis of the ORR demonstrated an ORR of 29% for the 5-day regimen and 53% in the 10-day regimen with an OS of 6.4 months and 11.3 months, respectively. The results of that study contrasted to those seen in a multicenter 10-day trial of decitabine plus bortezomib versus 10-day decitabine alone, where the ORR was 39% in the monotherapy arm with a median OS of 9.3 months in the upfront setting, a majority of which were without adverse cytogenetics and 31% of those responders received stem cell transplant in remission.26 Overall, our results in the upfront setting appear to be between those published in the 5 and 10-day studies, though a more detailed comparative analysis requires future randomized trials with responses stratified with respect to cytogenetics.
Additionally, the responses in the relapsed and refractory setting for heavily pretreated patients that have not been treated with prior decitabine featured a CR of 8.3% and an ORR of 25.0%, with most of these responses being CRi rather than CR. This compares to the limited data that has been reported for relapsed or refractory AML treated with a hypomethylating agent in combination with the BCL-2 antagonist, venetoclax, featuring an ORR (CR + CRi) of 22.5% and an OS of 6.6 months.24 Smaller studies with hypomethylating agents and venetoclax in the relapsed setting have showed less convincing responses (ORR of 21.4%, OS 4.7 months).27 Our data suggest that 10-day decitabine features a comparable ORR and OS to venetoclax-based therapy in the relapsed/refractory setting, particularly for the intermediate-risk cohort. However, the combination of decitabine and venetoclax appears superior in the upfront setting, with an ORR of 67% and a median overall survival of 17.5 months, though patients with unfavorable cytogenetics and those aged 75 or older had lower response rates ranging from 60–65%.28
Patients previously treated with decitabine that had disease progression or relapse on maintenance therapy and underwent re-escalation to 10-day cycles had an overall response rate of 0%. Though this eight-patient cohort is small, these findings suggest that the response to decitabine re-escalation is minimal and accompanied by high mortality, even when patients had an excellent initial overall response. This is supported by the overall upfront response to decitabine at 62.5% in the cohort of eight patients (2 CR and 3 CRi), which fell to 0% in the relapsed setting after decitabine re-induction. These findings suggest that non-decitabine regimens should be considered after decitabine failure.
Given the acceptable toxicity profile combined with the relatively low-to-similar 30 and 60-day mortality rates in the upfront setting compared to intensive chemotherapy, these results suggest utilization of a decitabine backbone is feasible when selecting therapy, even in elderly patients with unfavorable cytogenetics. Studies that have compared intensive induction chemotherapy to 10-day decitabine in patients with unfavorable cytogenetics showed a trend toward improved overall survival for intensive chemotherapy, but the results were not statistically significant after accounting for baseline differences in patient characteristics and stem-cell transplant.29,30 There is a preconception that hypomethylating therapy is associated with less toxicity than intensive chemotherapy, but prior studies have not shown a statistical difference in treatment-related mortality between these two groups.29 Toxicity data presented in this study for single-agent decitabine are in accordance with current published findings.31,32 A study comparing 10-day decitabine to intensive induction chemotherapy in elderly patients is currently underway (NCT02172872).
These findings, along with the studies of several others on the utility of hypomethylating agents in AML, illustrate the need for building upon induction therapy in the upfront and relapsed settings - particularly for elderly patients with unfavorable cytogenetics. The response rate and median OS of 10-day decitabine in the upfront setting appears shorter outside of a clinical trial when compared to prior published literature. Since toxicity and adverse events appear acceptable, building upon a decitabine backbone to augment induction therapy is a reasonable next step that is currently being investigated. Further combinations undergoing investigation include synergistic use the BCL-2 inhibitor venetoclax,33 the histone deacetylase inhibitors such as panobinostat or pracinostat,34 the isocitrate dehydrogenase inhibitors ivosidenib and enasidenib,35 combination chemoimmunotherapy with PD-1/PD-L1 or CTLA4 monoclonal antibodies,36 novel therapies targeting actionable driver mutations, and chimeric antigen receptor (CAR)-T cells.
The current study is limited by the fact that the data were collected retrospectively and the study was conducted with data from a single institution. Given that a large proportion of our patients had unfavorable cytogenetics and secondary AML, the results should be carefully interpreted with regards to generalizability. Since our data comes from real-world experience, there is inherently some selection bias in the overall cohort of patients, as the patients selected to receive hypomethylating therapy were representative of a population skewed toward unfavorable cytogenetics, an overall less fit and elderly cohort, and those otherwise not eligible for more intensive therapies. Lastly, the sample sizes in our subgroup analyses when stratified with respect to cytogenetic risk were relatively small.
Altogether, the results of this study alongside prior retrospective studies suggest that 10-day decitabine features response rates between those published in prior studies of 5 and 10-day regimens in the upfront setting, though the ORRs and overall survival appears comparable to decitabine and venetoclax in the relapsed and refractory setting. Further studies are needed to improve response rates and survival, especially in patients with unfavorable cytogenetics. In the meantime, a 10-day decitabine regimen is reasonable and well tolerated. In these cases, decitabine may represent a building block for future regimens in select subsets of AML.
Highlights.
Real world outcomes using the 10 day decitabine regimen in AML are lacking
10- day decitabine had an ORR of 36.1% in AML in the upfront setting
10- day decitabine had an ORR of 25% in AML in the relapsed setting
Median survival was 6.6 months in the upfront setting and 4.8 in relapsed disease
The 30 day mortality was 5.6% in the upfront setting and 8.3% in relapsed disease
Real world response data was comparable to clinical trials but survival was lower
Acknowledgements:
This work was supported by the Frances P. Tutwiler Fund, the Doug Coley Foundation for Leukemia Research, The McKay Cancer Research Foundation, and the National Institute of Health (TSP is supported by NCI 1R01CA197991–01A1 and SI is supported by NCI P30CA012197). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Footnotes
The authors have no relevant conflicts of interest to disclose.
Conflicts
The authors have no conflicts to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Licht JD, Sternberg DW. The molecular pathology of acute myeloid leukemia. Hematology Am Soc Hematol Educ Program 2005:137–42. [DOI] [PubMed] [Google Scholar]
- 2.Lindsley RC, Mar BG, Mazzola E, et al. Acute myeloid leukemia ontogeny is defined by distinct somatic mutations. Blood 2015;125:1367–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ball B, Zeidan A, Gore SD, Prebet T. Hypomethylating agent combination strategies in myelodysplastic syndromes: hopes and shortcomings. Leuk Lymphoma 2017;58:1022–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Christman JK. 5-Azacytidine and 5-aza-2’-deoxycytidine as inhibitors of DNA methylation: mechanistic studies and their implications for cancer therapy. Oncogene 2002;21:5483–95. [DOI] [PubMed] [Google Scholar]
- 5.Hollenbach PW, Nguyen AN, Brady H, et al. A comparison of azacitidine and decitabine activities in acute myeloid leukemia cell lines. PLoS One 2010;5:e9001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Datta J, Ghoshal K, Motiwala T, Jacob ST. Novel Insights into the Molecular Mechanism of Action of DNA Hypomethylating Agents: Role of Protein Kinase C delta in Decitabine-Induced Degradation of DNA Methyltransferase 1. Genes Cancer 2012;3:71–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huls G, Suciu S, Wijermans P, Kicinski M, Lubbert M. 10-day vs 5-day decitabine: equivalence cannot be concluded. Lancet Haematol 2019;6:e177. [DOI] [PubMed] [Google Scholar]
- 8.Short NJ, Kantarjian HM, Loghavi S, et al. Treatment with a 5-day versus a 10-day schedule of decitabine in older patients with newly diagnosed acute myeloid leukaemia: a randomised phase 2 trial. Lancet Haematol 2019;6:e29–e37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Short NJ, Ravandi F. 10-day vs 5-day decitabine: equivalence cannot be concluded - Authors’ reply. Lancet Haematol 2019;6:e178. [DOI] [PubMed] [Google Scholar]
- 10.Blum W, Garzon R, Klisovic RB, et al. Clinical response and miR-29b predictive significance in older AML patients treated with a 10-day schedule of decitabine. Proc Natl Acad Sci U S A 2010;107:7473–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ritchie EK, Feldman EJ, Christos PJ, et al. Decitabine in patients with newly diagnosed and relapsed acute myeloid leukemia. Leuk Lymphoma 2013;54:2003–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhatnagar B, Duong VH, Gourdin TS, et al. Ten-day decitabine as initial therapy for newly diagnosed patients with acute myeloid leukemia unfit for intensive chemotherapy. Leuk Lymphoma 2014;55:1533–7. [DOI] [PubMed] [Google Scholar]
- 13.Welch JS, Petti AA, Miller CA, et al. TP53 and Decitabine in Acute Myeloid Leukemia and Myelodysplastic Syndromes. N Engl J Med 2016;375:2023–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Michalski JM, Lyden ER, Lee AJ, et al. Intensity of chemotherapy for the initial management of newly diagnosed acute myeloid leukemia in older patients. Future Oncol 2019;15:1989–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kantarjian HM, Thomas XG, Dmoszynska A, et al. Multicenter, randomized, open-label, phase III trial of decitabine versus patient choice, with physician advice, of either supportive care or low-dose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukemia. J Clin Oncol 2012;30:2670–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thol F, Schlenk RF, Heuser M, Ganser A. How I treat refractory and early relapsed acute myeloid leukemia. Blood 2015;126:319–27. [DOI] [PubMed] [Google Scholar]
- 17.Dohner H, Weisdorf DJ, Bloomfield CD. Acute Myeloid Leukemia. N Engl J Med 2015;373:1136–52. [DOI] [PubMed] [Google Scholar]
- 18.Vardiman JW, Thiele J, Arber DA, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood 2009;114:937. [DOI] [PubMed] [Google Scholar]
- 19.Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016;127:2391. [DOI] [PubMed] [Google Scholar]
- 20.Dohner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 2017;129:424–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Farina L, Bruno B, Patriarca F, et al. The hematopoietic cell transplantation comorbidity index (HCT-CI) predicts clinical outcomes in lymphoma and myeloma patients after reduced-intensity or non-myeloablative allogeneic stem cell transplantation. Leukemia 2009;23:1131–8. [DOI] [PubMed] [Google Scholar]
- 22.Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol 2011;173:676–82. [DOI] [PubMed] [Google Scholar]
- 23.Creutzig U, Kaspers GJ. Revised recommendations of the International Working Group for diagnosis, standardization of response criteria, treatment outcomes, and reporting standards for therapeutic trials in acute myeloid leukemia. J Clin Oncol 2004;22:3432–3. [DOI] [PubMed] [Google Scholar]
- 24.Wang YW, Tsai CH, Lin CC, et al. Cytogenetics and mutations could predict outcome in relapsed and refractory acute myeloid leukemia patients receiving BCL-2 inhibitor venetoclax. Ann Hematol 2020. [DOI] [PubMed] [Google Scholar]
- 25.He PF, Zhou JD, Yao DM, et al. Efficacy and safety of decitabine in treatment of elderly patients with acute myeloid leukemia: A systematic review and meta-analysis. Oncotarget 2017;8:41498–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roboz GJ, Mandrekar SJ, Desai P, et al. Randomized trial of 10 days of decitabine +/− bortezomib in untreated older patients with AML: CALGB 11002 (Alliance). Blood Adv 2018;2:3608–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gaut D, Burkenroad A, Duong T, Feammelli J, Sasine J, Schiller G. Venetoclax combination therapy in relapsed/refractory acute myeloid leukemia: A single institution experience. Leuk Res 2020;90:106314. [DOI] [PubMed] [Google Scholar]
- 28.DiNardo CD, Pratz K, Pullarkat V, et al. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood 2019;133:7–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quintas-Cardama A, Ravandi F, Liu-Dumlao T, et al. Epigenetic therapy is associated with similar survival compared with intensive chemotherapy in older patients with newly diagnosed acute myeloid leukemia. Blood 2012;120:4840–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gupta N, Miller A, Gandhi S, et al. Comparison of epigenetic versus standard induction chemotherapy for newly diagnosed acute myeloid leukemia patients >/=60 years old. Am J Hematol 2015;90:639–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Issa JP, Garcia-Manero G, Giles FJ, et al. Phase 1 study of low-dose prolonged exposure schedules of the hypomethylating agent 5aza-2’-deoxycytidine (decitabine) in hematopoietic malignancies. Blood 2004;103:1635–40. [DOI] [PubMed] [Google Scholar]
- 32.Ali AM, Weisel D, Gao F, et al. Patterns of infectious complications in acute myeloid leukemia and myelodysplastic syndromes patients treated with 10-day decitabine regimen. Cancer Med 2017;6:2814–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nguyen LXT, Troadec E, Kalvala A, et al. The Bcl-2 inhibitor venetoclax inhibits Nrf2 antioxidant pathway activation induced by hypomethylating agents in AML. J Cell Physiol 2019;234:14040–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bewersdorf JP, Shallis R, Stahl M, Zeidan AM. Epigenetic therapy combinations in acute myeloid leukemia: what are the options? Ther Adv Hematol 2019;10:2040620718816698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wei AH, Tiong IS. Midostaurin, enasidenib, CPX-351, gemtuzumab ozogamicin, and venetoclax bring new hope to AML. Blood 2017;130:2469–74. [DOI] [PubMed] [Google Scholar]
- 36.Masarova L, Kantarjian H, Ravandi F, Sharma P, Garcia-Manero G, Daver N. Update on Immunotherapy in AML and MDS:Monoclonal Antibodies and Checkpoint Inhibitors Paving the Road for Clinical Practice. Adv Exp Med Biol 2018;995:97–116. [DOI] [PubMed] [Google Scholar]