SUMMARY
The preimplantation bovine embryo displays sexual dimorphism in glucose sensitivity and interferon-tau (IFNT) secretion that are negated by inhibition of the pentose phosphate pathway, suggesting that the association between glucose metabolism and IFNT likely underpins the selective loss of female embryos. The aim of this study was to determine if altered glucose metabolism, through glucose supplementation and/or uncoupling oxidative phosphorylation with 2,4-dinitrophenol (DNP), affected embryo development. Bovine blastocyst development, sex, and IFNT production were examined in embryos cultured in the presence or absence of glucose (0, 1.5, 4 mM) with or without exposure to DNP (0, 10, 100 μM) between Days 5 and 8 post-fertilization. The absence or presence of high (4 mM) glucose reduced blastocyst development and favored the development of male embryos (P < 0.001). DNP at 10 μM had no effect, whereas 100 μM had a negative impact on blastocyst development. Notably, in the presence or even absence of glucose, supplementation with 10 μM DNP further skewed the sex ratio toward males (P < 0.05). Sexually dimorphic IFNT production was maintained in these conditions, although total production was reduced in the presence of high glucose and DNP, irrespective of embryo sex. These data suggest that the pentose phosphate pathway can modulate embryonic sex ratio and development. Therefore, bovine embryo culture should be undertaken in a low glucose (<2.5 mM) medium to minimize potential embryonic stress, as higher concentrations have sexually dimorphic effects on development and an embryo’s ability to signal to the maternal reproductive tract.
INTRODUCTION
The production of mitochondrial adenosine triphosphate (ATP) via oxidative phosphorylation (OXPHOS) is essential for mammalian embryo development, particularly during compaction and blastocyst formation, when the demand for ATP increases substantially (reviewed by Gardner and Harvey, 2015). At these stages, glucose utilization increases in order to meet the requirements for ATP. This switch involves the metabolism of glucose through glycolysis and the pentose phosphate pathway. Partial inhibition of mitochondrial respiration, via low concentrations of the OXPHOS uncoupler 2,4-dinitrophenol (DNP), around the time of compaction can improve the in vitro development of bovine (Thompson et al., 2000) and porcine (Machaty et al., 2001) embryos. The mechanism by which DNP benefits early embryogenesis is not well defined, although an improvement in the balance between cytoplasmic and mitochondrial ATP production by this compound is postulated to enhance development (Thompson et al., 2000), as mediated by changes in metabolism (Rieger et al., 2002), reduced reactive oxygen species (ROS) production (Okuda et al., 1992; Korshunov et al., 1997), and reduced accumulation of medium-sized lipid droplets (De La Torre-Sanchez et al., 2006a).
Comparable numbers of male and female bovine embryos develop in vivo during the preimplantation period (Kimura et al., 2004a). The number of male-to-female embryos can be altered by environmental factors, including stress and/or diet (Rosenfeld et al., 2003; Rosenfeld and Roberts, 2004; Alexenko et al., 2007; Green et al., 2008). In vitro culture can similarly skew development, with the presence of high concentrations of D-glucose (glucose) in bovine embryo culture medium favoring the development of males (Bredbacka and Bredbacka, 1996; Gutierrez-Adan et al., 2001; Larson et al., 2001; Peippo et al., 2001; Kimura et al., 2005). Female bovine embryos display a greater sensitivity to glucose within the medium, as their development can be blocked at the morula stage when cultured in suboptimal glucose concentrations (Gutierrez-Adan et al., 2001; Larson et al., 2001; Peippo et al., 2001; Bermejo-Alvarez et al., 2012). Sexually dimorphic differences in metabolism likely underlie this sensitivity. Glucose uptake is reported to be twofold higher in male versus female bovine embryos, whereas female mouse embryos consume more glucose than their male counterparts (Lane and Gardner, 1996). Pentose phosphate pathway activity, on the other hand, is reported to be fourfold higher in female than in male bovine embryos (Tiffin et al., 1991a). Substitution of glucose with D-fructose reduces the selective in vitro loss of female bovine embryos (Kimura et al., 2005) and inhibition of the pentose phosphate pathway can likewise correct the bias (Kimura et al., 2005), suggesting that the metabolism of glucose, and not fructose or possibly other hexose sugars used as an energy source, underpins this selective pressure.
X-chromosome dosage has been shown to underlie differences in embryo metabolism (Tiffin et al., 1991a; Lane and Gardner, 1996), gene transcription (Bermejo-Alvarez et al., 2010), and epigenetic regulation (Bermejo-Alvarez et al., 2008). The X-chromosome carries several hundred genes, including glucose-6-phosphate dehydrogenase (G6PDH), which encodes the enzyme responsible for the rate-limiting step of the pentose phosphate pathway. G6PDH expression is significantly higher in in vitro-cultured female embryos (Gutierrez-Adan et al., 2000; Wrenzycki et al., 2002), even though dosage compensation is initiated prior to or at the blastocyst stage (Peippo et al., 2002; Wrenzycki et al., 2002). The expression of several X-linked genes, including pyruvate dehydrogenase (PDK) and X-inactive specific transcript (XIST), is significantly higher in in vitro-cultured blastocysts than those derived in vivo (Wrenzycki et al., 2002; Nino-Soto et al., 2007). Furthermore, in vitro embryo culture can affectX-linked gene transcription, particularly at the 8–16 cell stage, coincident with embryonic genome activation (Nino-Soto et al., 2007). Thus, in vitro environmental perturbations can directly impact gene expression patterns regulated by X-chromosome dosage, thereby differentially influencing male and female embryos. Just as sub-optimal culture conditions are revealed in embryo ultrastructure (Rizos et al., 2002a), metabolism (Thompson 2000; Absalon-Medina et al., 2014; Krisher et al., 2015), gene expression (Wrenzycki et al., 2001; Rizos et al., 2002b; Lonergan et al., 2003; Harvey et al., 2004), and viability (Bertolini et al., 2002), poor tolerance for excess glucose may reflect the presence of two activeX-chromosomes in female blastocysts, at least through to the morula stage, when random inactivation of oneX-chromosome is initiated within the trophectoderm (De La Fuente et al., 1999; Dupont and Gribnau, 2013).
The secretion of interferon-tau (IFNT) also differs between male and female bovine blastocysts, with female expanded blastocysts producing about twice the amount as males (Larson et al., 2001; Bermejo-Alvarez et al., 2011b). This difference has been observed irrespective of whether embryos are cultured in vitro or derived in vivo (Kimura et al., 2004a), suggesting a common sexually dimorphic mechanism. Indeed, sexually dimorphic IFNT transcript abundance is evident as early as Day 7 in vivo (Kubisch et al., 2004) but is lost by Day 14 (Kimura et al., 2004a), which corresponds with the completion of X-inactivation (De La Fuente et al., 1999). Macromolecule supplementation of in vitro culture medium can also alter IFNT secretion (Kubisch et al., 2001). IFNT is an autosomal gene (Ryan and Womack, 1993), yet its higher expression in female embryos suggests that it is regulated by X-chromosome dosage mechanisms that may be linked to the expression of G6PDH (Kimura et al., 2005) and/or O-linked N-acetylglucosamine transferase (OGT) (Shafi et al., 2000; Kimura et al., 2008). Differential transcription of autosomal genes is extensively documented in male and female bovine embryos (Bermejo-Alvarez et al., 2010), and may result from sexually dimorphic cellular redox states. Alternatively, differential expression of other genes, either on the X-chromosome or elsewhere within the genome, may modulate IFNT.
Bovine embryo sex is skewed toward males if glucose concentrations are above 2.5 mM in the culture medium (Kimura et al., 2005). Yet the interaction between glucose availability and metabolic-pathway modulation and the potential link between glucose utilization and sexually dimorphic IFNT production in regulating sex skewing of bovine embryos have not been investigated. Here, we hypothesize that altered glucose utilization following treatment with DNP and/or supplementation with a high glucose concentration (>2.5 mM) will further skew embryo development rates toward males, while maintaining sexually dimorphic IFNT production because the pentose phosphate pathway is fundamental to the regulation of sex ratio through G6PDH dosage, an effect further exacerbated by sub-optimal culture environments. The current study specifically investigated whether or not inhibition of mitochondrial respiration by the addition of DNP at 0, 10, or 100 μM, in the absence or presence of D-glucose (0, 1.5, or 4 mM) between Days 5 and 8 post-fertilisation, affected the development, sex ratio, and sexually dimorphic IFNT production of bovine embryos.
RESULTS
Glucose and DNP Impact Bovine Embryo Development at Day 8
Sex ratio on Day 3 of development (8-cell stage) was not significantly different from the expectation of a 1:1 ratio of male-to-female embryos (n = 114; P > 0.1; male 0.50, female 0.50). No significant difference in sex ratio was evident on Day 5 either (n = 852; P > 0.1; male 0.52, female 0.48). Progression of embryo development from Day 3 to Day 5 (the morula stage) was also not affected by glucose concentration (P > 0.1; 0 mM 94.2% [n = 280], 1.5 mM 96.1% [n = 281], 4 mM 91.8% [n = 291]).
Glucose concentration did significantly affect the number of blastocysts on Day 8 (Table 1) (P < 0.001), with more blastocysts developing in 1.5 mM glucose than in either 0 or 4 mM glucose (P < 0.0001). Fewer blastocysts were present in 4 mM glucose than in the absence of glucose (0 mM), although this difference was not significant (P = 0.085). No beneficial effect of DNP supplementation on development was identified at any stage (Table 1); in fact, the number of blastocysts formed in the presence of 100 μM DNP was significantly compromised compared to the other two groups (P < 0.0001), although the negative effect of 100 μM DNP was lower with 1.5 mM glucose (P < 0.05).
TABLE 1.
Blastocyst Development on Day 8 of Culture With Glucose (0, 1.5, or 4 mM) and/or DNP (0, 10, or 100 μM)
| Number of blastocysts (%) | |||||
|---|---|---|---|---|---|
|
| |||||
| Glucose (mM) | DNP (μM) | Day 5 number treated | Pre-expanding and expanding blastocysts (n) | Expanded and hatched blastocysts (n) | Total (n) |
|
| |||||
| 0 | 0 | 400 | 51.7 ± 5.2 (103)abcd | 48.3 ± 5.2 (88)abc | 47.4 ± 2.8 (191)ab |
| 10 | 325 | 48.1 ± 5.6 (78)acd | 51.9 ± 5.6 (94)abc | 50.9 ± 4.3 (172)b | |
| 100 | 350 | 57.1 ± 11.3 (55)b | 42.9 ± 11.3 (44)abc | 26.2 ± 4.2 (99)c | |
| 1.5 | 0 | 350 | 60.7 ± 6.0 (158)abc | 39.3 ± 6.0 (102)a | 74.3 ± 2.2 (260)d |
| 10 | 350 | 61.5 ± 5.3 (152)abc | 38.5 ± 5.3 (97)a | 71.1 ± 2.8 (249)d | |
| 100 | 350 | 65.2 ± 4.0 (165)ab | 34.8 ± 4.0 (82)a | 71.4 ± 3.5 (247)d | |
| 4 | 0 | 325 | 38.2 ± 4.5 (52)cd | 61.8 ± 4.5 (91)bc | 44.4 ± 2.9 (143)ab |
| 10 | 325 | 35.4 ± 5.9 (44) d | 64.6 ± 5.9 (78)b | 34.7 ± 2.0 (123)ac | |
| 100 | 325 | 51.3 ± 7.3 (44)abc | 48.7 ± 7.3 (56)ac | 26.7 ± 4.9 (100)c | |
Percentages development of pre-expanding and expanding, as well as expanded and hatched blastocyst, were calculated based on total blastocysts with in a treatment. Data were arcsine transformed prior to statistical analysis by GLM, with culture (n = 26) included as a random factor. Superscripts represent statistically significant differences between treatments within a developmental stage (P < 0.05). Data are presented as mean ± standard error of the mean.
The proportion of expanding versus expanded and hatched blastocysts at Day 8 was significantly affected by glucose concentration in the medium (Table 1) (P < 0.05): more blastocysts were classified as pre-expanded or expanding (stages 3–5) in 1.5 mM glucose than in 4 mM glucose (62.8% vs. 38.3%, respectively). While fewer pre-expanded or expanding blastocysts were evident in 0 mM glucose (51.1%), this treatment did not significantly differ from either the 1.5 mM or 4 mM treatments (P > 0.05). Although the percentage of pre-expanded/expanding blastocysts in 100 μM DNP was higher (59.2%) compared with those in 10 μM (50.4%) or 0mM (52.7%) DNP (P < 0.05), no overall beneficial effect was identified for DNP (Table 1).
A significant effect of glucose concentration on expanded and hatched blastocyst development (classified as stages 6–8) was also noted (Table 1) (P < 0.01). Even though fewer embryos reached the blastocyst stage by Day 8 in 4 mM glucose, the percentage of expanded/hatched blastocysts from the 4 mM glucose treatment was higher than those cultured in 1.5 mM glucose (61.5% and 37.2%, respectively; P < 0.01), but not significantly different from those cultured in 0 mM glucose (48.9%; P > 0.1). Similar to pre-expanded/expanding blastocyst percentages, no effect of DNP was observed (P > 0.1); indeed, in the presence of 4 mM glucose, 100 μM DNP resulted in a lower percentage of expanded/hatched blastocysts compared with those cultured with 10 μM DNP (Table 1) (P < 0.05), although this value was not significantly different to those cultured in the absence of DNP (P > 0.1).
No difference in the quality of blastocysts cultured in either 0 mM or 4 mM glucose was detected for either transferrable (grade 1 and 2) (0 mM, 83.1 ± 2.3%; 4 mM, 84.9 ± 3.6%) or non-transferrable (grade 3) (0mM, 16.7 ± 2.4%; 4 mM 15.2 ± 3.6%) quality embryos (P > 0.1). Similarly, DNP did not significantly affect blastocyst quality (P > 0.1) (transferrable: 0 μM, 82.6 ± 2.2%; 10 μM, 84.5 ± 2.8%; 100 μM, 85.3 ± 5.6% and non-transferrable: 0 μM, 17.4 ± 2.3%; 10 μM, 15.5 ± 2.8%; 100 μM, 14.7 ± 5.6%).
Glucose and DNP Skew the Bovine Embryo Sex Ratio
The Day-8 bovine blastocyst sex ratio deviated significantly from the expected 50:50 ratio, favoring males in the presence of 4 mM glucose (Table 2) (P < 0.05). An additive effect on male skewing was also identified (P < 0.01), irrespective of glucose concentration, in the presence of 10 μM DNP. In the absence of glucose, 10 μM DNP skewed the sex ratios toward male embryos (P < 0.01). No bias toward males was detected in pre-expanding and expanding blastocysts in the presence of glucose, although male skewing was evident later, in expanded and hatched blastocysts, in the presence of both 1.5 mM and 4 mM glucose (Table 2) (P < 0.05); this phenotype was exacerbated at each glucose concentration by the addition of 10 μM DNP (P < 0.01).
TABLE 2.
Sex Ratio of Pre-Expanded and Expanding, As Well As Expanded and Hatched Blastocysts, on Day 8 of Culture With Glucose (0, 1.5, or 4 mM) and/or DNP (0, 10, or 100 μM)
| Glucose (mM) | DNP (μM) | Pre-expanded and expanding blastocysts | Expanded and hatched blastocysts | Total (n) |
|---|---|---|---|---|
|
| ||||
| 0 | 0 | 0.60 | 0.42 | 0.52 (153) |
| 10 | 0.63z | 0.59 | 0.61y (150) | |
| 100 | 0.44 | 0.63 | 0.53 (95) | |
| 1.5 | 0 | 0.51 | 0.62z | 0.55 (260) |
| 10 | 0.55 | 0.66y | 0.59y (249) | |
| 100 | 0.52 | 0.62z | 0.55 (247) | |
| 4 | 0 | 0.58 | 0.63z | 0.60# (94) |
| 10 | 0.67 | 0.74y | 0.71y (51) | |
| 100 | 0.67* | 0.60 | 0.63z (70) | |
Number of cultures (n = 26). Deviation from the expected 0.50 ratio is denoted by superscripts:
P < 0.01
P < 0.05
P = 0.06; and
P = 0.07.
Glucose and DNP Impact Bovine Embryo Development at Day 10
Embryos selected for IFNT analysis were of comparable developmental stage on Day 8 (0 h, n = 190), such that no statistical difference was apparent (P > 0.1). Later development was not affected by glucose after culturing for an additional 24 hr (P > 0.05; average developmental score 6.8 ± 1.1, 7.4 ± 1.1, and 6.9 ± 1.3 for 0, 1.5, and 4 mM glucose, respectively), or 48 hr (P > 0.05; average developmental score 7.5 ± 0.9,7.7 ± 0.9, and 7.3 ± 1.3 for 0,1.5, and 4 mM glucose, respectively). In contrast, when these Day-8 embryos were exposed to DNP, later development at 24 hr was reduced in 100 μM DNP-treated blastocysts compared to those cultured without DNP (P < 0.05; average developmental score 6.6 ± 1.3 and 7.3 ± 1.1, respectively) but was not significantly different from those cultured in 10 μM DNP (P = 0.07; 7.1 ± 1.1). This reduction in development tended to be maintained through 48 h (P = 0.058; 7.7 ± 0.8 [n = 67], 7.5 ± 1.0 [n = 73], and 7.2 ± 1.2 [n = 50] for 0, 10, and 100 μM DNP, respectively).
IFNT Antiviral Activity of Culture Media on Days 9 and 10
IFNT is generally regarded as the major signal for maternal recognition of pregnancy in ruminant ungulates, and its production has been proposed as an indicator of embryo viability (Hernandez-Ledezma et al., 1992). Accordingly, the amount of IFNT released by the embryo into the medium during in vitro culture between Days 8 and 10 was measured by its antiviral activity (Tables 3 and 4). Evaluation of male or female embryos alive at the end of the culture period revealed no significant longitudinal interactions between DNP or glucose treatments. IFNT production at both 24 hr and 48 hr, however, was significantly affected by glucose concentration (Fig. 1; Table 3), DNP concentration (Fig. 1 and Table 4), and blastocyst sex (all P < 0.0001). IFNT abundance was higher in embryos cultured in 1.5 mM glucose than in those cultured in its absence at 24 hr (P < 0.0001), a difference that was maintained through to 48 hr (P < 0.05). By 48 hr, IFNT production by blastocysts cultured in 4 mM glucose was also significantly less than for blastocysts cultured without glucose (P < 0.01). Irrespective of glucose concentration, the presence of 100 μM DNP also significantly decreased IFNT production relative to 0 μM and 10 μM DNP, at both 24 hr and 48 hr (P < 0.0001). Sexual-dimorphic production of IFNT was maintained in all conditions (Tables 3 and 4), with female embryos producing more IFNT than males (P < 0.0001).
TABLE 3.
IFNT Production of Day-8 Bovine Blastocysts After 24 hr (Day 9) and 48 hr (Day 10) of Culture, Following Treatment With Glucose (0, 1.5, or 4 mM)
| Glucose (mM) | Sex | n | IFNT 24 hr (units) | IFNT 48 hr (units) |
|---|---|---|---|---|
|
| ||||
| 0 | F | 28 | 1.91 ± 0.15a | 3.53 ± 0.19ac |
| M | 27 | 0.91 ± 0.13b | 2.13 ± 0.17bd | |
| 1.5 | F | 25 | 2.79 ± 0.19c | 4.08 ± 0.24c |
| M | 28 | 2.04 ± 0.18a | 2.78 ± 0.23ab | |
| 4 | F | 17 | 1.84 ± 0.21a | 2.88 ± 0.27ab |
| M | 19 | 0.79 ± 0.17b | 1.45 ± 0.22d | |
Main effect shown as mean ± standard error of the mean. Different superscripts represent statistically significant differences within a time point. n = 144 embryos from three biological replicates.
TABLE 4.
IFNT Production of Day-8 Bovine Blastocysts After 24 hr (Day 9) and 48 hr (Day 10) of Culture, Following Treatment With DNP (0, 10, or 100 μM)
| DNP (μM) | Sex | n | IFNT 24 hr (units) | IFNT 48 hr (units) |
|---|---|---|---|---|
|
| ||||
| 0 | F | 27 | 2.48 ± 0.17a | 3.84 ± 0.22a |
| M | 28 | 1.70 ± 0.15b | 2.68 ± 0.19b | |
| 10 | F | 25 | 2.52 ± 0.17a | 3.78 ± 0.22a |
| M | 32 | 1.42 ± 0.14b | 2.56 ± 0.18b | |
| 100 | F | 18 | 1.54 ± 0.20b | 2.86 ± 0.26ab |
| M | 14 | 0.63 ± 0.19c | 1.14 ± 0.25c | |
Main effect shown as mean ± standard error of the mean. Different superscripts represent statistically significant differences within a time point. n = 144 embryos from three biological replicates.
Figure 1.
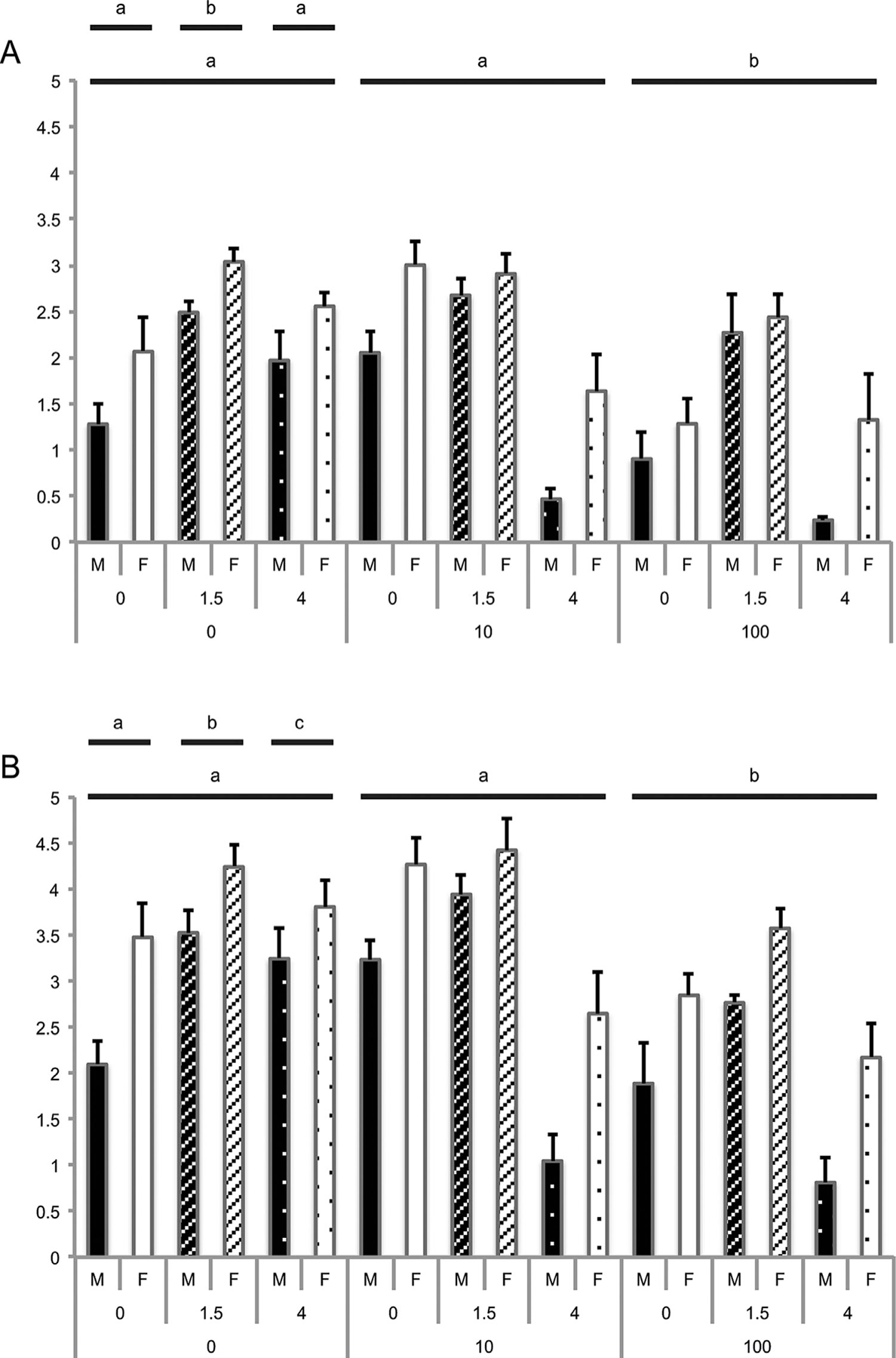
IFNT production of Day-8 male (M) and female (F) blastocysts (n = 190) after (A) 24 hr (Day 9) and (B) 48 hr (Day 10) of culture following treatment with D-glucose (0, 1.5, or 4 mM) and/or DNP (0, 10, or 100 μM). Means ± standard errors of the mean are shown. Different superscripts indicate statistically significant differences within a treatment (P < 0.05).
DISCUSSION
We determined how bovine embryo development was affected by supplemental glucose (0, 1.5, and 4 mM), between Days 3 and 8 post-fertilization, and DNP (0, 10, and 100 μM), in the presence or absence of supplemental glucose between Days 5 and 8. Glucose concentration had the biggest overall impact on development, sex ratio, and sexual-dimorphic IFNT production. The absence of glucose (0 mM) or its presence at a relatively high concentration (4 mM) reduced blastocyst development, with the latter condition also skewing the sex of surviving embryos toward males, for example, the male ratio achieved up to 0.71 in 4 mM glucose. Partial inhibition of mitochondrial respiration, using the uncoupler of oxidative phosphorylation, DNP, to alter glucose metabolism, did not improve blastocyst development; in fact 100 μM DNP negatively impacted blastocyst development. While 10 μM DNP did not affect Day-8 blastocyst development, this concentration still increased the proportion of males, even in the absence of supplemental glucose. In contrast, 4 mM glucose plus 100 μM DNP did not further increase the sex ratio. Sexual dimorphic production of IFNT, on the other hand, was maintained under all conditions tested, although total production was impaired in embryos cultured with 4 mM glucose and 100 μM DNP. These findings further link the availability and metabolism of glucose with the selective loss of female embryos, and highlight how critical optimal in vitro glucose supplementation is for the development of embryo populations without a biased sex ratio.
The highest rates of blastocyst development and IFNT production were observed with 1.5 mM glucose. Higher glucose (4 mM) substantially reduced bovine blastocyst development on Day 8, which is consistent with previous studies (Gutierrez-Adan et al., 2001; Larson et al., 2001; Peippo et al., 2001; Kimura et al., 2005) but did not affect embryo quality. This loss of embryos in high-glucose conditions likely results from the Crabtree effect—where provision of high concentrations of glucose redirects much of the monosaccharides into the pentose phosphate pathway—and may be exacerbated by DNP, which uncouples OXPHOS activity in the cell. In contrast to these in vitro concentrations, 0.02–0.2 mM glucose is present in the bovine reproductive tract (Carlson et al., 1970), and lower in vivo oviductal glucose concentrations are associated with viable pregnancy in cattle (Green et al., 2005). Together, these observations support the use of lower glucose concentrations during in vitro culture.
Mitochondrial activity and changes in glucose utilization, through glycolysis and the pentose phosphate pathway, are likely mediated by cellular redox and ROS, whereby low ROS levels are required for normal embryo development (Harvey et al., 2002). Based on this relationship, the high glucose concentrations (>2.5 mM) used in this study plausibly modulated metabolic pathway activity, resulting in the generation of ROS levels above those required for normal development, thus perturbing cellular redox status and triggering a stress response. Indeed, this is consistent with the report that bovine embryo development under high-glucose (4.5 mM) conditions is improved in the presence of the antioxidants superoxidase dismutase and mannitol (Iwata et al., 1998).
DNP, as an uncoupler of OXPHOS, decreases ROS production via the electron transport chain (Okuda et al., 1992; Korshunov et al., 1997) and modulates nicotinamide adenine dinucleotide (phosphate) ratios (NAD(P) H-to-NAD(P) ratio) (Sibille et al., 1995; Leverve et al., 1998; Rex et al., 1999; Hoffmann et al., 2001), thereby altering the cellular redox state to increase ATP production by glycolysis (O’Fallon and Wright 1986; Hewitson et al., 1996). Conflicting reports exist as to whether or not DNP benefits development, though. In the current study, which is similar to that of Harvey et al. (2004), no beneficial effect was evident, even in the presence of 1.5 mM glucose or under conditions that likely elicit stress (0 or 4 mM glucose). This outcome contrasts with previous reports of improved development of blastocysts cultured from the morula stage in the presence of 1.5 mM glucose and 10 μM DNP (Thompson et al., 2000; Machaty et al., 2001; Rieger et al., 2002; Barcelo-Fimbres and Seidel, 2007). This disparity in outcome following DNP treatment may be related to the composition of embryo culture media (De La Torre-Sanchez et al., 2006b) or with differing embryo density. Indeed, the content of culture medium significantly impacts embryo development and likely influences whether or not an embryo produces excess ROS, the range of which can lead to varying developmental outcomes. Thus, depending on the basal medium used, a partial (subacute) reduction in mitochondrial ATP production may either improve or have little effect on overall bovine embryo development. On the other hand, a large reduction in mitochondrial ATP production at higher DNP concentrations (100 μM) instead causes detrimental effects to embryo development, likely due to DNP-elicited changes in metabolism and cellular redox.
In the present study, the reduced embryo development in high-glucose and DNP concentrations may be partly attributed to the selective loss of female embryos prior to blastocyst formation or a delay in development because the sex ratio was skewed. More males were present following culture in 4 mM glucose, an effect that was compounded by the addition of 10 μM DNP. These results are consistent with previous studies that highlighted the relationship between stressful conditions, such as high glucose (>2.5 mM), and selective loss of female embryos (King et al., 1991; Bermejo-Alvarez et al., 2011a), resulting in a bias toward males (Gutierrez-Adan et al., 2001; Kimura et al., 2005). Even brief (24 hr) exposure to glucose immediately after fertilization is sufficient to skew development toward males (Peippo et al., 2001). Notably, our present experiments demonstrated that DNP tilts the sex ratio toward males even in the absence of glucose, suggesting that over-activation of the pentose phosphate pathway by DNP may be responsible for modulating sex ratio, resulting in a disproportionate failure of female embryos to flourish. This is supported by our previous data showing that addition of pentose-phosphate-pathway inhibitors can normalise the sex ratio in the presence of 4 mM glucose and prevent the sexually dimorphic production of IFNT (Kimura et al., 2005). Thus, DNP—in the absence or presence of glucose above 2.5 mM—likely represents an additional stressor that can affect the NAD(P)+-to-NAD(P)H ratio, resulting in a bias toward the survival of male embryos. These data also emphasize the importance of knowing the sex of an embryo when undertaking metabolic studies, as this parameter may confound findings and may account for the absence of significant changes in metabolic pathway activity previously reported (De La Torre-Sanchez et al., 2006a). This is particularly relevant considering that G6PDH, the rate limiting enzyme in the pentose phosphate pathway, is more highly expressed (Gutierrez-Adan et al., 2000) and has greater enzymatic activity in female than male bovine embryos (Tiffin et al., 1991b). Glucosamine supplementation after embryonic genome activation can likewise skew development toward males, via X-linked O-linked N-acetylglucosamine transferase activity (Kimura et al., 2008), suggesting that glucosamine metabolism may also be involved in early embryogenesis. Further studies are thus required to elucidate the combinatory effect of glucose and DNP on ATP production, as well as gene expression patterns in male and female blastocysts, and at stages prior to embryonic loss.
IFNT is a biomarker of embryo viability (Hernandez-Ledezma et al., 1992; Stojkovic et al., 1999; Neira et al., 2007). Production of IFNT is related to changes in glucose metabolism and the silencing of one of the two active-chromosomes (De La Fuente et al., 1999; Bermejo-Alvarez et al., 2011b). Sexually dimorphic production of IFNT was observed in both in vivo-derived and in vitro-produced bovine blastocysts, with female embryos synthesizing higher levels than their male counterparts (Larson et al., 2001; Kimura et al., 2004a). Inhibition of the pentose phosphate pathway abolishes sexually dimorphic IFNT production (Kimura et al., 2004b), thereby establishing a direct link between glucose metabolism and IFNT production in the developing embryo. In the present study, neither glucose nor DNP supplementation caused a loss in the sexually dimorphic production of IFNT; there was simply a reduction in IFNT production at high glucose and DNP concentrations. As the current study only uncoupled
OXPHOS, not the pentose phosphate pathway, this observation is consistent with our hypothesis that IFNT synthesis is associated with glucose production via the pentose phosphate pathway. The change in the amount of IFNT production between glucose concentrations, however, was unexpected and interesting. IFNT production was highest in the 1.5-mM-glucose treatment. Combined with reduced development rates associated with culture in 0 or 4 mM glucose, these data suggest conditions that compromise development result in reduced IFNT production that may hinder an embryo’s normal dialogue with the maternal reproductive tract during the perimplantation period.
CONCLUSION
Bovine blastocyst development was negatively affected by high glucose (4 mM) and the OXPHOS uncoupler DNP (100 μM). Sexually dimorphic production of IFNT was still evident, irrespective of glucose or DNP concentration, with optimal production observed at 1.5 mM glucose. DNP skewed the sex ratio toward males even in the absence of glucose, and had an additive effect, further favoring males when glucose was present. These data support the hypothesis that the pentose phosphate pathway contributes to sex bias during bovine embryo development. Together with previous reports, these findings suggest that culturing bovine post-compaction embryos in the presence of high glucose (>2.5 mM) or supplementation with DNP preferentially favor the survival of males rather than females. The potential benefits of DNP supplementation are likely only evident in sub-optimal media systems. Therefore, culturing embryos under low-glucose (1.5 mM) conditions should help minimize bovine embryo stress, thus reducing male sex bias by minimizing the selective loss or delayed development of female embryos.
MATERIALS AND METHODS
All chemicals were purchased from Sigma–Aldrich (St. Louis, MO), unless otherwise stated.
In Vitro Maturation, Fertilization, and Culture of Bovine Embryos
Oocytes (n = 9662) recovered from slaughterhouse-derived ovaries were supplied by BoMed Inc. (Madison, WI) and matured in TCM 199 with 10% (v/v) fetal bovine serum (FBS), 0.5 μg/mL follicle-stimulating hormone (Folltropin V; BIONICHE Animal Health Canada, Belleville, ON), 1 μg/mL 17β-estradiol, and 0.2 mM sodium pyruvate. Maturation was undertaken for 24 hr under mineral oil in a humidified 5% CO2 in atmospheric air.
Matured oocytes were fertilized as previously described (Kubisch et al., 1998; Kimura et al., 2004b) using frozen-thawed semen at a final concentration of 1 × 106 per mL in IVF-TL supplemented with 0.6 % (w/v) bovine serum albumin (BSA) and 20 μg/mL heparin. The day of fertilization was defined as Day 0. Presumptive zygotes were recovered after 18 hr, and cumulus cells were removed by vortexing and pipetting vigorously in 0.02% hyaluronidase-supplemented medium. Zygotes were subsequently cultured in 25-μL drops (n = 25 zygotes per drop) of D-glucose-free, modified synthetic oviduct fluid supplemented with amino acids and 0.4% BSA (mSOFaa) (Takahashi and First, 1992) at 39°C in 5% CO2, 5% O2, 90% N2. Eight-cell stage embryos were collected at 72 hr post-insemination, and a subset from each culture was randomly removed to analyse sex ratio. The remaining 8-cell stage embryos were used for the experimentsdescribed below.
Experimental Design
2,4-dinitrophenol (DNP), an inhibitor of mitochondrial respiration that uncouples OXPHOS from electron transport, was added to the medium at two concentrations (10 and 100 μM) based on previous studies (Thompson et al., 2000; Rieger et al., 2002). DNP does not dissolve in water, so stock solutions were made by dissolving the compound in acetone, which were then diluted in culture medium to produce the working dilutions (10 and 100 μM) with a final concentration of 0.01% acetone in these and control (0 μM DNP) cultures. Embryo quality was evaluated using a 1–4 scale (Kubisch et al., 1998; Larson et al., 2001), while embryo development was assessed using an expanded classification scale that has been previously described by Kimura et al. (2005): 1 = early morula; 2 = compact morula; 3 = early blastocyst; 4 = mid blastocyst; 5 = expanded blastocyst <2 times the normal diameter of normal embryo; 6 = expanded blastocyst >2 times the normal diameter; 7 = hatching blastocyst; and 8 = hatched blastocyst.
At 72 hr post-insemination (Day 3), 8-cell embryos were cultured in 25 μL drops (n = 25 embryos per drop) of mSOFaa containing 0, 1.5, or 4 mM of D-glucose at 39°C under mineral oil in 5% CO2, 5% O2, 90% N2 until 192 hr post-insemination (Day8). At 120 hr post-insemination (Day 5), the development and quality of morula-stage embryos were recorded. Morulae were randomly transferred into new drops containing 0, 1.5, or 4 mM of D-glucose supplemented with one of three DNP concentrations (0, 10, or 100 μM). At 19 2hr post-insemination, embryo development and quality were again assessed prior to collection and freezing of embryos for sex determination.
Individual Embryo Culture for the Analysis of IFNT
Following developmental and quality scoring at 192 hr post-insemination, a cohort of grade-1 to −3 embryos were individually cultured in a 55-μL drop of buffalo rat liver-conditioned TCM 199 media supplemented with 10% FBS at 39°C and 5% CO2, 5% O2, 90% N2. After 24 hr and 48 hr, embryos were re-assessed for developmental stage and quality, and the media collected to assess IFNT concentration at each time point. At the end of the 48 hr culture, embryos were snap frozen at −80°C for later sex determination.
Embryo culture medium was analyzed for antiviral activity via a cytopathic reduction assay (n = 13) involving Madin-Darby Bovine Kidney (MDBK) cells challenged with a vesicular stomatitis virus (Hernandez-Ledezma et al., 1992). The standard used was recombinant bovine IFNT1A with a known antiviral activity (7.5 × 107 IU mg−1) (Alexenko et al., 2000) that had been standardized against human IFNA (PBL Biomedical Laboratories; Piscataway, NJ). The assay protocol used has previously been described in detail (Larson et al., 2001).
Embryo Sexing
Preimplantation embryo DNA was extracted in 5 μL of lysis buffer (20 mM Tris-HCl, 0.9% Tween-20, 0.9% NP-40, pH 7.5 with Proteinase K at a final concentration of 0.4 mg/mL) (Canseco et al., 1994) at 55°C for 30 min followed by 10 min at 98°C. Sexing was performed by PCR amplification of a Y-specific DNA sequence (Peura et al., 1991), as described by Larson et al. (2001). Non-sex-specific bovine satellite primers (Peura et al., 1991) were included in the reaction mixture to ensure that amplification was successful. Bovine male and female genomic DNA (20 pg), as well as water samples were included as controls and treated exactly the same as embryonic DNA. Amplicons were visualized by electrophoresis on a 4% agarose gel. Amplification of bovine DNA by satellite oligonucleotides yielded a 216-bp band, and the Y-specific oligonucleotides yielded a 301-bp band.
Statistical Analyses
IFNT antiviral values were natural-log transformed to reduce variability. Embryo development rates and blastocyst developmental stage, as well as quality in each replicate, were arcsine transformed prior to analysis. A generalized linear mixed model followed by Tukey’s post-hoc comparison was used to investigate the effect of treatment (both DNP and glucose concentrations) and sex on development, quality, and IFNT production of embryos. Interactions between the main effects were also examined with culture replicate included as a random factor in the model. All analyses were run using the Proc Mix Glimmix procedure of SAS software version 9.1 (SAS institute, Cary, NC). Sex ratio (proportion that were male) was compared, with an expected 1:1 ratio by a corrected χ2 procedure as well as by binomial analysis. Results are presented as the least squared mean ± standard error of the mean, unless otherwise stated. Significance was determined at the level of P < 0.05.
ACKNOWLEDGMENTS
The authors thank Select Sires (11740 U.S. 42, P.O. Box 143, Plain City, OH 43064-0143), for donation of frozen semen. The research was supported by a grant from the USDA/CSREES/NRI (grant number: 2001-35203-10693) and the NIH (grant number: HD21896) awarded to RMR. Additional salary support was provided by Scientific Research/JSPS (grant number: 16780208) to KK and a Life Sciences Molecular Biology Fellowship, University of Missouri (grant number: 45211) to MPG.
Grant sponsor: USDA/CSREES/NRI; Grant number: 2001-35203-10693; Grant sponsor: NIH; Grant number: HD21896; Grant sponsor: Scientific Research/JSPS; Grant number: 16780208; Grant sponsor: Life Sciences Molecular Biology Fellowship, University of Missouri; Grant number: 45211
Abbreviations:
- ATP
adenosine triphosphate
- DNP
2,4 dinitrophenol
- G6PDH
glucose 6-phosphate dehydrogenase
- IFNT
interferon tau
- NAD[P]H
nicotinamide adenine dinucleotide [phosphate]
- OXPHOS
oxidative phosphorylation
- ROS
reactive oxygen species
REFERENCES
- Absalon-Medina VA, Butler WR, Gilbert RO. 2014. Preimplantation embryo metabolism and culture systems: Experience from domestic animals and clinical implications. J Assist Reprod Genet 31:393–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexenko AP, Ealy AD, Bixby JA, Roberts RM. 2000. A classification for the interferon-tau. J Interferon Cytokine Res 20:817–822. [DOI] [PubMed] [Google Scholar]
- Alexenko AP, Mao J, Ellersieck MR, Davis AM, Whyte JJ, Rosenfeld CS, Roberts RM. 2007. The contrasting effects of ad libitum and restricted feeding of a diet very high in saturated fats on sex ratio and metabolic hormones in mice. Biol Reprod 77:599–604. [DOI] [PubMed] [Google Scholar]
- Barcelo-Fimbres M, Seidel GE Jr. 2007. Effects of either glucose or fructose and metabolic regulators on bovine embryo development and lipid accumulation in vitro. Mol Reprod Dev 74: 1406–1418. [DOI] [PubMed] [Google Scholar]
- Bermejo-Alvarez P, Rizos D, Lonergan P, Gutierrez-Adan A. 2011a. Transcriptional sexual dimorphism during preimplantation embryo development and its consequences for developmental competence and adult health and disease. Reproduction 141:563–570. [DOI] [PubMed] [Google Scholar]
- Bermejo-Alvarez P, Rizos D, Lonergan P, Gutierrez-Adan A. 2011b. Transcriptional sexual dimorphism in elongating bovine embryos: Implications for XCI and sex determination genes. Reproduction 141:801–808. [DOI] [PubMed] [Google Scholar]
- Bermejo-Alvarez P, Rizos D, Rath D, Lonergan P, Gutierrez-Adan A. 2008. Epigenetic differences between male and female bovine blastocysts produced in vitro. Physiol Genomics 32: 264–272. [DOI] [PubMed] [Google Scholar]
- Bermejo-Alvarez P, Rizos D, Rath D, Lonergan P, Gutierrez-Adan A. 2010. Sex determines the expression level of one third of the actively expressed genes in bovine blastocysts. Proc Natl Acad Sci USA 107:3394–3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermejo-Alvarez P, Roberts RM, Rosenfeld CS. 2012. Effect of glucose concentration during in vitro culture of mouse embryos on development to blastocyst, success of embryo transfer, and litter sex ratio. Mol Reprod Dev 79:329–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolini M, Mason JB, Beam SW, Carneiro GF, Sween ML, Kominek DJ, Moyer AL, Famula TR, Sainz RD, Anderson GB. 2002. Morphology and morphometry of in vivo- and in vitro-produced bovine concepti from early pregnancy to term and association with high birth weights. Theriogenology 58: 973–994. [DOI] [PubMed] [Google Scholar]
- Bredbacka K, Bredbacka P. 1996. Glucose controls sex-related growth rate differences of bovine embryos produced in vitro. J Reprod Fertil 106:169–172. [DOI] [PubMed] [Google Scholar]
- Canseco RS, Sparks AE, Page RL, Russell CG, Johnson JL, Velander WH, Pearson RE, Drohan WN, Gwazdauskas FC. 1994. Gene transfer efficiency during gestation and the influence of co-transfer of non-manipulated embryos on production of transgenic mice. Transgenic Res 3:20–25. [DOI] [PubMed] [Google Scholar]
- Carlson D, Black DL, Howe GR. 1970. Oviduct secretion in the cow. J Reprod Fertil 22:549–552. [DOI] [PubMed] [Google Scholar]
- De La Fuente R, Hahnel A, Basrur PK, King WA. 1999. X inactive-specific transcript (Xist) expression and X chromosome inactivation in the preattachment bovine embryo. Biol Reprod 60:769–775. [DOI] [PubMed] [Google Scholar]
- De La Torre-Sanchez JF, Gardner DK, Preis K, Gibbons J, Seidel GEJ. 2006a. Metabolic regulation of in vitro-produced bovine embryos. II. Effects of phenazine ethosulfate, sodium azide and 2,4-dinitrophenol during post-compaction development on glucose metabolism and lipid accumulation. Reprod Fertil Dev 18:597–607. [DOI] [PubMed] [Google Scholar]
- De La Torre-Sanchez JF, Preis K, Seidel GEJ. 2006b. Metabolic regulation of in-vitro-produced bovine embryos. I. Effects of metabolic regulators at different glucose concentrations with embryos produced by semen from different bulls. Reprod Fertil Dev 18:585–596. [DOI] [PubMed] [Google Scholar]
- Dupont C, Gribnau J. 2013. Different flavors of X-chromosome inactivation in mammals. Curr Opin Cell Biol 25:314–321. [DOI] [PubMed] [Google Scholar]
- Gardner DK, Harvey AJ. 2015. Blastocyst metabolism. Reprod Fertil Dev 27:638–654. doi: 10.1071/rd14421 [DOI] [PubMed] [Google Scholar]
- Green MP, Hunter MG, Mann GE. 2005. Relationships between maternal hormone secretion and embryo development on day 5 of pregnancy in dairy cows. Anim Reprod Sci 88:179–189. [DOI] [PubMed] [Google Scholar]
- Green MP, Spate LD, Parks TE, Kimura K, Murphy CN, Williams JE, Kerley MS, Green JA, Keisler DH, Roberts RM. 2008. Nutritional skewing of conceptus sex in sheep: Effects of a maternal diet enriched in rumen-protected polyunsaturated fatty acids (PUFA). Reprod Biol Endocrinol 6:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez-Adan A, Granados J, Pintado B, De La Fuente J. 2001. Influence of glucose on the sex ratio of bovine IVM/IVF embryos cultured in vitro. Reprod Fertil Dev 13:361–365. [DOI] [PubMed] [Google Scholar]
- Gutierrez-Adan A, Oter M, Martinez-Madrid B, Pintado B, De La Fuente J. 2000. Differential expression of two genes located on the X chromosome between male and female in vitro-produced bovine embryos at the blastocyst stage. Mol Reprod Dev 55:146–151. [DOI] [PubMed] [Google Scholar]
- Harvey AJ, Kind KL, Pantaleon M, Armstrong DT, Thompson JG. 2004. Oxygen-regulated gene expression in bovine blastocysts. Biol Reprod 71:1108–1119. [DOI] [PubMed] [Google Scholar]
- Harvey AJ, Kind KL, Thompson JG. 2002. REDOX regulation of early embryo development. Reproduction 123:479–486. [DOI] [PubMed] [Google Scholar]
- Hernandez-Ledezma JJ, Sikes JD, Murphy CN, Watson AJ, Schultz GA, Roberts RM. 1992. Expression of bovine trophoblast interferon in conceptuses derived by in vitro techniques. Biol Reprod 47:374–380. [DOI] [PubMed] [Google Scholar]
- Hewitson LC, Martin KL, Leese HJ. 1996. Effects of metabolic inhibitors on mouse preimplantation embryo development and the energy metabolism of isolated inner cell masses. Mol Reprod Dev 43:323–330. [DOI] [PubMed] [Google Scholar]
- Hoffmann A, Gloe T, Pohl U. 2001. Hypoxia-induced upregulation of eNOS gene expression is redox-sensitive: A comparison between hypoxia and inhibitors of cell metabolism. J Cell Physiol 188:33–44. [DOI] [PubMed] [Google Scholar]
- Iwata H, Akamatsu S, Minami N, Yamada M. 1998. Effects of antioxidants on the development of bovine IVM/IVF embryos in various concentrations of glucose. Theriogenology 50: 365–375. [DOI] [PubMed] [Google Scholar]
- Kimura K, Iwata H, Thompson JG. 2008. The effect of glucosamine concentration on the development and sex ratio of bovine embryos. Anim Reprod Sci 103:228–238. [DOI] [PubMed] [Google Scholar]
- Kimura K, Spate LD, Green MP, Roberts RM. 2005. Effects of D-glucose concentration, D-fructose, and inhibitors of enzymes of the pentose phosphate pathway on the development and sex ratio of bovine blastocysts. Mol Reprod Dev 72:201–207. [DOI] [PubMed] [Google Scholar]
- Kimura K, Spate LD, Green MP, Murphy CN, Seidel GE Jr., Roberts RM 2004a. Sexual dimorphism in interferon-tau production by in vivo-derived bovine embryos. Mol Reprod Dev 67:193–199. [DOI] [PubMed] [Google Scholar]
- Kimura K, Spate LD, Green MP, Roberts RM. 2004b. Effects of oxidative stress and inhibitors of the pentose phosphate pathway on sexually dimorphic production of IFN-tau by bovine blastocysts. Mol Reprod Dev 68:88–95. [DOI] [PubMed] [Google Scholar]
- King WA, Yadav BR, Xu KP, Picard L, Sirard MA, Verini Supplizi A, Betteridge KJ. 1991. The sex ratios of bovine embryos produced in vivo and in vitro. Theriogenology 36:779–788. [DOI] [PubMed] [Google Scholar]
- Korshunov SS, Skulachev VP, Starkov AA. 1997. High protonic potential actuates a mechanism of production of reactive oxygen species in mitochondria. FEBS Lett 416:15–18. [DOI] [PubMed] [Google Scholar]
- Krisher RL, Heuberger AL, Paczkowski M, Stevens J, Pospisil C, Prather RS, Sturmey RG, Herrick JR, Schoolcraft WB. 2015. Applying metabolomic analyses to the practice of embryology: Physiology, development and assisted reproductive technology. Reprod Fertil Dev 27:602–620. doi: 10.1071/rd14359 [DOI] [PubMed] [Google Scholar]
- Kubisch HM, Larson MA, Ealy AD, Murphy CN, Roberts RM. 2001. Genetic and environmental determinants of interferon-tau secretion by in vivo- and in vitro-derived bovine blastocysts. Anim Reprod Sci 66:1–13. [DOI] [PubMed] [Google Scholar]
- Kubisch HM, Larson MA, Roberts RM. 1998. Relationship between age of blastocyst formation and interferon-tau secretion by in vitro-derived bovine embryos. Mol Reprod Dev 49: 254–260. [DOI] [PubMed] [Google Scholar]
- Kubisch HM, Sirisathien S, Bosch P, Hernandez-Fonseca HJ, Clements G, Liukkonen JR, Brackett BG. 2004. Effects of developmental stage, embryonic interferon-tau secretion and recipient synchrony on pregnancy rate after transfer of in vitro produced bovine blastocysts. Reprod Domest Anim 39: 120–124. [DOI] [PubMed] [Google Scholar]
- Lane M, Gardner DK. 1996. Selection of viable mouse blastocysts prior to transfer using a metabolic criterion. Human Reprod 11:1975–1978. [DOI] [PubMed] [Google Scholar]
- Larson MA, Kimura K, Kubisch HM, Roberts RM. 2001. Sexual dimorphism among bovine embryos in their ability to make the transition to expanded blastocyst and in the expression of the signaling molecule IFN-tau. Proc Natl Acad Sci USA 98:9677–9682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leverve X, Sibille B, Devin A, Piquet MA, Espie P, Rigoulet M. 1998. Oxidative phosphorylation in intact hepatocytes: Quantitative characterization of the mechanisms of change in efficiency and cellular consequences. Mol Cell Biochem 184:53–65. [PubMed] [Google Scholar]
- Lonergan P, Rizos D, Gutierrez-Adan A, Moreira PM, Pintado B, de la Fuente J, Boland MP. 2003. Temporal divergence in the pattern of messenger RNA expression in bovine embryos cultured from the zygote to blastocyst stage in vitro or in vivo. Biol Reprod 69:1424–1431. [DOI] [PubMed] [Google Scholar]
- Machaty Z, Thompson JG, Abeydeera LR, Day BN, Prather RS. 2001. Inhibitors of mitochondrial ATP production at the time of compaction improve development of in vitro produced porcine embryos. Mol Reprod Dev 58:39–44. [DOI] [PubMed] [Google Scholar]
- Neira JA, Tainturier D, L’Haridon RM, Martal J. 2007. Comparative IFN-tau secretion after hatching by bovine blastocysts derived ex vivo and completely produced in vitro. Reprod Domest Anim 42:68–75. [DOI] [PubMed] [Google Scholar]
- Nino-Soto MI, Basrur PK, King WA. 2007. Impact of in vitro production techniques on the expression of X-linked genes in bovine (bos taurus) oocytes and pre-attachment embryos. Mol Reprod Dev 74:144–153. [DOI] [PubMed] [Google Scholar]
- O’Fallon JV, Wright RW Jr. 1986. Quantitative determination of the pentose phosphate pathway in preimplantation mouse embryos. Biology Reprod 34:58–64. [DOI] [PubMed] [Google Scholar]
- Okuda M, Lee HC, Kumar C, Chance B. 1992. Comparison of the effect of a mitochondrial uncoupler, 2,4-dinitrophenol and adrenaline on oxygen radical production in the isolated perfused rat liver. Acta Physiol Scand 145:159–168. [DOI] [PubMed] [Google Scholar]
- Peippo J, Farazmand A, Kurkilahti M, Markkula M, Basrur PK, King WA. 2002. Sex-chromosome linked gene expression in in-vitro produced bovine embryos. Mol Hum Reprod 8:923–929. [DOI] [PubMed] [Google Scholar]
- Peippo J, Kurkilahti M, Bredbacka P. 2001. Developmental kinetics of in vitro produced bovine embryos: The effect of sex, glucose and exposure to time-lapse environment. Zygote 9:105–113. [DOI] [PubMed] [Google Scholar]
- Peura T, Hyttinen JM, Turunen M, Janne J. 1991. A reliable sex determination assay for bovine preimplantation embryos using the polymerase chain reaction. Theriogenology 35:547–555. [DOI] [PubMed] [Google Scholar]
- Rex A, Pfeifer L, Fink F, Fink H. 1999. Cortical NADH during pharmacological manipulations of the respiratory chain and spreading depression in vivo. J Neurosci Res 57:359–370. [PubMed] [Google Scholar]
- Rieger D, McGowan LT, Cox SF, Pugh PA, Thompson JG. 2002. Effect of 2,4-dinitrophenol on the energy metabolism of cattle embryos produced by in vitro fertilization and culture. Reprod Fertil Dev 14:339–343. [DOI] [PubMed] [Google Scholar]
- Rizos D, Fair T, Papadopoulos S, Boland MP, Lonergan P. 2002a. Developmental, qualitative, and ultrastructural differences between ovine and bovine embryos produced in vivo or in vitro. Mol Reprod Dev 62:320–327. [DOI] [PubMed] [Google Scholar]
- Rizos D, Lonergan P, Boland MP, Arroyo-Garcia R, Pintado B, de la Fuente J, Gutierrez-Adan A. 2002b. Analysis of differential messenger RNA expression between bovine blastocysts produced in different culture systems: Implications for blastocyst quality. Biol Reprod 66:589–595. [DOI] [PubMed] [Google Scholar]
- Rosenfeld CS, Grimm KM, Livingston KA, Brokman AM, Lamberson WE, Roberts RM. 2003. Striking variation in the sex ratio of pups born to mice according to whether maternal diet is high in fat or carbohydrate. Proc Natl Acad Sci USA 100:4628–4632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld CS, Roberts RM. 2004. Maternal diet and other factors affecting offspring sex ratio: A review. Biol Reprod 71: 1063–1070. [DOI] [PubMed] [Google Scholar]
- Ryan AM, Womack JE. 1993. Type I interferon genes in cattle: Restriction fragment length polymorphisms, gene numbers and physical organization on bovine chromosome 8. Anim Genet 24:9–16. [DOI] [PubMed] [Google Scholar]
- Shafi R, Iyer SP, Ellies LG, O’Donnell N, Marek KW, Chui D, Hart GW, Marth JD. 2000. The O-GlcNAc transferase gene resides on the X chromosome and is essential for embryonic stem cell viability and mouse ontogeny. Proc Natl Acad Sci USA 97:5735–5739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibille B, Keriel C, Fontaine E, Catelloni F, Rigoulet M, Leverve XM. 1995. Octanoate affects 2,4-dinitrophenol uncoupling in intact isolated rat hepatocytes. Eur J Biochem 231:498–502. [DOI] [PubMed] [Google Scholar]
- Stojkovic M, Buttner M, Zakhartchenko V, Riedl J, Reichenbach HD, Wenigerkind H, Brem G, Wolf E. 1999. Secretion of interferon-tau by bovine embryos in long-term culture: Comparison of in vivo derived, in vitro produced, nuclear transfer and demiembryos. Anim Reprod Sci 55:151–162. [DOI] [PubMed] [Google Scholar]
- Takahashi Y, First NL. 1992. In vitro development of bovine one-cell embryos: Influence of glucose, lactate, pyruvate, amino acids and vitamins. Theriogenology 37:963–978. [DOI] [PubMed] [Google Scholar]
- Thompson JG. 2000. In vitro culture and embryo metabolism of cattle and sheep embryos—A decade of achievement. Anim Reprod Sci 60–61:263–275. [DOI] [PubMed] [Google Scholar]
- Thompson JG, McNaughton C, Gasparrini B, McGowan LT, Tervit HR. 2000. Effect of inhibitors and uncouplers of oxidative phosphorylation during compaction and blastulation of bovine embryos cultured in vitro. J Reprod Fertil 118:47–55. [PubMed] [Google Scholar]
- Tiffin GJ, Rieger D, Betteridge KJ, Yadav BR, King WA. 1991a. Glucose and glutamine metabolism in pre-attachment cattle embryos in relation to sex and stage of development. J Reprod Fertil 93:125–132. [DOI] [PubMed] [Google Scholar]
- Tiffin GJ, Rieger D, Betteridge KJ, Yadav BR, King WA. 1991b. Measurement of the activity of the pentose phosphate pathway in sexed bovine embryos. Theriogenology 33:339. [Google Scholar]
- Wrenzycki C, Herrmann D, Keskintepe L, Martins A Jr., Sirisathien S, Brackett B, Niemann H 2001. Effects of culture system and protein supplementation on mRNA expression in pre-implantation bovine embryos. Human Reprod 16:893–901. [DOI] [PubMed] [Google Scholar]
- Wrenzycki C, Lucas-Hahn A, Herrmann D, Lemme E, Korsawe K, Niemann H. 2002. In vitro production and nuclear transfer affect dosage compensation of the X-linked gene transcripts G6PD, PGK, and Xist in preimplantation bovine embryos. Biol Reprod 66:127–134. [DOI] [PubMed] [Google Scholar]


